1. Introduction
Rare earth resources are widely used in many technology fields, become essential key components in high-tech products, equipment and technology, including clean energy such as solar and wind energy, national security and defense science and technology [
1,
2]. As a crucial strategic rare metal resource, rare earth elements are important metal resource in the development of science, technology and industry, especially in the electric vehicles and renewable energy field [
3,
4,
5,
6]. For carmakers in Europe and the US, the growing production of electric vehicles is dependent on rare-earth permanent magnets made from Pr and Nd, as a raw material. Besides, Pr and Nd materials are widely used in a series of new energy industrial chains such as hydrogen storage and permanent magnet materials. In particular, Pr and Nd materials have become essential for the wind power and magnetic materials industries [
7,
8]. The weathered crust elution-deposited rare earth ore enriches rare earth elements, which is the main sources of Pr and Nd. Pr and Nd are often adsorbed as hydrated or hydroxyl hydrated ions with clay minerals, which mainly include kaolinite, montmorillonite and illite in the weathered crust elution-deposited rare earth ore [
9,
10].
At present, scientists have done a lot of research work on the occurrence law and mineralization mechanism of rare earth elements on the surface of clay minerals. The mineralization process of ion-adsorbed rare earth minerals was revealed through the adsorption experiment of La on the surface of kaolinite [
11]. The Langmuir model was the best for the isothermal adsorption of heavy metal ions by humic acid, and the adsorption capacity increased with the increase of temperature was concluded by adsorption selectivity tests on the heavy metal mixture system containing La and Nd [
12]. The Langmuir model of adsorption of rare earth ions on clay minerals had the highest correlation was found by used surface adsorption of different types of rare earth ions on clay minerals [
13]. Through the adsorption and desorption process of rare earth ions on clay minerals, the adsorption and desorption characteristics of rare earth ions are not representative of actual carrier ion rare earth minerals. The adsorption of heavy rare earth by amino-phosphonic acid resin was the best when the buffer solution of HOAc-NaOAc with pH=5.0, its static saturation adsorption capacity at 298 K could reach 332 mg/g [
14]. The complete adsorption law of rare earth elements was studied by kaolinite at different pH and ionic strength, and made a systematic comparison of the enrichment law of rare earth elements on kaolinite [
15]. However, there were few reports on the enrichment rules and adsorption characteristics of Pr and Nd elements with the typical clay minerals, and the systematic analysis of Pr and Nd element was absent.
In the paper, kaolinite and halloysite were used as adsorbents, the adsorption behavior of kaolinite and halloysite on Pr and Nd elements were explored. Through static adsorption experiments, the effects of initial concentration, pH, and experimental temperature on the adsorption process of Pr and Nd by kaolinite minerals were systematically analyzed, and the adsorption model of kaolinite minerals on Pr and Nd was established. To reveal the mechanism of kaolinite adsorption of Pr and Nd based on quantum chemistry theory, which provides a theoretical basis for the green and efficient mining of Pr and Nd, and is expected to enrich the utilization of resource in permanent magnets.
2. Materials and Methods
2.1. Experimental Materials
The ore samples used in the experiment were collected from the weathered crust elution-deposited rare earth ore in Yunnan, China. The chemical composition of the ore samples was analyzed by X-ray fluorescence spectroscopy, as shown in
Table 1.
Table 1 showed that SiO
2 was the main chemical component in the ore, accounting for 66.23%, followed by Al
2O
3, accounting for 18.14%, while the content of rare earth oxide accounts for 0.12%. All chemical reagents were analytically pure.
2.2. Experimental Methods
2.2.1. Adsorption Experiment
In the isothermal adsorption experiments, different concentrations of Pr and Nd solutions were prepared, and 25 mL of each concentration was taken into a 50 mL centrifuge tube and repeated for four groups. 0.5 g kaolinite and halloysite were added to each of the above four solutions, mixed evenly, and then shaken for 12 h with a constant temperature oscillator. At the end of the reaction, the mixed group was separated by centrifugation, and the supernatant was taken to determine the element content. The solution pH was adjusted with 0.05 mol/L NaOH and 0.1 mol/L H2SO4.
In the exploration of isothermal adsorption experiments with temperature factors, the constant temperature was adjusted. The adsorption data were characterized by the adsorption amount
Qe, µmol/g, which means the mass of Pr or Nd adsorbed by per gram clay mineral. The equation is shown in Equation (1):
In the formula: C0, initial rare earth solution concentration, µmol/L. C, concentration of rare earth solution at adsorption equilibrium, µmol /L. V, solution volume, L. m, mass of clay minerals, g.
2.2.2. Isothermal Adsorption Model
The adsorption isotherms of Pr
3+ and Nd
3+ on kaolinite and halloysite were investigated by batch experiments, and the isothermal adsorption model was fitted to the experimental data. In this paper, the classical Langmuir model and Freundlich model were selected for fitting to reflect the adsorption mechanism. The Langmuir model describes the monolayer adsorption on A uniform surface, and the linear expression is shown in (2):
In the formula: Ce, Equilibrium concentration of Pr3+ or Nd3+, µmol/L. Qe, adsorption capacity, µmol/g. K, Langmuir absorption constant. Qmax, maximum adsorption capacity, mg/g.
The Freundlich adsorption isothermal formula is an empirical formula summarized based on a large number of experimental data, which is suitable for physical adsorption of multi-molecular layers on non-uniform surfaces. The linear expression is shown in (3):
In the formula: KF, Freundlich absorption constant. n, constant.
3. Results
3.1. Clay Mineral Composition and Content
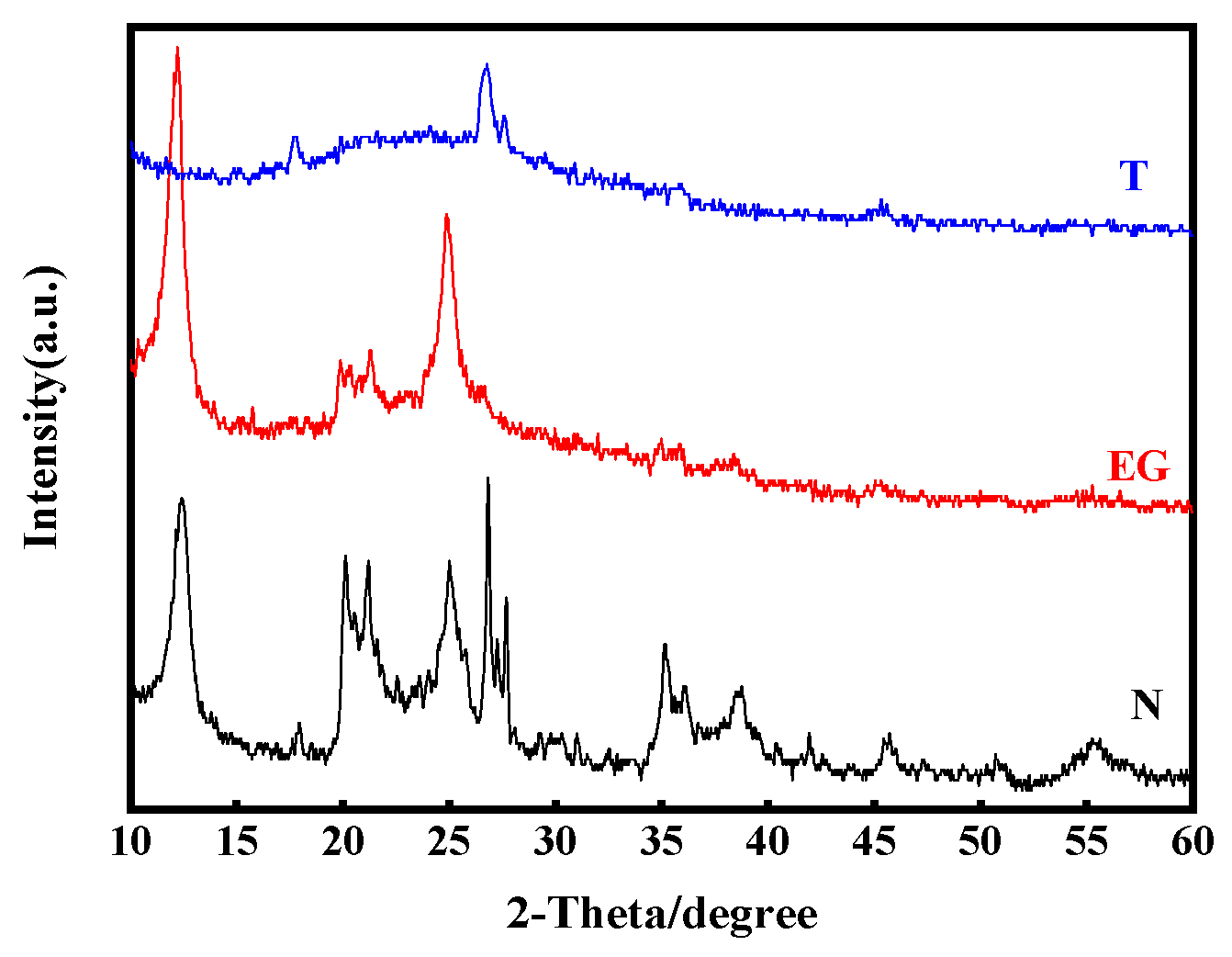
The clay mineral composition and content in the weathered crust elution-deposited rare earth ore is shown in the
Figure 1.
According to
Figure 1 and
Table 2, four clay minerals, kaolinite, halloysite, illite and montmorillonite, are mainly clay minerals in the ore, and the corresponding relative contents of these four clay minerals are 25.06%, 65.78%, 8.13% and 1.03%, respectively. It suggested that the clay minerals present in the rare earth formation are most abundant in halloysite, followed by kaolinite, with illite and montmorillonite being relatively less abundant.
3.2. Effects of Initial Solution Concentration on Adsorption Process
3.2.1. Effects of Kaolinite and Halloysite on Pr Adsorption Properties
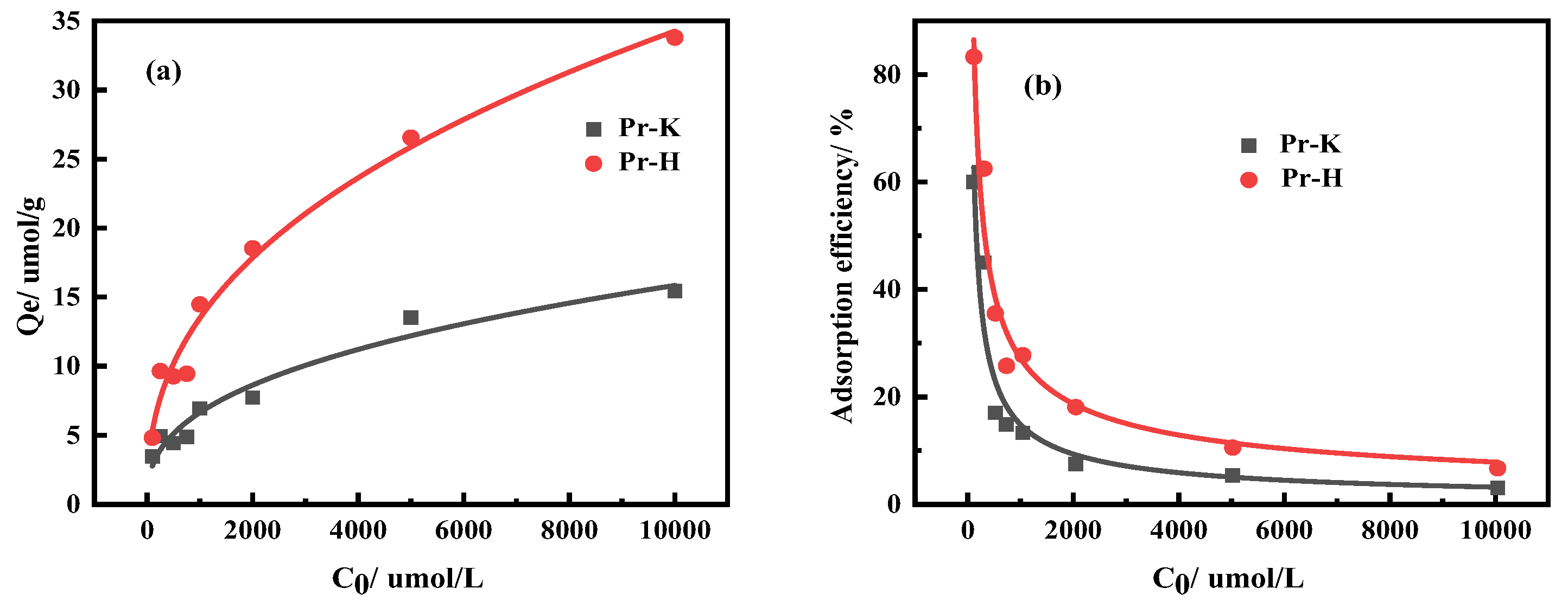
In order to research the effects of initial solution concentration on the adsorption process of Pr by kaolinite and halloysite, the content and efficiency of Pr adsorption by kaolinite and halloysite are shown in
Figure 2.
It can be seen from
Figure 2 that the adsorption capacity of kaolinite and halloysite for Pr increased with the enhancement in the rare earth solution concentration, while the adsorption efficiency for Pr decreased with the increase in the concentration of the solution. When the concentration of Pr
3+ was 10000 µmol/L, the adsorption capacity of kaolinite and halloysite was 15.45 µmol/g and 33.80 µmol/g, respectively. The adsorption efficiency was 6.73% and 3.08%, respectively. At this time, the adsorption process tended to be saturated, and the adsorption capacity was close to the maximum value. The experimental results showed that the adsorption capacity and adsorption efficiency of kaolinite to rare earth element Pr were lower than that of halloysite, which may be due to the fact that kaolinite has a silicon oxygen tetrahedral sheet and an aluminum oxygen octahedral sheet in the lattice structure, and the hydrogen bond between adjacent crystal layers prevents the expansion of kaolinite, which directly results in a small specific surface area of about 10-20 m
2/g, all on the outer surface. The possibility of isomorphous replacement occurring is very low and hence the cation exchange capacity is low [
16,
17]. However, halloysite showed excellent adsorption performance for rare earth element Pr due to its nano-hollow tubular morphology, abundant pore structure and external surface with tetrahedral siloxane groups [
18].
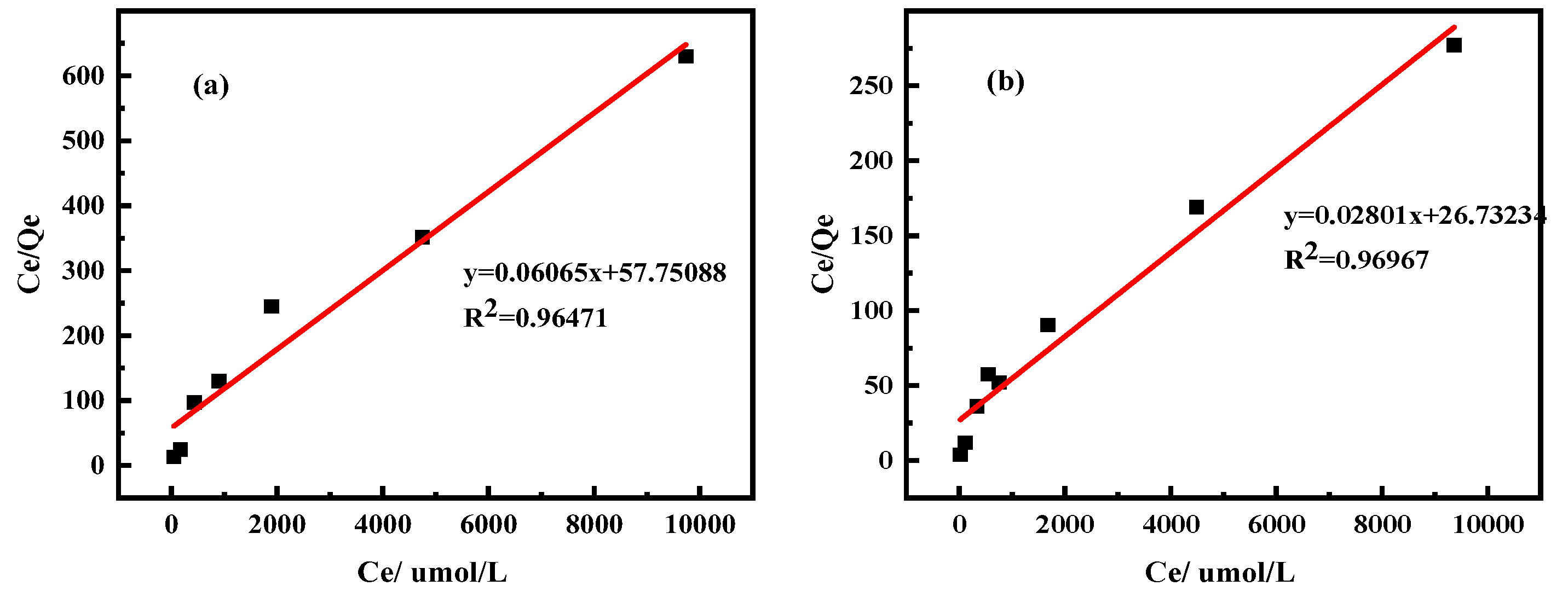
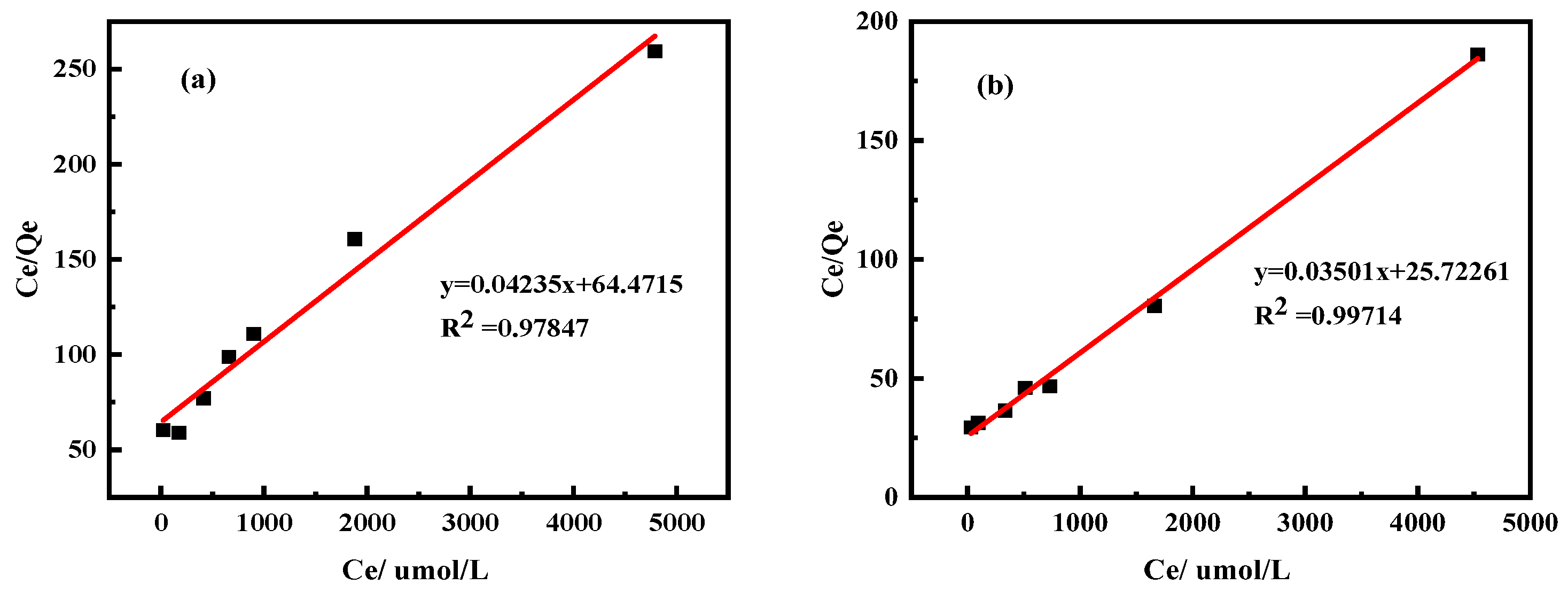
In order to further analyze the adsorption behaviors of Pr on kaolinite and halloysite, the isothermal adsorption model was fitted to the experimental data, and the fitting results are shown in
Figure 3, and the results of the relevant calculated parameters are listed in
Table 3.
As can be seen from
Table 3, the R
2 of the Langmuir model of kaolinite and halloysite adsorption Pr were 0.965 and 0.970, which were larger than the fitting parameters of the Freundlich model of 0.607 and 0.937. Therefore, the adsorption process of Pr
3+ by kaolinite and halloysite is more consistent with the Langmuir model, indicating that the adsorption process belongs to the monolayer adsorption, indicating that the monolayer adsorption is easier to adsorb the rare earth element Pr. The maximum adsorption capacity of kaolinite and halloysite for Pr was 16.488 µmol/g and 35.702 µmol/g calculated by Langmuir model fitting, which was consistent with the experimental data.
3.2.2. Effects of Kaolinite and Halloysite on Nd Adsorption Properties
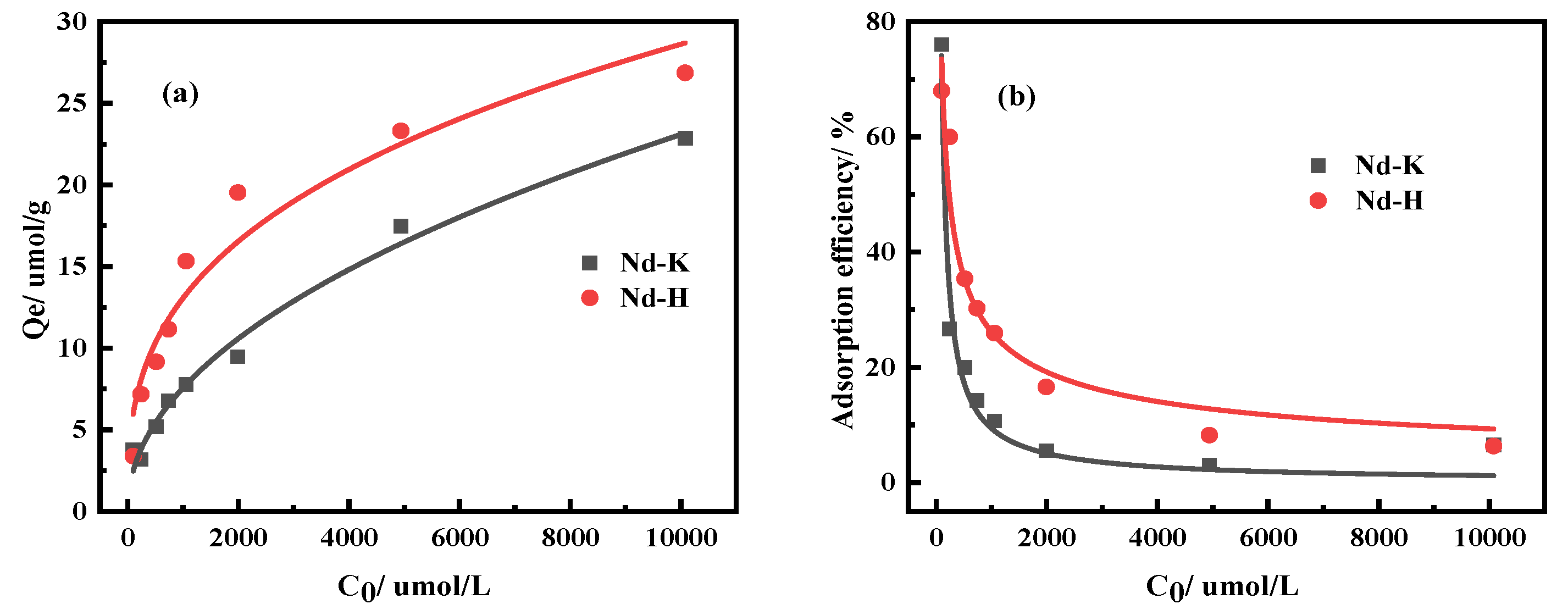
In order to study the effect of the solution concentration on the process of Nd adsorption of kaolinite and halloysite, the experiment was carried out and the experimental results are shown in
Figure 4.
According to the
Figure 4, with the rise of the concentration, the adsorption capacity of kaolinite and halloysite with Nd increased continuously, and the adsorption efficiency decreased gradually. In the
Figure 5, the adsorption capacity of Nd by kaolinite and halloysite reached 22.86 µmol/g and 26.86 µmol/g respectively when the concentration was 10000 µmol/L, the adsorption efficiencies were 6.52% and 6.32%, indicating that the adsorption process reached equilibrium, the adsorption capacity of Nd to kaolinite and halloysite also tends to be the maximum. According to two figures, the adsorption capacity and adsorption efficiency of halloysite and kaolinite to Nd were greater than kaolinite, indicating that halloysite showed better adsorption performance than kaolinite on Nd.
In order to further analyze the adsorption behaviors of neodymium on kaolinite and halloysite, the isothermal adsorption model was fitted to the experimental data, the results are shown in
Figure 5, and the calculated results are listed in
Table 4.
From the data in the
Table 4, it can be seen that the linear correlation coefficients of Langmuir model fitting for Nd adsorption of kaolinite and halloysite were 0.978 and 0.997, which are higher than the fitting parameters of Freundlich model, which is more consistent with the Langmuir model, indicating that the adsorption process is a single layer. These results indicate that Nd is more easily adsorbed by monolayer adsorption. According to the fitting parameters of the Langmuir model, it can also be seen that the maximum adsorption capacity of kaolinite to Nd was about
Qmax=23.613 µmol/g, and the maximum adsorption capacity of halloysite to Nd was about
Qmax=28.571 µmol/g, which was consistent with the experimental data. The effects of adsorption process on the La and Nd recovery can further strengthen the resources application.
3.3. Effects of pH on Adsorption Efficiency/Adsorption Capacity
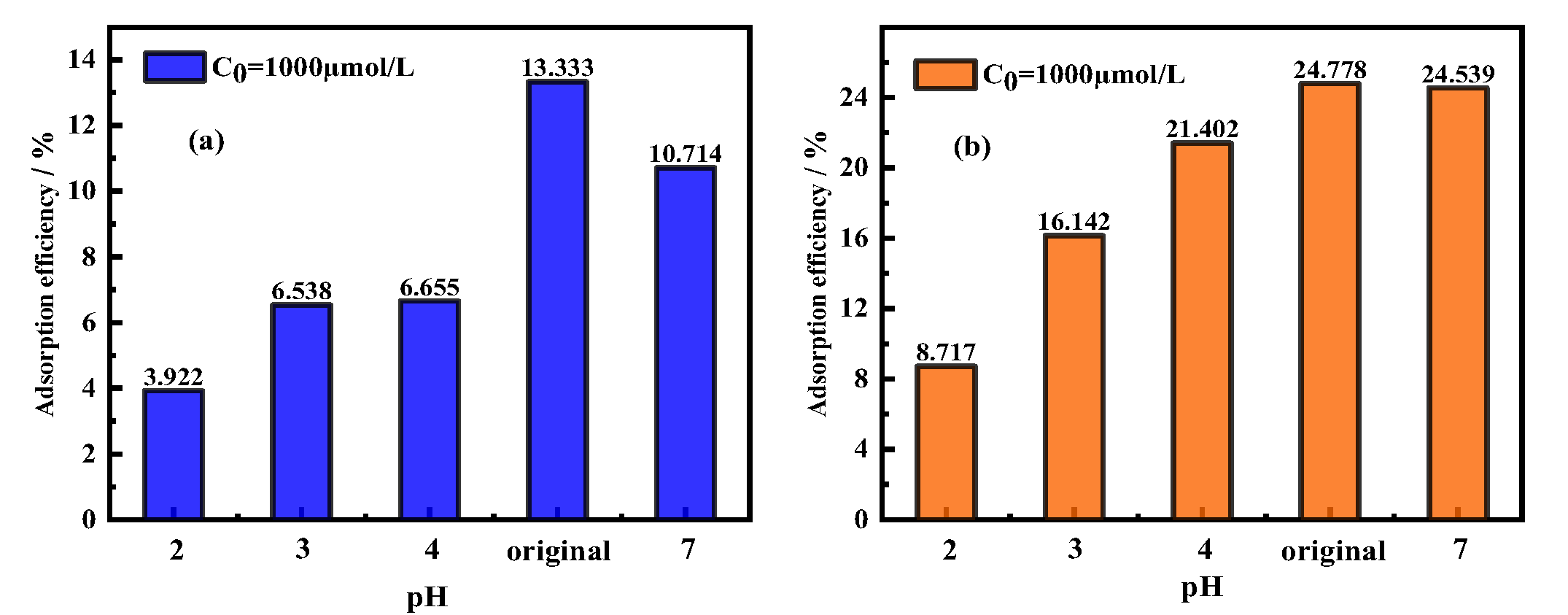
In order to analyze the effect of pH on the adsorption of rare earth element Pr by kaolinite and halloysite, static adsorption experiments were carried out under different pH conditions, and the experimental results are shown in
Figure 6.
As shown in
Figure 6, when the solution pH value ranged from 2 and 7, the adsorption efficiency of kaolinite and halloysite on Pr increased with the rise of pH, and the adsorption effect was better. When the pH at 7, the adsorption effects of kaolinite and halloysite on Pr become worse. This is because the variation of pH value will cause the change of surface charge of kaolinite and halloysite, and then lead the change of surface functional groups and structure. When the solution pH value was low, H
+ and Pr
3+ would compete for adsorption, and the adsorbent group would combine with H
+, so that the active site will be occupied and the adsorption rate of Pr
3+ will be reduced. The functional groups on the surface of kaolinite and halloysite might undergo degranulation reaction and form the co-precipitation of Pr [
19].
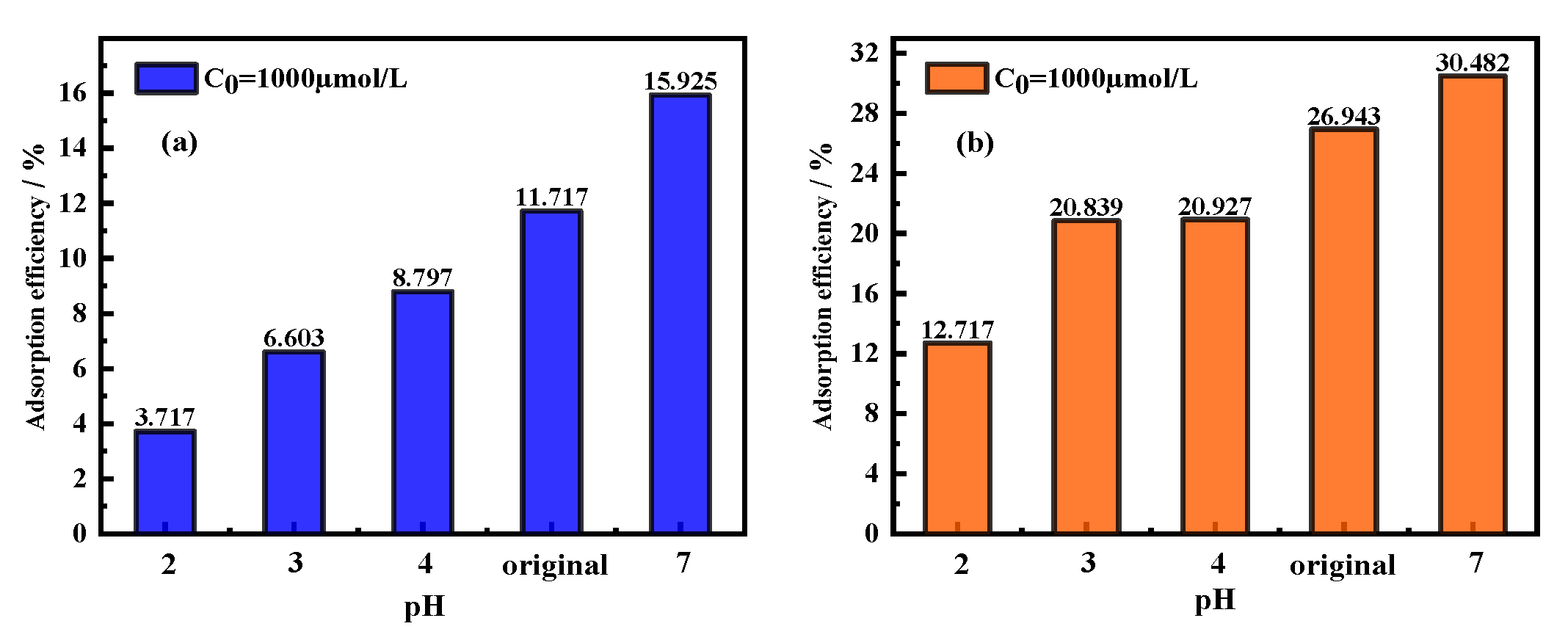
To explore the effects of pH on the adsorption of Nd by kaolinite and halloysite, static adsorption experiments were carried out by setting different pH, and the experimental results are shown in
Figure 7.
According to
Figure 7, when pH ranged from 4 and 7, the adsorption performance of kaolinite and halloysite on Nd was better with the increase of pH, which may be due to the increased adsorption effects of kaolinite and halloysite on Nd when the ionic strength was low and the pH increased without co-precipitation. The Nd was absorbed in the form of cation exchange on the surface of kaolinite and halloysite at low pH [
20]. Excessively high pH is not conducive to the existence of rare earth element ions and has a significant impact on the recovery of Pr and Nd. pH has a considerable influence on the adsorption of rare earth, and particular attention should be paid to the change of pH during the adsorption process for better recovery effect.
3.4. Effects of Temperature on Adsorption Efficiency/Adsorption Capacity
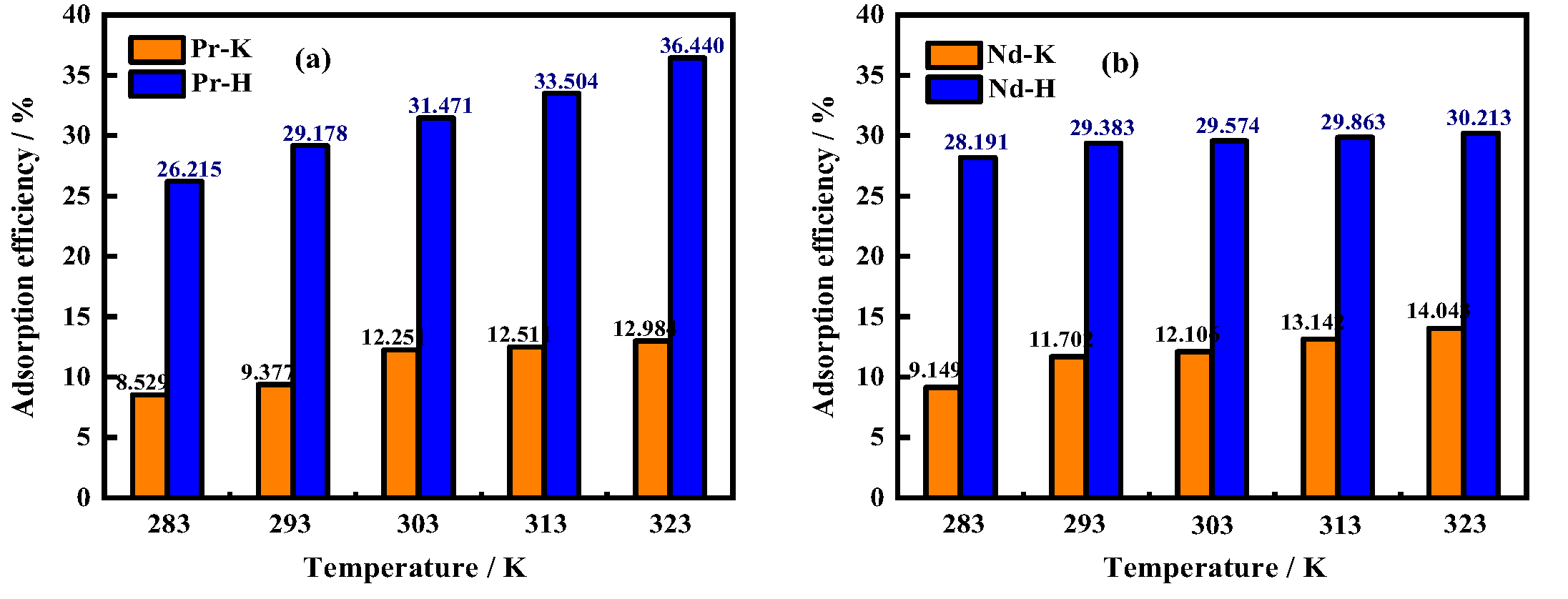
In order to study the effect of temperature on the adsorption process of Pr and Nd by kaolinite and halloysite, the static adsorption experiments of Pr and Nd elements were taken research at temperatures of 283 K, 293 K, 303 K, 313 K and 323 K, respectively, and the results are shown in
Figure 8.
As can be seen from
Figure 8, the adsorption efficiency of Pr and Nd by kaolinite and halloysite showed a gradual increase with the enhancement of the reaction temperature, indicating that the adsorption reaction is a heat-absorbing process, and the enhancement of the reaction temperature helps the adsorption of Pr
3+ and Nd
3+ by the clay minerals. However, the trend of increasing the equilibrium adsorption efficiencies of kaolinite and halloysite with rising temperature in the process was not obvious.
3.5. Adsorption Mechanism of Rare Earth Elements with Clay Minerals
3.5.1. SEM Analysis Results of Adsorption Process
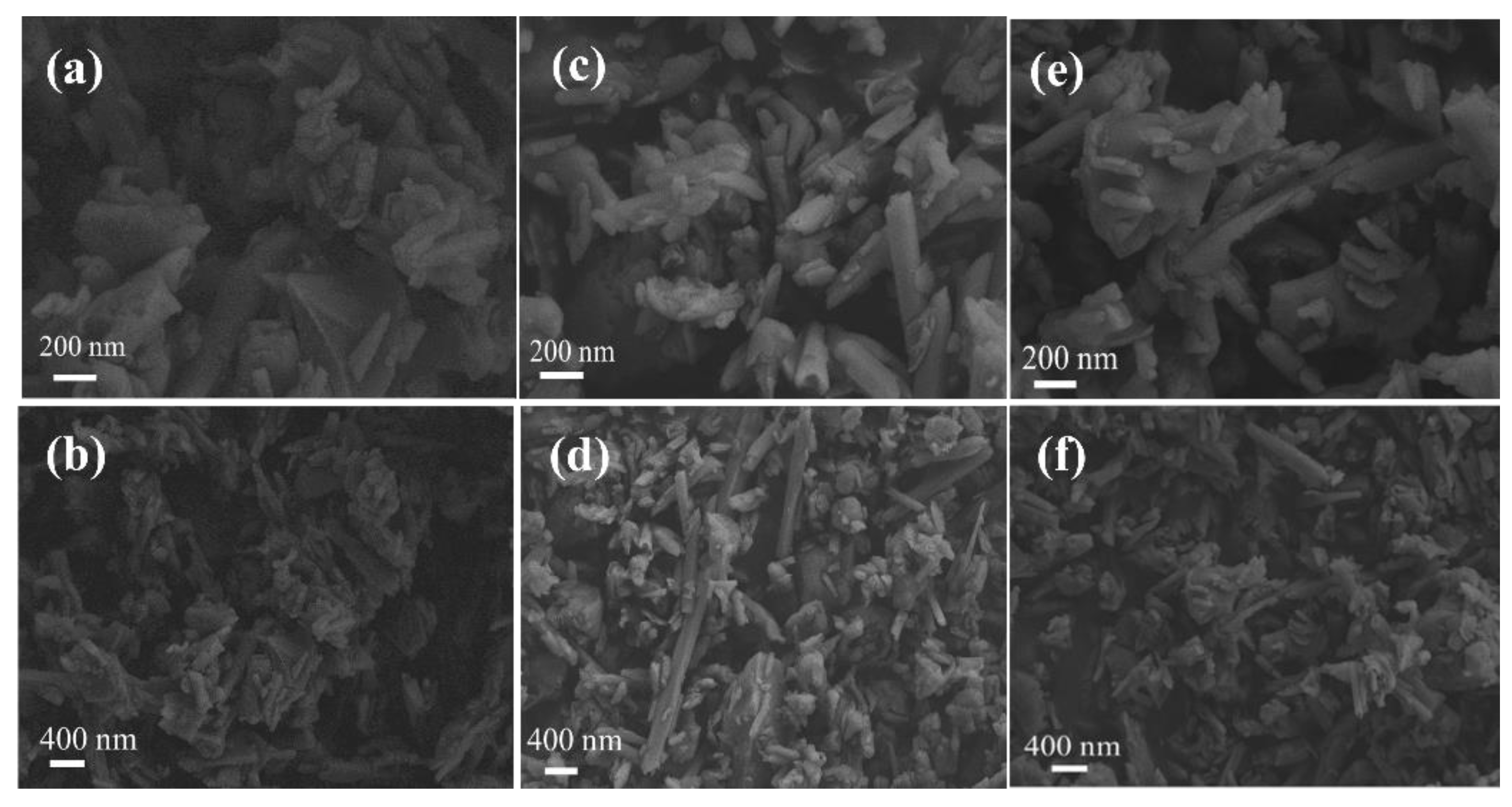
In order to explore the adsorption mechanism of Pr and Nd adsorbed by halloysite, the ore samples before and after the adsorption of Pr and Nd were analyzed by scanning electron microscopy, and the results are shown in
Figure 9.
Figure 9 showed that halloysite has a stacked tubular structure.
Figure 10(c)(d)(e)(f) shows that after Halloysite adsorbs Pr and Nd, except for the tubular halloysite, some granular substances can be clearly observed, which may be due to the fragmentation of halloysite nanotubes during the adsorption process, and the surface roughness of halloysite decreases after adsorption.
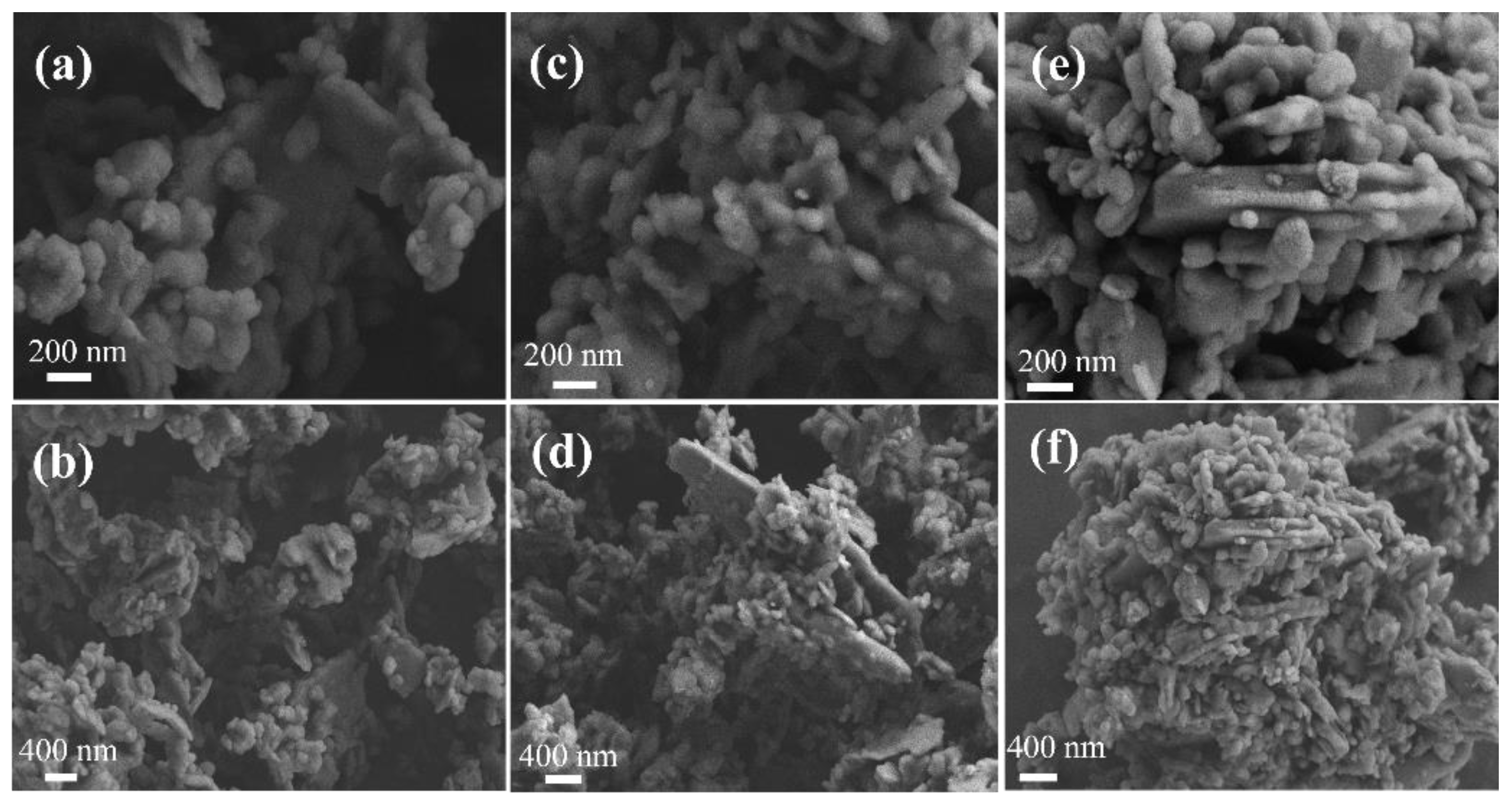
In order to explore the adsorption mechanism of rare earth elements Pr and Nd adsorbed by kaolinite, the ore samples before and after kaolinite adsorption of rare earth elements Pr and Nd were analyzed by scanning electron microscopy, and the results are shown in
Figure 10.
According to
Figure 10, kaolinite presents multi-layered stacking, and produces the phenomenon of cross-stacking of stacked bodies, and the overall appearance is a disorderly aggregation of particles of multiple layered stacked bodies.
Figure 10(c)(d)(e)(f) shows that after adsorption, the kaolinite stacks are denser, and more granular substances are gathered, which exist between the stacks, and the surface of the adsorbed kaolinite becomes rough.
3.5.2. Infrared Spectroscopy Analysis Results of Adsorption Process
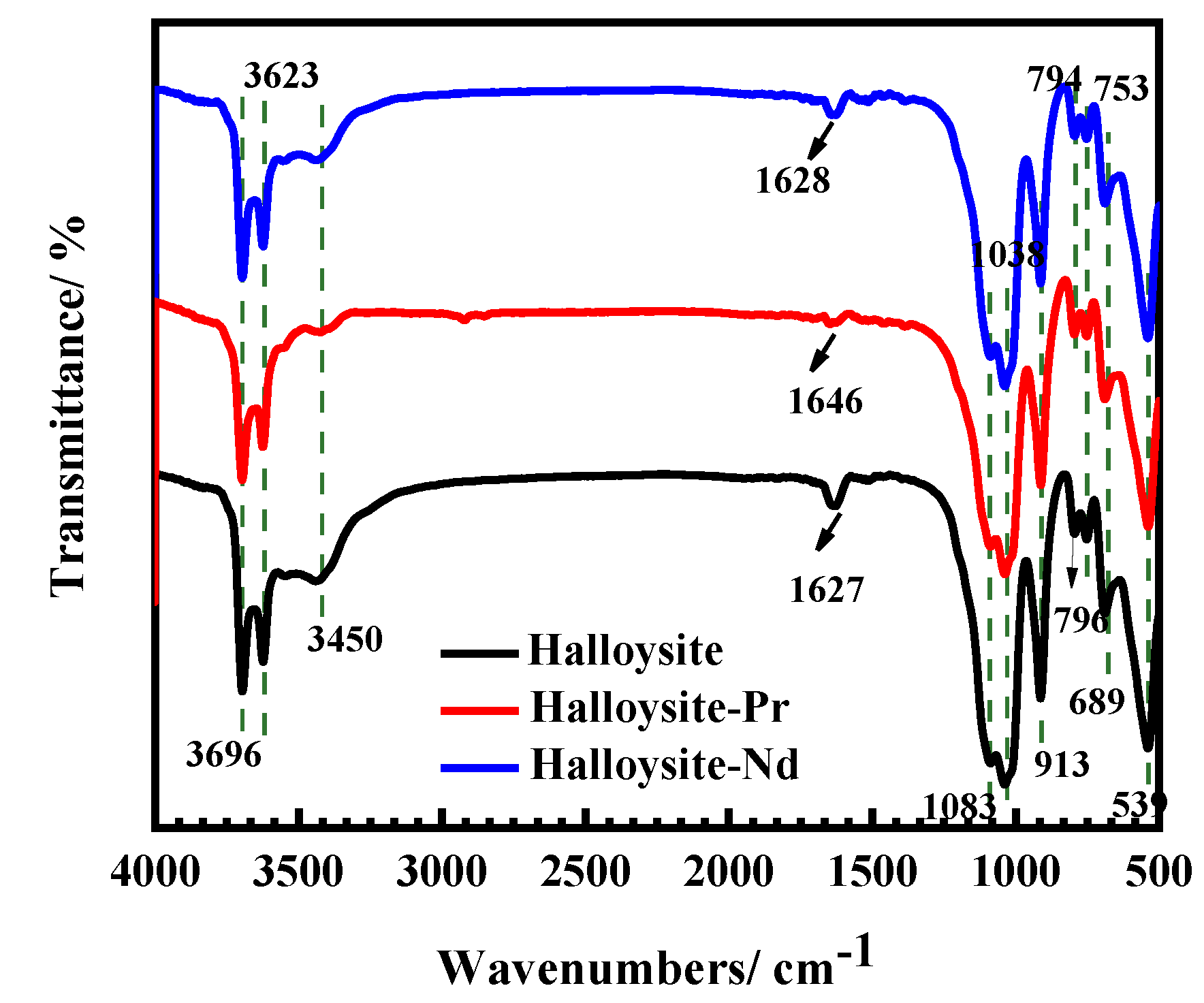
In order to explore the adsorption mechanism of rare earth elements Pr and Nd adsorbed by halloysite, the infrared spectra of adsorption samples of rare earth elements Pr and Nd were analyzed, and the results are shown in
Figure 11.
According to
Figure 11, the infrared absorption peaks with wave numbers of 3696 cm
-1 and 3623 cm
-1 correspond to the inner -OH and outer -OH stretching vibration peaks in the lamellar tube wall, respectively [
21]. The absorption peak at 3450 cm
-1 belonged to the stretching vibration of -OH, which is formed by the -OH on the surface of the halloysite nanotube through intramolecular hydrogen bonding. The absorption peak at the won-number 1628 cm
-1 was strengthened by the free water molecules contained in the halloysite nanotube [
22]. After the adsorption of rare earth element Pr, the absorption peak at 1628 cm
-1 was blue shifted to get the absorption peak at 1646 cm
-1 [
23], which indicated that there may be hydrogen bonding or surface complexation between Pr and halloysite. While Nd was redshifted from 1628 cm
-1 to 1627 cm
-1, it may be caused by hydrogen bonding between halloysite and Nd. The infrared absorption peak located at 1083 cm
-1 and 1038 cm
-1 belonged to the stretching vibration of Si-O, the absorption peak at 913 cm
-1 was attributed to the bending vibration of Al-OH, the vertical stretching peak of Si-O-Al and the bending vibration peak of Si-O at 753 cm
-1 and 539 cm
-1 were respectively. Both are characteristic absorption peaks of halloysite nanotubes [
24].
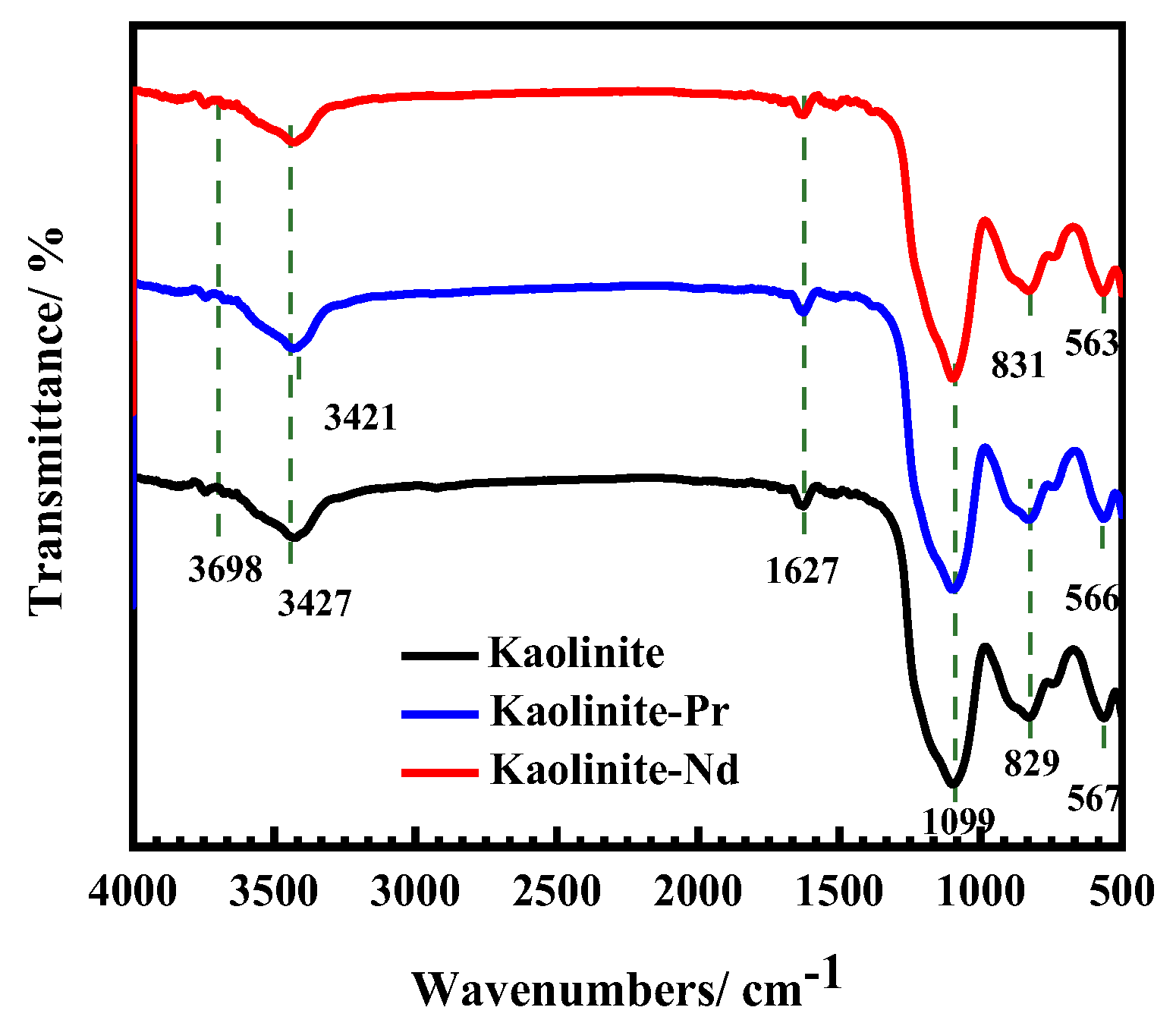
In order to explore the adsorption mechanism of rare earth elements Pr and Nd adsorbed by kaolinite, the ore samples before and after kaolinite adsorption of rare earth elements Pr and Nd were analyzed by scanning electron microscopy, and the results are shown in
Figure 12.
It can be observed from
Figure 12 that both 3698 cm
-1 and 3427 cm
-1 belonged to the hydroxyl group vibration on the inner surface of kaolinite [
25], while the stretching vibration peak at 3427 cm
-1 is redshifted to 3421 cm
-1 in the spectrum after the adsorption process, indicating that hydrogen bonding may occur between Pr and kaolinite. The stretching and bending vibration peaks at 1627 cm
-1 and 1099 cm
-1 did not change in the spectra, indicating that neither Pr nor Nd bonded with kaolinite. In the low frequency region, 831 cm
-1 and 563 cm
-1 were attributed to O-Al-OH vibration absorption. After the adsorption of Pr and Nd, the characteristic absorption peak at 831 cm
-1 was red shifted to 829 cm
-1, indicating that there may be hydrogen bonding between Pr and Nd with kaolinite. The characteristic absorption peak at 563 cm
-1 was blue shifted to 566 cm
-1 and 567 cm
-1, respectively, which further demonstrated that Pr and Nd were adsorbed between kaolinite layers and formed new hydrogen bonds.
3.5.3. Quantum Chemical Analysis Results of Adsorption Process
The adsorption process of rare earth elements Pr and Nd on clay minerals is affected by many factors, and the adsorption energy of different rare earth elements on the surface of the same clay minerals is different. In order to better explain the adsorption mechanism of kaolinite and halloysite on Pr and Nd, the strength of the binding effect of rare earth elements on the surface of clay minerals can be judged by adsorption energy. The adsorption energy of rare earth elements on the surface of clay minerals is calculated as shown in Equation (4):
where
EA/surf,
Esurf and
EA(g) are the energy of atom A adsorbed on the surface, the energy of cleaning the surface, the energy of an isolated A atom in A cubic periodic box of side length 20 A, and the 1×1×1 Monkhorst-Pack k point grid used for sampling the Briouin region, respectively. The negative value of adsorption energy indicates that adsorption can proceed spontaneously [
26].
According to
Table 5, the order of the adsorption energy of rare earth elements Pr and Nd on kaolinite followed E
ads(Pr)>E
ads(Nd), and both of them were negative values, which are -13.28eV and -13.39eV, respectively. Therefore, the adsorption reaction of elements Pr and Nd on kaolinite can be carried out spontaneously. The process of rare earth adsorption on the surface of kaolinite was actually the result of the competition between the trapping and retention of rare earth ions on the surface of kaolinite. The collecting process corresponds to the physical adsorption process of Pr and Nd ions on kaolinite surface, which mainly depends on the electrostatic interaction between molecules. The retention process mainly corresponds to the chemisorption process of Pr and Nd ions on the kaolinite surface, mainly by covalent interaction. The adsorption energy reflects the difficulty of the retention [
27,
28].
3.5.4. Model of the Microscopic Adsorption Structure of Pr and Nd by Kaolinite and Halloysite
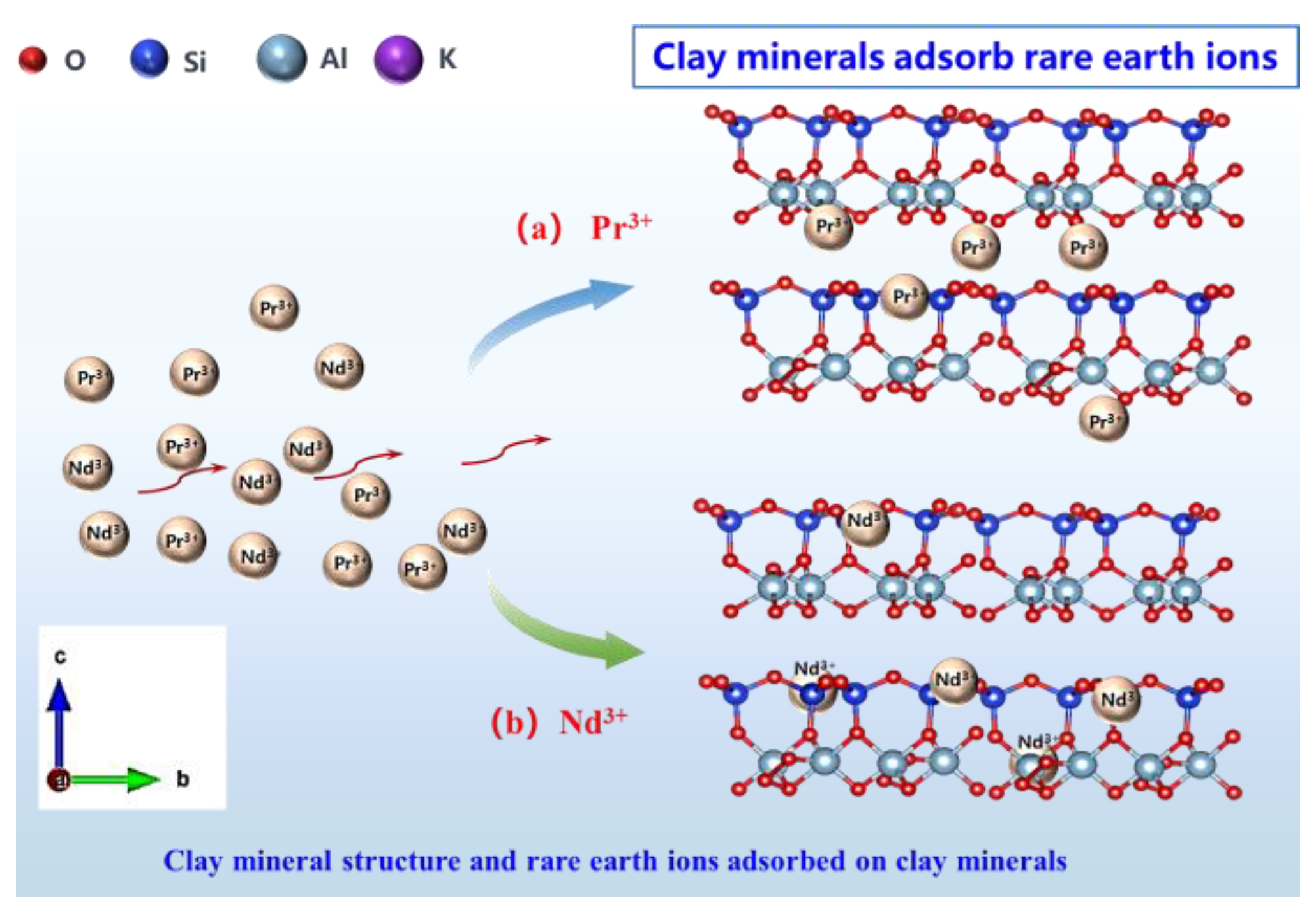
The clay minerals are the main carriers of rare earth elements in the weathered crust elution-deposited rare earth ore in southern Yunnan. Kaolinite and halloysite are the main clay minerals in the weathered crust elution-deposited rare earth ore in southern Yunnan, and the microscopic adsorption structure model of rare earth elements Pr and Nd is shown in
Figure 13.
Due to different adsorption energies, rare earth elements adsorbed on clay minerals showed different stability in the adsorption process, and the rare earth elements Pr and Nd continue to migrate to the upper part of the rare earth ore body. This continuous adsorption-desorption makes the Pr and Nd gradually enriched in rare earth ore bodies. It can provide the theoretic guidance to achieve the resources recycling.
3.6. The Relation Between the Adsorption Recovery and Rare Earths Resources Perspective
In recent years, praseodymium-neodymium metal become a key material for manufacturing the core component of new energy vehicles. With the deepening contradiction between the supply and demand of Pr and Nd, higher requirements have been put forward for the efficient and green extraction and separation of rare earth resources. Especially, the recovery of Pr and Nd resources from low-concentration rare earth solutions such as rare earth ore beneficiation wastewater, refining wastewater, seawater and hot springs has become a research hotspot. Compared with traditional extraction technologies, the adsorption method captures rare earth ions on the surface of adsorbents through physical or chemical adsorption. Due to its characteristics of large processing capacity, wide processing concentration range and short processing flow, the adsorption recovery of Pr and Nd resources from low-concentration rare earth leaching solutions can meet the urgent needs of industrial application. It is necessary to optimize the single-factor adsorption conditions of the adsorption process to enhance the adsorption process and increase the recovery rate of praseodymium-neodymium. Therefore, the research results are expected to provide a reference for the efficient utilization of rare earth resources.
4. Conclusions
Based on the adsorption experiments of Pr and Nd ions with kaolinite and halloysite, the adsorption characteristics of different clay minerals for different Pr and Nd were studied, and the effects of initial rare earth elements concentration, pH and experimental temperature on the adsorption of Pr and Nd ions in clay minerals were discussed. The results showed that the adsorption effect of clay minerals on Pr and Nd becomes worse with the rise of clay mineral concentration. The adsorption efficiency of kaolinite for Pr first increased and then decreased with the increase of pH, and the adsorption effect was the best when the adsorption situation pH was 6. The adsorption efficiency of kaolinite for Nd and that of halloysite for Pr and Nd increased with the increase of pH. When the temperature increased, the adsorption efficiency of clay minerals for Pr and Nd increased, thus the overall rise trend was not significant. The isothermal adsorption model was used to fit the experimental data, and the adsorption of kaolinite and halloysite for Pr and Nd were consistent with the Langmuir model, which presented as single molecular layer adsorption. The research results can provide beneficial theoretical guidance and technical support for the recovery of Pr and Nd from rare earth leaching solution to achieve resources recycling. It is of significant to strengthen the comprehensive utilization of rare earth resources, enhance the research and development of technologies for adsorption, it can effectively alleviate the shortage of praseodymium and neodymium resources, promote the efficient recycling and utilization of secondary rare earth resources, and avoid waste of rare earth resources.
Author Contributions
Conceptualization, Z.C.(Zhuo Chen) and Z.Z.(Zhenyue Zhang); methodology, H.W.(Han Wang); software, Z.C.; validation, Z.C., H.W. and R.C.(Ruan Chi); formal analysis, Z.C.; investigation, H.W.; resources, Z.C.; data curation, Z.C.; writing—original draft preparation, Z.C.; writing—review and editing, Z.Z.; visualization, H.W.; supervision, R.C.; project administration, Z.Z.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Nature Science Foundation of China (92475206 U24A2096, and 52304293).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Yang XJ, Lin A, Li XL, Wu YD, Zhou WB, Chen ZH. China's ion-adsorption rare earth resources, mining consequences and preservation. Environmental Development. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Sun ZS, Zhao YM, Yan GH, Yuan HT, Zhang MR, Zhang B. A novel method for low-rank coal drying using steam transient flash evaporation. Fuel. 2023, 354: 129238.
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Frontiers. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Gao YQ, Zhang SM, Zhao KY, Wang ZW, Xu SX, Liang ZP, Wu K. Adsorption of La3+ and Ce3+ by poly-γ-glutamic acid crosslinked with polyvinyl alcohol. J. Rare Earths. 2015, 33, 884–891. [Google Scholar] [CrossRef]
- Goodenough KM, Wall F, Merriman D. The rare earth elements: demand, global resources, and challenges for resourcing future generations. Natural Resources Research. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Izatt RM, Izatt SR, Bruening RL, Izatt NE, Moyer BA. Challenges to achievement of metal sustainability in our high-tech society. Chem. Soc. Reviews. 2014, 43, 2451–2475. [Google Scholar] [CrossRef]
- Lewis LH, Jiménez-Villacorta F. Perspectives on permanent magnetic materials for energy conversion and power generation. Metallurgical and Materials Transactions A. 2013, 44, 2–20. [Google Scholar] [CrossRef]
- Wilburn, DR. Wind energy in the United States and materials required for the land-based wind turbine industry from 2010 through 2030. Washington, DC: US Department of the Interior, US Geological Survey. 2011.
- Xiao YF, Feng ZY, Hu GH, Huang L, Huang XW, Chen YY, Li ML. Leaching and mass transfer characteristics of elements from ion-adsorption type rare earth ore. Rare Metals. 2015, 34, 357–365. [Google Scholar] [CrossRef]
- Huang SX, Feng J, Liu JQ, Yu JX, Chi RA. Adsorption and desorption characteristics of clay minerals on NH4+ in the leaching agent of weathered crust elution-deposited rare earth ore. China Min. Magazine. 2021, 30, 152–158. [Google Scholar]
- Deng ZX, Qin L, Wang GS, Luo SH, Peng CL, Li Q. Metallogenic process of ion adsorption REE ore based on the occurrence regularity of La in kaolin. Ore Geology Reviews. 2019, 112, 103022. [Google Scholar] [CrossRef]
- Jia L, Zhang ZL. Adsorption of La3+, Nd3+ and some heavy metal ions by humic acid in amulti-metal-ion system. J. Chin. Soc. Rare Earths. 2009, 27, 816–821. [Google Scholar]
- Xiao, YF. Study on the green and efficient leaching technology for ion-adsorption type rare earths ore with magnesium salt system. Northeastern University. 2015.
- Jiao YF, He XL, Liao CF, Jiang PG. Studies on the sorption of amino methylene phosphonic acid resin for heavy rare earth metals. Ion Exch. Adsorption. 2009, 25, 306–312. [Google Scholar]
- Yang MJ, Liang XL, Ma LY, Huang J, He HP, Zhu JX. Adsorption of REEs on kaolinite and halloysite: A link to the REE distribution on clays in the weathering crust of granite. Chemical Geology. 2019, 525: 210–217.
- Xue WL, Li FH, Liu Y, Li BY, Huang BB. Mixing soil with bentonite to amend its microstructure and permeability. J. Irrig. Drainage. 2022, 41, 85–92. [Google Scholar]
- Shen W, Wang Z. Research evolvement of treatment technology of Pb2+ in water with clay mineral. Guangzhou Chem. Industry. 2009, 37, 60–62. [Google Scholar]
- Xie, JJ. Structure and physical-chemistry property evolution of heat-treatment palygorskite as well as adsorption for phosphorus. Hefei University of Technology. 2014.
- Tang YN, Cai YT, Yang PJ, Chang KK. Ferric oxide on U(VI) adsorption. Biol. Chem. Engineering. 2016, 2, 23–24. [Google Scholar]
- Coppin F, Berger G, Bauer A, Castet S, Loubet M. Sorption of lanthanides on smectite and kaolinite. Chem. Geology. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Peixoto AF, Fernandes AC, Pereira C, Pires J, Freire C. Physicochemical characterization of organosilylated halloysite clay nanotubes. Microporous and Mesoporous Materials. 2016, 219, 145–154. [Google Scholar] [CrossRef]
- Li WY, Liu J, Chen H, Deng Y, Zhang B, Wang Z, Zhang X, Hong S. Application of oxalic acid cross-linking activated alumina/chitosan bio composites in defluorination from aqueous solution. Investigation of adsorption mechanism. Chemical engineering journal. 2013, 225, 865–872. [Google Scholar]
- Xu JC, Wu HD, Zhou ZH, Yao PK, Zhang QP. Intercalation-hydrothermal preparation of submicron 13X zeolite with coal-measure kaolin. Bull. Chin. Ceram. Society. 2018, 37, 1188–1194. [Google Scholar]
- Ma WS, Shi JJ, Wang W, Ning P. Surface modification of long-chain alkyl silane on hnts. Silicone Material. 2011, 25, 248–252. [Google Scholar]
- Zhang A, Kang LL, Zhang YM, Ding DQ, Zhang YF. Effect of Kaolinite Particle Size on lts Crystal Structure and Thermal Evolution Behaviors. Bull. Chin. Ceram. Society. 2019, 38, 3964–3971. [Google Scholar]
- Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MIJ, Refson K, Payne MC. First principles methods using CASTEP. Zeitschrift für kristallographie-crystalline materials. 2005, 220 567–570.
- Li, HT. First-principles study on microstructures and elastic properties of clay mineral. Taiyuan University of Technology. 2016.
- Wang GS, Lai YM, Peng CL. Adsorption of rare earth yttrium and ammonium ions on kaolinite surfaces: a DFT study. Theor. Chem. Accounts. 2018, 137, 1–11. [Google Scholar]
Figure 1.
XRD pattern of clay minerals (N: original sample, EG: Saturated sample with ethylene glycol, T: Sample was heated to 550°C for 2 hours).
Figure 1.
XRD pattern of clay minerals (N: original sample, EG: Saturated sample with ethylene glycol, T: Sample was heated to 550°C for 2 hours).
Figure 2.
Isothermal adsorption curve of Pr3+ adsorption by clay minerals(a) and adsorption efficiency diagram of Pr3+ adsorption by clay minerals(b).
Figure 2.
Isothermal adsorption curve of Pr3+ adsorption by clay minerals(a) and adsorption efficiency diagram of Pr3+ adsorption by clay minerals(b).
Figure 3.
Kaolinite fitting of Langmuir thermodynamic model(a) and halloysite fitting of Langmuir thermodynamic mode (b) for the adsorption of Pr3+.
Figure 3.
Kaolinite fitting of Langmuir thermodynamic model(a) and halloysite fitting of Langmuir thermodynamic mode (b) for the adsorption of Pr3+.
Figure 4.
Isothermal adsorption curve of Nd3+ adsorption by clay minerals(a) and adsorption efficiency diagram of Nd3+ adsorption by clay minerals(b).
Figure 4.
Isothermal adsorption curve of Nd3+ adsorption by clay minerals(a) and adsorption efficiency diagram of Nd3+ adsorption by clay minerals(b).
Figure 5.
Kaolinite fitting of Langmuir thermodynamic model(a) and halloysite fitting of Langmuir thermodynamic model(b) for the adsorption of Nd3+.
Figure 5.
Kaolinite fitting of Langmuir thermodynamic model(a) and halloysite fitting of Langmuir thermodynamic model(b) for the adsorption of Nd3+.
Figure 6.
Adsorption efficiency of Pr3+ by kaolinite(a) and Pr3+ by halloysite(b) with various pH.
Figure 6.
Adsorption efficiency of Pr3+ by kaolinite(a) and Pr3+ by halloysite(b) with various pH.
Figure 7.
Adsorption efficiency of Nd3+ by kaolinite(a) and Nd3+ by halloysite(b) with various pH.
Figure 7.
Adsorption efficiency of Nd3+ by kaolinite(a) and Nd3+ by halloysite(b) with various pH.
Figure 8.
Adsorption efficiency diagram of Pr3+ adsorbed by clay minerals at temperature(a) and Nd3+ adsorbed by clay minerals(b).
Figure 8.
Adsorption efficiency diagram of Pr3+ adsorbed by clay minerals at temperature(a) and Nd3+ adsorbed by clay minerals(b).
Figure 9.
SEM images of halloysite (a)(b), halloysite adsorbed Pr (c)(d), halloysite adsorbed Nd (e)(f).
Figure 9.
SEM images of halloysite (a)(b), halloysite adsorbed Pr (c)(d), halloysite adsorbed Nd (e)(f).
Figure 10.
SEM images of kaolinite(a)(b), kaolinite adsorbed Pr(c)(d), kaolinite adsorbed Nd(e)(f).
Figure 10.
SEM images of kaolinite(a)(b), kaolinite adsorbed Pr(c)(d), kaolinite adsorbed Nd(e)(f).
Figure 11.
FT-IR plots of halloysite, halloysite adsorbed Pr, halloysite adsorbed Nd.
Figure 11.
FT-IR plots of halloysite, halloysite adsorbed Pr, halloysite adsorbed Nd.
Figure 12.
FT-IR plots of kaolinite, kaolinite adsorbed Pr, kaolinite adsorbed Nd.
Figure 12.
FT-IR plots of kaolinite, kaolinite adsorbed Pr, kaolinite adsorbed Nd.
Figure 13.
The micro adsorption model between clay minerals and rare earth elements.
Figure 13.
The micro adsorption model between clay minerals and rare earth elements.
Table 1.
Chemical composition of rare earth ores (wt %).
Table 1.
Chemical composition of rare earth ores (wt %).
| Ingredient |
REO |
SiO2
|
Al2O3
|
MnO |
K2O |
Na2O |
| Content |
0.12 |
66.23 |
18.14 |
0.13 |
4.21 |
0.57 |
| Ingredient |
CaO |
TiO2
|
P2O5
|
Fe2O3
|
FeO |
Loss |
| Content |
0.04 |
0.05 |
0.09 |
1.60 |
0.6 |
7.1 |
Table 2.
Relative content of clay minerals (wt %).
Table 2.
Relative content of clay minerals (wt %).
| Specimen |
Kaolinite |
Halloysite |
Illite |
Montmorillonite |
| Ledge |
25.06 |
65.78 |
8.13 |
1.03 |
Table 3.
Thermodynamic model fitting parameters of Pr adsorption by clay minerals.
Table 3.
Thermodynamic model fitting parameters of Pr adsorption by clay minerals.
| Equations |
Freundlich equation |
Langmuir equation |
| In KF |
1/n |
R2
|
1/(QmaxKL) |
Qm(µmol /g) |
R2
|
| Clay minerals |
Kaolinite |
0.083 |
0.269 |
0.607 |
57.751 |
16.488 |
0.965 |
| Halloysite |
0.590 |
0.311 |
0.937 |
26.732 |
35.702 |
0.970 |
Table 4.
Thermodynamic model fitting parameters of Nd adsorption by clay minerals.
Table 4.
Thermodynamic model fitting parameters of Nd adsorption by clay minerals.
| Equations |
Frendlich equation |
Langmuir equation |
| In KF
|
1/n |
R2
|
1/(QmaxKL) |
Qm(µmol /g) |
R2
|
| Clay minerals |
Kaolinite |
-0.091 |
0.292 |
0.558 |
64.471 |
23.613 |
0.978 |
| Halloysite |
0.177 |
0.358 |
0.951 |
25.723 |
28.571 |
0.997 |
Table 5.
The adsorption energy of rare earth on the kaolinite.
Table 5.
The adsorption energy of rare earth on the kaolinite.
| Single atoms |
Model 1: kaolinite (001) |
| atom |
Etot (eV) |
adsorbate |
Etot (eV) |
Eads (eV) |
| Pr |
-0.157 |
Pr |
-737.186 |
-13.28 |
| Nd |
-0.156 |
Nd |
-737.295 |
-13.39 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).