1. Introduction
Proteoglycans (PGs) are complex macromolecules that are found on cell surface and in the extracellular matrix [
1]. They are formed by the covalent attachment of one or more glycosaminoglycan (GAG) chains onto the core protein. PG-GAG chains interact with a large number of ligands including growth factors and morphogens and their receptors as well as extracellular matrix structural molecules where they regulate activity and ligand stability [
2,
3]. PGs regulate several biological processes including cell-cell and cell-matrix interactions, development, signaling, proliferation, differentiation and various pathological conditions such as inflammation, tumor progression and viral and bacterial infection, [
4,
5,
6,
7]. Most commonly occurring PGs are chondroitin-sulphate PGs (CSPGs) and heparan-sulphate PGs (HSPGs). These PGs share a common tetrasaccharide linker region (GlcAβ1-3Galβ1-3Galβ1-4Xyl-O-Ser) for the attachment of the GAG chain onto the PG core protein. The first step in the synthesis of the tetrasaccharide primer is the addition of xylose on specific serine residues of the core protein. This initial and limiting step in GAG synthesis is catalyzed by xylosyltransferase I and II [
8,
9,
10]. Subsequently, two galactose residues are attached to xylose by β1,4-Galactosyltransferase 7 and β1,3-Galactosyltransferase 6 (β4GalT7 and β3GalT6), and one glucuronic acid by β1,3-Glucuronosyltransferase (GlcAT-I), therefore completing the synthesis of the terasaccharide primer. HS- and CS-GAG chains of PGs are built up on this linkage tetrasaccharide region by the alternate addition of
N-acetylhexosamine and glucuronic acid residues [
11]. The specificity of interaction between ligands and GAG chains is influenced by the fine structure of the GAG chain, primarily its sulfation pattern [
12]. The tetrasaccharide primer also undergoes various modifications including sulfation of galactose residues and phosphorylation of xylose [
13,
14].
Fam20 (family of sequence similarity 20) contains three members Fam20A, Fam20B and Fam20C [
15]. Their function has recently been established [
16,
17]. The substrates for Fam20A are unknown; however, Fam20A is important for the enamel biomineralization and tooth eruption [
17,
18]. Mutations in Fam20A are associated with various tooth disorders named human amelogenesis imperfecta and gingival hyperplasia syndrome [
19], and in some cases the renal calcification are also involved along with tooth disorders such as in Enamel Renal Syndrome [
18,
20]. Fam20C is a Golgi casein kinase and is mainly expressed in the biomineralized tissues. Fam20C phosphorylates casein and the Small Integrin-Binding Ligand, N-linked Glycoproteins (SIBLINGs) which are critical for biomineralization. Mutations in Fam20C are associated with Raine syndrome, characterized by a lethal osteosclerotic bone dysplasia [
16,
21,
22]. Recently, Fam20C from the C. elegans (ceFam20C) have been crystallized [
23]. The 3D structure revealed the presence of protein kinase-like fold with five disulfide and two Asparagine residues for N-linked glycosylation. The ceFam20C also contains a DFG (Asp-Phe-Gly) variant motif, in which the aspartate residue Asp
387 co-ordinates the divalent cation required for catalysis and a catalytic segment that includes the putative catalytic aspartate residue, Asp
366. The Asp
387 and Asp
366 residues are conserved in all the Fam20 family [
23].
Fam20B is a kinase that phosphorylates the xylose in the tetrasaccharide linkage region of PGs [
24,
25]. Genetic studies showed that loss-of-function mutations in
Fam20B in zebra fish decreases the amount of cellular GAG chains and cause cartilage and skeleton defects [
26]. Fam20B knockout in mice results in embryonic lethality and embryos showed multisystem organ hypoplasia and delayed development [
17]. On the other hand, it has been shown that gain-of-function of XYLP, the 2-phosphoxylose phosphatase that de-phosphorylates xylose in the tetrasaccharide linker region, increases the amounts of GAGs in Hela cells and inversely, loss-of-function of XYLP decreases GAGs synthesis [
27].
The aim of the present study was to investigate the potential regulatory function of FAM20B in the synthesis of PG. Here, we demonstrate that Fam20B gain-of-function inhibits the synthesis of PGs and showed that both CS/DS- and HS-PGs are affected. Interestingly, we found that Fam20B inhibition of PG synthesis is dose-dependent and is rescued by the phosphatase XYLP. Furthermore, based on the crystal structure of ceFam20C, we showed that aspartic acid residues D289 and D309 are essentials for Fam20B suppression of the synthesis of CS and HS-attached PGs.
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
The Chinese hamster ovary cells (CHO-K1), CHO galactosyltransferase I deficient cell line pgsB-618, human embryonic kidney cells HEK293, human brain tumor T98G, human lung adenocarcinoma cells A549 and human primary skin fibroblasts were cultured in DMEM (4.5 mg/ml glucose) or DMEM-F12 medium supplemented with 2 mM glutamin, 100 IU/ml penicillin, 100 µg/ml streptomycin and 10% fetal bovine serum (FBS, Dutscher Brumath, France) at 37°C with humidified atmosphere in a 5% CO2.
2.2. Vector Constructions and Cell Transfection
Fam20B was amplified from human placenta cDNA library (Takara Bio) by PCR using5’GAATTCCACCATGAAGCTAAAGCAGCGAGTCGTG3’ (forward) including EcoRI site and 5’GGATCCTTACAAGTGTGAGAGAGCCATCCT3’ (reverse) primers including BamHI site, using Advantage GC 2 Polymerase Mix (Takara Bio). XYLP was cloned by PCR using 5’GAATTCCACCATGCT TTTCCGCAACCGCTTC 3’ (forward) including EcoRI site and 5’CTCGAGTTAGAATCCTTCCCTGTGACATGC 3’ (reverse) primers including XhoI site. The amplified Fam20B and XYLP products were ligated into pCR2.1-TOPO vector (Invitrogen). Flag-XYLP and HA-XYLP cDNAs were generated by PCR. The coding region for Fam20B was excised and ligated into the pCMV vector (Stratagene, Valencia, CA) by double digestion with EcoRI and BamHI to generate pCMV-Fam20B. The coding region for Flag-XYLP and HA-XYLP were excised and ligated into the pCMV vector by double digestion with EcoRI and XhoI to generate pCMV-Flag-XYLP and pCMV-HA-XYLP. Decorin and HA-syndecan 4 cDNAs were generated by PCR and cloned into EcoRI and BamHI or EcoRI and XhoI sites of pCMV empty vector to generate pCMV-DCN and pCMV-HA-SDC4, respectively.
For transfection, cells were seeded in 6-well culture plate until 80% confluency and transfected with 250 ng of either pCMV-DCN or pCMV-HA-SDC4 in combination with 1 µg pCMV-Fam20B, pCMV-HA-XYLP, pCMV-Flag-XYLP, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV-empty vector using lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Expression of decorin and of syndecan 4 was analyzed at 48 h post-transfection by Western blotting.
2.3. Fam20B Knockdown
Sense 5’CACCGTATAGCCGAGACCATGTGG3’ and antisense 5’AA ACCCACA TGGTCTCGGCTATAC3’ oligonucleotides containing 20 bp sequence (underlined) targeting Fam20B exon 3 and cohesive ends (bold) with the vector were annealed and ligated into BbsI sites of pUC57-attbU6 sgRNA vector which express gRNA using avian-derived U6 promoter. Cells were transfected with empty pUc57-attbU6 (control), pUc57 attbU6/Fam20BgRNA in association with pspCas9 plasmid expressing Cas9. To facilitate screening, cells were co-transfected with pSVneo plasmid which express the neomycin resistance. Positive cells were screened using resistance to neomycin and gene mutation was determined by sequence analysis. The Fam20B genomic region targeted was amplified by PCR using specific primers. Sequencing results indicated that FAM20B gene harboured frameshift mutation mediated by deletion of two nucleotides at the sites targeted by gRNA, creating premature stop codon that prevents the translation of full length FAM20B. All analyzed sequences from the transfected cell clones exhibited the same deletions in FAM20B genomic region.
2.4. Site-Directed Mutagenesis
Site directed mutagenesis of the residues Asp289 to Ala289 and Asp309 to Ala309 in Fam20B were performed using the QuikChange XLII (Agilent, CA, USA) according to the recommendations of the manufacturer. pCMV-Fam20B expression vector was used as template. Sense and antisense oligonucleotides introducing the desired mutations were for D289A: 5’CTGATTGGCAATGCTGCCCGCATCACTAT GAG3’ (forward) and 5’CTCATAGTGATGGCGGGCAGCATTGCCAATCAG3’ (reverse) and for D309A: 5’TGCTCATCCTTCTTGCTAATGCCAAAAGCTTTGG3’ (forward) and 5’C CAAAGCTTTTGGCATTAGCAAGAAGGATGAGC A3’ (reverse). Full length mutated cDNA Fam20BD289A and Fam20BD309A were checked by DNA sequencing.
2.5. N-Glycosylation Analysis
Cells were grown in 6-well culture plate at 80% confluency then transfected with pCMV-Fam20B expression vector. At 24 h post-transfection the cells were lysed in the HEPES buffer and protein concentration was measured by the Bradford method [
28]. Twenty µg of protein was digested with PNGase F (New England Biolabs), which cleaves asparagine-linked (N-linked) oligosaccharides, according to the manufacturer’s instructions.
2.6. Western Blotting
Total protein from cells was extracted using RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1% deoxycholate, 0.1% SDS, 1% Triton X-100) supplemented with protease and phosphatase inhibitors (Roche Diagnostics, Indianapolis, IN, USA). Cell lysates were sonicated on ice and protein concentration of the samples was determined by the Bradford method. Proteins from culture medium and from cell lysates (50 μg/lane) were separated on 10% SDS-PAGE gels, transferred to a PVDF membrane (Bio-RAD), and subsequently blocked in PBS-Tween 20 containing 5% nonfat milk or 5% BSA. Membranes were then incubated overnight with primary antibodies directed against Fam20B (Cat# HPA 007409, 1/1000, Sigma-Aldrich), decorin (Cat# MAB143, 1:1000, R&D Systems), HA (Cat# 901501, 1:10000, BioLegend), M2-Flag (Cat# F1804, Sigma-Aldrich) or βactin (Cat#3700, 1/2000, CST) followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Cat# 7074, 1:2000, CST or Cat# 7076, 1:2000 CST). Antibodies were diluted in 5% BSA/0.01% tween 20 in PBS. The blots were then developed using Clarity Western ECL substrate (BIO-RAD, Hercules, CA) according to the instructions of the manufacturer.
2.7. Metabolic Labelling of GAG Chains
Metabolic labelling of PG-GAG chains was carried out using
35S-sulfate incorporation method as described by De Vries et al, (1986) [
29]. Briefly, cells were grown in 6-well culture plate and transfected with pCMV-Fam20B, pCMV-Fam20B
D289A, pCMV-Fam20B
D309A, or pCMV empty vector. Cells were then radiolabeled with 10 µCi/ml of
35S-sulfate (Perkin Elmer) in sulfate free media containing 2% dialyzed FBS. After overnight incubation, the culture medium was collected, digested with papain (1 mg/ml) and
35S-labeled GAGs were precipitated by cetylpyridinium chloride (CPC) as described by Bronson et al., (1987) [
30]. The CPC precipitated radiolabeled GAGs were separated by SDS-PAGE on a 4-12% Nu-PAGE gel. The gel was dried and exposed to autoradiography film. To measure the rate of sulfate incorporation into GAG chains of PGs, HEK293 transfected with pCMV empty vector or pCMV-Fam20B were radiolabeled with 10 µCi/ml of [
35S]-sulfate for 6 h then, conditioned culture medium was collected and digested with papain (1mg/ml). [
35S]-labeled GAG chains were precipitated by CPC dissolved in solvable and mixed in scintillation fluid (Perkin Elmer, MA, USA). The radioactivity associated with GAGs was measured by liquid scintillation counting (Packard, Rungis, France).
2.8. Immunofluorescence Analysis
The CHO-K1 cells were grown on glass coverslips and transfected with Fam20B expression vector pCMV-Fam20B or with pCMV empty vector (control). At 36 h after transfection, cells were fixed with 3% (w/v) paraformaldehyde in PBS for 20 min and were permeabilized by treatment with 0.1% (w/v) Triton X-100/PBS solution for 4 min. After extensive washing in 0.2% (w/v) fish skin gelatin in PBS, cells were then incubated with primary antibodies anti-Fam20B (Cat# HPA 007409, 1/100 Sigma-Aldrich) and anti-HS (Cat# 370255-1, 1:100, AMSBIO) for 20 min. Cells were washed several times in 0.2% (w/v) fish skin gelatin in PBS and incubated with secondary antibodies coupled with Alexa Fluor 555 and Alexa Fluor 488 (Cat# A-21428 or Cat# A-11017, Molecular Probes) for 20 min. Cells were washed with PBS and nuclei were stained with Hoechst/PBS solutions then coverslips were mounted with Moviol (National Diagnostics, U.K.) containing 1% propylgallate (Sigma-Aldrich). Digital images were captured with an inverted microscope Lieca DMI3000 B (Leica Microsystems, Germany).
2.9. Data Analysis and Statistical Procedures
Each experiment was repeated at least three times independently. Quantitative data were expressed as mean ± S.D. Statistical analysis was performed with an unpaired two-tailed Student's t-test, and effects were considered statistically significant at *P<0.05. One representative immunoblot of three independent experiments was shown in results.
4. Discussion
In this study we showed that Fam20B gain-of-function induced a strong reduction in overall PG-GAG chains produced in the cell. By using decorin and syndecan 4 as reporter proteins for CS and HS-attached PGs, we showed that Fam20B gain-of-function negatively regulates the synthesis of both CS- and HS-attached PGs. This has been further confirmed using human skin and lung fibroblasts expressing high amount of endogenous decorin in the medium. Fam20B gain-of-function blocks the synthesis of decorin in both skin and lung fibroblast cells. Similar results were obtained for endogenous HSPGs. By using anti-HS 10E4 antibody which is commonly used to detect HS chains of PGs [
31,
32,
33], we showed that Fam20B gain-of-function strongly attenuates the expression of cell surface HSPGs. Remarkably, we showed that Fam20B gain-of-function reduced the synthesis of both CS/DS-attached decorin and HS-attached syndecan 4 in a dose dependent manner and induces intracellular accumulation of GAG-free decorin and syndecan 4. Therefore, suggesting that lack of GAG elongation prevents PGs from being secreted. Interestingly, reduced secretion in culture medium of decorin and syndecan 4 in Fam20B expressing cells was accompanied with increased amounts of intracellular non elongated decorin and syndecan 4 that are probably not competent for secretion and accumulates in the cells. Altogether, these data clearly demonstrate that Fam20B gain-of-function negatively regulates the synthesis of PGs of both types CS and HS. As the synthesis of CS and HS GAGs share a common tetrasaccharide primer, Fam20B gain-of-function affects the synthesis of the primer. Indeed, blocking of any step in the synthesis of this primer will obviously impact the synthesis of both CS and HS.
Of note, it has been reported that stable expression of Fam20B in Hela cells increased the amounts of GAGs with particularly augmented short CS chains [
24]. On the other hand, it has been shown that loss-of-function of XYLP, the 2-phosphoxylose phosphatase that de-phosphorylates xylose of PG primer region, decreased HS and CS synthesis and inversely, gain-of-function increased their synthesis [
27]. Using transient expression of Fam20B in several cell lines as well as primary fibroblast cells we found that Fam20B gain-of-function reduced the synthesis of GAG-attached PGs in all the cells tested. The discrepancy may be due to the selection of stable clones or to an unknown factor that has to be identified. In the other hand, in agreement with our study, it has been shown the knockout of Fam20B in bone osteosarcoma cells U2OS led to reduction in the synthesis of GAGs [
25]. This study also showed that GalTII present higher activity towards phosphorylated Gal-Xylose, compared to its unphosphorylated counterpart, thus loss of Fam20B causes impaired GalTII activity resulting in the formation of incomplete linkage tetrasaccharides capped with sialic acid and cannot be elongated [
25].
During the physiological conditions there is a balance between the phosorylation and dephosphorylation of the xylose by Fam20B and XYLP, respectively. Therefore, gain- or loss-of-function of either Fam20B or XYLP may disturb this balance thus affect the rate of GAG synthesis. How Fam20B inhibits the synthesis of GAG chains is unknown, however one can hypothesize that Fam20B gain-of-function may lead to sustained phosphorylation of xylose residue on the core protein, therefore competing with XYLP phosphatase that de-phosphorylates xylose of PG linker region, a process that may be required prior to subsequent GAG elongation. Noteworthy, a working model for the role of Fam20B in the synthesis of the tetrasaccharide linkage and subsequent GAG elongation suggests that xylose phosphorylation by Fam20B occurs predominantly after addition of the first galactosyl residue to the growing linkage region and that the removal of the xylose 2-phosphate moiety by the xylose 2-phosphatase, XYLP occurs following completion of the tetrasaccharide linkage to allow GAG elongation [
34]. If the phosphate is not removed by XYLP, EXTL2 transfers the GlcNAc residue
via an α1,4-linkage on the phosphorylated tetrasaccharide, leading to synthesis of phosphorylated pentasaccharide structure unable to serve as primer for CS/HS polymerization [
27]. Whereas it has been reported that phosphorylated tetrasaccharide is a preferred substrate for ChGn-1 enzyme that transfers a GalNAc residue to the phosphorylated tetrasaccharide in the protein linkage region of CS.
Interestingly, it has been shown that phosphorylation of xylose before addition of the first galactosyl residue occurs. Indeed, analysis of [
32P]-labelled O-linked glycan chains and stubs attached to intracellular decorin, revealed that 52% of Xyl-decorin was phosphorylated, therefore indicating that phosphorylation of xylose occurs before addition of galactose residue on Xyl-decorin and continues when the Gal residues are added and is complete at the trisaccharide stage [
34,
35]. In a previous study, we have reported that β4GalT7 efficiently catalyzes the transfer of galactose residue onto the non-phosphorylated xyloside analog, whereas the phosphorylated xylose analog at position 2-O was not substrate, suggesting that the 2-O phosphorylation precludes the transfer of the galactose on xylose [
13]. In line with this, Siegbahn et al., showed that most modifications of position 2 in xylose, rendered analogs less prone to galactosylation by β4GalT7 [
36] and showed that xylose analogs carrying modification on the 2-O position were not able to prime GAG synthesis in human cell lines [
37]. These data indicate that the hydroxyl in position 2 might act as a hydrogen bond acceptor. The crystal structure of the β4GalT7 has been published recently and revealed that the Tyr
177, Tyr
179, Trp
207, and Leu
209 are important for the hydrophobic binding of the xylose and that the Asp
211 forms a strong hydrogen bond with the OH present in the 4
th position of the acceptor xylose. Whereas, the Asp
212 interacts via a hydrogen bond with the OH present at 2
nd position of the xylose [
38]. This, suggested that phosphorylation of xylose before addition of Gal residue by Fam20B may lead to premature termination of the tetrasaccharide primer synthesis and subsequently to unelongated GAGs. This may constitute a mechanism to counteract excessive synthesis of elongated GAG chains.
All the members of the Fam20 family contains a conserved C-terminal domain including the catalytic domain DRHHYE and a DFG motif which is crucial for the metal ion binding. It has been shown that mutation of the aspartic acid residues in the catalytic segment and in the DFG motif of Fam20C abolished the enzyme activity [
21]. These aspartic acid residues are conserved in all members of the Fam20 family and correspond to Asp
289 and Asp
309 of the human Fam20B. Mutation of either of the two aspartic residues to alanine abolished the ability of Fam20B to block the synthesis of both CS- and HS-attached PGs, suggesting that mutation of Asp
289 and Asp
309 impaired Fam20B activity, consistent with the putative role of theses residues in catalysis and in the binding of the divalent cation Mn
2+, respectively.
On the other hand, analysis of biological process including cell proliferation and migration indicated that both cell proliferation and migration were impaired in Fam20B-transfected cells. Expression of Fam20B in glioblastoma cells led to a significant reduction in proliferation and in the ability of cells to migrate in scratch-wound healing assays. The importance of PGs and their GAG chains in cell proliferation and migration is well established [
39] and numerous PGs have been implicated in tumor growth and metastasis [
40]. Therefore, Fam20B-dependent inhibition of PG-GAG synthesis could contribute to the cell proliferation and migration defects observed in glioblastoma cells.
Figure 1.
Fam20B blocks the synthesis of PG-GAG chains. HEK293 cells were transfected with pCMV-Fam20B or pCMV empty vector (control) and (A) Fam20B was detected by Western blot using anti-Fam20B antibodies. β-actin was used as loading control. (B) Western blot analysis of the sensitivity of Fam20B to digestion with PNGase F. (C) Analysis of the level of PG-GAG chain synthesis in HEK293 cells transfected with either Fam20B expression vector or empty plasmid (control) using 35S-sulfate incorporation method. (D) SDS-PAGE analysis of radiolabeled GAG chains produced in culture medium of HEK293 cells transfected with either Fam20B expression vector or empty plasmid (control) using 35S-sulfate incorporation method. The amounts of 35S-sulfate GAGs were normalized to DNA and relative to control. (E) The bar graph represents the quantification of GAG chains on the autoradiography of the SDS-PAGE. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
Figure 1.
Fam20B blocks the synthesis of PG-GAG chains. HEK293 cells were transfected with pCMV-Fam20B or pCMV empty vector (control) and (A) Fam20B was detected by Western blot using anti-Fam20B antibodies. β-actin was used as loading control. (B) Western blot analysis of the sensitivity of Fam20B to digestion with PNGase F. (C) Analysis of the level of PG-GAG chain synthesis in HEK293 cells transfected with either Fam20B expression vector or empty plasmid (control) using 35S-sulfate incorporation method. (D) SDS-PAGE analysis of radiolabeled GAG chains produced in culture medium of HEK293 cells transfected with either Fam20B expression vector or empty plasmid (control) using 35S-sulfate incorporation method. The amounts of 35S-sulfate GAGs were normalized to DNA and relative to control. (E) The bar graph represents the quantification of GAG chains on the autoradiography of the SDS-PAGE. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
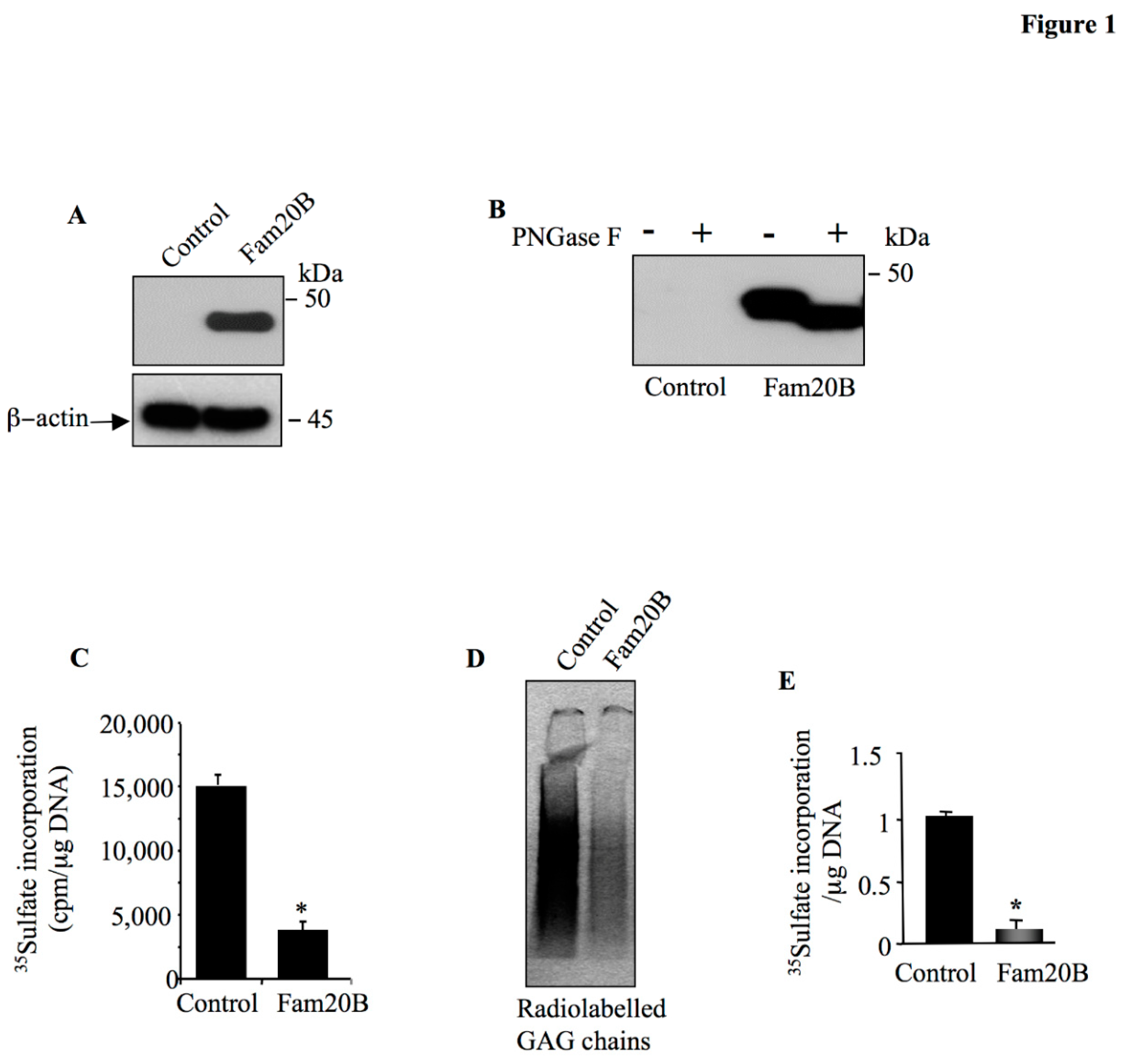
Figure 2.
Fam20B suppression of GAG chain synthesis is dose-dependent. (A) HEK293 cells were co-transfected with pCMV-DCN along with pCMV-Fam20B or empty pCMV vector (control) and the expression of DCN was analyzed in medium by Western blot using anti-DCN antibodies. (B) CHO PgsB-618 cells were co-transfected with pCMV-DCN along with empty pCMV (control), pCMV-Fam20B, pCMV-β4GalT7 or pCMV-Fam20B and pCMV-β4GalT7. DCN produced in the medium was analyzed by Western blot using anti-DCN antibodies. (C) Western blot analysis of Fam20B and DCN produced in the medium HEK293 cells transfected with pCMV-DCN and increased concentrations of Fam20B. (D) The bar graph represents the quantification of DCN in the Western blot. (E) Detection of intracellular DCN in cells transfected with pCMV-DCN and increased concentrations of Fam20B. β-actin was used as loading control. (F) The bar graph represents the quantification of DCN in the Western blot. (G) Western blot analysis of secreted DCN in the medium of human primary skin fibroblasts transfected with pCMV-Fam20B or empty vector (control). (H) The bar graph represents the quantification of DCN in the Western blot. (I) Detection of secreted DCN in the medium of A549 cells transfected with pCMV-Fam20B or empty vector (control). (J) The bar graph represents the quantification of DCN in the Western blot. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
Figure 2.
Fam20B suppression of GAG chain synthesis is dose-dependent. (A) HEK293 cells were co-transfected with pCMV-DCN along with pCMV-Fam20B or empty pCMV vector (control) and the expression of DCN was analyzed in medium by Western blot using anti-DCN antibodies. (B) CHO PgsB-618 cells were co-transfected with pCMV-DCN along with empty pCMV (control), pCMV-Fam20B, pCMV-β4GalT7 or pCMV-Fam20B and pCMV-β4GalT7. DCN produced in the medium was analyzed by Western blot using anti-DCN antibodies. (C) Western blot analysis of Fam20B and DCN produced in the medium HEK293 cells transfected with pCMV-DCN and increased concentrations of Fam20B. (D) The bar graph represents the quantification of DCN in the Western blot. (E) Detection of intracellular DCN in cells transfected with pCMV-DCN and increased concentrations of Fam20B. β-actin was used as loading control. (F) The bar graph represents the quantification of DCN in the Western blot. (G) Western blot analysis of secreted DCN in the medium of human primary skin fibroblasts transfected with pCMV-Fam20B or empty vector (control). (H) The bar graph represents the quantification of DCN in the Western blot. (I) Detection of secreted DCN in the medium of A549 cells transfected with pCMV-Fam20B or empty vector (control). (J) The bar graph represents the quantification of DCN in the Western blot. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
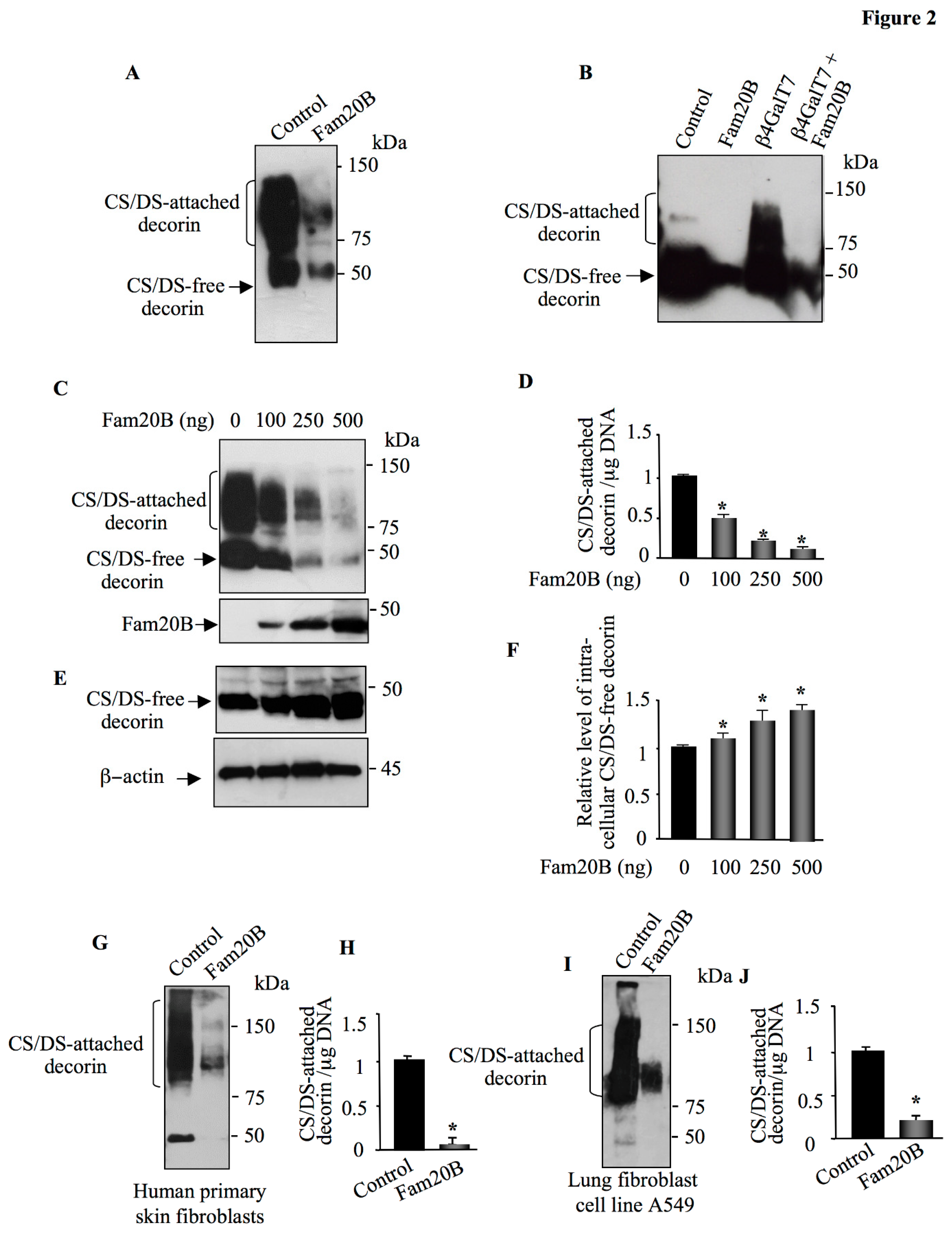
Figure 3.
Fam20B blocks the synthesis of HSPGs. (A) HEK293 cells were co-transfected with pCMV-HA-SDC4 and either pCMV-Fam20B or empty pCMV (control) and SDC4 was analyzed in cell lysate by Western blot using anti-HA antibodies. β-actin was used as loading control. (B) The bar graph represents the quantification of the Western blots. (C) Western blot analysis of SDC4 and of Fam20B in cell lysate of HEK293 cells transfected with increased concentrations of Fam20B. β-actin was used as loading control. (D) The bar graph represents the quantification of HS-attached SDC4 in the Western blot. (E) The bar graph represents the quantification of HS-free SDC4 in the Western blot. (F) CHO-K1 cells were transfected with pCMV-Fam20B or empty pCMV (control) vector and expression of cell surface HSPGs was examined by immunofluorescence using anti-heparan sulfate antibody 10E4 (green). The expression of Fam20B was analyzed using anti-Flag antibody (red). Nuclei were stained (blue) using Hoechst/PBS solution. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
Figure 3.
Fam20B blocks the synthesis of HSPGs. (A) HEK293 cells were co-transfected with pCMV-HA-SDC4 and either pCMV-Fam20B or empty pCMV (control) and SDC4 was analyzed in cell lysate by Western blot using anti-HA antibodies. β-actin was used as loading control. (B) The bar graph represents the quantification of the Western blots. (C) Western blot analysis of SDC4 and of Fam20B in cell lysate of HEK293 cells transfected with increased concentrations of Fam20B. β-actin was used as loading control. (D) The bar graph represents the quantification of HS-attached SDC4 in the Western blot. (E) The bar graph represents the quantification of HS-free SDC4 in the Western blot. (F) CHO-K1 cells were transfected with pCMV-Fam20B or empty pCMV (control) vector and expression of cell surface HSPGs was examined by immunofluorescence using anti-heparan sulfate antibody 10E4 (green). The expression of Fam20B was analyzed using anti-Flag antibody (red). Nuclei were stained (blue) using Hoechst/PBS solution. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
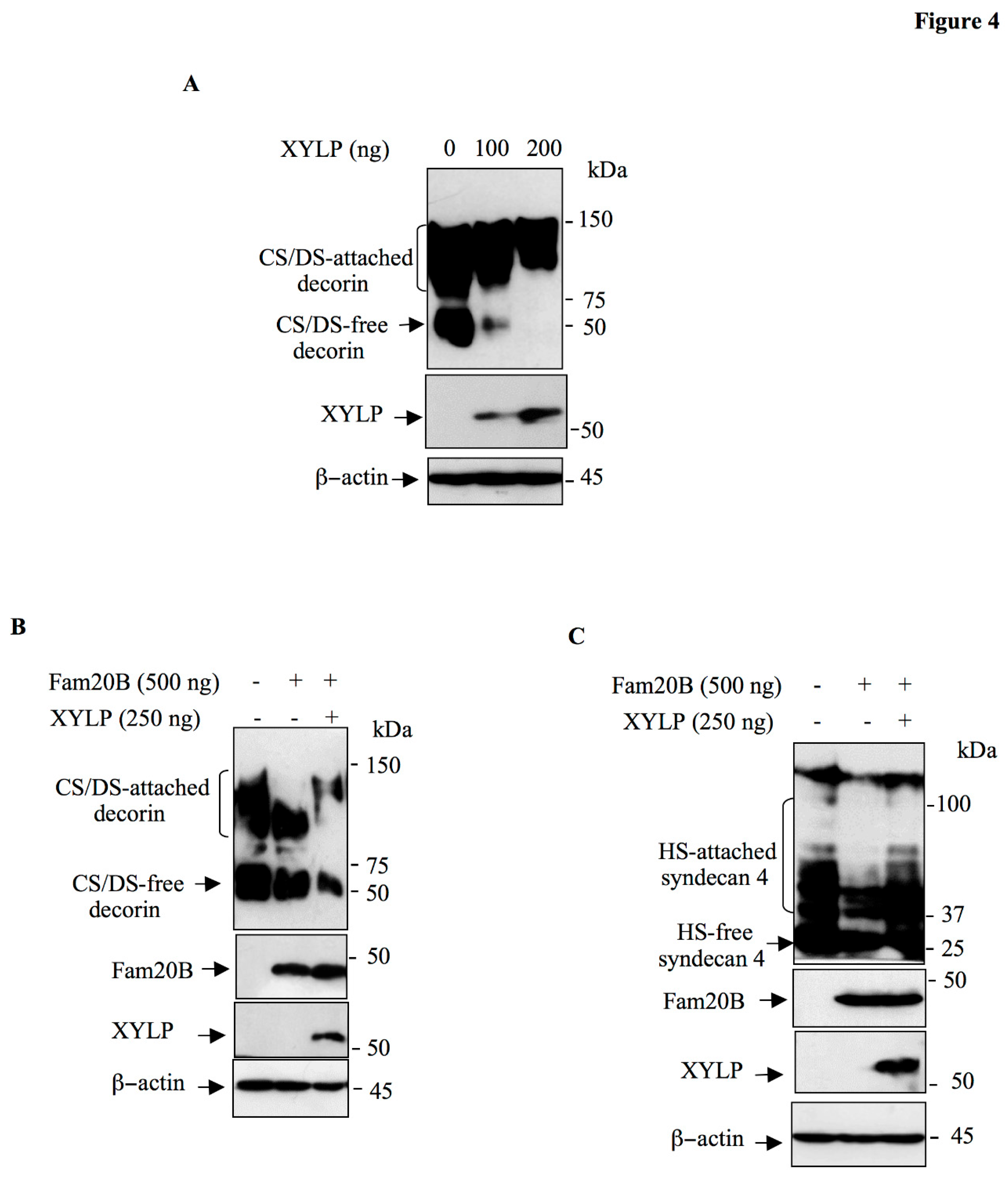
Figure 4.
XYLP rescue the synthesis of GAG-chains produced by Fam20B. (A) HEK293 Cells were transfected with pCMV-DCN together with increased concentrations of pCMV-HA-XYLP. Expression of DCN and XYLP was analyzed by Western blot using anti-DCN and anti-HA antibodies, respectively. β-actin was used as loading control. (B) HEK293 cells were transfected with pCMV-DCN (control), pCMV-DCN and pCMV-Fam20B or pCMV-DCN and pCMV-Fam20B together with pCMV-HA-XYLP. DCN, FAM20B and XYLP were analyzed by Western blot using anti-DCN, anti-Fam20B and anti-HA antibodies, respectively. β-actin was used as loading control. (C) HEK293 cells were transfected with pCMV-HA-SDC4, pCMV-HA-SDC4 and pCMV-Fam20B or pCMV-HA-SDC4 and pCMV-Fam20B together with pCMV-Flag-XYLP. SDC4, FAM20B and XYLP were analyzed in cell lysate by Western blot using anti-HA, anti-Fam20B and anti-Flag antibodies, respectively. β-actin was used as loading control. Data were presented as mean ± SD of three separate experiments.
Figure 4.
XYLP rescue the synthesis of GAG-chains produced by Fam20B. (A) HEK293 Cells were transfected with pCMV-DCN together with increased concentrations of pCMV-HA-XYLP. Expression of DCN and XYLP was analyzed by Western blot using anti-DCN and anti-HA antibodies, respectively. β-actin was used as loading control. (B) HEK293 cells were transfected with pCMV-DCN (control), pCMV-DCN and pCMV-Fam20B or pCMV-DCN and pCMV-Fam20B together with pCMV-HA-XYLP. DCN, FAM20B and XYLP were analyzed by Western blot using anti-DCN, anti-Fam20B and anti-HA antibodies, respectively. β-actin was used as loading control. (C) HEK293 cells were transfected with pCMV-HA-SDC4, pCMV-HA-SDC4 and pCMV-Fam20B or pCMV-HA-SDC4 and pCMV-Fam20B together with pCMV-Flag-XYLP. SDC4, FAM20B and XYLP were analyzed in cell lysate by Western blot using anti-HA, anti-Fam20B and anti-Flag antibodies, respectively. β-actin was used as loading control. Data were presented as mean ± SD of three separate experiments.
Figure 5.
The aspartic acid residues Asp289 and Asp309 are essential for Fam20B function. (A) Detection of wild-type Fam20B and mutants in HEK293 cells transfected with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) using anti-FAM20B antibodies. β-actin was used as loading control. (B) HEK293 cells were co-transfected with pCMV-DCN along with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) and DCN produced in the medium was analyzed by Western blot using anti-DCN antibodies. (C) HEK293 cells were co-transfected with pCMV-HA-SDC4 along with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) and SDC4 was analyzed by Western blot using anti-HA antibodies. β-actin was used as loading control. (D) PG-GAG chains produced in the medium of HEK293 cells transfected with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) were metabolically labelled by 35S-sulfate incorporation and isolated by CPC precipitation then separated by SDS-PAGE and revealed by autoradiography. (E) The bar graph represents the quantification of 35S-sullfate radiolabelled GAG chains of the autoradiography. (F) A549 cells were transfected with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) and DCN secreted by the cells in the medium was analyzed by Western blot using anti-DCN antibodies. (G) The bar graph represents the quantification of the Western blot. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
Figure 5.
The aspartic acid residues Asp289 and Asp309 are essential for Fam20B function. (A) Detection of wild-type Fam20B and mutants in HEK293 cells transfected with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) using anti-FAM20B antibodies. β-actin was used as loading control. (B) HEK293 cells were co-transfected with pCMV-DCN along with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) and DCN produced in the medium was analyzed by Western blot using anti-DCN antibodies. (C) HEK293 cells were co-transfected with pCMV-HA-SDC4 along with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) and SDC4 was analyzed by Western blot using anti-HA antibodies. β-actin was used as loading control. (D) PG-GAG chains produced in the medium of HEK293 cells transfected with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) were metabolically labelled by 35S-sulfate incorporation and isolated by CPC precipitation then separated by SDS-PAGE and revealed by autoradiography. (E) The bar graph represents the quantification of 35S-sullfate radiolabelled GAG chains of the autoradiography. (F) A549 cells were transfected with pCMV-Fam20B, pCMV-Fam20BD289A, pCMV-Fam20BD309A or pCMV empty vector (control) and DCN secreted by the cells in the medium was analyzed by Western blot using anti-DCN antibodies. (G) The bar graph represents the quantification of the Western blot. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
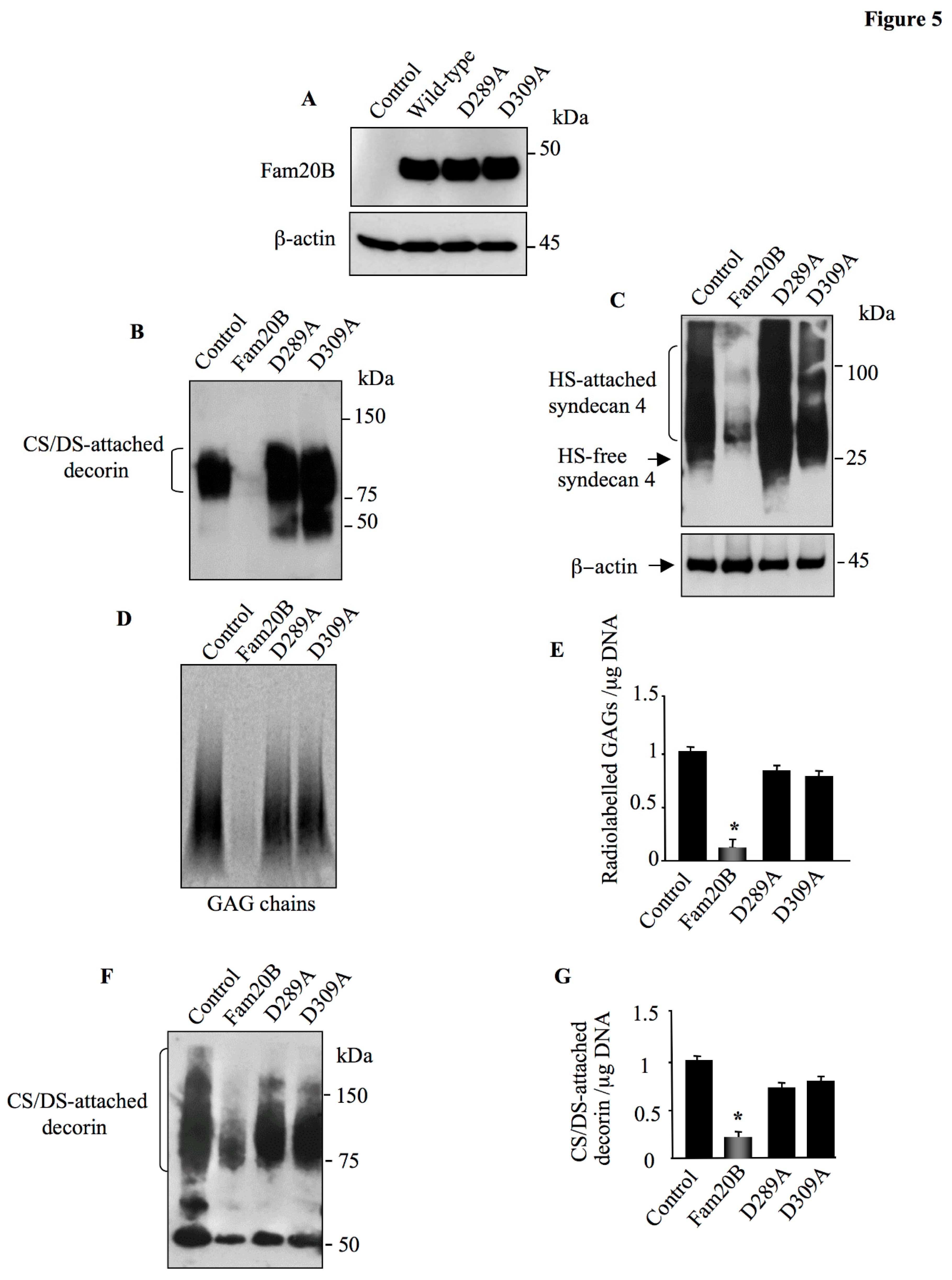
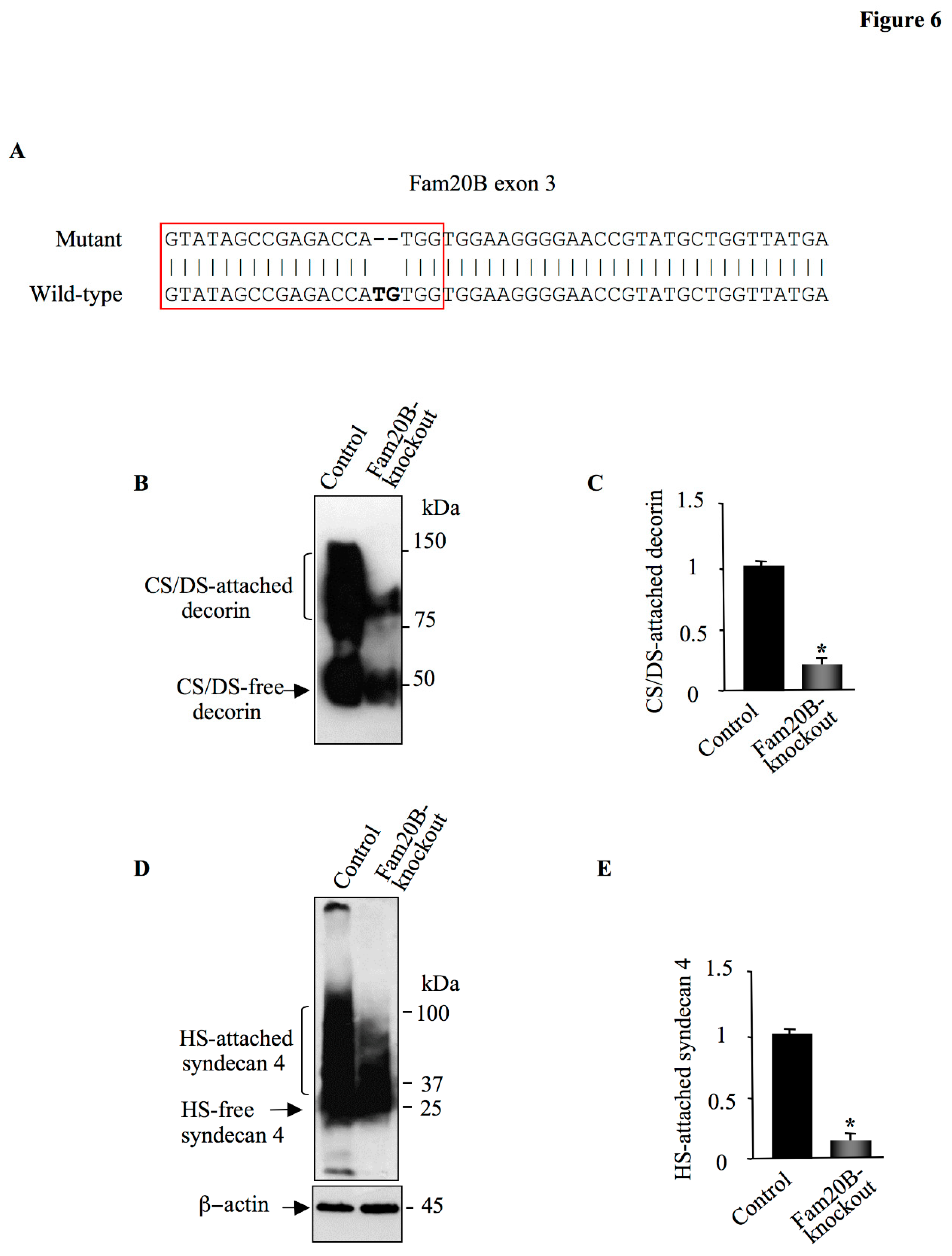
Figure 6.
CRISPR/Cas9 knockdown of Fam20B. (A) Alignment of Fam20B targeted sequence from wild-type and mutant HEK293 cells. (B). Detection of DCN in the culture medium of control and Fam20B-knockout HEK293 cells transfected with pCMV-DCN. (C) The bar graph represents the quantification of the Western blot. (D) Western blot analysis of SDC4 in cell lysate of HEK293 cells (control) and Fam20B-knockout HEK293 cells transfected with pCMV-HA-SDC4. β-actin was used as loading control. (E) The bar graph represents the quantification of the Western blot. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
Figure 6.
CRISPR/Cas9 knockdown of Fam20B. (A) Alignment of Fam20B targeted sequence from wild-type and mutant HEK293 cells. (B). Detection of DCN in the culture medium of control and Fam20B-knockout HEK293 cells transfected with pCMV-DCN. (C) The bar graph represents the quantification of the Western blot. (D) Western blot analysis of SDC4 in cell lysate of HEK293 cells (control) and Fam20B-knockout HEK293 cells transfected with pCMV-HA-SDC4. β-actin was used as loading control. (E) The bar graph represents the quantification of the Western blot. Data were presented as mean ± SD of three separate experiments. Statistical significance was evaluated using Student’s t test (*, P<0.05).
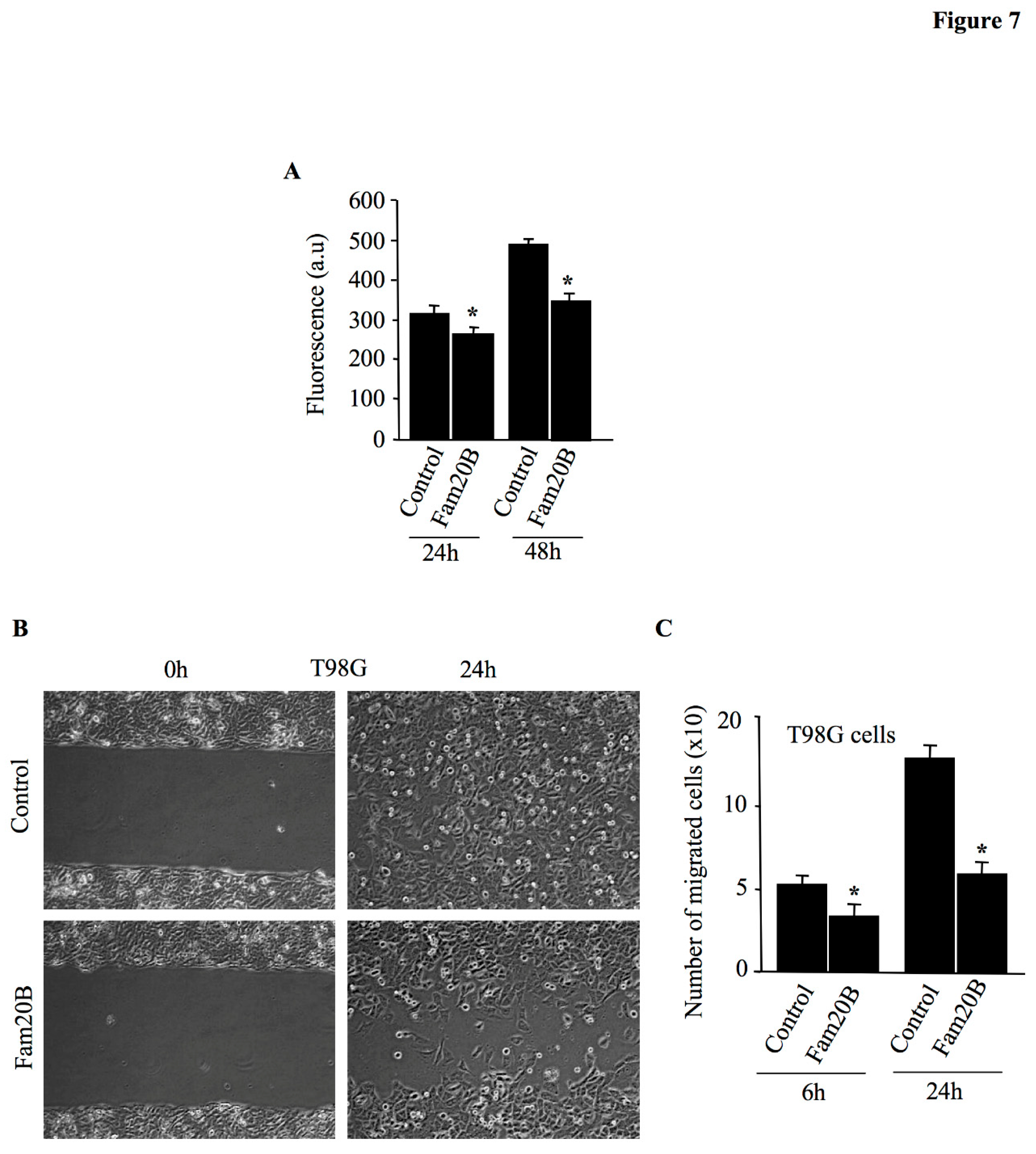
Figure 7.
Fam20B reduces the proliferation and migration of glioblastoma cells T98G. (A) Glioblastoma cells T98G were plated in 96 well plates and transfected with pCMV-Fam20B or empty pCMV vector (control) and cell proliferation was measured by CyQUANT NF Cell Proliferation Assay Kit at 24 h and 48 h after transfection. (B) Migration of glioblastoma cell line T98G transfected with pCMV-Fam20B or pCMV empty vector (control) was assessed using scratch wound-healing assay as described in Materials and Methods section and cell migration was photographed at 0 and 24h after scratch by phase-contrast microscopy. (C) The bar graph depicting the number of cells that migrate in the scratch area after 6 h and 24 h. Data were presented as mean ± SD of three separate experiments with three replicates each. Statistical significance was evaluated using Student’s t test (*, P<0.05).
Figure 7.
Fam20B reduces the proliferation and migration of glioblastoma cells T98G. (A) Glioblastoma cells T98G were plated in 96 well plates and transfected with pCMV-Fam20B or empty pCMV vector (control) and cell proliferation was measured by CyQUANT NF Cell Proliferation Assay Kit at 24 h and 48 h after transfection. (B) Migration of glioblastoma cell line T98G transfected with pCMV-Fam20B or pCMV empty vector (control) was assessed using scratch wound-healing assay as described in Materials and Methods section and cell migration was photographed at 0 and 24h after scratch by phase-contrast microscopy. (C) The bar graph depicting the number of cells that migrate in the scratch area after 6 h and 24 h. Data were presented as mean ± SD of three separate experiments with three replicates each. Statistical significance was evaluated using Student’s t test (*, P<0.05).