Submitted:
15 April 2025
Posted:
16 April 2025
You are already at the latest version
Abstract
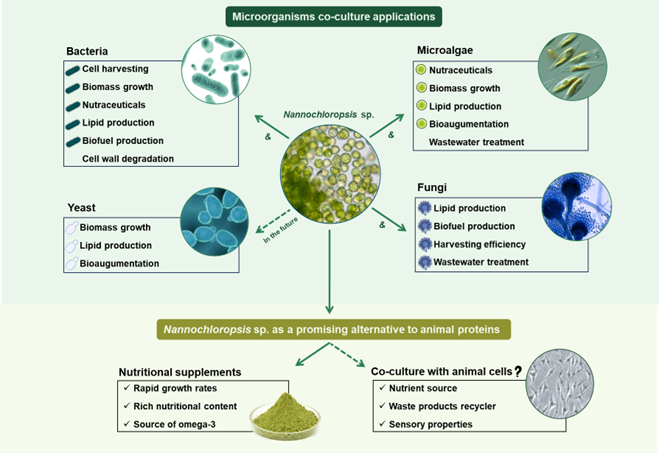
Keywords:
1. Introduction
2. Materials and Methods
3. Mechanisms of Interaction in Microbial Consortia
3.1. Metabolite Exchange
3.2. Chemical Interactions
3.3. Metabolomics of Nannochloropsis
4. Co-Culture of Nannochloropsis with Microorganisms
4.1. Microalgae and Microalgae
4.2. Microalgae and Bacteria
4.3. Microalgae and Fungi
Microalgae and Yeast
4.4. Co-Culture of Other Microalgae with Microorganisms
5.1. Microalgae in Food and Feed
5.2. The Potential of Nannochloropsis in the Alternative Proteins’ World
5.3. Toxicology, Safety and Regulatory Aspects of Nannochloropsis sp.
6. Current Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DHA | Docosahexaenoic Acid |
| DW | Dry Weight |
| EPA | Eicosapentaenoic Acid |
| EPS | Extracellular Polymeric Substances |
| EU | European Union |
| FA | Fatty Acid |
| IAA | Indole-3-acetic Acid |
| MUFA | Monounsaturated Fatty Acid |
| PUFA | Polyunsaturated Fatty Acid |
| SFA | Saturated Fatty Acid |
| TAG | Triacylglycerol |
| QS | Quorum Sensing |
| WWT | Wastewater Treatment |
References
- J. Wu et al. Bioactive substances and potentiality of marine microalgae. Food Sci Nutr 2021, 9, 5279–5292. [Google Scholar] [CrossRef] [PubMed]
- M. K. Anusree, K. M. K. Anusree, K. Manasa Leela, M. Sreehari, S. Raj, A. Sreenikethanam, and A. K. Bajhaiya. Marine microalgae: an emerging source of pharmaceuticals and bioactive compounds. New Horizons in Natural Compound Research, 2023. [Google Scholar] [CrossRef]
- S. M. Tibbetts, J. E. Milley, and S. P. Lall. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- J. C. Lee et al.. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int 2013, 13, 1–7. [Google Scholar] [CrossRef]
- V. Mimouni et al.. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr Pharm Biotechnol 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- P. K. Das, J. Rani, S. Rawat, and S. Kumar. Microalgal Co-cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. BioEnergy Research 2021, 15, 1–26. [Google Scholar] [CrossRef]
- Y. Ye, M. Liu, L. Yu, H. Sun, and J. Liu. Nannochloropsis as an Emerging Algal Chassis for Light-Driven Synthesis of Lipids and High-Value Products. Marine Drugs 2024, 22, 54. [Google Scholar] [CrossRef]
- Y. Ma, Z. Wang, C. Yu, Y. Yin, and G. Zhou. Evaluation of the potential of 9 Nannochloropsis strains for biodiesel production. Bioresour Technol 2014, 167, 503–509. [Google Scholar] [CrossRef]
- X. Wu, R. Ruan, Z. Du, and Y. Liu. Current Status and Prospects of Biodiesel Production from Microalgae. Energies 2012, 5, 2667–2682. [Google Scholar] [CrossRef]
- C. Celi, D. Fino, and F. Savorani. Phaeodactylum tricornutum as a source of value-added products: A review on recent developments in cultivation and extraction technologies. Bioresour Technol Rep 2022, 19, 101122. [Google Scholar] [CrossRef]
- Y. Chisti. Biodiesel from microalgae. Biotechnol Adv 2007, 25, 294–306. [Google Scholar] [CrossRef]
- N. Mishra. EXPLORING THE BIOLOGICALLY ACTIVE METABOLITES OF ISOCHRYSIS GALBANA IN PHARMACEUTICAL INTEREST: AN OVERVIEW. Int J Pharm Sci Res 2018, 2, 2162–2174. [Google Scholar] [CrossRef]
- W. K. Lee et al.. Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System. Mar Drugs 2021, 19, 314. [Google Scholar] [CrossRef]
- S. Mehariya et al.. A comprehensive review on versatile microalga Tetraselmis: Potentials applications in wastewater remediation and bulk chemical production. J Environ Manage 2024, 365, 121520. [Google Scholar] [CrossRef] [PubMed]
- G. de S. Celente, T. M. Rizzetti, Y. Sui, and R. de C. de S. Schneider. Potential use of microalga Dunaliella salina for bioproducts with industrial relevance. Biomass Bioenergy 2022, 167, 106647. [Google Scholar] [CrossRef]
- G. Chi et al.. Production of polyunsaturated fatty acids by Schizochytrium (Aurantiochytrium) spp. Biotechnol Adv 2022, 55, 107897. [Google Scholar] [CrossRef]
- Singh, P. S. Nigam, and J. D. Murphy. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour Technol 2011, 102, 10–16. [Google Scholar] [CrossRef]
- L. A. Meireles, A. C. Guedes, and F. X. Malcata. Lipid class composition of the microalga Pavlova lutheri: Eicosapentaenoic and docosahexaenoic acids. J Agric Food Chem 2003, 51, 2237–2241. [Google Scholar] [CrossRef]
- “The Biology of Nannochloropsis oceanica Suda & Miyashita (a microalga). Australian Government, 2019.
- M. W. Fawley, I. Jameson, and K. P. Fawley. The phylogeny of the genus Nannochloropsis (Monodopsidaceae, Eustigmatophyceae), with descriptions of N. australis sp. Nov. and Microchloropsis gen. Nov. Phycologia 2015, 54, 545–552. [Google Scholar] [CrossRef]
- M. R. Brown. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol 1991, 145, 79–99. [Google Scholar] [CrossRef]
- M. J. Scholz et al.. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef]
- M. M. Rebolloso-Fuentes, A. Navarro-Pérez, F. García-Camacho, J. J. Ramos-Miras, and J. L. Guil-Guerrero. Biomass Nutrient Profiles of the Microalga Nannochloropsis. J Agric Food Chem 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- L. Zanella and F. Vianello. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J Funct Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- G. Padmaperuma, R. V. Kapoore, D. J. Gilmour, and S. Vaidyanathan. Microbial consortia: a critical look at microalgae co-cultures for enhanced biomanufacturing. Crit Rev Biotechnol 2018, 38, 690–703. [Google Scholar] [CrossRef] [PubMed]
- L. Goers, P. Freemont, and K. M. Polizzi. Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface 2014, 11. [Google Scholar] [CrossRef]
- A. Corcoran and W. J. Boeing. Biodiversity Increases the Productivity and Stability of Phytoplankton Communities. PLoS One 2012, 7, e49397. [Google Scholar] [CrossRef]
- J. A. Christie-Oleza, D. Sousoni, M. Lloyd, J. Armengaud, and D. J. Scanlan. Nutrient recycling facilitates long-term stability of marine microbial phototroph-heterotroph interactions. Nat Microbiol 2017, 2, 17100. [Google Scholar] [CrossRef]
- D. M. Selegato and I. Castro-Gamboa. Enhancing chemical and biological diversity by co-cultivation. Front Microbiol 2023, 14, 1117559. [Google Scholar] [CrossRef]
- C. A. Santos and A. Reis. Microalgal symbiosis in biotechnology. Appl Microbiol Biotechnol 2014, 98, 5839–5846. [Google Scholar] [CrossRef]
- J. M. Uratani, R. Kumaraswamy, and J. Rodríguez. A systematic strain selection approach for halotolerant and halophilic bioprocess development: A review. Extremophiles 2014, 18, 629–639. [Google Scholar] [CrossRef]
- S. C. Baker, S. J. Ferguson, B. Ludwig, M. D. Page, O.-M. H. Richter, and R. J. M. van Spanning. Molecular genetics of the genus Paracoccus: metabolically versatile bacteria with bioenergetic flexibility. Microbiol Mol Biol Rev 1998, 62, 1046–1078. [Google Scholar] [CrossRef]
- J. Lian, P. Schimmel, S. Sanchez-Garcia, R. H. Wijffels, H. Smidt, and D. Sipkema. Different co-occurring bacteria enhance or decrease the growth of the microalga Nannochloropsis sp. CCAP211/78. Microb Biotechnol 2021, 14, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- G. Daly, V. Ghini, A. Adessi, M. Fondi, A. Buchan, and C. Viti. Towards a mechanistic understanding of microalgae–bacteria interactions: integration of metabolomic analysis and computational models. FEMS Microbiol Rev 2022, 46, 1–19. [Google Scholar] [CrossRef]
- J. L. Fuentes, I. Garbayo, M. Cuaresma, Z. Montero, M. González-Del-Valle, and C. Vílchez. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Marine Drugs 2016, 14, 100. [Google Scholar] [CrossRef]
- S. Chaïb, J. C. A. Pistevos, C. Bertrand, and I. Bonnard. Allelopathy and allelochemicals from microalgae: An innovative source for bio-herbicidal compounds and biocontrol research. Algal Res 2021, 54, 102213. [Google Scholar] [CrossRef]
- K. Montgomery, J. C. Charlesworth, R. LeBard, P. T. Visscher, and B. P. Burns. Quorum Sensing in Extreme Environments. Life 2013, 3, 131–148. [Google Scholar] [CrossRef]
- J. Zhou, Y. Lyu, M. L. Richlen, D. M. Anderson, and Z. Cai. Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. CRC Crit Rev Plant Sci 2016, 35, 81. [Google Scholar] [CrossRef]
- E. Segev et al.. Dynamic metabolic exchange governs a marine algal-bacterial interaction. Elife, 2016. [CrossRef]
- S. A. Amin et al.. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Y. Ye, M. Liu, L. Yu, H. Sun, and J. Liu. Nannochloropsis as an Emerging Algal Chassis for Light-Driven Synthesis of Lipids and High-Value Products. Mar Drugs 2024, 22, 54. [Google Scholar] [CrossRef]
- J. Li et al.. Choreography of Transcriptomes and Lipidomes of Nannochloropsis Reveals the Mechanisms of Oil Synthesis in Microalgae. Plant Cell 2014, 26, 1645–1665. [Google Scholar] [CrossRef]
- J. Yang et al.. PDAT regulates PE as transient carbon sink alternative to triacylglycerol in Nannochloropsis. Plant Physiol 2022, 189, 1345–1362. [Google Scholar] [CrossRef]
- W. A. Wan Razali, C. A. Evans, and J. Pandhal. Comparative Proteomics Reveals Evidence of Enhanced EPA Trafficking in a Mutant Strain of Nannochloropsis oculata. Front Bioeng Biotechnol 2022, 10, 838445. [Google Scholar] [CrossRef]
- M. M. Morris et al.. Bacterial community assembly, succession, and metabolic function during Nannochloropsis salina outdoor cultivation. 2021. Accessed: Mar. 29, 2025. Available online: https://www.osti. 1876.
- S. K, T. V, J. D, A. P, and R. K. Sarangi. Growth promoting studies on co-culturing Nannochloropsis oceanica with Halomonas aquamarina actively enhance the algal biomass and lipid production. Biocatal Agric Biotechnol 2020, 29, 101790. [Google Scholar] [CrossRef]
- N. Ferrer-Ledo, L. Stegemüller, M. Janssen, R. H. Wijffels, and M. J. Barbosa. Growth and fatty acid distribution over lipid classes in Nannochloropsis oceanica acclimated to different temperatures. Front Plant Sci 2023, 14, 1078998. [Google Scholar] [CrossRef]
- N. Gu, Q. Lin, G. Li, Y. Tan, L. Huang, and J. Lin. Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng Life Sci 2012, 12, 631–637. [Google Scholar] [CrossRef]
- Sukenik, Y. Carmeli, and T. Berner. REGULATION OF FATTY ACID COMPOSITION BY IRRADIANCE LEVEL IN THE EUSTIGMATOPHYTE NANNOCHLOROPSIS SP.1. J Phycol 1989, 25, 686–692. [Google Scholar] [CrossRef]
- W. Kim, M. G. Sung, K. Nam, M. Moon, J. H. Kwon, and J. W. Yang. Effect of monochromatic illumination on lipid accumulation of Nannochloropsis gaditana under continuous cultivation. Bioresour Technol 2014, 159, 30–35. [Google Scholar] [CrossRef]
- L. Xu, X. Cheng, and Q. Wang. Enhanced lipid production in Chlamydomonas reinhardtii by co-culturing with azotobacter chroococcum. Front Plant Sci 2018, 9, 338772. [Google Scholar] [CrossRef]
- L. A. Leyva, Y. Bashan, and L. E. de-Bashan. Activity of acetyl-CoA carboxylase is not directly linked to accumulation of lipids when Chlorella vulgaris is co-immobilised with Azospirillum brasilense in alginate under autotrophic and heterotrophic conditions. Ann Microbiol 2015, 65, 339–349. [Google Scholar] [CrossRef]
- J. Sharma et al.. Microalgal consortia for municipal wastewater treatment – Lipid augmentation and fatty acid profiling for biodiesel production. J Photochem Photobiol B 2020, 202, 111638. [Google Scholar] [CrossRef]
- R. W. Davis, A. J. Siccardi, N. D. Huysman, N. B. Wyatt, J. C. Hewson, and T. W. Lane. Growth of mono- and mixed cultures of Nannochloropsis salina and Phaeodactylum tricornutum on struvite as a nutrient source. Bioresour Technol 2015, 198, 577–585. [Google Scholar] [CrossRef]
- M. Maglie, C. Baldisserotto, A. Guerrini, A. Sabia, L. Ferroni, and S. Pancaldi. A co-cultivation process of Nannochloropsis oculata and Tisochrysis lutea induces morpho-physiological and biochemical variations potentially useful for biotechnological purposes. J Appl Phycol 2021, 33, 2817–2832. [Google Scholar] [CrossRef]
- L. Thurn, J. Schobel, and D. Weuster-Botz. Photoautotrophic Production of Docosahexaenoic Acid- and Eicosapentaenoic Acid-Enriched Biomass by Co-Culturing Golden-Brown and Green Microalgae. Fermentation 2024, 10, 220. [Google Scholar] [CrossRef]
- N. A. T. Tran, B. Tamburic, C. R. Evenhuis, and J. R. Seymour. Bacteria-mediated aggregation of the marine phytoplankton Thalassiosira weissflogii and Nannochloropsis oceanica. J Appl Phycol 2020, 32, 3735–3748. [Google Scholar] [CrossRef]
- A. Corcoran et al.. Scale-dependent enhancement of productivity and stability in xenic Nannochloropsis cultures. Algal Res 2022, 68, 102892. [Google Scholar] [CrossRef]
- R. J. Powell and R. T. Hill. Rapid Aggregation of Biofuel-Producing Algae by the Bacterium Bacillus sp. Strain RP1137. Appl Environ Microbiol 2013, 79, 6093–6101. [Google Scholar] [CrossRef]
- C. Muñoz, C. Hidalgo, M. Zapata, D. Jeison, C. Riquelme, and M. Rivas. Use of cellulolytic marine bacteria for enzymatic pretreatment in microalgal biogas production. Appl Environ Microbiol 2014, 80, 4199–4206. [Google Scholar] [CrossRef]
- Z. Y. Du et al.. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- D. Wrede et al.. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS One 2014, 9, e113497. [Google Scholar] [CrossRef]
- T. Ishika, N. R. Moheimani, and P. A. Bahri. Sustainable saline microalgae co-cultivation for biofuel production: A critical review. Renewable and Sustainable Energy Reviews 2017, 78, 356–368. [Google Scholar] [CrossRef]
- M. Maglie, C. Baldisserotto, A. Guerrini, A. Sabia, L. Ferroni, and S. Pancaldi. A co-cultivation process of Nannochloropsis oculata and Tisochrysis lutea induces morpho-physiological and biochemical variations potentially useful for biotechnological purposes. J Appl Phycol 2021, 33, 2817–2832. [Google Scholar] [CrossRef]
- L. Thurn, A. Stock, S. Gerwald, and D. Weuster-Botz. Simultaneous photoautotrophic production of DHA and EPA by Tisochrysis lutea and Microchloropsis salina in co-culture. Bioresour Bioprocess 2022, 9, 1–13. [Google Scholar] [CrossRef]
- L. Thurn, J. Schobel, and D. Weuster-Botz. Photoautotrophic Production of Docosahexaenoic Acid- and Eicosapentaenoic Acid-Enriched Biomass by Co-Culturing Golden-Brown and Green Microalgae. Fermentation 2024, 10, 220. [Google Scholar] [CrossRef]
- Ray, M. Nayak, and A. Ghosh. A review on co-culturing of microalgae: A greener strategy towards sustainable biofuels production. Science of The Total Environment 2022, 802, 149765. [Google Scholar] [CrossRef]
- W. Ding, X. Zhou, M. He, W. Jin, Y. Chen, and J. Sun. Pollutant removal and resource recovery of co-cultivated microalgae Chlorella sp. and Phaeodactylum tricornutum for marine aquaculture wastewater. Journal of Water Process Engineering 2024, 67, 106182. [Google Scholar] [CrossRef]
- M. Padri, N. Boontian, C. Piasai, and M. S. Tamzil. Construction of co-culture of microalgae with microorganisms for enhancing biomass production and wastewater treatment: a review. IOP Conf Ser Earth Environ Sci 2021, 623, 012024. [Google Scholar] [CrossRef]
- S. K, T. V, J. D, A. P, and R. K. Sarangi. Growth promoting studies on co-culturing Nannochloropsis oceanica with Halomonas aquamarina actively enhance the algal biomass and lipid production. Biocatal Agric Biotechnol 2020, 29, 101790. [Google Scholar] [CrossRef]
- Y. Y. Wang et al.. Co-cultivation of Isochrysis galbana and Marinobacter sp. can enhance algal growth and docosahexaenoic acid production. Aquaculture 2022, 556, 738248. [Google Scholar] [CrossRef]
- M. Chorazyczewski, I. S. Huang, H. Abdulla, X. Mayali, and P. V. Zimba. The Influence of Bacteria on the Growth, Lipid Production, and Extracellular Metabolite Accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 2021, 57, 931–940. [Google Scholar] [CrossRef]
- D. Wrede et al.. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS One 2014, 9, e113497. [Google Scholar] [CrossRef]
- N. Uduman, Y. N. Uduman, Y. Qi, M. K. Danquah, G. M. Forde, and A. Hoadley. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. Journal of Renewable and Sustainable Energy, 2010; 1. [Google Scholar] [CrossRef]
- N. A. T. Tran, B. Tamburic, C. R. Evenhuis, and J. R. Seymour. Bacteria-mediated aggregation of the marine phytoplankton Thalassiosira weissflogii and Nannochloropsis oceanica. J Appl Phycol 2020, 32, 3735–3748. [Google Scholar] [CrossRef]
- R. Hajjar, G. Ambaraghassi, H. Sebajang, F. Schwenter, and S. H. Su. Raoultella ornithinolytica: Emergence and Resistance. Infect Drug Resist 2020, 13, 1091. [Google Scholar] [CrossRef] [PubMed]
- Z. Y. Du et al.. Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata. Biotechnol Biofuels 2018, 11, 1–16. [Google Scholar] [CrossRef]
- Dias, J. Santos, A. Reis, and T. Lopes da Silva. Yeast and microalgal symbiotic cultures using low-cost substrates for lipid production. Bioresour Technol Rep 2019, 7, 100261. [Google Scholar] [CrossRef]
- N. Arora, A. Patel, J. Mehtani, P. A. Pruthi, V. Pruthi, and K. M. Poluri. Co-culturing of oleaginous microalgae and yeast: paradigm shift towards enhanced lipid productivity. Environmental Science and Pollution Research 2019, 26, 16952–16973. [Google Scholar] [CrossRef]
- R. K. Naidoo, Z. F. Simpson, J. R. Oosthuizen, and F. F. Bauer. Nutrient exchange of carbon and nitrogen promotes the formation of stable mutualisms between chlorella sorokinianaand saccharomyces cerevisiae under engineered synthetic growth conditions. Front Microbiol 2019, 10, 424255. [Google Scholar] [CrossRef]
- J. Ling, S. Nip, W. L. Cheok, R. A. de Toledo, and H. Shim. Lipid production by a mixed culture of oleaginous yeast and microalga from distillery and domestic mixed wastewater. Bioresour Technol 2014, 173, 132–139. [Google Scholar] [CrossRef]
- Dias, A. Reis, J. A. L. Santos, L. Gouveia, and T. Lopes da Silva. Primary brewery wastewater as feedstock for the yeast Rhodosporidium toruloides and the microalga Tetradesmus obliquus mixed cultures with lipid production. Process Biochemistry 2022, 113, 71–86. [Google Scholar] [CrossRef]
- W. Bahafid, N. Tahri Joutey, H. Sayel, I. Boularab, and N. El Ghachtouli. Bioaugmentation of chromium-polluted soil microcosms with Candida tropicalis diminishes phytoavailable chromium. J Appl Microbiol 2013, 115, 727–734. [Google Scholar] [CrossRef]
- S. Cai, C. Hu, and S. Du. Comparisons of Growth and Biochemical Composition between Mixed Culture of Alga and Yeast and Monocultures. J Biosci Bioeng 2007, 104, 391–397. [Google Scholar] [CrossRef]
- Azarpour, S. Zendehboudi, O. Mohammadzadeh, A. R. Rajabzadeh, and I. Chatzis. A review on microalgal biomass and biodiesel production through Co-cultivation strategy. Energy Convers Manag 2022, 267, 115757. [Google Scholar] [CrossRef]
- L. Leng et al.. Co-culture of fungi-microalgae consortium for wastewater treatment: A review. Bioresour Technol 2021, 330, 125008. [Google Scholar] [CrossRef]
- P. K. Das, J. Rani, S. Rawat, and S. Kumar. Microalgal Co-cultivation for Biofuel Production and Bioremediation: Current Status and Benefits. BioEnergy Research 2021, 15, 1–26. [Google Scholar] [CrossRef]
- Y. Tong, K. Honda, and C. J. C. Derek. A review on microalgal-bacterial co-culture: The multifaceted role of beneficial bacteria towards enhancement of microalgal metabolite production. Environ Res 2023, 228, 115872. [Google Scholar] [CrossRef] [PubMed]
- R. Naseema Rasheed et al.. Microalgal co-cultivation -recent methods, trends in omic-studies, applications, and future challenges. Front Bioeng Biotechnol 2023, 11, 1193424. [Google Scholar] [CrossRef]
- W. Ding, X. Zhou, M. He, W. Jin, Y. Chen, and J. Sun. Pollutant removal and resource recovery of co-cultivated microalgae Chlorella sp. and Phaeodactylum tricornutum for marine aquaculture wastewater. Journal of Water Process Engineering 2024, 67, 106182. [Google Scholar] [CrossRef]
- W. -W. Huang, B.-Z. Dong, Z.-P. Cai, and S.-S. Duan. Growth effects on mixed culture of Dunaliella salina and Phaeodactylum tricornutum under different inoculation densities and nitrogen concentrations. Afr J Biotechnol 2011, 10, 13164–13174. [Google Scholar] [CrossRef]
- N. L. Kadalag, P. R. Pawar, and G. Prakash. Co-cultivation of Phaeodactylum tricornutum and Aurantiochytrium limacinum for polyunsaturated omega-3 fatty acids production. Bioresour Technol 2022, 346, 126544. [Google Scholar] [CrossRef]
- M. Chorazyczewski, I. S. Huang, H. Abdulla, X. Mayali, and P. V. Zimba. The Influence of Bacteria on the Growth, Lipid Production, and Extracellular Metabolite Accumulation by Phaeodactylum tricornutum (Bacillariophyceae). J Phycol 2021, 57, 931–940. [Google Scholar] [CrossRef]
- Škufca, *!!! REPLACE !!!*; et al. . Interaction between Microalgae P. tricornutum and Bacteria Thalassospira sp. for Removal of Bisphenols from Conditioned Media. Int J Mol Sci 2022, 23, 8447. [Google Scholar] [CrossRef]
- T. T. Vuong, B. R. Kwon, J. I. Eom, B. K. Shin, and S. M. Kim. Interaction between marine bacterium Stappia sp. K01 and diatom Phaeodactylum tricornutum through extracellular fatty acids. J Appl Phycol 2020, 32, 71–82. [Google Scholar] [CrossRef]
- P. V. Phatarpekar, R. A. Sreepada, C. Pednekar, and C. T. Achuthankutty. A comparative study on growth performance and biochemical composition of mixed culture of Isochrysis galbana and Chaetoceros calcitrans with monocultures. Aquaculture 2000, 181, 141–155. [Google Scholar] [CrossRef]
- H. Wang et al.. Treatment of fishery wastewater by co-culture of Thalassiosira pseudonana with Isochrysis galbana and evaluation of their active components. Algal Res 2021, 60, 102498. [Google Scholar] [CrossRef]
- Y. Y. Wang et al.. Co-cultivation of Isochrysis galbana and Marinobacter sp. can enhance algal growth and docosahexaenoic acid production. Aquaculture 2022, 556, 738248. [Google Scholar] [CrossRef]
- S. V. Sandhya and K. K. Vijayan. Symbiotic association among marine microalgae and bacterial flora: a study with special reference to commercially important Isochrysis galbana culture. J Appl Phycol 2019, 31, 2259–2266. [Google Scholar] [CrossRef]
- M. Kawaroe, T. Prartono, A. Sunuddin, and D. Saputra. Marine Microalgae Tetraselmis suecica as Flocculant Agent of Bio-flocculation Method. Hayati 2016, 23, 62–66. [Google Scholar] [CrossRef]
- J. Park et al.. Phycospheric native bacteria Pelagibaca bermudensis and Stappia sp. Ameliorate biomass productivity of Tetraselmis striata (KCTC1432BP) in co-cultivation system through mutualistic interaction. Front Plant Sci 2017, 8, 244337. [Google Scholar] [CrossRef]
- J. Han, L. Zhang, S. Wang, G. Yang, L. Zhao, and K. Pan. Co-culturing bacteria and microalgae in organic carbon containing medium. Journal of Biological Research 2016, 23, 8. [Google Scholar] [CrossRef]
- N. Muradov et al.. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol Biofuels, 2015. [CrossRef]
- J. Zhang, B. Huang, and T. Tang. Effect of co-culture with Halomonas mongoliensis on Dunaliella salina growth and phenol degradation. Front Bioeng Biotechnol 2022, 10, 1072868. [Google Scholar] [CrossRef]
- T. Butler. The diatom Phaeodactylum tricornutum as a sustainable microalgal cell factory: towards a biorefinery approach. 2021.
- Kusmayadi, Y. K. Leong, H. W. Yen, C. Y. Huang, and J. S. Chang. Microalgae as sustainable food and feed sources for animals and humans – Biotechnological and environmental aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Dineshbabu, G. Goswami, R. Kumar, A. Sinha, and D. Das. Microalgae–nutritious, sustainable aqua- and animal feed source. J Funct Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- L. R. Martínez-Córdova, M. Emerenciano, A. Miranda-Baeza, and M. Martínez-Porchas. Microbial-based systems for aquaculture of fish and shrimp: an updated review. Rev Aquac 2015, 7, 131–148. [Google Scholar] [CrossRef]
- Tyus. Discovering Potential Protein, Carbohydrate, and Lipid Based Food Ingredients in a Co-Culture of Microalgae. Louisiana State University and Agricultural & Mechanical College. ProQuest Dissertation, s & Theses, 2019, Accessed: Feb. 08, 2025.
- J. Espinosa-Ramírez, A. C. Mondragón-Portocarrero, J. A. Rodríguez, J. M. Lorenzo, and E. M. Santos. Algae as a potential source of protein meat alternatives. Front Nutr 2023, 10, 1254300. [Google Scholar] [CrossRef] [PubMed]
- M. Kent, H. M. Welladsen, A. Mangott, and Y. Li. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS One 2015, 10, e0118985. [Google Scholar] [CrossRef]
- N. Rojas-Tavara and A. J. Donayre-Torres. Microalgae in lab-grown meat production. https://cjfs.agriculturejournals.cz/doi/10.17221/69/2023-CJFS.html CJFS 2023, 41, 406–418. [Google Scholar] [CrossRef]
- S. Hubalek, M. J. Post, and P. Moutsatsou. Towards resource-efficient and cost-efficient cultured meat. Curr Opin Food Sci 2022, 47, 100885. [Google Scholar] [CrossRef]
- Y. Okamoto, Y. Haraguchi, N. Sawamura, T. Asahi, and T. Shimizu. Mammalian cell cultivation using nutrients extracted from microalgae. Biotechnol Prog 2020, 36, e2941. [Google Scholar] [CrossRef]
- Y. Haraguchi and T. Shimizu. Microalgal culture in animal cell waste medium for sustainable ‘cultured food’ production. Arch Microbiol 2021, 203, 5525–5532. [Google Scholar] [CrossRef]
- Y. Haraguchi and T. Shimizu. Three-dimensional tissue fabrication system by co-culture of microalgae and animal cells for production of thicker and healthy cultured food. Biotechnol Lett 2021, 43, 1117–1129. [Google Scholar] [CrossRef]
- Babuskin Srinivasan, Krishnan K. R., Sivarajan Meenatchisundaram, and Sukumar Muthusamy. Functional foods enriched with marine microalga Nannochloropsis oculata as a source of ω-3 fatty acids. Food Technol Biotechnol 2014, 52, 292–299, Accessed online: https://wwwcabidigitallibraryorg/doi/full/105555/20143328456. [Google Scholar]
- L. Zanella and F. Vianello. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J Funct Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- J. Ji, L. Shi, J. Ampofo, and Lord Abbey. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- M. C. Mendes et al.. Algae as Food in Europe: An Overview of Species Diversity and Their Application†. Foods 2022, 11, 1871. [Google Scholar] [CrossRef]
- J. R. Centre et al., Microalgae-based products for the food and feed sector – An outlook for Europe. Publications Office, 2014. [CrossRef]
- “Algae as Novel Food in Europe. European Algae Biomass Association (EABA).
- “Food and Feed Information Portal Database | FIP.” Accessed: Aug. 05, 2024. Online.. Available: https://ec.europa.
- U. Al Hoqani, U. U. Al Hoqani, U. Al-Hoqani, R. Young, and S. Purton. Article in Perspectives in Phycology. 2016. [Google Scholar] [CrossRef]
- L. Goers, P. L. Goers, P. Freemont, and K. M. Polizzi. Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface, 2014; 96. [Google Scholar] [CrossRef]
| Marine microalgae Specie | Bioactive compounds |
Total Lipid content | Applications | Reference |
|---|---|---|---|---|
| Nannochloropsis spp. | PUFAs, EPA | 30% - 60% | Biofuel, nutraceuticals, feed and food | [7,8] |
|
Phaeodactylum tricornutum |
PUFAs, EPA, fucoxanthin | 20 - 30% | Nutraceuticals, pharmaceuticals, biofuel, cosmetics | [9,10] |
| Isochrysis galbana | PUFAs, DHA and EPA | 25 - 30 % | Aquaculture feed, nutraceuticals | [11,12] |
| Tetraselmis spp. | PUFAs | 12 - 15 % | Aquaculture feeds, biofuel and wastewater treatment | [13,14] |
| Dunaliella salina | PUFAs, Beta-carotene, lutein | 7 % | Biofuel, cosmetics, nutraceuticals and pharmaceuticals | [15] |
| Schizochytrium spp. | PUFAs, DHA | (up to 60% under stress) | DHA-rich oils, oral vaccines | [16,17] |
| Pavlova lutheri | PUFAs, DHA, EPA | 50 - 70 % | Aquaculture feed, biofuel | [18] |
| Co-culture type | Microalgae Specie | Co-culture Species | Application | Reference |
|---|---|---|---|---|
|
Microalgae- microalgae |
Nannochloropsis sp. | MAC11: Chlorella sp., Chlamydomonas reinhardtii, Scenedesmus bijugatus, and Oscillatoria MAC2: Chlorella sp., Kirchnella, Scenedesmus dimorphus, and Microcoleus* |
- Sewage wastewater treatment - Heavy metal removal by bioaugmentation |
[53] |
| M. salina |
Phaeodactylum tricornutum |
- Wastewater treatment | [54] | |
| N. oculata | Tisochrysis lutea | - Biomass growth and lipid production | [55] | |
| N. oceanica | Isochrysis galbana | - Biomass growth and lipid production | [56] | |
|
Microalgae- bacteria |
N. oceanica | Halomonas aquamarina | - Biomass growth and lipid production | [46] |
| N. oceanica | Bacterial isolates from the Rhodobacterales, Flavobacteriales and Sphingomonadale orders | - Facilitate biomass aggregation | [57] | |
| N. oceanica | Genera Algoriphagus, Oceanicaulis, and Marinobacter | - Enhance productivity and stability of cell cultures | [58] | |
| N. oceanica | Bacillus sp. | - Cell aggregation for biofuel production | [59] | |
| M. gaditana | Raoultella ornithinolytica | - Cell wall degradation for biofuel production | [60] | |
| Microalgae-fungi | N. oceanica | Mortierella elongata | - Biofuel production - Harvesting efficiency |
[61,62] |
| N. oculata | Aspergillus fumigatus | - Wastewater Treatment - Lipid production - Harvesting efficiency |
[62] |
| Marine microalgae specie | Co-culture type | Co-culture partners | Application | Reference |
|---|---|---|---|---|
|
Phaeodactylum tricornutum |
Microalgae- microalgae |
Chlorella sp. | - Marine aquaculture wastewater treatment | [90] |
| Dunaliella salina | - Biomass growth, lipid and chlorophyll production | [91] | ||
| Aurantiochytrium limacinum | - EPA and DHA production | [92] | ||
| Microalgae-bacteria | Marinobacter sp. | - Biomass growth and lipid production | [93] | |
| Thalassospira sp. | - Bisphenols removal from media | [94] | ||
| Stappia sp. | - Biomass growth, lipid and carotenoid production | [95] | ||
|
Isochrysis galbana |
Microalgae- microalgae |
Chaetoceros calcitrans | - Added-value metabolites production | [96] |
| Microalgae-bacteria | Thalassiosira pseudonana | - Fishery wastewater treatment | [97] | |
| Marinobacter sp. | - Biomass growth and DHA production | [98] | ||
| Alteromonas sp. | - Biomass growth and metabolites production | [99] | ||
| Labrenzia sp. | - Biomass growth and metabolites production | [99] | ||
| Microalgae-yeast | Ambrosiozyma cicatricosa | - Biomass growth | [84] | |
| Tetraselmisspp. | Microalgae- microalgae |
T. lutea and Microchloropsis salina | - EPA and DHA production | [65] |
|
T. sueccia and Chlorella sp., Nannochloropsis sp. |
- Bio-flocculation for cell harvesting | [100] | ||
| Microalgae-bacteria |
T. striata and Pelagibaca bermudensis, Stappia sp. |
Biomass growth and lipid production | [101] | |
| T. chuii and Muricauda sp. | - Biomass growth | [102] | ||
| Microalgae-fungi |
T. suecica and Aspergillus fumigatus |
- Bio-flocculation for cell harvesting, biomass growth and lipid production | [103] | |
|
Dunaliella salina |
Microalgae-bacteria | Halomonas mongoliensis | - Bisphenols removal from wastewater | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





