1. Introduction
Breast cancer is one of the most common malignant tumors in humans and animals, breast cancer has a high rate of morbidity and mortality [
1,
2]. Drug resistance in advanced breast cancer limits the therapeutic effectiveness of treatment [
3]. Drug combination application is one of the crucial strategies to develop new cancer therapies and solve multi-drug resistance [
4], and "new use of old drugs" can reduce the development risk cost and shorten the development time [
5]. IVM is a broad-spectrum antiparasitic drug that has recently been shown to exhibit strong anticancer action. This effect may be mediated by a variety of pathways that impact the proliferation and metastasis of cancer cells. IVM can treat various cancer kinds, including breast cancer [
6], ovarian cancer [
7], colon cancer [
8], carcinoma of urinary bladder [
9], and so on, Diao H et al. found that ivermectin inhibited the growth of canine breast tumor cell lines in a dose - and time-dependent manner, and significant inhibition of ivermectin on tumor growth was observed in canine breast tumor xenografts [
10].
In the therapeutic treatment of type 2 diabetes, MET is a first-line medication. It plays a hypoglycemic role by inhibiting gluconeogenesis and glucose synthesis in the liver by activating AMPK signal transduction in liver cells [
11]. Its hypoglycemic mechanism is similar to that of anti-tumor mechanism, and both type 2 diabetes and breast cancer are metabolic diseases. It has been in clinical use for decades, and its safety has been widely recognized. Recent studies have shown that MET exerts anti-tumor effects in various types of cancer, such as breast cancer [
12], thyroid cancer, [
13] and prostate cancer [
14].Fan Y et al. found that metformin could inhibit the proliferation of canine breast tumor (CMGT) cells through cell cycle arrest, induction of apoptosis, activation of AMPK and inhibition of AKT/mTOR signaling pathway [
15]. However, the body may resist the effects of anti-tumor medications in tumour patients treated with a single medication for an extended period of time due to gene mutations, changes in protein expression, and loss of stem cell target expression, which presents serious hurdles for the treatment of cancer [
16].
On the contrary, the new target or protein expression regulation changes initiated by the drug combination may avoid the body's tolerance to the drug, improve efficacy, and reduce toxicity [
17]. From the perspective of cell homeostasis, autophagy is one of the important mechanisms for coping with oxidative stress. Autophagy can reduce cell damage by clearing ROS, damaged biomoleculins, and mitochondria to meet cells' metabolic needs and cope with specific stress states [
18]. However, when ROS accumulation is excessive, or the duration is too long, autophagy may not be able to clear excess ROS, resulting in a large number of mitochondrial autophagy and insufficient cell energy, which will lead to autophagic cell death [
19].
This study investigates, for the first time, the combined use of IVM and MET to determine whether the excessive accumulation of intracellular ROS inhibits the activation of the PI3K/AKT/mTOR signaling pathway, inducing a significant number of autophagosomes in breast cancer cells. This process ultimately leads to autophagic cell death, providing a new basis for the potential use of IVM combined with MET as an autophagy inducer and a novel treatment method for breast cancer.
2. Materials and Methods
2.1. Reagents
IVM (purity ≥98%) was purchased from Sigma company, MET (purity ≥98%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. The use of primary antibodies as follows: β-actin (ABclonal, AS014), LC3B (ABclonal, A19665), Beclin1 (ABclonal, A21191), P62 (protein tech, 18420-1-AP), Bcl-2 (ABclonal, A19693), PI3 Kinase p85 (ABclonal, A4992), Phospho-PI3K P85α (ABclonal, Abclonal, AP0854), AKT (ABclonal, A20779), Anti-AKT (Wanleibio, WLP001), mTOR (ABclonal, A11345), and Phospho-mTOR (ABclonal, AP0115). HRP goat anti-rabbit protein (no clone), Alexa Fluor 594-goat anti-rabbit protein (Abbox, Jiangsu, China), Cy3 goat anti-rabbit protein (H+L) (no clone), and FITC goat anti-rabbit protein (Wuhan, China) were used as secondary antibodies for western blot or immunofluorescence.
2.2. Cell Culture
CMT-1211 cell line used in this study was isolated from a canine breast tumor and donated by Professor Lin Degui, China Agricultural University laboratory. 4T1 cell line was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cell lines were grown in DMEM medium (Gibco) containing 10% fetal bovine serum (VISTECH) and 2% penicillin-streptomycin (Sigma) and cultured in a cell incubator at 37℃ and 5%CO2 saturated humidity. When the cell density reached more than 80%, the cells were either passaged or used for the next experiment.
2.3. Cell Viability Was Detected by the CCK-8 Method
Suspended CMT-1211 and 4T1 cells were inoculated in 96-well plates with 100μL per well, ensuring at least 2×10³ cells per well. Five replicates were set for each drug concentration. Cells were cultured in a 5% CO2 incubator at 37℃ for 12 hours until they reached 50% confluence. Different concentrations of IVM, MET, and IVM+MET were then added. After 12, 24, or 48 hours of further incubation, the culture medium was replaced with DMEM and CCK-8 solution (9:1 ratio, 100μL per well). Plates were incubated at 37℃ for 30 minutes, and absorbance was measured using a Bio-Rad enzyme-labelled instrument measured cell viability by absorbance (optical density) at 450nm.
2.4. Scratch Test
Horizontal lines were marked on the bottom of a six-well plate. A single-cell suspension was inoculated into each well (2mL per well) and cultured in a CO2 incubator at 37℃ for 24h. Once cells formed a monolayer, vertical lines were drawn with a yellow pipette tip, and cells were washed three times with PBS to remove detached cells. Serum-free DMEM, IVM, MET, and IVM+MET solutions were added, and cells were cultured for another 24h. Cell proliferation was observed using an inverted microscope (Olympus, CKX41, Tokyo, Japan), and the scratch area was analyzed using ImageJ software.
2.5. Cell Invasion Experiment
Prepare a 24-well plate and Transwell chamber. Dilute glue A (1×) 12 times with buffer C (0.5×) at 37℃ (Biozellen Inc). Add 100µL of this diluted glue A to each upper chamber and incubate at 4℃ for 2h. Suspend 7.5×10⁴ cells, treated with 0, IVM, MET, or IVM+MET for 24h in 100µL culture medium. Add 1mL DMEM with 10% fetal bovine serum to the upper and lower chambers to promote cell migration and invasion. After incubating the plates in a 5% CO2 incubator at 37℃ for 24h, remove the medium, wash twice with PBS, fix with 1mL 100% methanol for 30min, and stain with 1mL 0.1% crystal violet for 30min. Wash twice with PBS, place the upper chamber upside down on a slide, and observe cell migration under an inverted light microscope.
2.6. RNA Sequencing Analysis
To investigate the response mechanism of CMT-1211 and 4T1 cells to IVM, MET, and IVM+MET, Wuhan Kangce test Technology Co., Ltd. conducted 12 eukaryotic UID-mRNA sequencing analyses (RNA-Seq). RNA seq data is uploaded to the SRA, and the submission number is SRX25668925.Total RNA was extracted, ensuring OD values between 1.8 and 2.2 and concentrations of at least 100ng/μL, with a minimum sample amount of 5μg. Data quality control was performed using fastp (version 0.23.0). The library was constructed and sequenced using an Illumina kit. Genes with an absolute logFC >1 and P<0.05 were considered differentially expressed. Gene enrichment analyses were conducted using GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes).
2.7. Western Blot for Autophagy-Related Proteins
IVM, MET, and IVM+MET were introduced to CMT-1211 and 4T1 cell lines after they were administered with 2×10⁵ cells per well in 6-well plates and grown for an additional night. After a day, the cells were centrifuged for 20 minutes at 12,000 rpm after being lysed on ice for 30 minutes in RIPA buffer containing 1% PMSF. A BCA protein quantification kit was used to measure the protein concentration. Samples were electrophoresed using SDS-PAGE, then transferred to a PVDF membrane and blocked for 2h with 5% skim milk. After an overnight incubation period at 4°C, primary antibodies were 3X washed with TBST. For 2h, the secondary antibody was incubated at room temperature. The Fusion gel imaging equipment was used to take pictures of protein expression, and ImageJ was used to analyze the results.
2.8. Transmission Electron Microscope (TEM)
Cells (CMT-1211 and 4T1) were immobilized in 4% glutaraldehyde (Sigma). Prepare ultra-thin slices after dewatering with the sorvallMT5000 (Dupont Instruments, MT5000). The autophagy vacuoles in the cytoplasmic region were calculated using ImageProPlus v.3, stained with lead citrate or 1% uranyl acetate. After IVM, MET, and IVM+MET treated cells for 24h, (2×105) ~ (1×106) cells were collected, and the supernatant was discarded by centrifugation. Wash with PBS once, centrifuge, and discard the supernatant. Fix 2.5% glutaraldehyde above 20 times the sample volume with PBS for 4h, rinse with 0.1mol/LPBS (dosage 0.5-1.0 ml) twice for 15min each time, fixed with 1% osmic acid fixing solution for 1h, and rinse with ddH2O twice for 10-15min each time. The cells were fixed/stained with 2% uranium acetate for 30min, then dehydrated by ethanol gradient, permeated, embedded, and polymerized. The cells were sliced with an ultrathin micrograph and stained with uranium acetate and lead citrate. Autophagosome formation was observed under TEM.
2.9. Measurement of Intracellular ROS Production
ROS production in CMT-1211 and 4T1 cells was measured using a ROS assay kit (Hyhcezmbio, Wuhan, China). Cells were inoculated in 6-well plates at 1×10⁵ cells/well and cultured for 24h. After treatment, cells were collected, centrifuged at 1200 rpm for 5min, washed twice with PBS, and resuspended in 1mL of 50mg/mL DCFH-DA probe per well. Cells were incubated at 37℃ in a CO2 incubator for 35min, then centrifuged, washed with PBS, and resuspended. Rosup (50mg/mL) was used as a positive control. At least 10,000 live cells were analyzed using flow cytometry (CytoFLEX, Huazhong Agricultural University), and the results were analyzed using FlowJo software.
2.10. In Situ Breast Cancer Model in Mice
Balb/c mice female (5-6 weeks) were purchased from the Experimental Animal Center of Huazhong Agricultural University and acclimated for one week. The Animal Ethics Committee of Huazhong Agricultural University approved all studies (HZAUMO-2024-0222). CMT-1211 cells suspended in PBS were transplanted into the fourth mammary fat pad of Balb/c mice to establish the in-situ breast cancer model. Once tumor volume reached 100mm³, mice were randomly divided into 6 groups (5 mice each) and treated with PBS (0.1mL), IVM (0.2mg/kg, Sigma), MET (100mg/kg, Shanghai Maclin), IVM+MET (0.1mg/mL IVM + 50mg/kg MET), or paclitaxel (10mg/kg). IVM was administered subcutaneously (0.1mL/mouse every 2 days), and MET was given via intragastric administration (0.1mL/mouse daily). Tumor volume (V=0.5× length × width²) and weight were measured every 2 days. After four weeks, mice were euthanized, and tumor tissue and internal organs were either frozen in liquid nitrogen or fixed in 4% paraformaldehyde for further analysis.
2.11. H&E Dyeing
The tissue organs (heart, liver, spleen, lung, and kidney) and mouse mammary tumors were dehydrated, fixed in formaldehyde for 48 hours, and then embedded in paraffin wax. After that, they were sliced into 5μm slices for staining with hematoxylin and eosin (H&E). To capture images, an optical microscope is employed.
2.12. Immunofluorescence
CMT-1211 and 4T1 cells were cultured and treated with IVM, MET, and IVM+MET. Following a 24-hour incubation period at 37℃ in a 5% CO2 incubator, the cells were rinsed with PBS, treated with 4% paraformaldehyde for 30 minutes to fix them, permeabilized with 0.2% Triton X-100 for 15 minutes, and then blocked with 5% BSA for 2 hours. The cells were incubated with primary antibodies (LC3B and P62), followed by incubation with FITC-labeled or Cy3-labeled goat anti-rabbit IgG for 2 hours in a dark environment. The cells were subjected to DAPI staining for a duration of 8 minutes and were thereafter rinsed with PBS after each stage. Alternatively, tumour sections were treated with xylene and a mixture of ethanol with varying concentrations to remove the wax. Then, they were submerged in a solution containing citric acid to restore the antigen, with a pH level of 6.0. Finally, the sections were heated in a microwave to retrieve the antigen. Sections were subjected to identical methods such as cellular immunofluorescence. The images were observed using a fluorescence microscope.
2.13. Statistical Analysis
The statistical analysis was conducted using GraphPadPrism8. The data are presented as the mean ± standard deviation (Mean±S.D) of a minimum of three independently conducted studies. Group differences were determined using either bidirectional variance analysis or unpaired T-test.*p<0.05,**p<0.01, ***p<0.001,****p<0.0001 indicates statistical significance.
4. Discussion
Breast cancer develops primarily because of abnormal proliferation, inhibited apoptosis, and accelerated invasion of breast cells. Inhibiting the proliferation and invasion of cancer cells and inducing their autophagy and death is one of the important mechanisms of drug therapy for breast tumors. Recent studies have shown that IVM can exert anti-tumor effects through multiple pathways, such as inhibiting the WNT-TCF signaling pathway, modulating PAK1, and inducing mitochondrial dysfunction [
22,
23,
24]. The tumor-specific enhancement of IVM can also be attributed to the Warburg effect and high dependence on glycolysis
in vivo hypoxic tumor environment [
25]. As a first-line drug in treating diabetes, MET's anti-cancer potential has also been confirmed by many studies, especially in breast cancer and other tumors with significant inhibitory effects. Recent studies have confirmed that MET can reduce the risk of tumor development and tumor-related mortality in patients with type 2 diabetes [
26]. K Saeki et al found that metformin significantly inhibited tumor growth in xenografted metastatic CMGT cells [
27]. Because of the anti-tumor effects of both drugs, this study, for the first time, used the two drugs in combination to evaluate the therapeutic effect of IVM combined with MET on canine breast cancer and explore its underlying mechanism.
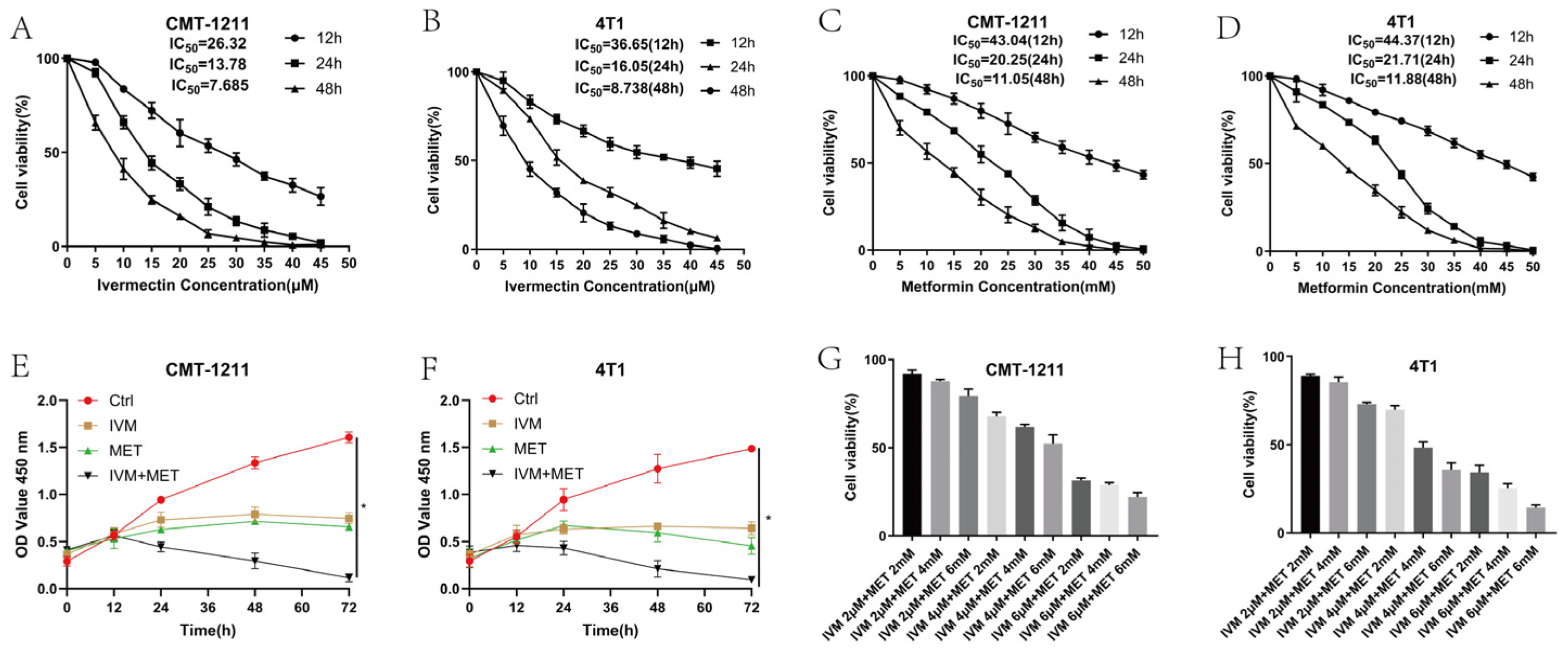
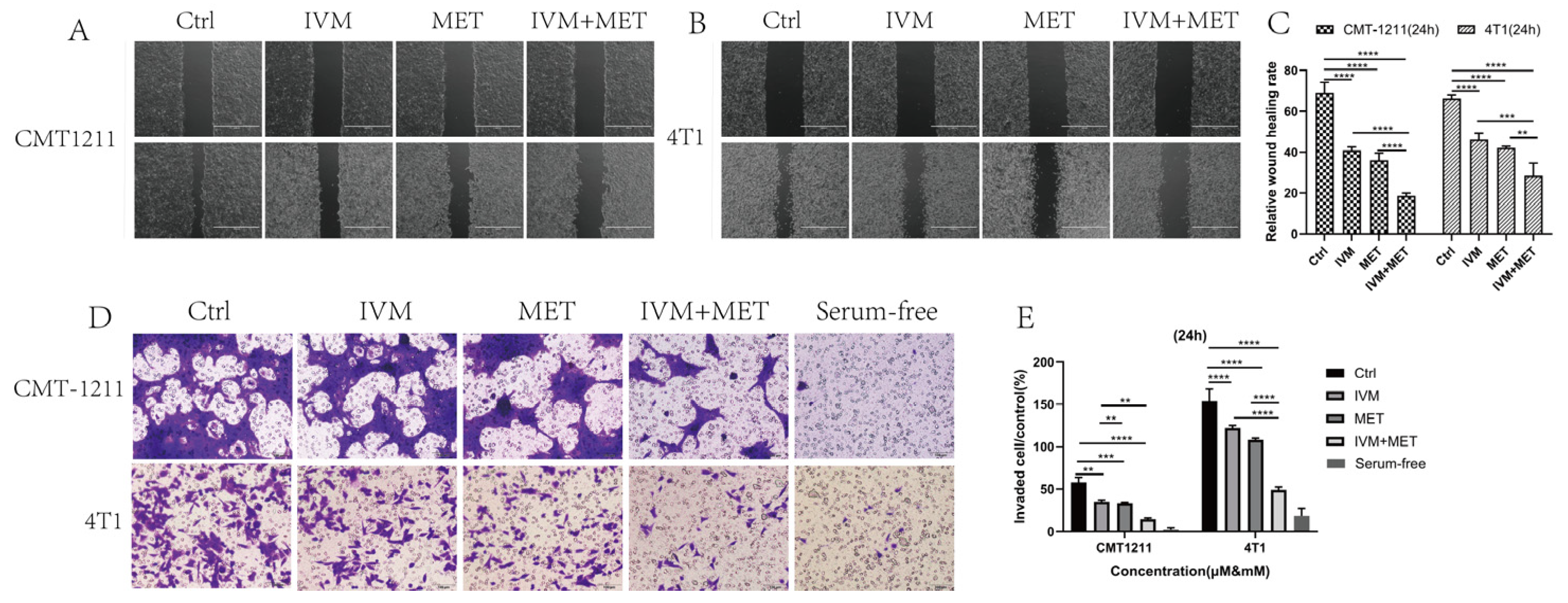
This study, the CCK-8 method was used to evaluate the growth of two breast cancer cell lines (CMT-1211, 4T1) treated with different drug groups. It was found that the cell viability of breast cancer cell lines treated with IVM or MET alone tended to decrease in a time - and dose-dependent manner. However, the activity of breast tumor cells in any single treatment group was higher than in the combined treatment group, indicating that the combination of IVM and MET had a strong synergistic effect. This can also be verified from scratch tests and invasion assay experiments.
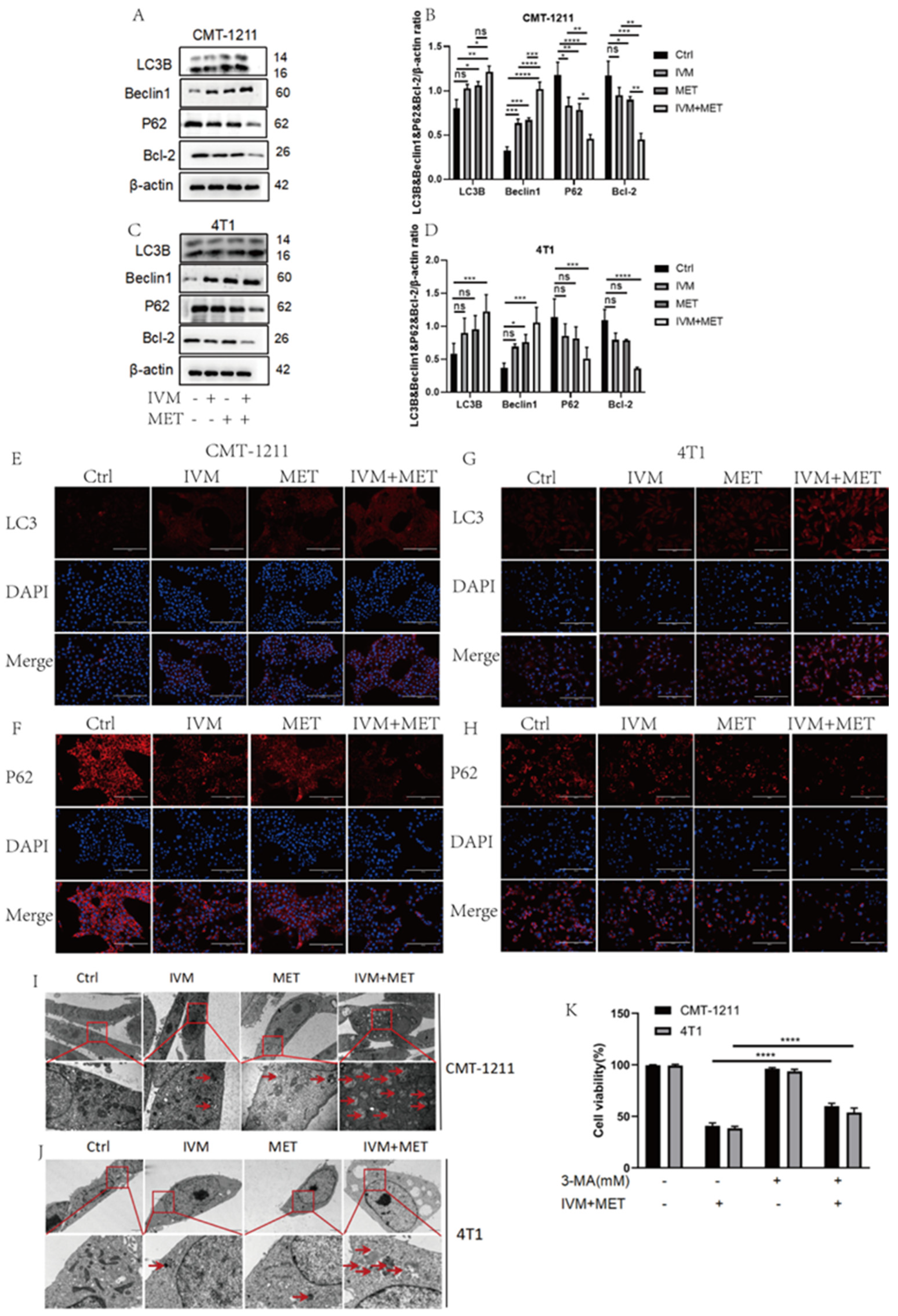
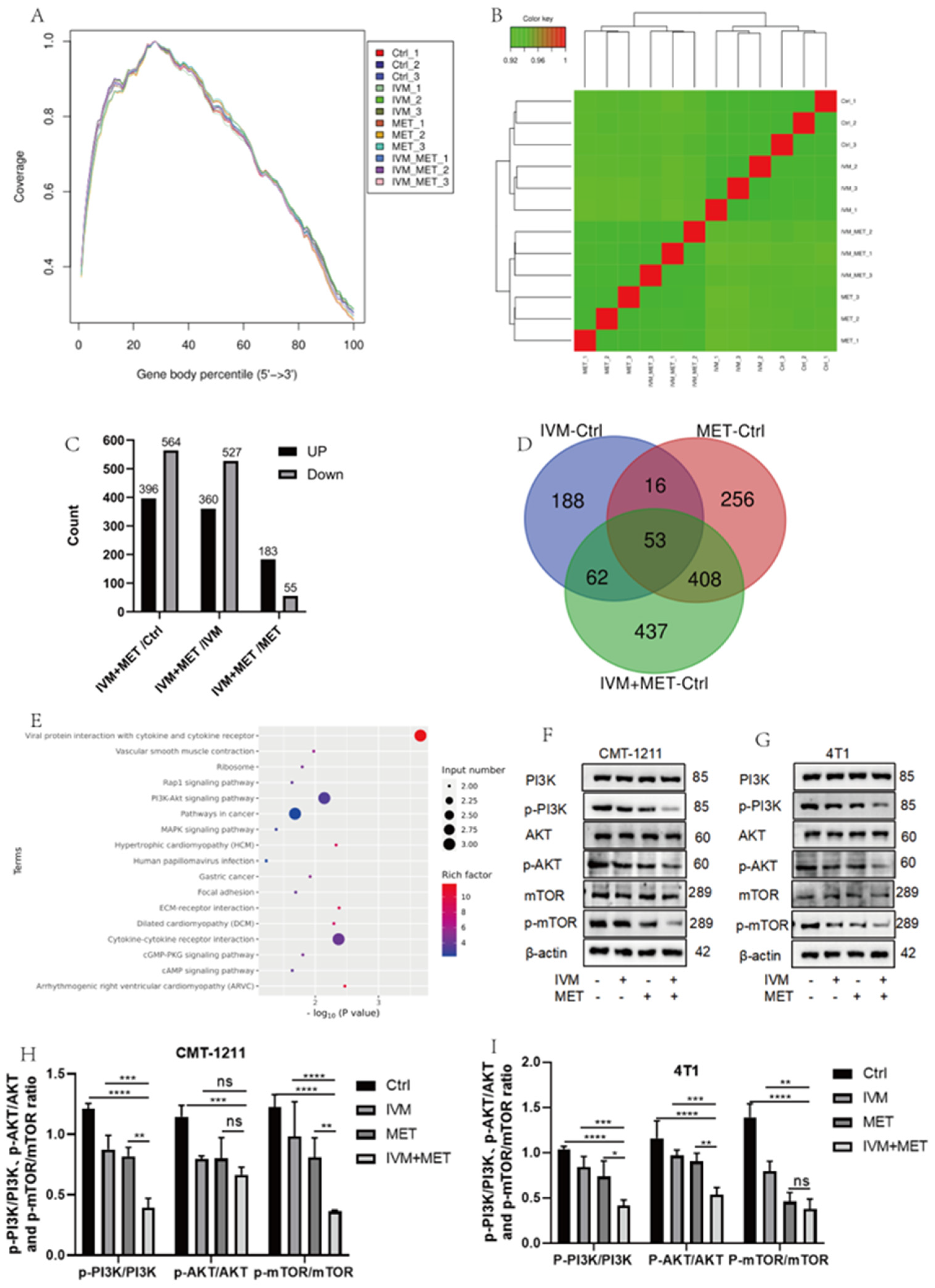
In this study, transcriptome analysis was carried out based on the observation that drugs greatly decrease the activity of breast tumor cells. KEGG analysis showed that IVM combined with MET mainly involved the PI3K/AKT signaling pathway; GO analysis showed that combining the two drugs could affect the regulation of protein catabolism, polyamine metabolism, glycolysis, etc. These effects are directly or indirectly related to autophagy. The PI3K/AKT/mTOR pathway is closely associated with cell proliferation, differentiation, metabolism, autophagy, and other functions. Then, we explored the phosphorylation level of the PI3K/AKT/mTOR signaling pathway based on transcriptomic analysis. Western blot results showed that, compared with the single treatment group, the phosphorylation levels of the two cell pathway proteins showed a significantly decreased trend after combined treatment, indicating that IVM combined with MET could dramatically inhibit the activation of PI3K/AKT/mTOR signaling pathway.
Autophagy functions critically in tumor cells and the surrounding matrix [
28]. Autophagy can inhibit tumor growth in some cellular environments and induce cell death [
29]. In this study, we found that IVM combined with MET can induce autophagy of breast tumor cells, thus inhibiting breast tumor growth. Studies have shown that reactive oxygen species, a byproduct of REDOX homeostasis, play an important role in cell signaling processes and are essential for regulating the balance between autophagy and apoptosis in cancer cells after different drug treatments and gene modifications [
30]. As a signal molecule, ROS can participate in signal transduction pathways, regulate cell growth, differentiation, and survival of cells, and participate in inflammation and immune response [
31]. However, excessive accumulation of ROS will cause damage to DNA and cells' proteins, resulting in a slowdown of cell metabolism, reduced activity, and even death [
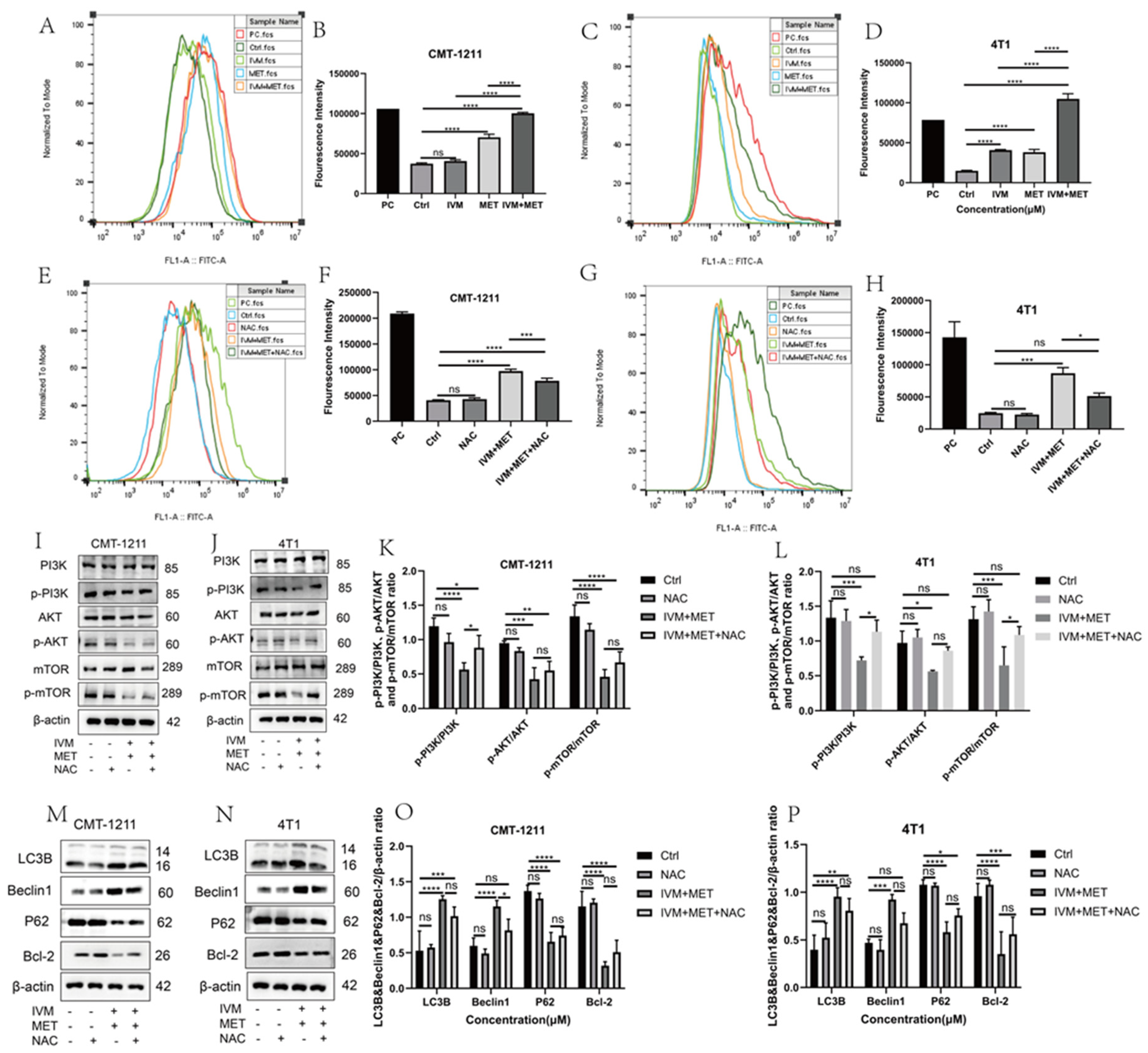
32] . In our study, ROS levels in the IVM+MET group were significantly higher than in other medication groups. ROS levels in the IVM+MET group were significantly reduced after using ROS scavenger NAC. The interaction between autophagy and ROS may play an essential role in resisting the proliferation of breast tumor cells. The PI3K/AKT/mTOR pathway is an important signaling pathway in cells, and it regulates cell growth, proliferation, metabolism, and survival. Cell growth and proliferation will be inhibited, which may lead to autophagic cell death.
Studies have shown that ROS can activate autophagy by inhibiting PI3K/AKT/mTOR [
33,
34]. LC3 is one of the signature proteins of autophagy formation [
35], and the final mature autophagosome fuses with the lysosome under the action of the selective autophagy adapter protein p62 and degrades the autophagosome contents in the lysosome [
36]. Our experiments showed that in both CMT-1211 and 4T1 cell lines, the IVM+MET group promoted the expression of LC3B and Beclin1 while decreasing the expression of P62 and Bcl-2. The application of NAC could partially mitigate this change.
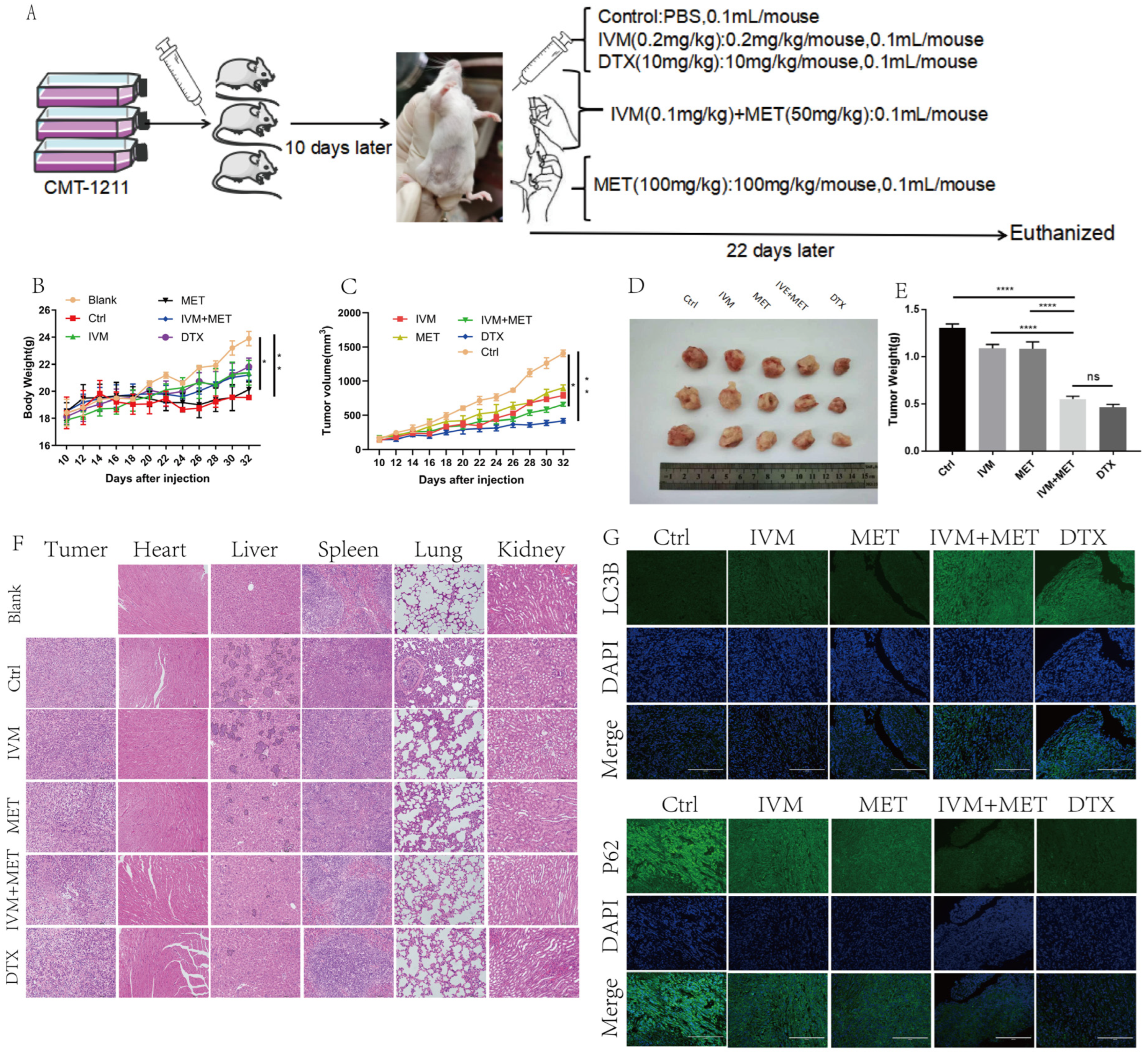
Meanwhile, canine breast tumor cells CMT-1211 were subcutaneously injected into the breast fat pad of Balb/c mice using a xenograft breast cancer model. The results showed that the growth rate of xenograft tumors in mice treated with IVM combined with MET was slower than in the control group. IVM+MET treatment increased the necrosis of tumor cells to a certain extent. In addition to observing the effect of drugs on the tumor itself, we also paid attention to the inhibitory effect of drugs on the distant metastasis of tumor cells. We conducted paraffin sections and H&E staining on mouse tumors and essential organs, and the results showed that the combined drug group reduced lung and liver metastasis of breast cancer cells. Immunofluorescence detection of mouse tumor tissues showed that LC3B fluorescence intensity of IVM combined with MET was significantly enhanced compared with the control group. In contrast, P62 fluorescence intensity was significantly decreased compared with the control group. These data suggest that IVM combined with MET can significantly inhibit the growth of canine breast tumor xenografts in vivo.
Figure 1.
Cytotoxic effects of IVM, MET and their combination on CMT-1211 and 4T1 cells. (A-B) CCK-8 assay detected CMT-1211 and 4T1 cell viability at 12h, 24h, and 48h after IVM treatment. (C-D) CCK-8 assay detected CMT-1211 and 4T1 cell viability at 12h, 24h, and 48h after MET treatment. (E-F) CCK-8 was used to evaluate the activity of CMT-1211 and 4T1 cells after 0h, 12h, 24h, 36h, 48h, 60h, and 72h in different treatment groups. (G-H) IVM of 2μM, 4μM, and 6μM were combined with MET of 2 mm, 4 mm, and 6 mm, respectively. CCK-8 method was used to evaluate the cell viability of CMT-1211 and 4T1 after treatment with different drug groups.
Figure 1.
Cytotoxic effects of IVM, MET and their combination on CMT-1211 and 4T1 cells. (A-B) CCK-8 assay detected CMT-1211 and 4T1 cell viability at 12h, 24h, and 48h after IVM treatment. (C-D) CCK-8 assay detected CMT-1211 and 4T1 cell viability at 12h, 24h, and 48h after MET treatment. (E-F) CCK-8 was used to evaluate the activity of CMT-1211 and 4T1 cells after 0h, 12h, 24h, 36h, 48h, 60h, and 72h in different treatment groups. (G-H) IVM of 2μM, 4μM, and 6μM were combined with MET of 2 mm, 4 mm, and 6 mm, respectively. CCK-8 method was used to evaluate the cell viability of CMT-1211 and 4T1 after treatment with different drug groups.
Figure 2.
Effects of IVM combined with MET on migration and invasion of breast cancer cells. The (A-C) wound healing test was used to measure the migration capacity of tumor cells for 24 hours on A scale of 1000µm. (D-E) The invasive ability of CMT-1211 and 4T1 cells treated with different treatment groups for 24h was determined using Transwell chambers precoated with matrix gel. Scale: 100µm.
Figure 2.
Effects of IVM combined with MET on migration and invasion of breast cancer cells. The (A-C) wound healing test was used to measure the migration capacity of tumor cells for 24 hours on A scale of 1000µm. (D-E) The invasive ability of CMT-1211 and 4T1 cells treated with different treatment groups for 24h was determined using Transwell chambers precoated with matrix gel. Scale: 100µm.
Figure 3.
Analysis of transcriptome results. (A) The uniform distribution curve of Reads on the reference genome. (B) Hierarchical clustering diagram of gene expression levels in samples. (C) Differentially expressed genes screened by IVM+MET/Ctrl group、IVM+MET/IVM group、IVM+MET/METgroup. (D) Venn diagram of differentially expressed genes. (E)KEGG enrichment pathway. (F-H) Western blotting was used to analyze the expression levels different groups of PI3K, p-PI3K, AKT, p-AKT, mTOR, p-mTOR in CMT-1211 and 4T1 cells. All the experiment results are the mean ± standard deviation of three independent experiments.*p<0.05;**p<0.001;***p<0.0001;****p<0.00001;ns=not significant.
Figure 3.
Analysis of transcriptome results. (A) The uniform distribution curve of Reads on the reference genome. (B) Hierarchical clustering diagram of gene expression levels in samples. (C) Differentially expressed genes screened by IVM+MET/Ctrl group、IVM+MET/IVM group、IVM+MET/METgroup. (D) Venn diagram of differentially expressed genes. (E)KEGG enrichment pathway. (F-H) Western blotting was used to analyze the expression levels different groups of PI3K, p-PI3K, AKT, p-AKT, mTOR, p-mTOR in CMT-1211 and 4T1 cells. All the experiment results are the mean ± standard deviation of three independent experiments.*p<0.05;**p<0.001;***p<0.0001;****p<0.00001;ns=not significant.
Figure 5.
IVM+MET induces autophagy in CMT-1211 and 4T1 by promoting excessive ROS accumulation. (A-D) Flow cytometry was used to detect the ROS levels of CMT-1211 and 4T1 cells after different treatment groups. (E-H) After being treated with 0mM, NAC (10mM), IVM+MET, and IVM+MET+NAC, the ROS levels of CMT-1211 and 4T1 cells were detected by flow cytometry. (I-L) After 0µM, NAC (10mM), IVM+MET, IVM+MET+NAC, Western blotting was used to analyze the expression levels of PI3K, pPI3K, AKT, p-AKT, mTOR, p-mTOR in CMT-1211 and 4T1 cells. (M-P) After 0µM, NAC (10mM), IVM+MET, IVM+MET+NAC, Western blotting was used to analyze the expression levels of autophagy related proteins LC3B, Beclin, P62 and Bcl-2 in CMT-1211 and 4T1 cells.
Figure 5.
IVM+MET induces autophagy in CMT-1211 and 4T1 by promoting excessive ROS accumulation. (A-D) Flow cytometry was used to detect the ROS levels of CMT-1211 and 4T1 cells after different treatment groups. (E-H) After being treated with 0mM, NAC (10mM), IVM+MET, and IVM+MET+NAC, the ROS levels of CMT-1211 and 4T1 cells were detected by flow cytometry. (I-L) After 0µM, NAC (10mM), IVM+MET, IVM+MET+NAC, Western blotting was used to analyze the expression levels of PI3K, pPI3K, AKT, p-AKT, mTOR, p-mTOR in CMT-1211 and 4T1 cells. (M-P) After 0µM, NAC (10mM), IVM+MET, IVM+MET+NAC, Western blotting was used to analyze the expression levels of autophagy related proteins LC3B, Beclin, P62 and Bcl-2 in CMT-1211 and 4T1 cells.
Figure 6.
IVM combined with MET can inhibit the growth of canine breast tumor xenografts in vivo.(A) Establishing a mouse tumor model and a schematic diagram of the treatment plan. (B) Changes in body weight of mice in each group during treatment. (C) Changes in tumor volume of mice in each group during treatment. (D-E) After euthanizing the mice, the tumors of the mice were collected and weighed. (F) H&E staining of mouse tumor tissues and organs (heart, liver, spleen, lung, kidney). (G) Immunofluorescence staining of LC3B and P62 in tumors. Scale: 200µM.
Figure 6.
IVM combined with MET can inhibit the growth of canine breast tumor xenografts in vivo.(A) Establishing a mouse tumor model and a schematic diagram of the treatment plan. (B) Changes in body weight of mice in each group during treatment. (C) Changes in tumor volume of mice in each group during treatment. (D-E) After euthanizing the mice, the tumors of the mice were collected and weighed. (F) H&E staining of mouse tumor tissues and organs (heart, liver, spleen, lung, kidney). (G) Immunofluorescence staining of LC3B and P62 in tumors. Scale: 200µM.