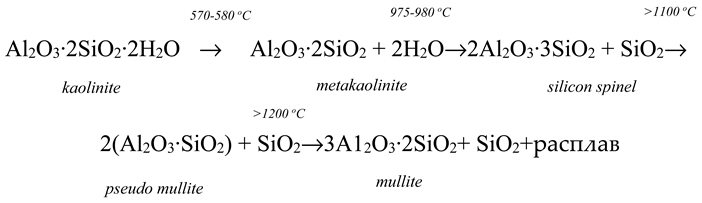3. Results and Discussion
The work shows the mineral-forming effect of the joint participation of two unusual in physical and chemical properties mineral types of raw materials that have been little studied in this technology - wollastonite of contact-metasomatic origin and quartz of finely ground to a powdery state by natural exogenous processes. The emerging features of the formation and transformation of phases, structure and physical and technical properties of the synthesized electrical porcelain were investigated.
A series of laboratory studies were carried out In the course of searching for more rational compositions of masses. New compositions were selected based on calculations and experiments. The standard composition 1SC with the following components was adopted as the starting point: kaolin (27.5%), white-burning clay (22.5%), quartz sand (18.0%) and feldspar (32,0 %) [
9].
In order to establish and analyze the dynamics of changes in the characteristics of this ceramics in the process of introducing selected components for experiments, three potentially interesting compositions were found based on preliminary results – 2СМ, 3СW and 4СМW. In the 2 СМ composition, traditional quartz sand (18%) is completely replaced by marshalite, in 3СW wollastonite (2%) is used, and in 4СМW, where quartz sand (18%) is also completely replaced by marshalite, wollastonite is also introduced (2 %).
To obtain a preliminary assessment of the sinterability of the masses, melting curves were constructed using the phase diagrams of the K2O-Al2O3-SiO2and Na2O-Al2O3-SiO2 systems. The choice of two systems is explained by the insignificant difference in the К2O and Na2O contents in the masses.
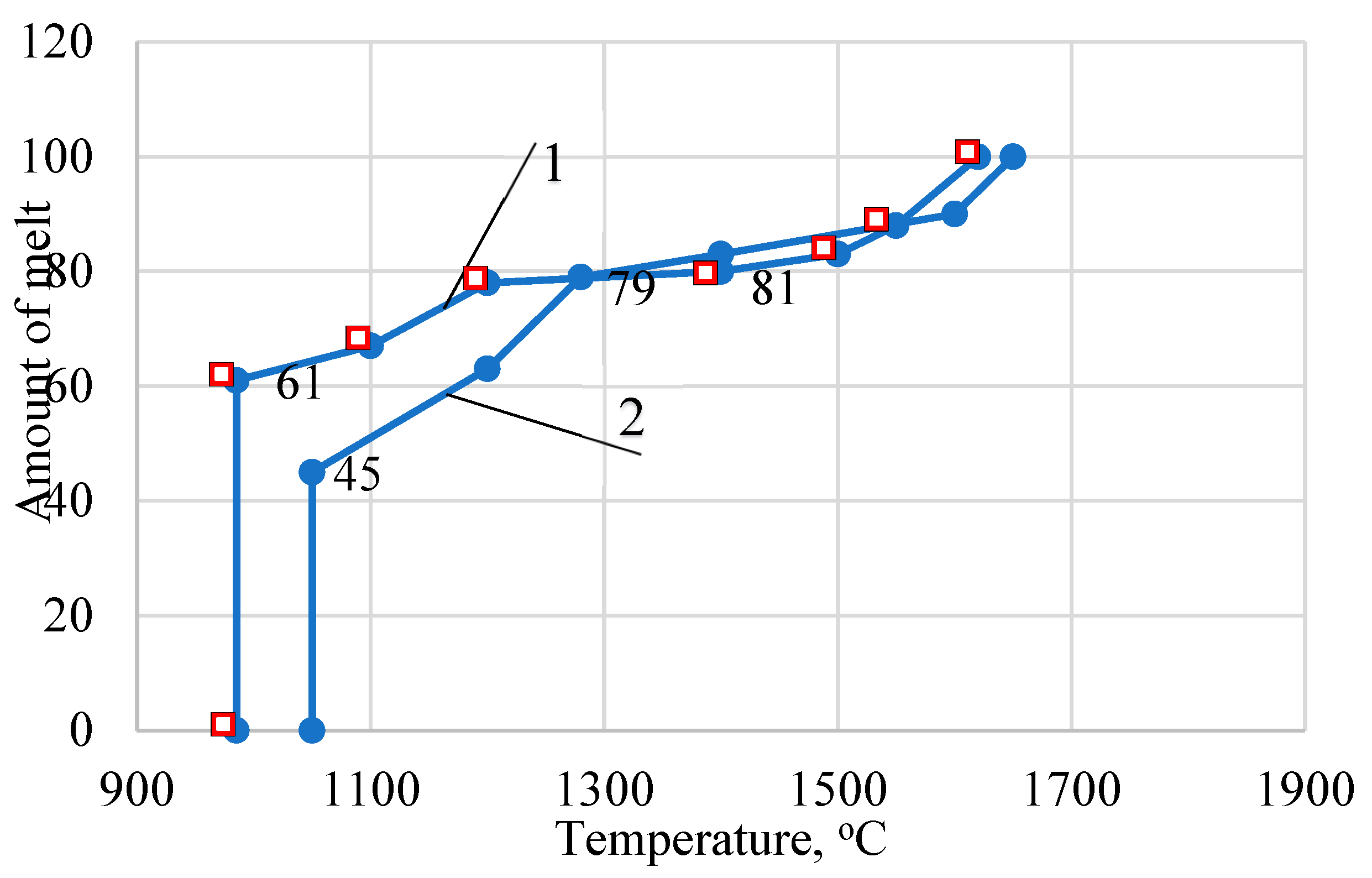
The positive value of the phase diagram is that it makes it possible to determine both the sequence of separation of solid phases and the limiting state to which the system tends. The analysis of the equilibrium states of porcelain masses at different temperatures in the Na2O-Al2O3-SiO2 and K2O-Al2O3-SiO2 systems is due to the fact that the contents of other oxides do not exceed 0.5% and in total amount to 1.38%. The developed compositions under consideration are located within the SiO2 – 3Al2O3∙2SiO2– К2О∙Al2O3∙6SiO2 triangle.
The amount of primary melt formed in the masses in the Na
2O-Al
2O
3-SiO
2 system is 45% (1050
oC), and in the K
2O-Al
2O
3-SiO
2 system – 61% (985
oС). According to the equilibrium melting curves of the above systems, at a firing temperature of 1270–1350 °C, 79–81% of the melt is formed (
Figure 1). The actual amount of melt during firing of the studied masses does not correspond to the amount of melt calculated from the equilibrium melting curves. Ternary eutectics are not formed in systems at temperatures of 985 – 1050 °C. This is due to the fact that mullite is not synthesized below a temperature of 1200 °C.
Insignificant shrinkage (5–6%) in porcelain masses up to a temperature of 1000 °C is associated with solid-phase sintering and the appearance of a primary melt in small quantities due to binary eutectics. The equilibrium amount of eutectic melt in the K
2O-SiO
2 system at a temperature of 767
oC is 6.55%, the amount of eutectic melt in the equilibrium state in the Na
2O-SiO
2 system at a temperature of 793
oC is 4.07 % [
9,
10,
11,
12]. The total amount of melt in the equilibrium state at a temperature of 793 °C is 10.62% (
Table 1).
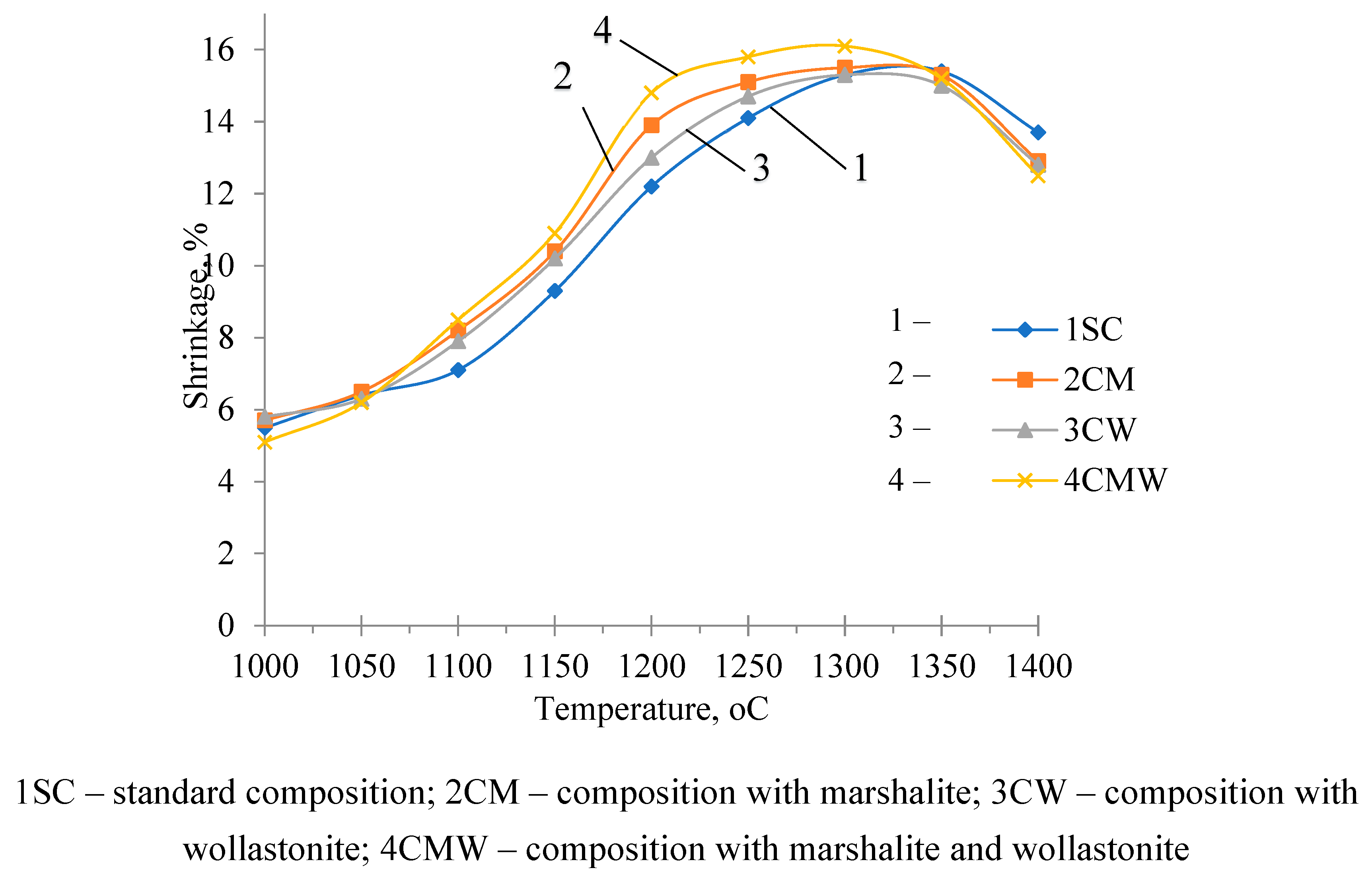
On the shrinkage curves, intensive sintering is recorded from a temperature of 1070
oC, shrinkage at this temperature increases from 5 – 6% to maximum values (14 – 16%) at firing temperatures of 1250 – 1300
oC (
Figure 2).
When the studied mass is fired in the solid phase, the decomposition of clay minerals occurs, mainly kaolinite, which makes up 42,5 %. Its decomposition occurs at 42 570-580
oС, and at a temperature of 975-980
oC, a spinel phase is formed according to the reaction scheme [
9,
12]:
The oxide system in this case does not reach equilibrium due to the fact that the mullitization process takes place at temperatures above 1200 °C. Interaction with components and dissolution of quartz is expanded in time and temperature. for activation of phase formation with the participation of silica, quartz sand in the masses was completely replaced by marshalite. Marshallite is a natural highly dispersed silica raw material, where the proportion of particles smaller than 0.01 mm is 80-85%, while the average particle size of quartz sand in the mass, even after grinding, is 25-30 microns.
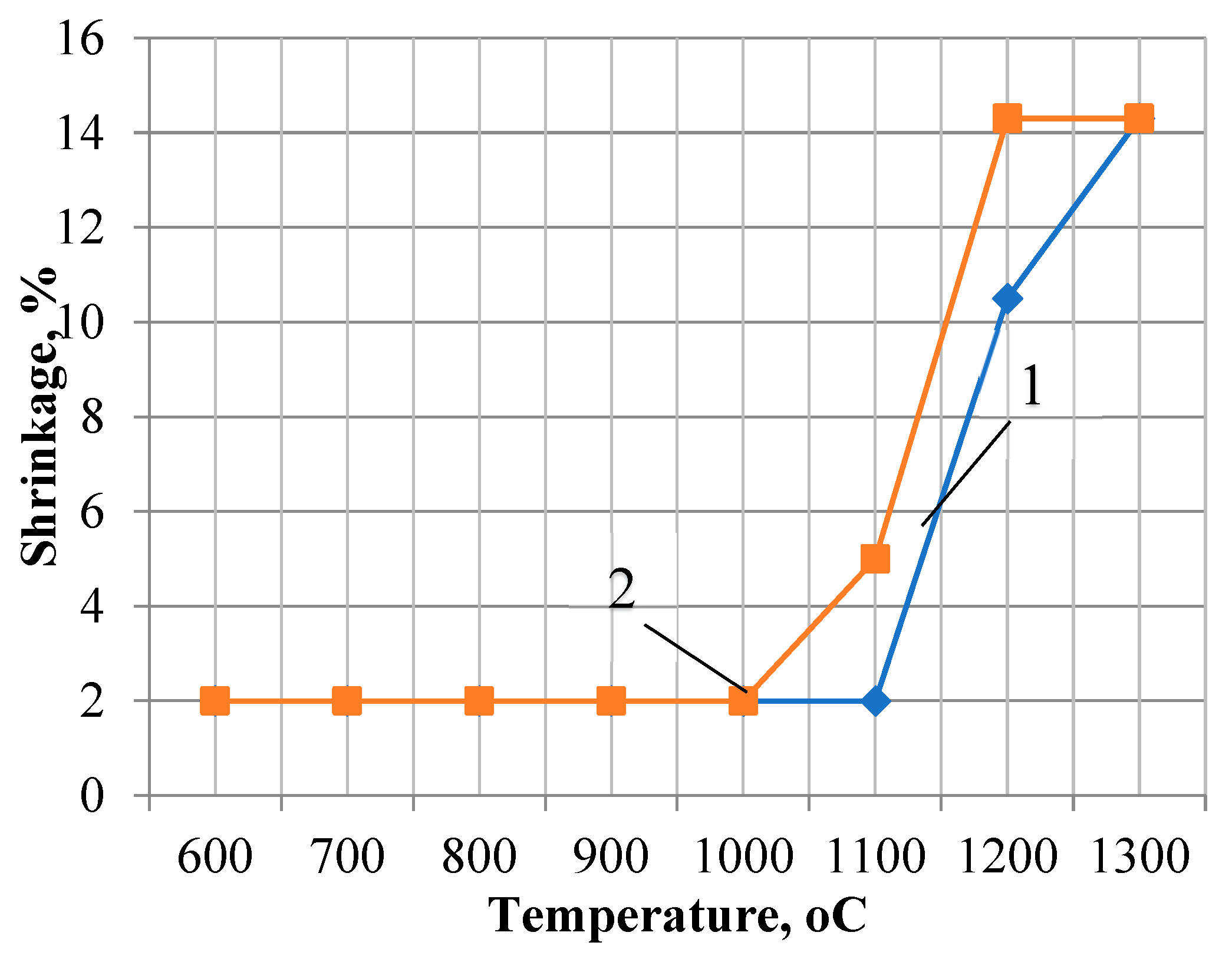
The influence of the dispersion factor on the sintering processes, accompanied by active mineral formation, is demonstrated in the shrinkage and water absorption curves of the «feldspar-quartz sand» and «feldspar-marshallite» compositions we studied [
8,
9,
12]. The preparation of the investigation samples was carry out using the same technology as the studied porcelain masses. The burning of these samples was carried out at temperatures of 600-1300 °C every 100
oС. The shrinkage curves of the samples of the studied compositions are presented in
Figure 3. In the temperature range of 600-1000 °C, changes in linear dimensions are insignificant, which is characterized on the graph by a straight line corresponding to 2% shrinkage. An increase in the shrinkage value for compositions with marshalite is observed starting from a firing temperature of 1000
oС. The maximum value (14.3%) of shrinkage is reached at a firing temperature of 1200
oC, and this value remains unchanged at a temperature of 1300
oС [
9,
10,
11].
The shrinkage of samples of compositions with quartz sand up to a temperature of 1100
oC does not exceed 2%, a further increase in temperature is accompanied by an increase in shrinkage values up to 14% at a temperature of 1300
oС. Analysis of the shrinkage curves of the studied compositions showed that sintering of the compositions with marshalite begins at a temperature 1100
oС, which is almost 100 °C lower than the sintering temperature of the quartz-feldspar composition [
9,
10,
11,
12].
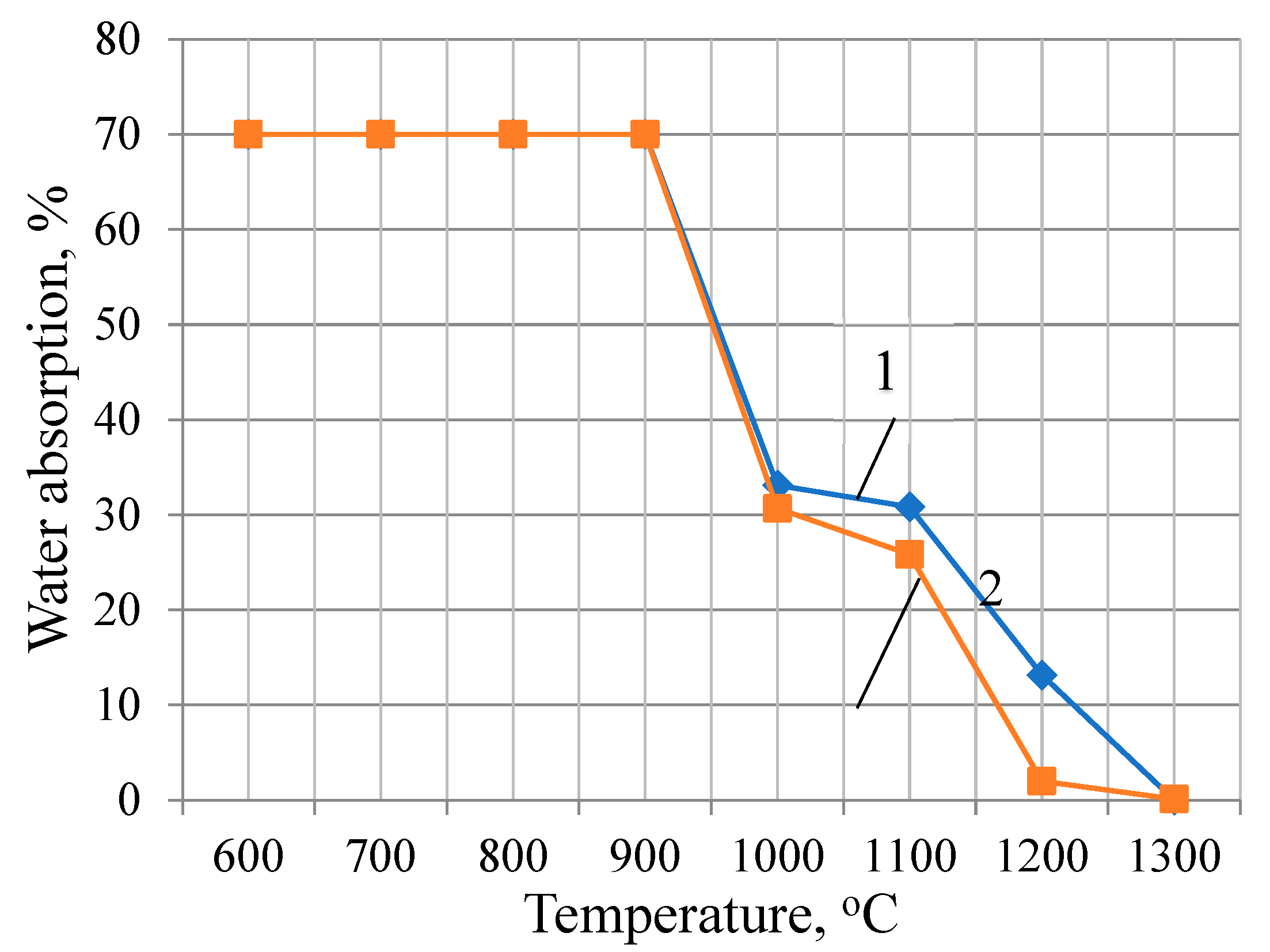
The determination of water absorption of samples from the studied compositions becomes possible starting from a firing temperature of 1000 oC, when they begin to acquire monolithicity.
Analysis of the dynamics of water absorption of the samples of the studied compositions showed that the values of this indicator of the feldspar-quartz sand composition decrease uniformly, starting from a temperature of 1100 °C, then reach zero at a temperature of 1300 °C (
Figure 4). The water absorption of the feldspar-marshallite composition approaches zero (2.0%) at a temperature of 1200°C, while that of the feldspar-quartz sand composition at this temperature is equal to 13,2 %. The obtained results show that finely dispersed marshalite actively interacts with feldspar, starting already from 1000
oС [
9,
10,
11,
12].
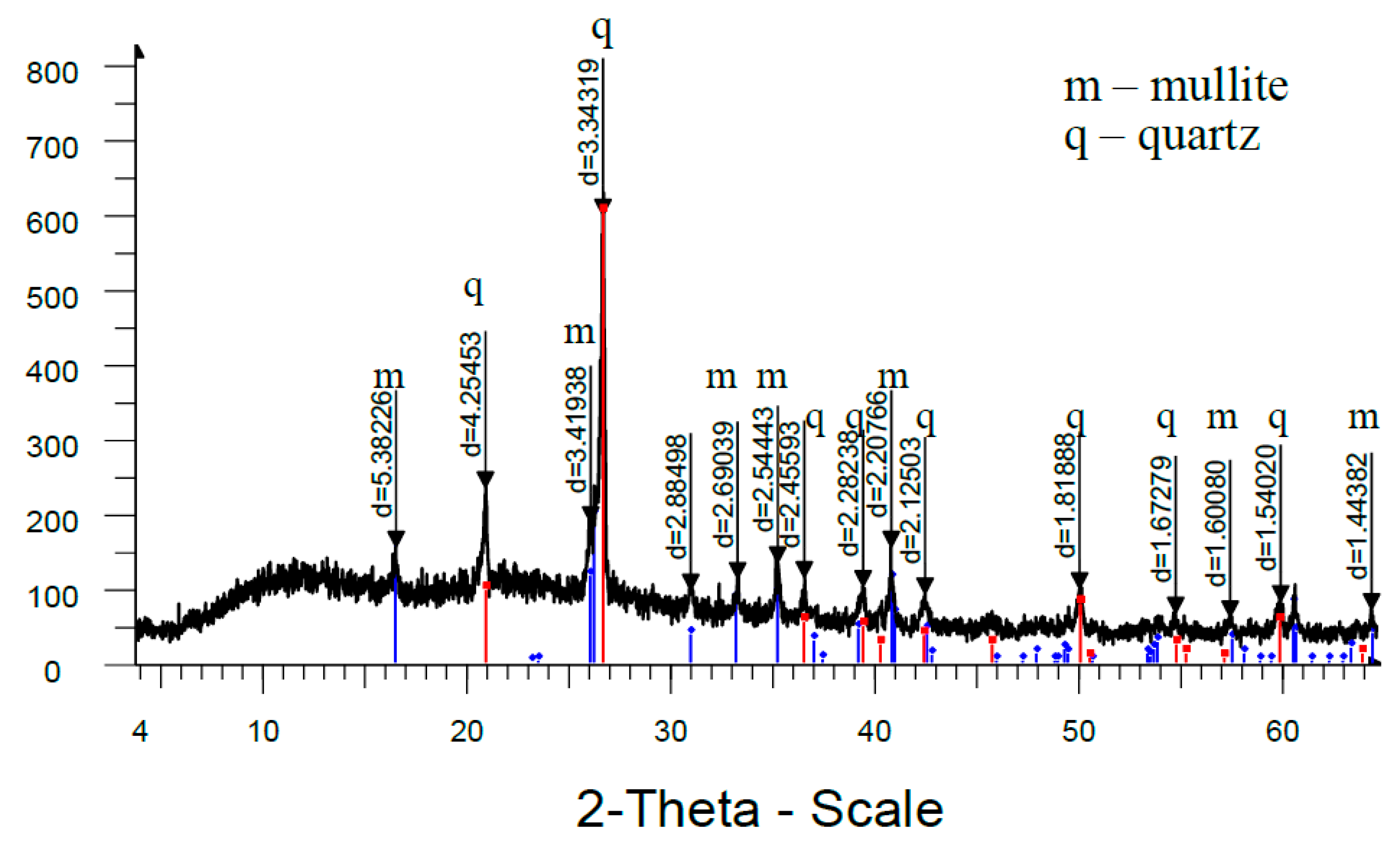
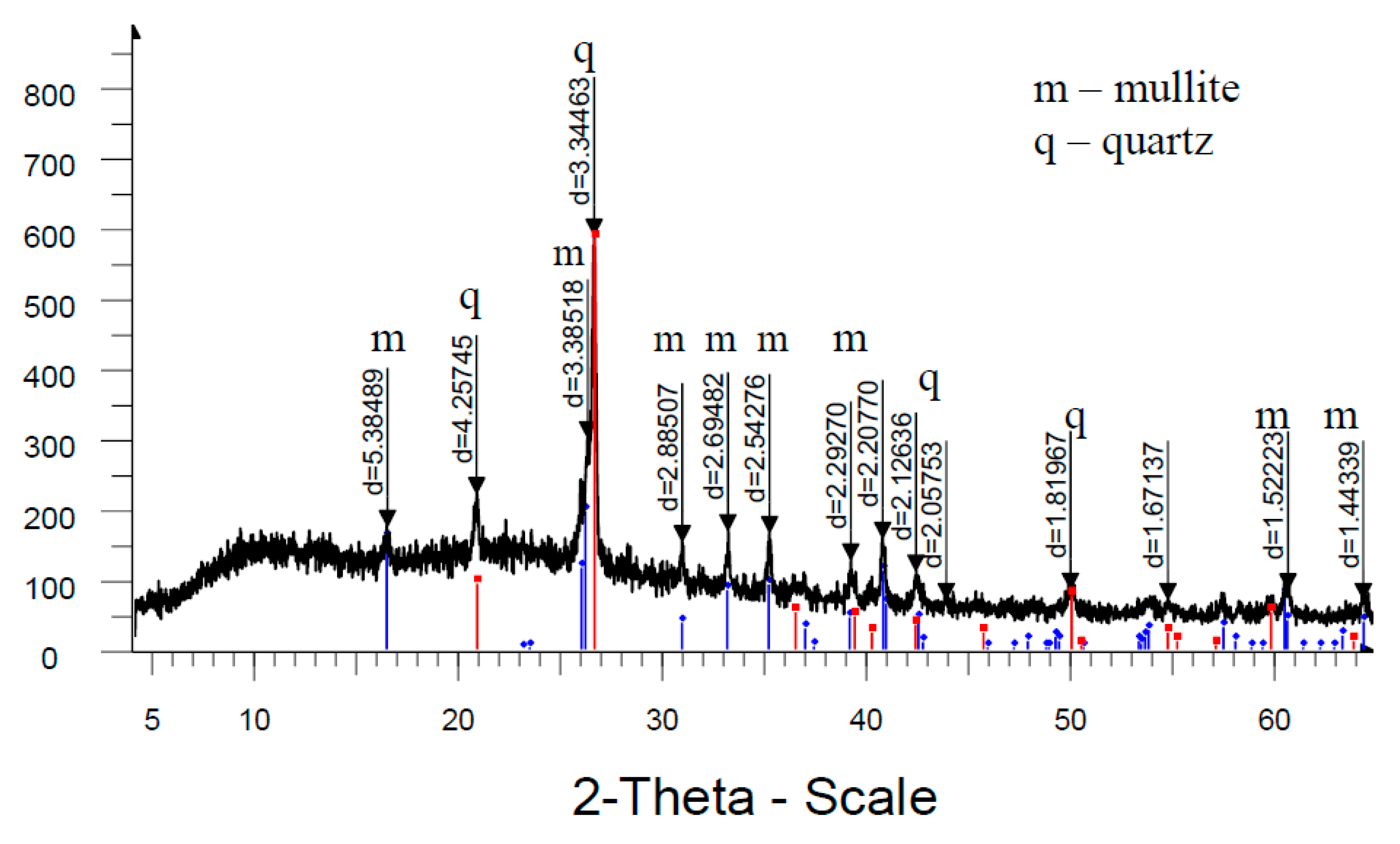
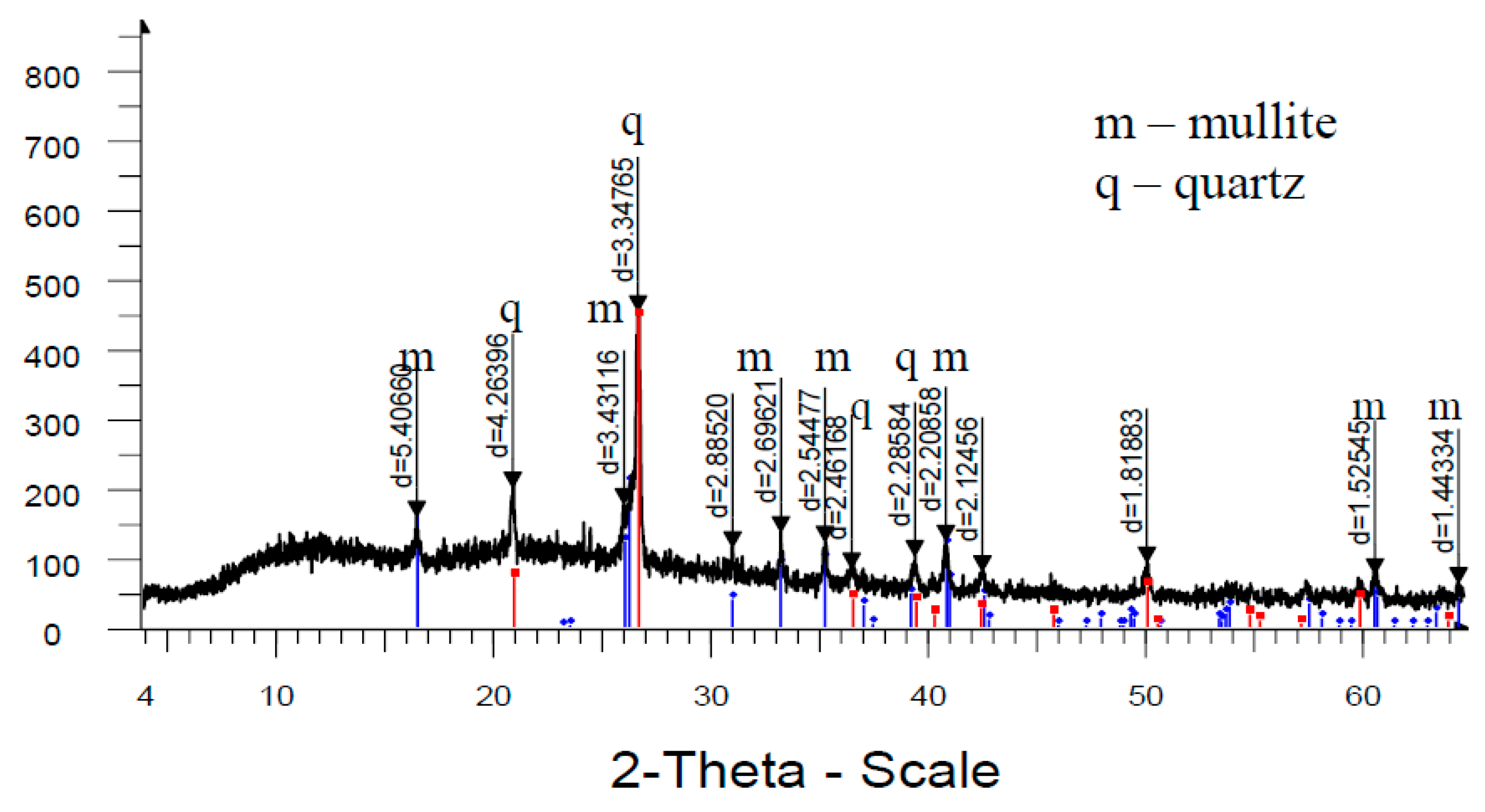
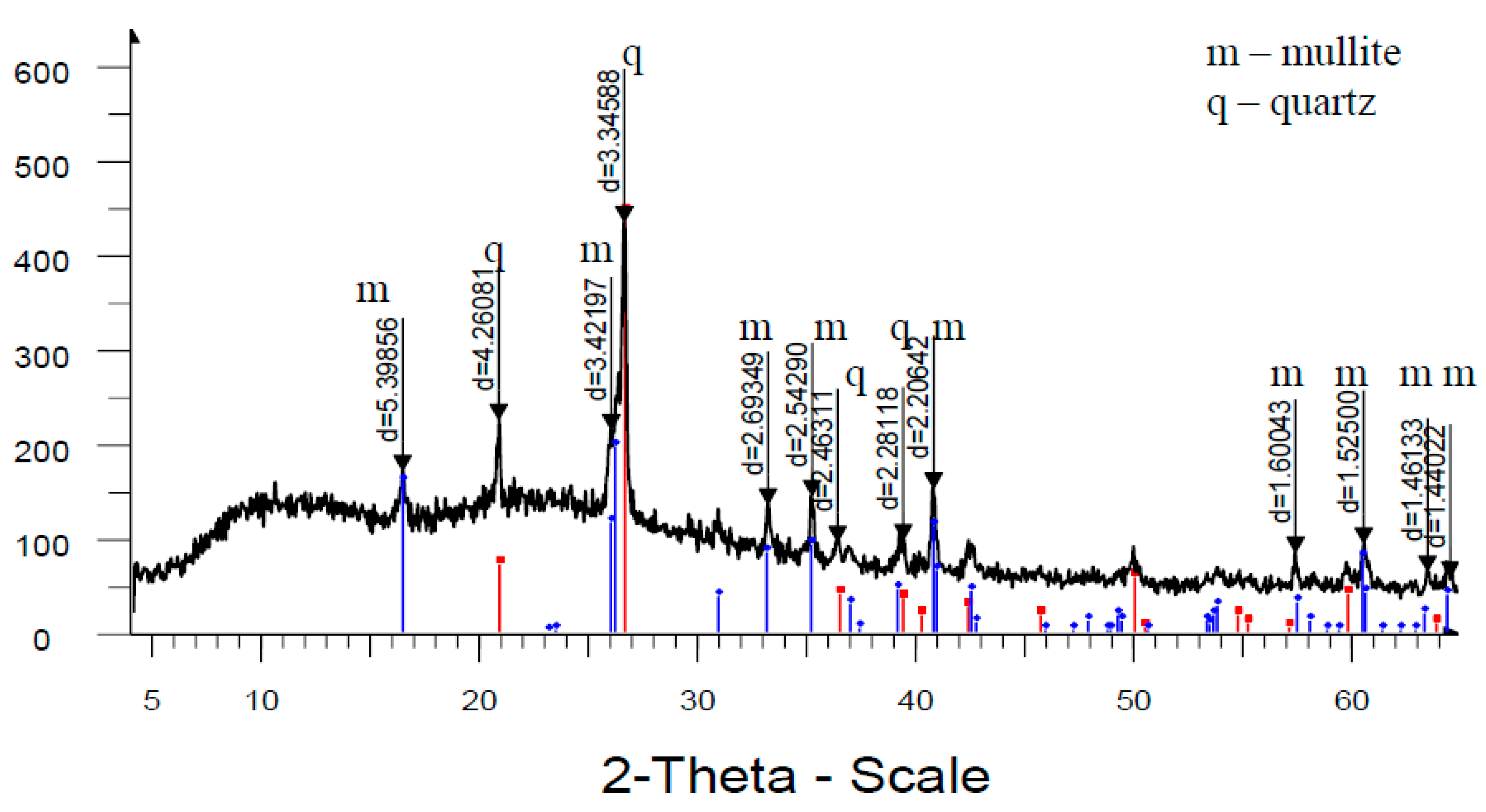
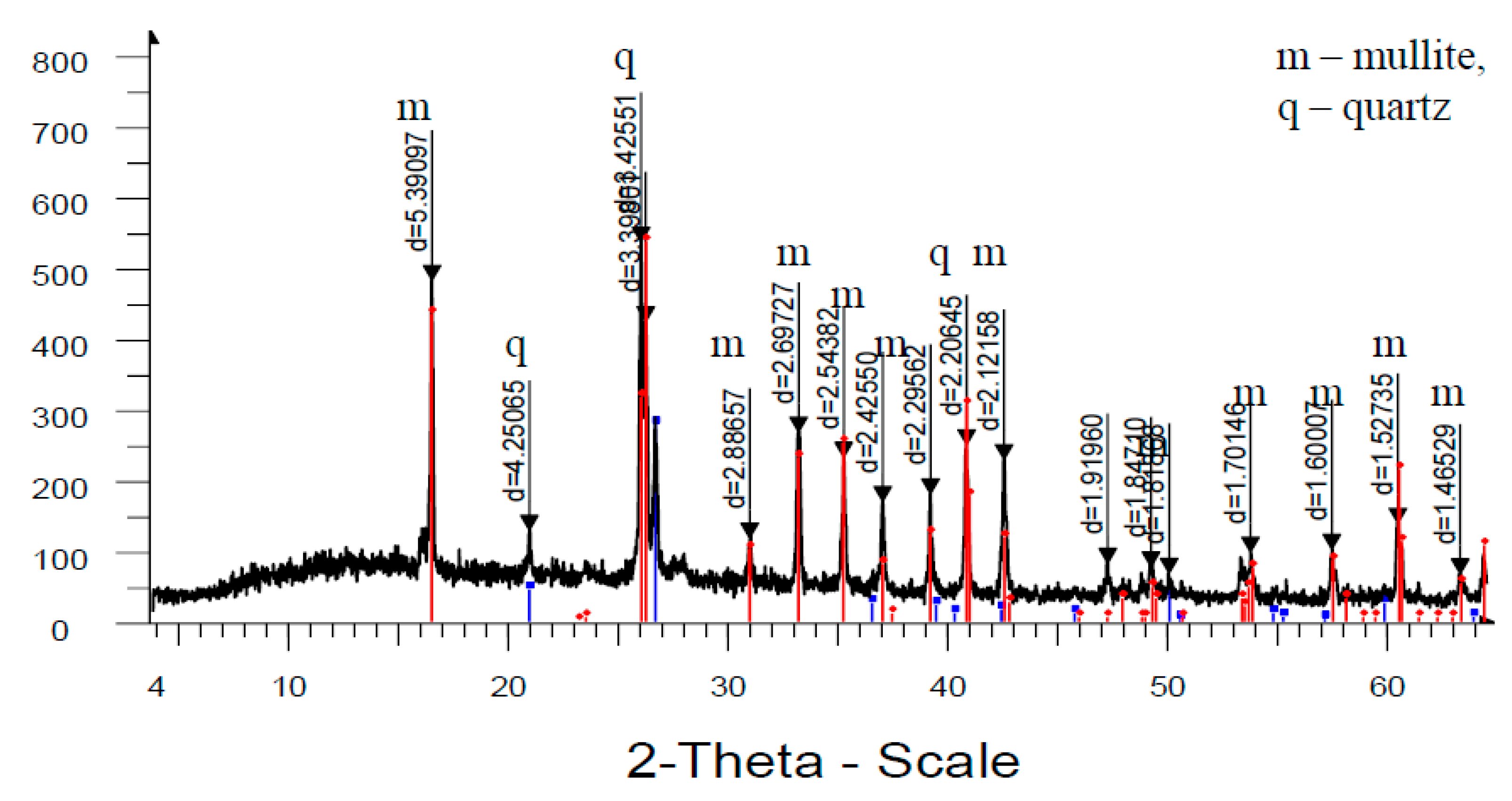
Analysis of high-temperature phase transformations in the developed mass compositions according to X-ray diffraction patterns (
Figure 5,
Figure 6,
Figure 7 and
Figure 8) shows that the diffraction maximum of quartz in the mass with marshalite is less than the similar indicator of the mass with quartz sand. This is due to the fact that the amount of melt formed with the participation of finely dispersed silica in the mass with marshalite increases.
The increase of the glass phase content in the mass with marshalite is also confirmed by the presence of diffraction scattering in the corresponding X-ray patterns. The appearance of the largest amount of glass phase is recorded in the mass with marshalite and wollastonite.
For clarity of the comparative assessment,
Figure 9 shows an X-ray pattern of reference synthetic mullite. The X-ray diffraction pattern shows slight diffraction scattering, indicating a negligible presence of glass phase. According to the data of the factory laboratory, in this sample the proportion of mullite in the crystalline phase reaches more than 92%, and quartz more than 7%.
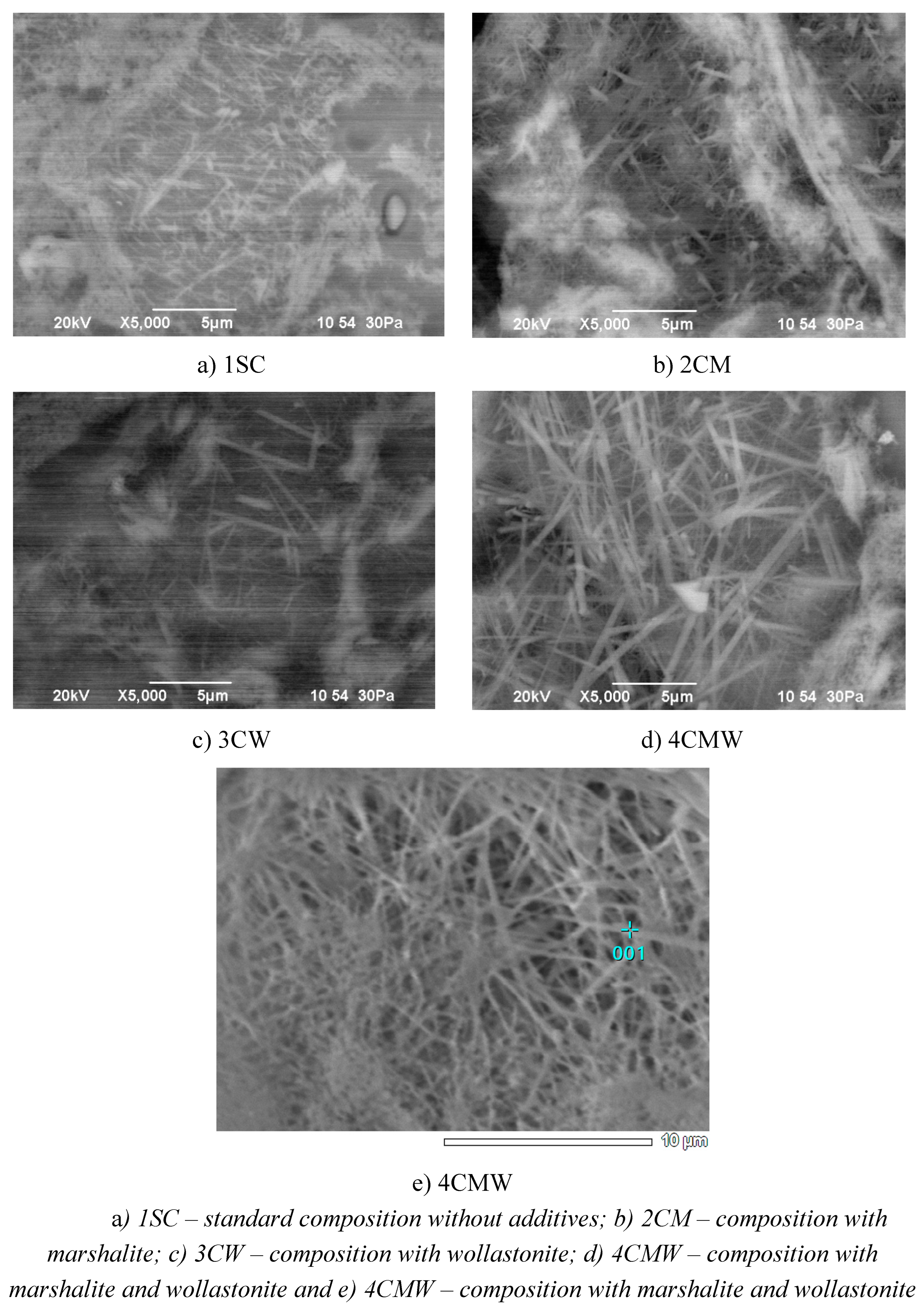
Using the capabilities of scanning electron microscopy, it was possible to obtain additional results on the phase composition, the nature of crystalline neoplasms and the structure of synthesized materials based on the compositions 1SС, 2СМ, 3СW and 4СМW, subjected to firing at the found optimal temperatures (
Figure 10).
Figure 10a shows one of the electron microscopic images of a chip of porcelain from a standard mass – 1SC. The sample has a fairly dense structure, its general appearance is represented by clearly distinguishable relics of feldspar from glass and mullite, grains of residual quartz, surrounded by halos of high-silica glass. Traces of the crystallization process of the feldspar-silica melt are clearly visible, which allowed the growth of mullite aggregates, a mineral that is not typical for the earth's crust for genetic reasons [
9,
10,
11].
There are areas penetrated by fairly large well-developed columnar crystals and areas in which there are dense interlacing of clearly distinguishable mullite needles. The sizes of these crystals of new formations of aluminum silicate are mostly 2 - 3 microns.
In the pictures of the 2CM sample chip (
Figure 10b) show a noticeable decrease in the size and amount of residual quartz, which confirms the increased reactivity of finely dispersed marshalite. Wares obtained on the basis of 2CM composition have a more uniform and dense structure. Their main and constant part, in which the crystalline phases are dispersed, is a transparent base, i.e. structurally glassy phase of the 2CM composition is a system intergrown with submicroscopic and uniformly dispersed mullite crystals [
9,
10,
11].
The microstructure of the samples obtained on the basis 3CW composition (
Figure 10c) is a complex heterogeneous system consisting of crystalline, glassy and gas phases. Glassy-mullitic regions are distinguished within the original feldspar particles and almost undecomposed particles of clay minerals. The noted mullite formations due to feldspar are completely different in size and growth pattern. Large mullite needles grow from the surface inward as the composition changes due to alkali diffusion in feldspar relics. The presence of quartz grains with surrounding shell structure of glass mass is characteristic. The quartz surface is usually corroded by the feldspar melt. Mullitization extends right up to the outer edge of the glass phase zone.
The study of electron microscopic materials of samples of the 4CMW composition (
Figure 10 g, d) showed that the structure of the developed samples containing marshalite and wollastonite is characterized by a denser structure and is characterized by the highest degree of mullitization. There are practically no pores in the products. The number and overall dimensions of the needle-shaped mullite crystals in the products, compared to other compositions, have noticeably increased due to the complex initiating effect of marshalite and wollastonite on the course of mineral formation [
9,
10,
11,
12].
Experiments show that in this ceramics, replacing traditional quartz sand with marshalite helps to reduce the temperature of the liquid phase appearance, and wollastonite has a positive effect on the structure and properties of the binary eutectic melt. At the same time, diffusion processes involving the melt are activated, and its dissolving capacity increases. As a result, the amount of dissolved primary mullite increases and it is transformed into a crystalline phase in the form of secondary mullite, which is mostly represented in feldspar areas in the form of shagreen and thick felted fabric. There are areas with densely intertwined and intergrow mullite needles that form a three-dimensional framework (
Figure 10d). All this ensures an increase in thermal resistance, physical, mechanical and electrical strength of porcelain. Porcelain samples of composition 4SMW have a good degree of maturity [
9,
10,
11].
The achieved transformations in the composition and structure of the developed ceramic materials had a positive effect on all the properties included in the standards (
Table 2).
It should be noted that the firing temperature for the developed compositions has been reduced to a useful level, it is in the range of 1270-1300 °C, while for the standard sample it is 1340 °C.
Water absorption, shrinkage and density for all developed samples are normal and stable.
The electrical strength of products made from composition 1SC meets the requirements for ceramic electrical materials of group 100, and the highest indicator in this regard is for 4SMW composition. This is the result of the complex effect of marshalite and wollastonite. The electrical strength of products made from composition 1SC meets the requirements for ceramic electrical materials of group 100, and the highest indicator in this regard is for 4SMW composition. This is the result of the complex effect of marshalite and wollastonite. Replacing quartz sand in the mass with marshalite, which easily forms eutectics with orthoclase and albite, helps to reduce the melt appearance temperature. Entering the ceramic masses from the wollastonite structure Ca
2+ ions (1.04 Å) activate the crystallization ability of mullite from this eutectic melt and replace Na
+ (0.98 Å) and K
+ (1.33 Å) ions. As a result, electrical strength increases. An increase in the number and length of mullite crystals due to the action of wollastonite also has a positive effect on the mechanical strength of porcelain. The bending strength of samples made of masses with marshalite and wollastonite is the highest and amounts to 81.7 MPa [
9,
10,
11,
12].
Thermal stability of the samples of the studied porcelain masses was determined by performing successive cycles - heating in an electric furnace with a 30-minute soak at a given temperature and abrupt cooling in water for 15 minutes. The results of determining the thermal stability of the samples showed full compliance of their values with the requirements of State Standards. The highest thermal stability indicators were found in samples of 2CM and 4CMW compositions.
Thus, the introduction of original natural infusible wollastonite and marshalite into ceramic masses, justified in crystal chemistry, very effectively affects the processes of mineral formation and the formation of the structure of ceramic stone.