1. Introduction
Wine quality is shaped by a complex interplay of biochemical and physical processes, many of which are initiated or influenced during alcoholic fermentation. Among the various parameters that affect the final profile of red wines, color and aroma stand out as critical sensory attributes, not only for consumer acceptance but also for their connection to varietal typicity, aging potential, and overall perception of quality. These attributes are primarily governed by the presence and transformation of phenolic compounds and volatile organic compounds (VOCs), both of which are highly sensitive to the physicochemical environment during fermentation [
1,
2].
Color in red wines is mainly derived from anthocyanins, flavonols, and tannins extracted from grape skins and seeds during maceration. These compounds undergo a range of chemical transformations—such as oxidation, polymerization, and copigmentation—that determine the stability and intensity of the color. At the same time, the volatile fraction of wine, which includes esters, alcohols, acids, and terpenes, originates from both grape precursors and fermentative metabolism, with yeast activity being a key modulator of the aromatic profile. The successful management of both phenolic and volatile compounds is, therefore, fundamental to producing high-quality red wines [
3,
4].
One of the most influential but complex factors in the regulation of these transformations is oxygen. Although excessive exposure to oxygen can result in degradation of sensitive compounds and undesirable sensory attributes, a controlled supply at specific stages of vinification has proven to be beneficial. In particular, low-dose oxygenation during fermentation has been shown to stimulate yeast activity, promote the stabilization of color through the formation of polymeric pigments, and enhance the production of favorable aroma compounds. As a result, oxygen management has become a central component in modern enology [
5,
6].
Among the most common techniques for controlled oxygen delivery is micro-oxygenation (MOX), which was first developed in the 1990s and has since been widely implemented in both traditional and innovative winemaking practices. Micro-oxygenation involves the gradual introduction of precise amounts of oxygen—typically in the range of 1 to 5 mg/L per month—into must or wine, either during or after alcoholic fermentation. Its application has been associated with multiple benefits, including increased color stability, smoother tannin structure, and improved integration of aromatic compounds. Numerous studies have highlighted its effectiveness, especially in red grape varieties with high phenolic potential, where MOX can contribute to the polymerization of flavanols and anthocyanins, leading to improved chromatic and organoleptic properties [
7,
8].
Nevertheless, the efficacy of micro-oxygenation depends on a number of interacting factors, such as the timing and duration of application, the composition of the matrix, temperature, and notably, the solubility and diffusion of oxygen in the liquid phase. Under standard atmospheric pressure, the dissolution rate of oxygen in must or wine is limited, and the uniform distribution of oxygen across the fermentation matrix can be difficult to achieve. This is particularly relevant during the active fermentation phase, where metabolic activity of yeasts, temperature gradients, and CO₂ evolution further complicate oxygen management [
9,
10].
To overcome these limitations, recent research has begun to explore the use of physical interventions that can modify the behavior of oxygen in the medium. One such approach is the use of hyperbaric conditions—applying elevated pressures during fermentation to increase the solubility of gases, including oxygen. This concept is well-known in food processing technologies aimed at enhancing mass transfer, microbial inactivation, or extraction efficiency, but its application in winemaking remains largely unexplored. Preliminary studies in model systems have demonstrated that increased pressure can significantly enhance the rate of oxygen dissolution and alter redox potential in the matrix, potentially offering a more effective and controllable environment for phenolic transformations and aroma development [
11,
12].
The use of non-thermal, pressure-based technologies is gaining attention within the wine sector, as it aligns with the growing demand for innovative and energy-efficient processing methods. Techniques such as high-pressure processing (HPP), pulsed electric fields (PEF), or ultrasound-assisted maceration are increasingly being tested for their ability to modulate extraction, microbial stability, and product quality without relying on high temperatures or chemical additives. Within this broader technological context, hyperbaric micro-oxygenation emerges as a promising candidate for improving fermentation performance, particularly in red musts where phenolic content is high and oxygen management is critical [
13,
14].
In this regard, the grape variety
Vitis vinifera L. cv. Monastrell (also known as Mourvèdre in France) presents a compelling model. Widely cultivated in Mediterranean regions, Monastrell holds particular significance in southeastern Spain—especially in the Region of Murcia—where it is the dominant local variety and deeply adapted to the specific combination of soil, climate, and traditional cultivation practices. Characterized by its thick skin, high phenolic content, and susceptibility to oxidation, Monastrell grapes yield wines with deep color and intense aromas. However, these wines often require careful oxygen management to maintain the stability of their sensory attributes over time. Furthermore, the typical climatic conditions of Monastrell-producing areas—marked by high solar radiation, low rainfall, and elevated temperatures—frequently result in grapes with high sugar content and potentially unbalanced phenolic ripeness, adding further complexity to the winemaking process. Therefore, refining fermentation protocols through targeted interventions such as micro-oxygenation could contribute to optimizing the sensory and chemical profile of wines produced from this emblematic variety [
15,
16]
Despite the widespread use of micro-oxygenation and the theoretical advantages of hyperbaric environments, few studies have investigated their combined application during active fermentation, especially in commercial-scale conditions and using authentic grape musts from Mediterranean cultivars. Most available data focus on post-fermentation stages or employ model solutions, limiting their direct applicability to real-world winemaking scenarios. Furthermore, the interactions between oxygen availability, pressure, yeast metabolism, and phenolic chemistry remain insufficiently understood, making it difficult to predict the outcomes of such treatments without empirical validation [
17,
18]
.
Taken together, this study explores an emerging technological alternative for optimizing key enological processes: the application of micro-oxygenation under hyperbaric conditions. This strategy aims to modulate the color of grape must by stabilizing anthocyanins and tannins during fermentation, influence the volatile compound profile through altered yeast metabolic pathways under controlled oxygen and pressure conditions, and assess the impact on sensory characteristics to determine the potential of this technique to improve overall product quality. The integration of non-conventional technologies, such as hyperbaric treatment, at a critical stage of the enological process represents an innovative approach that could open new avenues for improving must fermentation in a controlled and reproducible manner [
19,
20].
2. Materials and Methods
2.1. Samples
Grapes from
Vitis vinifera L. cv. Monastrell were hand-harvested at optimal ripeness from Casa Rojo Winery and Vineyards (Jumilla, Spain;
https://www.casarojo.com/). The harvest was conducted under dry conditions, selecting healthy and homogeneous grape clusters. After harvesting, the grapes were transported to the winery in 15 kg boxes to avoid crushing and were stored in cold chambers at 2 °C for 48 hours to prevent premature oxidation and initiate cold maceration.
For must preparation, the grape clusters were destemmed and gently crushed upon arrival at the winery. The resulting must, along with the solid parts (skins and seeds), was transferred into temperature-controlled stainless-steel tanks. No corrections were made to the must, which underwent a pre-fermentative maceration at 10 °C for 24 hours to enhance the initial extraction of phenolic and aromatic compounds.
Alcoholic fermentation was initiated by inoculating the must with selected commercial yeasts (Saccharomyces cerevisiae). Fermentation was conducted in stainless-steel tanks at a controlled temperature of 24–26 °C. Daily pump-overs were carried out during the first days to optimize anthocyanin extraction.
Samples were taken and several physicochemical parameters were measured at three key stages: initially, at mid-fermentation, and at the end of fermentation (
Table 1). Measurements of density, pH, alcohol content, residual sugars, probable alcohol, and total acidity were performed at 20 °C. Samples were previously filtered using a Sartorius Ministart filter with a pore size of 1.2 µm, and analyzed with an infrared analyzer (OenoFoss, serial number 91791632).
2.2. Experimental Treatments
Two fermentation treatments were designed to evaluate the effects of micro-oxygenation under hyperbaric conditions on the chromatic, volatile, and sensory characteristics of Vitis vinifera L. cv. Monastrell grape must. Treatments were defined based on the application (MOX) or absence of micro-oxygenation (CON) and the stage of fermentation at which measurements were taken: mid-fermentation (Day 3) and end of fermentation (Day 13). All fermentations were carried out under identical temperature and vessel conditions, differing only in the oxygenation regime.
MOX: Must treated with hyperbaric micro-oxygenation
CON: Untreated control must (no micro-oxygenation).
The micro-oxygenation treatment was applied using a custom-designed hyperbaric chamber that allowed precise oxygen dosing under elevated pressure. For MOX samples, controlled oxygen injection was initiated at fermentation onset and maintained at consistent pressure and flow parameters throughout the experimental period.
Sampling and analysis were performed at three key time points:
Initial must (Day 0): Baseline before fermentation.
Day 3 (M1): Mid-fermentation point where anthocyanin extraction is actively progressing.
Day 13 (M2): End of alcoholic fermentation, representing maximum phenolic transformation and aroma development.
All physicochemical (
Table 1), colorimetric (
Table 2), volatile (
Table 3), and sensory (
Table 4) evaluations were conducted at these stages. The use of this time-structured design allowed the assessment of both the immediate and cumulative effects of micro-oxygenation under hyperbaric conditions on wine quality parameters.
2.3. Color Measurement
Color measurements were conducted at 25 ± 1 °C using a HunterLab ColorFlex spectrophotometer (HunterLab, Reston, VA, USA). The instrument was operated with a D65 standard illuminant and a 10° standard observer, in accordance with established colorimetric protocols. A dedicated sample holder for reflectance analysis was employed (internal diameter: 5.9 cm; height: 3.8 cm), providing a fixed optical path length of 10 mm. Baseline (blank) measurements were obtained by filling the sample cup with distilled water and recording the reflectance against a calibrated white reference background.
Color parameters were expressed using the CIELAB color space, which describes color as a position within a three-dimensional coordinate system. The L* coordinate corresponds to lightness, ranging from 0 (black) to 100 (white), representing the achromatic axis. The chromatic components, a* and b*, denote the red-green and yellow-blue axes, respectively. Specifically, positive a* values indicate a shift towards red, while negative values indicate a shift towards green; conversely, positive b* values correspond to yellow hues, and negative values to blue. Chroma (C*) was calculated as C* = √ (a² + b²), reflecting the intensity or saturation of the color, with higher values indicating greater color vividness. The hue angle (hab), representing the qualitative aspect of color, was determined using the expression hab = arctangent(b*/a*) and is expressed in degrees, measured counterclockwise from the +a* axis (0° = red, 90° = yellow, 180° = green, 270° = blue) [
21].
Color differences (
) between two color points in the CIELAB space are calculated as the Euclidean distance between their locations in the three-dimensional space defined by L*, a* and b*. Thus, mathematically, they are calculated by applying formula:
2.3. Volatile Organic Compounds (VOCs)
Ten grams of sample (must and wine) were added to a hermetic vial with a polypropylene cap and PTFE (polytetrafluoroethylene)/silicone septa, along with 1 g NaCl. The extraction of the volatile compounds of the samples was carried out using the headspace solid-phase microextraction (HS-SPME) method with Shimadzu AOC-6000 Plus autosampler (Shimadzu Scientific Instruments, Inc., Columbia, MD, USA). The sample was maintained at 40 °C with constant orbital agitation for 10 minutes to stabilize the headspace of the vial. After this time, a DVB/CAR/PDMS chromatographic fiber (1 cm) was introduced and kept in the headspace of the vial for 30 minutes. Once the extraction and absorption of volatile compounds were completed, the fiber was injected into the Shimadzu GC2030 gas chromatograph with an SLB-5ms column (30 m, 0.25 mm, and 0.25 μm). Helium was the carrier gas, with a split ratio of 1:10, a purge flow in the injector of 6 ml/min, total column flow of 0.6 ml/min, and temperature of injector of 230 ◦C. The oven program was the following: (i) initial temperature of 50 ◦C, and hold 1 min, (ii) ramp of 2 ◦C/min up to 100 ◦C, (iii) ramp of 3 ◦C/min up to 180 ◦C, and (iv) ramp of 20 ◦C/min up to 230 ◦C and hold 5 min. Same method were used previously in wine samples by [
22].
2.4. Descriptive Sensory Analysis
Ten trained panelists (4 males and 6 females) from the Food Quality and Safety Group (CIAGRO-UMH) selected, trained and validated according to ISO standard 8586-1 [
23], performed the sensory analyses. Lexicon used were based on the [
24] with some modifications. The panel analyzed the following descriptors:
Odor: alcohol, fruity, floral, herbal, spicy, woody, roasted, ripe fruit, tropical fruit, red fruit, citric, animal, balsamic, coffee and nuts.
Flavor: alcohol, fruity, floral, herbal, spicy, woody, roasted, sweet, sour, bitter, astringency, persistence, ripe fruit, tropical fruit, red fruit, citric, animal, balsamic, coffee, nuts and defects.
Panelists used a scale of 0 to 10 points for the evaluation, where 10 was extremely high intensity and 0 was extremely low intensity or not noticeable. 35 mL of sample was served in a black cup, randomly served coded with 3-digit numbers, at temperature of 14–16 °C. The analyses were conducted in a standardized tasting room equipped with 24 sensory booths. The room was maintained at a temperature of 22 °C. The panelists were provided with water and breadsticks to clean their palates between samples.
2.5. Statistical Analysis
The statistical analysis was conducted using IBM SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA). The data included physicochemical parameters, CIELAB color coordinates, visible absorbance spectra, volatile compound concentrations, and sensory descriptors.
Each treatment condition was replicated five times (n = 5), and every replicate was analyzed in technical triplicate. Mean values and standard deviations were calculated for all variables. To evaluate the effect of treatment and sampling time, a one-way analysis of variance (ANOVA) was performed. When statistically significant differences were found (p < 0.05), Tukey’s Honest Significant Difference (HSD) test was used for pairwise comparisons.
Before applying ANOVA, the underlying assumptions of the model were verified. The normality of residuals was assessed using the Shapiro–Wilk test and normal Q–Q plots. The homogeneity of variances was evaluated with Levene’s test. The independence of observations was guaranteed by the design of the experiment and sample processing protocol.
For the analysis of volatile organic compounds (VOCs), concentrations of individual compounds as well as grouped families (alcohols, acids, esters, and terpenes) were analyzed separately using the same statistical approach. This allowed the detection of treatment-related changes not only in specific aroma compounds but also in broader aromatic profiles relevant to wine quality.
Regarding the descriptive sensory analysis, data from ten trained panelists were treated as independent observations. Each aroma and flavor descriptor were analyzed individually by ANOVA followed by Tukey’s HSD test. This provided detailed insight into the sensory dimensions most affected by the treatments, particularly in relation to aromatic intensity, varietal character, and flavor persistence.
3. Results and Discussions
3.1. Color Measurements
3.1.1. CIELab
The data obtained show clear differences in the color parameters measured in the CIELab color space between the base must (Must) and the treated samples. In the Must sample, the values for lightness (L*), red coordinate (a*), and blue coordinate (b*) were lower than those observed in the treated samples (
Table 2).
M1_MOX (micro-oxygenated on day 3 under hyperbaric conditions) exhibited a notable increase in L* (10.59 compared to 5.94 in Must) and a* (18.12 vs. 13.17), indicating greater lightness and an intensification of the red hue. This behavior suggests that micro-oxygenation under hyperbaric conditions promotes the extraction and stabilization of phenolic compounds responsible for color, in agreement with findings reported in recent studies [
25,
26]. M2_MOX (micro-oxygenated on day 13) showed even higher a* values (24.54) and an increase in b* (5.25), which may be associated with controlled oxidation processes and anthocyanin polymerization, resulting in a distinct chromatic profile and potentially greater complexity in the final product [
7,
27]. In contrast, the control samples (M1_CON and M2_CON), which were not subjected to micro-oxygenation, showed less pronounced changes compared to Must. For example, M1_CON exhibited a slightly higher L* value (6.98), and M2_CON reached 9.71, but without the increases in a* and b* observed in the MOX samples. This suggests that the absence of micro-oxygenation limits the evolution of the chromatic profile, highlighting the role of the treatment in modulating color-related compounds [
20]. These results are consistent with recent publications demonstrating that micro-oxygenation—particularly under hyperbaric conditions—can enhance the extraction and stabilization of anthocyanins and other phenolic compounds, leading to products with greater color intensity and stability [
7,
26].
The values of chroma (C*) quantify color saturation or intensity. Higher C* values correspond to a more vivid or intense perceived color [
28,
29]. In red musts and wines, elevated C* is typically associated with a higher concentration of anthocyanins and polymeric pigments that are key contributors to color [
26,
30]. In the data presented in
Table 2, a significant evolution of chroma (C*) is observed across the different samples. The base must (Must), with a C* value of 13.40, serves as the initial reference, where the color intensity is moderate due to the natural anthocyanin concentration and the absence of any technological intervention.
Upon application of hyperbaric micro-oxygenation, a substantial increase in color saturation is observed. For instance, M1_MOX (day 3) showed a C* value of 18.23, indicating that controlled oxygen supply facilitated the early extraction and stabilization of color compounds. This effect became more pronounced in M2_MOX (day 13), where C* reached 25.10, suggesting that prolonged treatment time promotes anthocyanin–tannin polymerization and copigmentation reactions, resulting in a more vibrant and saturated color.
By contrast, the control samples (M1_CON and M2_CON), which were not subjected to micro-oxygenation, displayed a less marked chromatic evolution. While M1_CON reached a C* value of 13.97 (slightly above the base must), M2_CON increased to 18.42 at day 13, demonstrating that although time alone may enhance color saturation, the absence of controlled oxygen input limits the development of reactions that intensify chroma [
20,
26]. This contrast highlights the effectiveness of hyperbaric micro-oxygenation in promoting a faster and more pronounced evolution of chroma compared to conventional aging or maturation without such treatment.
Micro-oxygenation under hyperbaric conditions plays a fundamental role in enhancing color intensity and stability in red musts and wines. Under these conditions, the increased solubility of oxygen favors both enzymatic and non-enzymatic reactions that promote anthocyanin polymerization and the formation of stable complexes with tannins—processes known to intensify chroma [
29]. The presence of controlled oxygen enables effective copigmentation, whereby anthocyanins combine with other phenolic compounds to form more robust and degradation-resistant pigments. This stabilization translates into a notable increase in C*, as observed in the micro-oxygenated samples, where chroma values progressively increased from 18.23 in M1_MOX to 25.10 in M2_MOX.
Moreover, hyperbaric micro-oxygenation minimizes the risks associated with excessive oxidation, as the oxygen supply is precise and controlled, optimizing the formation of polymeric pigments without degradation of color compounds [
7,
20]. Consequently, this treatment not only enhances the visual appearance of the product but also contributes to its long-term color stability, offering an advanced technological solution to improve the sensory quality of the final product. Current evidence supports that strategic oxygen management is key to achieving products with greater color saturation and durability—an essential factor for both enological acceptance and commercial appeal [
31,
32].
The hue angle (h°) in the CIELab color space represents the direction of the color vector in the plane defined by the a* (red–green) and b* (yellow–blue) axes. It is a key parameter for interpreting the tone and color evolution in enological samples. This angle is typically calculated using the arctangent function arctan(b*/a*), and it helps determine whether the perceived color leans more toward reddish, orange, or purplish hues. From a sensory standpoint, variations in hue may reflect changes in pigment composition, particularly regarding the form and stability of anthocyanins and other phenolic compounds.Recent studies have emphasized that, in addition to chroma, hue is crucial for a comprehensive evaluation of color, as shifts in hue can influence perceptions of ripeness and oxidative evolution in wine [
33,
34].
As shown in
Table 2, the base must (Must) presented a hue angle of 10.36°, serving as the initial reference for color tone. Samples subjected to hyperbaric micro-oxygenation exhibited notable variations: M1_MOX, measured on day 3, had a hue of 5.82°, indicating a shift toward tones perceived as more intense or concentrated. M2_MOX, measured on day 13, reached a hue of 12.05°, suggesting a subtle tonal shift likely associated with pigment evolution and the formation of polymeric compounds. On the other hand, the control samples (M1_CON and M2_CON) showed hue values of 4.20° and 2.69°, respectively, indicating that in the absence of micro-oxygenation, hue shifts were even more pronounced—possibly due to lower anthocyanin stabilization and a different evolution of the phenolic matrix. These differences in hue, when considered alongside chroma behavior, reinforce the hypothesis that hyperbaric micro-oxygenation not only enhances color saturation but also subtly modulates the final color tone, improving chromatic stability and, consequently, the sensory quality of the product [
8,
32].
To further quantify chromatic evolution during fermentation, the color difference (ΔEab) was calculated between the initial must and the samples collected on day 3 and day 13, both with and without micro-oxygenation. This parameter represents the Euclidean distance in the CIELAB color space and is widely used to assess perceptible changes in color. On day 3 of fermentation, the micro-oxygenated sample (M1_MOX) showed a ΔEab of 6.81 compared to the initial must, while the untreated control (M1_CON) reached 3.97. By day 13, the ΔEab for the treated sample (M2_MOX) increased to 12.69, whereas the control (M2_CON) recorded a value of 6.61. These results indicate that micro-oxygenation accelerates and amplifies chromatic changes during the fermentation process. From a sensory perspective, ΔEab values above 3.0 are generally considered noticeable to the human eye, and values exceeding 5.0 are regarded as clearly perceptible. Therefore, the differences observed are not only analytically significant but also visually relevant. The higher ΔEab values in MOX samples suggest enhanced extraction and stabilization of phenolic pigments, likely due to increased oxygen availability under hyperbaric conditions. These observations are consistent with previous research highlighting the role of micro-oxygenation in stabilizing wine color. For example, [
32] demonstrated that applying micro-oxygenation before malolactic fermentation led to more stable color characteristics. Similarly, [
35,
36] reported that micro-oxygenation during barrel aging, particularly in combination with oak chips, promoted anthocyanin polymerization and improved color brilliance. Other studies have also shown that early application of oxygen can prevent the typical decrease in color following malolactic fermentation. In addition, recent proposals have suggested the use of updated classification systems for red wine color based on CIELAB parameters, enabling more objective comparisons across wine types [
28,
37]. In this context, ΔEab provides a robust and interpretable metric for monitoring color development and assessing the impact of technological interventions on visual wine quality.
3.1.2. Absorbance Measurements in the Visible Spectrum
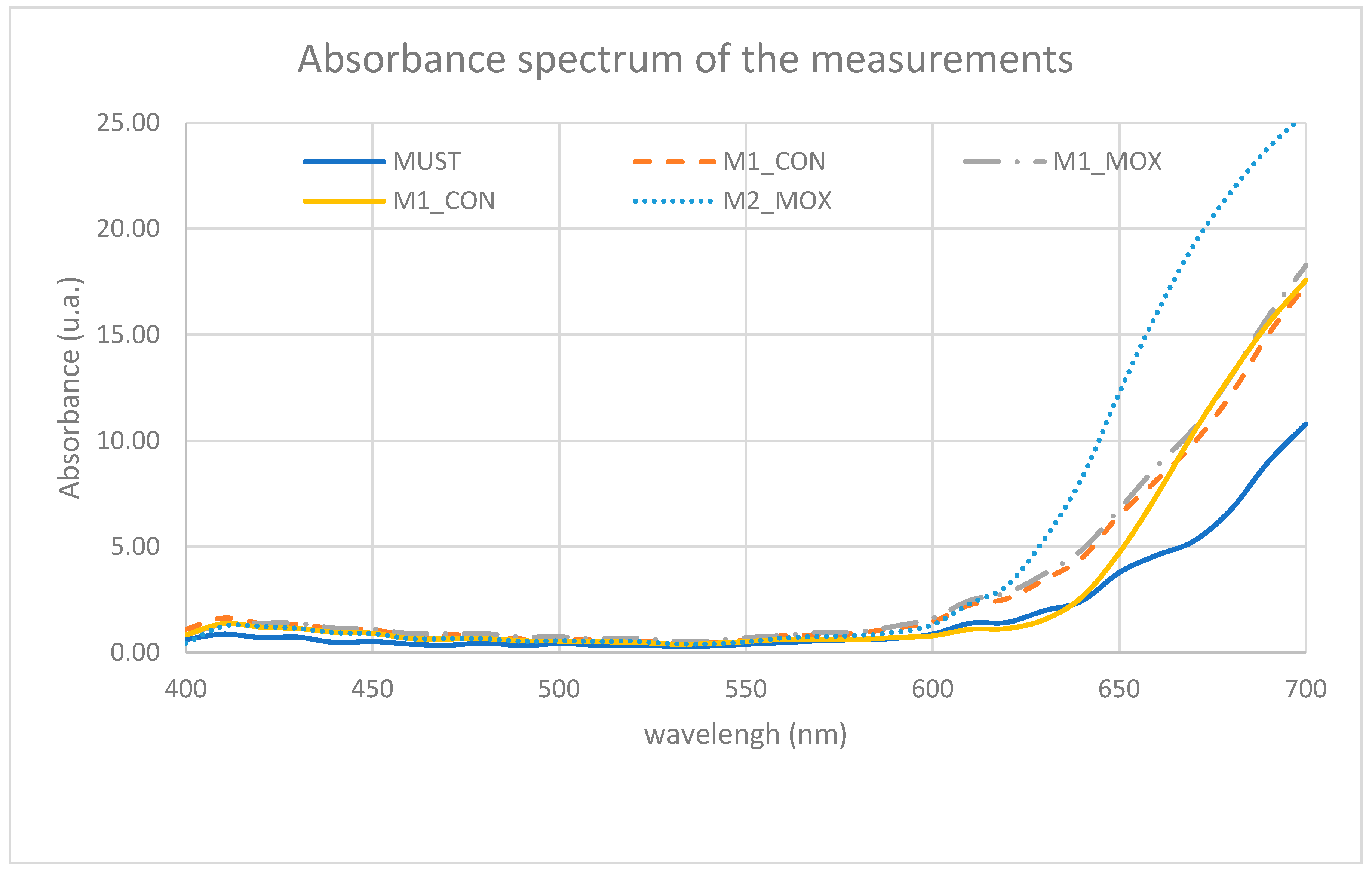
In
Figure 1, the results of absorbance measurements in the visible spectrum (400–700 nm) are shown, illustrating the evolution of phenolic compounds responsible for color in
Vitis vinifera L. cv. Monastrell, with a focus on the effect of micro-oxygenation under hyperbaric conditions.
The most relevant regions of the visible spectrum include 400–420 nm (yellow), associated with flavonoid-type compounds and the onset of oxidized anthocyanin absorption; 520–540 nm (red), representing the main absorption peak of free anthocyanins and, in part, early polymerized pigments; and 600–620 nm (blue-violet), indicating the presence of anthocyanin-derived pigments, tannins, and more stable polymeric complexes.
Overall, the M1_MOX and M2_MOX samples exhibited higher absorbance values in the red region (around 520 nm) and extending up to 600–650 nm, suggesting an intensification and stabilization of color-related compounds. In contrast, the Must sample—as expected—and the control samples (M1_CON and M2_CON) showed lower absorbance values, indicating a lower degree of extraction and/or stabilization of color compounds (
Figure 1).
The increase in absorbance observed around 520–620 nm in the MOX samples, particularly in M2_MOX (
Figure 1), is associated with the formation of more stable pigments (polymeric pigments) and greater anthocyanin retention, promoted by the availability of oxygen under controlled conditions. Recent studies indicate that micro-oxygenation enhances controlled polymerization and the formation of bonds that are more resistant to pH changes and oxidation, thereby improving chromatic stability [
17,
38].
The effects of micro-oxygenation under hyperbaric conditions are linked to higher oxygen solubility and controlled reactivity, which increases oxygen availability in the must/wine matrix. This facilitates polymerization and copigmentation reactions more efficiently and rapidly than under normal atmospheric conditions [
7,
17]. However, as it is a controlled environment, excessive oxidation and anthocyanin degradation are avoided.
This process is also reflected in the intensification of color and the formation of stable pigments. The M1_MOX sample (measured on day 3) shows an initial increase in absorbance (
Figure 1), whereas the M2_MOX sample (measured on day 13) presents the highest peak in the 520–620 nm region, suggesting progressive anthocyanin polymerization and their integration with tannins [
39]. This temporal sequence aligns with previous studies reporting that oxygen supplied during early fermentation and/or maturation phases contributes to the formation of stable color compounds [
40].
In comparison, the control samples M1_CON and M2_CON (not micro-oxygenated) exhibited a more limited chromatic evolution, with lower absorbance in the red region. This indicates that in the absence of controlled oxygen input, color stabilization processes are slower or less efficient, underscoring the importance of micro-oxygenation during the fermentation or maturation phase to optimize color quality [
20,
41].
3.2. Volatile Compounds
To understand the influence of the microoxygenation treatment on the volatile profile of the red wine samples, the 17 aromatic compounds found in the highest concentration in the samples under study were analyzed (
Table 3). These include alcohols, acids, esters, and terpenes.
Alcohols are primarily formed during alcoholic fermentation, when yeasts convert the sugars present in grapes into alcohol and carbon dioxide. 1-Propanol is an alcohol found in small quantities in most red wines. Its presence contributes to the subsequent formation of esters during fermentation. The compound 1-hexanol is an alcohol that contributes to the herbaceous and green aromas in red wine. 2-Methylpropan-1-ol, also known as isobutanol, is an alcohol that contributes to the secondary aromas of wine, providing slightly alcoholic and fruity notes, while 3-Methylbutan-1-ol, also known as isoamyl alcohol, imparts banana and ripe fruit aromas. Both compounds are products of alcoholic fermentation. Finally, the compound 2-phenylethanol is highly valued in red wine for presenting sensory notes reminiscent of flowers, particularly roses.
The acid family is represented by the hexanoic acid compound. This is produced during the metabolism of yeasts and bacteria in the fermentation process, contributing to the complexity of the wine, although at high concentrations it gives rancid notes.
The esters analyzed, Ethyl acetate, Ethyl butyrate, Isoamyl acetate, Ethyl hexanoate, Hexyl acetate, Ethyl octanoate, Ethyl decanoate, Ethyl dodecanoate, are formed through reactions between the alcohols and acids present in the wine. For example, ethyl acetate is formed when ethanol reacts with acetic acid. These reactions can occur during fermentation and during wine aging. Their presence is commonly associated with pleasant notes, apart from ethyl acetate, which can be associated with glue-like notes. Commonly, ethyl esters C6, C8, and C10 are related to higher quality aromas in wines. Finally, terpenes (Linalool, Geraniol, β-Ionone) are aromatic compounds naturally found in grapes and released during fermentation. They mainly contribute floral aromas to the wine [
42,
43].
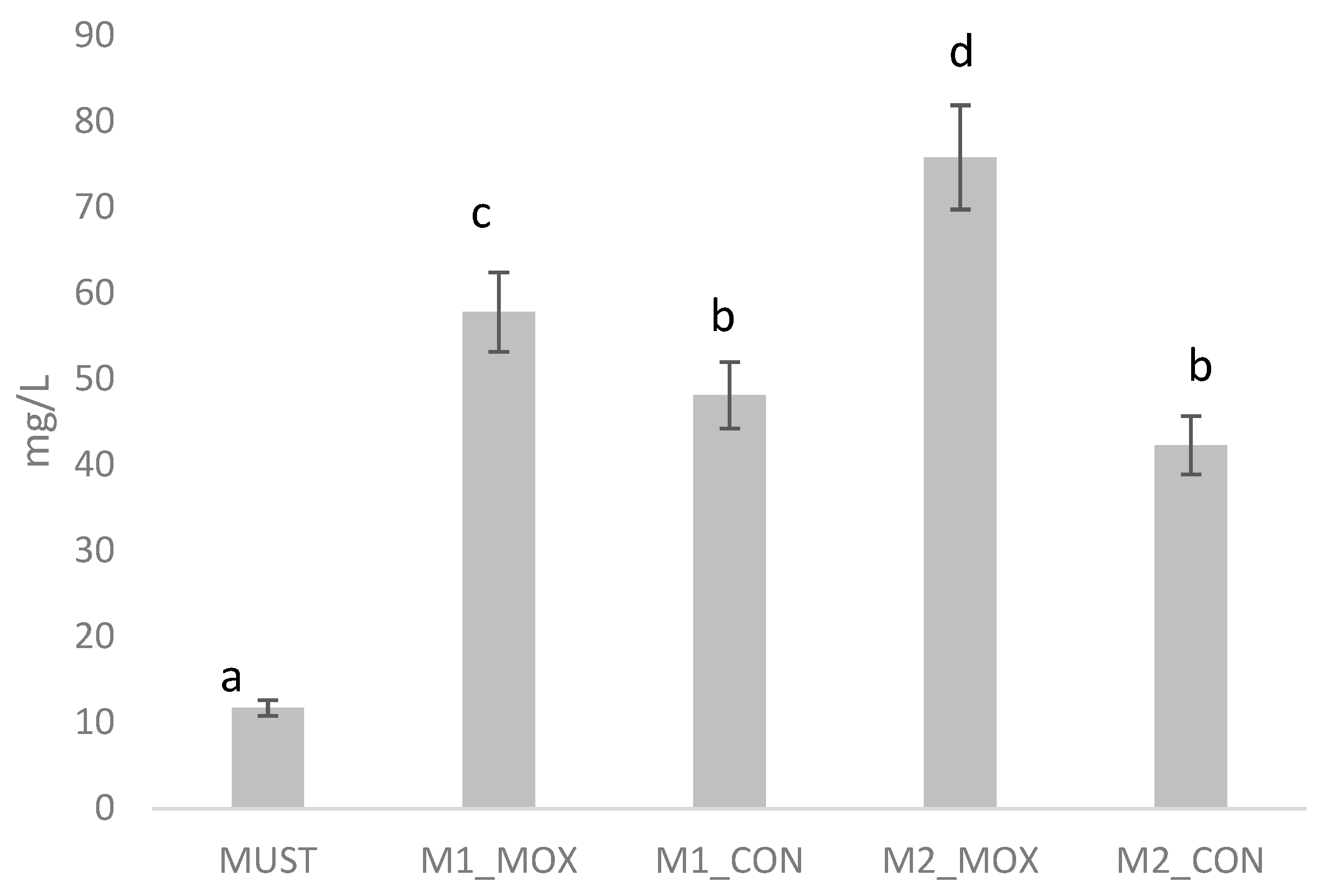
In the case of the must, the compounds found in the highest concentration were ethyl acetate (~3.4 mg/L), 1-hexanol (~1.9 mg/L), hexyl acetate (~1.4 mg/L), and ethyl dodecanoate (~1.0 mg/L). The rest of the compounds had concentrations below 1 mg/L, with ethyl butyrate being found in the lowest concentration (0.003 mg/L). When analyzing the results obtained from the wine analysis, the compounds found in the highest concentration were the esters ethyl octanoate (~22 mg/L) and ethyl decanoate (~8 mg/L). In this case, the compound found in the lowest concentration was β-ionone (~0.07 mg/L). In general, samples treated with microoxygenation had higher concentrations of volatile compounds where statistically significant differences were found. At the first sampling time (M1), MIC samples had higher concentrations of hexyl acetate, ethyl octanoate, and ethyl decanoate. As mentioned earlier, these three compounds are characterized by contributing aromas related to greater structure and/or quality in wine. At the second sampling time (M2), these differences were magnified, with the MIC samples having higher concentrations in 9 of the 17 aromatic compounds analyzed, being also 9 of the 10 most abundant compounds.
In
Figure 2, it can be observed how the content of aromatic compounds increases when the must ferments and wine is produced. It can be seen again that the MOX samples, regardless of the sampling time, had the highest content of aromatic compounds. Main differences occurred in esters family (
Table 3). Samples obtained by microoxygenation increase the concentration of these compounds, compared to control samples. Same results were obtained in wines made by Pinot noir, Cabernet Sauvignon and Dornfelder grapes under microoxygenation techniques [
44]. Wines treated with MOX led to larger differences in certain volatile compounds such as terpenes and ethyl esters in wines produced by Tempranillo and Cabernet Sauvignon, before malolactic fermentation, however, these differences became smaller with prolonged aging [
45].
3.3. Descriptive Sensory Analysis
Statistically significant differences were found in 21 of the 36 sensory descriptors analyzed. As shown in
Table 4 and as expected, the must had higher intensity of fruity, floral, herbal, and red fruit aromas and flavors compared to the wine samples. Additionally, as expected, must samples have higher sweetness
When comparing treatments at each sampling time, we can observe that in M1 the CON sample reduced the fruity descriptor (odor and flavor) more intensely (compared to the intensity detected in the initial must). No statistically significant differences were found between the samples in the rest of the descriptors. However, in M2 the differences between treatments became more noticeable. The CON sample showed fewer intensities in 12 of the 14 descriptors where differences were found. This sample had less intensity of sweetness, acidity, and bitterness, which can be translated as a lower flavor intensity. Furthermore, it had fewer intensities of alcohol aroma and fruity, floral, herbal, spicy, ripe fruit, and red fruit (odor and flavor). Additionally, it had higher bitterness intensity and a slight defect (slight Brett aroma).
These results are consistent with those obtained after the volatile analysis of the samples, as it was also found that samples treated with microoxygenation have higher aromatic concentration.
It has been demonstrated that MOX can improve the tannin composition, total phenol concentration, flavanols, proanthocyanidins, and, therefore, structure of red wine due to linkages between catechin moieties [
15,
46,
47]. This allows the wine to have greater structure and complexity, making its passage through the mouth more sensorially acceptable.
On the other hand, [
48] demonstrated that microoxygenation had a positive effect on the integration of fruity and varietal aromas in red wines (Tinta de Toro and Mencía), and, even, a reduction of some compounds related with off-flavors, like furfural compounds.
4. Conclusions
The application of micro-oxygenation under hyperbaric conditions in Vitis vinifera L. cv. Monastrell grape must led to significant modifications in physicochemical, chromatic, aromatic, and sensory parameters during fermentation. Treated samples (M1_MOX and M2_MOX) showed a clear enhancement in color attributes measured in the CIELab space, with substantial increases in lightness (L*), redness (a*), and chroma (C*), as well as moderated shifts in hue angle (h°). These effects suggest improved extraction, stabilization, and polymerization of phenolic compounds such as anthocyanins and tannins.
Visible absorbance spectra (400–700 nm) reinforced these observations, with MOX-treated samples exhibiting increased absorbance in the 520–620 nm region, indicating the formation of more stable polymeric pigments. The effect was more pronounced in M2_MOX, where longer exposure under hyperbaric conditions resulted in stronger color intensity and stability. In contrast, control samples (M1_CON and M2_CON) showed more limited chromatic evolution, reinforcing the role of controlled oxygen availability in modulating phenolic dynamics.
In terms of volatile composition, wines from hyperbarically micro-oxygenated musts showed higher concentrations of esters and terpenes, particularly ethyl octanoate, ethyl hexanoate, and geraniol, which are associated with fruity and floral aromas. At the final sampling time (M2), MOX samples concentrated 9 of the 10 most abundant volatile compounds at significantly higher levels than controls. This aligns with previous findings on the effect of oxygen on yeast metabolism and ester formation.
Sensory analysis confirmed the analytical results. Wines produced under hyperbaric micro-oxygenation retained more intense fruity, floral, and varietal aromas, and were perceived as more flavorful and persistent. M2_MOX samples, in particular, were characterized by greater aromatic complexity and structure, while M2_CON samples showed reduced aroma intensity and even slight defects (e.g., Brett character), likely due to the absence of oxygen modulation.
In summary, hyperbaric micro-oxygenation accelerates and enhances phenolic extraction, color stabilization, and aroma development in Monastrell grape must, resulting in wines with more intense color, higher aromatic complexity, and improved sensory profiles. The combination of increased oxygen solubility under pressure and precise oxygen management offers a promising technological approach for optimizing red wine fermentation. These findings support the potential of this technique to improve the quality and stability of wines produced from phenolic-rich grape varieties under warm climate conditions.
Author Contributions
Conceptualization, AJPL, PM and AVL.; methodology, AJPL, PM and AVL.; software, X.X.; validation AJP, LNA, PN, PM, AVL and JRAM; formal analysis, AJPL, LNA.; investigation, AJP, LNA, PN and JRAM.; resources, AJPL, LN, PM, AVL and JRAM.; data curation, AJP, LNA and JRAM; writing—original draft preparation, AJP, LNA and JRAM.; writing—review and editing, AJP, LNA and JRAM.; visualization AJP, LNA and JRAM.; supervision, AJP, LNA, PN, PM, AVL and JRAM.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Du, Y.H.; Ye, Y.Q.; Hao, Z.P.; Tan, X.Y.; Ye, M.Q. Research on wine flavor: A bibliometric and visual analysis (2003-2022). Food Chem. Adv 2024, 4, 100717. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Yang, H. , Sun, L.; Xia, H.; Sun, W.; Wang, Z.; Zhang, J. Bacterial communities related to aroma formation during spontaneous fermentation of ‘Cabernet Sauvignon’wine in Ningxia, China. Foods, 2022, 11, 2775. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ji, M.; Gong, J.; Chitrakar, B. The formation of volatiles in fruit wine process and its impact on wine quality. Appl. Microbiol. Biotechnol 2024, 108, 420. [Google Scholar]
- Martínez-Moreno, A.; Toledo-Gil, R.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Yuste, J.E.; Vallejo, F. Exploring the Impact of Extended Maceration on the Volatile Compounds and Sensory Profile of Monastrell Red Wine. Fermentation 2024, 10, 343. [Google Scholar] [CrossRef]
- González-Sanjosé, M.L.; Ortega-Heras, M.; Pérez-Magariño, S. Microoxygenation Treatment and Sensory Properties of Young Red Wines. Food Science and Technology International. 2008, 14, (5_suppl):123-130.
- Cejudo-Bastante, M.J.; Pérez-Coello, M.S.; Hermosín-Gutiérrez, I. Effect of wine micro-oxygenation treatment and storage period on colour-related phenolics, volatile composition and sensory characteristics. Lwt - Food Science and Technology. 2011, 44, 866-874.
- Sánchez-Gómez, R.; del Alamo-Sanza, M.; Martínez-Gil, A.M.; Nevares, I. Red Wine Aging by Different Micro-Oxygenation Systems and Oak Wood—Effects on Anthocyanins, Copigmentation and Color Evolution. Processes 2020, 8, 1250. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Cano-López, M. A review on micro-oxygenation of red wines: Claims, benefits and the underlying chemistry. Food Chem 2011, 125, 1131–1140. [Google Scholar] [CrossRef]
- Aceituno, F.F.; Orellana, M.; Torres, J.; Mendoza, S. Oxygen Response of the Wine Yeast Saccharomyces cerevisiae EC1118 Grown under Carbon-Sufficient, Nitrogen-Limited Enological Conditions. Appl Environ Microbiol 2012, 78, 8340–8352. [Google Scholar] [CrossRef] [PubMed]
- Nevares, I.; Fernández-Díaz, A.; del Alamo-Sanza, M. Characterization and Control of Hidden Micro-Oxygenation in the Winery: Wine Racking. Foods, 2021, 10, 386.
- Guerrini, L.; Masella, P.; Angeloni, G.; Sacconi, A.; Calamai, L.; Parenti, A. Effects of a Small Increase in Carbon Dioxide Pressure during Fermentation on Wine Aroma. Foods 2020, 9, 1496. [Google Scholar] [CrossRef]
- Lukić, K.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K. Phenolic and Aroma Changes of Red and White Wines during Aging Induced by High Hydrostatic Pressure. Foods 2020, 9, 1034. [Google Scholar] [CrossRef]
- Kumar, Y.; Marangon, M.; Mayr Marangon, C. The Application of Non-Thermal Technologies for Wine Processing, Preservation, and Quality Enhancement. Beverages 2023, 9, 30. [Google Scholar] [CrossRef]
- Comuzzo, P. , & Calligaris, S. Potential Applications of High Pressure Homogenization in Winemaking: A Review. Beverages 2019, 5, 56. [Google Scholar]
- Cano-López, M.; Pardo-Mínguez, F.; Schmauch, G.; Saucier, C.; Teissedre, P.-L.; López-Roca, J.M.; Gómez-Plaza, E. Effect of micro-oxygenation on color and anthocyanin-related compounds of wines with different phenolic contents. J Agric Food Chem, 2008, 56, 5932–5941. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Intrigliolo, D.S.; Almajano, M.P. Effects of water deficit irrigation on phenolic composition and antioxidant activity of Monastrell grapes under semiarid conditions. Antioxidants, 2021, 10, 1301. [Google Scholar] [CrossRef]
- Schmidtke, L.M. , Clark, A.C.; Scollary, G.R. Micro-Oxygenation of Red Wine: Techniques, Applications, and Outcomes. Crit Rev Food Sci Nutr 2011, 51, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Januszek, M. Effect of Musts Oxygenation at Various Stages of Cider Production on Oenological Parameters, Antioxidant Activity, and Profile of Volatile Cider Compounds. BioMolecules 2020, 10, 890. [Google Scholar] [CrossRef]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef]
- Carrasco-Quiroz, M.; del Alamo-Sanza, M.; Martínez-Gil, A.M.; Nevares, I. (2023). Influence of Oxygen Management on Color and Phenolics of Red Wines. Molecules 2023, 28, 459. [Google Scholar] [CrossRef]
- Pérez-López, A.J.; Beltran, F.; Serrano-Megías, M.; López, D.S.; Carbonell-Barrachina, A.A. Changes in orange juice color by addition of mandarin juice. Eur Food Res Technol 2006, 222, 516–520. [Google Scholar] [CrossRef]
- Issa-Issa, H.; Hernández, F.; Lipan, L.; López-Lluch, D.; Carbonell-Barrachina, Á.A. Quality, Nutritional, Volatile and Sensory Profiles and Consumer Acceptance of Fondillón, a Sustainable European Protected Wine. Agronomy 2021, 11, 1701. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques, 4rd ed.; Taylor Francis (CRC Press): Boca Raton, USA, 2006; p. 416. [Google Scholar]
- Issa-Issa, H.; Noguera-Artiaga, L.; Sendra, E.; Pérez-López, A.J.; Burló, F.; Carbonell-Barrachina, A.A.; López-Lluch, D. Volatile Composition, Sensory Profile, and Consumers’ Acceptance of Fondillón. J Food Qual 2019, 1, 5981762. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Cebrián-Tarancón, C.; Martínez-Gil, A.M.; Nevares, I.; Alonso, G.L.; Salinas, M.R.; Del Alamo-Sanza, M. Effect of micro-oxygenation on color of wines made with toasted vine-shoots. J Sci Food Agric. 2025, 105(3), 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.; Lerno, L.; Sari, S.; Fanzone, M.; Casassa, L.F.; Oberholster, A. Impact of micro-oxygenation timing and rate of addition on color stabilization and chromatic characteristics of Cabernet Sauvignon wines. LWT - Food Sci Technol 2021, 149, 111776. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, T.; Gao, J.; Han, X.; Huang, W.; You, Y.; Zhan, J. Color myth: anthocyanins reactions and enological approaches achieving their stabilization in the aging process of red wine. Food Innov Adv 2023, 2, 255–271. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Y.; Li, Y. A New Approach for Quantitative Classification of Red Wine Color from the Perspective of Micro and Macro Levels. Fermentation 2023, 9, 519. [Google Scholar] [CrossRef]
- Uysal, R.S.; Issa-Issa, H.; Sendra, E.; Carbonell-Barrachina, Á A. Changes in anthocyanin pigments, trans-resveratrol, and colorimetric characteristics of Fondillón wine and other “Monastrell” wines during the aging period. Eur Food Res Technol 2023, 249, 1821–1831. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Lyu, X.; Fang, X.; Cao, X. Metabolomic analysis to unravel the composition and dynamic variations of anthocyanins in bayberry-soaked wine during the maceration process. Food Chem 2024, 21, 101175. [Google Scholar] [CrossRef]
- Dai, L.; Sun, Y.; Qian, M. Influence of Oxygen Management during the Post-Fermentation Stage on Acetaldehyde, Color, and Phenolics of Vitis vinifera L. Cv. Cabernet Sauvignon Wine. Molecules 2022, 27, 6692. [Google Scholar] [CrossRef]
- Yang, Y.; Deed, R.C.; Araujo, L.D.; Kilmartin, P.A. The Influence of Micro-oxygenation on the Long-term Ageing Ability of Pinot noir Wine. S. Afr J Enol Vitic 2022, 43, 1–9. [Google Scholar] [CrossRef]
- Baris, F.; Castro Marin, A.; Chinnici, F. Oxidative Evolution of Different Model Rosé Wines Affected by Distinct Anthocyanin and Tannin Contents. Beverages 2024, 10, 43. [Google Scholar] [CrossRef]
- Pelonnier-Magimel, E.; Chira, K.; Teissèdre, P.-L.; Jourdes, M.; Barbe, J.-C. (2023). Color Characterization of Bordeaux Red Wines Produced without Added Sulfites. Foods 2023, 12, 2358. [Google Scholar] [CrossRef]
- Oberholster, A.; Elmendorf, B.L.; Lerno, L.A.; King, E.S.; Heymann, H.; Brenneman, C.E.; Boulton, R.B. Barrel maturation, oak alternatives and micro-oxygenation: influence on red wine aging and quality. Food Chem 2015, 173, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Iglesias, M.; González-Sanjosé, M.L.; Pérez-Magariño, S.; Ortega-Heras, M.; González-Huerta, C. Effect of micro-oxygenation and wood type on the phenolic composition and color of an aged red wine. J Agric Food Chem 2009, 57, 11498–11509. [Google Scholar] [CrossRef]
- Durner, D.; Hensel, M. Is there a need to re-define the methods to evaluate wine color. 44th World Congress of Vine and Wine. BIO Web Conf. 68, 2023.
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M. J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed]
- Gambuti, A.; Rinaldi, A.; Ugliano, M.; Moio, L. Evolution of phenolic compounds and astringency during aging of red wine: effect of oxygen exposure before and after bottling. J Agric Food Chem 2013, 61, 1618–1627. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Role of lees in wine production: A review. Food Chem 2008, 111, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Sánchez-Iglesias, M.; Ortega-Heras, M.; González-Huerta, C.; González-Sanjosé, M.L. (2007). Colour stabilization of red wines by microoxygenation treatment before malolactic fermentation. Food Chem 2007, 101, 881–893. [Google Scholar] [CrossRef]
- Prusova, B.: Humaj, J.; Sochor, J.; Baron, M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation 2022, 8, 93.
- Zhang, D.; Wei, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef]
- Schmarr, H.G.; Bernhardt, J.; Fischer, U.; Stephan, A.; Müller, P.; Durner, D. Two-dimensional gas chromatographic profiling as a tool for a rapid screening of the changes in volatile composition occurring due to microoxygenation of red wines. Anal Chim Acta 2010, 672, 114–123. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Lapeña, A.C.; Escudero, A.; Astrain, J.; Baron, C.; Pardo, I.; Polo, L.; Ferrer, S.; Cacho, J.; Ferreira, V. Effect of micro-oxygenation on the evolution of aromatic compounds in wines: Malolactic fermentation and ageing in wood. LWT - Food Sci Technol 2009, 42, 391–401. [Google Scholar] [CrossRef]
- Drinkine, J.; Glories, Y.; Saucier, C. (+)-Catechin−Aldehyde Condensations: Competition between Acetaldehyde and Glyoxylic Acid. J Agric Food Chem 2005, 53, 7552–7558. [Google Scholar] [CrossRef]
- Geldenhuys, L.; Oberholster, A.; du Toit, W.J. Monitoring the effect of micro-oxygenation before malolactic fermentation on South African pinotage red wine with different colour and phenolic analyses. S. Afr J Enol Vitic 2012, 33, 150–160. [Google Scholar] [CrossRef]
- Heras, M.O.; Rivero-Pérez, M.D.; Pérez-Magariño, S.; González-Huerta, C.; González-Sanjosé, M.L. Changes in the volatile composition of red wines during aging in oak barrels due to microoxygenation treatment applied before malolactic fermentation. Eur Food Res Technol 2008, 226, 1485–1493. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).