Submitted:
24 April 2025
Posted:
25 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. The Yogi (Subject)
2.2. Experimental Timeline
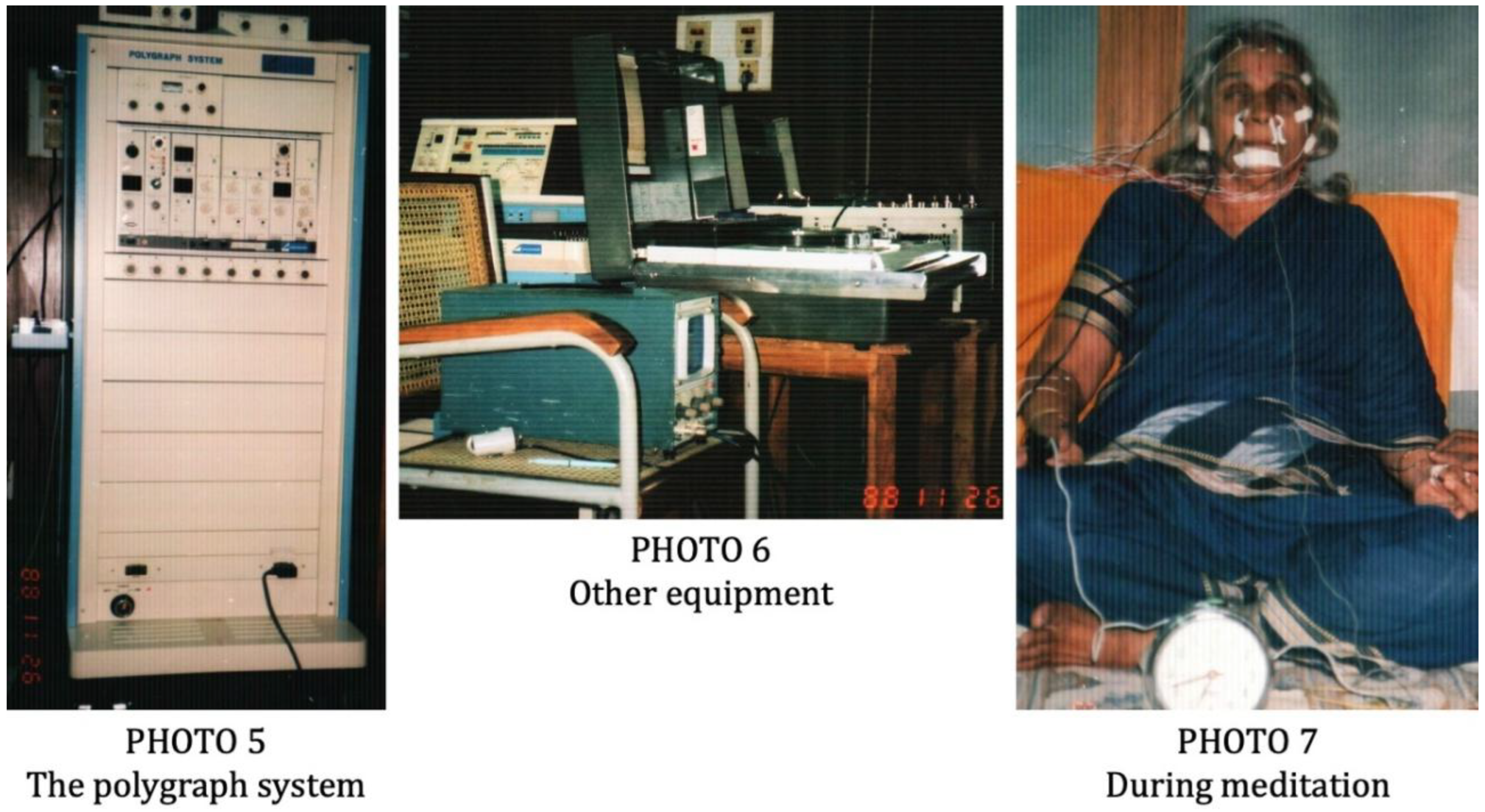
2.3. Instruments Used and Signals Recorded
2.4. Methodological Details of the Experiments
2.4.1. Experiment-1 (1985)
4.2.2. Experiment-2 (1988)
3. Results and Discussion
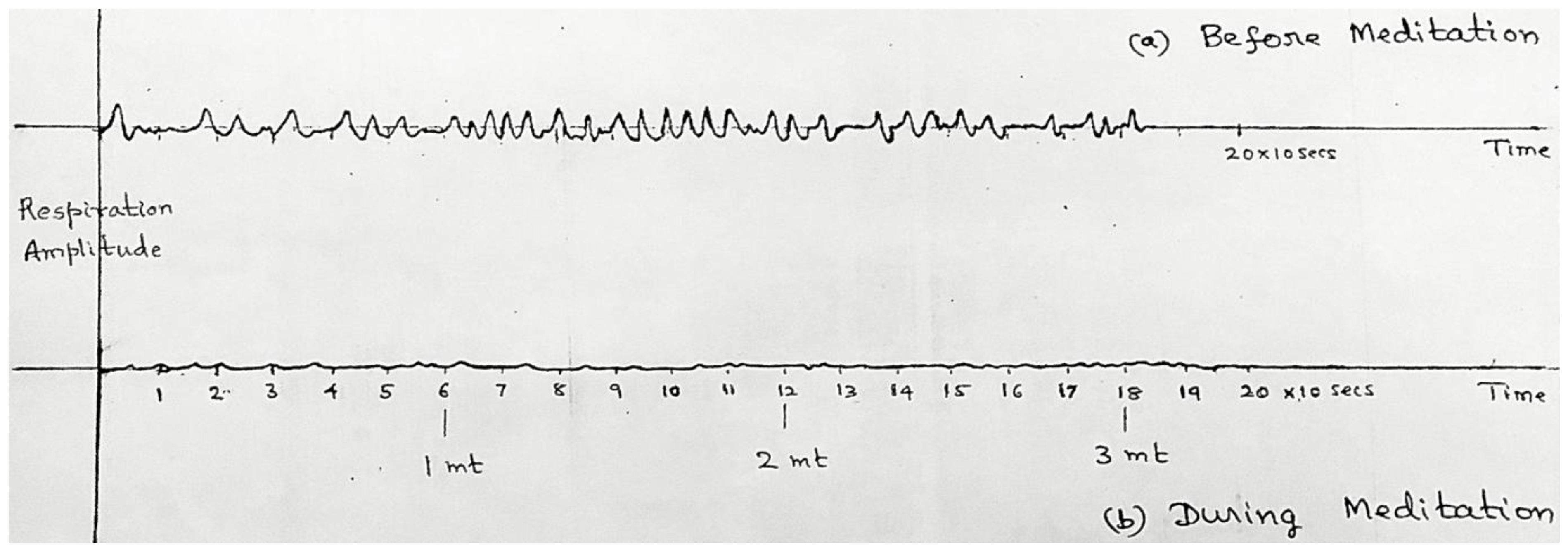
3.1. Respiration Results (Both Experiments)
3.2. Electrical Activity of the Muscles (EMG Results) (Both Experiments)
3.3. Electrical Activity of the Heart (ECG Results) (Both Experiments)
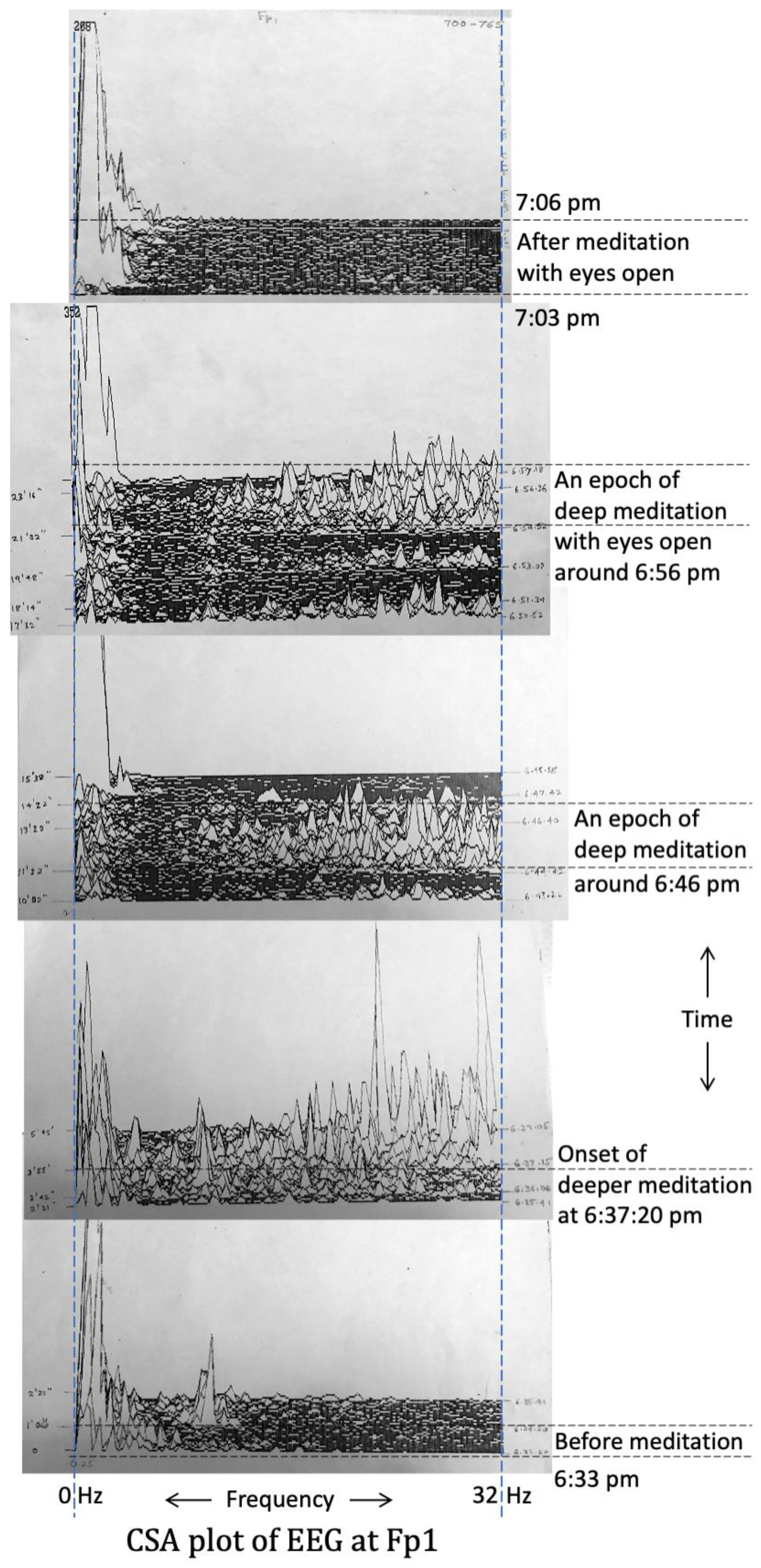
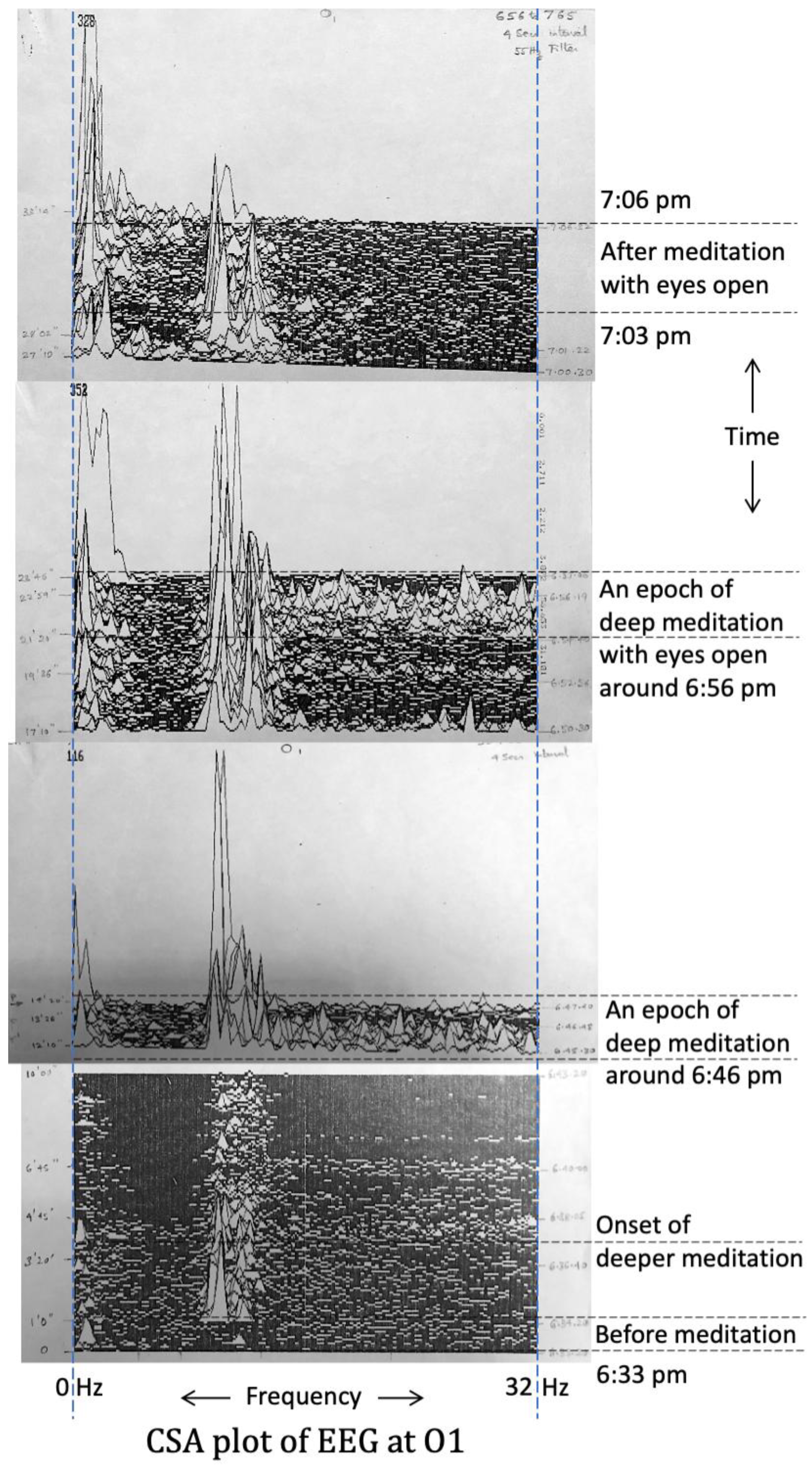
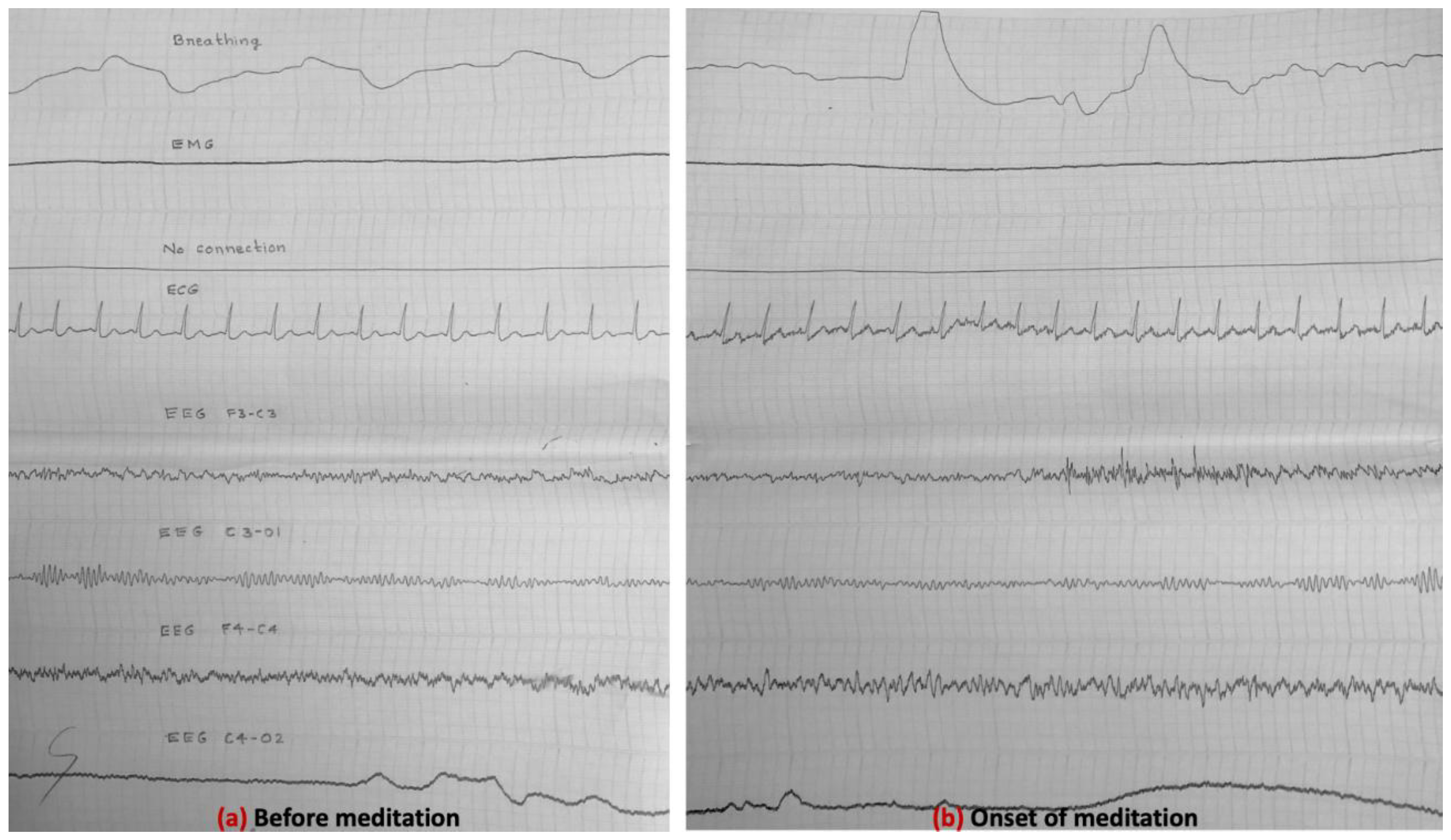
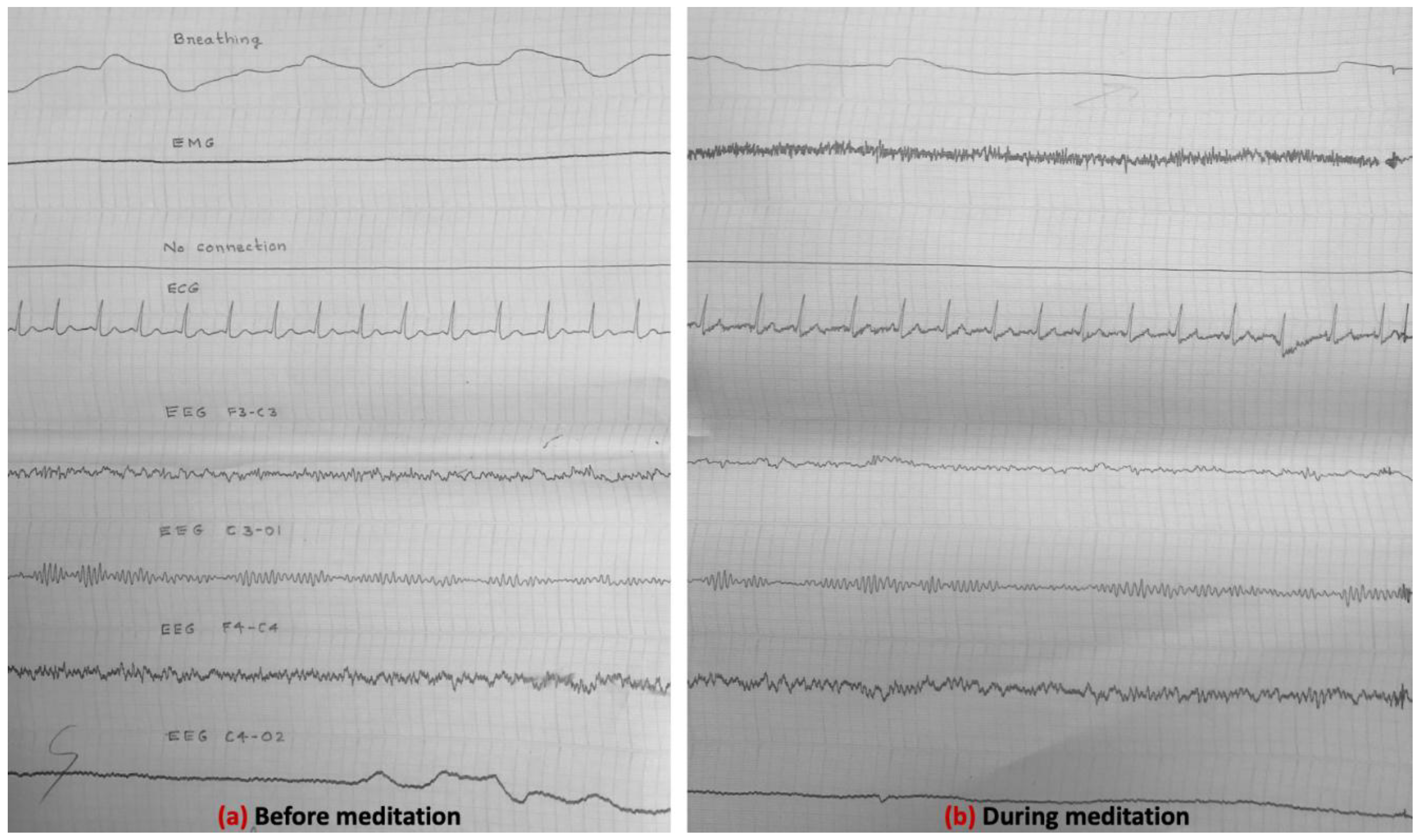
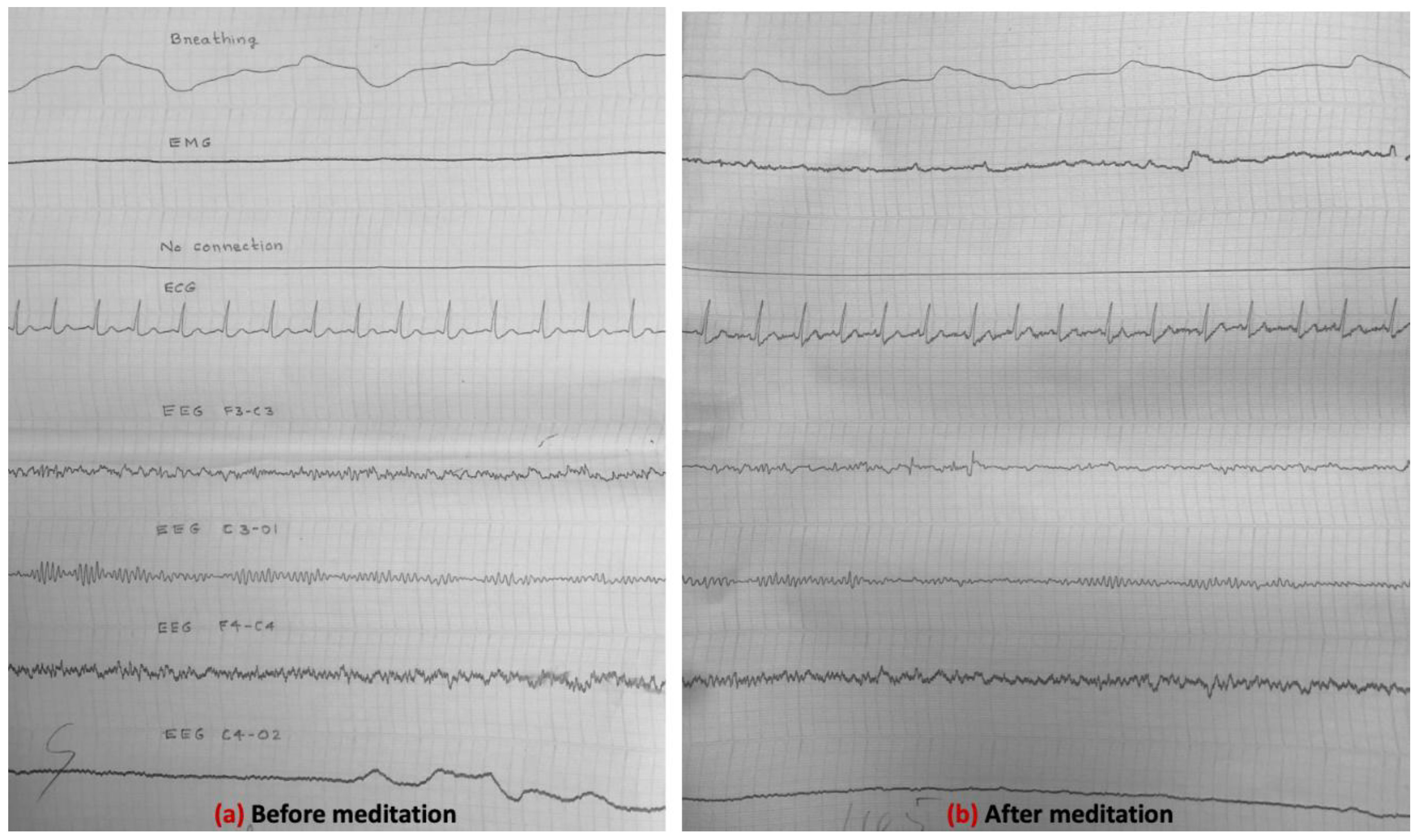
3.4. Electrical Activity of the Brain (EEG Results) (Experiment-1, 1985)
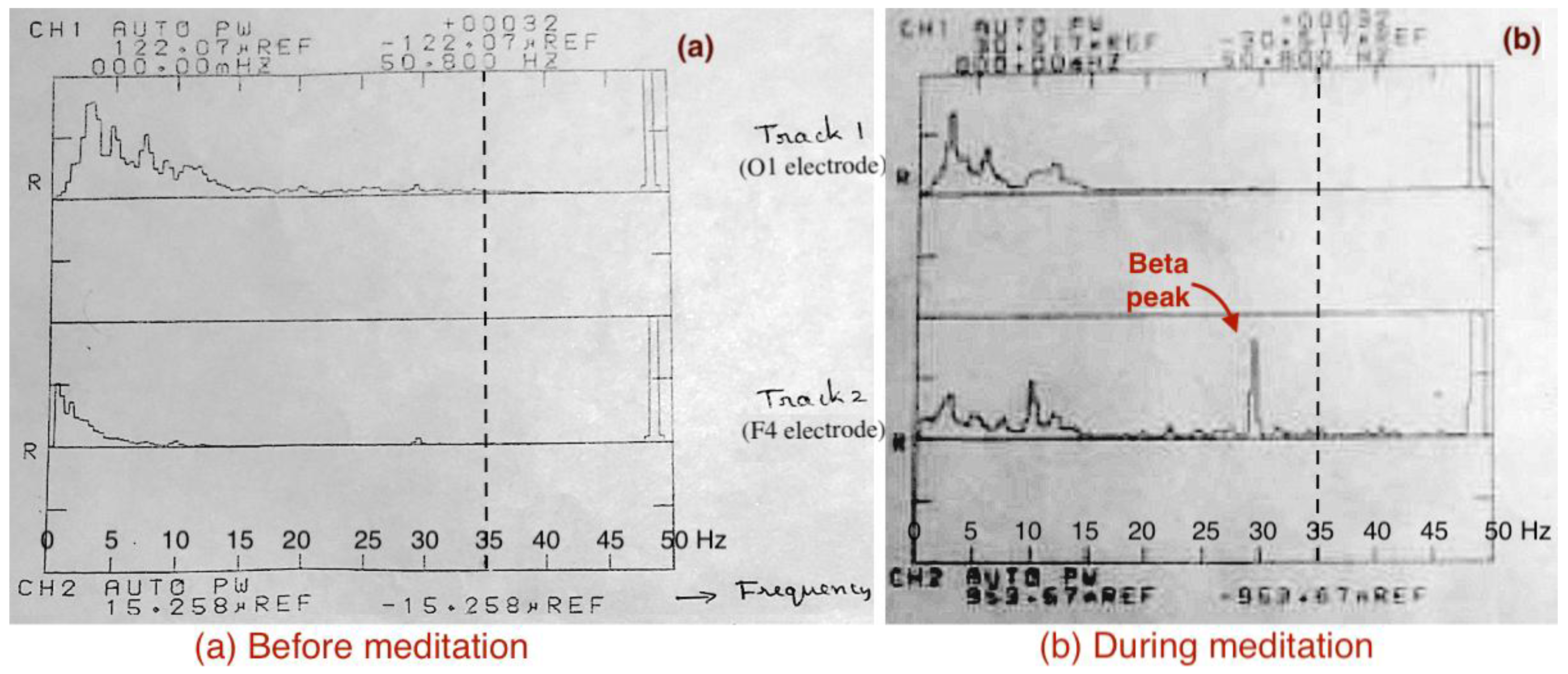
3.5. EEG Results (Experiment-2, 1988)
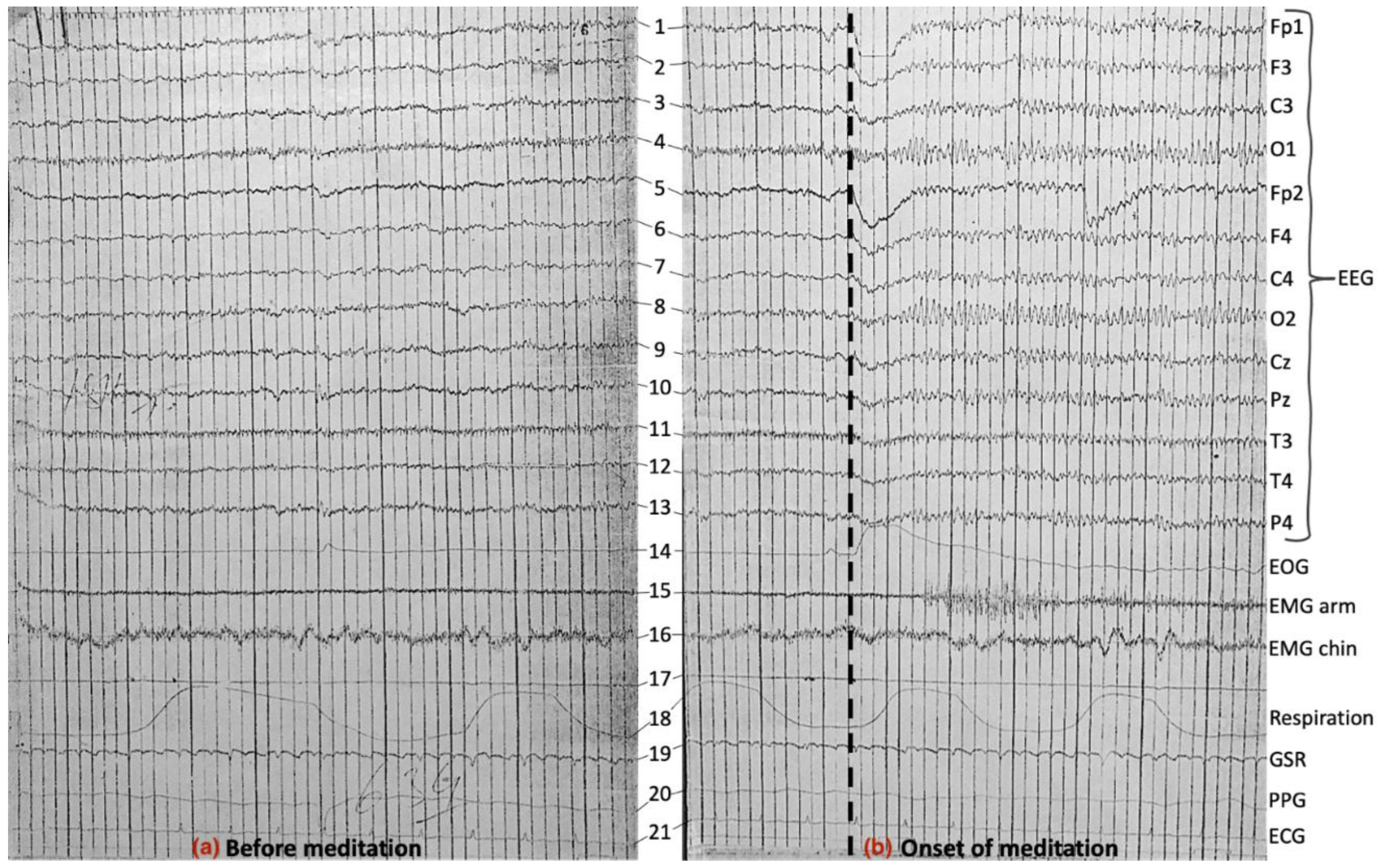
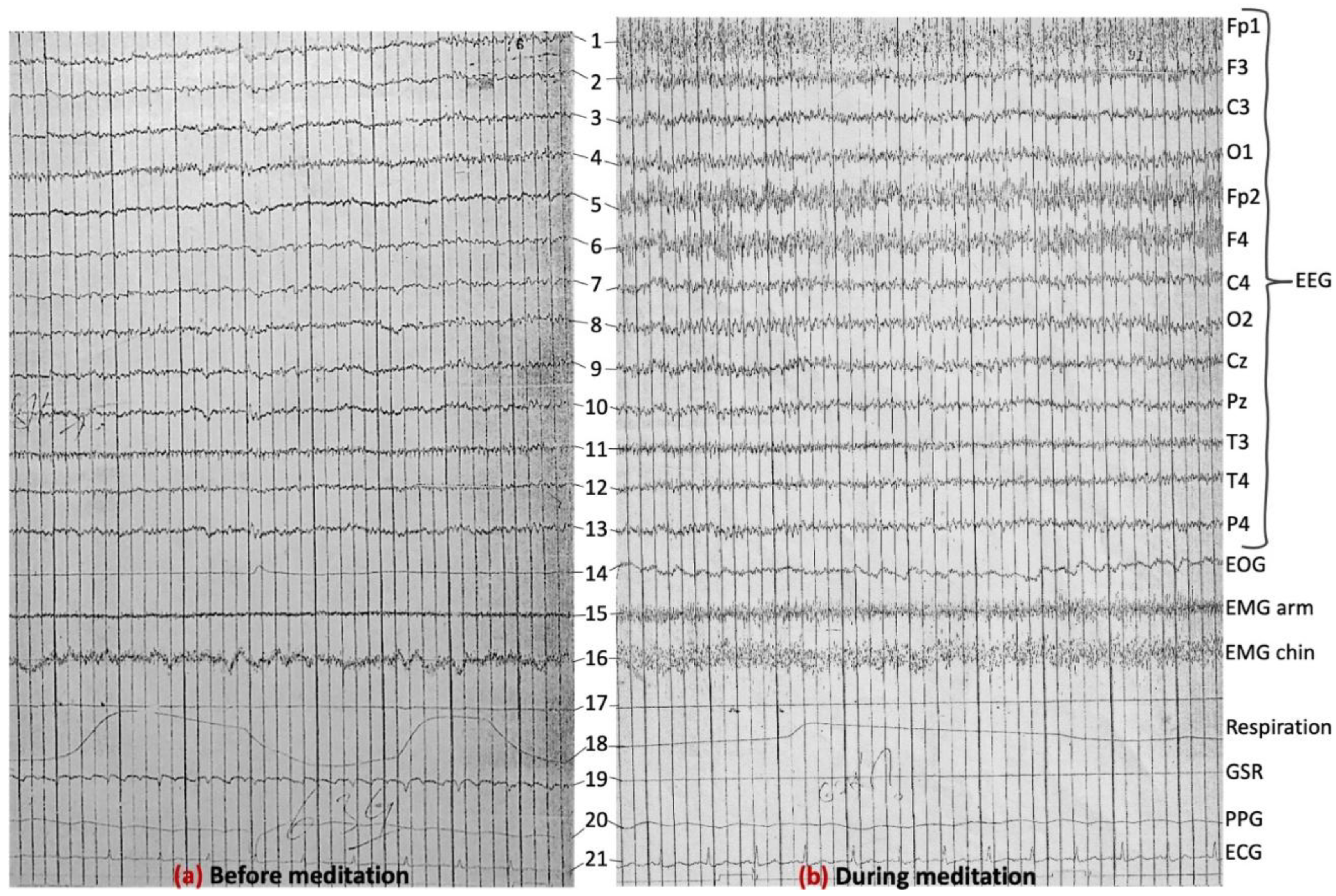
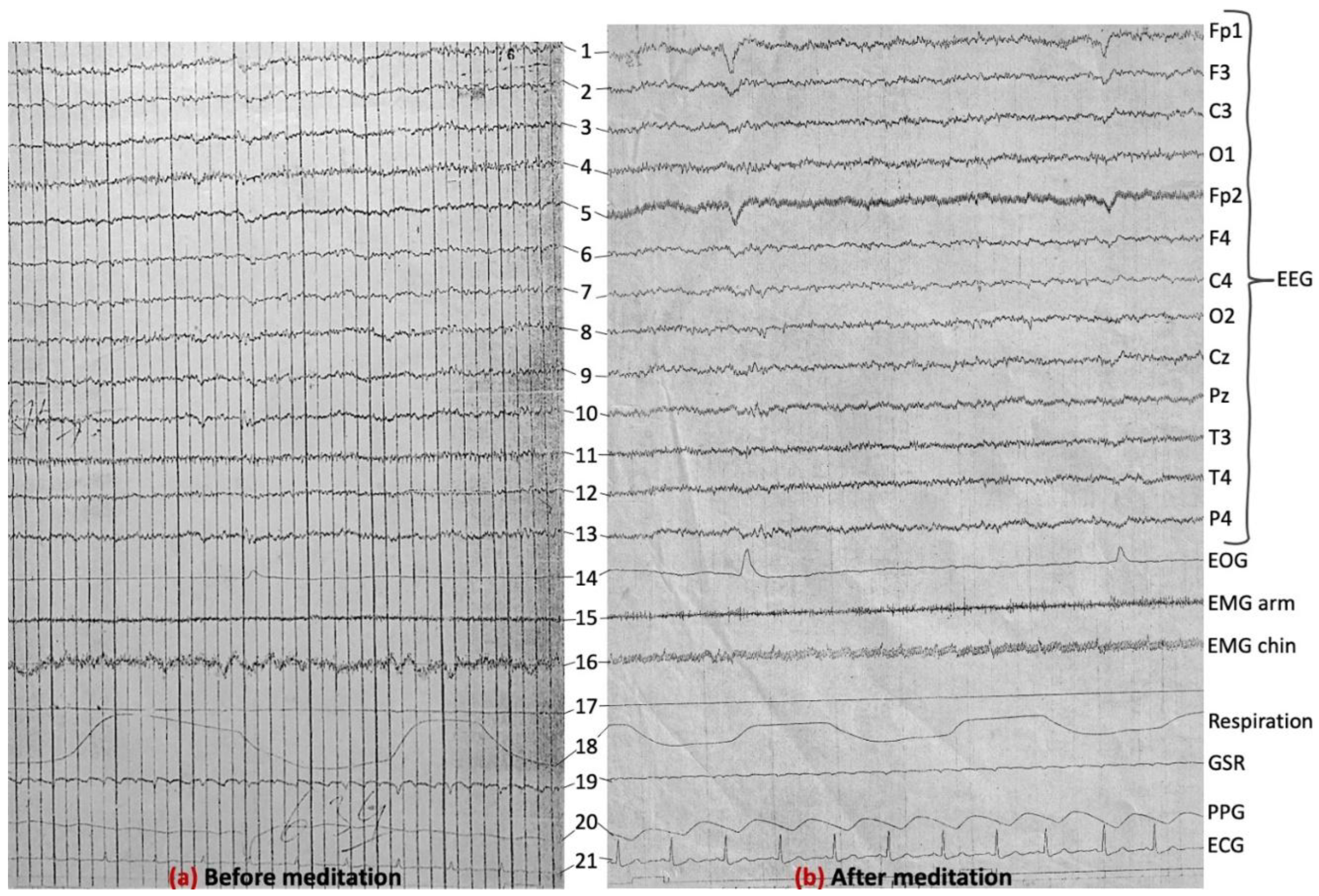
3.6. Further Discussion and Scope for Future Work
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Historical Context
Appendix A.2. Details of the Signals Recorded
References
- S. H. Aranya, Introduction: Yoga Philosophy of Patanjali with Bhasvati, Calcutta, India: University of Calcutta, 2000, pp. xxiii-xxiv.
- J. Mallinson and M. Singleton, Roots of Yoga, London, UK: Penguin Books, 2017.
- R. E. Hume, The Thirteen Principal Upanishads, Oxford, UK: Oxford University Press, 1921. [CrossRef]
- F. Tola, C. Dragonetti and K. D. Prithipaul, The Yogasūtras of Patañjali on concentration of mind, Delhi, India: Motilal Banarsidass, 1987.
- S. V. Bharati, Yoga Sutras of Patanjali: With the Exposition of Vyasa, Delhi, India: Motilal Banarsidas, 2001.
- S. Mahaguru, Amaravani - A compilation of the lectures of Sriranga Mahaguru on Ayurveda-Yoga-NadiShastra: Volume 6 (in Kannada language), Mysore, India: Ashtanga Yoga Vijnana Mandiram, 1988.
- M. Washburn, The Ego and the Dynamic Ground: A Transpersonal Theory of Human Development, Albany, NY, USA: State University of New York Press, 1988.
- G. Maehle, Ashtanga Yoga: Practice and Philosophy, Novato, CA, USA: New World Library, 2007.
- S. D. Goswami, Readings in Vedic Literature: The Tradition Speaks for Itself, Mumbai, India: Bhaktivedanta Book Trust, 1976.
- P. Yogananda, Autobiography of a Yogi, Los Angeles, CA, USA: Self-Realization Fellowship, 1946.
- S. Vivekananda, Complete Works of Swami Vivekananda, Hollywood, CA, USA: Vedanta Press, 1922.
- S. Rama, Living with the Himalayan Masters, Honesdale, PA, USA: Himalayan Institute Press, 1978.
- Sivarupa, Yogis of India: Timeless Stories of Their Lives and Wisdom, Delhi, India: Wisdom Tree Publishers, 2012.
- B. Ramamurthi, "The fourth state of consciousness: the Thuriya Avastha.," Psychiatry and clinical neurosciences, vol. 49, no. 2, p. 107–110, 1995. [CrossRef]
- B. K. Anand, G. S. Chhina and B. Singh, "Studies on Shri Ramanand Yogi during his stay in an air-tight box.," The Indian journal of medical research, vol. 49, p. 1, 1961.
- M. A. Wenger and B. K. Bagchi, "Studies of autonomic functions in practitioners of Yoga in India.," Behavioral science, vol. 6, p. 312–323, 1961. [CrossRef]
- M. A. Wenger, B. K. Bagchi and B. K. Anand, "Experiments in India on "voluntary" control of the heart and pulse.," Circulation, vol. 24, p. 1319–1325, 1961. [CrossRef]
- L. K. Kothari, A. Bardia and O. P. Gupta, "The yogic claim of voluntary control over the heart beat: an unusual demonstration.," American heart journal, vol. 86, no. 2, p. 282–284, 1973. [CrossRef]
- P. Arambula, E. Peper, M. Kawakami and K. H. Gibney, "The physiological correlates of Kundalini Yoga meditation: a study of a yoga master.," Applied psychophysiology and biofeedback, vol. 26, no. 2, p. 147–153, 2001. [CrossRef]
- D. S. Shannahoff-Khalsa, B. B. Sramek, M. B. Kennel and S. W. Jamieson, "Hemodynamic observations on a yogic breathing technique claimed to help eliminate and prevent heart attacks: a pilot study.," Journal of alternative and complementary medicine, vol. 10, no. 5, p. 757–766, 2004.
- R. Kakigi, H. Nakata, K. Inui, N. Hiroe, O. Nagata, M. Honda, S. Tanaka, N. Sadato and M. Kawakami, "Intracerebral pain processing in a Yoga Master who claims not to feel pain during meditation.," European journal of pain, vol. 9, no. 5, p. 581–589, 2005. [CrossRef]
- D. T. Lott, T. Yeshi, N. Norchung, S. Dolma, N. Tsering, N. Jinpa, T. Woser, K. Dorjee, T. Desel, D. Fitch, .. ... and R. J. Davidson, "No Detectable Electroencephalographic Activity After Clinical Declaration of Death Among Tibetan Buddhist Meditators in Apparent Tukdam, a Putative Postmortem Meditation State.," Frontiers in psychology, vol. 11, p. 599190, 2021.
- B. K. Anand, G. S. Chhina and B. Singh, "Some aspects of electroencephalographic studies in Yogis," Electroencephalography and Clinical Neurophysiology, vol. 13, no. 3, pp. 452-456, 1961. [CrossRef]
- S. Jois, The light that lit up for a hundred years: A brief depiction of the divine personality of Sri Vijayalakshmi Srimataji (original published in Kannada language: Nooru Samvatsara Belagida Nandadeepa), Mysore, India: Ashtanga Yoga Vijnana Mandiram, 2019.
- S. V. Chamu, Sri Ranga Mahaguru (in Kannada language), Mysore, India: Ashtanga Yoga Vijnana Mandiram, 1972.
- S. V. Chamu, Sri Ranga Sadguru: A short Biography of a Yogi, Mysore, India: Ashtanga Yoga Vijnana Mandiram, 1992.
- J. K. Cooper, "Electrocardiography 100 years ago. Origins, pioneers, and contributors.," The New England journal of medicine, vol. 315, no. 7, p. 461–464, 1986.
- M. B. Raez, M. S. Hussain and F. Mohd-Yasin, "Techniques of EMG signal analysis: detection, processing, classification and applications.," Biological procedures online, vol. 8, p. 11–35, 2006.
- M. Brown, M. Marmor, Vaegan, E. Zrenner, M. Brigell, M. Bach and ISCEV, "ISCEV Standard for Clinical Electro-oculography (EOG) 2006.," Documenta ophthalmologica. Advances in ophthalmology, vol. 113, no. 3, p. 205–212, 2006. [CrossRef]
- E. Jovanov, D. Raskovic and R. Hormigo, "Thermistor-based breathing sensor for circadian rhythm evaluation.," Biomedical sciences instrumentation, vol. 37, p. 493–497, 2001.
- S. Palva and J. M. Palva, "New vistas for alpha-frequency band oscillations.," Trends in neurosciences, vol. 30, no. 4, p. 150–158, 2007.
- G. Buzsáki, Rhythms of the Brain., New York, NY, USA: Oxford University Press, 2006.
- S. Upadhyaya, Nadi Vijnana: Ancient Pulse Science, New Delhi, India: Chaukhamba Sanskrit Pratishthan, 1986.
- V. D. Lad, Secrets of the Pulse: The Ancient Art of Ayurvedic Pulse Diagnosis, Delhi, India: Motilal Banarsidass Publishing House, 1996.
- T. V. Ananthapadmanabha, Inner workings during yoga practice, Bangalore, India: Anugraha Publishers, 1999.
- P. V. G. Kumar, S. Deshpande and H. R. Nagendra, "Traditional practices and recent advances in Nadi Pariksha: A comprehensive review.," Journal of Ayurveda and integrative medicine, vol. 10, no. 4, p. 308–315, 2019. [CrossRef]
- L. Mishra, B. B. Singh and S. Dagenais, "Healthcare and disease management in Ayurveda.," Alternative therapies in health and medicine, vol. 7, no. 2, p. 44–50, 2001.
- C. S. Hu, Y. F. Chung, C. C. Yeh and C. H. Luo, "Temporal and spatial properties of arterial pulsation measurement using pressure sensor array.," Evidence-based complementary and alternative medicine, p. 745127, 2012. [CrossRef]
- H. Kim, J. Y. Kim, Y. J. Park and Y. B. Park, "Development of pulse diagnostic devices in Korea.," Integrative medicine research, vol. 2, no. 1, p. 7–17, 2013. [CrossRef]
- P. Wang, W. Zuo and D. Zhang, "A Compound Pressure Signal Acquisition System for Multichannel Wrist Pulse Signal Analysis," IEEE Transactions on Instrumentation and Measurement, vol. 63, no. 6, pp. 1556-1565, 2014. [CrossRef]
- D. Rangaprakash and D. Narayana Dutt, "Study of wrist pulse signals using time domain spatial features.," Computers & Electrical Engineering, vol. 45, pp. 100-107, 2015. [CrossRef]
- D. Wang, D. Zhang and G. Lu, "A Novel Multichannel Wrist Pulse System With Different Sensor Arrays.," IEEE Transactions on Instrumentation and Measurement, vol. 64, no. 7, pp. 2020-2034, 2015. [CrossRef]
- Y.-J. Lee, J. Lee and J.-Y. Kim, "A study on characteristics of radial arteries through ultrasonic waves.," Annual International Conference of the IEEE Engineering in Medicine and Biology Society, p. 2453–2456, 2008.
- J. U. Kim, Y. J. Lee, J. Lee and J. Y. Kim, "Differences in the Properties of the Radial Artery between Cun, Guan, Chi, and Nearby Segments Using Ultrasonographic Imaging: A Pilot Study on Arterial Depth, Diameter, and Blood Flow.," Evidence-based complementary and alternative medicine, p. 381634, 2015.
- B. J. Lee, Y. J.B. J. Lee, Y. J. Jeon, B. Ku, J. U. Kim, J. H. Bae and J. Y. Kim, "Association of hypertension with physical factors of wrist pulse waves using a computational approach: a pilot study.," BMC complementary and alternative medicine, vol. 15, p. 222, 2015. [CrossRef]
- W. Zuo, P. Wang and D. Zhang, "Comparison of Three Different Types of Wrist Pulse Signals by Their Physical Meanings and Diagnosis Performance.," IEEE journal of biomedical and health informatics, vol. 20, no. 1, p. 119–127, 2016. [CrossRef]
- A. Joshi, A. Kulkarni, S. Chandran, V. K. Jayaraman and B. D. Kulkarni, "Nadi Tarangini: a pulse based diagnostic system.," Annual International Conference of the IEEE Engineering in Medicine and Biology Society, p. 2207–2210, 2007.
- D. Wang, D. Zhang and G. Lu, "An Optimal Pulse System Design by Multichannel Sensors Fusion.," IEEE journal of biomedical and health informatics, vol. 20, no. 2, p. 450–459, 2016. [CrossRef]
- D. Lin, A. Zhang, J. Gu, X. Chen, Q. Wang, L. Yang, Y. Chou, G. Liu and J. Wang, "Detection of multipoint pulse waves and dynamic 3D pulse shape of the radial artery based on binocular vision theory.," Computer methods and programs in biomedicine, vol. 155, p. 61–73, 2018. [CrossRef]
- C. Chen, Z. Li, Y. Zhang, S. Zhang, J. Hou and H. Zhang, "A 3D Wrist Pulse Signal Acquisition System for Width Information of Pulse Wave.," Sensors (Basel, Switzerland), vol. 20, no. 1, p. 11, 2019. [CrossRef]
- Q. Zhang, J. Zhou and B. Zhang, "Graph Based Multichannel Feature Fusion for Wrist Pulse Diagnosis.," IEEE journal of biomedical and health informatics, vol. 25, no. 10, p. 3732–3743, 2021. [CrossRef]
- Guo, Z. Jiang, H. He, Y. Liao and D. Zhang, "Wrist pulse signal acquisition and analysis for disease diagnosis: A review.," Computers in biology and medicine, vol. 143, p. 105312, 2022. [CrossRef]
- S. Kumar, S. Kumar and K. Veer, "Pulse (Nadi) Analysis for Disease Diagnosis: A Detailed Review.," Proceedings of the National Academy of Sciences, India Section A: Physical Sciences, vol. 93, p. 135–145, 2023.
- R. G. Bickford, J. Brim, L. Berger and M. Aung, "Application of compressed spectral array in clinical EEG," in Automation of clinical Electroencephalo-graphy, New York, Raven Press, 55-64, p. 1973.
- L. M. Moura, M. M. Shafi, M. Ng, S. Pati, S. S. Cash, A. J. Cole, D. B. Hoch, E. S. Rosenthal and M. B. Westover, "Spectrogram screening of adult EEGs is sensitive and efficient.," Neurology, vol. 83, no. 1, p. 56–64, 2014. [CrossRef]
- D. L. Bailey, D. W. Townsend, P. E. Valk and M. N. Maisy, Positron Emission Tomography: Basic Sciences, Secaucus, NJ, USA: Springer-Verlag, 2003. [CrossRef]
- E. F. Bryant, The Yoga sūtras of Patañjali : a new edition, translation, and commentary with insights from the traditional commentators., Albany, NY, USA: North Point Press, 2009.
- P. Ren, A. Barreto, Y. Gao and M. Adjouadi, "Comparison of the use of pupil diameter and galvanic skin response signals for affective assessment of computer users.," Biomedical sciences instrumentation, vol. 48, p. 345–350, 2012.
- B. Bernard, The Essence of Yoga: Reflections on the Yoga Sūtras of Patañjali., Delhi, India: Sri Satguru Publications, 1997.
- G. Thiene, S. Rizzo and C. Basso, "Chapter 11 - Pathology of sudden death, cardiac arrhythmias, and conduction system," in Cardiovascular Pathology, 5th edition ed., Cambridge, MA, USA, Academic Press, 2022, pp. 447-534.
- A. L. Goldberger, Z. D. Goldberger and A. Shvilkin, Goldberger's Clinical Electrocardiography., 9th edition ed., Cambridge, MA, USA: Academic Press, 2017.
- E. Kirmizi-Alsan, Z. Bayraktaroglu, H. Gurvit, Y. H. Keskin, M. Emre and T. Demiralp, "Comparative analysis of event-related potentials during Go/NoGo and CPT: decomposition of electrophysiological markers of response inhibition and sustained attention.," Brain research, vol. 1104, no. 1, p. 114–128, 2006. [CrossRef]
- R. J. Barry, A. R. Clarke, S. J. Johnstone, C. A. Magee and J. A. Rushby, "EEG differences between eyes-closed and eyes-open resting conditions.," Clinical neurophysiology, vol. 118, no. 12, p. 2765–2773, 2007. [CrossRef]
- C. Müller-Axt, C. Eichner, H. Rusch, L. Kauffmann, P. L. Bazin, A. Anwander, M. Morawski and K. von Kriegstein, "Mapping the human lateral geniculate nucleus and its cytoarchitectonic subdivisions using quantitative MRI.," NeuroImage, vol. 244, p. 118559, 2021. [CrossRef]
- J. R. Polimeni, B. Fischl, D. N. Greve and L. L. Wald, "Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1.," NeuroImage, vol. 52, no. 4, p. 1334–1346, 2010. [CrossRef]
- J. L. Prince and J. Links, Medical imaging signals and systems, 2nd edition ed., New York, NY, USA: Pearson, 2014.
- N. Pradhan and D. Narayana Dutt, "An analysis of dimensional complexity of brain electrical activity during meditation.," in Proceedings of the First Regional Conference on IEEE Engineering in Medicine and Biology Society and the 14th Conference of the Biomedical Engineering Society of India, New Delhi, India, 1995.
- C. M. Michel, G. Lantz, L. Spinelli, R. G. De Peralta, T. Landis and M. Seeck, "128-channel EEG source imaging in epilepsy: clinical yield and localization precision.," Journal of clinical neurophysiology, vol. 21, no. 2, p. 71–83, 2004. [CrossRef]
- N. K. Logothetis, "What we can do and what we cannot do with fMRI.," Nature, vol. 453, no. 7197, p. 869–878, 2008.
- J. F. Jansen, W. H. Backes, K. Nicolay and M. E. Kooi, "1H MR spectroscopy of the brain: absolute quantification of metabolites.," Radiology, vol. 240, no. 2, p. 318–332, 2006. [CrossRef]
- S. Petcharunpaisan, J. Ramalho and M. Castillo, "Arterial spin labeling in neuroimaging.," World journal of radiology, vol. 2, no. 10, p. 384–398, 2010.
- V. J. Wedeen, P. Hagmann, W. Y. Tseng, T. G. Reese and R. M. Weisskoff, "Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging.," Magnetic resonance in medicine, vol. 54, no. 6, p. 1377–1386, 2005. [CrossRef]
- Y. Y. Tang, Q. Lu, X. Geng, E. A. Stein, Y. Yang and M. I. Posner, "Short-term meditation induces white matter changes in the anterior cingulate.," Proceedings of the National Academy of Sciences of the United States of America, vol. 107, no. 35, p. 15649–15652, 2010.
- B. Fischl and A. M. Dale, "Measuring the thickness of the human cerebral cortex from magnetic resonance images.," Proceedings of the National Academy of Sciences of the United States of America, vol. 97, no. 20, p. 11050–11055, 2010. [CrossRef]
- S. W. Lazar, C. E. Kerr, R. H. Wasserman, J. R. Gray, D. N. Greve, M. T. Treadway, M. McGarvey, B. T. Quinn, J. A. Dusek, H. Benson, S. L. Rauch, C. I. Moore and B. Fischl, "Meditation experience is associated with increased cortical thickness.," Neuroreport, vol. 16, no. 17, p. 1893–1897, 2005. [CrossRef]
- N. Coquelet, X. De Tiège, F. Destoky, L. Roshchupkina, M. Bourguignon, S. Goldman, P. Peigneux and V. Wens, "Comparing MEG and high-density EEG for intrinsic functional connectivity mapping.," NeuroImage, vol. 210, p. 116556, 2020. [CrossRef]
- S. Ganesan, E. Beyer, B. Moffat, N. T. Van Dam, V. Lorenzetti and A. Zalesky, "Focused attention meditation in healthy adults: A systematic review and meta-analysis of cross-sectional functional MRI studies.," Neuroscience and biobehavioral reviews, vol. 141, p. 104846, 2022. [CrossRef]
- K. C. Fox, M. L. Dixon, S. Nijeboer, M. Girn, J. L. Floman, M. Lifshitz, M. Ellamil, P. Sedlmeier and K. Christoff, "Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations.," Neuroscience and biobehavioral reviews, vol. 65, p. 208–228, 2016. [CrossRef]
- C. Gururaja, D. Rangaprakash and G. Deshpande, "Research Trends in the Application of Yoga to Human Health: A Data Science Approach.," International journal of public mental health and neurosciences, vol. 7, no. 1, p. 8–13, 2020.
- K. C. Fox, S. Nijeboer, M. L. Dixon, J. L. Floman, M. Ellamil, S. P. Rumak, P. Sedlmeier and K. Christoff, "Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners.," Neuroscience and biobehavioral reviews, vol. 43, p. 48–73, 2014. [CrossRef]
- N. P. Gothe, I. Khan, J. Hayes, E. Erlenbach and J. S. Damoiseaux, "Yoga Effects on Brain Health: A Systematic Review of the Current Literature.," Brain plasticity, vol. 5, no. 1, p. 105–122, 2019. [CrossRef]
- A. Venkatraman, R. Nandy, S. S. Rao, D. H. Mehta, A. Viswanathan and R. Jayasundar, "Tantra and Modern Neurosciences: Is there any Correlation?.," Neurology India, vol. 67, no. 5, p. 1188–1193, 2019. [CrossRef]
- J. van Aalst, J. Ceccarini, G. Schramm, D. Van Weehaeghe, A. Rezaei, K. Demyttenaere, S. Sunaert and K. Van Laere, "Long-term Ashtanga yoga practice decreases medial temporal and brainstem glucose metabolism in relation to years of experience.," EJNMMI research, vol. 10, no. 1, p. 50, 2020. [CrossRef]
- E. R. Gizewski, R. Steiger, M. Waibel, S. Pereverzyev, P. J. D. Sommer, C. Siedentopf, A. E. Grams, L. Lenhart and N. Singewald, "Short-term meditation training influences brain energy metabolism: A pilot study on 31 P MR spectroscopy.," Brain and behavior, vol. 11, no. 1, p. e01914, 2021.
- M. G. R. Babu, R. Kadavigere, P. Koteshwara, B. Sathian and K. S. Rai, "Rajyoga meditation induces grey matter volume changes in regions that process reward and happiness.," Scientific reports, vol. 10, no. 1, p. 16177, 2020. [CrossRef]
- S. Kajimura, N. Masuda, J. K. L. Lau and K. Murayama, "Focused attention meditation changes the boundary and configuration of functional networks in the brain.," Scientific reports, vol. 10, no. 1, p. 18426, 2020. [CrossRef]
- H. Kaur, S. Chaudhary, S. Mohanty, G. Sharma, S. S. Kumaran, N. Ghati, R. Bhatia, A. Nehra and R. M. Pandey, "Comparing cognition, coping skills and vedic personality of individuals practicing yoga, physical exercise or sedentary lifestyle: a cross-sectional fMRI study.," Integrative medicine research, vol. 11, no. 1, p. 100750, 2022. [CrossRef]
- D. Chetry, S. Telles and A. Balkrishna, "A PubMed-Based Exploration of the Course of Yoga Research from 1948 to 2020.," International journal of yoga therapy, vol. 31, no. 1, p. Article_22, 2021. [CrossRef]
- T. Jao, C. W. Li, P. E. Vértes, C. W. Wu, S. Achard, C. H. Hsieh, C. H. Liou, J. H. Chen and E. T. Bullmore, "Large-Scale Functional Brain Network Reorganization During Taoist Meditation.," Brain connectivity, vol. 6, no. 1, p. 9–24, 2016. [CrossRef]
- C. Kaur and P. Singh, "EEG Derived Neuronal Dynamics during Meditation: Progress and Challenges.," Advances in preventive medicine, p. 614723, 2015. [CrossRef]
- H. Nakata, K. Sakamoto and R. Kakigi, "Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus.," Frontiers in psychology, vol. 5, p. 1489, 2014.
- K. B. Bærentsen, "Patanjali and neuroscientific research on meditation.," Frontiers in psychology, vol. 6, p. 915, 2015. [CrossRef]
- J. S. K. Reddy and S. Roy, "Commentary: Patanjali and neuroscientific research on meditation.," Frontiers in psychology, vol. 9, p. 248, 2018. [CrossRef]
- S. S. Rangapriya Sri Srih, Insights into Sanatana Dharma, Mysore, India: Ashtanga Yoga Vijnana Mandiram, 2010.
- V. Chayapati, Food, Health and Spirituality (in kannada language: Aahara Aarogya Aadhyatma), Ashtanga Yoga Vijnana Mandiram: Mysore, India, 2006.
- S. S. Rangapriya Sri Srih, Indian Festivals (in Kannada language: Bharatiyara Habba Haridinagalu), Bangalore, India: Bharatha Samskruthi Prakashana, 2013.
- S. Mahaguru, Amaravani - A compilation of the lectures of Sriranga Mahaguru on Samskara: Volume 9 (in Kannada language), Mysore, India: Ashtanga Yoga Vijnana Mandiram, 1999.
- S. S. Rangapriya Sri Srih, Rites and Rituals: What, Why and How (in Kannada language: Samskaragalu enu eke hege), Mysore, India: Ashtanga Yoga Vijnana Mandiram, 2010.
- S. S. Rangapriya Sri Srih, A Hand-Book of Sandhyavandanam, Mysore, India: Ashtanga Yoga Vijnana Mandiram, 2022.
- S. Mahaguru, Amaravani - A compilation of the lectures of Sriranga Mahaguru on life (jeevana): Volume 1 (in Kannada language), Mysore, India: Ashtanga Yoga Vijnana Mandiram, 1983.
- V. Tripathi and P. Bharadwaj, "Neuroscience of the yogic theory of consciousness.," Neuroscience of consciousness, vol. 2021, no. 2, p. niab030, 2021. [CrossRef]
- E. Niedermeyer and F. L. da Silva, Electroencephalography: Basic Principles, Clinical Applications, and Related Fields., Philadelphia, PA, USA: Lippincott Williams & Wilkins, 2004.
- S. Ismail, I. Siddiqi and U. Akram, "Localization and classification of heart beats in phonocardiography signals —a comprehensive review.," EURASIP Journal on Advances in Signal Processing, p. 26, 2018. [CrossRef]
- S. Telles, R. Nagarathna and H. R. Nagendra, "Breathing through a particular nostril can alter metabolism and autonomic activities.," Indian journal of physiology and pharmacology, vol. 38, no. 2, p. 133–137, 1994.
- R. Kahana-Zweig, M. Geva-Sagiv, A. Weissbrod, L. Secundo, N. Soroker and N. Sobel, "Measuring and Characterizing the Human Nasal Cycle.," PloS one, vol. 11, no. 10, p. e0162918, 2016. [CrossRef]
- K. Singh, H. Bhargav and T. M. Srinivasan, "Effect of uninostril yoga breathing on brain hemodynamics: A functional near-infrared spectroscopy study.," International journal of yoga, vol. 9, no. 1, p. 12–19, 2016. [CrossRef]
- E. F. Du Bois, "The basal metabolism in fever.," Journal of the American Medical Association, vol. 77, no. 5, p. 352–355, 1921.
- S. Mahaguru, "Vijnyana Mandiram: Its Aims and Programme," 24 October 1947. [Online]. Available: https://www.ayvm.in/aboutus_page1. [Accessed 2023].
- M. N. Rusalova, "Frequency-amplitude characteristics of the EEG at different levels of consciousness.," Neuroscience and behavioral physiology, vol. 36, no. 4, p. 351–358, 2006. [CrossRef]
- J. Pillai and M. R. Sperling, "Interictal EEG and the diagnosis of epilepsy.," Epilepsia, vol. 47, no. Suppl 1, p. 14–22, 2006. [CrossRef]
- H. Jasper, "Report of the committee on methods of clinical examination in electroencephalography," Electroencephalography and Clinical Neurophysiology, vol. 10, no. 2, p. 370–375, 1958.
- G. H. Klem, H. O. Lüders, H. H. Jasper and C. Elger, "The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology.," Electroencephalography and clinical neurophysiology. Supplement, vol. 52, p. 3–6, 1999.
- T. Alotaiby, F. E. A. El-Samie, S. A. Alshebeili and I. Ahmad, "A review of channel selection algorithms for EEG signal processing.," EURASIP Journal of Advances in Signal Processing, p. 66, 2015. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




