1. Introduction
There are about 250 species of
Gardenia (
Gardenia jasminoides) in the world.
Gardenia is suitable for growing in warm and humid environments, mainly distributed in tropical and subtropical regions. It is also found in temperate regions [
1]. Gardenia belongs to the four seasons evergreen plant. The flowers have ornamental, medicinal, tea, dye extraction, oil extraction, spices and other uses. The fruits have gallbladder, liver protection, blood pressure reduction, swelling and sedation, antipyretic, anti-inflammatory and other functions [
2].
Low temperature is a natural disaster often encountered in the process of crop growth and development, which will cause physiological damage to crops, resulting in yield decline and economic losses. Short-term low temperature stress will slow the growth and development of crops and reduce the protoplasmic capacity. With the intensification of low temperature stress, various tissues and organs of crops will be damaged, growth and metabolic activities will be inhibited, and even lead to cell death [
3]. The root system is an important organ for plants to absorb water and nutrients [
4,
5]. After different degrees of low temperature stress, the root system will show different changes. Under mild low temperature, the root surface area and root volume increase, and the root tip cell structure is intact. Under heavy and low temperature conditions, root growth stops, cell wall begins to disintegrate, and root tip cells become loose and seriously cause death [
6].
When plants are subjected to low temperature stress, a large number of reactive oxygen species will accumulate in the body, which will destroy the dynamic balance between the production and removal of original free radicals in the cell. A large amount of reactive oxygen species will not only induce membrane lipid peroxidation, which will destroy the cell membrane, but also cause serious damage to some macromolecules. Or a series of physiological and biochemical reactions in the body can cause serious effects [
7]. Studies have confirmed that low temperature stress significantly increases the production rate of reactive oxygen species in maize seedlings, resulting in dynamic imbalance of free radicals in plants [
8]. Therefore, improving the ability to remove reactive oxygen species in plants is an important way to improve the resistance of crops. Under stress conditions, plants can improve their resistance by self-regulation of protective enzyme system to resist the toxic effects of free radicals. Superoxide dismutase (SOD), catalase (POD) and peroxidase (CAT) are a class of enzymatic systems that can effectively remove reactive oxygen species from plants [
8]. Previous studies have shown that low temperature stress significantly decreased the activities of three antioxidant enzymes (SOD, POD, CAT) in
Phalaenopsis leaves, and high intensity low temperature inhibited or destroyed the intracellular antioxidant enzyme system [
9]. Under normal growth conditions, free radicals in rice are in a dynamic level of continuous generation and elimination, but under low temperature conditions, the free radical generation rate in rice is far greater than the removal rate [
10]. Within a certain range, antioxidant substances such as SOD increase, but after strong low temperature stress, the antioxidant oxidase activity in rice rapidly increases and then significantly decreases. This indicates that strong low temperature destroys the protease synthesis mechanism in rice, affects the normal life activities, and ultimately affects the yield [
10].
Osmotic adjustment ability is an important reflection of plant adaptation to low temperature. The normal metabolic activities of plant cells will change under stress, forcing the increase of osmoregulatory substances in the body. Osmoregulatory substances can not only maintain cell turgor pressure and prevent excessive water loss of protoplasm, but also stabilize organelle structure, thereby regulating certain physiological functions and alleviating the damage to plants under stress [
11]. Osmoregulatory substances in plants mainly include proline, soluble protein and soluble sugar [
11]. With the change of external environment, the contents of the three substances in plants will change significantly, and their contents are related to the low temperature tolerance of plants [
11]. Research results confirmed that soluble protein content in plants was positively correlated with their ability to resist low temperature [
12]. As a protective substance of plant tissues, the increase of soluble protein content can improve the water retention ability of cells, reduce the freezing point of cells, and alleviate the damage caused by low temperature to plants [
12]. As an important osmoregulatory substance in plants, proline can prevent water loss and protect membrane proteins, and its content will change greatly under the influence of temperature, and it is of great significance for maintaining the integrity of cell membranes [
13]. As an important osmoregulatory substance, soluble sugar content is the most sensitive index to reflect plant metabolism under low temperature stress [
14]. When plants are stressed by stress, they will actively accumulate osmoregulatory substances such as soluble protein, proline and soluble sugar through osmoregulation, improve the water holding capacity of cells and enhance plant stress resistance [
11].
Photosynthesis can promote the cold resistance of plants. Sugars (such as glucose) and amino acids produced by photosynthesis can directly increase the concentration of cell fluid, reduce the freezing point, and prevent the formation of intracellular ice crystals [
15]. For example, cactus produces ‘cactin’ through photosynthesis, which can both resist drought and stabilize cell membrane structure to resist low temperature damage [
16]. The fatty acid composition (such as cis-unsaturated fatty acid content) of chloroplast membrane is closely related to cold resistance. High content of cis-unsaturated fatty acids can maintain the fluidity of chloroplast membrane and ensure normal photosynthesis at low temperatures, thus enhancing cold tolerance [
17]. Spraying potassium dihydrogen phosphate and other fertilizers can improve photosynthetic efficiency of leaves and increase cell fluid concentration [
18]. Brassinolides promote the accumulation of photosynthates by regulating endogenous hormone balance, and increase cold resistance by more than 8 times [
19]. Photosynthesis is not only the material basis of plant cold resistance (through sugar accumulation and membrane structure optimization), but also dynamically adjusted by low temperature environment. Exogenous substances supplement, such as the addition of trehalose, can jointly improve the photosynthetic efficiency and cold resistance of plants, forming a virtuous cycle [
20,
21].
Trehalose is a typical stress metabolite, which can form a unique protective film on the cell surface under harsh environmental conditions such as high temperature, high cold, high osmotic pressure and dry water loss, effectively protecting the structure of biomolecules from being destroyed, so as to maintain the life process and biological characteristics of living organisms. Current studies have shown that trehalose biosynthesis mainly consists of two reactions: first, trehalose 6-phosphate is produced by uridine diphosphate glucose and 6-phosphate glucose under the catalysis of trehalose 6-phosphate synthetase, and then trehalose 6-phosphate trehalose is produced under the hydrolysis of trehalose 6-phosphatase [
22]. Trehalose is a safe, stable and very reliable natural sugar, which has a non-specific protective effect on biological macromolecules and organisms, especially under adverse conditions such as low temperature, drought, salt damage and high heat, and has an efficient protective effect on a series of biological macromolecules that maintain normal plant life activities [
23].
Exogenous trehalose spray can protect plant proteins from damage under abiotic stress conditions such as low temperature, drought and salt damage [
24]. Studies have shown that exogenous trehalose spray can reduce MDA content and relative permeability of plasma membrane under salt stress, and increase SOD and POD activities [
25]. Under high temperature stress, exogenous trehalose spray increased ascorbic acid content, enhanced CAT and ascorbate peroxidase activities, and decreased MDA and hydrogen peroxide content in wheat seedlings [
26]. At the same time, exogenous spray of trehalose can improve POD and CAT activities and ascorbic acid content of maize under drought stress, and alleviate oxidative damage under drought stress [
27]. Under the condition of low temperature treatment, exogenous trehalose spray increased the relative water content, proline content and soluble sugar content of wheat, decreased MDA content, and alleviated the damage caused by low temperature stress [
28]. Other studies have shown that trehalose has a promoting effect under salt stress, and salt stress leads to a significant decrease in plant height, stem diameter, dry and fresh weight, strong seedling index, etc., of muskmelon seedlings. However, after applying different concentrations of trehalose, various indexes of muskmelon seedlings are improved, and root activity and soluble sugar content are increased, indicating that within a certain concentration range of trehalose, It also has a alleviating effect on physiological and biochemical characteristics of plants and seedlings [
29].
As a landscape plant, gardenia is suitable for growing in a warm and humid environment, but the harsh climate such as cold spring occurs frequently, which seriously affects the growth of gardenia. Trehalose, as the stress metabolite of plants adapted to environmental stress, can enhance the stress resistance of plants and ensure the normal growth and development of crops. The main purpose of this study was to explore the effect and mechanism of trehalose on the growth and cold resistance of gardenia seedlings under low temperature stress. The main significance of this study was to provide new technology and theoretical support for cold resistance cultivation of gardenia from the perspective of exogenous trehalose.
2. Materials and Methods
2.1. Plant Material and Experimental Design
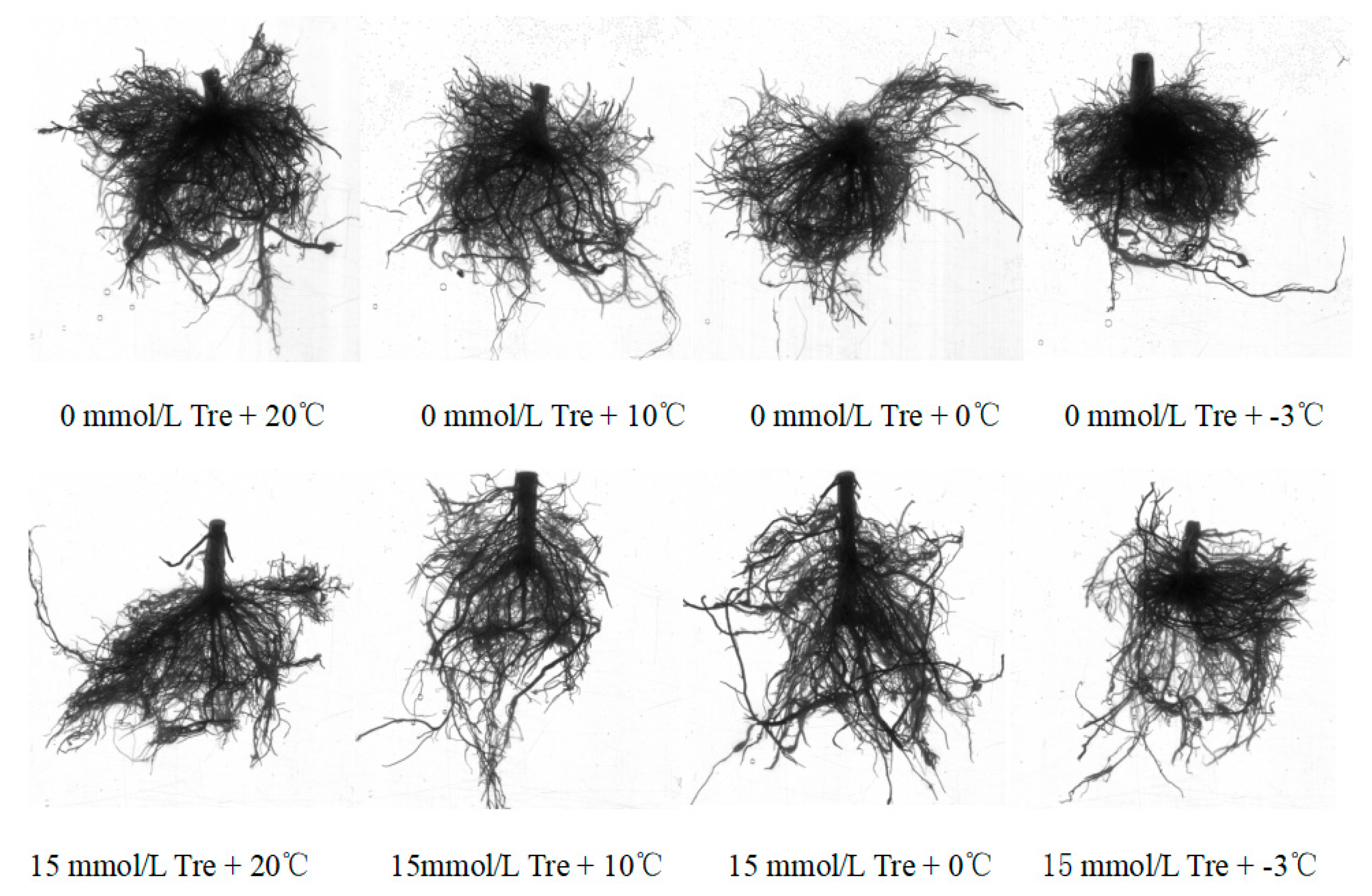
The experiment adopted a two-factor design: (1) trehalose treatment (including 0 mmol/L Tre and 15 mmol/L Tre); (2) Low temperature treatment (20℃, 10℃, 0℃ and -3℃). The experiment consisted of 8 treatments with 5 replicates per treatment group (1 basin per replicate) and a total of 40 POTS. In this experiment, gardenia seedlings were transplanted into plastic POTS on April 12, 2023, and then the plastic POTS were placed in light incubators at four temperatures (20℃, 10℃, 0℃ and -3℃). Hoaglang nutrient solution was irrigated from the day of planting, and the nutrient solution with corresponding concentration of trehalose was irrigated once every three days. Plants were harvested on June 11, 2023 after 2 months of trehalose treatment at 4 temperatures and corresponding concentrations.
2.2. Plant Growth Index and Root System Configuration
Before harvest, the plant height was measured with a ruler (cm), and the number of fully unfolded leaves was measured by counting method. After harvest, the total weight of plants, fresh weight of above-ground parts and underground parts were measured by electronic balance. After the plants were harvested, root images were obtained by Epson V700 color image scanner, and root configuration parameters (root length, number of lateral roots, total root surface area, root volume, etc.) were obtained by WinRHIZO root analyzer.
2.3. Chlorophyll in Leaves, Chlorophyll Fluorescence Parameters and Photosynthetic Intensity Parameters
Multifunctional leaf measuring instrument was used to measure the content of chlorophyll in plants. Before harvesting plants, healthy and fully unfolded leaves were selected, and the content of chlorophyll a, chlorophyll b and total chlorophyll were measured after wiping the leaves with a clean wet cloth. The chlorophyll fluorescence parameters were determined by IMAGING-PAM, a German M-series modulated chlorophyll fluorescence meter. Before harvesting, Gardenia of different treatments were completely unfolded and measured from 09:00-11:00. After dark adaptation treatment for 20 min, the blades were fixed on the loading platform. The actual photochemical efficiency (φPSII), maximum photochemical efficiency (Fv'/Fm'), non-photochemical quenching coefficient (NPQ) and photochemical quenching coefficient (qP) in the blades were measured to evaluate the light energy utilization efficiency. Before harvesting plants, leaf photosynthetic parameters were determined by Li-6400 photosynthesator. Functional leaves at the 4th to 5th positions with good physiological status were selected as the measurement objects, and parameters such as transpiration rate (Tr), net photosynthetic rate (Pn), intercellular CO2 concentration (Ci) and stomatal conductance (Gs) were obtained.
2.4. Activities of Reactive Oxygen Species and Antioxidant Enzymes and Contents of Osmotic Substances in Roots
By hydroxylamine oxidation method, hydroxylamine (NH2OH) and O2⁻· specific reoxidation reaction, determination of absorbance at 540 nm wavelength, calculate the concentration of NO2- by the standard curve, can reflect the content of superoxide anion. Hydrogen peroxide (H2O2) is analyzed by titanium sulfate colorimetry. Titanium sulfate reacts with hydrogen peroxide to produce yellow precipitate. The precipitate can detect the concentration of hydrogen peroxide by measuring OD value at 415 nm.
The activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) were determined by nitrogen blue tetrazole photoreduction method, guaiacol color development method and ammonium molybdate color development method.
The proline content was determined by sulfosalicylic acid extraction and acid indanhydrin color development method. The product was obtained by this method and placed at 520 nm wavelength, and then the concentration of proline was converted by detecting OD value of the wavelength. The soluble sugar was detected by anthrone color development method. The product obtained by this method was placed at 620 nm wavelength, and then the content of soluble sugar could be converted by detecting the OD value of the wavelength. The content of malondialdehyde was determined by thiobarbituric acid (TBA) color development method. The concentration of malondialdehyde was obtained by detecting OD600, OD532 and OD450 of the reaction products on the spectrophotometer. Soluble protein content was determined by Coomasil bright blue (G-250) staining method. 20 μL extract solution (enzyme solution) was added to 80 μL, 0.05 mol·L-1, pH 7.8 phosphate buffer, then 2.9 mL Coomasil bright blue solution was added, and OD595 was measured after reaction for 2 min. The soluble protein concentration can be obtained by calculating the value.
2.5. Endogenous Hormones, Malic Acid and Succinic Acid in Roots
Determination of endogenous hormones in roots using kit (Nanjing Jiancheng Bioengineering Research Institute Co., LTD.) They were plant growth hormone (IAA) ELISA kit, plant trans-zeaxin nucleoside (tZR) ELISA kit, plant abscisic acid (ABA) ELISA kit and plant gibberellin (GA3) ELISA kit. The above kits are based on the principle of double antibody sandwich method to detect hormone content in plant samples. Malic acid and succinic acid contents were determined by high performance liquid chromatography (HPLC) : 1.00 g of gardenia root was accurately weighed and mixed by a blender, ground with 4 mL of extraction solution, centrifuged for 10 min at 10000 r/min, and the residue was added with 2 mL of extraction solution and then extracted, combined with the supernatant, dried in a water bath at 90℃, at a fixed volume of 10 mL, and then extracted with a disposable syringe after whirlpool mixing. It was filtered by 0.45 μm filter membrane and analyzed by machine.
2.6. Statistical Analysis
We performed a variance analysis (ANOVA) (SAS software 8.1v, SAS Institute, Gaston County, North Carolina, USA) to statistically analyze the data. Microsoft Excel (Version 2013, Microsoft Institute, Redmond, WA, USA) was used for data processing and graphing, and Duncan’s multirange experiment compared significant differences between treatments with p < 0.05.
4. Discussion
The growth of
Gardenia is regulated not only by cultivation measures, but also by exogenous substances, which is one of the effective means for plants to resist the stress of adversity. At present, few studies have reported the regulation of exogenous trehalose on the growth of gardenia seedlings under low temperature stress, but the regulation of other crops and adversity has been reported. Based on low-temperature stress experiments on
Catharanthus roseus as the experimental material, Wei et al. [
30] discovered that exogenous application of trehalose effectively increased plant growth, leaves’ chlorophyll content and antioxidant enzyme activity, and then improve its cold resistance. Aldesuquy et al. [
31] found that the exogenous application of trehalose appeared to mitigate the damage effect of drought with different magnitude throughout counteracting the negative effects of water stress on all growth criteria of wheat root and improving wheat leaf turgidity by decreasing the rate of transpiration, increasing relative water content and decreasing saturation water deficit as well as increasing water use efficiency for wheat economic yield. In this study, exogenous application of trehalose solution under low temperature stress significantly improved the growth potential of gardenia seedlings, such as plant height, leaf number, total plant weight, above-ground fresh weight, underground fresh weight, root total length, lateral root number, total root surface area and root volume, etc., especially under -3℃ low temperature stress. Exogenous trehalose had the best effect on restoring the growth potential of
Gardenia. This is similar to the results of Raza et al. [
32] study on exogenous trehalose's effect on cold tolerance of Rapeseed (
Brassica napus L.) seedlings under low temperature stress.
Photosynthesis of plants requires the participation of many pigments, and photosynthetic pigments are a crucial component. Photosynthetic pigments mainly include chlorophyll and carotenoids, and light energy is transferred and transformed in the body after being absorbed by them during photosynthesis [
33]. Chlorophyll is divided into chlorophyll a and chlorophyll b, which are responsible for the capture and transfer of light energy. When plants encounter low temperature stress, it will have adverse effects on their own photosynthesis process. It is generally believed that the original chlorophyll will be destroyed by low temperature, and the chlorophyll content will be forced to decrease, which will eventually weaken the photosynthetic capacity of plants and inhibit the carbon assimilation pathway, resulting in slow plant growth [
34]. Tang et al. [
35] proved that exogenous trehalose (10 mmol·L
-1) could significantly increase the contents of chlorophyll a, chlorophyll b and total chlorophyll in the leaves of wheat seedlings under low temperature stress. This is consistent with the results of this study: low temperature stress inhibited the synthesis of chlorophyll in leaves of
Gardenia to varying degrees, and exogenous trehalose of 15 mmol/L could effectively alleviate the inhibition of low temperature stress on chlorophyll synthesis in leaves of
Gardenia, and moderately restored the contents of chlorophyll a, chlorophyll b and total chlorophyll.
When plant growth is subjected to abiotic stress, the inner membrane of chloroplast will be destroyed, thus affecting plant photosynthesis and growth and development [
36]. The photosynthetic mechanism PSII on chloroplast thylakoid membrane is the most sensitive to environmental changes [
36]. The chlorophyll fluorescence parameters φPSII, Fv '/Fm', qP and NPQ can represent the initial photochemical capacity of PSII and are important indicators to reflect the effects of environmental stress on photosynthesis [
37]. In the photosynthetic apparatus PSII, Fv '/Fm' is decreased when plants are subjected to photoinhibition, which indicates that photosystem II is destroyed [
37]. Pilon-Smits [
38] showed that trehalose treatment significantly improved the Fv '/Fm' value in leaves under abiotic stress and restored it to the control level, effectively alleviating the damage of abiotic stress on PSII reaction center, indicating that trehalose can alleviate or even restore the damage of abiotic stress on PSII. This results also showed that trehalose increased φPSII in leaves under abiotic stress, indicating that the actual photochemical utilization efficiency of leaves increased [
38]. Trehalose also slows down the decrease of qP and ETR in leaves caused by abiotic stress, and decreases NPQ, indicating that trehalose can alleviate the problems such as the decrease of photochemical efficiency and fluorescence yield in leaves caused by abiotic stress [
38]. The above results were similar to the present study: φPSII, Fv '/Fm' and qP values of
Gardenia leaves decreased to varying degrees under low temperature stress, and exogenous trehalose treatment could effectively restore these parameters to the control level, so as to improve the adverse effect of low temperature stress on PSII reaction center.
Photosynthesis is very sensitive to abiotic stress, including strong light, water stress, high temperature, salt damage, etc., which will reduce plant photosynthetic efficiency and thus affect the normal growth of plants [
39]. The photosynthetic intensity parameters can accurately reflect the photosynthetic intensity of plants, which mainly include net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci) and transpiration rate (Tr) [
40]. Plants mainly rely on stomata to exchange with external gases (CO
2 and H
2O
2), and CO
2 is the substrate of plant photosynthesis, so the level of Ci and Tr will be affected by Gs, further affecting the strength of photosynthesis [
40]. Tr represents the amount of water evaporated per unit leaf area of plant leaves within a certain period of time, and the transpiration pull generated by Tr can absorb and transport water, providing material sources for plant photosynthesis process [
40]. It has been reported that stress can affect the normal level of various photosynthetic intensity parameters [
41]. With the aggravations of stress, Pn, Tr and Gs of leaves of
Leymus chinensis showed a gradual decline, while Ci showed an upward trend [
41]. In order to reduce the damage caused by stress on plant growth and development, researchers adopted the addition of exogenous substances (such as trehalose) to alleviate the adverse effects of stress on plant photosynthesis. Studies have shown that the exogenous addition of trehalose can affect the parameters of photosynthetic intensity in wheat leaves under drought stress, and the appropriate concentration of trehalose can significantly increase the chlorophyll content and enhance the values of Pn, Tr and Gs in wheat leaves [
42]. Razzaq et al. [
43] showed that chromium (Cr) stress (100 uM) significantly reduced the Pn, Gs and Tr in
Zea mays leaves, and the increase of Cr concentration (500 uM) further exacerbated this adverse effect. However, exogenous trehalose treatment can effectively reduce these adverse effects caused by Cr stress on
Zea mays leaves, and the effect of trehalose of 50 mM is better than that of trehalose of 25 mM. These results are consistent with the results of this study: low temperature stress inhibited the photosynthetic intensity parameters and photosynthetic efficiency of
Gardenia, especially the low temperature stress of -3℃ significantly decreased Pn, Gs, Ci and Tr, while 15 mmol/L trehalose effectively mitigated the inhibition effects of low temperature stress on the photosynthetic intensity and photosynthetic efficiency of
Gardenia leaves.
Plants will produce reactive oxygen species (ROS) when they are subjected to environmental stress. When ROS is generated too much but cannot be removed in time, it will destroy macromolecular substances such as DNA, proteins and membrane structures in plant tissues [
44,
45,
46]. In order to avoid the damage caused by excessive ROS accumulation to cells, plants will relieve the damage by antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione reductase (GR) and ascorbate peroxidase (APX) [
47,
48,
49,
50]. Luo and Li [
51] has shown that heat stress can significantly increase ROS content (hydrogen peroxide, superoxide anion radical, etc.) in wheat, while exogenous trehalose can scavenge ROS content (hydrogen peroxide, superoxide anion, etc.) by increasing the activities of antioxidant enzymes such as APX, SOD, and CAT, that alleviate the damage caused by abiotic stress in wheat. Trehalose treatment significantly enhanced the activities of antioxidant-related enzymes such as APX, CAT, SOD, and GR, as well as the transcription levels
AsA-GSH cycle related gene, which led to the reduction of ROS (such as hydrogen peroxide) content in peach during cold storage [
52]. According to the study of Akram et al. [
53] in radish (
Raphanus sativus L.), spraying trehalose (25 mM) can alleviate the damage caused by water stress on seedlings by enhancing the activities of SOD and POD. Zheng et al. [
54] also confirmed that exogenous trehalose (5.0 mM) could induce the increase of antioxidant enzyme activity (such as SOD and POD) in tea plant under heat stress, indicating that trehalose could further stimulate the enzymatic defense system of tea seedling under heat stress, enhance the antioxidant capacity of plants, and alleviate the damage to cell membrane caused by high-temperature stress. These results are consistent with the results of this study: low temperature stress promotes the production of excessive ROS in
Gardenia, and exogenous trehalose can effectively enhance the activity of antioxidant enzymes in
Gardenia under low temperature stress, improve its antioxidant defense ability to reduce the ROS content in vivo, maintain the reoxygen-reduction balance in cells, and protect the structure and function of cell membranes.
Plants under abiotic stress will produce a large number of osmoregulatory substances. Osmoregulatory substances can not only maintain cell turgor pressure, prevent excessive water loss of protoplasm, but also stabilize organelle structure, regulate some physiological functions, and alleviate the damage to plants under stress. Proline (Pro), malondialdehyde (MDA), soluble protein and soluble sugar are osmoregulatory substances of plants. Hasanuzzaman et al. [
55] has confirmed that drought increased Pro and MDA contents along with altered antioxidant and glyoxalase systems in three Brassica species (
B. napus,
B. campestris and
B. juncea), while trehalose reduced MDA and Pro contents, and LOX activity, that further enhanced its drought tolerance capability. It is consistent with the conclusions of this study: low temperature stress increased the contents of osmoregulatory substances (Pro and MDA) in the roots of
Gardenia, and exogenous trehalose could effectively alleviate the abnormal accumulation of osmoregulatory substances induced by low temperature, reduce the damage of membrane lipid peroxidation, and improve the cold resistance of
Gardenia. In addition, this study also found that soluble protein and soluble sugar were not affected by low temperature stress and exogenous trehalose. However, our results are not quite the same as Zheng et al. [
54]: the contents of PRO and soluble sugar exhibited a significant increase, while MDA content decreased following treatment with 5.0 mM trehalose under 24 h high-temperature stress (38 °C/29 °C, 12 h/12 h). This may be due to the different responses of trehalose to different plants under different abiotic stress conditions, and the specific reasons need to be further explored.
Plant hormones are widely involved in plant stress signaling substances, play an important role in plant stress response, and can cause adaptive regulatory responses in plants [
56,
57,
58]. When plants encounter abiotic stress such as low temperature, high temperature, drought, salt and alkali, they can cope with environmental stress by regulating related hormones in the plants [
59,
60]. Cui et al. [
61] treated
Cabernet Sauvignon seedlings with 15 mmol·L
-1 trehalose and determined and analyzed the contents of four endogenous hormones (tZR, GA3, IAA and ABA) under low temperature stress. Compared with the control group, the contents of tZR, GA3 and ABA in trehalose treatment group at -3 ℃ were increased by 80.03%, 27.66% and 39.14%, respectively, while the contents of IAA were decreased to 0.94 ng·g
-1 [
61]. This is consistent with the findings of this study: low temperature stress decreased IAA content but increased tZR, GA3 and ABA contents in roots of
Gardenia; and 15 mmol/L trehalose treatment alleviated the decreasing effect of low temperature stress on IAA content in roots of
Gardenia, and weakened the increasing effect of low temperature stress on tZR and GA3 contents, but had no significant effect on ABA. It can be concluded from the above that trehalose treatment has a certain protective effect on plant hormone synthesis system under low temperature stress, especially can restore the auxin content to a certain extent to restore plant growth.
The normal development of respiratory metabolism plays a vital role in the process of plant growth and development [
62]. When plants face abiotic stress, the appropriate amount of intermediate metabolites is the basis for their adaptation to low temperature [
62]. As intermediate products of plant respiratory metabolism, succinic acid and malic acid are closely related to plant metabolism [
63]. The succinic acid produced during the tricarboxylic acid cycle, under the action of SDH, produces fumaric acid, which is converted into malic acid by hydration [
63]. Previous study has shown that the concentration of root respiratory metabolites is significantly correlated with root activity in rhizosphere soil, and malic acid and succinic acid as root respiratory metabolites can enhance root activity and promote plant growth [
64]. However, trehalose treatment in this study increased the content of malic acid in roots of gardenia, but decreased the content of succinic acid. This may be different from the response of different plants to trehalose stimulation. Gardenia may respond to the stimulation of exogenous trehalose on root respiration through malic acid. Therefore, the application of appropriate concentration of trehalose can promote the production of root respiratory metabolites, enhance root vitality, promote plant growth, and improve plant resistance to low temperature.