1. Introduction
Microcephaly with pontine and cerebellar hypoplasia (MICPCH) syndrome is a severe neurodevelopmental disorder with numerous symptoms associated with microcephaly and pontocerebellar hypoplasia, usually found in girls, and lethality in boys [
1,
2,
3]. MICPCH syndrome is caused by the deficiency of the X-linked gene calcium/calmodulin-dependent serine protein kinase (CASK) that encodes a membrane associated guanylate kinase (MAGUK) family protein, containing a CaMK domain, two L27 domains, a PDZ domain, an SH3 domain, and a C-terminal guanylate kinase domain [
4]. CASK has been originally identified as an intracellular binding partner for Neurexins [
5] and considered to function for regulating formation and maintenance of synapse and neurotransmitter release in the brain [
6]. CASK knockout (KO) mice showed significant developmental abnormalities and lethality [
7]. The excitatory/inhibitory (E/I) balance is disrupted in cultured neurons derived from CASK-KO mice. Female CASK heterozygote KO (CASK-hKO) mice may be the models that mimic the genetic background of most MICPCH syndrome. These mice are subjected to the effects of X-chromosome inactivation, resulting in a mosaic distribution of CASK-expressing and CASK-KO cells in the cerebral cortex [
8]. Patch-clamp electrophysiology in acute brain slices has shown that excitatory synaptic input to CASK-KO pyramidal neurons is increased, while inhibitory synaptic input is reduced in CASK-hKO mice [
8]. This is attributed to insufficient transcription of Grin2B, which encodes the GluN2B subunit of the N-methyl-D-aspartate (NMDA) receptor, due to a deficiency in the complex formed between guanylate kinase domain of CASK and the T-box transcription regulatory factor TBR1 [
8].
Mutations in CASK identified in the human patients have been shown to affect brain development and function.
In vitro experiments demonstrated these mutations cause neurodegeneration and apoptosis in cerebellar granule cells (CG cells) [
9]. However, the detailed interaction between the CASK deficiency and the degeneration of CG cells has not been completely investigated. A better understanding of the role of CASK in maintenance of the cerebellar structure may provide insights into the prevention of the cerebellar hypoplasia in MICPCH syndrome.
Ding et.al., reported that the knockdown of CASK in hepatocellular carcinoma (HCC) activates the Jun N-terminal kinase (JNK), a kinase that belongs to the family of stress-activated serine/threonine protein kinases of the mitogen-activated protein kinase (MAPK) pathway [
10]. JNK signaling plays a key role in cell death by regulating reactive oxygen species (ROS) [
11,
12], and the administration of the JNK inhibitor significantly attenuates CASK deficiency-mediated autophagic cell death [
10]. This finding suggests that CG cell death in CASK-KO mice may be also involved in the JNK and MAPK pathways, and inhibiting such pathways may rescue CG cell survival.
To investigate the molecular mechanism underlying cerebellar neurodegeneration caused by CASK deficiency, we performed RNA-sequencing (RNA-seq) analysis on CASK-KO CG cell culture. We identified that the JNK pathway-related genes were upregulated in these cells. The administration of JNK inhitibor rescued the CASK-KO CG cells in both in vitro and in vivo. These results suggest that JNK inhibitors have the potential to serve as a therapeutic strategy for cerebellar neurodegeneration in MICPCH syndrome.
2. Materials/Subjects and Methods
2.1. Animals and Housing Conditions
Floxed CASK (CASK
flox/flox) mice and wild type mice (CASK
+/+)(B6;129-Casktm1Sud/J, JAX Stock #006382) were as described previously[
9], Hprt-eGFP mice was obtained by other members in our laboratory. Male homozygous CASK KO (CASK
-/Y) mice and female heterozygous CASK KO mice (CASK
+/- mice) were obtained by crossing CASK
flox/flox mice and ZP3-Cre mice [
13] (C57BL/6-Tg (Zp3-cre)93Knw/J, JAX Stock #003651). Heterozygous CASK KO with Hprt-eGFP (CASK
+/floxHprt
eGFP/+) mice was obtained by crossing female CASK
+/- mice and Hprt-eGFP mice.
All animals were maintained with a 12:12 light-dark cycle with food and water ad libitum. The room temperature was maintained at 23 ± 2◦C. Animal experiments were reviewed by the Committee for Animal Experiments and were approved by the president of Shinshu University No.021045. The methods were carried out in accordance with the Regulations for Animal Experimentation of Shinshu University and the ARRIVE guidelines 2.0.
2.2. Plasmid and Lentivirus Preparation
Lentivirus-CTL: EGFP was removed by digestion with BamHI and EcoRI from pFSy(1.1)GW (Addgene #27232) and self-ligated. pFSyGW-iCre (lentivirus-iCre): BamHI-iCre-EcoRI was cloned into the BamHI/EcoRI site of pFSy (1.1)GW. Empty lentivirus (pFSy-control, lentivirus-CTL) was used as control. Construction of all other plasmid used in this study was described previously[
9].
2.3. Cell Culture
Primary CG cells were prepared from wild-type C57BL/6, CASK
floxed/floxed, Hprt
eGFP/eGFP, or CASK
+/floxHprt
eGFP/+ mice at P6, as described previously[
9,
14]. In short, isolated cerebella were incubated with phosphate-buffered saline (PBS) containing 1% trypsin (Sigma-Aldrich, St. Louis, MO, USA) and 0.1% DNase I (Sigma-Aldrich) for 3 min and dissociated by passing through a fire-polished Pasteur pipette in PBS containing 0.05% DNase I, 0.03% trypsin inhibitor (Sigma-Aldrich), and 2 mM MgCl
2.Cells were plated on coverslips coated with mouse laminin (ThermoFisher Scientific, Waltham, MA, USA) and poly-L-lysine (Sigma-Aldrich) at a density of 2 × 10
6cells/well, cultured in Neurobasal-A (ThermoFisher Scientific) supplemented with 2% B-27 supplement (ThermoFisher Scientific), 5% fetal bovine serum (FBS) (Biowest, Nuaillé, France), 100 U/mL of Antibiotic-Antimycotic Mixed (Nacalai tesque, Kyoto, Japan), and 2 mM GlutaMAX I (ThermoFisher Scientific) for 24 h at 37
◦C in a 5% CO
2 atmosphere, then cultured in the same medium without FBS.
The cultured cells were infected with either an empty lentivirus (pFSy(1.1)GW) or the lentivirus-iCre (pFSyGW iCre) at DIV1. After the first infections, the culture medium was replaced with the fresh medium, followed by infection with an empty lentivirus or a lentivirus expressing CASK. For drug effect testing, after the initial infections, the culture medium was replaced with the fresh medium, followed by a 0.1 μM, 1 μM, 10 μM, 20 μM, 50 μM or 100 μM of inhibitors (Table S2) or DMSO (0.1%) as a control. The cells were harvested in DIV4 and prepared for RNA extraction. The cells were fixed in DIV7 by 4%PFA+4% sucrose for further experiments.
2.4. RNA Extraction and Purification
Total RNA was extracted using TRIzol (Ambion, Carlsbad, CA, USA) followed by the manufacturer’s instructions, purified with the PureLink™ RNA Mini Kit (ThermoFisher Scientific). After purification, the total RNA was stored at -80◦C for further study.
2.5. RNA-Sequencing Analysis
RNA-seq was mainly completed by Takara Bio (TAKARA BIO INC., Shiga, Japan). Briefly, the reverse transcription was performed with the SMART (Switching Mechanism at 5' End of RNA Template) method with the SMART-Seq Stranded Kit. Then the PCR product purification was conducted using AMpure XP (Beckman Coulter, California, USA). The RNA-seq library was prepared by Nextera XT DNA Library Prep Kit. Sequence was performed with NovaSeq 6000 (Illumina, San Diego, USA). The results normalized by using the TPM (Transcripts Per Million), data visualization was performed using Rstudio[
15] (R 4.3.0; Posit Software; BOSTON, USA), gene ontology analysis was performed with the Metascape[
16] and gene set enrichment analysis (GSEA)[
17,
18].
2.6. Stereotaxic Surgery
P6 CASK+/floxHprteGFP/+, CASK+/+HprteGFP/+, or CASK+/+ mice were anesthetized on ice. Using Neuros syringes (65460-02, Hamilton, USA) and approximately 10 mg/ml (2% DMSO, 30% PEG 300, 5% Tween 80, 63% ddH2O) JNK-IN-8 or DMSO, 6.6 mg/kg was injected bilaterally in the following stereotactic coordinates of the cerebellum-anterioposterior (AP) - 1.2 mm, mediolateral (ML) ± 1.0 mm, and dorsoventral (DV) - 1.3 mm, taking lambda as the reference point using a stereotaxic device (Narishige, Tokyo, Japan). Each mouse injected 2ul drugs and 1ul 0.4%trypan blue. After the surgery, keep the heater on for 30 min before moving back to the mother. The mice were kept for 2 weeks after injection, before further experiments.
2.7. Limb-Clasping Scoring
One week before scoring, mice were allowed to acclimate to the vivarium. Mice were held by the tail for 60 s and recorded by the GoPro 9 (GoPRO, San Mateo, USA). During tail suspension, limb-clasping behavior was evaluated with the following standards: 0 - No limb clasping. Normal escape extension: 1 - 1 hind limb exhibits incomplete splay and loss of mobility. Toes exhibit normal splay; 2 - Both hind limbs exhibit incomplete splay and loss of mobility. Toes exhibit normal splay; 3 - Both hind limbs exhibit clasping with curled toes and immobility; 4 - Forelimbs and hind limbs exhibit clasping and are crossed, curled toes and immobility [
19].
2.8. Mouse Foot Gait Analysis by Ink
Habituate mice to the testing room and gait tunnel with the room light. Place the food at the surface level at the end of the gait tunnel to serve as a goal box. Mice’s forelimbs are coated with red ink, and the hindlimbs are coated with blue ink before they walk across a white paper. The papers with paw prints were used to measure the toe spread, stride length, and stride width [
20].
2.9. Data Visualization and Statistical Analyses
GraphPad (version 10.2.3(403), GraphPad Software; San Diego, CA, USA) and RStudio (R 4.3.0) were used for data visualization, and the quantitative variables are expressed as the mean ± standard error of the mean (SEM). Data statistical analyses were performed using GraphPad (version 10.2.3(403) with two-way analysis of variance (2-way ANOVA) followed by Tukey’s correction, p<0.05 viewed as statistical significance between each group.
2.10. Additional Materials and Methods
Additional methods are described in Supplementary Methods. Sequences of primers are listed in Table S1. Reagents and antibodies cat numbers are listed in Table S2. Original blot images are shown in Original Data 1.
3. Results
3.1. CASK Deletion Causes Activation of JNK Signaling and Apoptosis Pathway in CG Cells
To investigate the molecular mechanism underlying cerebellar hypoplasia in MICPCH syndrome, we first studied postnatal day 0 (P0) CASK-/Y mice. We found the body size of CASK-/Y mice was significantly smaller than that of CASK+/Y mice (Figure S1A). Morphological abnormality was also observed in the H&E stained cerebellar slices in CASK-/Y mice (Figure S1B). Since CASK-/Y mice die at P0, the capability of in vivo analysis in CASK-/Y mice was limited.
We then decided to study gene expression profiling in CASK deficient cells in comparison with that in wild-type cells. For this, we performed RNA-seq analysis in cultured CG cells. We prepared dissociated CG cell cultures form CASK
flox/flox and CASK
+/+ mice and infected them with lentiviruses expressing iCre at day
in vitro (DIV) 1 to generate CASK-KO and wild-type control CG cell cultures (
Figure 1A). Since CASK-KO GC cells started dying at DIV4, we collected mRNA from CASK-KO and control CG cell cultures at DIV4. The expression of CASK in CASK
flox/flox iCre CG cells was significantly (
p <0.001) decreased compared to that in CASK
+/+ iCre CG cell in the RNA-seq results, as expected (
Figure 1B). We found the expression levels of JNK signaling and apoptosis-related genes such as c-Jun (
p <0.01), Fosl2 (
p <0.01), Gem (
p <0.01) and Cxcl2 (
p <0.05) were significantly increased in CASK
flox/flox iCre CG cells compared to those in CASK
+/+ iCre CG cells. We selected genes of which expression were significantly changed in CASK-KO CG cells compared to that in \wild-type cells and performed Gene Ontology analysis (Figures S2A, 430 in total). The most significantly changed ones were related to the regulation of the neurotransmitter transport pathway (
Figure 1C) and the localization process (
Figure 1D). This suggests that the deletion of CASK may cause abnormality in neurotransmission, highlight the significance of CASK in the synaptic function also in CG cells. GSEA indicated that cell death related signaling such as apoptosis and Reactive Oxygen Species (ROS) pathway were enhanced in CASK
flox/flox iCre CG cells (
Figure 1E). To further investigate the molecular interaction, we conducted IPA signaling analysis for the representative genes and found that the CASK deletion caused upregulation of the apoptosis and inflammation signaling, such as IL-1β and Tnf, (
Figure 1F). These results suggest that the CASK deletion causes the activation of JNK signaling and apoptosis pathway in CG cells.
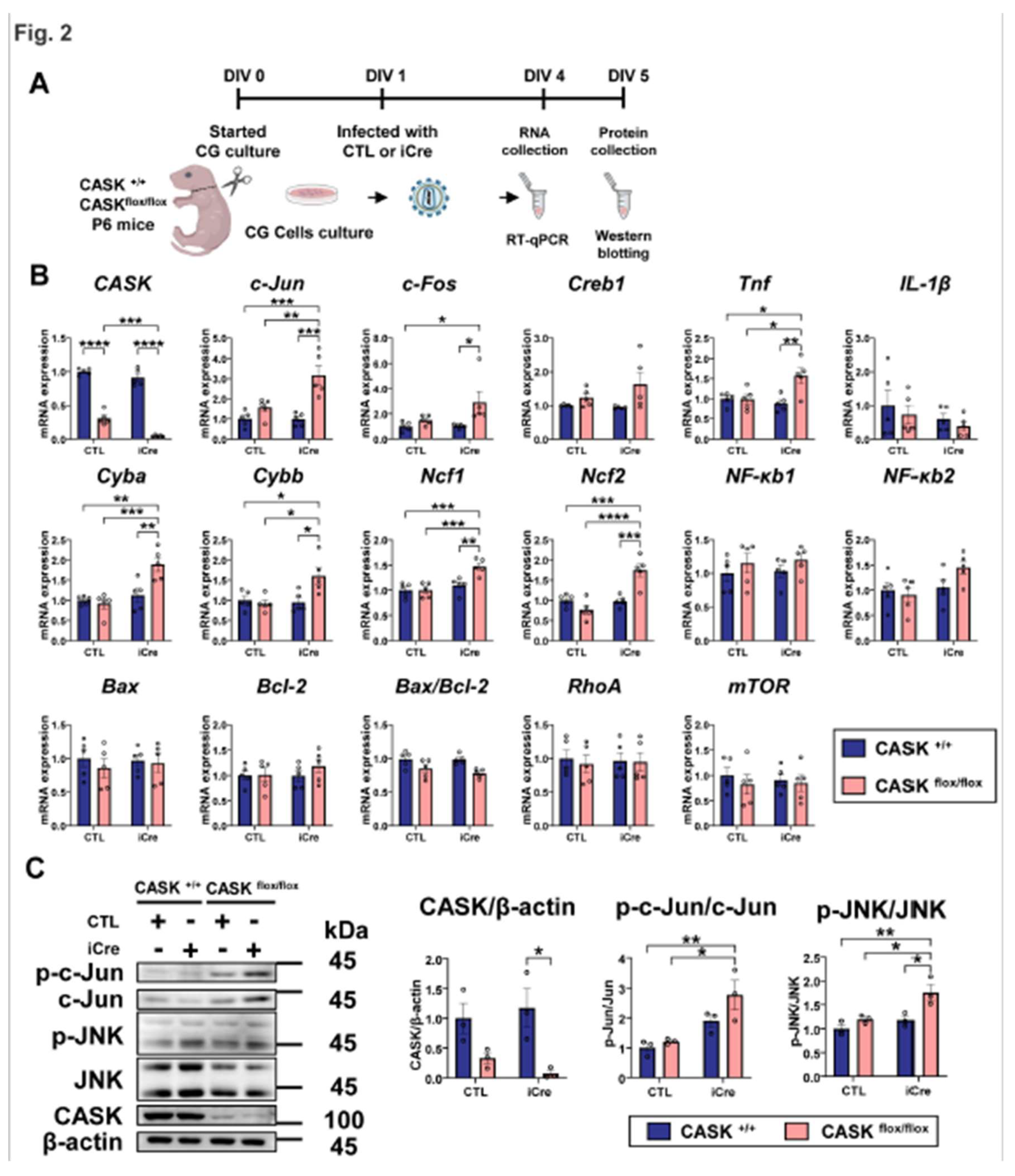
3.2. The Levels of p-c-Jun and p-JNK in CASKflox/flox iCre CG Cells Are Significantly Increased
To confirm the RNA-seq results, we performed realtime-qPCR and western blotting analysis in the extracts from CG cell cultures infected with lentivirus expressing iCre (
Figure 2A). The expression of CASK in CASK
flox/flox iCre was almost undetectable in these experiments indicating gene removal by Cre recombination was successful (
Figure 2B). The expression of genes related to JNK signaling such as c-Jun (
p <0.001) and c-Fos (
p <0.001) in CASK
flox/flox iCre CG cells were significantly upregulated compared to the CASK
+/+ iCre CG cells. The expression of ROS related genes such as Cyba, Cybb, Ncf1 and Ncf2 was also significantly upregulated in CASK
flox/flox iCre infected CG cells. On the other hand, gene expressions such as IL-1β, NF-κb1, NF-κb2, and Bax/Bcl-2 were not significantly changed between each group (
Figure 2B). Therefore, we decided to focus on the protein phosphorylation changes in JNK signaling and ROS pathway.
In western blotting analysis on these cells, we found the phosphorylated c-Jun (
p <0.05) and JNK (
p=0.1725) increased in CASK
flox/flox iCre CG cells compared to the CASK
+/+ CTL CG cells (
Figure 2C). This suggests the knockout of CASK promotes phosphorylation signaling of JNK, that will cause cell apoptosis in CG cells. These results indicate that the inhibition of JNK signaling may be effective for preventing cell death in CASK deficient CG cells.
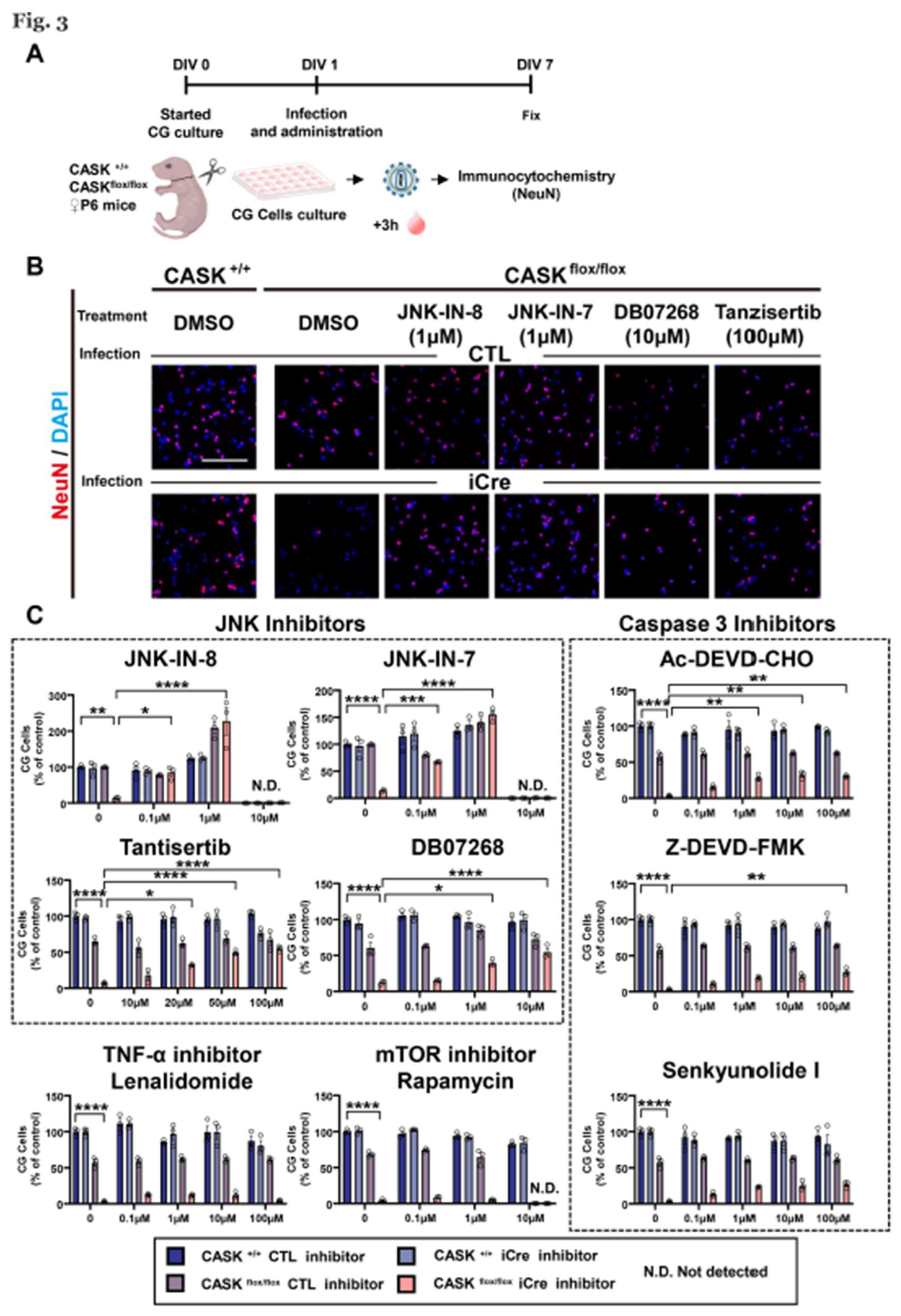
3.3. Administration of JNK Inhibitors Promotes the Survival Rate in CASKflox/flox iCre CG Cells In Vitro
To investigate the effects of JNK inhibition on CG cells, we administrated JNK inhibitors on CG cells with different concentrations and stained the cells with NeuN and DAPI to evaluate the survival rate changes (
Figure 3A). Without administration, the survival rate of CASK
flox/flox iCre CG cells was significantly lower than the CASK
+/+ CTL CG cells (
Figure 3B). We found the administration of 1 µM of JNK-IN-8 and JNK-IN-7 significantly (
p<0.0001) increased the survival rate of CASK
flox/flox iCre CG cells compared to those untreated. However, administration of 10 µM of these drugs showed cytotoxicity. The administration of Tantisertib, DB07268, and SP600125, another JNK inhibitors, increased the survival rate of CASK KO CG cells as the concentration increased (
Figure 3C, S3A, B). These results indicate that the inhibition of JNK signaling promotes the survival rate in CASK
flox/flox iCre CG Cells.
To verify the result, we also administrated JNK signaling related inhibitors, such as the inhibitors of Caspase 3, TNF, mTOR, and p38 signaling (
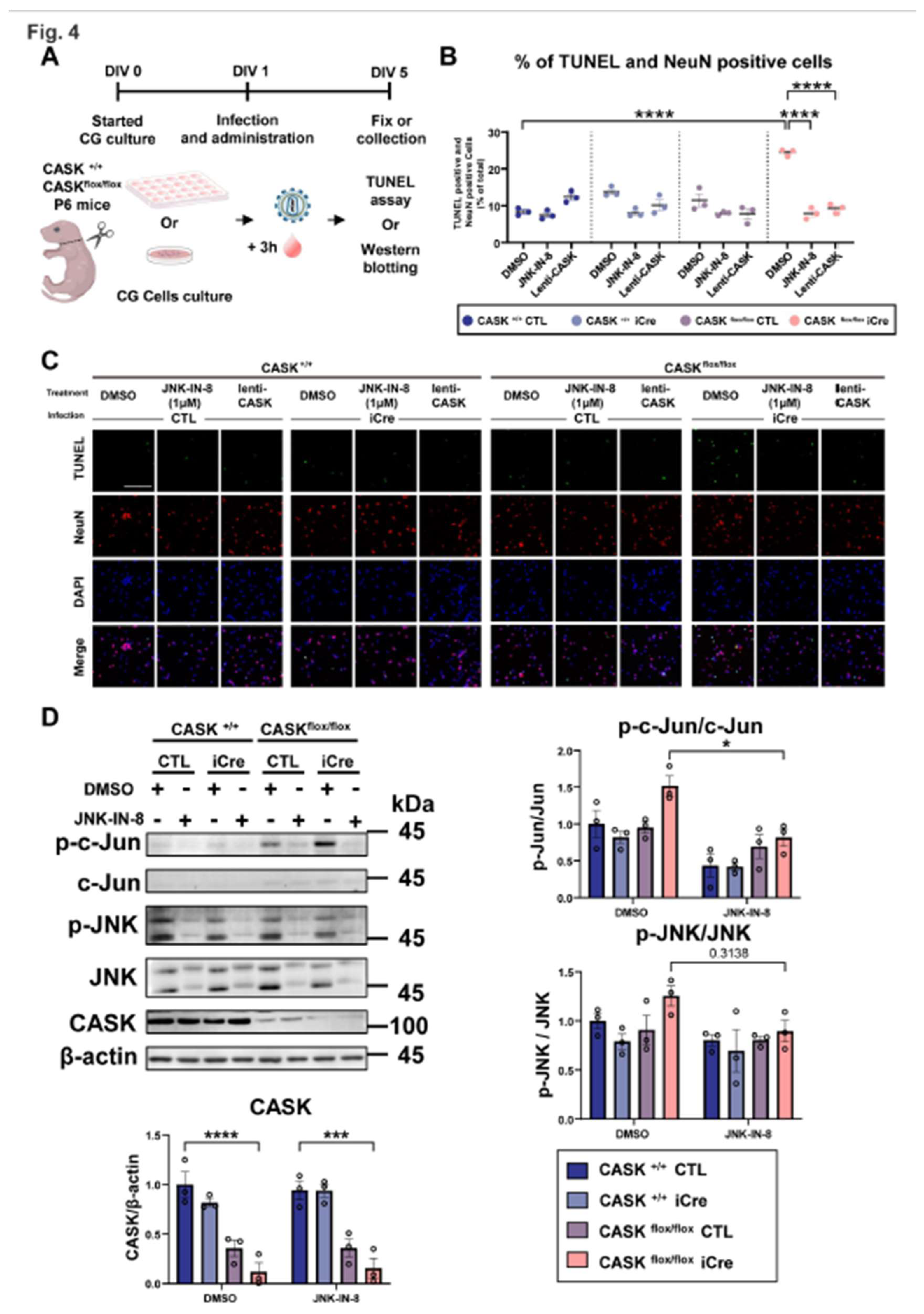
Figure 3C and Figure SS3A,B). Those inhibitors showed a minor effect on the survival rate of CASK-KO CG cells, but not comparative to the effect of JNK inhibitors. These indicate that the JNK inhibition has the strongest relationship with CG cell death caused by CASK deletion. JNK-IN-8 showed strongest rescue effects on CASK deficient CG cell survival among JNK inhibitors we tested. To investigate the effect on apoptosis, we administrated the JNK-IN-8 on CG cells and performed TUNEL staining (
Figure 4A). We found the number of TUNEL and NeuN double positive cells was significantly (
p<0.0001) increased in CASK
flox/flox iCre CG cells compared to CASK
+/+ CTL CG cells (
Figure 4B,C). After the administration of JNK-IN-8, the number of double positive cells was significant (
p<0.0001) reduced in CASK
flox/flox iCre CG cells. As expected, the number of TUNEL and NeuN double positive cells were decreased in CASK
flox/flox iCre CG cells after the infection with Lenti-CASK (
p<0.0001). The result of TUNEL indicated that the administration of JNK-IN-8 promoted the survival rate in CASK
flox/flox iCre CG cells, by preventing the apoptosis caused by the deletion of CASK.
We further verified the inhibition effect of JNK-IN-8 on JNK signaling in CG cells (
Figure 4D). The p-c-Jun/c-Jun and p-JNK/JNK were suppressed by the JNK-IN-8 administration. We also tested the effect of Lenti-CASK infection on CASK
+/+ CG cells. Although Lenti-CASK infection reduced p-JNK/JNK compared to the uninfected control, the effect was smaller than that of the JNK-IN-8 administration (Figure S3C).
3.4. Gene Expression Analyses Indicates that JNK Inhibition Specifically Reduced Apoptosis in CASKflox/flox iCre CG Cells
To further confirm the JNK inhibitor’s effect on the alteration of the gene expression in CG cells, we examined the expression levels of those genes in JNK-IN-8- or DMSO-treated CG cells by realtime-qPCR (
Figure 5A and Figure S4). We found that the expression levels of c-Jun (
p<0.0001), c-Fos (p<0.01), and Tnf (p<0.001) were significantly reduced after the administration of JNK-IN-8 in CASK
flox/flox iCre CG cells. In addition, the levels of ROS and ROS related genes were also reduced by the administration of JNK-IN-8 in CASK
flox/flox iCre CG cells (
Figure 5A,B). By RNA-seq analysis, we found apoptosis genes such as Gem and Cxcl2 were significantly reduced in JNK-IN-8 treated CASK
flox/flox iCre CG cells compared to those in DMSO treated CASK
flox/flox iCre CG cells (
Figure 5C,D). From GESA analysis (
Figure 5E), cell death related signaling such as apoptosis and ROS pathway was suppressed by the administration of JNK-IN-8. However, the other apoptosis signaling related genes such as Bax and Bcl2 were not significantly affected by the administration of JNK-IN-8 (
Figure 5D), which was also verified by realtime-qPCR (Figure S4), indicating that JNK signaling plays a specific role in suppressing apoptosis and ROS caused by CASK deletion. By inhibiting JNK signaling, CG cell survival was improved in CASK deficient CG cells by suppressing the expression of apoptotic genes
in vitro. This indicates application of JNK inhibitors may be the method for preventing cerebellar hypoplasia in MICPCH syndrome.
3.5. The Administration of the JNK Inhibitor In Vivo Shows Preventative Effect Against Neurodegeneration
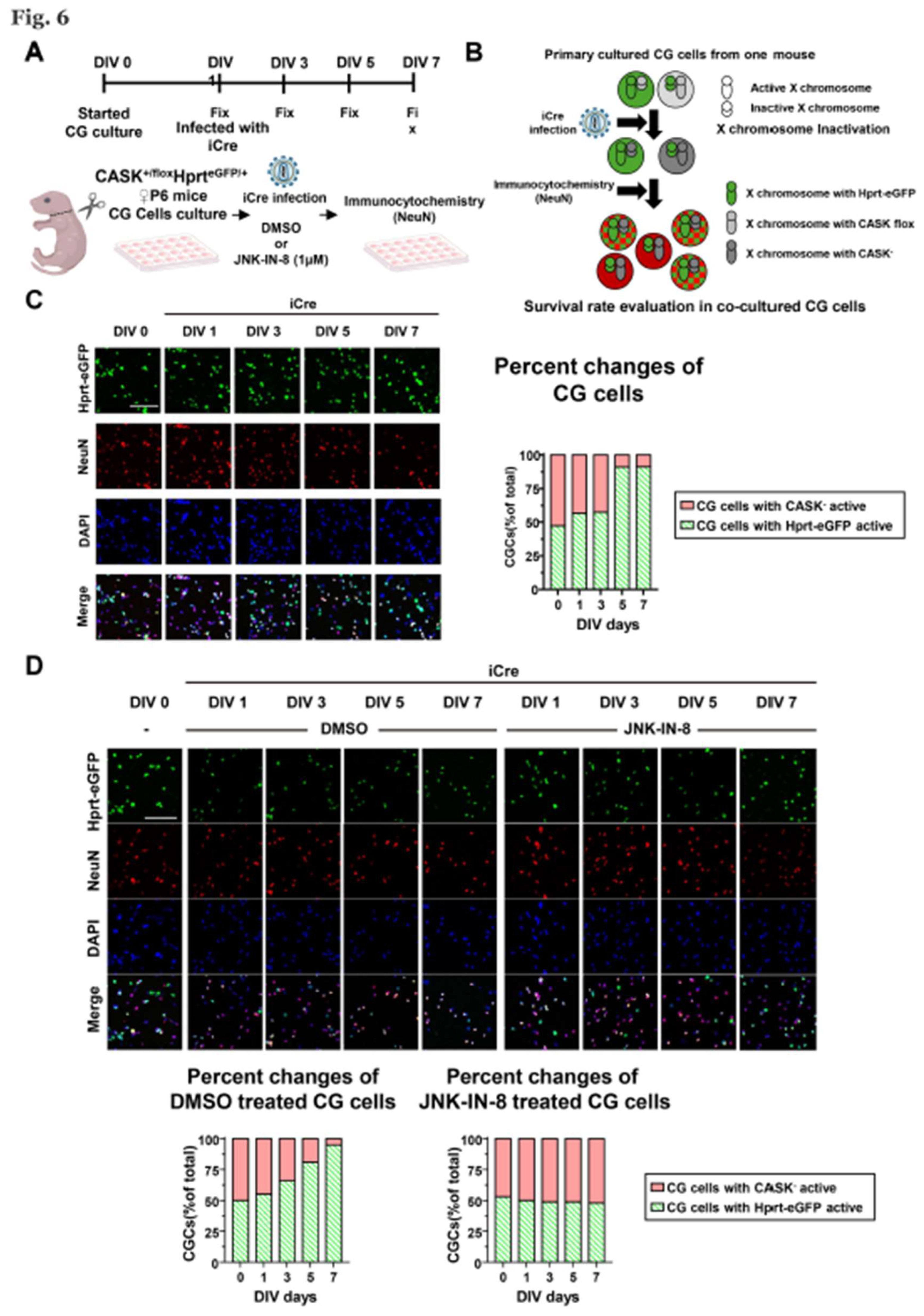
Due to the perinatal lethality of CASK knockout, we evaluated the effect of JNK inhibition on the cerebellar hypoplasia
in vivo in female CASK heterozygote knockout mice, of which genetic background is representative to the majority of MICPCH syndrome. Our previous study demonstrated that X-chromosome inactivation produced mosaic distribution of the CASK expressing and CASK non-expressing neurons in the brain of female CASK heterozygote knockout mice [
8]. To investigate whether the CG cell death in female CASK heterozygote knockout mice is specific to CASK knockout cells or not, we utilized Hprt-eGFP mice to visualize the pattern of X-chromosome inactivation. In Hprt-eGFP mice, the eGFP expression cassette under the control of CAG promoter was knocked in the hprt locus on the X-chromosome [
21]. We generated CASK flox and Hprt-eGFP trans-heterozygote mice (CASK
flox; Hprt
eGFP ) by crossing CASK flox and Hprt-eGFP mice. In these mice, the cells expressing eGFP indicate that the X-chromosome inherited from Hprt-eGFP mice is intact and express wild-type CASK. In contrast, the cells that are not expressing eGFP indicate of which X-chromosome inherited from Hprt-eGFP is inactivated and have active X-chromosome with CASK flox allele. We prepared CG cell culture from CASK
flox; Hprt
eGFP mice and infected with lentivirus expressing iCre to create CASK KO and CASK intact culture (Figure S5). We examined the survival rate by NeuN and DAPI staining (
Figure 6A,B). By analyzing immuno
cytochemistry-stained images, we found the percentage of the CG cells without eGFP expression is gradually reduced in these cultures, indicating CASK knockout cells die selectively without affecting CASK intact cells (
Figure 6C). We administered JNK-IN-8 or DMSO in the co-cultured CG cells 3 hours after the iCre infection. We found the DMSO administration did not increase the percentage of CASK knockout CG cells (
Figure 6D). In contrast, JNK-IN-8 administration rescued the reduction of CASK knockout cells and the ratio between eGFP positive and negative cells was almost 1:1 (
Figure 6D). These results suggest that apoptosis occurs selectively in CASK knockout CG cells and it can be prevented by the administration of JNK-IN-8.
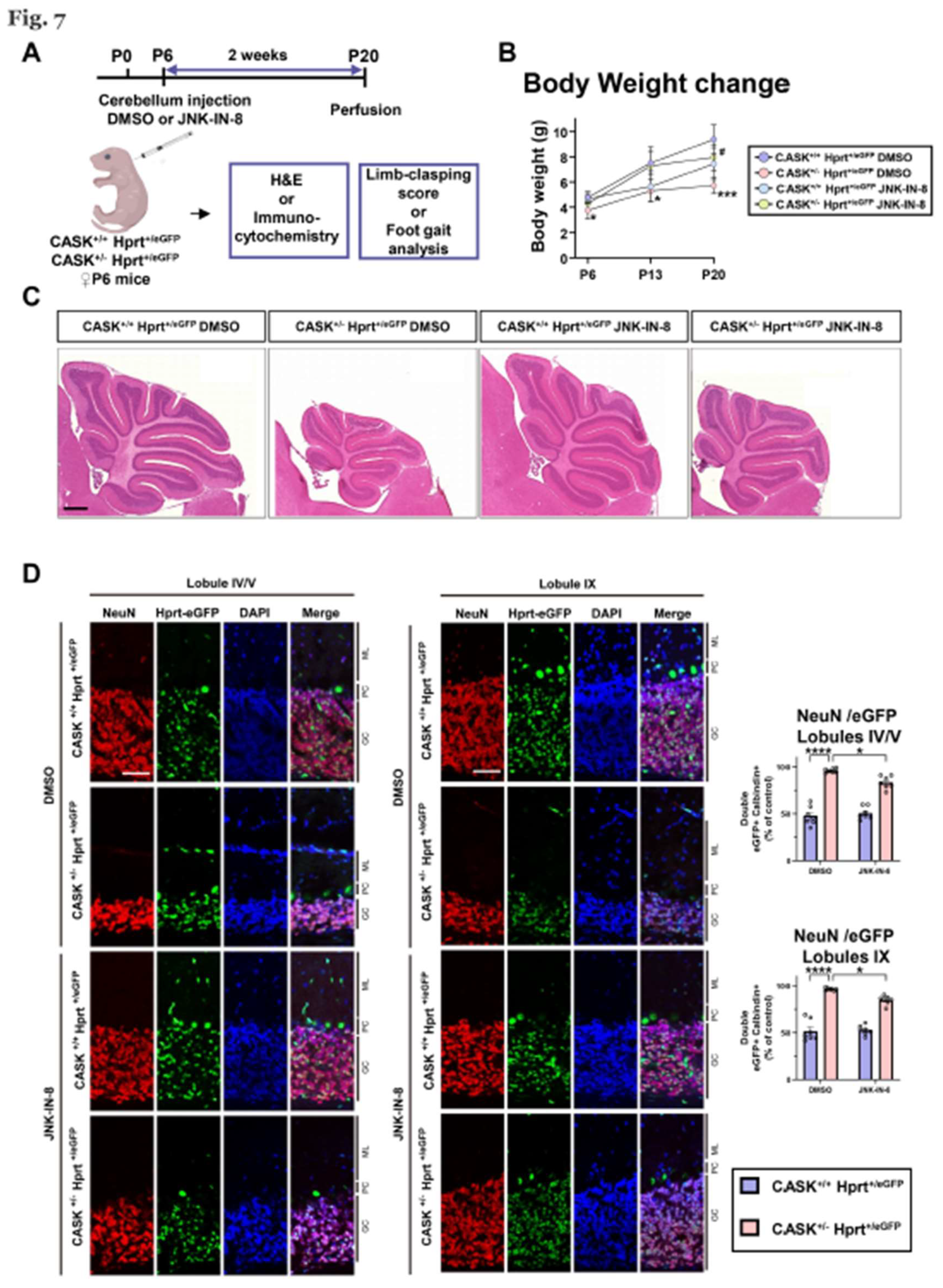
Next, to evaluate the effect of JNK-IN-8 on heterozygous CASK knockout mice
in vivo, we administrated JNK-IN-8 in the cerebellum of P6 CASK
+/-Hprt
eGFP/+ and CASK
+/+Hprt
eGFP/+ mice and analyzed 2 weeks after the injection (
Figure 7A). Before injection, the body weight of CASK
+/-Hprt
eGFP/+ mice was significantly (P6:
p<0.05; P13:
p<0.05; P20:
p<0.001) lower than CASK
+/+Hprt
eGFP/+ mice (
Figure 7B and Figure S6A). The JNK-IN-8 administration significantly (
p<0.05) increased the body weight of CASK
+/-Hprt
eGFP/+ mice, 2 weeks after the injection. In H&E stained cerebellum slices, we found the size of the cerebellum in CASK
+/-Hprt
eGFP/+ mice was smaller than that of CASK
+/+Hprt
eGFP/+ mice at P20. The administration of JNK-IN-8 increased the size in CASK
+/-Hprt
eGFP/+ mice (
Figure 7C). Moreover, by immuno
histochemistry staining or H&E stained on slices from Lobules IX and Lobules IV/V, we found granular cell (GC) layer of the CASK
+/-Hprt
eGFP/+ was thinner and the cell density was lower than those of CASK
+/+Hprt
eGFP/+ (
Figure 7D and Figure S6B). Almost 100% of cells in CASK
+/-Hprt
eGFP/+ GC layer are NeuN and eGFP double positive, indicating that only those CASK expressing CG cells were survival. After the JNK-IN-8 administration, the ratio of such double positive cells significantly (
p<0.05) reduced in both Lobules IX and Lobules IV/V. Indicating that some CASK knockout CG cells were recovered in those layers. We found similar results in Purkinje cell (PC) layer (Figure S7). These morphological results indicated that JNK-IN-8 administration
in vivo prevents cerebellar cell loss in of CASK
+/-Hprt
eGFP/+ mice.
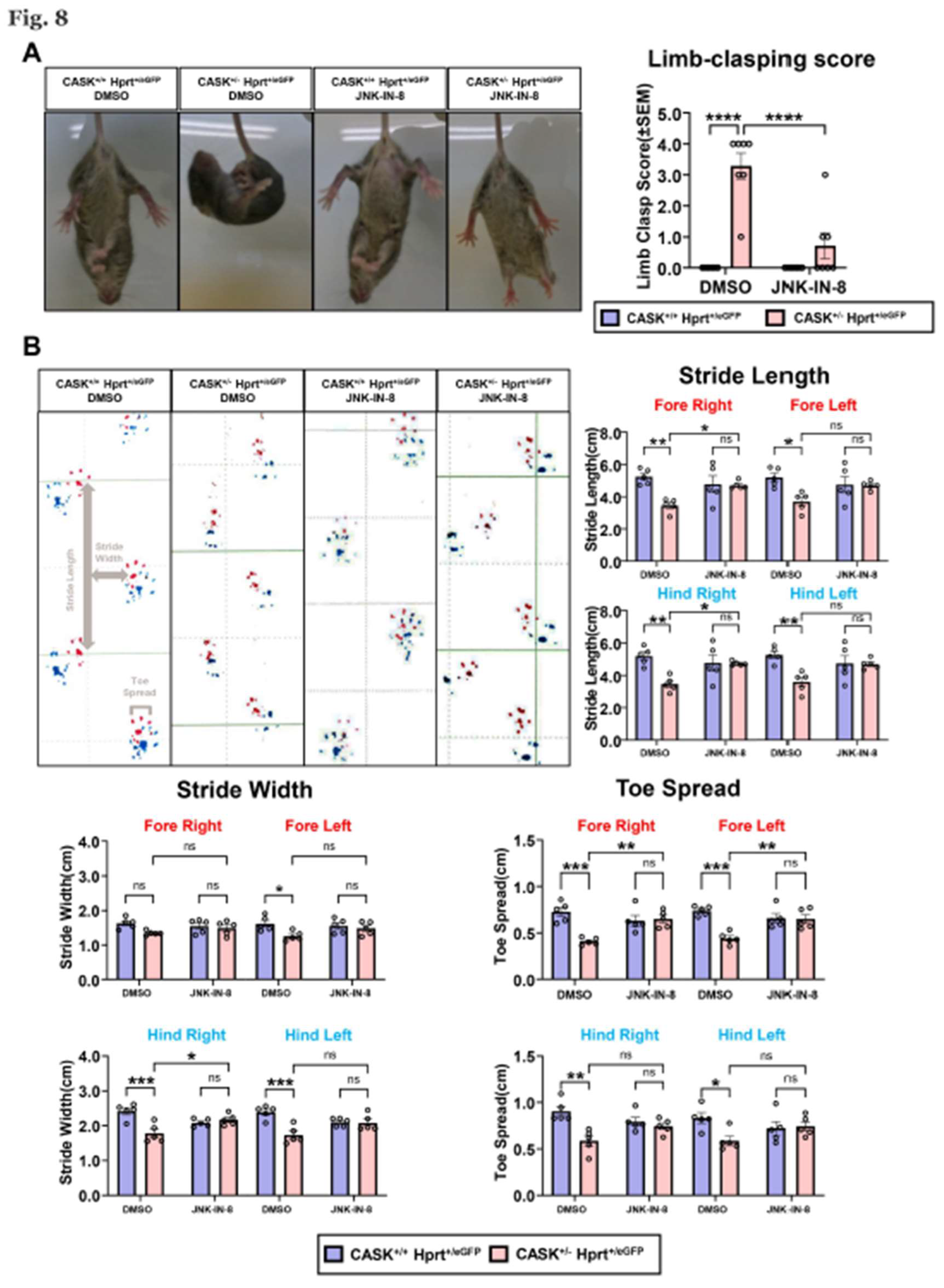
Lastly, we examined the effect of the administration of the JNK inhibitor on the behavior of CASK
+/-Hprt
eGFP/+ mice. We performed limb clasping test on these mice that can monitor cerebellar ataxia. CASK
+/-Hprt
eGFP/+ mice exhibited clasping and crossing of their limbs and immobilized indicating they have cerebellar ataxia (
Figure 8A). However, after the JNK-IN-8 injection into cerebellum at P6, the mobility was improved, and the limb-clasping score was significantly decreased (
p<0.0001) compared to untreated mice. We also performed gait analysis on these mice. We found the stride length and toe spread of CASK
+/-Hprt
eGFP/+ mice were significantly shorter than CASK
+/+Hprt
eGFP/+ (
Figure 8B). These behavioral analyses indicated that the cerebellar function of CASK
+/-Hprt
eGFP/+ mice was improved by the administration of JNK-IN-8.
4. Discussion
Although apoptosis caused by CASK deletion has been well documented in previous neurological studies [
2,
22,
23], the specific molecular pathways mediating CG cell death have remained largely unexplored. Our study is the first to perform bulk RNA-seq and pathway-level analysis on cerebellar granule (CG) cells from CASKflox/flox iCre mice, revealing that CASK deficiency induces significant activation of the JNK signaling and reactive oxygen species (ROS) pathways. This result was further confirmed by qPCR, western blotting, and TUNEL assays, supporting the hypothesis that CASK plays a crucial role in maintaining cellular homeostasis in the cerebellum.
Our findings are consistent with previous reports linking JNK activation and ROS accumulation to neurodegenerative processes [
25,
26]. The upregulation of apoptosis-related genes such as c-Jun, Fosl2, Gem, and Cxcl2, alongside the increased phosphorylation of JNK and c-Jun, strongly suggest that the JNK pathway is a key effector of CG cell death in the absence of CASK. Importantly, JNK-IN-8 treatment effectively suppressed both the JNK phosphorylation and the transcriptional activation of ROS-related genes, thereby rescuing cell survival. Among the JNK inhibitors tested, JNK-IN-8 showed the most robust and selective effect, highlighting its potential as a therapeutic compound for CASK-related neurodegeneration.
Moreover, our in vivo experiments in heterozygous CASK knockout mice (CASK+/-HprteGFP/+) provided compelling evidence that JNK inhibition alleviates cerebellar hypoplasia and motor deficits. Notably, eGFP-negative cells—representing the CASK-deficient population due to X-inactivation—were selectively lost in untreated mice, but their survival was preserved following JNK-IN-8 administration. Behavioral improvements in limb-clasping and gait analysis further confirmed the functional recovery of cerebellar circuits, particularly in the hemisphere receiving JNK-IN-8 injections.
Nevertheless, our study has several limitations. First, while CG cells were the primary focus, the potential involvement of other cerebellar cell types, such as Purkinje cells and interneurons, was not fully explored. Second, our omics analysis focused primarily on apoptosis and oxidative stress pathways; other altered processes, including metabolism-related changes [
31], may also play a role and warrant future investigation. In addition, while bulk RNA-seq provided valuable global insight, single-cell resolution would help distinguish cell-type–specific responses and mosaic expression patterns due to X-inactivation. Further studies utilizing single-cell RNA-seq or spatial transcriptomics would be particularly informative.
Lastly, while our results strongly support the use of JNK inhibitors to rescue CASK-dependent phenotypes, the long-term safety and efficacy of such compounds in vivo remain to be established. The possibility of off-target effects or compensation by parallel pathways cannot be excluded. Nonetheless, our work provides a molecular framework that bridges CASK deficiency and cell death signaling in the cerebellum and opens the door for targeted therapeutic strategies in MICPCH syndrome.
5. Conclusions
Our study demonstrates that CASK deletion in cerebellar granule cells leads to apoptosis and neurodegeneration through activation of the JNK signaling and ROS pathways. Using RNA-seq, molecular assays, and behavioral analysis in vitro and in vivo, we showed that pharmacological inhibition of JNK signaling—particularly via JNK-IN-8—effectively suppresses CG cell death and alleviates cerebellar ataxia in a mouse model of MICPCH syndrome. These findings identify the JNK pathway as a critical mediator of CASK-related cerebellar pathology and support the potential utility of JNK inhibitors as therapeutic agents for neurodevelopmental disorders involving CASK dysfunction.
Declaration of competing interest
We have no conflicts to declare.
Availability of Data and Materials
The RNA-seq data and other raw datasets are available at [
https://] (link to be inserted), or from Q.G. upon request (gq_2222@126.com). Materials used in the study are available from K.T. upon request (ktabuchi@shinshu-u.ac.jp).
Funding statements
This work was supported by grants KAKENHI 23H02575 (K.T.); the Grant-in-Aid for Transformative Research Areas (A) 23H04227 (K.T.); and SENSHIN Medical Research Foundation (K.T.).
Author Contributions
Conceptualization, K.T.; methodology, Q.G., E.K.S., Y.S. and K.T.; data acquisition, Q.G. and E.K.S.; data curation, Q.G.; formal analysis and investigation, Q.G. and E.K.S.; project administration, E.K.S., Y.S.; resources, E.K.S.; supervision, E.K.S., Y.S. and K.T.; writing – original draft, Q.G.; writing – review & editing, E.K.S., Y.S. and K.T.; funding acquisition, K.T.
Acknowledgments
We thank all the lab members of Department of Molecular and Cellular Physiology of Shinshu University for helpful suggestions. We also thank members of Research Center for Advanced Sci-ence and Technology of Shinshu University for their technical supports.
References
- Nishio, Y.; Kidokoro, H.; Takeo, T.; Narita, H.; Sawamura, F.; Narita, K.; et al. The eldest case of MICPCH with CASK mutation exhibiting gross motor regression. Brain Dev 2021, 43, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Piluso, G.; D'Amico, F.; Saccone, V.; Bismuto, E.; Rotundo, I.L.; Di Domenico, M.; et al. A missense mutation in CASK causes FG syndrome in an Italian family. Am J Hum Genet 2009, 84, 162–177. [Google Scholar] [CrossRef]
- Moog, U.; Kutsche, K.; Kortum, F.; Chilian, B.; Bierhals, T.; Apeshiotis, N.; et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet 2011, 48, 741–751. [Google Scholar] [CrossRef]
- Hsueh, Y.P. Calcium/calmodulin-dependent serine protein kinase and mental retardation. Ann Neurol 2009, 66, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Butz, S.; Sudhof, T.C. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci 1996, 16, 2488–2494. [Google Scholar] [CrossRef] [PubMed]
- Olsen, O.; Moore, K.A.; Nicoll, R.A.; Bredt, D.S. Synaptic transmission regulated by a presynaptic MALS/Liprin-alpha protein complex. Curr Opin Cell Biol 2006, 18, 223–227. [Google Scholar] [CrossRef]
- Atasoy, D.; Schoch, S.; Ho, A.; Nadasy, K.A.; Liu, X.; Zhang, W.; et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A 2007, 104, 2525–2530. [Google Scholar] [CrossRef]
- Mori, T.; Kasem, E.A.; Suzuki-Kouyama, E.; Cao, X.; Li, X.; Kurihara, T.; et al. Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by down-regulating GluN2B. Mol Psychiatry 2019, 24, 1079–1092. [Google Scholar] [CrossRef]
- Guo, Q.; Kouyama-Suzuki, E.; Shirai, Y.; Cao, X.; Yanagawa, T.; Mori, T.; Tabuchi, K. Structural Analysis Implicates CASK-Liprin-alpha2 Interaction in Cerebellar Granular Cell Death in MICPCH Syndrome. Cells 2023, 12. [Google Scholar] [CrossRef]
- Ding, B.; Bao, C.; Jin, L.; Xu, L.; Fan, W.; Lou, W. CASK Silence Overcomes Sorafenib Resistance of Hepatocellular Carcinoma Through Activating Apoptosis and Autophagic Cell Death. Front Oncol 2021, 11, 681683. [Google Scholar] [CrossRef]
- Shen, H.M.; Liu, Z.G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 2006, 40, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.H.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ 2006, 13, 730–737. [Google Scholar] [CrossRef]
- Wu, H.; Luo, J.; Yu, H.; Rattner, A.; Mo, A.; Wang, Y.; et al. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron 2014, 81, 103–119. [Google Scholar] [CrossRef]
- Uemura, T.; Suzuki-Kouyama, E.; Kawase, S.; Kurihara, T.; Yasumura, M.; Yoshida, T.; et al. Neurexins play a crucial role in cerebellar granule cell survival by organizing autocrine machinery for neurotrophins. Cell Rep 2022, 39, 110624. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Guo, Q.; Wang, P.; Li, P.; Du, Q.; et al. Sophoricoside reduces inflammation in type II collagen-induced arthritis by downregulating NLRP3 signaling. Biochemistry and Biophysics Reports 2024, 40, 101867. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Zhao, Y.; Sakurai, T.; Kamiyoshi, A.; Tanaka, M.; Ichikawa-Shindo, Y.; Kawate, H.; et al. Adrenomedullin 2/Intermedin Exerts Cardioprotective Effects by Regulating Cardiomyocyte Mitochondrial Function. Hypertension 2025. [CrossRef]
- Miedel, C.J.; Patton, J.M.; Miedel, A.N.; Miedel, E.S.; Levenson, J.M. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-beta and Tau Neuropathology. J Vis Exp 2017. [Google Scholar] [CrossRef]
- Wertman, V.; Gromova, A.; La Spada, A.R.; Cortes, C.J. Low-Cost Gait Analysis for Behavioral Phenotyping of Mouse Models of Neuromuscular Disease. J Vis Exp 2019. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hosoi, Y.; Shiura, H.; Yamagata, K.; Takahashi, S.; Fujihara, Y.; et al. Live imaging of X chromosome reactivation dynamics in early mouse development can discriminate naive from primed pluripotent stem cells. Development 2016, 143, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Tarpey, P.S.; Smith, R.; Pleasance, E.; Whibley, A.; Edkins, S.; Hardy, C.; et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat Genet 2009, 41, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.S.; Hong, C.J.; Yen, T.Y.; Huang, H.Y.; Ou, Y.; Huang, T.N.; et al. Transcriptional modification by a CASK-interacting nucleosome assembly protein. Neuron 2004, 42, 113–128. [Google Scholar] [CrossRef]
- Patel, P.A.; Hegert, J.V.; Cristian, I.; Kerr, A.; LaConte, L.E.W.; Fox, M.A.; et al. Complete loss of the X-linked gene CASK causes severe cerebellar degeneration. J Med Genet 2022, 59, 1044–1057. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Borsello, T.; Forloni, G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des 2007, 13, 1875–1886. [Google Scholar] [CrossRef]
- Busquets, O.; Parcerisas, A.; Verdaguer, E.; Ettcheto, M.; Camins, A.; Beas-Zarate, C.; et al. c-Jun N-Terminal Kinases in Alzheimer's Disease: A Possible Target for the Modulation of the Earliest Alterations. J Alzheimers Dis 2021, 82, S127–S139. [Google Scholar] [CrossRef]
- Ahmed, T.; Zulfiqar, A.; Arguelles, S.; Rasekhian, M.; Nabavi, S.F.; Silva, A.S.; Nabavi, S.M. Map kinase signaling as therapeutic target for neurodegeneration. Pharmacol Res 2020, 160, 105090. [Google Scholar] [CrossRef]
- Murata, H.; Phoo, M.T.Z.; Ochi, T.; Tomonobu, N.; Yamamoto, K.I.; Kinoshita, R.; et al. Phosphorylated SARM1 is involved in the pathological process of rotenone-induced neurodegeneration. J Biochem 2023, 174, 533–548. [Google Scholar] [CrossRef]
- Yadav, R.K.; Minz, E.; Mehan, S. Understanding Abnormal c-JNK/p38MAPK Signaling in Amyotrophic Lateral Sclerosis: Potential Drug Targets and Influences on Neurological Disorders. CNS Neurol Disord Drug Targets 2021, 20, 417–429. [Google Scholar]
- Zhang, Z.; Li, W.; Yang, G.; Lu, X.; Qi, X.; Wang, S.; et al. CASK modulates the assembly and function of the Mint1/Munc18-1 complex to regulate insulin secretion. Cell Discov 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; McMillan, R.; Willis, J.; Clark, H.; Chavan, V.; Liang, C.; et al. X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta Neuropathol Commun 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Tibbe, D.; Pan, Y.E.; Reissner, C.; Harms, F.L.; Kreienkamp, H.J. Functional analysis of CASK transcript variants expressed in human brain. PLoS One 2021, 16, e0253223. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
RNA-seq analysis showed activation of JNK signaling and apoptosis pathway in CASKflox/flox iCre CG cells A. Experimental design of the RNA-seq analysis. P6 CASKflox/flox and CASK+/+ mice were used for CG cell culture. Cells were infected with iCre in DIV1, RNA was collected in DIV4 and Protein was collected in DIV5. (n=3 in each group) B. Volcano map of gene expression difference between CASK flox/flox iCre CG cells and CASK+/+ iCre CG cells. Red indicated genes upregulated in CASK flox/flox iCre CG cells, blue indicated genes downregulated in CASK flox/flox iCre CG cells. (Threshold: p<0.05, Fold change over2) C. The top 10 Gene Ontology enrichment pathways in CASK flox/flox iCre CG cells analyzed by Metascape. D. The top 10 Gene Ontology enrichment process in CASK flox/flox iCre CG cells analyzed by Metascape. E. Representative Gene Set Enrichment Analysis (GESA) results of apoptosis related pathways. F. Upregulated signaling in CASK flox/flox iCre CG cells was analyzed using IPA analysis, and the heatmap of the related gene expression detected by RNA-seq.
Figure 1.
RNA-seq analysis showed activation of JNK signaling and apoptosis pathway in CASKflox/flox iCre CG cells A. Experimental design of the RNA-seq analysis. P6 CASKflox/flox and CASK+/+ mice were used for CG cell culture. Cells were infected with iCre in DIV1, RNA was collected in DIV4 and Protein was collected in DIV5. (n=3 in each group) B. Volcano map of gene expression difference between CASK flox/flox iCre CG cells and CASK+/+ iCre CG cells. Red indicated genes upregulated in CASK flox/flox iCre CG cells, blue indicated genes downregulated in CASK flox/flox iCre CG cells. (Threshold: p<0.05, Fold change over2) C. The top 10 Gene Ontology enrichment pathways in CASK flox/flox iCre CG cells analyzed by Metascape. D. The top 10 Gene Ontology enrichment process in CASK flox/flox iCre CG cells analyzed by Metascape. E. Representative Gene Set Enrichment Analysis (GESA) results of apoptosis related pathways. F. Upregulated signaling in CASK flox/flox iCre CG cells was analyzed using IPA analysis, and the heatmap of the related gene expression detected by RNA-seq.
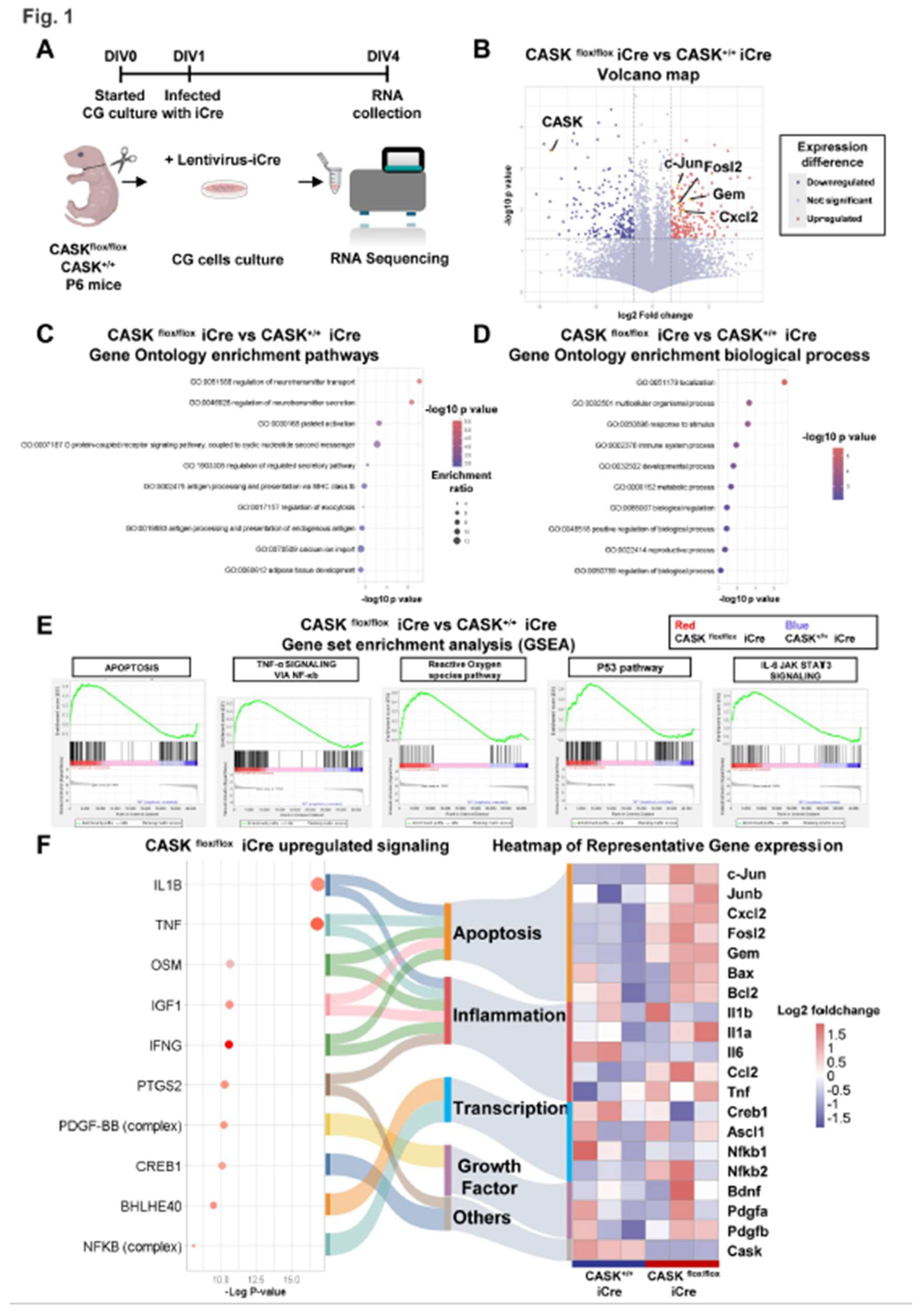
Figure 2.
Realtime-qPCR analysis and western blotting confirmed the activation of JNK signaling in CASKflox/flox iCre CG cells A. Experimental design of realtime-qPCR and western blotting. CG cells were infected with lentivirus-iCre and lentivirus-CTL in DIV1, RNA and protein samples were collected in DIV4. B. Realtime-qPCR analysis on CG cells. (n=5 in each group) C. Western blotting analysis on CG cells. (n=3 in each group) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 2.
Realtime-qPCR analysis and western blotting confirmed the activation of JNK signaling in CASKflox/flox iCre CG cells A. Experimental design of realtime-qPCR and western blotting. CG cells were infected with lentivirus-iCre and lentivirus-CTL in DIV1, RNA and protein samples were collected in DIV4. B. Realtime-qPCR analysis on CG cells. (n=5 in each group) C. Western blotting analysis on CG cells. (n=3 in each group) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 3.
Administration of JNK inhibitors promoted the in vitro survival rate in CG cells A. Experimental design of the intracellular signaling inhibitors administration on CG cells. CG cells were infected with lentivirus-iCre and lentivirus-CTL in DIV1, 3 hours later the inhibitors were administrated. In DIV 7, the CG cells were fixed and stained with NeuN and DAPI. B. Images of CG cells stained with NeuN and DAPI, scale bar =100 µm. C. Changes in cell survival rate calculated by the NeuN and DAPI double positive cells. And the group of CASK+/+ CTL CG cells with DMSO was used as the control. (n=3) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 3.
Administration of JNK inhibitors promoted the in vitro survival rate in CG cells A. Experimental design of the intracellular signaling inhibitors administration on CG cells. CG cells were infected with lentivirus-iCre and lentivirus-CTL in DIV1, 3 hours later the inhibitors were administrated. In DIV 7, the CG cells were fixed and stained with NeuN and DAPI. B. Images of CG cells stained with NeuN and DAPI, scale bar =100 µm. C. Changes in cell survival rate calculated by the NeuN and DAPI double positive cells. And the group of CASK+/+ CTL CG cells with DMSO was used as the control. (n=3) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 4.
JNK inhibitor reduced apoptosis and JNK signaling in CASKflox/flox iCre CG cells A. Experimental design for TUNEL assay and western blotting. CG cells was infected with lentivirus-iCre and lentivirus-CTL in DIV1, 3 hours later the inhibitors were administrated. In DIV5, the CG cells were fixed for TUNEL assay staining or collected for western blotting. B. Percentage of TUNEL and NeuN double positive cells. (n=3) C. Images of CG cells with TUNEL, NeuN, and DAPI triple staining, scale bar =100 µm. D. Western blotting analysis on CG cells with or without JNK-IN-8 administration. (n=3 in each group) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 4.
JNK inhibitor reduced apoptosis and JNK signaling in CASKflox/flox iCre CG cells A. Experimental design for TUNEL assay and western blotting. CG cells was infected with lentivirus-iCre and lentivirus-CTL in DIV1, 3 hours later the inhibitors were administrated. In DIV5, the CG cells were fixed for TUNEL assay staining or collected for western blotting. B. Percentage of TUNEL and NeuN double positive cells. (n=3) C. Images of CG cells with TUNEL, NeuN, and DAPI triple staining, scale bar =100 µm. D. Western blotting analysis on CG cells with or without JNK-IN-8 administration. (n=3 in each group) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 5.
JNK inhibitor reduced apoptosis and reactive oxygen species pathway. A. Realtime-qPCR analysis on JNK-IN-8 or DMSO treated CG cells. (n=5 in each group) B. Reactive oxygen species visualization and quantification of fluorescence intensity, scale bar =100 µm. (n=3) C. Volcano map of gene expression difference between JNK-IN-8 treated CASK flox/flox iCre CG cells and DMSO treated CASK flox/flox iCre CG cells. Red indicated genes upregulated in JNK-IN-8 treated CG cells, blue indicated genes downregulated in JNK-IN-8 treated CG cells. Detected by RNA-seq. (Threshold: p<0.05, Fold change over2)D. Heatmap of the representative gene expression detected by RNA-seq.E. Representative Gene Set Enrichment Analysis (GESA) results of apoptosis related pathways.* indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 5.
JNK inhibitor reduced apoptosis and reactive oxygen species pathway. A. Realtime-qPCR analysis on JNK-IN-8 or DMSO treated CG cells. (n=5 in each group) B. Reactive oxygen species visualization and quantification of fluorescence intensity, scale bar =100 µm. (n=3) C. Volcano map of gene expression difference between JNK-IN-8 treated CASK flox/flox iCre CG cells and DMSO treated CASK flox/flox iCre CG cells. Red indicated genes upregulated in JNK-IN-8 treated CG cells, blue indicated genes downregulated in JNK-IN-8 treated CG cells. Detected by RNA-seq. (Threshold: p<0.05, Fold change over2)D. Heatmap of the representative gene expression detected by RNA-seq.E. Representative Gene Set Enrichment Analysis (GESA) results of apoptosis related pathways.* indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 6.
Survival rate evaluation on heterozygous CASK knockout CG cells A. Experimental design on CASK+/floxHprteGFP/+ CG cells. B. Schematic diagram showing how survival rate evaluation was conducted on different X chromosome activated CG cells in co-cultivation. And the CG cells with double positive of eGFP and NeuN were viewed as the CG cells with Hprt-eGFP X chromosome active. The NeuN positive CG cells without eGFP florescent light were viewed as the CG cells with CASK knockout (CASK-) X chromosome active. C. Immunocytochemistry stained Images of eGFP, NeuN, and DAPI in CG cells. And the percent changes of CG cells were evaluated by those images, scale bar =100 µm. (n=3) D. Immunocytochemistry stained Images of eGFP, NeuN, and DAPI in JNK-IN-8 or DMSO administrated CG cells. And the percent changes of CG cells were evaluated, scale bar =100 µm. (n=3).
Figure 6.
Survival rate evaluation on heterozygous CASK knockout CG cells A. Experimental design on CASK+/floxHprteGFP/+ CG cells. B. Schematic diagram showing how survival rate evaluation was conducted on different X chromosome activated CG cells in co-cultivation. And the CG cells with double positive of eGFP and NeuN were viewed as the CG cells with Hprt-eGFP X chromosome active. The NeuN positive CG cells without eGFP florescent light were viewed as the CG cells with CASK knockout (CASK-) X chromosome active. C. Immunocytochemistry stained Images of eGFP, NeuN, and DAPI in CG cells. And the percent changes of CG cells were evaluated by those images, scale bar =100 µm. (n=3) D. Immunocytochemistry stained Images of eGFP, NeuN, and DAPI in JNK-IN-8 or DMSO administrated CG cells. And the percent changes of CG cells were evaluated, scale bar =100 µm. (n=3).
Figure 7.
JNK-IN-8 administration in vivo cerebellum development of CASK+/-HprteGFP/+ mice A. Experimental design on CASK+/-HprteGFP/+ and CASK+/-HprteGFP/+ mice. B. Body Weight changes in CASK+/-HprteGFP/+ and CASK+/-HprteGFP/+ mice. (n=5)(* indicates difference between CASK+/- Hprt+/eGFP DMSO and CASK+/+ Hprt+/eGFP DMSO, # indicates difference between CASK+/- Hprt+/eGFP JNK-IN-8 and CASK+/- Hprt+/eGFP DMSO) C. Photography of H&E stained cerebellum slices, scale bar= 500µm. D. Immunohistochemistry stained Lobules IV/V slices and Lobules IX. ML, molecular layer; PC, Purkinje cell layer; GC, granular cell layer. The ratio of double positive cells of NeuN and eGFP was calculated. (n=7) * indicates difference between the groups. *, # indicate p<0.05; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 7.
JNK-IN-8 administration in vivo cerebellum development of CASK+/-HprteGFP/+ mice A. Experimental design on CASK+/-HprteGFP/+ and CASK+/-HprteGFP/+ mice. B. Body Weight changes in CASK+/-HprteGFP/+ and CASK+/-HprteGFP/+ mice. (n=5)(* indicates difference between CASK+/- Hprt+/eGFP DMSO and CASK+/+ Hprt+/eGFP DMSO, # indicates difference between CASK+/- Hprt+/eGFP JNK-IN-8 and CASK+/- Hprt+/eGFP DMSO) C. Photography of H&E stained cerebellum slices, scale bar= 500µm. D. Immunohistochemistry stained Lobules IV/V slices and Lobules IX. ML, molecular layer; PC, Purkinje cell layer; GC, granular cell layer. The ratio of double positive cells of NeuN and eGFP was calculated. (n=7) * indicates difference between the groups. *, # indicate p<0.05; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 8.
behavioral analyses indicated that JNK-IN-8’s administration improved cerebellum function. A. Photography on P20 CASK+/-HprteGFP/+ and CASK+/-HprteGFP/+ mice, treated with or without JNK-IN-8. Limb-clasping scores were calculated by their behavioral performance. (n=7) B. Gait analysis record and the stride length, stride width, and toe spread were measured separately from each limb. (n=5) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
Figure 8.
behavioral analyses indicated that JNK-IN-8’s administration improved cerebellum function. A. Photography on P20 CASK+/-HprteGFP/+ and CASK+/-HprteGFP/+ mice, treated with or without JNK-IN-8. Limb-clasping scores were calculated by their behavioral performance. (n=7) B. Gait analysis record and the stride length, stride width, and toe spread were measured separately from each limb. (n=5) * indicates difference between the groups. * indicates p<0.05; ** indicates p<0.01; *** indicates p<0.001 and **** indicates p<0.0001.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).