1. Introduction
1.1. Histology of the Small Intestine
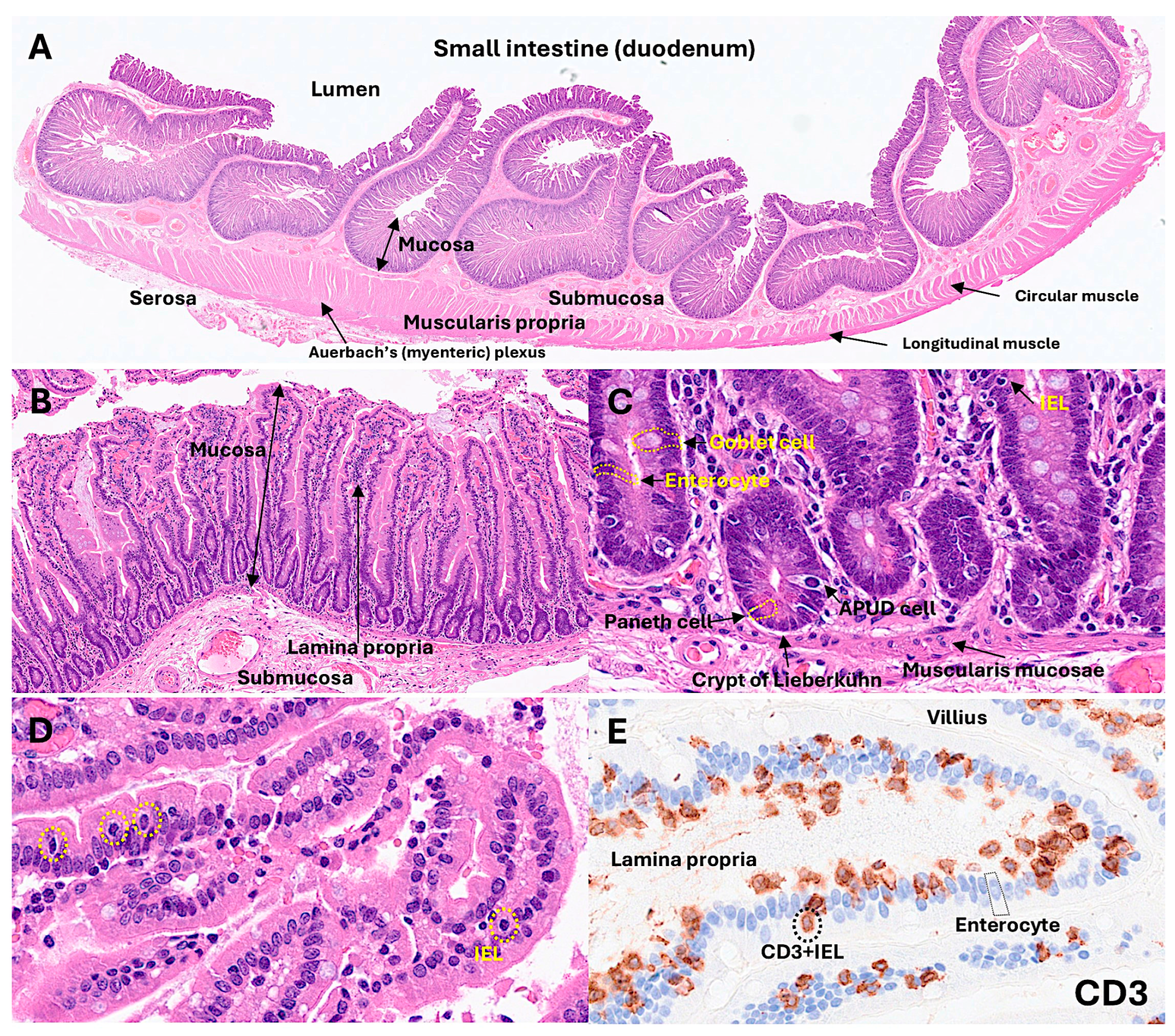
The normal intestinal mucosa has a defined architecture, including the villi, crypts, lamina propria, and muscularis mucosae.
The villi exhibit a digitiform shape with a 3:1 ratio between the height of the villi and the depth of the glandular crypts. The glandular crypts comprise several cell subtypes, including epithelial, Paneth, goblet, and endocrine cells. Each of these cells has different functions.
Intestinal epithelial cells (IECs) line the surface of the intestine and are responsible for aliment digestion, nutrient absorption, and infection protection by creating a physical barrier and modulating the immune response [
1]. Intestinal epithelial cells are sensitive to the nutrients in the diet [
1]. Paneth cells secrete alfa defensins, which are broad-spectrum microbicides that control gut microbiota and intestinal homeostasis. In hematoxylin and eosin (H&E) staining, Paneth cells display bright red cytoplasmic granules [
2]. Goblet cells produce mucus and are intimately involved in controlling the mucosal immune system [
3]. Goblet cells sample luminal antigens to initiate the adaptive immune response. There are several subtypes of goblet cells with different localization and gene expression [
3]. There are several types of endocrine cells in the small intestine [
4]: EC cells produce serotonin (5-HT) [
5,
6,
7]; L cells, GLP-1, GLP-2, and peptide YY [
8,
9,
10]; K cells, GIP and 5-HT [
11,
12]; I cells, cholecystokinin and 5-HT [
13,
14]; D cells, somatostatin [
15,
16]; G cells, gastrin [
17,
18,
19]; N cells, neurotensin [
20,
21]; M cells, motilin [
22,
23]; and S cells, secretin [
24,
25]. The main functions of endocrine cells are gut motility, appetite control, insulin release, cell proliferation control, gastric acid motility, pancreatic enzyme secretion, and intestinal absorption [
4].
The lamina propria is a thin connective tissue layer located below the epithelial basement membrane. The lamina propria is rich in fibroblasts, myofibroblasts, vascular and lymphatic vessels, elastic fibers, smooth muscle fascicles, and immune cells, including lymphocytes, plasma cells, macrophages, eosinophils, and mast cells [
26].
The muscularis mucosa is composed of a very thin layer of smooth muscular cells with motor activity, which are linked to mucosal absorption and secretion functions [
27].
The submucosa contains blood and lymphatic vessels and nerves of the parasympathetic system, including the submucous plexus, also known as Meissner’s plexus [
28]. The submucosal extracellular matrix is minimally immunogenic [
28]. The muscularis propria [
29] comprises an inner circular and outer longitudinal layer, and Auerbach’s (myenteric) plexus.
1.2. Instraepithelial Lymphocytes
IELs are found in the epithelium of the skin, genitourinary tract, respiratory tract, and intestinal tract [
30]. IELs are a first line of defense against pathogens that have attacked the epithelial surface. The typical phenotype is of cytotoxic T-lymphocytes, being CD3- and CD8-positive [
31]. The T-cell receptor (TCR) can be alphabeta (αβ) or gammadelta (γδ)-positive. Some IELs present with self-reactive TCR, suggesting an extrathymic origin [
30,
32,
33,
34,
35,
36].
IELs are specialized immune cells that colonize the intestinal mucosa. Although B and innate cell populations may also transit inside this compartment, T-lymphocytes comprise the majority of intestinal IELs. IELs represent one of the largest lymphocyte populations in the intestine and contribute to epithelial homeostasis and barrier integrity, including tolerance, resistance, and tissue protection [
37]. There are several subsets of IELs. However, all strains share common characteristics, including restricted TCR diversity, epithelium-adapted profile, innate-like properties, and cytotoxic potential [
37]. Human IELs can recognize modified self-antigens using both natural killer (NK) receptors and foreign antigens using the TCR [
31]. The main characteristics of IELs are as follows:
- (1)
IELs permanently reside in the epithelial tissue and do not recirculate because of the expression of CD103 [
38,
39] that binds to E-cadherin [
39,
40,
41]. CD103 is also known as ITGAE (Integrin, Alpha E, and Human Mucosal Lymphocyte Antigen 1). E-cadherin is also known as Cadherin-1 (CDH1), and CD324.
- (2)
The mucosal epithelial environment is highly immunogenic, with constant activation and tolerance that prevents tissue damage. Therefore, IELs express several T-cell co-inhibitory molecules and NK inhibitory receptors [
42,
43] and downregulate TCR co-stimulatory molecules.
- (3)
The TCR diversity of IELs is limited compared to peripheral T-lymphocytes [
44,
45] and specific to conserved microbial or dietary antigens [
46].
- (4)
IELs have innate-like properties enabling rapid TCR-independent responses to stress signals [
42,
47].
- (5)
IELs have cytotoxic activity [
47,
48,
49,
50], and an alteration may be associated with several gastrointestinal diseases, such as celiac disease and inflammatory bowel disease (IBD) [
50,
51,
52,
53,
54].
- (6)
IELs are stratified into natural IELs (nIELs) and peripherally induced IELs (pIELs) [
55,
56,
57,
58]. nIELs are generated in the thymus and migrate to the intestine. In contrast, pIELs are derived from CD4-positive or CD8-positive T-cells at inductive sites, such as gut-associated lymph nodes, in response to dietary and microbial antigens [
31,
37,
55,
56,
57,
58,
59,
60,
61].
- (7)
IELs can be further subclassified according to their TCR subtype: (I) TCRγδ+nIELs (tissue surveillance and repair), (II) TCRαβ+CD8αα+nIELs (regulation), (III) TCRαβ+CD8αβ+pIELs (effector memory, cytotoxicity), (IV) TCRαβ+CD4+pIELs (regulation, cytotoxicity) [
31,
37]. Subtypes I and II may recognize self-antigens using their TCR, are present at birth, and are microbiota-independent. Subtypes III and IV may recognize microbial, viral, and dietary antigens using TCRs, are absent at birth, increase with age, and are microbiota- and diet-dependent [
31,
37]. CD4+FOXP3+regulatory T-lymphocytes (Tregs) can undergo CD4+CD8αα+ IEL differentiation in the intestinal epithelium [
62,
63].
- (8)
CD8αα+ is an indication of intestinal IELs. Conventional CD8+T-cells express the CD8αβ heterodimer that is a TCR coreceptor, and enhance the TCR-MHC-I interactions during antigen presentation. Most IELs express CD8αα homodimer that decreases TCR sensitivity and prevents IEL hyperactivation via the mechanism of CD8αα homodimer interaction with thymus leukemia (TL) antigen [
64], which is expressed by intestinal epithelial cells. Therefore, TL expression plays a critical role in maintaining IEL effector functions. in a genetic model of inflammatory bowel disease, TL deficiency was associated with colitis [
65].
- (9)
IELs contribute to chronic intestinal inflammatory disease pathogenesis. Inflammatory bowel disease (IBD) includes Crohn disease and ulcerative colitis. Dysregulated intestinal immune response to microbiota is a cause of IBD [
66,
67]. IELs could play a regulatory role in IBD [
65,
66,
67,
68,
69,
70,
71,
72]. Preserved villous architecture and increased IELs characterize microscopic colitis [
73,
74,
75,
76]. Celiac disease is an autoimmune disease triggered by dietary gliadin and is characterized by villous atrophy, crypt hyperplasia, and chronic inflammation of the lamina propria [
77,
78,
79,
80]. In celiac disease, there are increased CD8αβ+ pIELs and TCRγδ+ nIELs [
31]. IELs can undergo neoplastic transformation into enteropathy-associated T-cell lymphoma, a rare complication in patients with celiac disease who are unresponsive to gluten-free diet and treatment [
81,
82,
83,
84].
Figure 1.
Histology of the small intestine. The small intestine comprises several layers, including the mucosa, submucosa, muscularis propria, and serosa. In the mucosa, intraepithelial lymphocytes (IELs) are found between epithelial cells (enterocytes). Using CD3 immunostaining, IELs can be easily identified under the optical microscope.
Figure 1.
Histology of the small intestine. The small intestine comprises several layers, including the mucosa, submucosa, muscularis propria, and serosa. In the mucosa, intraepithelial lymphocytes (IELs) are found between epithelial cells (enterocytes). Using CD3 immunostaining, IELs can be easily identified under the optical microscope.
1.3. Celiac Disease
Celiac disease is a common immune-related disease with a prevalence of approximately 1% in most populations [
85]. The incidence of celiac disease has increased in recent years; the reason for this is unknown, but it may be related to environmental factors associated with the loss of tolerance to dietary gluten [
85].
The pathogenesis of celiac disease is multifactorial. The pathogenesis includes a genomic background with the presence of several genetic factors [
86], such as the close association with HLA-DR3-DQ2 and/or DR4-Dq8 gene locus, which is highly present in patient with celiac disease [
87]. Other gene loci related to metabolism and immune system have also been identified using genome-wide association studies (GWAS), such as 3p21.31 (
CCR3 and
CCR2), 4p27 (
KIAA1109, ADAD1, IL2, and
IL21), 6q15 (
BACH2), 6q25.3 (
TAGAP), 1q24.3 (
FASLG, TNFSF18, and
TNFSF4), 6q22.31 (
NKAIN2), 10p15.1 (
PFKFB3 and
PRKCQ), and 17q21.32 (
HOXB9) [
88]. Genome-wide gene expression studies have also highlighted similar biomarkers, including
APOC3, CYP3A4, OCLN, MAD2L1, MKI67, CXCL11, and
IL17A [
89].
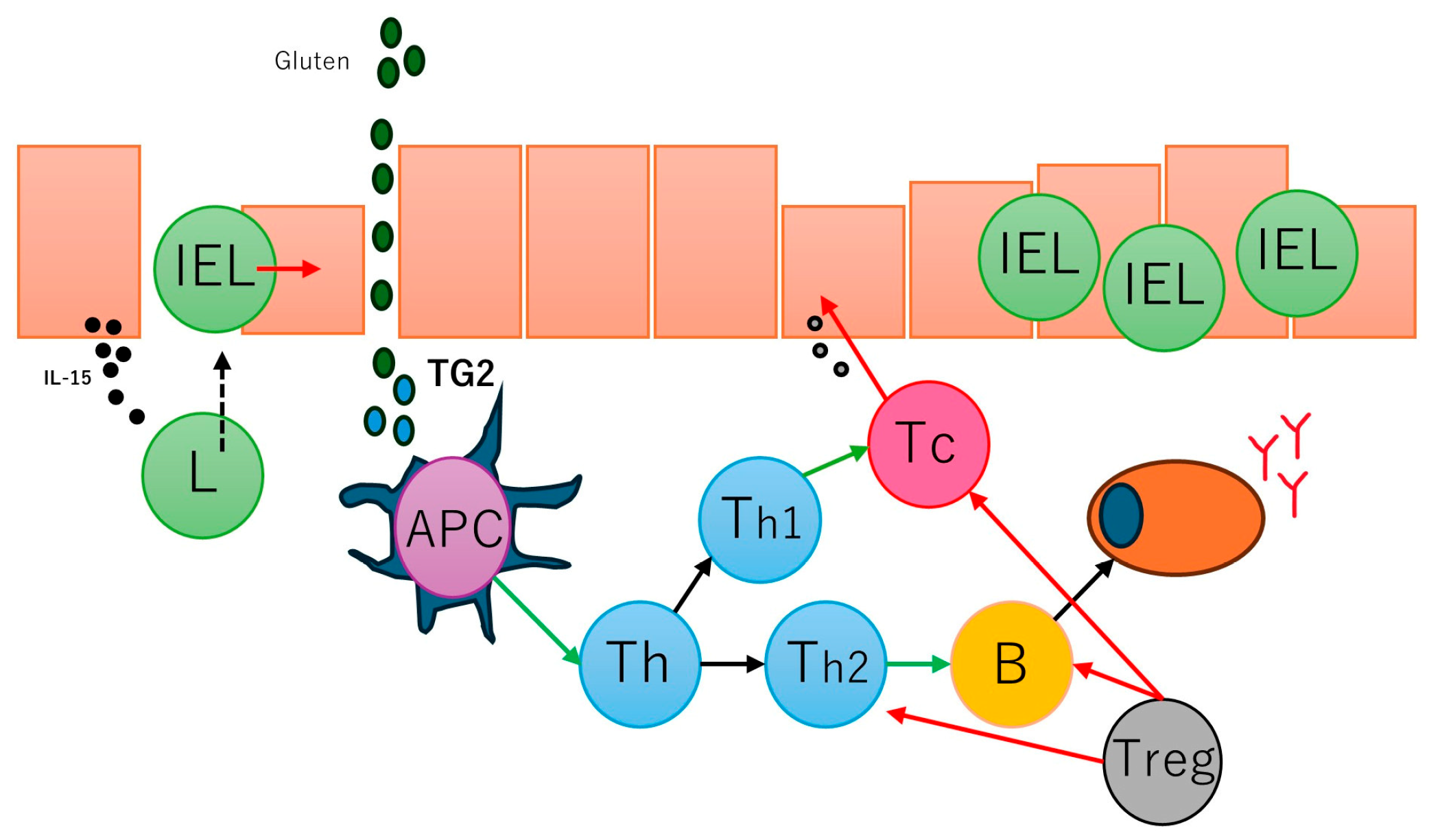
Celiac disease is characterized by an abnormal mucosal immune response to gliadin fractions, resulting in chronic inflammatory infiltration of the lamina propria and epithelium and villous atrophy [
90]. Regarding the adaptive immune response, the key factors are peptide 56-89 (α-gliadin) (
Figure 2), which is resistant to gastrointestinal peptidases [
91,
92], tissue transglutaminase, and gliadin-reactive T-cells.
In active and gluten-sensitive celiac disease, the number of intraepithelial lymphocytes increases, and these cells express interferon gamma and IL-10 [
93]. The gammadelta T-cell receptor (γδTCR) is also found to be increased in intraepithelial lymphocytes [
94] in addition to the common alfa-beta T-cell receptor (αβTCR); in case of refractive celiac disease, intraepithelial lymphocytes may have an aberrant phenotype and restricted gene rearrangement [
95,
96]. Several antibodies are found in the serum of patients with celiac disease, including anti-gliadin antibodies (anti-AGA), anti-deamidated gliadin peptide antibodies (anti-DGP), anti-transglutaminase 2 antibodies (anti-TG2), anti-R1-type reticulin antibodies (anti-ARA), and anti-endomysia antibodies (anti-EMA) [
97]. Gluten peptides also activate innate immune responses, such as IL-15, intraepithelial lymphocytes, type 1 interferon (gamma), macrophages, monocytes, and dendritic cells, and induce dysbiosis [
98]. A summary of the pathogenesis of celiac disease has been presented in our previous publications [
77,
78] (
Figure 3).
Table 1 summarizes the epidemiology, pathogenesis, and clinical manifestations of celiac disease in adults.
The diagnosis of celiac disease includes a serologic evaluation, endoscopy with small bowel biopsy (duodenum), and HLA testing in selected patients [
146,
147]. The diagnostic approach of celiac disease depends on the individual’s disease probability. Individuals with low celiac disease probability should undergo serologic testing, and endoscopy and biopsy if positive. When there is a high probability (highly suggestive clinical presentation and presence of risk factors), should undergo both serologic testing and biopsy. Tissue transglutaminase (tTG)-IgA antibody is the single preferred test for detection of celiac disease in adults. Serum antibody assays include autoantibodies, antigliadin, anti-endomysia, and anti-tissue transglutaminase [
97,
146,
147,
148].
The endoscopic characteristics of celiac disease have low sensitivity and include atrophic mucosa with loss of folds, fissures, nodularity and prominent submucosal vascularity [
149,
150].
The histological features of celiac disease range from mild alteration with increased IELs to severe atrophy with loss of villi, high epithelial apoptosis, and crypt hyperplasia.
Table 2 presents the histological classification based on Marsh [
151].
The definition of celiac disease is a condition in which a chronic inflammation of the mucosa improves morphologically in a gluten-free diet and relapses when it is reintroduced. Therefore, the treatment consists on lifelong adherence to a gluten-free diet and identification and treatment of nutritional deficiencies. Investigational approaches include the use of transglutaminase inhibitors [
152,
153,
154,
155].
1.4. LAIR1
Leukocyte-associated immunoglobulin like receptor 1 (LAIR1), also known as CD305, is an immune-inhibitory receptor found on mature hematopoietic cells, particularly on immune cells such as mononuclear cells, natural killer cells, and T- and B-lymphocytes [
156].
The gene is located in the 19q13.4 region and is known as the leukocyte receptor cluster, which contains several genes that encode leukocyte receptors of the immunoglobulin superfamily. LAIR1 induces cell death, inhibits cytokine release and the activation of the NFKB pathway in myeloid leukemia [
157,
158,
159]. Figure 4 shows the structure of LAIR1. It is a type I glycoprotein comprising 287 amino acids belonging to the family IR [
160].
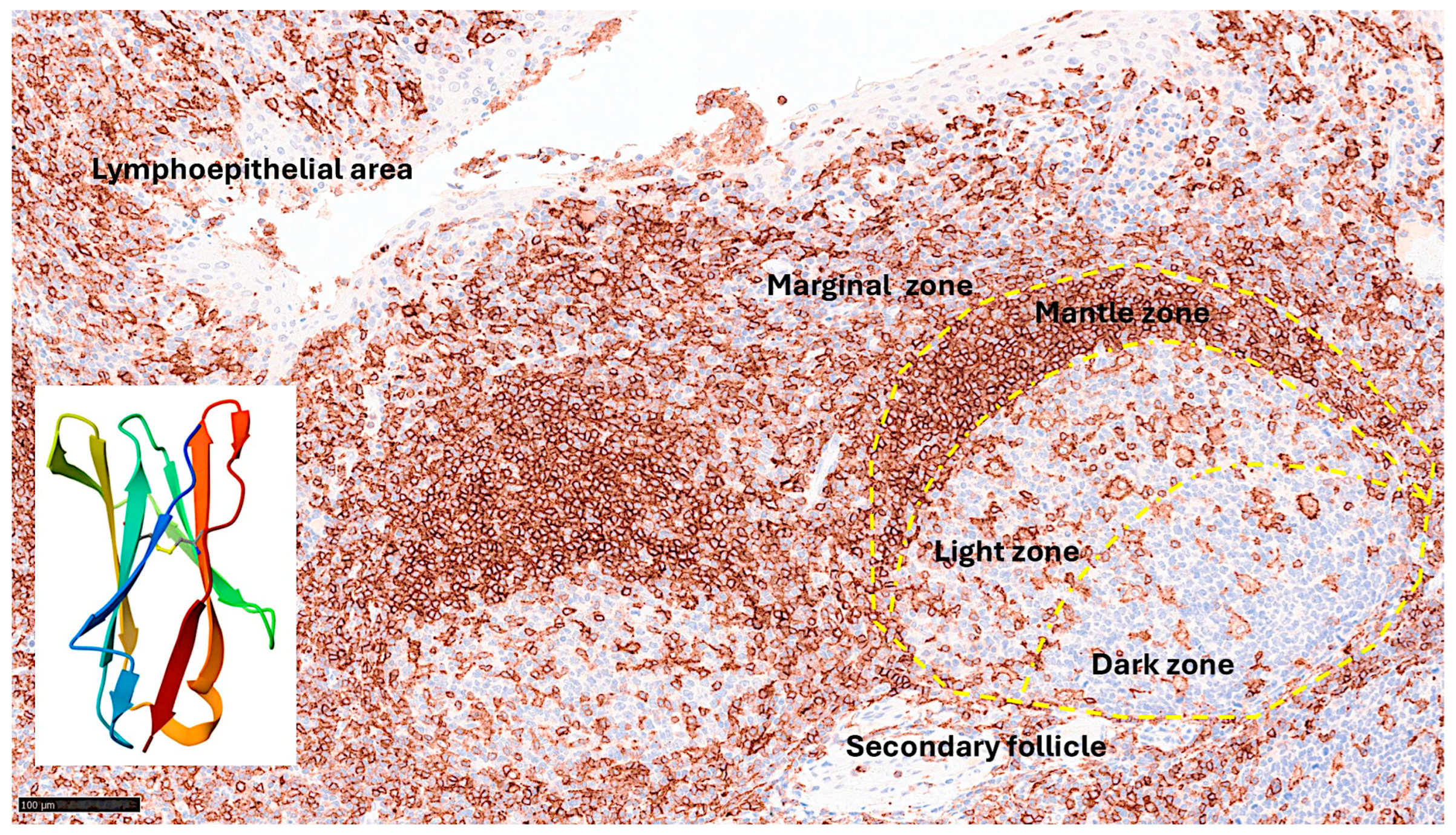
Figure 4.
LAIR1 expression in reactive tonsils and human LAIR1 crystal structure. Immunohistochemistry staining of LAIR1 in reactive tonsil controls from human samples. LAIR1 was expressed in both follicles and interfollicular areas of reactive tonsils. In the secondary follicles, the pattern was characteristic of macrophage/dendritic cells in the germinal center, and of naïve B-lymphocytes in the mantle zone. In the interfollicular area, the pattern was compatible with dendritic cells, including in the lymphoepithelial area. Because LAIR1+IELs were found, this research study aimed to characterize LAIR1 expression in IELs of the intestinal mucosa, with a focus on the small intestine, and Celiac Disease as a pathological counterpart. The crystal structure of human LAIR1 in the C2 space group is also shown; experimental data using the X-ray diffraction method (
https://www.rcsb.org/structure/3RP1 ; accessed on March 25, 2025). Tissue: human reactive tonsil (Department of Pathology, Tokai University Hospital).
Figure 4.
LAIR1 expression in reactive tonsils and human LAIR1 crystal structure. Immunohistochemistry staining of LAIR1 in reactive tonsil controls from human samples. LAIR1 was expressed in both follicles and interfollicular areas of reactive tonsils. In the secondary follicles, the pattern was characteristic of macrophage/dendritic cells in the germinal center, and of naïve B-lymphocytes in the mantle zone. In the interfollicular area, the pattern was compatible with dendritic cells, including in the lymphoepithelial area. Because LAIR1+IELs were found, this research study aimed to characterize LAIR1 expression in IELs of the intestinal mucosa, with a focus on the small intestine, and Celiac Disease as a pathological counterpart. The crystal structure of human LAIR1 in the C2 space group is also shown; experimental data using the X-ray diffraction method (
https://www.rcsb.org/structure/3RP1 ; accessed on March 25, 2025). Tissue: human reactive tonsil (Department of Pathology, Tokai University Hospital).
A comprehensive review of the role of LAIR1 in immune cell responses and neoplasia was recently been performed by Poggi A. et al. [
161], and the association with immune disorders and hematological neoplasms was reported by Van Laethem F. et al. [
156]. Immune cell function can be modulated using inhibitory receptors. Many of these inhibitory receptors recognize a limited number of specific ligands. However, a subgroup of inhibitory receptors, called inhibitory pattern recognition receptors (iPRRs) [
163], can bind a large number of ligands of structural similarity [
162] . LAIR1 belongs to the iPRR group and recognizes common structural patterns in collagens and collagen domain-containing proteins [
164].
Autoimmune diseases are characterized by a pathological response to self- or autoantigens. These disorders can be either systemic (such as systemic lupus erythematosus and vasculitis), or organ-specific (such as autoimmune thyroiditis, and multiple sclerosis); and can be either acute or chronic [
165]. The pathogenesis of autoimmune diseases is complex [
165] and involve breakdown or defects in immune tolerance, defects in active regulation and control of autoreactivity (FOXP3+Tregs, IL-10, CTLA-4, TGF-beta) [
166], defects in regulation of autoimmune B-cell responses (autoreactive B cells) [
167], targeting of cell surface and soluble antigens and immune complex formation, immune complexes (between autoantibodies and the corresponding autoantigen present in the circulation and/or on cell surfaces) [
168], effector T-cell-mediated injury (cytotoxic cells) [
169], innate immune mechanisms (pattern recognition receptors) [
170], specific T-cell subsets (Th1, Th2, Th17, Tregs), cytokines, internalization of autoantibodies (myopathies), and dysregulation of apoptosis (autoimmune lymphoproliferative syndrome (ALPS) [
171], systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis) [
172]. Regarding ulcerative colitis, we have recently described that steroid-requiring patients had high infiltration of LAIR1+ cells in the lamina propria [
173].
LAIR1 has been associated with the pathogenesis of several autoimmune diseases, including systemic lupus erythematosus (SLE) [
174], rheumatoid arthritis (RA) [
175], allergy (airway hyper-reactivity and asthma) [
176], graft rejection (kidney transplant) [
177], and chronic hepatitis [
178]. Regarding neoplasia of the hematopoietic and lymphoid system, we have recently described the role of LAIR1 in the pathogenesis of follicular lymphoma [
179] and diffuse large B-cell lymphoma [
180].
Epidemiological studies continue to identify celiac disease-associated diseases, such as inflammatory arthritis and irritable bowel disease [
181]. Celiac disease is a systemic immunological disorder caused by gluten (gliadin and other prolamin) in genetically predisposed individuals. The pathogenesis is complicated and remains a subject of research. The basic treatment is elimination of products that may contain gluten from the diet. However, new therapeutic strategies are being developed, such as supplementation with exogenous endopeptidases, immune response modification, and the use of zonulin and transglutaminase 2 inhibitors [
182]. Due to the relevance of LAIR1 immune-inhibitory receptor on the function and activation of mature hematopoietic cells, particularly on immune cells such as mononuclear cells, natural killer cells, and T and B-lymphocytes, the role of LAIR1 in intestinal mucosa (IELs) and celiac disease pathogenesis warrants analysis.
1.5. Aim of the Study
This study aimed to analyze the phenotype of intraepithelial lymphocytes (IELs) and the lamina propria in the small intestine, including LAIR1, and to confirm the expression of LAIR1 in celiac disease.
Highlights:
- ●
In small intestine control, IELs exhibited a cytotoxic T-cell phenotype and were positive for CD3, CD8, CD103, TCRβ, and LAIR1.
- ●
CD was characterized by higher LAIR1-positive cells than the small intestine control (P = 0.004).
- ●
Higher intestinal lesions evaluated by Marsh scoring were correlated with higher LAIR1 (P < 0.001).
- ●
CD was characterized by gene-set enrichment of LAIR1 pathway using an independent transcriptomic dataset.
2. Materials and Methods
2.1. Patients and Samples
This study included 16 cases of celiac disease (total number of biopsies n = 57), 18 cases of small intestine control, 11 reactive lymphoid tissue, and 3 lymphoma cases (used as immunohistochemical internal control). The celiac cases were selected from the Department of Pathology of Hospital Clinic Barcelona, Spain, as described in our previous publications [
77,
78]. The patients were diagnoses with celiac disease following the conventional diagnosis, with clinical criteria, positive celiac serology, and histological criteria, including the presence of increased intraepithelial lymphocytes with crypt hyperplasia (Marsh type 2) or villous atrophy (Marsh type 3). The detailed data are presented in
Appendix A, Table A1.
This study was conducted according to the principles of the Declaration of Helsinki for human experimentation. Ethical Committee of Tokai University approved this study (IRB14R-080 and IRB20-156).
2.2. Immunohistochemistry
Several immunohistochemical markers were analyzed in the tissue samples using a Leica Bond Max automated stainer according to the manufacturer’s instructions. The primary antibodies that were used were the following: CD3 (clone LN10, Leica Biosystems, Leica K.K., Tokyo, Japan), CD4 (4B12, Leica), CD8 (4B11, Leica), CD103 (EP206, Leica), granzyme B (11F1, Leica), TCRβ (TRBC1/TCRβ constant region 1 (E6Z3S) Rabbit mAb #79485, Cell Signaling Technology K.K., Tokyo, Japan), TCRδ (TRDC/TCRδ (E2E9T) XP® Rabbit mAb #55750, Cell Signaling), CD56 (CD56-504-L-CE, Leica), CD16 (CD16-L-CE, Leica), LAIR1 (CD305, JAVI82A, created by Giovanna Roncador, Spanish National Cancer Research Center (CNIO)), PD-L1 (73-10, Leica), PD1 (CD279, NAT105, CNIO), BTLA (CD272, FLO67B, CNIO), TOX2 (TOM924D, CNIO), HVEM (TNFRSF14, ab47677), CD163 (CD163-L-CE, Leica), HLA-DP-DQ (JS76, CNIO), IL4I1 (BALI265E,543H,573B, CNIO), and FOXP3 (236A, CNIO). The details of the primary antibody details are presented in
Table 3.
Confocal microscopy was performed as described previously [
183] using a Fluoview FV3000 confocal laser scanning microscope (Olympus K.K, Hachioji, Japan) with Alexa Fluor 488 and 594 and DAPI dyes.
The immunohistochemical expression of BTLA in celiac disease was imported from our previous publication, including the histological slides, and reanalyzed [
77].
LAIR1 was evaluated in a semiquantitative manner as low (1+, 20%), intermediate (2+, 20-50%), and high (3+, >50%) for the statistical purposes.
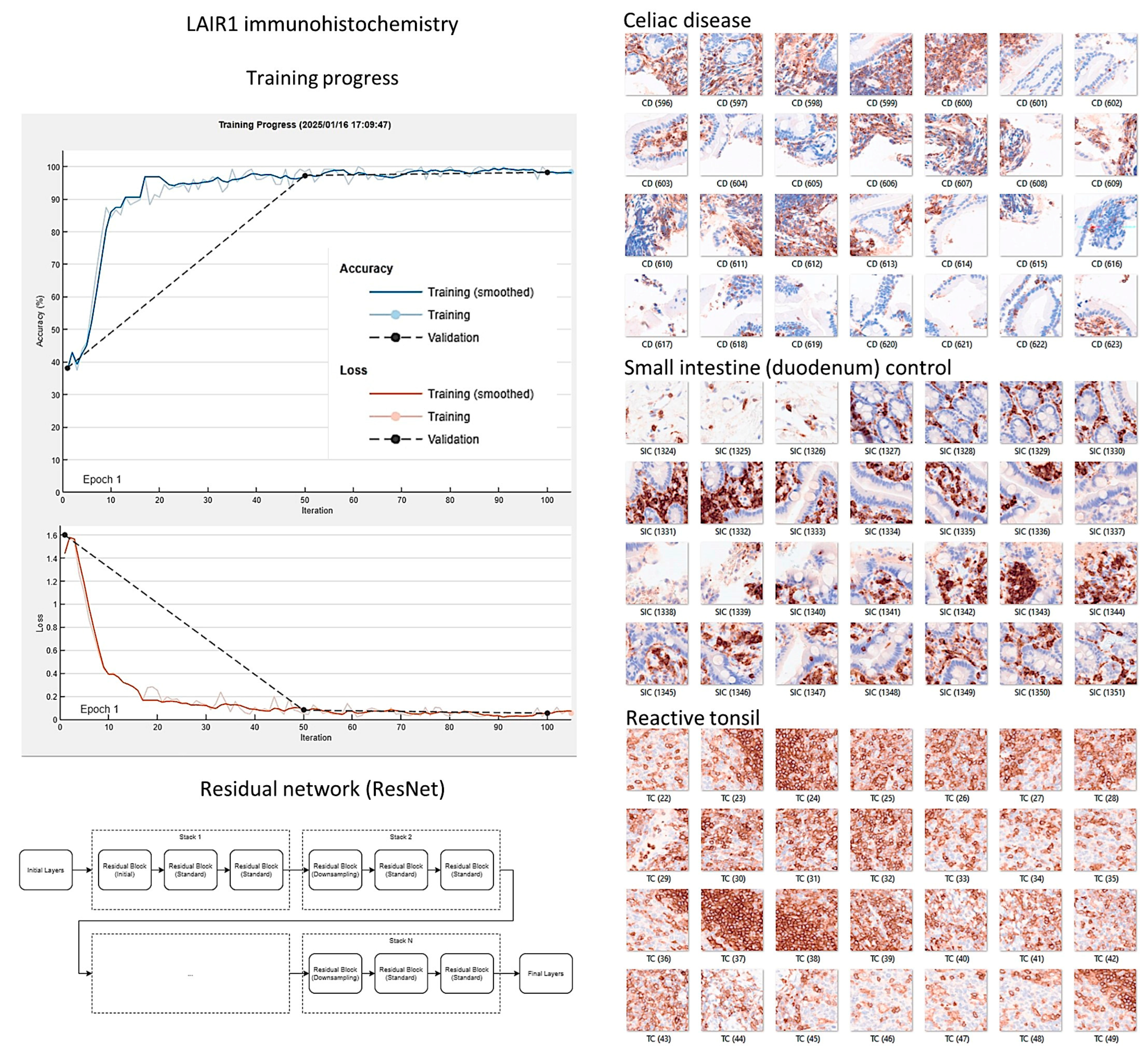
2.3. Image Classification
Image classification based on the immunohistochemical expression of LAIR1 was performed using transfer learning and the ResNet18 deep learning model, as recently described [
77,
184,
185]. All histological slides were scanned using a NanoZoomer S360 digital slide scanner (Hamamatsu Photonics K.K., Hamamatsu City, Japan). After visualization using the NDP.view2 image viewing software U12388-01 (Hamamatsu Photonics K.K.), the whole-tissue sections were exported into a jpeg file at 200× magnification and 150 dpi. The images were split into images patches of 224 × 224 × 3 using PhotoScape v3.7 (
http://www.photoscape.org/ ; last accessed on July 31, 2025). All image patches were manually revised to exclude artifacts such as broken, folded, and nondiagnostic images. Image patches of not 224 × 224 size, without tissue, or tissue less than 20% were automatically discarded. The image patches were pooled into 3 different folders, and the data were split into 3 sets: training set (70%) for training the network, validation set (10%) for testing its performance during training, and test set (20%) used after training to assess how well the network performed on new data. Grad-CAM analysis was used as an explainable AI method to visualize which areas of the input image were most important for model prediction and image classification. The methodology was performed as previously described in our previous publications [
78,
173,
184]. All analyses were performed using a desktop equipped with an AMD Ryzen 9 5900X 12-Core Processor 3.70 GHz, 48.0 GB of RAM, an NVIDIA GeForce RTX 4080 SUPER (16 GB) GPU, and MATLAB R2023b Update 10 (23.2.0.2859533) 64-bit (win64) 27 January 2025 (MathWorks, Natick, MA, USA).
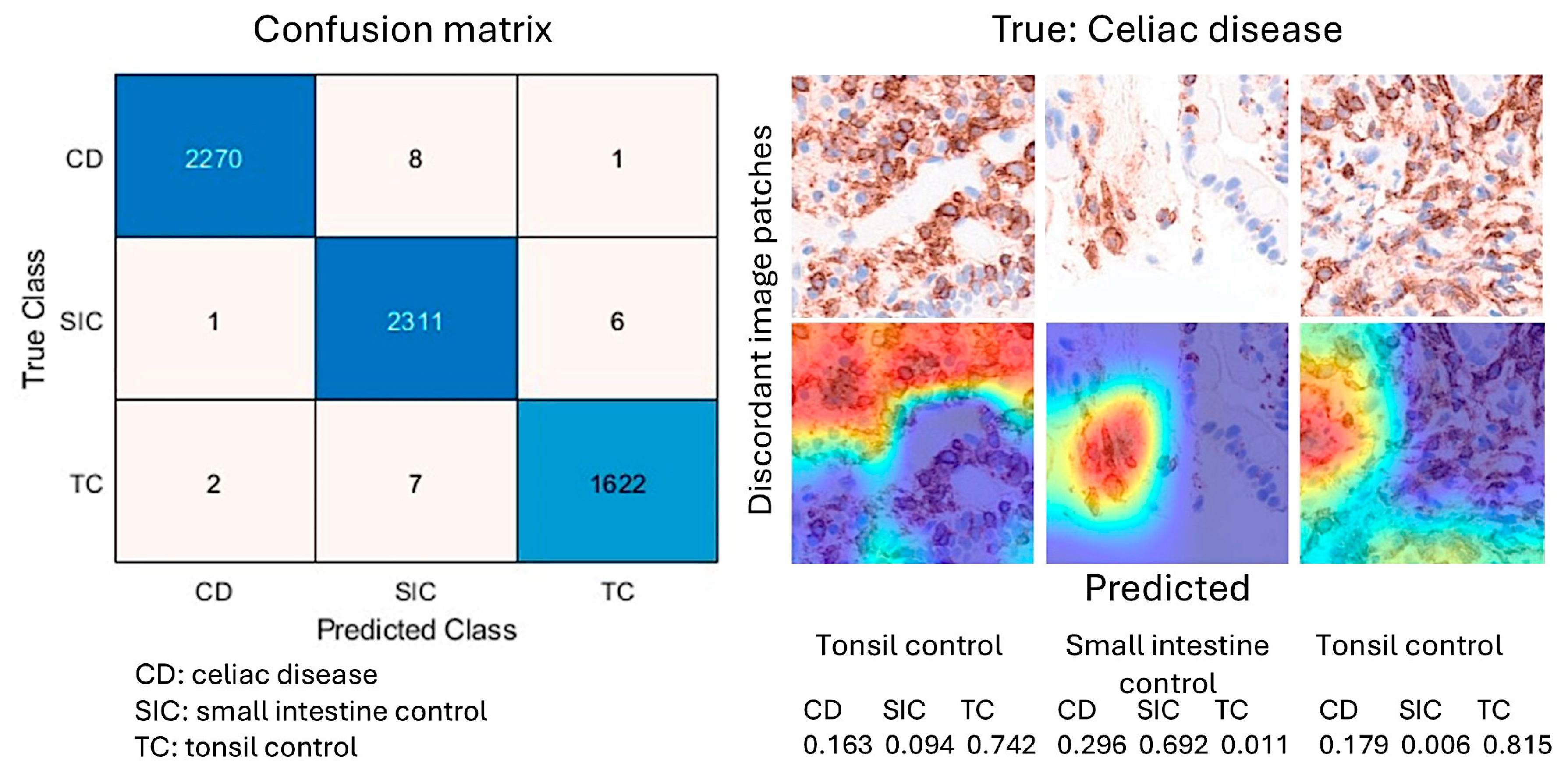
In the confusion matrix, the image-patches were recorded as true positive (TP), false positive (FP), false negative (FN), and true negative (TN). The accuracy performance parameter was calculated as follows: Accuracy = (TP + TN)/(TP + TN + FP + FN).
The relevant code functions were as follows: [imdsTrain, imdsVal, imdsTest] = splitEachLabel(imds, 0.7, 0.1, “randomized”); imdsTrain = shuffle(imdsTrain); YPred = classify(trainedNetwork_1, imdsTestAug); accuracy = sum(YPred == imdsTest.Labels) / length(YPred); scores = predict(trainedNetwork_1, imdsTestAug); confusionchart(YPred, imdsTest.Labels); wronglyPredicted = find(YPred~= imdsTest.Labels); imdsTest.Files{wronglyPredicted}
2.4. Gene Expression Analysis
A suitable independent series of celiac disease was searched in the Gene Expression Omnibus database, and the public dataset published by Dr Worf J et al. [
186] was selected. This dataset includes transcriptome analysis of 48 duodenal biopsies of 26 children and adolescents diagnosed with celiac disease, and 22 children without celiac disease as controls. Frozen tissue biopsies were obtained, and total RNA was extracted using a Qiagen AllPrep® DNA/RNA Microkit. Gene expression was assessed using the Illumina HumanHT-12 v4.0 beadchip [
186].
Gene set enrichment analysis (GSEA) was performed using GSEA software version 4.4.0 (build 18) from Broad Institute, Inc. (USA). The analysis procedure was as previously described [
77]. STRING version 12.0 (SIB, Swiss Institute of Bioinformatics; CPR, Novo Nordisk Foundation Center Protein Research; and EMBL, European Molecular Biology Laboratory) was used for the functional network association analysis [
187].
2.5. Statistical Analyses
All statistical analyses were performed using IBM SPSS version 27.0.1.0 (64-bit edition; IBM Corporation, Armonk, New York, NY, USA). Comparison between groups was performed using crosstabulation and chi-square test, and Mann–Whitney U nonparametric test. Spearman’s rho test was used to determine nonparametric correlations between genes. P values ≤0.05 were considered statistically significant.
3. Results
3.1. Immunophenotype of IELs in Intestinal Mucosa Control
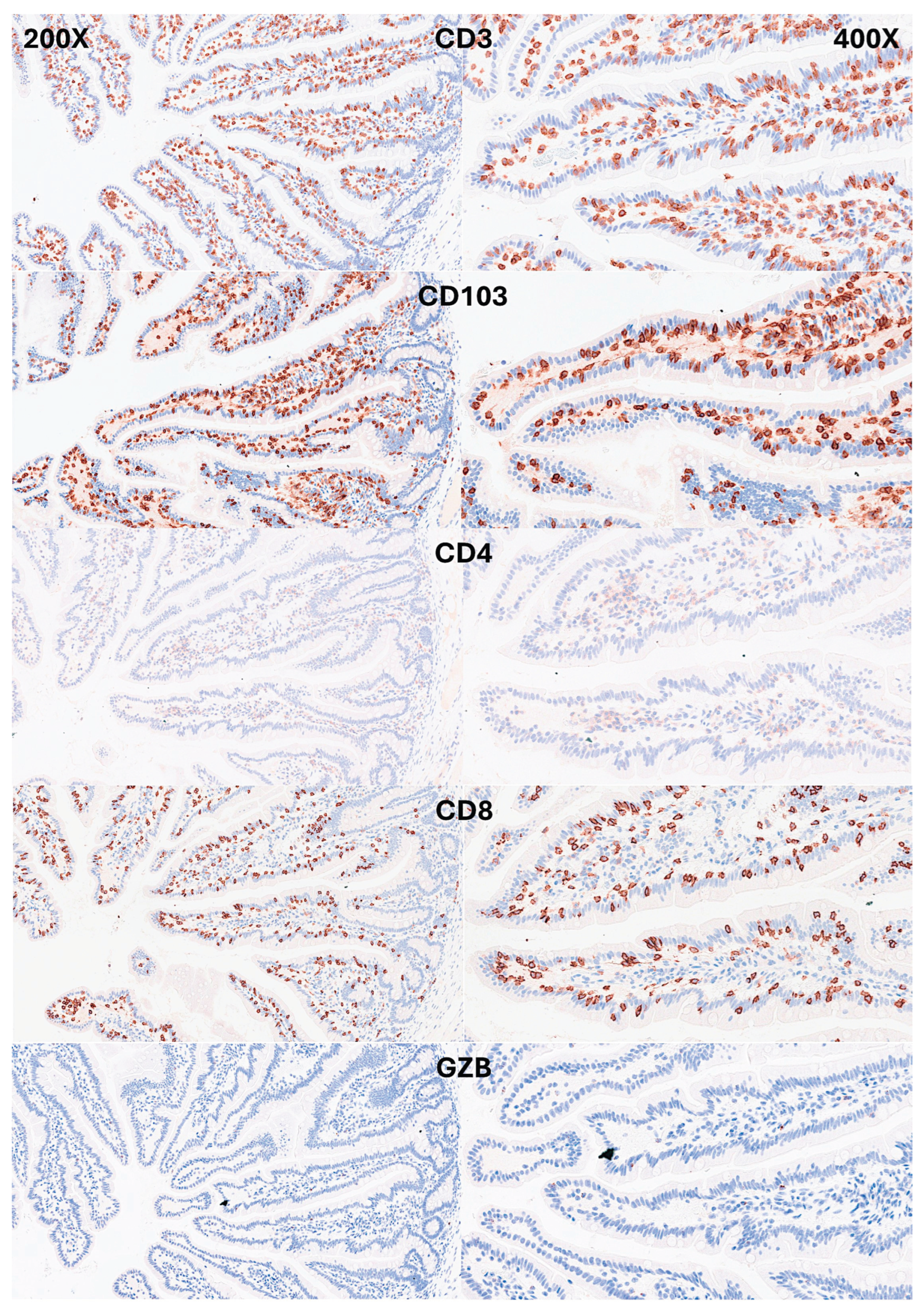
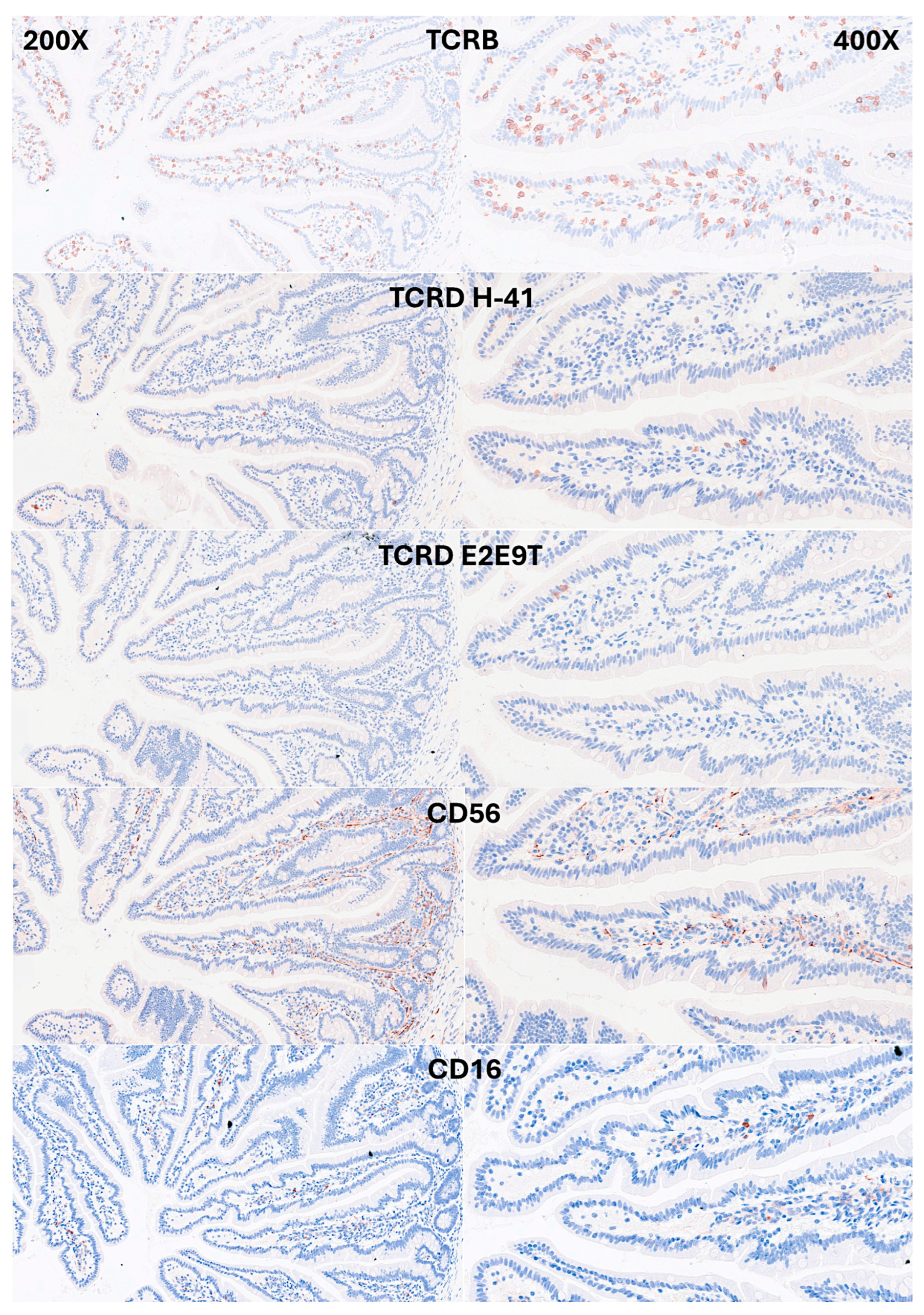
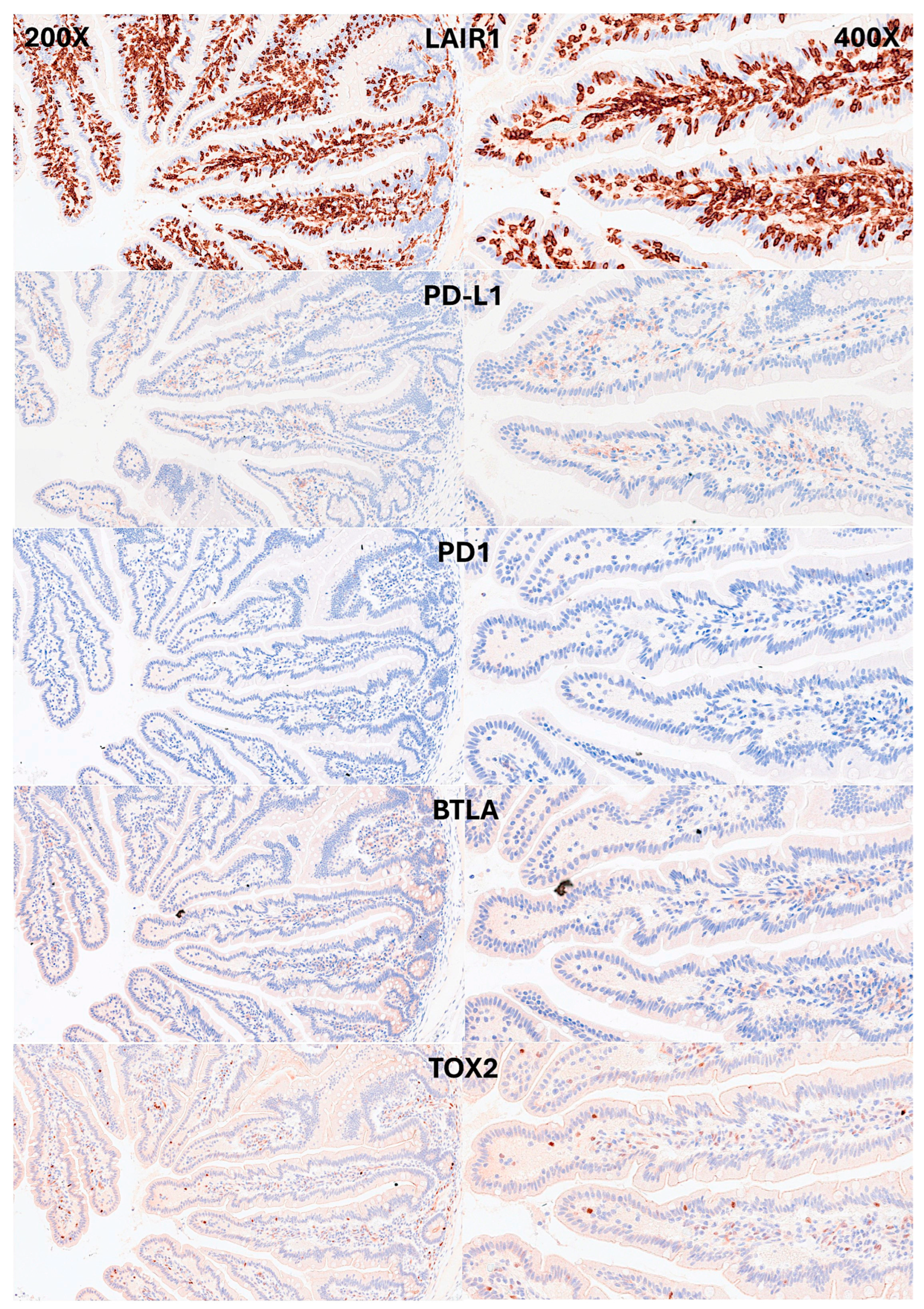
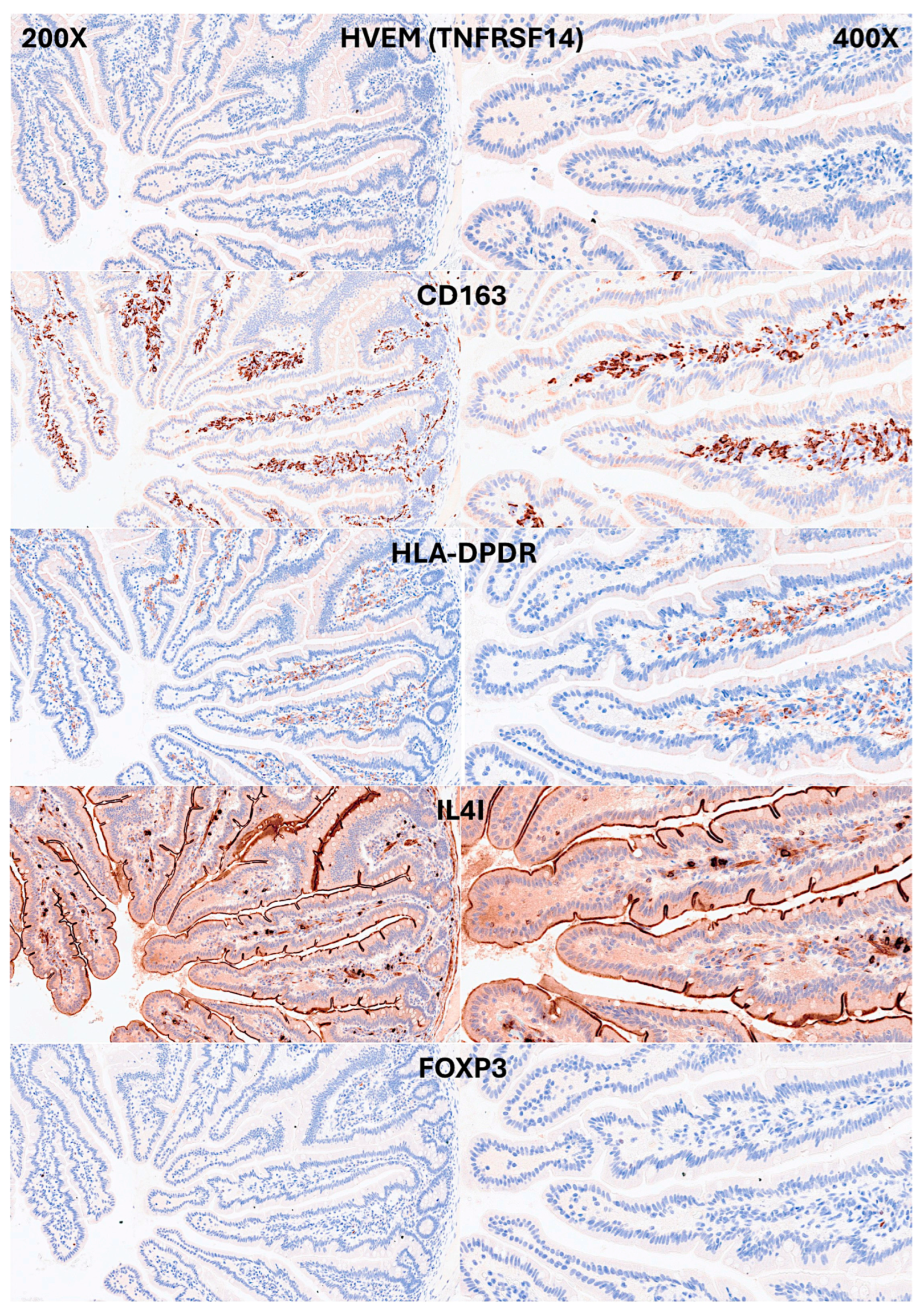
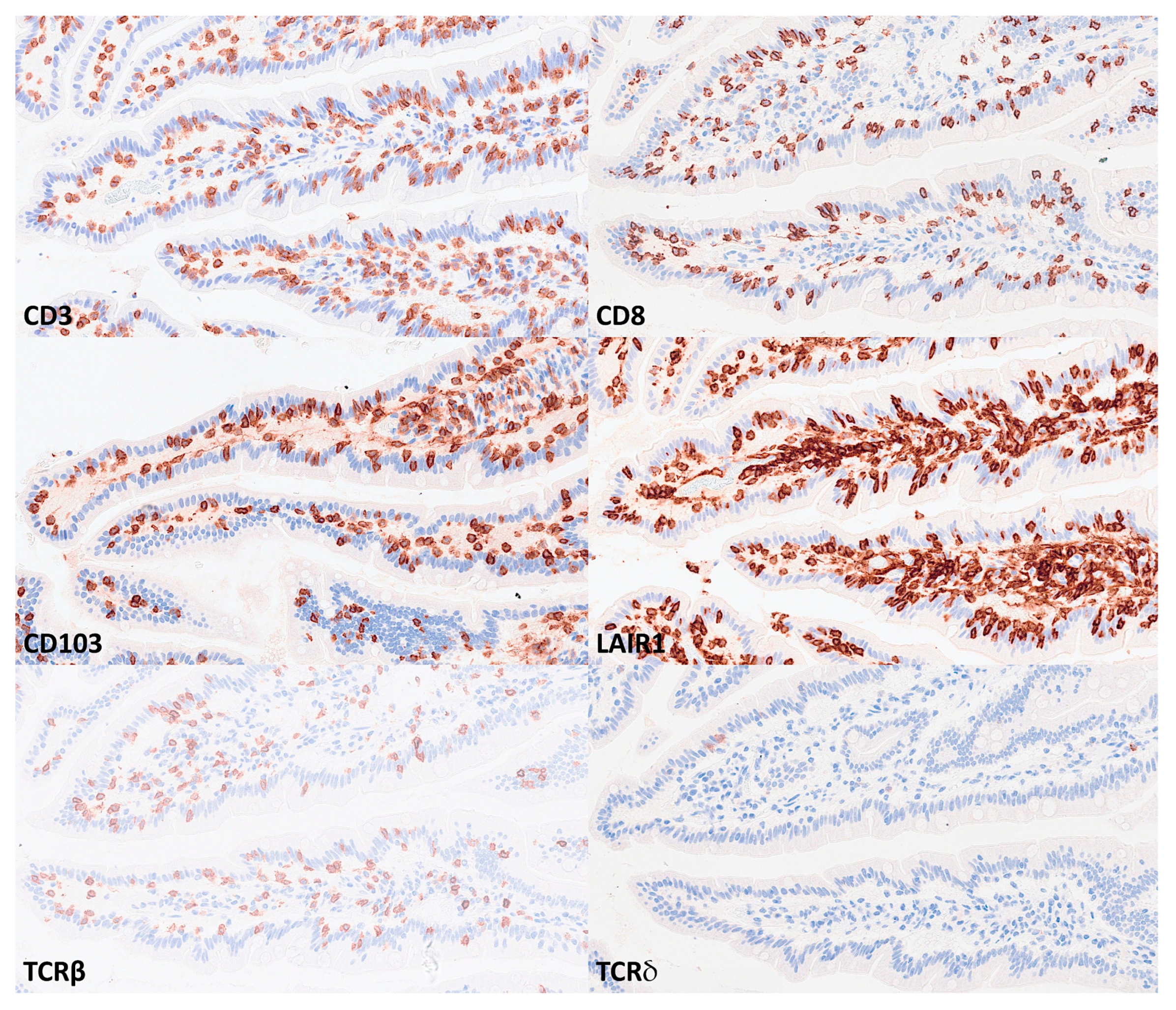
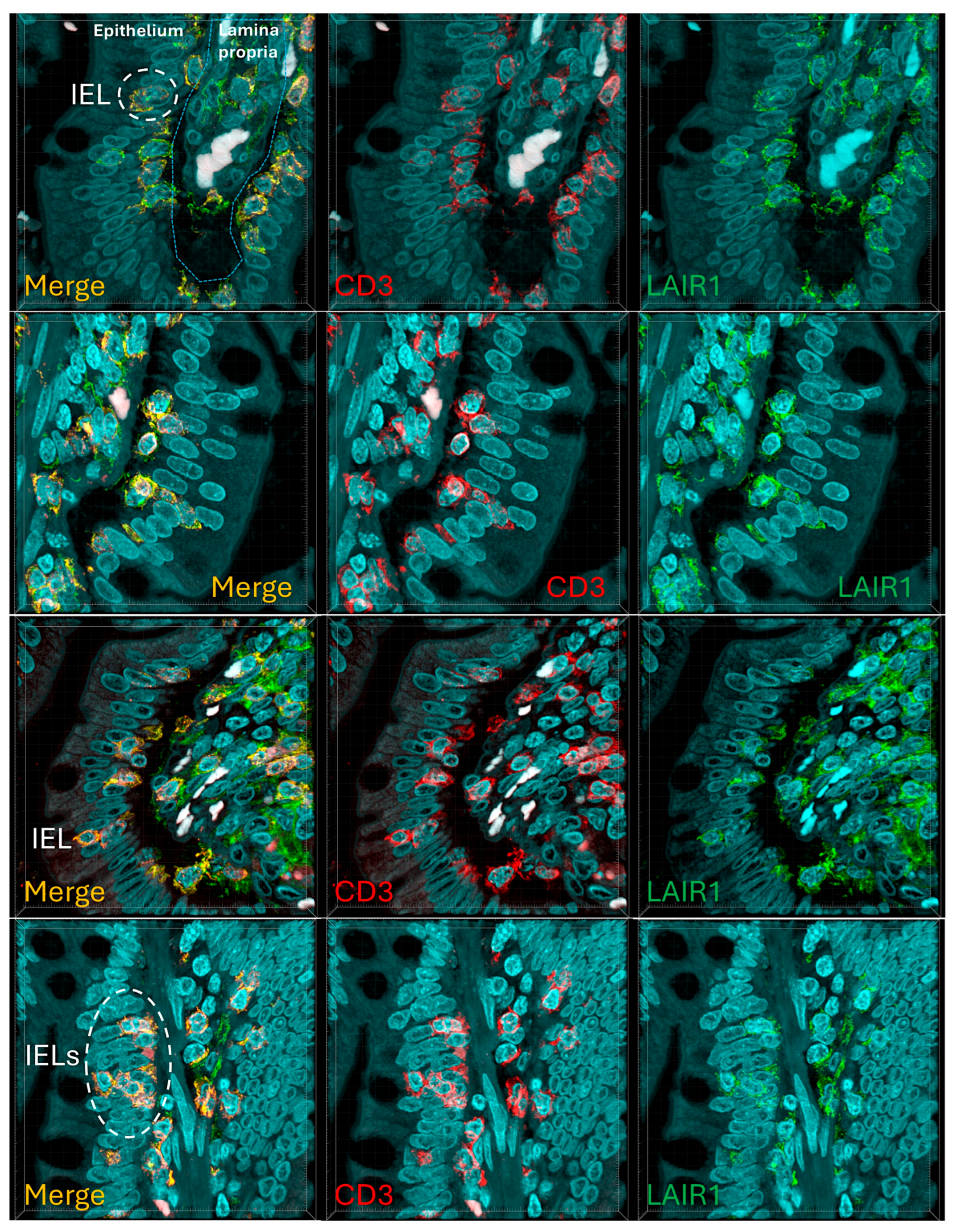
IELs were defined as lymphoid cells within the mucosal epithelial layer. CD3 staining was used as a reference. Under control physiological conditions, IELs were characterized by a T cell phenotype that is positive for CD3 and a cytotoxic phenotype with CD8 expression. Cytotoxic granules were occasionally identified by granzyme B staining. Most IELs were positive for CD103/ITGAE. Most IELs expressed TCRβ chains; therefore, expressed the TCRαβ chains. CD56+IELs, TCRδ chain+IELs (i.e. TCRγδ+IELs), and TOX2+IELs were occasionally found. Notably, all IELs were LAIR1 positive by immunohistochemistry.
Abundant CD163+macrophages/dendritic cells were found in the lamina propria, which also expressed HLA-DP-DQ. CD4+cells were mainly found in the lamina propria; however, clusters attached below the epithelial basal membrane were found. BTLA+cells were found in the lamina propria, which is consistent with our previous results [
77]. PD-L1 expression was limited to the lamina propria in a pattern compatible with APC (macrophages, dendritic cells). Regulatory T-lymphocytes were identified in the lamina propria using the FOXP3 marker.
Table 4 summarizes the findings. Characteristic images are shown in Figure 4,
Figure 5,
Figure 6 and
Figure 7.
Figure 8 confirms that the IELs are CD3 and LAIR1 double-positive using confocal microscopy. Notably, LAIR1 staining revealed that many cells of the lamina propria were LAIR1-positive (Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8 and
Figure 9).
Figure 4.
Immunophenotype characterization of IELs in the intestinal mucosa control. Most IELs were CD3, CD103, and CD8-positive. CD4-positive cells were mainly found in the lamina propria.
Figure 4.
Immunophenotype characterization of IELs in the intestinal mucosa control. Most IELs were CD3, CD103, and CD8-positive. CD4-positive cells were mainly found in the lamina propria.
Figure 5.
Immunophenotype characterization of IELs in the intestinal mucosa control. Most IELs expressed TCRβ chains; therefore, expressed the TCRαβ chains. Occasionally, CD56+IELs were found, as well as TCRδ chain-positive IELs (i.e. TCRγδ+IELs).
Figure 5.
Immunophenotype characterization of IELs in the intestinal mucosa control. Most IELs expressed TCRβ chains; therefore, expressed the TCRαβ chains. Occasionally, CD56+IELs were found, as well as TCRδ chain-positive IELs (i.e. TCRγδ+IELs).
Figure 6.
Immunophenotype characterization of IELs in the intestinal mucosa control. IELs were diffusely and strongly positive for LAIR1. LAIR1 also marked the inflammatory infiltrate of the lamina propria. The expression of PD-L1 and BTLA was limited in the lamina propria. Occasional PD1+ cells were identified, and TOX2+ IELs were occasionally found.
Figure 6.
Immunophenotype characterization of IELs in the intestinal mucosa control. IELs were diffusely and strongly positive for LAIR1. LAIR1 also marked the inflammatory infiltrate of the lamina propria. The expression of PD-L1 and BTLA was limited in the lamina propria. Occasional PD1+ cells were identified, and TOX2+ IELs were occasionally found.
Figure 7.
Immunophenotype characterization of IELs in intestinal mucosa control. Antigen-presenting cells (APCs), mainly macrophages and dendritic cells, were identified using CD163 and HLA-DPDR in the lamina propria. Few FOXP3+Tregs were identified in the lamina propria.
Figure 7.
Immunophenotype characterization of IELs in intestinal mucosa control. Antigen-presenting cells (APCs), mainly macrophages and dendritic cells, were identified using CD163 and HLA-DPDR in the lamina propria. Few FOXP3+Tregs were identified in the lamina propria.
Figure 8.
Main phenotype of IELs in control intestinal mucosa. This figure summarizes the main immunophenotypes of IELs, including CD3+, CD8+, CD103+, LAIR+, and TCRβ. An area with aggregation of IELs and immune cells in the lamina propria are shown.
Figure 8.
Main phenotype of IELs in control intestinal mucosa. This figure summarizes the main immunophenotypes of IELs, including CD3+, CD8+, CD103+, LAIR+, and TCRβ. An area with aggregation of IELs and immune cells in the lamina propria are shown.
Figure 9.
Confocal microscopy showing double immunofluorescence between CD3 (red) and LAIR1(green) in the control small intestine. The IELs were double-positive for CD3 and LAIR1.
Figure 9.
Confocal microscopy showing double immunofluorescence between CD3 (red) and LAIR1(green) in the control small intestine. The IELs were double-positive for CD3 and LAIR1.
3.2. Multicolor Analysis of LAIR1 and Other Immune Markers
We performed quadruple and triple immunofluorescence analyses using confocal microscopy. The combinations that also included nuclear staining were as follows: PD1 (cyan), CD163 (green), and LAIR1 (red); and CD4 (green), CD8 (cyan), and LAIR1 (red).
In the human mucosa, CD4- and probably CD8-positive cells were positive for LAIR1. PD1-positive cells also expressed LAIR1. CD163-positive cells (M2-like macrophages) were partially positive for LAIR1 in the interfollicular area and/or lamina propria. Mantle zone B-lymphocytes were LAIR1-positive, but not in the germinal centers (
Figure 10).
3.3. Analysis of LAIR1 Expression in Patients with Celiac Disease
Table 5 shows the distribution of cases according to the Marsh histological classification. In celiac disease, Mash 0-2 accounted for 31.3%, and Marsh 3 for 68.7% of the cases. As expected, celiac disease cases had higher values in the Marsh classification (P < 0.001).
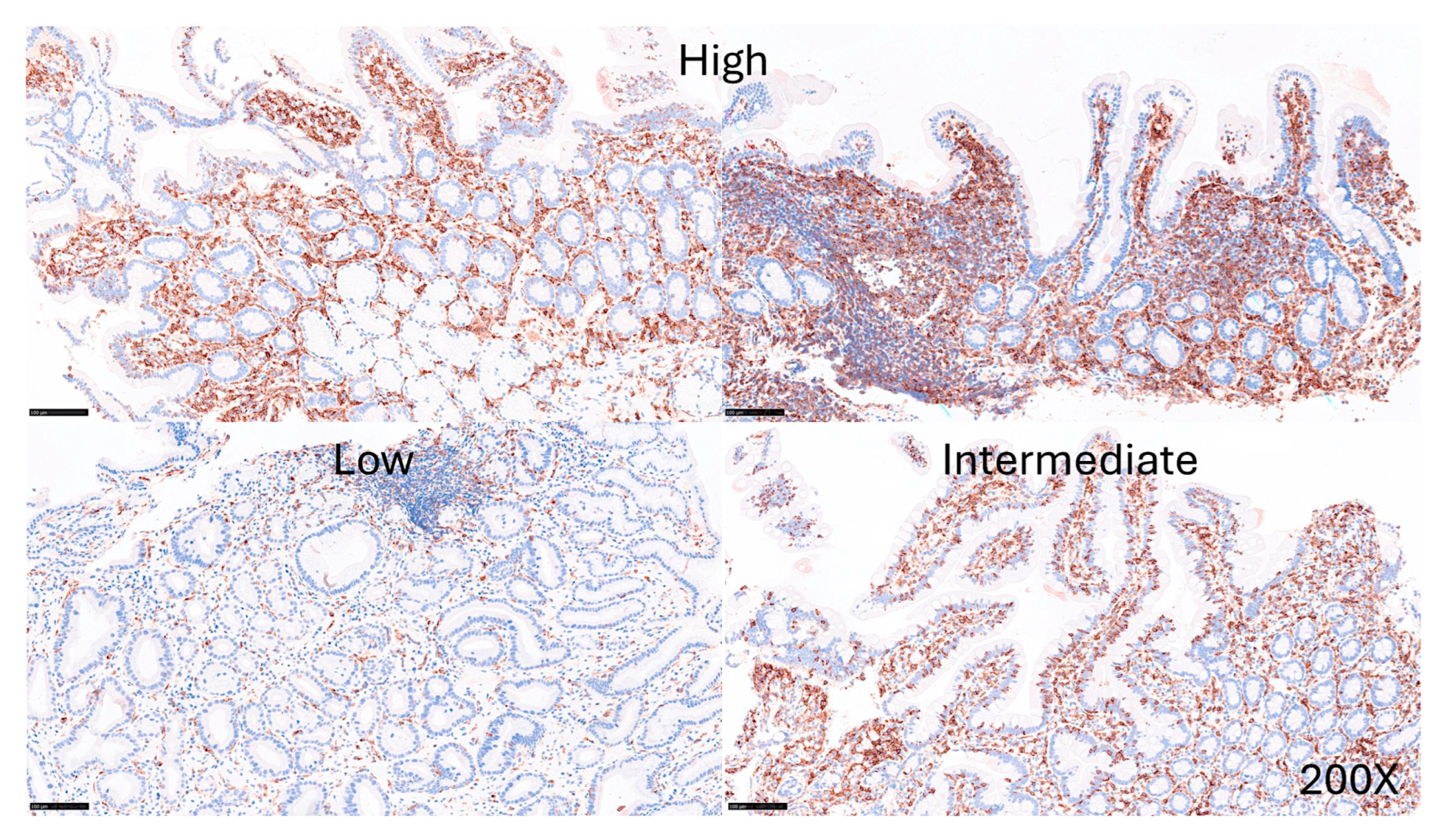
The protein expression of LAIR1 using immunohistochemistry was evaluated in the celiac disease biopsies. LAIR1 was expressed in the IELs, but also in the inflammatory infiltrate of the lamina propria, and the distribution of LAIR1+IELs was heterogeneous within and between biopsies.
LAIR1 expression in the mucosa control was low (1+) in 6/18 (33.3%) or intermediate (2+) in 12/18 (66.7%). In celiac disease, LAIR1 expression ranged from low (1+, 1/16, 6.3%), intermediate (2+, 8/16, 50%), and high (3+, 7/16, 43.8%). Therefore, celiac disease was characterized by higher LAIR1 expression (P = 0.004) (
Table 6).
The histological features of celiac disease ranged from mild alteration with increased IELs to severe atrophy with loss of villi, high epithelial apoptosis, and crypt hyperplasia.
Table 7 shows the correlation between Marsh histological classification and LAIR1. Higher histological lesions were correlated with higher LAIR1 expression (P < 0.001).
Figure 11.
Expression of LAIR1 in celiac disease. LAIR1 expression in the lamina propria ranged from low (1+, <20%, 1/16, 6.3%), intermediate (2+, 20-50%, 8/16, 50%), and high (3+, >50%, 7/16, 43.8%). Higher histological alterations correlated with higher values in the Marsh classification (P < 0.001).
Figure 11.
Expression of LAIR1 in celiac disease. LAIR1 expression in the lamina propria ranged from low (1+, <20%, 1/16, 6.3%), intermediate (2+, 20-50%, 8/16, 50%), and high (3+, >50%, 7/16, 43.8%). Higher histological alterations correlated with higher values in the Marsh classification (P < 0.001).
Figure 12.
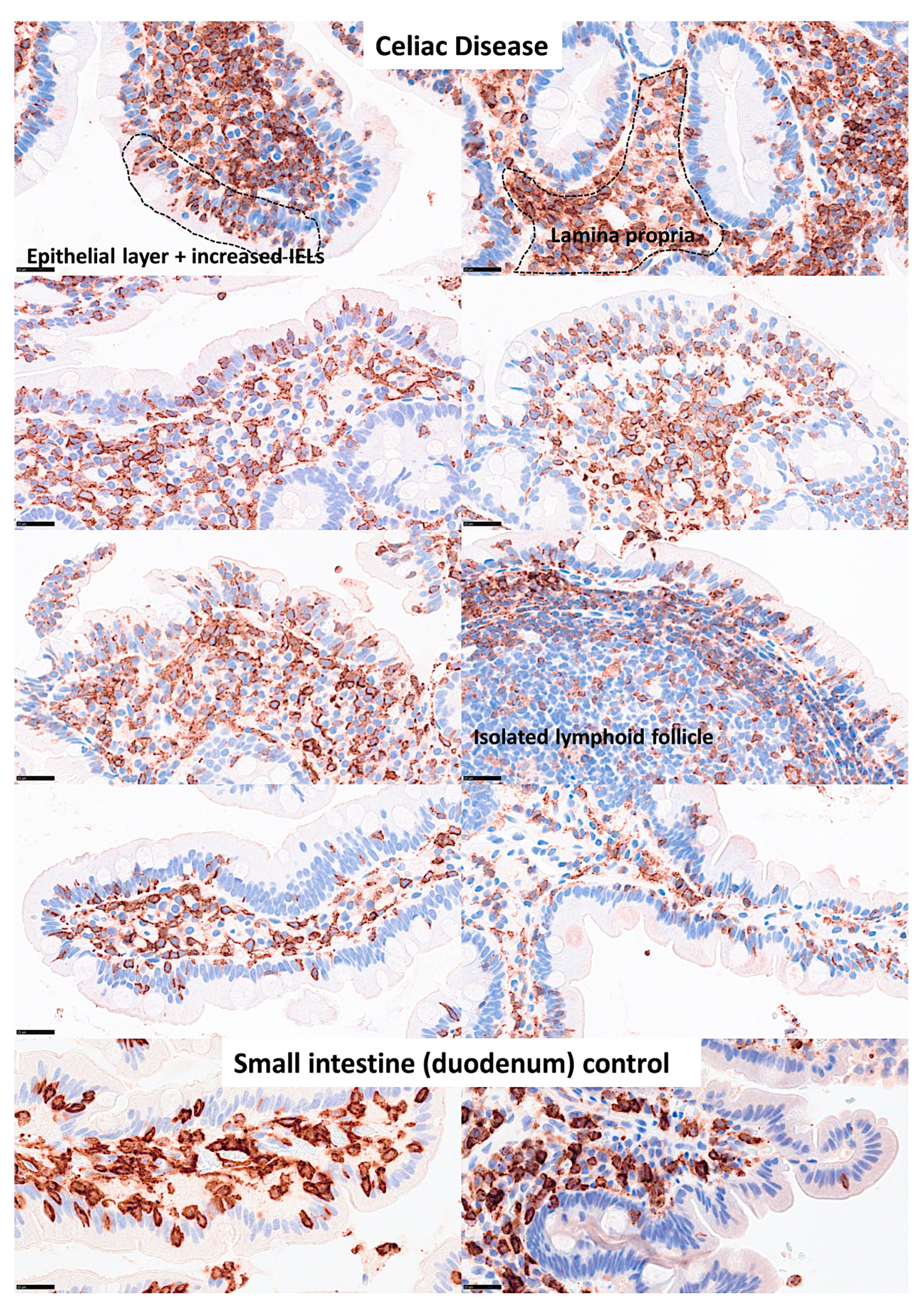
Additional LAIR1 images. Additional images of celiac disease and small intestine control (duodenum) stained with LAIR1 marker are shown. In the small intestine control, LAIR1 expression was limited to the lamina propria, and in IELs when present. In celiac disease, the infiltration of LAIR1+ cells in the lamina propria was variable, but was high (3+) or intermediate (2+) in most cases. Increased numbers of LAIR1+ IELs were found in the epithelial layer of celiac disease cases. In addition to immune system cells, celiac disease showed architectural changes such as villus atrophy and crypt hyperplasia. Original magnification 800x.
Figure 12.
Additional LAIR1 images. Additional images of celiac disease and small intestine control (duodenum) stained with LAIR1 marker are shown. In the small intestine control, LAIR1 expression was limited to the lamina propria, and in IELs when present. In celiac disease, the infiltration of LAIR1+ cells in the lamina propria was variable, but was high (3+) or intermediate (2+) in most cases. Increased numbers of LAIR1+ IELs were found in the epithelial layer of celiac disease cases. In addition to immune system cells, celiac disease showed architectural changes such as villus atrophy and crypt hyperplasia. Original magnification 800x.
3.4. Image Classification of Celiac Disease, Small Intestine Control, and Reactive Tonsil Control Based on LAIR1 Immunohistochemical Expression
Images of LAIR1 protein expression analyzed by immunohistochemistry in celiac disease, small intestine control, and reactive tonsils were used as input data in a ResNet18 model [
185].
The ResNet18 model comprises 18 layers, including convolutional layers and residual blocks [
188,
189,
190]. The series included 11,367 image patches of celiac disease, 11,630 small intestine control, and 8147 reactive tonsil control. The image patches were pooled into 3 different folders, and the data were split into 3 sets: training set (70%) for training the network, validation set (10%) for testing its performance during training, and test set (20%) used after training to assess how well the network performed on new data.
After 5 epochs in the training, the validation accuracy was 99.5%. After image patch classification using the test (holdout) series, the accuracy was 99.6%. The confusion matrix shows the distribution of image patches, including correctly and misclassified patches (Supplementary data). The Grad-CAM technique was used to understand why the deep learning network made its classification decisions in incorrectly classified cases (
Figure 13 and
Figure 14).
3.5. Analysis of LAIR1 in Celiac Disease Using Gene Expression Data
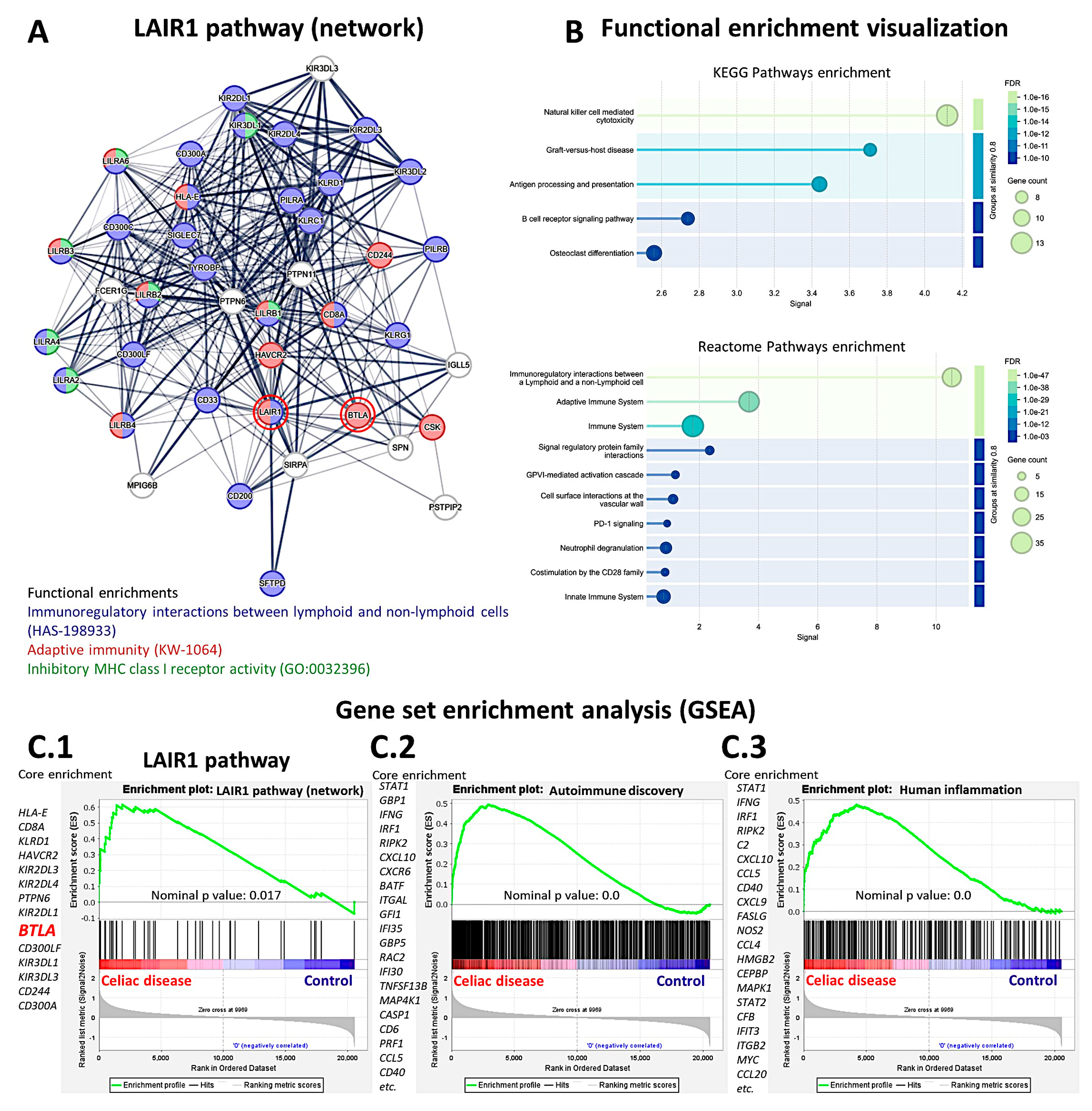
A suitable independent series of celiac disease was used to validate the findings of LAIR1 at the gene expression level. A functional network association analysis approach was used to define the LAIR1 pathway. The result of the network analysis is shown in
Figure 15 A: in the network (i.e., the LAIR1 pathway), the partners are shown and are classified in different colors according to their immunoregulatory interactions, adaptive immunity, and MHC class I inhibitory function. Functional enrichment visualization confirmed the immunoregulatory function of LAIR1 and its partners (
Figure 15 B), and the association (i.e., enrichment, upregulation) of the
LAIR1 pathway in patients with celiac disease was confirmed in the gene set enrichment analysis (GSEA) (
Figure 15 C.1). The enrichment of autoimmune and human inflammation-associated genes was also confirmed by GSEA (Figures C.2 and C.3). Of note, both network analysis and GSEA highlighted the
BTLA marker (Figures 15 A and C.1). BTLA was close to LAIR1 in the network, and in the GSEA BTLA was found within the core enrichment.
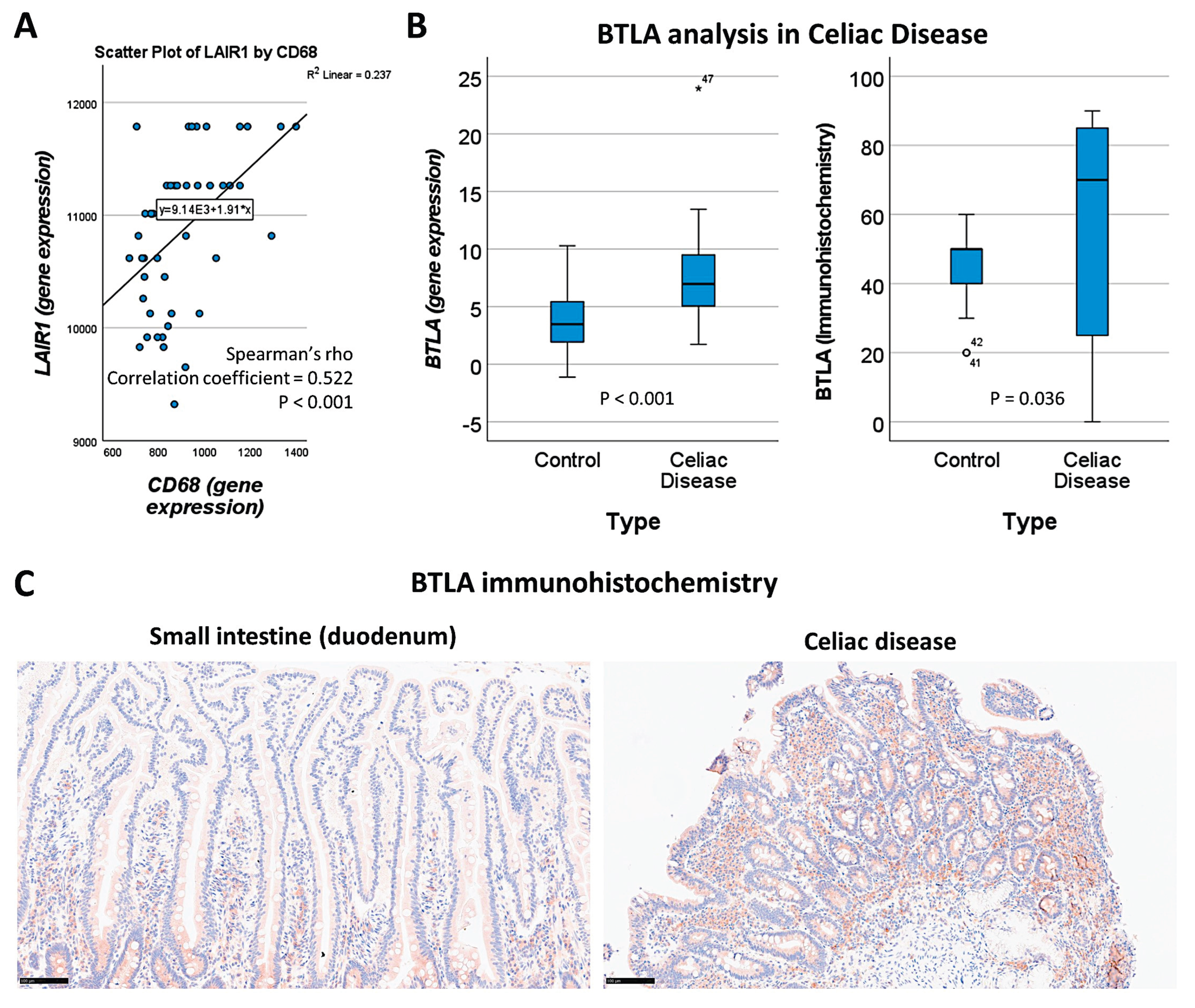
Our study results showed that LAIR1 identified CD163+ cells in the lamina propria (
Section 3.2,
Figure 10). By gene expression,
LAIR1 correlated with CD68, a pan-macrophage marker (
Figure 16A).
BTLA was further analyzed, and gene expression levels were confirmed to be overexpressed in celiac disease (P < 0.001) (
Figure 16 B). At the protein level in our series, high BTLA expression was confirmed in celiac disease cases (P = 0.036) (
Figure 16 B and C).
Figure 15.
Functional network and gene set enrichment analysis (GSEA). The LAIR1 network and pathway were analyzed using functional network association analysis and GSEA. The analysis highlighted the importance of LAIR1 and its partners in immune regulation (A). Functional enrichment analysis confirmed these findings using KEGG and Reactome pathways (B). GSEA confirmed the enrichment (overexpression) of the LAIR1 pathway in celiac disease patients (C.1), as well as other autoimmune (C.2) and human inflammation genes (C.3). Of note, BTLA was highlighted both in the network and GSEA analysis.
Figure 15.
Functional network and gene set enrichment analysis (GSEA). The LAIR1 network and pathway were analyzed using functional network association analysis and GSEA. The analysis highlighted the importance of LAIR1 and its partners in immune regulation (A). Functional enrichment analysis confirmed these findings using KEGG and Reactome pathways (B). GSEA confirmed the enrichment (overexpression) of the LAIR1 pathway in celiac disease patients (C.1), as well as other autoimmune (C.2) and human inflammation genes (C.3). Of note, BTLA was highlighted both in the network and GSEA analysis.
Figure 16.
Correlation between LAIR1 and CD68+ macrophages, and BTLA analysis. By gene expression, LAIR1 correlated with CD68 pan-macrophage marker, as shown in the immunofluorescence confocal analysis (A). LAIR1 network and GSEA analysis pinpointed BTLA marker, which is another immune co-inhibitory marker. BTLA was confirmed to be overexpressed both at RNA and protein levels in patients with celiac disease (B). An example of the different expression of BTLA in the mucosa of control and celiac disease is shown (C).
Figure 16.
Correlation between LAIR1 and CD68+ macrophages, and BTLA analysis. By gene expression, LAIR1 correlated with CD68 pan-macrophage marker, as shown in the immunofluorescence confocal analysis (A). LAIR1 network and GSEA analysis pinpointed BTLA marker, which is another immune co-inhibitory marker. BTLA was confirmed to be overexpressed both at RNA and protein levels in patients with celiac disease (B). An example of the different expression of BTLA in the mucosa of control and celiac disease is shown (C).
4. Discussion
Celiac disease is a gluten-sensitive enteropathy and common immune-mediated inflammatory condition of the small intestine caused by sensitivity to dietary gluten and related proteins in genetically predisposed individuals [
191]. In western countries, celiac disease is estimated to affect approximately 1% of the population. Celiac disease clinically presents as heterogeneous; therefore, it continues to be underestimated [
191].
There are several phenotypes of celiac disease. Symptomatic diseases include classic and nonclassic celiac diseases. Classic celiac disease is a gluten-sensitive enteropathy characterized by diarrhea, signs and symptoms of malabsorption, villous atrophy, and resolution upon withdrawal from gluten-containing foods [
79]. Nonclassic celiac disease is also known as “atypical” and the patients lack the classic symptoms of malabsorption and only present with minor gastrointestinal complaints. However, duodenal biopsies show villous atrophy, the production of celiac autoantibodies, such as anti-tissue transglutaminase, and extraintestinal manifestations [
123,
192].
The other phenotypes include subclinical or asymptomatic, potential, latent, and refractory celiac disease. Refractory celiac disease is defined as the persistence of clinical symptoms and villous atrophy despite adherence to a gluten-free diet. Failure to improve on a gluten-free diet is mostly due to noncompliance. However, in few cases, a pure refractory condition is found: refractory celiac disease type 1 (normal population of IELs), the semi-malignant inflammatory condition (refractory type 2; aberrant immunophenotype and T-cell receptor clonality analysis of IELs), transformation to enteropathy-associated T-cell lymphoma (EATL), collagenous sprue, or alternative diagnosis of autoimmune enteropathy [
126,
127,
193].
The cause of refractory disease is unknown, and immunosuppression has been the treatment of choice. Traditional glucocorticoids, such as intravenous hydrocortisone and oral prednisolone are used. Alternative immunosuppressant therapies include azathioprine, 6-mercaptopurine, and thioguanine [
126,
194,
195,
196,
197]. A monoclonal antibody therapy using anti-CD52 (alemtuzumab) was reported [
198].
Badran YR et al. reported eight cases of immune checkpoint inhibitor-associated celiac disease, suggesting that the drugs disrupted gut immune homeostasis and tolerance mechanism [
199]. In that study, immunohistochemical analysis of several markers included CD3, CD8, TCRγδ, PD1, CD68, PD-L1, and quantification of IELs [
199]. In our study, we analyzed several immuno-oncology markers in small intestine control, and later LAIR1 expression in celiac disease. Our findings showed that in the small intestine, LAIR1 expression is found not only in IELs but also in lamina propria immune cells. LAIR1 was diffusely expressed in celiac disease.
LAIR1 belongs to the family of immune-inhibitory receptors and is expressed by mature hematopoietic cells, particularly in natural killer (NK) and T/B-lymphocytes immune cells [
198]. Beyond the physiological function of immune homeostasis and immune tolerance, LAIR1 has been involved in several autoimmune and inflammatory conditions and neoplasia [
156]: allergy [
200], systemic lupus erythematosus [
201], rheumatoid arthritis [
175,
202,
203], graft rejection [
204], breast carcinoma [
205], glioma [
206], solid tumors [
207], hepatocellular carcinoma [
208], among others.
We recently demonstrated the usefulness of using deep learning to analyze gene expression and classify images of celiac disease [
77,
78] and ulcerative colitis [
173,
184,
209]. In this study, deep learning was used to classify LAIR1 image patches between celiac disease, small intestine control, and reactive tonsils. The proposed network managed to classify images with good performance. However, the aim was to conduct a proof-of-concept analysis, not to create a trained network production or commercial applications. Narrow artificial intelligence is not ready to take over the job of pathology-trained medical doctors because histological biopsies obtained from endoscopic examinations may be associated with other diseases. Notably, other research groups, such as Denholm et al., Molder et al., Scheppach et al., and Schreiber et al. (among others), have successfully used deep learning in celiac disease [
210,
211,
212,
213,
214,
215,
216,
217].
This study analyzed the phenotype of intraepithelial lymphocytes (IELs) and the lamina propria in the small intestine, including LAIR1; and confirmed the LAIR1 expression in celiac disease. In celiac disease, both IELs and lamina propria cells were positive for LAIR1. Compared with the small intestine control, the lamina propria infiltration in celiac disease was higher. Finally, as a proof-of-concept AI analysis, a convolutional neural network classified LAIR1-stained image patches between the 3 diagnoses of small intestine control, celiac disease, and reactive tonsils with high accuracy. Therefore, IELs are positive for LAIR1. The LAIR1 marker is relevant in intestinal mucosa immunology, and celiac disease.
Of note, one clinical trial targeting LAIR1 is listed in the website of clinicaltrials.gov (last accessed on July 31, 2025): A Safety, Tolerability and Efficacy Study of NC525 in Subjects With Advanced Myeloid Neoplasms (Id. NCT05787496, NextCure, Inc., drug NC525, Monoclonal antibody specific for LAIR-1).
5. Conclusions
This study used several immuno-oncology and immune-phenotype markers to characterize the intraepithelial lymphocytes and the lamina propria of small intestine control and to confirm the expression of LAIR1 in celiac disease. In small intestine mucosa control, IELs exhibited a cytotoxic T-cell phenotype and were CD3, CD8, CD103, TCRβ, and LAIR1-positive. In celiac disease, both IELs and many lamina propria cells were LAIR1-positive. In comparison to small intestine control, LAIR1 lamina propria infiltration in celiac disease was higher. This study also successfully performed a proof-of-concept deep learning histological analysis of LAIR1 between small intestine control, celiac disease, and reactive tonsils. A convolutional neural network classified LAIR1-stained image patches between the 3 diagnoses of small intestine control, celiac disease, and reactive tonsils with high accuracy (99.6%).
In conclusion, IELs are LAIR1 positive. High LAIR1 expression in IELs and lamina propria immune cells characterize CD.
Supplementary Materials
The following supporting information (output file from CNN ResNet image classification) can be downloaded at the website of this paper posted on Preprints.org.
Funding
This research was funded to J.C. by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS), grant numbers KAKEN 15K19061, 18K15100, and 23K06454.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of TOKAI UNIVERSITY, SCHOOL OF MEDICINE (protocol codes IRB14R-080 and IRB20-156).
Informed Consent Statement
Informed consent was obtained from study participants.
Data Availability Statement
All data and methodology are available upon request to Dr Joaquim Carreras (joaquim.carreras@tokai.ac.jp), and are also uploaded to the Zenodo open repository: Carreras, J. (2025). LAIR1 Celiac Disease for CNN (examples) (Version 1) [Data set]. Zenodo.
https://doi.org/10.5281/zenodo.16451426 ; Carreras, J. (2025). LAIR1 images Celiac Disease and controls (Version 1) [Data set]. Zenodo.
https://doi.org/10.5281/zenodo.16911678 .
Conflicts of Interest
The author have no conflict of interest to declare.
Abbreviations
The following abbreviations are used in this manuscript:
| IELs |
Intraepithelial lymphocytes |
| EATL |
Enteropathy-associated T-cell lymphoma |
| LAIR1 |
Leukocyte-associated immunoglobulin like receptor 1 |
Appendix A
Appendix A1. Table A1. Clinicopathological Characteristics of Patients with Celiac Disease.
| Age |
Sex |
Biopsy Location |
Diagnosis |
Marsh |
LAIR1 |
| 70 |
Male |
Duodenum |
Celiac Disease |
3a |
3+ |
| 62 |
Male |
Pylorus/duodenum |
Celiac Disease/Chronic gastritis |
2 |
1+ |
| 62 |
Male |
Duodenum |
Celiac Disease |
2 |
2+ |
| 78 |
Female |
Duodenum |
Celiac Disease |
3b |
3+ |
| 59 |
Male |
Duodenum |
Celiac Disease |
3a |
2+ |
| 44 |
Female |
Duodenum |
Celiac Disease |
2 |
2+ |
| 17 |
Female |
Duodenum |
Celiac Disease |
3b |
3+ |
| 56 |
Female |
Duodenum |
Celiac Disease |
3a |
2+ |
| 54 |
Female |
Duodenum |
Celiac Disease |
2 |
2+ |
| 58 |
Female |
Duodenum |
Celiac Disease |
3b |
3+ |
| 61 |
Female |
Duodenum |
Celiac Disease |
3c |
3+ |
| 45 |
Male |
Duodenum |
Celiac Disease |
3a |
2+ |
| 70 |
Female |
Duodenum |
Celiac Disease |
2 |
2+ |
| 40 |
Female |
Duodenum |
Celiac Disease |
3a |
2+ |
| 61 |
Female |
Duodenum |
Celiac Disease |
3c |
3+ |
| 44 |
Female |
Duodenum |
Celiac Disease |
3a |
3+ |
| 63 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
1+
++ |
| 64 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 64 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 64 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
1+ |
| 72 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 72 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 63 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 63 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 68 |
Female |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 68 |
Female |
Small intestine control |
Reactive lymphoid tissue |
0 |
1+ |
| 63 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
1+ |
| 53 |
Female |
Small intestine control |
Reactive lymphoid tissue |
0 |
1+ |
| 64 |
Male |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 73 |
Female |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 73 |
Female |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 73 |
Female |
Small intestine control |
Reactive lymphoid tissue |
0 |
2+ |
| 76 |
Male |
Small intestine control |
Duodenum, reactive lymphoid tissue |
0 |
2+ |
| 59 |
Male |
Small intestine control |
Jejunum, reactive lymphoid tissue |
0 |
1+ |
| 55 |
Male |
Tonsil (AI analysis) |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 51 |
Male |
Lymph node (axilla) |
Hodgkin lymphoma (IHC control*) |
N/A |
N/A |
| 55 |
Male |
Left testicle |
Diffuse large B-cell lymphoma (IHCcontrol*) |
N/A |
N/A |
| 42 |
Male |
Left testicle |
Diffuse large B-cell lymphoma (IHC control*) |
N/A |
N/A |
| 66 |
Female |
Lymph node (neck) |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 28 |
Female |
Tonsil |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 30 |
Female |
Tonsil |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 28 |
Male |
Tonsil |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 61 |
Female |
Tonsil |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 45 |
Male |
Tonsil |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 55 |
Female |
Lymph node (neck) |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 26 |
Male |
Tonsil |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 76 |
Female |
Appendix |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| 21 |
Male |
Lymph node (abdomen) |
Reactive lymphoid hyperplasia |
N/A |
N/A |
| Marsh, Marsh-Oberhuber classification; N/A, non-assessable/applicable; IHC, immunohistochemistry; * Not used for analysis, only as LAIR1 immunohistochemical staining internal control. |
References
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. Biomed Res Int 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Shen, J. The roles and functions of Paneth cells in Crohn’s disease: A critical review. Cell Prolif 2021, 54, e12958. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Atanga, R.; Singh, V.; In, J.G. Intestinal Enteroendocrine Cells: Present and Future Druggable Targets. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S.M. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J Clin Med Res 2009, 1, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol (Oxf) 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Cinci, L.; Faussone-Pellegrini, M.S.; Rotondo, A.; Mule, F.; Vannucchi, M.G. GLP-2 receptor expression in excitatory and inhibitory enteric neurons and its role in mouse duodenum contractility. Neurogastroenterol Motil 2011, 23, e383–392. [Google Scholar] [CrossRef]
- Guan, X.; Shi, X.; Li, X.; Chang, B.; Wang, Y.; Li, D.; Chan, L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab 2012, 303, E853–864. [Google Scholar] [CrossRef]
- Overton, H.A.; Fyfe, M.C.; Reynet, C. GPR119, a novel G protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br J Pharmacol 2008, 153 Suppl 1, S76–81. [Google Scholar] [CrossRef]
- Martin, B.; Lopez de Maturana, R.; Brenneman, R.; Walent, T.; Mattson, M.P.; Maudsley, S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular Med 2005, 7, 3–36. [Google Scholar] [CrossRef]
- Szewczyk, J.R.; Laudeman, C. CCK1R agonists: a promising target for the pharmacological treatment of obesity. Curr Top Med Chem 2003, 3, 837–854. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Katsuma, S.; Adachi, T.; Koshimizu, T.A.; Hirasawa, A.; Tsujimoto, G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn Schmiedebergs Arch Pharmacol 2008, 377, 523–527. [Google Scholar] [CrossRef]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol 2013, 34, 228–252. [Google Scholar] [CrossRef]
- Harda, K.; Szabo, Z.; Juhasz, E.; Dezso, B.; Kiss, C.; Schally, A.V.; Halmos, G. Expression of Somatostatin Receptor Subtypes (SSTR-1-SSTR-5) in Pediatric Hematological and Oncological Disorders. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Schmassmann, A.; Reubi, J.C. Cholecystokinin-B/gastrin receptors enhance wound healing in the rat gastric mucosa. J Clin Invest 2000, 106, 1021–1029. [Google Scholar] [CrossRef]
- Larsson, L.I. Developmental biology of gastrin and somatostatin cells in the antropyloric mucosa of the stomach. Microsc Res Tech 2000, 48, 272–281. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Zhang, X.Y.; Liu, X.; Liu, X.; Wu, X.; Jose, P.A.; Duan, S.; Xu, F.J.; Yang, Z. Intestinal Gastrin/CCKBR (Cholecystokinin B Receptor) Ameliorates Salt-Sensitive Hypertension by Inhibiting Intestinal Na(+)/H(+) Exchanger 3 Activity Through a PKC (Protein Kinase C)-Mediated NHERF1 and NHERF2 Pathway. Hypertension 2022, 79, 1668–1679. [Google Scholar] [CrossRef]
- Xiao, Y.; Yan, W.; Lu, Y.; Zhou, K.; Cai, W. Neurotensin contributes to pediatric intestinal failure-associated liver disease via regulating intestinal bile acids uptake. EBioMedicine 2018, 35, 133–141. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Yan, B.; Weiss, H.L.; Weiss, L.T.; Gao, T.; Evers, B.M. Neurotensin differentially regulates bile acid metabolism and intestinal FXR-bile acid transporter axis in response to nutrient abundance. FASEB J 2021, 35, e21371. [Google Scholar] [CrossRef]
- Takeshita, E.; Matsuura, B.; Dong, M.; Miller, L.J.; Matsui, H.; Onji, M. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol 2006, 41, 223–230. [Google Scholar] [CrossRef]
- Miedzybrodzka, E.L.; Foreman, R.E.; Lu, V.B.; George, A.L.; Smith, C.A.; Larraufie, P.; Kay, R.G.; Goldspink, D.A.; Reimann, F.; Gribble, F.M. Stimulation of motilin secretion by bile, free fatty acids, and acidification in human duodenal organoids. Mol Metab 2021, 54, 101356. [Google Scholar] [CrossRef]
- Modvig, I.M.; Andersen, D.B.; Grunddal, K.V.; Kuhre, R.E.; Martinussen, C.; Christiansen, C.B.; Orskov, C.; Larraufie, P.; Kay, R.G.; Reimann, F.; Gribble, F.M.; Hartmann, B.; Bojsen-Moller, K.N.; Madsbad, S.; Wewer Albrechtsen, N.J.; Holst, J.J. Secretin release after Roux-en-Y gastric bypass reveals a population of glucose-sensitive S cells in distal small intestine. Int J Obes (Lond) 2020, 44, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Kobayashi, K.; Kusakizako, T.; Iida, W.; Kato, M.; Shihoya, W.; Nureki, O. Structure of the human secretin receptor coupled to an engineered heterotrimeric G protein. Biochem Biophys Res Commun 2020, 533, 861–866. [Google Scholar] [CrossRef]
- Roulis, M.; Flavell, R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016, 92, 116–131. [Google Scholar] [CrossRef]
- Uchida, K.; Kamikawa, Y. Muscularis mucosae - the forgotten sibling. J Smooth Muscle Res 2007, 43, 157–177. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, H.; Sun, L.; Tong, J.; Cui, C.; Bai, Z.; Yan, J.; Qin, D.; Liu, Y.; Wang, J.; Wu, X.; Li, B. The application of small intestinal submucosa in tissue regeneration. Mater Today Bio 2024, 26, 101032. [Google Scholar] [CrossRef]
- Lai, S.; Yu, W.; Wallace, L.; Sigalet, D. Intestinal muscularis propria increases in thickness with corrected gestational age and is focally attenuated in patients with isolated intestinal perforations. J Pediatr Surg 2014, 49, 114–119. [Google Scholar] [CrossRef]
- Beagley, K.W.; Husband, A.J. Intraepithelial lymphocytes: origins, distribution, and function. Crit Rev Immunol 1998, 18, 237–254. [Google Scholar] [CrossRef]
- Mayassi, T.; Jabri, B. Human intraepithelial lymphocytes. Mucosal Immunol 2018, 11, 1281–1289. [Google Scholar] [CrossRef]
- Lin, T.; Matsuzaki, G.; Kenai, H.; Nakamura, T.; Nomoto, K. Thymus influences the development of extrathymically derived intestinal intraepithelial lymphocytes. Eur J Immunol 1993, 23, 1968–1974. [Google Scholar] [CrossRef]
- Matsuzaki, G.; Lin, T.; Nomoto, K. Differentiation and function of intestinal intraepithelial lymphocytes. Int Rev Immunol 1994, 11, 47–60. [Google Scholar] [CrossRef]
- Lin, T.; Matsuzaki, G.; Kenai, H.; Kishihara, K.; Nabeshima, S.; Fung-Leung, W.P.; Mak, T.W.; Nomoto, K. Characteristics of fetal thymus-derived T cell receptor gamma delta intestinal intraepithelial lymphocytes. Eur J Immunol 1994, 24, 1792–1798. [Google Scholar] [CrossRef]
- Trejdosiewicz, L.K. Intestinal intraepithelial lymphocytes and lymphoepithelial interactions in the human gastrointestinal mucosa. Immunol Lett 1992, 32, 13–19. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Page, S.T.; Pullen, A.M. Distinct methylation states of the CD8 beta gene in peripheral T cells and intraepithelial lymphocytes. J Immunol 1997, 159, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.; Mucida, D.; Bilate, A.M. Intraepithelial Lymphocytes of the Intestine. Annu Rev Immunol 2024, 42, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, R.; Nemoto, Y.; Yonemoto, Y.; Tanaka, S.; Takei, Y.; Oshima, S.; Nagaishi, T.; Tsuchiya, K.; Nozaki, K.; Mizutani, T.; Nakamura, T.; Watanabe, M.; Okamoto, R. Intraepithelial Lymphocytes Suppress Intestinal Tumor Growth by Cell-to-Cell Contact via CD103/E-Cadherin Signal. Cell Mol Gastroenterol Hepatol 2021, 11, 1483–1503. [Google Scholar] [CrossRef]
- Hartl, C.; Finke, J.; Hasselblatt, P.; Kreisel, W.; Schmitt-Graeff, A. Diagnostic and therapeutic challenge of unclassifiable enteropathies with increased intraepithelial CD103(+) CD8(+) T lymphocytes: a single center case series. Scand J Gastroenterol 2021, 56, 889–898. [Google Scholar] [CrossRef]
- Dietz, S.B.; Whitaker-Menezes, D.; Lessin, S.R. The role of alpha E beta 7 integrin (CD103) and E-cadherin in epidermotropism in cutaneous T-cell lymphoma. J Cutan Pathol 1996, 23, 312–318. [Google Scholar] [CrossRef]
- Xu, W.; Bergsbaken, T.; Edelblum, K.L. The multifunctional nature of CD103 (alphaEbeta7 integrin) signaling in tissue-resident lymphocytes. Am J Physiol Cell Physiol 2022, 323, C1161–C1167. [Google Scholar] [CrossRef]
- Yomogida, K.; Trsan, T.; Sudan, R.; Rodrigues, P.F.; Ulezko Antonova, A.; Ingle, H.; Luccia, B.D.; Collins, P.L.; Cella, M.; Gilfillan, S.; Baldridge, M.T.; Oltz, E.M.; Colonna, M. The transcription factor Aiolos restrains the activation of intestinal intraepithelial lymphocytes. Nat Immunol 2024, 25, 77–87. [Google Scholar] [CrossRef]
- Jabri, B.; de Serre, N.P.; Cellier, C.; Evans, K.; Gache, C.; Carvalho, C.; Mougenot, J.F.; Allez, M.; Jian, R.; Desreumaux, P.; Colombel, J.F.; Matuchansky, C.; Cugnenc, H.; Lopez-Botet, M.; Vivier, E.; Moretta, A.; Roberts, A.I.; Ebert, E.C.; Guy-Grand, D.; Brousse, N.; Schmitz, J.; Cerf-Bensussan, N. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology 2000, 118, 867–879. [Google Scholar] [CrossRef]
- Melandri, D.; Zlatareva, I.; Chaleil, R.A.G.; Dart, R.J.; Chancellor, A.; Nussbaumer, O.; Polyakova, O.; Roberts, N.A.; Wesch, D.; Kabelitz, D.; Irving, P.M.; John, S.; Mansour, S.; Bates, P.A.; Vantourout, P.; Hayday, A.C. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol 2018, 19, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Girardi, M.; Lewis, J.M.; Filler, R.B.; Hayday, A.C.; Tigelaar, R.E. Environmentally responsive and reversible regulation of epidermal barrier function by gammadelta T cells. J Invest Dermatol 2006, 126, 808–814. [Google Scholar] [CrossRef]
- Nakandakari-Higa, S.; Canesso, M.C.C.; Walker, S.; Chudnovskiy, A.; Jacobsen, J.T.; Bilanovic, J.; Parigi, S.M.; Fiedorczuk, K.; Fuchs, E.; Bilate, A.M.; Pasqual, G.; Mucida, D.; Pritykin, Y.; Victora, G.D. Universal recording of cell-cell contacts in vivo for interaction-based transcriptomics. bioRxiv 2023. [Google Scholar]
- Hariss, F.; Delbeke, M.; Guyot, K.; Zarnitzky, P.; Ezzedine, M.; Certad, G.; Meresse, B. Cytotoxic innate intraepithelial lymphocytes control early stages of Cryptosporidium infection. Front Immunol 2023, 14, 1229406. [Google Scholar] [CrossRef]
- Zhou, C.; Qiu, Y.; Yang, H. CD4CD8alphaalpha IELs: They Have Something to Say. Front Immunol 2019, 10, 2269. [Google Scholar] [CrossRef]
- Yakou, M.H.; Ghilas, S.; Tran, K.; Liao, Y.; Afshar-Sterle, S.; Kumari, A.; Schmid, K.; Dijkstra, C.; Inguanti, C.; Ostrouska, S.; Wilcox, J.; Smith, M.; Parathan, P.; Allam, A.; Xue, H.H.; Belz, G.T.; Mariadason, J.M.; Behren, A.; Drummond, G.R.; Ruscher, R.; Williams, D.S.; Pal, B.; Shi, W.; Ernst, M.; Raghu, D.; Mielke, L.A. TCF-1 limits intraepithelial lymphocyte antitumor immunity in colorectal carcinoma. Sci Immunol 2023, 8, eadf2163. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, A.; Botella, T.; Moon, C.S.; Rao, S.; Gelbs, J.; Cheng, L.; Miller, J.; Bacarella, A.M.; Garcia-Vilas, J.A.; Vargas, J.; Yu, X.; Krupska, I.; Bush, E.; Garcia-Carrasquillo, R.; Lebwohl, B.; Krishnareddy, S.; Lewis, S.; Green, P.H.R.; Bhagat, G.; Yan, K.S.; Han, A. Gluten induces rapid reprogramming of natural memory alphabeta and gammadelta intraepithelial T cells to induce cytotoxicity in celiac disease. Sci Immunol 2023, 8, eadf4312. [Google Scholar] [CrossRef]
- Russell, G.J.; Nagler-Anderson, C.; Anderson, P.; Bhan, A.K. Cytotoxic potential of intraepithelial lymphocytes (IELs). Presence of TIA-1, the cytolytic granule-associated protein, in human IELs in normal and diseased intestine. Am J Pathol 1993, 143, 350–354. [Google Scholar]
- Abadie, V.; Discepolo, V.; Jabri, B. Intraepithelial lymphocytes in celiac disease immunopathology. Semin Immunopathol 2012, 34, 551–566. [Google Scholar] [CrossRef]
- Iijima, H.; Takahashi, I.; Kiyono, H. Mucosal immune network in the gut for the control of infectious diseases. Rev Med Virol 2001, 11, 117–133. [Google Scholar] [CrossRef]
- Santiago, L.; Castro, M.; Pardo, J.; Arias, M. Mouse Model of Colitis-Associated Colorectal Cancer (CAC): Isolation and Characterization of Mucosal-Associated Lymphoid Cells. Methods Mol Biol 2019, 1884, 189–202. [Google Scholar]
- Gui, Y.; Cheng, H.; Zhou, J.; Xu, H.; Han, J.; Zhang, D. Development and function of natural TCR(+) CD8alphaalpha(+) intraepithelial lymphocytes. Front Immunol 2022, 13, 1059042. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Hummel, J.F.; Faller, L.; d’Hargues, Y.; Ebert, K.; Tanriver, Y. A committed postselection precursor to natural TCRalphabeta(+) intraepithelial lymphocytes. Mucosal Immunol 2018, 11, 333–344. [Google Scholar] [CrossRef]
- Harada, Y.; Sujino, T.; Miyamoto, K.; Nomura, E.; Yoshimatsu, Y.; Tanemoto, S.; Umeda, S.; Ono, K.; Mikami, Y.; Nakamoto, N.; Takabayashi, K.; Hosoe, N.; Ogata, H.; Ikenoue, T.; Hirao, A.; Kubota, Y.; Kanai, T. Intracellular metabolic adaptation of intraepithelial CD4(+)CD8alphaalpha(+) T lymphocytes. iScience 2022, 25, 104021. [Google Scholar] [CrossRef] [PubMed]
- Morrow, N.M.; Morissette, A.; Mulvihill, E.E. Immunomodulation and inflammation: Role of GLP-1R and GIPR expressing cells within the gut. Peptides 2024, 176, 171200. [Google Scholar] [CrossRef]
- Canesso, M.C.C.; Lemos, L.; Neves, T.C.; Marim, F.M.; Castro, T.B.R.; Veloso, E.S.; Queiroz, C.P.; Ahn, J.; Santiago, H.C.; Martins, F.S.; Alves-Silva, J.; Ferreira, E.; Cara, D.C.; Vieira, A.T.; Barber, G.N.; Oliveira, S.C.; Faria, A.M.C. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation. Mucosal Immunol 2018, 11, 820–834. [Google Scholar] [CrossRef]
- Gao, J.; Xu, C.; Zhang, M.; Liu, J.; Wu, X.; Cui, C.; Wei, H.; Peng, J.; Zheng, R. Functional fiber enhances the effect of every-other-day fasting on insulin sensitivity by regulating the gut microecosystem. J Nutr Biochem 2022, 110, 109122. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, A.; Miyake, S.; Saga, R.; Chiba, A.; Mochizuki, H.; Yamamura, T. Gut environment-induced intraepithelial autoreactive CD4(+) T cells suppress central nervous system autoimmunity via LAG-3. Nat Commun 2016, 7, 11639. [Google Scholar] [CrossRef] [PubMed]
- Sujino, T.; London, M.; Hoytema van Konijnenburg, D.P.; Rendon, T.; Buch, T.; Silva, H.M.; Lafaille, J.J.; Reis, B.S.; Mucida, D. Tissue adaptation of regulatory and intraepithelial CD4(+) T cells controls gut inflammation. Science 2016, 352, 1581–1586. [Google Scholar] [CrossRef]
- London, M.; Bilate, A.M.; Castro, T.B.R.; Sujino, T.; Mucida, D. Stepwise chromatin and transcriptional acquisition of an intraepithelial lymphocyte program. Nat Immunol 2021, 22, 449–459. [Google Scholar] [CrossRef]
- Olivares-Villagomez, D.; Van Kaer, L. TL and CD8alphaalpha: Enigmatic partners in mucosal immunity. Immunol Lett 2010, 134, 1–6. [Google Scholar] [CrossRef]
- Olivares-Villagomez, D.; Mendez-Fernandez, Y.V.; Parekh, V.V.; Lalani, S.; Vincent, T.L.; Cheroutre, H.; Van Kaer, L. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc Natl Acad Sci U S A 2008, 105, 17931–17936. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc 2019, 94, 155–165. [Google Scholar] [CrossRef]
- Hung, C.T.; Ma, C.; Panda, S.K.; Trsan, T.; Hodel, M.; Frein, J.; Foster, A.; Sun, S.; Wu, H.T.; Kern, J.; Mishra, R.; Jain, U.; Ho, Y.C.; Colonna, M.; Stappenbeck, T.S.; Liu, T.C. Western diet reduces small intestinal intraepithelial lymphocytes via FXR-Interferon pathway. Mucosal Immunol 2024, 17, 1019–1028. [Google Scholar] [CrossRef]
- Tougaard, P.; Skov, S.; Pedersen, A.E.; Krych, L.; Nielsen, D.S.; Bahl, M.I.; Christensen, E.G.; Licht, T.R.; Poulsen, S.S.; Metzdorff, S.B.; Hansen, A.K.; Hansen, C.H. TL1A regulates TCRgammadelta+ intraepithelial lymphocytes and gut microbial composition. Eur J Immunol 2015, 45, 865–875. [Google Scholar] [CrossRef]
- Abuquteish, D.; Putra, J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review. World J Gastroenterol 2019, 25, 1928–1935. [Google Scholar] [CrossRef]
- Hu, M.D.; Edelblum, K.L. Sentinels at the frontline: the role of intraepithelial lymphocytes in inflammatory bowel disease. Curr Pharmacol Rep 2017, 3, 321–334. [Google Scholar] [CrossRef]
- Patterson, E.R.; Shmidt, E.; Oxentenko, A.S.; Enders, F.T.; Smyrk, T.C. Normal villous architecture with increased intraepithelial lymphocytes: a duodenal manifestation of Crohn disease. Am J Clin Pathol 2015, 143, 445–450. [Google Scholar] [CrossRef]
- van Hemert, S.; Skonieczna-Zydecka, K.; Loniewski, I.; Szredzki, P.; Marlicz, W. Microscopic colitis-microbiome, barrier function and associated diseases. Ann Transl Med 2018, 6, 39. [Google Scholar] [CrossRef]
- Miehlke, S.; Verhaegh, B.; Tontini, G.E.; Madisch, A.; Langner, C.; Munch, A. Microscopic colitis: pathophysiology and clinical management. Lancet Gastroenterol Hepatol 2019, 4, 305–314. [Google Scholar] [CrossRef]
- Yuan, L.; Wu, T.T.; Zhang, L. Microscopic colitis: lymphocytic colitis, collagenous colitis, and beyond. Hum Pathol 2023, 132, 89–101. [Google Scholar] [CrossRef]
- Burke, K.E.; D’Amato, M.; Ng, S.C.; Pardi, D.S.; Ludvigsson, J.F.; Khalili, H. Microscopic colitis. Nat Rev Dis Primers 2021, 7, 39. [Google Scholar] [CrossRef]
- Carreras, J. Artificial Intelligence Analysis of Celiac Disease Using an Autoimmune Discovery Transcriptomic Panel Highlighted Pathogenic Genes including BTLA. Healthcare (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Carreras, J. Celiac Disease Deep Learning Image Classification Using Convolutional Neural Networks. J Imaging 2024, 10. [Google Scholar] [CrossRef]
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac disease. Lancet 2022, 399, 2413–2426. [Google Scholar] [CrossRef]
- Villanacci, V.; Vanoli, A.; Leoncini, G.; Arpa, G.; Salviato, T.; Bonetti, L.R.; Baronchelli, C.; Saragoni, L.; Parente, P. Celiac disease: histology-differential diagnosis-complications. A practical approach. Pathologica 2020, 112, 186–196. [Google Scholar] [CrossRef]
- Al Somali, Z.; Hamadani, M.; Kharfan-Dabaja, M.; Sureda, A.; El Fakih, R.; Aljurf, M. Enteropathy-Associated T cell Lymphoma. Curr Hematol Malig Rep 2021, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Marchi, E.; Craig, J.W.; Kalac, M. Current and upcoming treatment approaches to uncommon subtypes of PTCL (EATL, MEITL, SPTCL, and HSTCL). Blood 2024, 144, 1898–1909. [Google Scholar] [CrossRef]
- Abdullah, S.A.A.; Goa, P.; Vandenberghe, E.; Flavin, R. Update on the Pathogenesis of Enteropathy-Associated T-Cell Lymphoma. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brais, R.; Lavergne-Slove, A.; Jeng, Q.; Payne, K.; Ye, H.; Liu, Z.; Carreras, J.; Huang, Y.; Bacon, C.M.; Hamoudi, R.A.; Save, V.; Venkatraman, L.; Isaacson, P.G.; Woodward, J.; Du, M.Q. Continual monitoring of intraepithelial lymphocyte immunophenotype and clonality is more important than snapshot analysis in the surveillance of refractory coeliac disease. Gut 2010, 59, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- Cerqueira, J.X.M.; Saavalainen, P.; Kurppa, K.; Laurikka, P.; Huhtala, H.; Nykter, M.; L, L.E.K.; Yohannes, D.A.; Kilpelainen, E.; Shcherban, A.; Palotie, A.; Kaukinen, K.; Lindfors, K. Independent and cumulative coeliac disease-susceptibility loci are associated with distinct disease phenotypes. J Hum Genet 2021, 66, 613–623. [Google Scholar] [CrossRef]
- Liu, E.; Lee, H.S.; Aronsson, C.A.; Hagopian, W.A.; Koletzko, S.; Rewers, M.J.; Eisenbarth, G.S.; Bingley, P.J.; Bonifacio, E.; Simell, V.; Agardh, D.; Group, T.S. Risk of pediatric celiac disease according to HLA haplotype and country. N Engl J Med 2014, 371, 42–49. [Google Scholar] [CrossRef]
- Garner, C.; Ahn, R.; Ding, Y.C.; Steele, L.; Stoven, S.; Green, P.H.; Fasano, A.; Murray, J.A.; Neuhausen, S.L. Genome-wide association study of celiac disease in North America confirms FRMD4B as new celiac locus. PLoS One 2014, 9, e101428. [Google Scholar] [CrossRef]
- Bragde, H.; Jansson, U.; Jarlsfelt, I.; Soderman, J. Gene expression profiling of duodenal biopsies discriminates celiac disease mucosa from normal mucosa. Pediatr Res 2011, 69, 530–537. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Yao, J.; Lebwohl, B.; Green, P.H.R.; Yuan, S.; Leffler, D.A. Coeliac disease: complications and comorbidities. Nat Rev Gastroenterol Hepatol 2025. [Google Scholar] [CrossRef]
- Shan, L.; Molberg, O.; Parrot, I.; Hausch, F.; Filiz, F.; Gray, G.M.; Sollid, L.M.; Khosla, C. Structural basis for gluten intolerance in celiac sprue. Science 2002, 297, 2275–2279. [Google Scholar] [CrossRef]
- Sakly, W.; Thomas, V.; Quash, G.; El Alaoui, S. A role for tissue transglutaminase in alpha-gliadin peptide cytotoxicity. Clin Exp Immunol 2006, 146, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, G.; Hernell, O.; Melgar, S.; Israelsson, A.; Hammarstrom, S.; Hammarstrom, M.L. Paradoxical coexpression of proinflammatory and down-regulatory cytokines in intestinal T cells in childhood celiac disease. Gastroenterology 2002, 123, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Eggesbo, L.M.; Risnes, L.F.; Neumann, R.S.; Lundin, K.E.A.; Christophersen, A.; Sollid, L.M. Single-cell TCR sequencing of gut intraepithelial gammadelta T cells reveals a vast and diverse repertoire in celiac disease. Mucosal Immunol 2020, 13, 313–321. [Google Scholar] [CrossRef]
- de Mascarel, A.; Belleannee, G.; Stanislas, S.; Merlio, C.; Parrens, M.; Laharie, D.; Dubus, P.; Merlio, J.P. Mucosal intraepithelial T-lymphocytes in refractory celiac disease: a neoplastic population with a variable CD8 phenotype. Am J Surg Pathol 2008, 32, 744–751. [Google Scholar] [CrossRef]
- Soderquist, C.R.; Lewis, S.K.; Gru, A.A.; Vlad, G.; Williams, E.S.; Hsiao, S.; Mansukhani, M.M.; Park, D.C.; Bacchi, C.E.; Alobeid, B.; Green, P.H.; Bhagat, G. Immunophenotypic Spectrum and Genomic Landscape of Refractory Celiac Disease Type II. Am J Surg Pathol 2021, 45, 905–916. [Google Scholar] [CrossRef]
- Caja, S.; Maki, M.; Kaukinen, K.; Lindfors, K. Antibodies in celiac disease: implications beyond diagnostics. Cell Mol Immunol 2011, 8, 103–109. [Google Scholar] [CrossRef]
- Kim, S.M.; Mayassi, T.; Jabri, B. Innate immunity: actuating the gears of celiac disease pathogenesis. Best Pract Res Clin Gastroenterol 2015, 29, 425–435. [Google Scholar] [CrossRef]
- Londei, M.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Quaratino, S.; Maiuri, L. Gliadin as a stimulator of innate responses in celiac disease. Mol Immunol 2005, 42, 913–918. [Google Scholar] [CrossRef]
- Kelly, C.P.; Murray, J.A.; Leffler, D.A.; Getts, D.R.; Bledsoe, A.C.; Smithson, G.; First, M.R.; Morris, A.; Boyne, M.; Elhofy, A.; Wu, T.T.; Podojil, J.R.; Miller, S.D.; Group, T.A.K.S. TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Gastroenterology 2021, 161, 66–80 e68. [Google Scholar] [CrossRef]
- Dodero, V.I.; Herrera, M.G. Oligomerization of 33-mer Gliadin Peptides: Supramolecular Assemblies in Celiac Disease. ChemMedChem 2025, 20, e202400789. [Google Scholar] [CrossRef]
- Barone, M.V.; Salvatore, A. Pro-Inflammatory Nutrient: Focus on Gliadin and Celiac Disease. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Smigoc Schweiger, D.; Mendez, A.; Kunilo Jamnik, S.; Bratanic, N.; Bratina, N.; Battelino, T.; Brecelj, J.; Vidan-Jeras, B. High-risk genotypes HLA-DR3-DQ2/DR3-DQ2 and DR3-DQ2/DR4-DQ8 in co-occurrence of type 1 diabetes and celiac disease. Autoimmunity 2016, 49, 240–247. [Google Scholar] [CrossRef]
- Cerda-Contreras, E.; Ramirez-Cervantes, K.L.; Granados, J.; Mena, L.; Nunez-Alvarez, C.; Uscanga, L. Is celiac disease better identified through HLA-DQ8 than through HLA-DQ2 in Mexican subjects? Rev Gastroenterol Mex (Engl Ed) 2018, 83, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.J.; Cuthbertson, D.; Steck, A.K.; Herold, K.C.; Oram, R.; Atkinson, M.; Brusko, T.M.; Parikh, H.M.; Krischer, J.P.; Onengut-Gumuscu, S.; Rich, S.S.; Sosenko, J.M.; Type 1 Diabetes TrialNet Study, G. Characteristics of autoantibody-positive individuals without high-risk HLA-DR4-DQ8 or HLA-DR3-DQ2 haplotypes. Diabetologia 2025, 68, 588–601. [Google Scholar] [CrossRef]
- Kaur, G.; Sarkar, N.; Bhatnagar, S.; Kumar, S.; Rapthap, C.C.; Bhan, M.K.; Mehra, N.K. Pediatric celiac disease in India is associated with multiple DR3-DQ2 haplotypes. Hum Immunol 2002, 63, 677–682. [Google Scholar] [CrossRef]
- Bolognesi, E.; Karell, K.; Percopo, S.; Coto, I.; Greco, L.; Mantovani, V.; Suoraniemi, E.; Partanen, J.; Mustalahti, K.; Maki, M.; Momigliano-Richiardi, P. Additional factor in some HLA DR3/DQ2 haplotypes confers a fourfold increased genetic risk of celiac disease. Tissue Antigens 2003, 61, 308–316. [Google Scholar] [CrossRef]
- Lundin, K.E.; Scott, H.; Fausa, O.; Thorsby, E.; Sollid, L.M. T cells from the small intestinal mucosa of a DR4, DQ7/DR4, DQ8 celiac disease patient preferentially recognize gliadin when presented by DQ8. Hum Immunol 1994, 41, 285–291. [Google Scholar] [CrossRef]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: a comprehensive current review. BMC Med 2019, 17, 142. [Google Scholar] [CrossRef]
- Lundin, K.E.; Wijmenga, C. Coeliac disease and autoimmune disease-genetic overlap and screening. Nat Rev Gastroenterol Hepatol 2015, 12, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Crimi, R.; Cenit, M.C.; Nunez, C. The genetics of celiac disease: A comprehensive review of clinical implications. J Autoimmun 2015, 64, 26–41. [Google Scholar] [CrossRef]
- Gnodi, E.; Meneveri, R.; Barisani, D. Celiac disease: From genetics to epigenetics. World J Gastroenterol 2022, 28, 449–463. [Google Scholar] [CrossRef]
- Dubois, P.C.; Trynka, G.; Franke, L.; Hunt, K.A.; Romanos, J.; Curtotti, A.; Zhernakova, A.; Heap, G.A.; Adany, R.; Aromaa, A.; Bardella, M.T.; van den Berg, L.H.; Bockett, N.A.; de la Concha, E.G.; Dema, B.; Fehrmann, R.S.; Fernandez-Arquero, M.; Fiatal, S.; Grandone, E.; Green, P.M.; Groen, H.J.; Gwilliam, R.; Houwen, R.H.; Hunt, S.E.; Kaukinen, K.; Kelleher, D.; Korponay-Szabo, I.; Kurppa, K.; MacMathuna, P.; Maki, M.; Mazzilli, M.C.; McCann, O.T.; Mearin, M.L.; Mein, C.A.; Mirza, M.M.; Mistry, V.; Mora, B.; Morley, K.I.; Mulder, C.J.; Murray, J.A.; Nunez, C.; Oosterom, E.; Ophoff, R.A.; Polanco, I.; Peltonen, L.; Platteel, M.; Rybak, A.; Salomaa, V.; Schweizer, J.J.; Sperandeo, M.P.; Tack, G.J.; Turner, G.; Veldink, J.H.; Verbeek, W.H.; Weersma, R.K.; Wolters, V.M.; Urcelay, E.; Cukrowska, B.; Greco, L.; Neuhausen, S.L.; McManus, R.; Barisani, D.; Deloukas, P.; Barrett, J.C.; Saavalainen, P.; Wijmenga, C.; van Heel, D.A. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet 2010, 42, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Santisteban, I.; Romero-Garmendia, I.; Cilleros-Portet, A.; Bilbao, J.R.; Fernandez-Jimenez, N. Celiac disease susceptibility: The genome and beyond. Int Rev Cell Mol Biol 2021, 358, 1–45. [Google Scholar] [PubMed]
- Verdu, E.F.; Schuppan, D. Co-factors, Microbes, and Immunogenetics in Celiac Disease to Guide Novel Approaches for Diagnosis and Treatment. Gastroenterology 2021, 161, 1395–1411 e1394. [Google Scholar] [CrossRef] [PubMed]
- Serena, G.; Lima, R.; Fasano, A. Genetic and Environmental Contributors for Celiac Disease. Curr Allergy Asthma Rep 2019, 19, 40. [Google Scholar] [CrossRef]
- Romanos, J.; van Diemen, C.C.; Nolte, I.M.; Trynka, G.; Zhernakova, A.; Fu, J.; Bardella, M.T.; Barisani, D.; McManus, R.; van Heel, D.A.; Wijmenga, C. Analysis of HLA and non-HLA alleles can identify individuals at high risk for celiac disease. Gastroenterology 2009, 137, 834–840, 840 e831–833. [Google Scholar] [CrossRef]
- Trynka, G.; Zhernakova, A.; Romanos, J.; Franke, L.; Hunt, K.A.; Turner, G.; Bruinenberg, M.; Heap, G.A.; Platteel, M.; Ryan, A.W.; de Kovel, C.; Holmes, G.K.; Howdle, P.D.; Walters, J.R.; Sanders, D.S.; Mulder, C.J.; Mearin, M.L.; Verbeek, W.H.; Trimble, V.; Stevens, F.M.; Kelleher, D.; Barisani, D.; Bardella, M.T.; McManus, R.; van Heel, D.A.; Wijmenga, C. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-kappaB signalling. Gut 2009, 58, 1078–1083. [Google Scholar] [CrossRef]
- Hunt, K.A.; Zhernakova, A.; Turner, G.; Heap, G.A.; Franke, L.; Bruinenberg, M.; Romanos, J.; Dinesen, L.C.; Ryan, A.W.; Panesar, D.; Gwilliam, R.; Takeuchi, F.; McLaren, W.M.; Holmes, G.K.; Howdle, P.D.; Walters, J.R.; Sanders, D.S.; Playford, R.J.; Trynka, G.; Mulder, C.J.; Mearin, M.L.; Verbeek, W.H.; Trimble, V.; Stevens, F.M.; O’Morain, C.; Kennedy, N.P.; Kelleher, D.; Pennington, D.J.; Strachan, D.P.; McArdle, W.L.; Mein, C.A.; Wapenaar, M.C.; Deloukas, P.; McGinnis, R.; McManus, R.; Wijmenga, C.; van Heel, D.A. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet 2008, 40, 395–402. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Ludvigsson, J.F.; Brantner, T.L.; Murray, J.A.; Everhart, J.E. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012, 107, 1538–1544, quiz 1537, 1545.. [Google Scholar] [CrossRef]
- Choung, R.S.; Larson, S.A.; Khaleghi, S.; Rubio-Tapia, A.; Ovsyannikova, I.G.; King, K.S.; Larson, J.J.; Lahr, B.D.; Poland, G.A.; Camilleri, M.J.; Murray, J.A. Prevalence and Morbidity of Undiagnosed Celiac Disease From a Community-Based Study. Gastroenterology 2017, 152, 830–839 e835. [Google Scholar] [CrossRef]
- Rubin, C.E.; Brandborg, L.L.; Phelps, P.C.; Taylor, H.C., Jr. Studies of celiac disease. I. The apparent identical and specific nature of the duodenal and proximal jejunal lesion in celiac disease and idiopathic sprue. Gastroenterology 1960, 38, 28–49. [Google Scholar] [CrossRef]
- Sahin, Y. Celiac disease in children: A review of the literature. World J Clin Pediatr 2021, 10, 53–71. [Google Scholar] [CrossRef]
- Hujoel, I.A.; Reilly, N.R.; Rubio-Tapia, A. Celiac Disease: Clinical Features and Diagnosis. Gastroenterol Clin North Am 2019, 48, 19–37. [Google Scholar] [CrossRef]
- Troncone, R.; Greco, L.; Mayer, M.; Paparo, F.; Caputo, N.; Micillo, M.; Mugione, P.; Auricchio, S. Latent and potential coeliac disease. Acta Paediatr Suppl 1996, 412, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Soderquist, C.R.; Bhagat, G.; Cerf-Bensussan, N. Advances in Nonresponsive and Refractory Celiac Disease. Gastroenterology 2024, 167, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.R.; Paski, S.; Ko, C.W.; Rubio-Tapia, A. AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review. Gastroenterology 2022, 163, 1461–1469. [Google Scholar] [CrossRef]
- Malamut, G.; Cellier, C. Refractory Celiac Disease. Gastroenterol Clin North Am 2019, 48, 137–144. [Google Scholar] [CrossRef]
- Soldera, J.; Salgado, K.; Pegas, K.L. Refractory celiac disease type 2: how to diagnose and treat? Rev Assoc Med Bras (1992) 2021, 67, 168–172. [Google Scholar] [CrossRef]
- Perfetti, V.; Brunetti, L.; Biagi, F.; Ciccocioppo, R.; Bianchi, P.I.; Corazza, G.R. TCRbeta clonality improves diagnostic yield of TCRgamma clonality in refractory celiac disease. J Clin Gastroenterol 2012, 46, 675–679. [Google Scholar] [CrossRef]
- Branchi, F.; Wiese, J.J.; Heldt, C.; Manna, S.; Dony, V.; Loddenkemper, C.; Bojarski, C.; Siegmund, B.; Schneider, T.; Daum, S.; Hummel, M.; Moos, V.; Schumann, M. The combination of clinical parameters and immunophenotyping of intraepithelial lymphocytes allows to assess disease severity in refractory celiac disease. Dig Liver Dis 2022, 54, 1649–1656. [Google Scholar] [CrossRef]
- Nasr, I.; Nasr, I.; Campling, H.; Ciclitira, P.J. Approach to patients with refractory coeliac disease. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Rejeski, J.; Conway, J.; Zhou, Y. Collagenous Sprue. Am J Med Sci 2020, 359, 310–311. [Google Scholar] [CrossRef]
- Scarpignato, C.; Bjarnason, I. Drug-Induced Small Bowel Injury: a Challenging and Often Forgotten Clinical Condition. Curr Gastroenterol Rep 2019, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Hamdeh, S.; Micic, D.; Hanauer, S. Review article: drug-induced small bowel injury. Aliment Pharmacol Ther 2021, 54, 1370–1388. [Google Scholar] [CrossRef]
- Reunala, T.; Hervonen, K.; Salmi, T. Dermatitis Herpetiformis: An Update on Diagnosis and Management. Am J Clin Dermatol 2021, 22, 329–338. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Kim, S.J. Dermatitis Herpetiformis: An Update on Diagnosis, Disease Monitoring, and Management. Medicina (Kaunas) 2021, 57. [Google Scholar] [CrossRef]
- Micic, D.; Rao, V.L.; Semrad, C.E. Celiac Disease and Its Role in the Development of Metabolic Bone Disease. J Clin Densitom 2020, 23, 190–199. [Google Scholar] [CrossRef]
- Kondapalli, A.V.; Walker, M.D. Celiac disease and bone. Arch Endocrinol Metab 2022, 66, 756–764. [Google Scholar] [CrossRef]
- Xing, Y.; Morgan, S.L. Celiac disease and metabolic bone disease. J Clin Densitom 2013, 16, 439–444. [Google Scholar] [CrossRef]
- Freeman, H.J. Iron deficiency anemia in celiac disease. World J Gastroenterol 2015, 21, 9233–9238. [Google Scholar] [CrossRef]
- Talarico, V.; Giancotti, L.; Mazza, G.A.; Miniero, R.; Bertini, M. Iron Deficiency Anemia in Celiac Disease. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Kirkineska, L.; Perifanis, V.; Vasiliadis, T. Functional hyposplenism. Hippokratia 2014, 18, 7–11. [Google Scholar] [PubMed]
- Marafini, I.; Monteleone, G.; Stolfi, C. Association Between Celiac Disease and Cancer. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Kyle, R.A.; Kaplan, E.L.; Johnson, D.R.; Page, W.; Erdtmann, F.; Brantner, T.L.; Kim, W.R.; Phelps, T.K.; Lahr, B.D.; Zinsmeister, A.R.; Melton, L.J., 3rd; Murray, J.A. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009, 137, 88–93. [Google Scholar] [CrossRef]
- Shiha, M.G.; Chetcuti Zammit, S.; Elli, L.; Sanders, D.S.; Sidhu, R. Updates in the diagnosis and management of coeliac disease. Best Pract Res Clin Gastroenterol 2023, 64-65, 101843. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Losurdo, G.; Di Leo, M.; Santamato, E.; Arena, M.; Rendina, M.; Luigiano, C.; Ierardi, E.; Di Leo, A. Serologic diagnosis of celiac disease: May it be suitable for adults? World J Gastroenterol 2021, 27, 7233–7239. [Google Scholar] [CrossRef]
- McIntyre, A.S.; Ng, D.P.; Smith, J.A.; Amoah, J.; Long, R.G. The endoscopic appearance of duodenal folds is predictive of untreated adult celiac disease. Gastrointest Endosc 1992, 38, 148–151. [Google Scholar] [CrossRef]
- Dickey, W. Endoscopic markers for celiac disease. Nat Clin Pract Gastroenterol Hepatol 2006, 3, 546–551. [Google Scholar] [CrossRef]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Dotsenko, V.; Tewes, B.; Hils, M.; Pasternack, R.; Isola, J.; Taavela, J.; Popp, A.; Sarin, J.; Huhtala, H.; Hiltunen, P.; Zimmermann, T.; Mohrbacher, R.; Greinwald, R.; Lundin, K.E.A.; Schuppan, D.; Maki, M.; Viiri, K.; Investigators, C.E.C. Transcriptomic analysis of intestine following administration of a transglutaminase 2 inhibitor to prevent gluten-induced intestinal damage in celiac disease. Nat Immunol 2024, 25, 1218–1230. [Google Scholar] [CrossRef]
- Schuppan, D.; Maki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, O.; Lahdeaho, M.L.; Fusco, S.; Schumann, M.; Torok, H.P.; Kupcinskas, J.; Zopf, Y.; Lohse, A.W.; Scheinin, M.; Kull, K.; Biedermann, L.; Byrnes, V.; Stallmach, A.; Jahnsen, J.; Zeitz, J.; Mohrbacher, R.; Greinwald, R.; Group, C.E.C.T. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N Engl J Med 2021, 385, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Isola, J.; Maki, M.; Hils, M.; Pasternack, R.; Viiri, K.; Dotsenko, V.; Montonen, T.; Zimmermann, T.; Mohrbacher, R.; Greinwald, R.; Schuppan, D. The Oral Transglutaminase 2 Inhibitor ZED1227 Accumulates in the Villous Enterocytes in Celiac Disease Patients during Gluten Challenge and Drug Treatment. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Elhence, A.; Ghoshal, U.C. A Trial of a Transglutaminase 2 Inhibitor in Celiac Disease. N Engl J Med 2021, 385, e57. [Google Scholar] [PubMed]
- Van Laethem, F.; Donaty, L.; Tchernonog, E.; Lacheretz-Szablewski, V.; Russello, J.; Buthiau, D.; Almeras, M.; Moreaux, J.; Bret, C. LAIR1, an ITIM-Containing Receptor Involved in Immune Disorders and in Hematological Neoplasms. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Kang, X.; Lu, Z.; Cui, C.; Deng, M.; Fan, Y.; Dong, B.; Han, X.; Xie, F.; Tyner, J.W.; Coligan, J.E.; Collins, R.H.; Xiao, X.; You, M.J.; Zhang, C.C. The ITIM-containing receptor LAIR1 is essential for acute myeloid leukaemia development. Nat Cell Biol 2015, 17, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, S.; Puan, K.J.; Li, X.; Xu, C.; Fan, J.; Dou, Z.; Zhang, J.; Ju, D. Development of an anti-LAIR1 antibody-drug conjugate for acute myeloid leukemia therapy. Int J Biol Macromol 2025, 293, 139432. [Google Scholar] [CrossRef]
- Lovewell, R.R.; Hong, J.; Kundu, S.; Fielder, C.M.; Hu, Q.; Kim, K.W.; Ramsey, H.E.; Gorska, A.E.; Fuller, L.S.; Tian, L.; Kothari, P.; Paucarmayta, A.; Mason, E.F.; Meza, I.; Manzanarez, Y.; Bosiacki, J.; Maloveste, K.; Mitchell, N.; Barbu, E.A.; Morawski, A.; Maloveste, S.; Cusumano, Z.; Patel, S.J.; Savona, M.R.; Langermann, S.; Myint, H.; Flies, D.B.; Kim, T.K. LAIR-1 agonism as a therapy for acute myeloid leukemia. J Clin Invest 2023, 133. [Google Scholar] [CrossRef]
- Meyaard, L.; Adema, G.J.; Chang, C.; Woollatt, E.; Sutherland, G.R.; Lanier, L.L.; Phillips, J.H. LAIR-1, a novel inhibitory receptor expressed on human mononuclear leukocytes. Immunity 1997, 7, 283–290. [Google Scholar] [CrossRef]
- Poggi, A.; Matis, S.; Uras, C.R.M.; Raffaghello, L.; Benelli, R.; Zocchi, M.R. The Role of LAIR1 as a Regulatory Receptor of Antitumor Immune Cell Responses and Tumor Cell Growth and Expansion. Biomolecules 2025, 15, 866. [Google Scholar] [CrossRef] [PubMed]
- Rumpret, M.; von Richthofen, H.J.; Peperzak, V.; Meyaard, L. Inhibitory pattern recognition receptors. J Exp Med 2022, 219. [Google Scholar] [CrossRef]
- Zhang, C.C. A perspective on LILRBs and LAIR1 as immune checkpoint targets for cancer treatment. Biochem Biophys Res Commun 2022, 633, 64–67. [Google Scholar] [CrossRef]
- Keerthivasan, S.; Senbabaoglu, Y.; Martinez-Martin, N.; Husain, B.; Verschueren, E.; Wong, A.; Yang, Y.A.; Sun, Y.; Pham, V.; Hinkle, T.; Oei, Y.; Madireddi, S.; Corpuz, R.; Tam, L.; Carlisle, S.; Roose-Girma, M.; Modrusan, Z.; Ye, Z.; Koerber, J.T.; Turley, S.J. Homeostatic functions of monocytes and interstitial lung macrophages are regulated via collagen domain-binding receptor LAIR1. Immunity 2021, 54, 1511–1526 e1518. [Google Scholar] [CrossRef]
- Pisetsky, DS. Overview of autoimmunity. In: UpToDate, Rigby W FC, Case SM (Ed), Wolters Kluwer. (Accessed on July 31, 2025.). Website: www.uptodate.com Topic last updated: Feb 20, 2025.
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat Immunol 2018, 19, 665–673. [Google Scholar] [CrossRef]
- Du, W.; Han, M.; Zhu, X.; Xiao, F.; Huang, E.; Che, N.; Tang, X.; Zou, H.; Jiang, Q.; Lu, L. The Multiple Roles of B Cells in the Pathogenesis of Sjogren’s Syndrome. Front Immunol 2021, 12, 684999. [Google Scholar] [CrossRef]
- Accapezzato, D.; Caccavale, R.; Paroli, M.P.; Gioia, C.; Nguyen, B.L.; Spadea, L.; Paroli, M. Advances in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct Target Ther 2023, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Huang, M.; Tu, H.; Zhang, B.; Lin, X.; Xu, H.; Dong, C.; Hu, X. Innate and adaptive immune abnormalities underlying autoimmune diseases: the genetic connections. Sci China Life Sci 2023, 66, 1482–1517. [Google Scholar] [CrossRef]
- Lambert, M.P. Presentation and diagnosis of autoimmune lymphoproliferative syndrome (ALPS). Expert Rev Clin Immunol 2021, 17, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Elkon, K. Apoptosis and autoimmune disease. In: UpToDate, Rigby FC W., Case SM (Ed), Wolters Kluwer. (Accessed on July 31, 2025.). Website: www.uptodate.com Topic last updated: Nov 11, 2024.
- Carreras, J.; Roncador, G.; Hamoudi, R. Ulcerative Colitis, LAIR1 and TOX2 Expression, and Colorectal Cancer Deep Learning Image Classification Using Convolutional Neural Networks. Cancers (Basel) 2024, 16. [Google Scholar] [CrossRef]
- Son, M.; Diamond, B. C1q-mediated repression of human monocytes is regulated by leukocyte-associated Ig-like receptor 1 (LAIR-1). Mol Med 2015, 20, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, X.; Zhang, M.; Wu, T.; Liu, X.; Zhang, Y.; Xie, Z.; Liu, S.; Xia, T.; Wang, Y.; Wei, F.; Wang, H.; Xie, C. Efficient delivery of the lncRNA LEF1-AS1 through the antibody LAIR-1 (CD305)-modified Zn-Adenine targets articular inflammation to enhance the treatment of rheumatoid arthritis. Arthritis Res Ther 2023, 25, 238. [Google Scholar] [CrossRef]
- Helou, D.G.; Shafiei-Jahani, P.; Hurrell, B.P.; Painter, J.D.; Quach, C.; Howard, E.; Akbari, O. LAIR-1 acts as an immune checkpoint on activated ILC2s and regulates the induction of airway hyperreactivity. J Allergy Clin Immunol 2022, 149, 223–236 e226. [Google Scholar] [CrossRef]
- Ouyang, W.; Xue, J.; Liu, J.; Jia, W.; Li, Z.; Xie, X.; Liu, X.; Jian, J.; Li, Q.; Zhu, Y.; Yang, A.; Jin, B. Establishment of an ELISA system for determining soluble LAIR-1 levels in sera of patients with HFRS and kidney transplant. J Immunol Methods 2004, 292, 109–117. [Google Scholar] [CrossRef]
- Gu, Y.; Bi, Y.; Wei, H.; Li, J.; Huang, Z.; Liao, C.; Liao, W.; Huang, Y. Expression and clinical significance of inhibitory receptor Leukocyte-associated immunoglobulin-like receptor-1 on peripheral blood T cells of chronic hepatitis B patients: A cross-sectional study. Medicine (Baltimore) 2021, 100, e26667. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, H.; Carreras, J.; Kikuti, Y.Y.; Miyaoka, M.; Nagase, S.; Kondo, Y.; Ito, A.; Orita, M.; Tomita, S.; Hiraiwa, S.; Kawada, H.; Garcia, J.F.; Roncador, G.; Campo, E.; Nakamura, N. Comparison of the Mutational Profile between BCL2- and BCL6-Rearrangement Positive Follicular Lymphoma. J Mol Diagn 2025, 27, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Carreras, J.; Ikoma, H.; Kikuti, Y.Y.; Miyaoka, M.; Hiraiwa, S.; Tomita, S.; Kondo, Y.; Ito, A.; Nagase, S.; Miura, H.; Kawada, H.; Roncador, G.; Campo, E.; Hamoudi, R.; Nakamura, N. Mutational, immune microenvironment, and clinicopathological profiles of diffuse large B-cell lymphoma and follicular lymphoma with BCL6 rearrangement. Virchows Arch 2024, 484, 657–676. [Google Scholar] [CrossRef]
- Doyle, J.B.; Lebwohl, B. Celiac disease and nonceliac enteropathies. Curr Opin Gastroenterol 2024, 40, 464–469. [Google Scholar] [CrossRef]
- Kowalski, M.K.; Domzal-Magrowska, D.; Malecka-Wojciesko, E. Celiac Disease-Narrative Review on Progress in Celiac Disease. Foods 2025, 14. [Google Scholar] [CrossRef]
- Carreras, J.; Lopez-Guillermo, A.; Kikuti, Y.Y.; Itoh, J.; Masashi, M.; Ikoma, H.; Tomita, S.; Hiraiwa, S.; Hamoudi, R.; Rosenwald, A.; Leich, E.; Martinez, A.; Roncador, G.; Villamor, N.; Colomo, L.; Perez, P.; Tsuji, N.M.; Campo, E.; Nakamura, N. High TNFRSF14 and low BTLA are associated with poor prognosis in Follicular Lymphoma and in Diffuse Large B-cell Lymphoma transformation. J Clin Exp Hematop 2019, 59, 1–16. [Google Scholar] [CrossRef]
- Carreras, J.; Roncador, G.; Hamoudi, R. Dataset and AI Workflow for Deep Learning Image Classification of Ulcerative Colitis and Colorectal Cancer. Preprints 2024, 2024121201. [Google Scholar] [CrossRef]
- Carreras, J.; Ikoma, H.; Kikuti, Y.Y.; Nagase, S.; Ito, A.; Orita, M.; Tomita, S.; Tanigaki, Y.; Nakamura, N.; Masugi, Y. Histological Image Classification Between Follicular Lymphoma and Reactive Lymphoid Tissue Using Deep Learning and Explainable Artificial Intelligence (XAI). Cancers 2025, 17, 2428. [Google Scholar] [CrossRef]
- Wolf J., Willscher E., Loeffler-Wirth H., Schmidt M., Flemming G., Zurek M., Uhlig H.H., Händel N., Binder H. Deciphering the Transcriptomic Heterogeneity of Duodenal Coeliac Disease Biopsies. Int. J. Mol. Sci. 2021;22:2551. [CrossRef]
- Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, Bork P, Jensen LJ, von Mering C. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023 Jan 6;51(D1):D638-D646. [CrossRef] [PubMed] [PubMed Central]
- He, Kaiming, Xiangyu Zhang, Shaoqing Ren, and Jian Sun. “Deep Residual Learning for Image Recognition.” Preprint, submitted December 10, 2015. https://arxiv.org/abs/1512.03385.
- He, Kaiming, Xiangyu Zhang, Shaoqing Ren, and Jian Sun. “Identity Mappings in Deep Residual Networks.” Preprint, submitted July 25, 2016. https://arxiv.org/abs/1603.05027.
- He, Kaiming, Xiangyu Zhang, Shaoqing Ren, and Jian Sun. “Delving Deep into Rectifiers: Surpassing Human-Level Performance on ImageNet Classification.” In Proceedings of the 2015 IEEE International Conference on Computer Vision, 1026–34. Washington, DC: IEEE Computer Vision Society, 2015.
- Zingone, F.; Bai, J.C.; Cellier, C.; Ludvigsson, J.F. Celiac Disease-Related Conditions: Who to Test? Gastroenterology 2024, 167, 64–78. [Google Scholar] [CrossRef]
- Roshanzamir, N.; Zakeri, Z.; Rostami-Nejad, M.; Sadeghi, A.; Pourhoseingholi, M.A.; Shahbakhsh, Y.; Asadzadeh-Aghdaei, H.; Elli, L.; Zali, M.R.; Rezaei-Tavirani, M. Prevalence of celiac disease in patients with atypical presentations. Arab J Gastroenterol 2021, 22, 220–223. [Google Scholar] [CrossRef]
- Scarmozzino, F.; Pizzi, M.; Pelizzaro, F.; Angerilli, V.; Dei Tos, A.P.; Piazza, F.; Savarino, E.V.; Zingone, F.; Fassan, M. Refractory celiac disease and its mimickers: a review on pathogenesis, clinical-pathological features and therapeutic challenges. Front Oncol 2023, 13, 1273305. [Google Scholar] [CrossRef]
- Rolny, P.; Sigurjonsdottir, H.A.; Remotti, H.; Nilsson, L.A.; Ascher, H.; Tlaskalova-Hogenova, H.; Tuckova, L. Role of immunosuppressive therapy in refractory sprue-like disease. Am J Gastroenterol 1999, 94, 219–225. [Google Scholar] [CrossRef]
- Vaidya, A.; Bolanos, J.; Berkelhammer, C. Azathioprine in refractory sprue. Am J Gastroenterol 1999, 94, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Tack, G.J.; van Asseldonk, D.P.; van Wanrooij, R.L.; van Bodegraven, A.A.; Mulder, C.J. Tioguanine in the treatment of refractory coeliac disease--a single centre experience. Aliment Pharmacol Ther 2012, 36, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Maurino, E.; Niveloni, S.; Chernavsky, A.; Pedreira, S.; Mazure, R.; Vazquez, H.; Reyes, H.; Fiorini, A.; Smecuol, E.; Cabanne, A.; Capucchio, M.; Kogan, Z.; Bai, J.C. Azathioprine in refractory sprue: results from a prospective, open-label study. Am J Gastroenterol 2002, 97, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Vivas, S.; Ruiz de Morales, J.M.; Ramos, F.; Suarez-Vilela, D. Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T-cell lymphoma. N Engl J Med 2006, 354, 2514–2515. [Google Scholar] [CrossRef]
- Badran, Y.R.; Shih, A.; Leet, D.; Mooradian, M.J.; Coromilas, A.; Chen, J.; Kem, M.; Zheng, H.; Borowsky, J.; Misdraji, J.; Mino-Kenudson, M.; Dougan, M. Immune checkpoint inhibitor-associated celiac disease. J Immunother Cancer 2020, 8. [Google Scholar] [CrossRef]
- Omiya, R.; Tsushima, F.; Narazaki, H.; Sakoda, Y.; Kuramasu, A.; Kim, Y.; Xu, H.; Tamura, H.; Zhu, G.; Chen, L.; Tamada, K. Leucocyte-associated immunoglobulin-like receptor-1 is an inhibitory regulator of contact hypersensitivity. Immunology 2009, 128, 543–555. [Google Scholar] [CrossRef]
- Colombo, B.M.; Canevali, P.; Magnani, O.; Rossi, E.; Puppo, F.; Zocchi, M.R.; Poggi, A. Defective expression and function of the leukocyte associated Ig-like receptor 1 in B lymphocytes from systemic lupus erythematosus patients. PLoS One 2012, 7, e31903. [Google Scholar] [CrossRef]
- Myers, L.K.; Winstead, M.; Kee, J.D.; Park, J.J.; Zhang, S.; Li, W.; Yi, A.K.; Stuart, J.M.; Rosloniec, E.F.; Brand, D.D.; Tuckey, R.C.; Slominski, A.T.; Postlethwaite, A.E.; Kang, A.H. 1,25-Dihydroxyvitamin D3 and 20-Hydroxyvitamin D3 Upregulate LAIR-1 and Attenuate Collagen Induced Arthritis. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Spiliopoulou, A.; Iakovliev, A.; Plant, D.; Sutcliffe, M.; Sharma, S.; Cubuk, C.; Lewis, M.; Pitzalis, C.; Barton, A.; McKeigue, P.M. Genome-Wide Aggregated Trans Effects Analysis Identifies Genes Encoding Immune Checkpoints as Core Genes for Rheumatoid Arthritis. Arthritis Rheumatol 2025. [Google Scholar] [CrossRef]
- Agashe, V.V.; Jankowska-Gan, E.; Keller, M.; Sullivan, J.A.; Haynes, L.D.; Kernien, J.F.; Torrealba, J.R.; Roenneburg, D.; Dart, M.; Colonna, M.; Wilkes, D.S.; Burlingham, W.J. Leukocyte-Associated Ig-like Receptor 1 Inhibits T(h)1 Responses but Is Required for Natural and Induced Monocyte-Dependent T(h)17 Responses. J Immunol 2018, 201, 772–781. [Google Scholar] [CrossRef]
- Joseph, C.; Alsaleem, M.A.; Toss, M.S.; Kariri, Y.A.; Althobiti, M.; Alsaeed, S.; Aljohani, A.I.; Narasimha, P.L.; Mongan, N.P.; Green, A.R.; Rakha, E.A. The ITIM-Containing Receptor: Leukocyte-Associated Immunoglobulin-Like Receptor-1 (LAIR-1) Modulates Immune Response and Confers Poor Prognosis in Invasive Breast Carcinoma. Cancers (Basel) 2020, 13. [Google Scholar] [CrossRef]
- Tripathi, S.; Najem, H.; Dussold, C.; Pacheco, S.; Miska, J.; McCortney, K.; Steffens, A.; Walshon, J.; Winkowski, D.; Cloney, M.; Ordon, M.; Gibson, W.; Kemeny, H.; Youngblood, M.; Du, R.; Mossner, J.; Texakalidis, P.; Sprau, A.; Tate, M.; James, C.D.; Horbinski, C.M.; Wadhwani, N.R.; Lesniak, M.S.; Lam, S.; Sati, A.; Aghi, M.; DeCuypere, M.; Heimberger, A.B. Cancer-associated fibroblast-secreted collagen is associated with immune inhibitor receptor LAIR1 in gliomas. J Clin Invest 2024, 134. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Huang, J.; Gibson, L.; Fradette, J.J.; Chen, H.H.; Koyano, K.; Cortez, C.; Li, B.; Ho, C.; Ashique, A.M.; Lin, V.Y.; Crawley, S.; Roda, J.M.; Chen, P.; Fan, B.; Kim, J.; Sissons, J.; Sitrin, J.; Kaplan, D.D.; Gibbons, D.L.; Rivera, L.B. Antitumor Activity of a Novel LAIR1 Antagonist in Combination with Anti-PD1 to Treat Collagen-Rich Solid Tumors. Mol Cancer Ther 2024, 23, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Ke, X.; Qiu, J.; Ye, D.; Zhang, Z.; Zhang, X.; Luo, Y.; Yao, Y.; Wu, X.; Wang, X.; Tang, N. LAIR1-mediated resistance of hepatocellular carcinoma cells to T cells through a GSK-3beta/beta-catenin/MYC/PD-L1 pathway. Cell Signal 2024, 115, 111039. [Google Scholar] [CrossRef]
- Carreras, J. Artificial Intelligence Analysis of Ulcerative Colitis Using an Autoimmune Discovery Transcriptomic Panel. Healthcare (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Denholm, J.; Schreiber, B.A.; Evans, S.C.; Crook, O.M.; Sharma, A.; Watson, J.L.; Bancroft, H.; Langman, G.; Gilbey, J.D.; Schonlieb, C.B.; Arends, M.J.; Soilleux, E.J. Multiple-instance-learning-based detection of coeliac disease in histological whole-slide images. J Pathol Inform 2022, 13, 100151. [Google Scholar] [CrossRef]
- Molder, A.; Balaban, D.V.; Molder, C.C.; Jinga, M.; Robin, A. Computer-Based Diagnosis of Celiac Disease by Quantitative Processing of Duodenal Endoscopy Images. Diagnostics (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Scheppach, M.W.; Rauber, D.; Stallhofer, J.; Muzalyova, A.; Otten, V.; Manzeneder, C.; Schwamberger, T.; Wanzl, J.; Schlottmann, J.; Tadic, V.; Probst, A.; Schnoy, E.; Rommele, C.; Fleischmann, C.; Meinikheim, M.; Miller, S.; Markl, B.; Stallmach, A.; Palm, C.; Messmann, H.; Ebigbo, A. Detection of duodenal villous atrophy on endoscopic images using a deep learning algorithm. Gastrointest Endosc 2023, 97, 911–916. [Google Scholar] [CrossRef]
- Schreiber, B.A.; Denholm, J.; Gilbey, J.D.; Schonlieb, C.B.; Soilleux, E.J. Stain normalization gives greater generalizability than stain jittering in neural network training for the classification of coeliac disease in duodenal biopsy whole slide images. J Pathol Inform 2023, 14, 100324. [Google Scholar] [CrossRef]
- Stoleru, C.A.; Dulf, E.H.; Ciobanu, L. Automated detection of celiac disease using Machine Learning Algorithms. Sci Rep 2022, 12, 4071. [Google Scholar] [CrossRef]
- DiPalma, J.; Suriawinata, A.A.; Tafe, L.J.; Torresani, L.; Hassanpour, S. Resolution-based distillation for efficient histology image classification. Artif Intell Med 2021, 119, 102136. [Google Scholar] [CrossRef]
- Gruver, A.M.; Lu, H.; Zhao, X.; Fulford, A.D.; Soper, M.D.; Ballard, D.; Hanson, J.C.; Schade, A.E.; Hsi, E.D.; Gottlieb, K.; Credille, K.M. Pathologist-trained machine learning classifiers developed to quantitate celiac disease features differentiate endoscopic biopsies according to modified marsh score and dietary intervention response. Diagn Pathol 2023, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, E.; Rajaram, A.; Cote, K.; Farag, M.; Maleki, F.; Gao, Z.H.; Maedler-Kron, C.; Marcus, V.; Fiset, P.O. A Deep Learning-Based Approach to Estimate Paneth Cell Granule Area in Celiac Disease. Arch Pathol Lab Med 2024, 148, 828–835. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).