1. Introduction
In 1993, Victor Ambros and Gary Ruvkun first described microRNAs and their functions in the nematode
C. elegans, thereby initiating intensive research on the role of these molecules in living organisms. In their studies, researchers demonstrated that the
lin-4 gene encodes small RNA molecules whose sequences are complementary to the 3' UTR regions in the mRNA of the
lin-14 gene and thus regulate the translation of the transcript of this gene, through RNA-RNA interaction [
1,
2]. In 2024, they were awarded the Nobel Prize in Physiology or Medicine for their scientific discoveries.
MicroRNAs are small non-coding RNA molecules 20-24 nucleotides in length that are known for their ability to post-transcriptionally regulate gene expression. MicroRNA molecules can bind to mRNAs, which can lead to transcript degradation or translation inhibition [
3,
4]. Due to the tremendous impact of miRNAs on protein regulation, they are crucial for maintaining body homeostasis. Previous studies show that their dysregulated levels can cause many dangerous diseases. Among other things, it has been discovered that dysregulated miRNAs are associated with the occurrence of numerous heart diseases [
5], as well as autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis [
6]. However, special attention is drawn to the impact of miRNAs on the development and progression of various types of cancer, which, due to their higher incidence and high mortality rate, pose a serious challenge to the health care system [
7].
MicroRNAs are responsible for many cellular processes such as differentiation, proliferation, growth and development [
8], so these molecules are being studied for their role in tumorigenesis. One of the miRNAs implicated in cancer development is miR-28. Its role in tumorigenesis has been described by many research groups, and this article reviews the current literature on this topic. We also investigated the function of miR-28 in other non-oncological diseases, including immunological and neurodegenerative diseases.
2. MicroRNA Biogenesis
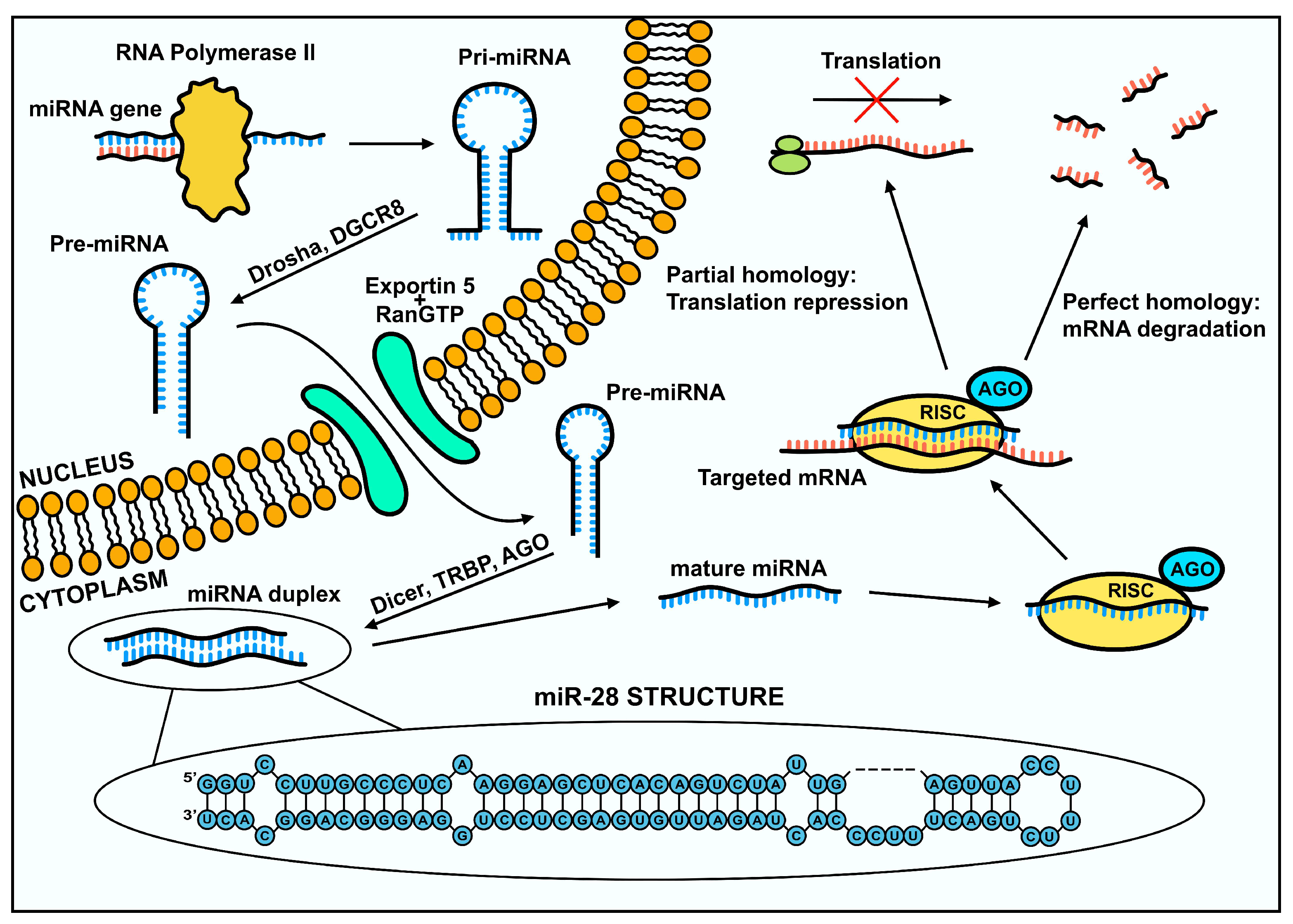
The synthesis of miRNA begins with the transcription of miRNA genes by RNA polymerase II, which forms molecules about 80 nucleotides long, containing a 3’ poly(A) tail and a 5' cap [
9]. This structure is a primary transcript called pri-miRNA. These transcripts are then recognized and cleaved by a nuclear ribonuclease, Drosha RNase III fused to the DiGeorge syndrome critical region 8 (DGCR8) protein, which together form a microprocessor complex. Drosha ribonuclease cleaves hairpin fragments from the pri-miRNA, to form pre-miRNA of about 60 nucleotides in length [
4,
10,
11]. The pre-miRNA is then transported from the cell nucleus into the cytoplasm via exportin-5/RanGTP [
11,
12]. Once the pre-miRNA is exported to the cytoplasm, it undergoes further processing by the RNase III endonuclease Dicer. After this step, a miRNA duplex is formed, and each strand already has a target length of about 20-24 nucleotides. Subsequently, the duplex containing the 5p and 3p strands is cleaved into two separate strands. The 5p strand is then usually incorporated into the miRISC (miRNA-induced silencing complex), and the 3p strand may be degraded or perform regulatory functions in the cell as well (
Figure 1) [
11,
13].
3. Mechanism of Gene Expression Regulation via miR-28
The fully formed miRISC is involved in regulating protein levels in the cell [
4]. The AGO (argonaute) family proteins, which bind to mature miRNAs, play a very important role in this complex [
14]. The microRNAs incorporated into RISC are used to recognize the complementary sequences in the 3'UTR region of the target mRNA [
15]. The type of regulation depends on the complementarity of the target sequence with the miRNA and the type of AGO protein incorporated into the complex. Typically, when the sequence is highly complementary, it results in mRNA degradation. When the nucleotide match is lower, inhibition of transcript translation occurs (
Figure 1) [
3]. The mechanism of transcript degradation is initiated by the attachment of miRISC to mRNA, which activates binding of PABP (Poly(A)-Binding Protein), PAN2-PAN3 (poly(A)-nuclease, poly(A) nuclease) and CCR4-NOT (carbon catabolite repression- negative on TATA) complexes, which are successively responsible for the process of poly(A) deadenylation. Subsequently, the DCP2 (decapping protein 2) protein with associated cofactors, removes the cap from the 5' end of the transcript. Deadenylation and decapping leads to degradation of the transcript facilitated by 5' to 3' exoribonuclease 1 (XRN1) [
14].
In mammalian cells, the process of translation inhibition is responsible for 6-26% of all gene expression regulatory processes [
14]. Various possible mechanisms of inhibition have been proposed. One of the possibilities is regulation through modification of the cap-dependent translation process and the poly(A) tail. Transcript translation is dependent on the eIF4F complex that binds the 5’ cap of mRNA. The complex consists of three subunits. One of these subunits is eIF4G factor, which interacts with PABP protein. Disruption of PABP binding and its interaction with eIF4G, as well as deadenylation of the poly(A) tail in mRNA results in translation inhibition [
16]. Transcripts that are regulated are then translocated to specific sites in the cell called P-bodies (processing bodies), where they can be stored or degraded [
17].
4. Significance of miR-28 in Cancer Progression
4.1. Mechanisms of Cancer Progression
The process of tumorigenesis is often associated with exposure to chemical agents, ionizing and UV radiation, as well as the result of inherited predispositions [
18,
19,
20]. Nevertheless, it may be also a consequence of unhealthy lifestyle: smoking, alcohol overconsumption, poor diet or physical inactivity [
18,
19].
The tumorigenic process is initiated by DNA damage, which consequently leads to the transformation of normal cells into cancer cells with increased survival, migration and invasion capabilities. Carcinogenesis also involves the formation of the tumor microenvironment through remodeling of the extracellular matrix (ECM) [
21,
22]. Tumor cells proliferate rapidly, leading to intense tumor growth. At the same time, cells acquire the ability to evade the immune system response by losing the MHC I antigen presentation mechanism. This allows tumor cells to evade recognition by CD8 T cells [
23]. Furthermore, angiogenesis processes are activated, which promotes disease progression [
24]. In subsequent stages of progression, tumor cells acquire the ability to migrate and invade other tissues. This is a result of the epithelial-mesenchymal transition (EMT), associated with the loss of E-cadherin, which is crucial for maintaining intercellular connections [
25].
MicroRNAs play an important role in the processes of carcinogenesis. They can act as both tumor suppressors by regulating oncogenes, as well as be responsible for inhibiting tumor suppressor genes, thereby enhancing oncogenic processes. MicroRNAs that induce tumorigenesis are called oncomiRs [
26]. Some miRNAs depending on the type of cancer, can act both as oncomiRs and suppressors [
26]. Dysregulation of these miRNAs affects key cellular processes, leading to increased proliferation, inhibition of apoptosis, and enhanced migration and invasion ability. Furthermore, miRNA dysregulation also promote angiogenesis necessary for further tumor growth [
27].
Numerous studies show that both upregulation and downregulation of miR-28, can cause changes in tumorigenesis related processes [
7]. Similar to other miRNAs, miR-28 can also act as both a suppressor and oncomiR [
7,
28]. Therefore, comprehensive studies are being conducted to understand the role of miR-28 in molecular mechanisms of tumorigenesis.
Figure 2 shows the key cellular processes regulated by miR-28.
4.2. Regulation of miR-28 in cancer - lncRNA and circRNA
Studies indicate that the levels of various miRNAs in cancer cells are dysregulated [
29]. These alterations may be driven by several factors, including long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), which interact with miRNAs in various ways to modulate gene expression [
30]. They can act as a miRNA sponges, preventing them from binding to mRNAs, thus protecting transcripts from degradation. In addition, they can modulate transcription factors, enabling their binding to promoters and regulating gene expression [
30].
Long non-coding RNAs (lncRNAs) belong to a class of RNA molecules, longer than 200 nucleotides that are usually not translated into proteins. LncRNAs affect gene expression, chromatin dynamics, and consequently differentiation and development [
31,
32]. Many types of lncRNAs modulate miR-28 expression in various types of cancer (
Table 1). The effect of lncRNAs has been observed, e.g., in pancreatic cancer, in which lncRNAs LOXL1- AS1 and LINC00514 caused miR-28 level disorders resulting in deregulation of expression of genes crucial for tumorigenesis [
33,
34]. A similar interaction was observed in melanoma, where lncLUADT1 and UCA1 affected tumor progression [
35,
36]. The infulence of lncRNAs on miR-28 levels has also been reported in breast cancer, where lncRNA MCM3AP-AS1 levels were associated with increased tumor progression. Similar effect has been observed in lung cancer and many other cancer types [
37,
38].
Circular RNAs are a class of single-stranded RNA molecules that have been identified in a variety of virsues and organisms, including plants and animals. Due to their circular structure, these molecules have increased stability and are more resistant to degradation than linear RNAs [
32,
39]. Their function is not yet well understood. Nevertheless, numerous studies show that severe circular RNAs can affect tumorigenesis by interacting with miR-28 (
Table 1). One of them is circ-CSNK1G1, which, through suppression of miR-28-5p, causes overexpression of the LDHA gene in breast cancer, leading to increased proliferation, migration and invasion [
40]. Another example is circ-002178, which participates in evasion of the immune response in adenocarcinoma by translocating via exosomes to CD8+ T cells. Binding of miR-28-5p to circ-002178 leads to increased expression of the PD1 receptor, which inhibits the immune anti-cancer response [
41]. On the other hand, circ-AHNAK is confirmed to act as an inhibitor of tumorigenesis. Its overexpression in ovarian cancer decrease proliferation and migration of tumor cells, while increasing apoptosis and preventing EMT. The effect of circ-AHNAK has been linked to its ability to sponge miR-28, which is overexpressed in ovarian cancer and acts as an oncomiR in this tumor [
42]. Other circular RNAs that affect miR-28 in cancer are circ-0001068 (ovarian cancer), circ-MYBL2 and circ-AGFG1 (NSCLC) [
43,
44,
45].
Table 1.
lncRNA and circRNA involvement in cancer types and associated pathways. The table summarizes the roles of lncRNAs and circRNAs in various cancer types, highlighting their association with key molecular targets and pathways. These ncRNAs play critical role in regulating cancer progression, including processes such as tumor growth, invasion, and immune evasion, by modulating specific targets and signaling pathways.
Table 1.
lncRNA and circRNA involvement in cancer types and associated pathways. The table summarizes the roles of lncRNAs and circRNAs in various cancer types, highlighting their association with key molecular targets and pathways. These ncRNAs play critical role in regulating cancer progression, including processes such as tumor growth, invasion, and immune evasion, by modulating specific targets and signaling pathways.
| lncRNA |
Cancer type |
Targets/Pathway |
References |
| LOXL1-AS1 |
Endometrial cancer |
miR-28-5p, RAP1B |
[46] |
| Pancreatic cancer |
miR-28-5p, SEMA7A / CD44/EGFR |
[46] |
| LINC00514 |
Pancreatic cancer |
miR-28-5p, RAP1B |
[33] |
| LUADT1 |
Melanoma |
miR-28-5p, RAP1B |
[36] |
| UCA1 |
Melanoma |
miR-28-5p, HOXB3 |
[47] |
| Colon cancer |
miR-28-5p, HOXB3 |
[48] |
| MCM3AP-AS1 |
Breast cancer |
miR-28-5p, CENPF |
[38] |
| CCAT1 |
Prostate cancer |
miR-28-5p, DDX5 |
[49] |
| CDKN2B-AS1 |
Colorectal cancer |
miR-28-5p, URGCP |
[50] |
| NORAD |
Lung cancer |
miR-28-3p, E2F2 |
[51] |
| CASC9 |
Papillary thyroid carcinoma |
miR-28-3p, BCL-2 / PI3K/AKT |
[52] |
| LINC02298 |
Hepatocellular Carcinoma |
miR-28-5p, CCDC6 |
[53] |
| circRNA |
Cancer type |
Targets/Pathway |
References |
| circ-CSNK1G1 |
Triple-negative breast cancer |
miR-28-5p, LDHA |
[40] |
| circ-002178 |
Lung adenocarcinoma |
miR-28-5p, PDL1/PD1 |
[41] |
| circ-AHNAK |
Ovarian cancer |
miR-28-5p, EIF2B5 / JAK2/STAT3 |
[42] |
| circ-0001068 |
Ovarian cancer |
miR-28-5p, PD1 |
[43] |
| circ-MYBL2 |
Non-small cell lung cancer |
miR-28-5p |
[44] |
| circ-AGFG1 |
Non-small cell lung cancer |
miR-28-5p, HIF-1α |
[45] |
5. The Role of miRNA-28 in Different Types of Cancer
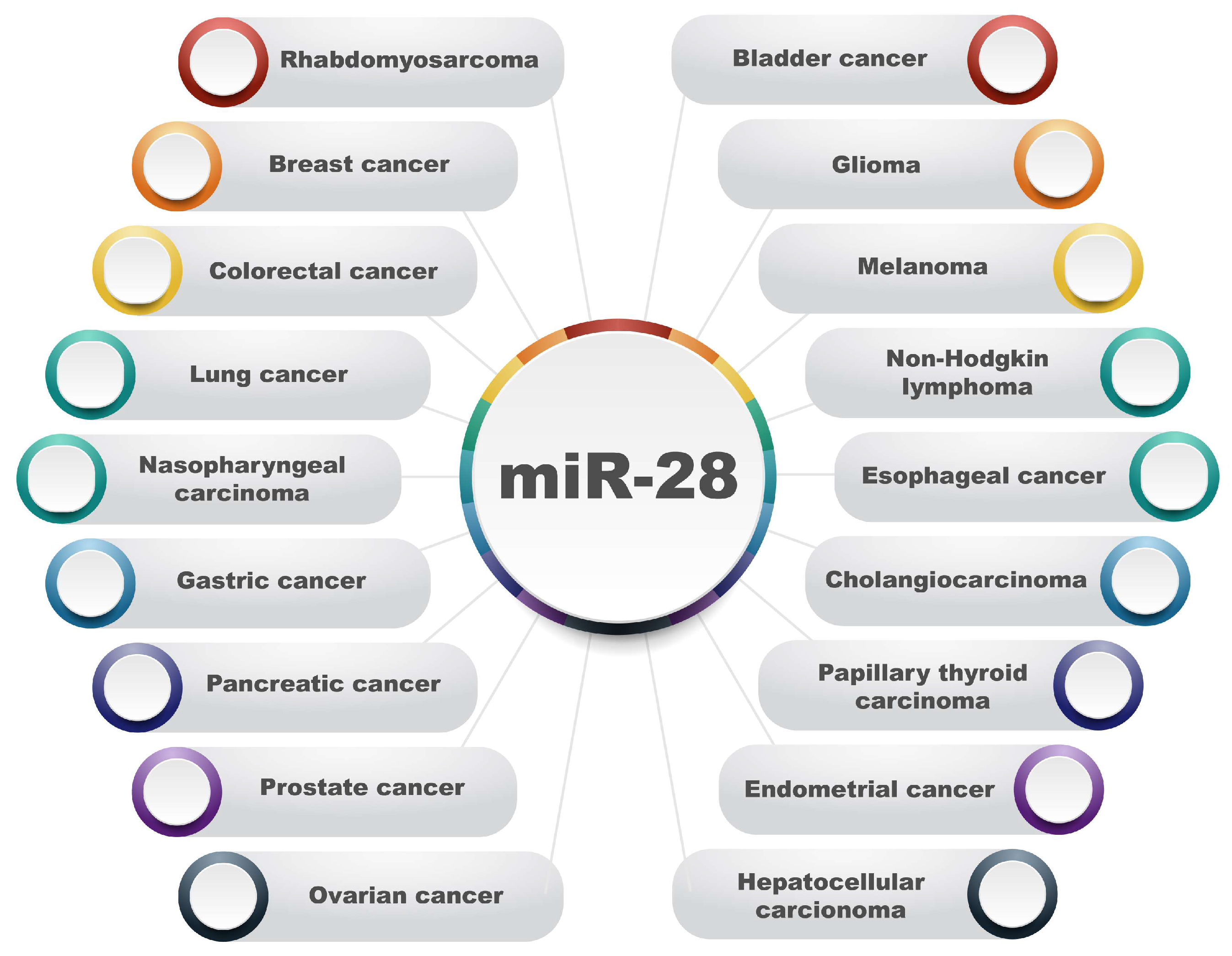
MicroRNA-28 plays an extremely important role in the development of various cancers (
Figure 3). Dysregulation in the levels of this miRNA has been demonstrated to induce tumorigenesis and play an active role in cancer progression by affecting the expression of genes related to oncogenesis. This section will describe the impact of miR-28 on the progression of various malignancies.
5.1. Nasopharyngeal Carcinoma (NPC)
Nasopharyngeal carcinoma (NPC) is a type of epithelial carcinoma that originates from the mucosal epithelium of the nasopharynx. It has been observed that this cancer occurs most frequently in Southeast Asian populations, with more than 70% of new cases being diagnosed in this region. [
54]. Anatomy-based staging classification is not sufficient to assess prognosis, so the use of molecular biomarkers, including microRNAs is essential [
54].
Reduced levels of miR-28-5p and miR-28-3p have been reported in this rare type of malignant tumor. Significant differences between function of strands -3p and -5p of this miRNA have been observed [
55]. In nasopharyngeal cancer, miR-28-5p plays an important role in cell cycle arrest, proliferation, and cell apoptosis in vitro. The same study also reported that miR-28-3p increases the ability of NPC cells to migrate and invade [
55]. The increase of migratory potential has been correlated with miR-28-3p's effect on down-regulating the expression of NM23-H1 and E-cadherin, which are crucial for epithelial-mesenchymal transition [
55,
56]. MicroRNA-28-5p affect on CCND1 expression, whose downregulation is associated with the development and progression of NPC [
54]. CCND1 encodes a cyclin D1 protein essential for the G1 to S phase transition in cell cycle. Its abnormal expression can lead to uncontrolled tumor growth [
55,
57].
5.2. Esophageal Cancer (EC)
Esophageal cancer is most common in East Asia, East and Southern Africa and the southern part of Europe. This indicates that the incidence is usually a combination of both genetic and ethnic factors, as well as lifestyle [
58]. To date, the only known precursor to this disease is Barrett's esophagus, which increases the risk of esophageal cancer by up to 40 times [
58].
Due to the difficult course of the disease and the unsatisfactory results of existing therapies, new diagnostic and therapeutic methods are being tested. Thus, research groups investigate the role of miRNAs in the pathogenesis of this cancer. Previous results suggest that miR-28-5p may be involved in the progression of esophageal cancer. It has been shown that miR-28-5p can promote tumor progression by increasing proliferation and inhibiting apoptosis via the supression of MTSS1 (Metastasis Suppressor 1), which acts as a tumor suppressor [
59].
5.3. Gastric Cancer (GCa)
Gastric cancer (GCa) is a malignant neoplasm with a high mortality rate, most diagnosed in male patients and the elderly individuals. Risk factors include smoking, overconsumption of salty and smoked foods and insufficient consumption of fruits and vegetables [
60,
61]. Despite the high mortality rate, early detection of this disease significantly increases the chances of successful therapy. Therefore, the search for molecular markers that would enable more accurate diagnosis is of paramount importance. A promising candidate for further study is miR-28, which may be involved in gastric cancer tumorigenesis.
Studies of miR-28-5p expression in GCa cells show reduced levels of this miRNA compared to normal cells. Moreover, patients with higher levels of miR-28-5p had a better prognosis [
61]. Expression of miR-28-5p in GCa correlates with the depth of tumor infiltration and lymph node metastasis. GCa cells with overexpression of miR-28-5p show reduced migration and invasion capacity, which consequently limits their ability to metastasize [
61]. The effect of miR-28-5p on cancer cell migration is associated with inhibition of serine/threonine protein kinase (AKT) phosphorylation, which is involved in the regulation of epithelial-mesenchymal transition (EMT) [
61]. Inhibition of the AKT signaling pathway in this case supresses EMT and, consequently, cell migration [
61,
62]. The results suggest that miR-28-5p may inhibit gastric cancer cell migration by affecting the AKT pathway.
Increased levels of the transcription factor NRF2 were noted in GCa, which correlated with poorer patient prognosis. Therefore, the effect of miR-28-5p on NRF2 levels has been investigated. Studies have proved that elevated levels of miR-28-5p leads to decreased NRF2 expression, limiting the ability of tumor cells to migrate [
63].
MicroRNA-28-5p in GCa is also closely associated with disease progression through regulation of the GAGE12I (G antigen 12I), which overexpression was associated with poor patient prognosis. It was shown that miR-28-5p downregulate GAGE12I, resulting in a reduced ability of cells to migrate. Moreover, 72 hours after transfection, there was a decrease in cell survival below 50%, indicating a strong inhibition of their growth rate [
64].
Studies indicate that deregulation of miR-28 levels in gastric cancer may also induce oncogenic effects. This is related to the regulation of the PTEN gene, whose decreased expression, caused by elevated miR-28-5p levels, leads to increased proliferation and invasiveness of tumor cells [
65].
5.4. Colorectal Cancer (CRC)
Colorectal cancer (CRC) accounts for about 10% of all human cancer cases and is currently the fourth deadliest cancer. There are about 900,000 CRC-related deaths worldwide each year. It ranks as the second most frequently diagnosed cancer among women and the third most frequently diagnosed among men. Modern eating habits, physical inactivity, obesity and smoking are major risk factors for CRC. The incidence of colorectal cancer is expected to increase in the coming years [
66].
In colorectal cancer, miR-28 regulates the expression of the SSRP1 gene, whose functions include transcriptional control and DNA damage repair [
67]. Similar to other cancers, overexpression of SSRP1 has been reported in CRC [
67,
68]. High expression of this gene correlates with faster disease recurrence [
68]. Furthermore, SSRP1 has been shown to promote tumor cell proliferation and metastasis and affect the epithelial-mesenchymal transition in CRC. Silencing of SSRP1 expression in CRC increases the efficacy of treatment with 5-fluorouracil and cisplatin [
68].
MicroRNA-28-5p is a negative regulator of SSRP1. The level of miR-28-5p is often downregulated in CRC cells, and transfection with miRNA mimics sequences leads to a decrease in SSRP1 expression, resulting in reduced proliferation, migration and invasiveness of cancer cells [
68]. A similar mechanism has been observed for CAMTA2, whose expression is also regulated by miR-28-5p, thereby limiting CRC progression through the Wnt/ β-catenin pathway [
69].
In colorectal cancer, miR-28-5p and miR-28-3p may exert opposing effects, as a result, overexpression of either strand may lead to different biological outcomes [
70]. Overexpression of miR-28-5p reduce the ability of cancer cells to migration and invasion, while the effect of miR-28-3p is the opposite - its increased expression significantly enhance the migration and invasiveness of CRC cells [
70]. These differences result from the strand-specific effects on different genes. MicroRNA-28-5p downregulate CCND1 (cyclin D1), a cell cycle regulatory protein considered as protooncogene, whose overexpression is characteristic for cancer cells [
57,
70]. Moreover, miR-28-5p decrease levels of HOXB3, a member of the HOX protein family that plays a crucial role in cancer initiation and progression [
71]. Therefore, it can be concluded that miR-28-5p contributes to the reduction of colorectal cancer progression by downregulation HOXB3 [
70,
71]. In contrast, the opposing effect of miR-28-3p in CRC may be due to its effect on the NM23-H1 gene [
70]. The NM23-H1 protein exhibits antimetastatic properties, including inhibiting the migration and proliferation of tumor cells [
72]. In colorectal cancer, miR-28-3p downregulate NM23-H1, resulting in an increased ability of CRC cells to migration and invasion [
70].
The effect of miR-28-5p on ferroptosis was also analyzed. Ferroptosis is a form of regulated cell death in which iron and lipid peroxides play a major role. [
73]. Ferroptosis can be activated by depletion of cysteine and glutathione in the cell or inhibition of glutathione peroxidase 4 (GPX4) activity. These metabolic changes result in the accumulation of reactive oxygen species (ROS), generated by a variety of mechanisms, including the Fenton reaction, which is catalyzed by iron. ROS accumulation then leads to lipid peroxidation, which is crucial for the induction of ferroptosis [
73,
74]. Induction of this process may provide an alternative method to eliminate treatment-resistant cancer cells. Studies show that overexpression of miR-28-5p enhances ferroptosis in colorectal cancer cells [
75]. The effect of miR-28-5p on this process may be used in the future to develop novel therapeutic strategies for the treatment of CRC.
5.5. Hepatocellular Carcinoma (HCC)
Hepatocellular carcinoma (HCC) may be a malignant neoplasm and its development is associated with many factors, such as viral infections (hepatitis B and C), alcohol overconsumption, smoking and non-alcoholic fatty liver disease [
76]. The effectiveness rate of HCC treatment is high only when the disease is detected on early stage [
77].
A study of HCC revealed that hepatocellular cancer stem cells exhibit downregulated expression of miR-28-5p. Knockdown of miR-28-5p enhances tumorigenesis and promotes self-renewal of cancer stem cells [
77]. Liver cancer stem cells play a pivotal role in tumor initiation and progression, as well as tumor recurrence and resistance to treatment [
77]. Overexpression of miR-28-5p in these cells inhibits tumorigenesis, thus HCC cells became more sensitive to Sorafenib treatment. The effect of miR-28-5p in CSCs and its impact on disease progression is due to the interaction of this miRNA with insulin-like growth factor 1 (IGF-1), which can promote the development of CSCs in HCC [
77].
5.6. Cholangiocarcinoma (CCA)
Cholangiocarcinoma is a cancer of epithelial origin, occurring more frequently in male than in female patients. The incidence rate for this type of cancer is higher in Asian and Hispanic populations, and lowest among white and black populations. A particularly high incidence has been reported in the Southeast Asian region (113 cases per 100,000 population), which is associated with frequent parasitic infections caused by the hepatobiliary flukes, Opisthorchis viverrini and Clonorchis sinensis [
78].
In cholangiocarcinoma, the anti-tumor effect of miR-28-5p is correlated with its effect on CD44. Increased expression of miR-28-5p results in inhibition of the cell cycle and proliferation, and reduced tumor growth in vivo. In addition, a reduced ability of cells to metastasis was observed [
79].
5.7. Pancreatic Cancer (PC)
In recent years, the incidence of pancreatic cancer (PC) has increased significantly, and it is one of the leading causes of cancer mortality worldwide. The incidence is the highest in developed countries, especially in North America, Europe and Australia, which is associated with increased life expectancy, an aging population and a higher prevalence of risk factors such as obesity, diabetes and alcohol overconsumption [
80].
Studies on the effect of miR-28-5p on pancreatic cancer progression have shown that there is a LOXL1-AS1/miR-28-5p/SEMA7A regulatory axis that is responsible for the progression of this cancer [
34]. The level of miR-28-5p was found to be significantly downregulated in PC cells. Regulation of this miRNA occurs through the lncRNA LOXL1-AS1, which sponges miR-28-5p, leading to increased expression of semaphorin 7A (SEMA7A) and inducing tumor progression. Overexpression of miR-28-5p inhibits the proliferation and migration of PC cells, suggesting that it functions as a tumor suppressor in this type of cancer. Understanding the relationship between the LOXL1-AS1/miR-28-5p/SEMA7A axis may serve to develop novel therapeutic strategies [
34].
It was also discovered that downregulation of miR-28-5p in PC is affected by lncRNA LINC00514, resulting in increased expression of RAP1B. Overexpression of this gene is associated with lung metastasis and accelerates tumor growth in vivo [
33].
5.8. Breast Cancer
In 2022, breast cancer was the most frequently diagnosed cancer among women. There has been a significant increase in the incidence of this cancer over the past four decades [
81,
82]. Despite the high incidence, the mechanisms underlying this disease still require detailed investigation [
81].
A tumor suppressor effect of miR-28 has been shwon in breast cancer, with studies demonstrating that miR-28-5p may reduce proliferation, as well as migration and tumor invasion [
83,
84]. Analysis of the expression level of miR-28-5p in breast cancer cells showed that the level of this miRNA was significantly lower than in normal tissue. This suggests that there is a link between miR-28-5p and tumorigenesis [
83]. In addition, it has been proved that low levels of miR-28-5p are also associated with lower patient survival [
83]. Studies conducted on MCF-7 breast cancer cell line have shown that increasing miR-28-5p levels by transfection, leads to a decrease in their ability to migrate. On the other hand, the use of miR-28-5p inhibitors increased their migration potential [
83]. In the same study, genes regulated by miR-28-5p and mechanisms of their effects on migration and tumor progression, have been determined by use of gene chip assay. It has been shown that one of the targets of miR-28-5p is the WSB2 gene. Its dysregulated expression can induce tumorigenesis, e.g., in human melanoma cells [
85]. Upregulation of WSB2 expression in breast cancer, may correlate with low levels of miR-28-5p. Increased levels of this miRNA lead to downregulated expression of WSB2, which induces breast cancer cell migration. The association of miR-28-5p with WSB2, may in the future contribute to the development of more effective therapies for this type of cancer [
83].
It has also been confirmed that miR-28 regulates the expression of NRF2 (NF-E2-related factor 2) [
86]. The NRF2/KEAP1 pathway plays a pivotal role in maintaining redox homeostasis within cells, thereby exhibiting significant anti-inflammatory and anti-tumor properties [
87]. In cancer, overexpression of NRF2 can promote cell proliferation and accelerate disease progression [
87]. Furthermore, NRF2 inhibits apoptosis, promotes angiogenesis and renewal of cancer stem cells, and enhances resistance to chemotherapy and radiotherapy [
87]. Consequently, elevated levels of this transcription factor may be associated with a poor prognosis. Its overexpression has also been reported in breast cancer compared to normal cells [
86]. In contarst, miR-28 upregulation reduces NRF2 levels [
86], which may inhibit tumor growth and improve the effectiveness of anti-cancer treatment.
A gene that may play an important role in breast cancer progression is CENPF, encoding centromere protein F, which is overexpressed in this type of cancer. Elevated levels of CENPF have been correlated with increased ability of tumor cells to migrate, invade, and accelerated proliferation. Expression of this gene is regulated by miR-28-5p, and reduced levels of this miRNA prevent tumor growth restriction. Abnormal levels of miR-28-5p have been observed to be caused by overexpression of the lncRNA MCM3AP-AS1, which sequester this microRNA and leads to further cellular dysfunction [
38].
Another example of the effect of miR-28-5p on breast cancer progression is its regulation of the LDHA gene, which is overexpressed in various types of cancer and is associated with a poorer patient prognosis [
88]. LDHA plays a pivotal role in the induction of tumorigenesis, and studies have shown that its overexpression in breast cancer correlates with increased cell proliferation and accelerated tumor growth [
89]. Elevated levels of miR-28-5p have been shown to inhibit proliferation, reduce migration and invasion of tumor cells, and promote apoptosis. The data obtained show that, through its tumor-suppressive effect on LDHA, miR-28-5p inhibits breast cancer development [
84].
5.9. Ovarian Cancer (OC)
Ovarian cancer is the eighth most prevalent type of cancer among women worldwide. In 2022, it accounted for 4.8% of all cancer-related deaths [
82]. Despite the high mortality rate, the prognosis depends on the stage at which therapy is initiated. In stage I, the 10-year survival rate is 73-92%, while in stage III and IV it drops to 21% and less than 6%, respectively [
90].
In ovarian cancer, miR-28-5p acts as an oncomiR and promotes the progression of this cancer. Its expression is significantly elevated compared to healthy tissue. Overexpression of miR-28-5p enhances proliferation, migration and invasion ability of cells, and affects the cell cycle. Moreover, miR-28-5p inhibits apoptosis and promotes tumor growth in vivo [
91]. It has also been found that miR-28-5p promotes epithelial-mesenchymal transition (EMT), further facilitating tumor progression. Furthermore, the mechanism of action of miR-28-5p in ovarian cancer has been linked to downregulation of N4BP1 protein, which is involved in protein degradation through ubiquitination [
91,
92].
5.10. Prostate Cancer (PCa)
Prostate cancer (PCa) is among the most prevalent forms of cancer diagnosed in males between the ages of 45 and 60, and it ranks second in terms of incidence among male cancer diagnoses [
93]. According to global statistics, in 2022 it accounted for approximately 14.2% of all cancer diagnoses and was the fifth most common cause of cancer-related deaths among men [
82].
Decreased expression of miR-28-3p was found in prostate cancer cells. Transfection of these cells with miRNA mimics led to inhibition of proliferation, while application of miR-28-3p inhibitors had the opposite effect – it increased the rate of cell division. These results confirm the involvement of miR-28-3p in regulating proliferation of prostate cancer cells [
94].
In addition, miR-28-3p has been shown to be a regulator of prostate cancer cell apoptosis. Upregulation of miR-28-3p led to increased apoptosis of cancer cells, which correlated with decreased expression of anti-apoptotic the BCL-2 protein and increased levels of pro-apoptotic Bax protein [
94]. The use of miR-28-3p inhibitors reversed this effect by reducing cell apoptosis [
94]. Moreover, it was found that miR-28-5p may also contribute to the induction of apoptosis by downregulating the E2F6 protein, known for its anti-apoptotic properties [
95].
MicroRNA-28-3p also regulates the expression of the ARF6 gene. Overexpression of ARF6 is associated with an increased ability of cancer to migrate and invade. This suggests that ARF6 may play a crucial role in prostate cancer metastasis. MicroRNA-28-3p may therefore act as a tumor suppressor miRNA and represent a potential target for anti-cancer therapy [
94].
The transcription factor SREBF2 is an oncogenic protein that, in prostate cancer, regulates proliferation, cell survival and the ability of cancer cells to migrate and invade [
96]. Downregulation of its expression leads to inhibition of the progression of this cancer. SREBF2 has been identified as a direct target of miR-28-5p. Exogenous introduction of this microRNA into cells results in decreased SREBF2 expression at both the mRNA and protein levels, demonstrating a tumor-suppressive role for miR-28-5p in prostate cancer [
96].
5.11. Bladder Cancer (BCa)
Bladder cancer is the most prevalent type of cancer affecting the urinary system [
97]. In approximately 75% of cases, the cancer is confined to the mucosa (non-muscle invasive bladder cancer). In 25-30% of cases, the tumor invades deeper layers of the bladder wall and infiltrates the muscle membrane or forms metastases [
97]. Despite advances in diagnosis and anti-cancer therapy, long-term survival rates have remained unchanged for many years [
97].
Numerous types of miRNAs have been identified in bladder cancer, suggesting their pivotal roles in disease progression, and thus these molecules may serve as valuable targets in medical applications. Overexpression of miR-28-5p has been detected in the urine of BCa patients, which may have important diagnostic implications. This may allow early non-invasive detection of cancer and rapid implementation of appropriate therapy [
98,
99].
5.12. Non-Small Cell Lung Cancer (NSCLC)
Non-small cell lung cancer (NSCLC) is a malignant tumor that accounts for a significant percentage of cancer-related deaths [
100,
101]. In the case of NSCLC, miR-28 acts as an oncomiR, as its elevated levels promote increased proliferation of tumor cells [
101]. MicroRNA-28 affects the PTEN gene, a key tumor suppressor, through mechanisms dependent and independent of the PI3K pathway [
101,
102]. Decreased PTEN activity leads to increased proliferation and survival of tumor cells and affects the microenvironment that promotes new tumor growth [
102]. Overexpression of miR-28 in NSCLC, therefore, may induce tumor cell proliferation by inhibiting PTEN expression (activating the PI3K/AKT pathway) [
101].
5.13. Glioma
Gliomas are the most common primary brain and spinal cord tumors [
103], for which no effective therapy has yet been developed [
104]. Numerous studies indicate that miR-28 has a significant impact on processes involved in the progression of gliomas, including regulation of tumorigenesis-related genes.
Studies showed that the human TRPM7 gene negatively regulates miR-28-5p expression, which affects glioma progression [
104]. Levels of miR-28-5p in glioma cells are significantly downregulated compared to normal cells. Increasing miR-28-5p expression by transfection of miRNA mimics resulted in inhibition of proliferation and reduced the ability of glioma cells to invade. The use of miR-28-5p inhibitors had the opposite effect, confirming the involvement of this microRNA in the mechanisms of glioma progression [
104]. Further analysis showed that miR-28-5p upregulates RAP1B, a protein that participates in numerous signaling pathways involved in the processes of cell proliferation, differentiation and apoptosis [
104,
105]. Considering the oncogenic nature of RAP1B in glioma and the ability of miR-28-5p to downregulate this protein, it can be inferred that miR-28-5p has an important function in the molecular mechanisms of progression in this cancer [
104].
Other studies indicate that miR-28-5p in glioma may also exhibit oncogenic properties. It has been shown that it can interact with the FOXO1 gene, whose reduced activity promotes the formation of tumor spheres, increases the viability of tumor cells and their ability to proliferate, which promotes disease progression [
106].
5.14. Rhabdomyosarcoma (RMS)
Rhabdomyosarcoma (RMS) is one of the most common soft tissue malignancies in individuals under 20 years of age. It accounts for approximately 7% of cancers in children and 1% in adults [
107]. RMS originate from impaired differentiation of stem cells or myogenic progenitor cells [
108]. It has been demonstrated that one miRNA with a significant role in the development and progression of RMS is miR-28, which is regulated by the transcription factor SNAIL [
109,
110].
Studies of two RMS subtypes: alveolar and embryonal, revealed that elevated levels of miR-28-3p caused a decrease in the number of S-phase cells and an increase in the G0/G1 phase and lead to inhibition of proliferation and diminished ability for uncontrolled cell division [
108]. Higher levels of miR-28-3p also significantly inhibits cell migration in the scratch assay, which suggests its effect on limiting the tumor's ability to metastasize [
108]. The effect of miR-28-3p on the expression of myogenic regulatory factors (MRFs) such as MYOG, MYOD, MEF2A, as well as MSTN was also observed in various RMS subtypes [
108].
5.15. Melanoma
Melanoma is a malignant tumor that mainly affects the skin, eyes and mucous membranes [
111]. It occurs most often in geographical regions where individuals with light skin pigmentation are exposed to excessive sun. The risk of developing the disease is much higher in people with a genetic predisposition, but it is not the most significant risk factor [
112].
In melanoma, miR-28-5p can be bound by the lncRNA LUADT1, leading to increased expression of the RAP1B gene. As a result, deregulation of the expression of this oncogene promotes intense cell proliferation [
36]. Understanding the relationship between the miR-28-5p/RAP1B axis and LUADT1 may help develop effective therapeutic strategies [
36]. Moreover, the lncRNA UCA1/miR-28-5p/HOXB3 axis has emerged as a key regulatory pathway [
35]. In this mechanism, HOXB3 functions as a miR-28-5p target gene, while UCA1, which is overexpressed in melanoma, can bind miR-28-5p. As a result, HOXB3 gene expression is increased, facilitating tumor progression [
35].
A common phenomenon observed in cancer is the presence of exhausted T cells within the tumor microenvironment. These cells are characterized by overexpression of inhibitory receptors (IRs), diminished production of effector cytokines, and reduced cytolytic activity, which prevents effective elimination of tumor cells [
113,
114]. According to studies, the exhausted T cells isolated from murine melanoma exhibit dysregulated levels of specific miRNAs, including miR-28 [
113]. It has been indicated that miR-28 can bind to the PD-1 (programmed cell death protein 1) receptor, leading to inhibition of anti-cancer immune response [
113,
115]. Transfection with miR-28 mimics to upregulate this miRNA has been shown to reduce PD-1 expression [
113]. The use of miR-28 to downregulate inhibitory receptor (IR) expression and prevent tumor cells from evading immune response mechanisms may represent a promising in anti-cancer therapies.
5.16. Non-Hodgkin lymphoma (NHL)
Non-Hodgkin's lymphomas (NHLs) are the most common hematological malignancy worldwide [
116]. They include B-cell lymphomas such as Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL) and T/NK-cell lymphomas such as T-cell acute lymphoblastic leukemia. Risk factors vary depending on the subtype of NHL [
116]. The incidence of these cancers is higher among men than women and among individuals over 65 years of age. Moreover, NHLs are often associated with autoimmune diseases, viral infections and hereditary predisposition to hematological malignancies [
116].
In Burkitt lymphoma (BL) and other types of non-Hodgkin lymphomas (NHL) originating from germinal centers, decreased expression of miR-28 was found, resulting from negative regulation of this miRNA by the MYC oncogene [
117,
118]. Restoration of normal miR-28 expression has been shown to impair tumor cell proliferation and clonogenic properties in BL. MicroRNA-28 acts as a tumor suppressor by affecting the MAD2L1 gene expression, which is a component of the spindle checkpoint, required to inhibit proliferation induced by this miRNA. In addition, miR-28 also interacts with BAG1, a BCL2-related factor that acts as an activator of the ERK signaling pathway [
117].
5.17. Papillary Thyroid Carcinoma (PTC)
Papillary thyroid carcinoma (PTC) is the most prevalent endocrine gland tumor, accounting for approximately 85% of all thyroid cancer cases. It is classified as an indolent cancer, which is characterized by a relatively favorable prognosis, with a 10-year survival rate of 93%. However, certain subtypes of PTC have been observed to exhibit higher malignancy and lower survival rates [
119].
In the tissues of patients diagnosed with PTC, elevated levels of the lncRNA CASC9 were found. CASC9 has been shown to influence the progression of this malignancy, through the sequestration of miR-28-3p. Thus, downregulating this lncRNA with siRNA resulted in increased levels of miR-28-3p. The analyses demonstrated that in the examined cell lines, increasing the level of this miRNA resulted in increased apoptosis, cell sensitivity to doxorubicin, and reduced migration and proliferation. In vivo studies in mice also showed that decreased CASC9 expression reduced tumor growth. The involvement of miR-28-3p in PTC progression is due to the upregulation of BCL-2 protein, which is known for its anti-apoptotic properties [
52].
The involvement of miR-28 in the pathogenesis and progression of various cancer types discussed in this review has been summarized in
Table 2.
6. MicroRNA-28 as a Therapeutic Target and Diagnostic Biomarker in Cancers
As previously discused in earlier sections of this publication, miR-28 has emerged as a promising biomarker, with the potential to facilitate early detection of numerous cancers. In many cases, early diagnosis has been shown to significantly enhance the efficacy of therapeutic interventions. Moreover, it can also be used to assess the clinical status of patients. Previous studies indicate that miR-28-3p and miR-28-5p can be used as biomarkers in the diagnosis of colorectal cancer and gastric cancer [
120,
121,
122,
123]. MicroRNA-28 has also been identified as one of four circulating miRNAs with diagnostic potential in diffuse large B-cell lymphoma (DLBCL). A prognostic model based on miRNA levels in patients’ serum provides a simple and effective method for assessing their clinical status [
124]. The model can also be used to diagnose the early stages of renal cell carcinoma, in which patients’ serum miR-28-5p levels are significantly reduced compared to healthy individuals [
125]. There is also the potential to use miR-28-5p as a biomarker in the non-invasive diagnosis of bladder cancer by analyzing patients' urine, which may facilitate screening [
98,
99].
The potential application of microRNAs in combination therapy with anticancer drugs is very promising. This approach may enhance treatment efficacy, while concurrently reducing the risk of treatment failure and limiting side effects. A notable example of this type of therapy is the combination of miR-28 and ibrutinib for the treatment of DLBCL [
126]. Similarly, combination of miR-28 with doxorubicin (adriamycin), enhances the efficacy of DLBCL treatment [
127]. These results suggest that strategies based on combination therapy may also be effective in the treatment of other cancer types [
126]. Furthermore, it has been shown that in DLBCL, cell proliferation can be decreased and apoptosis induced by curcumin, which can upregulate miR-28-5p [
128]. Another therapeutic approach is the use of antisense oligonucleotides (AMOs) and antagomiRs, which can inhibit the activity of mature miRNAs [
129]. The use of microRNA masks, designed to interfere with the interaction between specific microRNA and their target mRNAs, also shows great promise. Equally promising appears to be the use of miRNA mimics sequences that can compensate for miR-28 deficiencies in cancer cells [
129].
Despite promising results, there are several practical challenges like finding proper drug route of administration, controlling their stability in the organism, targeting specific tissues, and their efficacy at the intracellular level [
130,
131]. Several clinical trials are currently underway for the treatment of cancer, including hepatocellular carcinoma, melanoma and non-small cell lung cancer, using miRNA-based therapies [
130]. Moreover, novel technologies for targeted drug delivery to cancer cells are currently under development. The utilization of antibody-coated nanoparticles, which are designed to target specific tumor antigens, is a current area of research. The combination of these nanoparticles with microRNAs and anti-cancer drugs has the potential to facilitate the delivery of therapeutic agents into target cells [
132].
7. MicroRNA-28 in Non-Oncological Diseases
MicroRNAs can be used not only in cancer therapy and diagnostics but also in the treatment and monitoring of other serious non-oncological diseases. Numerous studies indicate the key role of miR-28 in regulating inflammatory processes and its involvement in the pathogenesis of autoimmune, neurodegenerative, cardiovascular, and metabolic diseases.
7.1. Infectious Diseases (Pathogenic)
MicroRNA-28 has been demonstrated to play a pivotal role in the immune response to a wide range of infections caused by various pathogens, including both viruses and bacteria. MicroRNA-28-3p can serve as a biomarker for identifying infections with Helicobacter pylori, the bacterium responsible for the development of gastric ulcers. Its presence among circulating miRNAs correlates with H. pylori infection may help diagnose infections with this pathogen [
133].
Furthermore, studies have demonstrated that miR-28-3p plays a crucial role in the suppression of HTLV-1 (human T-lymphotropic virus type 1) and HIV-1 replication, through the binding of miR-28-3p to specific sequences present in the viral mRNA [
134]. MicroRNA-28 is known as an anti-HIV-1 miRNA due to its involvement in the immune response against HIV-1 infection. The 3' end of this virus' mRNA is a molecular target for miR-28, and its expression affects the course of infection and disease progression [
135].
The virus pandemic SARS-CoV-2, which began in 2019, has led to approximately 777 million infections and 7 million deaths by February 2025 [
136,
137]. It has been shown that miR-28-3p may also be a therapeutic target in that disease. SARS-CoV-2 uses a spike protein (S-protein) to spread, which binds to the trans-membrane protein- angiotensin-converting enzyme 2 (ACE2). ACE2 receptors are present on cells of many organs, such as the heart, brain and lungs [
138]. In vitro studies have shown that in SARS-CoV-2 virus infection, miR-28-3p levels in cells treated with the virus' S protein are lower than in control cells. Moreover, it has been established that the molecular target for this miRNA may be the metalloproteinase ADAM17, which is involved in the mechanism of virus entry into cells via ACE2 receptors [
139].
7.2. Metabolic and Endocrine
The prevalence of lifestyle diseases has emerged as a pressing global health concern. One of them is obesity. Its impact on metabolism and the development of cardiovascular diseases is already evident in the youngest patients. Preventing obesity in childhood can significantly reduce the risk of related diseases in adulthood. Accordingly, studies revealed that circulating microRNAs, including miR-28-3p, can be used to diagnose and detect metabolic disorders in children with obesity [
140].
Diabetes mellitus is a common metabolic disorder occurring worldwide. Evidence suggest that in type 2 diabetes mellitus, miRNA levels are dysregulated, including miR-28-3p [
141]. This disease is also commonly associated with various complications. One of them is diabetic retinopathy (DR), which is the leading cause of vision loss among middle-aged economically active people [
142]. Nevertheless, there is still a lack of appropriate biomarkers for its early detection. Therefore, conducted studies identified miR-28-3p as one of three potential biomarkers of DR. In addition, it has been confirmed that miRNAs may be involved in pathogenesis by affecting the proliferation of human retinal microvascular endothelial cells (HREMECs) [
143].
Polycystic ovary syndrome (PCOS) is a hereditary condition that affects approximately 15% of women of reproductive age worldwide. The disease leads to ovulation disorders, infertility, obesity and type 2 diabetes [
144]. Furthermore, women diagnosed with PCOS have higher risk of developing endometrial cancer [
145]. Prokineticin 1 (PROK1) is regulated by miR-28-5p and may play a role in the pathogenesis of PCOS. PROK1 is associated with ovarian physiology, endometrial receptivity and embryo implantation. Studies in a rat model, indicated that PROK1 in PCOS promotes proliferation, limits cell cycle inhibition, and affects the process of apoptosis. Therefore, it is hypothesized that miR-28-5p may limit the progression of PCOS, and the miR-28-5p/PROK1 axis may be a potential therapeutic target in patients with this condition [
146].
An interesting example of the application of miR-28 is its use as a biomarker in the diagnosis of sarcopenia caused by Cushing's syndrome (CS) [
147]. Cushing's syndrome is associated with hypercortisolism resulting from overactive adrenal glands, pituitary gland or excessive secretion of adrenocorticotropic hormone (ACTH). More than one-third of patients with CS experience muscle weakness in the lower extremities and pelvic region. The symptoms are the result of the inhibitory effects of steroid hormones on protein synthesis, increased catabolism, and inhibition of myogenesis through effects on myogenin, a transcription factor essential for normal muscle differentiation [
148]. MicroRNA-28-5p plays a crucial role in the processes of muscle development, especially in myoblast differentiation and proliferation. Plasma levels of this miRNA are elevated in patients after CS remission. With this knowledge, it is possible to diagnose patients with an increased risk of sarcopenia, so that appropriate measures can be implemented to improve their quality of life and function [
147].
7.3. Neurological and Neurodegenerative Diseases
Dysregulated levels of miRNAs have also been observed in Parkinson's disease (PD), the second most common neurodegenerative disease worldwide. Its incidence is expected to rise in future generations, while early detection reamins a major challenge in modern medicine [
149]. Consequently, it is necessary to develop new diagnostic methods to detect PD more effectively. Studies have shown that miR-28-5p can act as a biomarker in the diagnosis and assessment of the progression of this disease. Analysis of the expression of this miRNA in the serum of patients may contribute to earlier diagnosis and better monitoring of the course of the disease [
150].
Neuropathic pain is defined as pain originating from damage or disease of the somatosensory nervous system [
151]. Research conducted on rats with chronic constriction injury (CCI) of the sciatic nerve has shown a reduction in the expression levels of miR-28-5p. In vivo analyses showed that miR-28-5p overexpression significantly reduced levels of the proinflammatory cytokines IL-6 and IL-1β, as well as cyclooxygenase Cox-2, which are involved in the progression of neuropathic pain [
152]. It has been shown that miR-28-5p can alleviate pain by downregulating the expression of the ZEB1 gene, which is a key regulator of neuroinflammatory conditions and is also involved in the development of neuropathic pain. The analyses performed indicate that the miR-28-5p/ZEB1 axis may be a potential therapeutic target for the treatment of neuropathic pain [
152].
7.4. Autoimmune and Inflammatory Diseases
Rheumatoid arthritis (RA) is an autoimmune disease that affects approximately 1% of the global population. RA is characterized by chronic inflammation of the joints, resulting in progressive joint damage. Moreover, RA can cause additional complications, including damage to the heart, kidneys, lungs and nervous system [
153]. The involvement of miRNAs in the pathogenesis of autoimmune diseases has been scientifically confirmed [
6]. Studies have shown that in RA, miR-28 can regulate the expression of genes involved in the pathogenic process. Chronic inflammation leads to exhaustion of immune cells such as NK cells and T cells. One of the mechanisms responsible for NK cell exhaustion is overexpression of the PD-1 receptor, leading to reduced proliferation, reduced cytokine production and impaired cytolytic activity. MicroRNA-28 may be involved in the regulation of PD-1 receptor expression, suggesting its involvement in RA-related pathogenic processes [
154].
7.5. Circulatory Diseases
Pulmonary embolism (PE) is the third most common death among hospitalized patients [
155]. Studies of plasma samples from PE patients indicate that miR-28-3p levels are elevated, suggesting its potential use as a non-invasive diagnostic biomarker [
156].
8. MicroRNA-28 in Stem Cells
The involvement of miR-28 in regulating the differentiation of human mesenchymal stem cells (MSCs) has also been demonstrated. MSCs isolated from bone marrow (BM-MSCs) are multipotent cells, with low immunogenicity, characterized by a high capacity for proliferation and differentiation, and therefore can be used to treat many diseases. In BM-MSCs undergoing osteogenesis, expression of miR-28 is increased, indicating that this miRNA is involved in osteogenic differentiation of these cells. It has been shown that miR-28 directly binds to the STAT1 mRNA, decreasing its expression, thus leading to overexpression of ACP and RUNX2, which in turn induces differentiation [
157]. Stem cells are important tools for modern therapies, so the study of microRNAs, including miR-28, may play an important role in the development of regenerative medicine.
9. Conclusions
MicroRNAs are key post-transcriptional regulators of gene expression, primarily modulating mRNA stability and translation. They participate in a wide range of biological processes, including cell proliferation, differentiation, and apoptosis. Among them, miR-28 plays a crucial regulatory role, and its dysregulation has been implicated in the pathogenesis of various diseases, including cancer. Notably, miR-28 exhibits a dualistic function, acting either as a tumor suppressor or an oncogene, which emphasizes the importance of elucidating its molecular mechanisms in disease development.
Recent studies highlight the potential of miR-28 as a biomarker for both diagnostic and prognostic purposes. MicroRNA-28 expression profiling offers a valuable tool for the early and non-invasive detection of pathological conditions. Ongoing research in the field of microRNAs further underscores the strategic relevance of miR-28 as a target for clinical translation. Moreover, the ability to pharmacologically modulate miR-28 levels opens a new approach for personalized therapies, particularly in oncology.
Author Contributions
Conceptualization, K.K., M.K. and K.S.; writing—original draft preparation, K.K.; data collection, K.K.; writing—review and editing, K.K., M.K. and K.S..; visualization, K.K. and M.K.; supervision, M.K., K.S..; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Jagiellonian University Medical College to K.S.: N41/DBS/000851 and M.K.: N41/DBS/001344.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
All figures in this manuscript, are original and were created by authors. Their preparation involved use of individual elements from freely available resources on Adobe Stock (under the Standard License) and self-drawn elements inspired by Servier Medical Art. The final illustrations were designed using Adobe Illustrator 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACE2 |
Angiotensin-converting enzyme 2 |
| AKT |
Serine/threonine - protein kinase |
| AMOs |
Anti-miRNA antisense inhibitors |
| ARF6 |
ADP-ribosylation factor 6 |
| BAG1 |
BAG family molecular chaperone regulator 1 |
| BAX |
Bcl-2-like protein 4 |
| BM-MSC |
Bone marrow mesenchymal stem cells |
| CCND1 |
Cycline D1 gene |
| CENPF |
Centromere protein F |
| CircRNA |
Circulating RNA |
| CSCs |
Cancer stem cells |
| E2F6 |
E2F Transcription Factor 6 |
| ECM |
Extracellular matrix |
| EMT |
Epithelial-Mesenchymal Transition |
| ERK |
Extracellular-signal-regulated kinase |
| FOXO1 |
Forkhead box protein O1 |
| GAGE12I |
G antigen 12I |
| GC |
Germinal center |
| HOXB3 |
Homeobox protein Hox-B3 |
| hREMECs |
Human retinal microvascular endothelial cells |
| IGF-1 |
Insulin-like growth factor 1 |
| LDHA |
Lactate dehydrogenase A |
| LncRNA |
Long non-coding RNA |
| MAD2L1 |
Mitotic spindle assembly checkpoint protein MAD2A |
| MEF2A |
Myocyte-specific enhancer factor 2A |
| miRNA |
MicroRNA |
| MRF |
Myogenic regulatory factors |
| MSTN |
Myostatin |
| MYOD |
Myoblast determination protein |
| MYOG |
Myogenin |
| N4BP1 |
NEDD4 Binding Protein 1 |
| NRF2 |
Nuclear factor erythroid 2-related factor 2 |
| PD |
Parkinson disease |
| PD1 |
Programmed cell death protein 1 |
| PI3K |
Phosphoinositide 3-kinases |
| PTEN |
Phosphatase and tensin homologue |
| RAP1B |
Ras-related protein Rap-1b |
| ROS |
Reactive oxygen species |
| RUNX2 |
Runt-related transcription factor 2 |
| SEMA7A |
Semaphorin 7A |
| SNAIL |
Zinc finger protein SNAI1 |
| SREBF2 |
Sterol regulatory element-binding protein 2 |
| SSRP1 |
Structure specific recognition protein 1 |
| STAT1 |
Signal transducer and activator of transcription 1 |
| TRPM7 |
Transient receptor potential cation channel subfamily M member 7 |
| WSB2 |
WD repeat and SOCS box-containing protein 2 |
| ZEB1 |
Zinc finger E-box-binding homeobox 1 |
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [CrossRef]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional Regulation of the Heterochronic Gene Lin-14 by Lin-4 Mediates Temporal Pattern Formation in C. Elegans. Cell 1993, 75, 855–862. [CrossRef]
- Tang, G. SiRNA and MiRNA: An Insight into RISCs. Trends Biochem. Sci. 2005, 30, 106–114. [CrossRef]
- Majorek, K.; Krzyżosiak, W.J. Role of MicroRNA in Pathogenesis, Diagnostics and Therapy of Cancer. Contemp. Oncol/Wspol. Onkol. 2006, 10, 359–366.
- Colpaert, R.M.W.; Calore, M. MicroRNAs in Cardiac Diseases. Cells 2019, 8. [CrossRef]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical Significance of MiRNAs in Autoimmunity. J Autoimmun. 2020, 109. [CrossRef]
- Hosseini, S.F.; Javanshir-giv, S.; Soleimani, H.; Mollaei, H.; Sadri, F.; Rezaei, Z. The Importance of Hsa-MiR-28 in Human Malignancies. Biomed. Pharmacother. 2023, 161, 114453. [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna. J. Med. Biotechnol. 2010, 2, 161.
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [CrossRef]
- Budzynski, M.; Grenda, A.; Filip, A.A. MicroRNA Molecules as a Significant Constituent in Gene Regulation Mechanisms Related to Cancer. Nowotwory. J. Oncol. 2014, 64, 48–60. [CrossRef]
- Koralewska, N.; Ciechanowska, K.; Pokornowska, M.; Figlerowicz, M.; Kurzynska-Kokorniak, A. Ludzka Rybonukleaza Dicer a Struktura i Funkcje Biologiczne. Postepy Biochem. 2019, 65, 173–182. [CrossRef]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear Export of MicroRNA Precursors. Science 2004, 303, 95–98. [CrossRef]
- Zajkowska A; Małecki M MicroRNAs ROLE IN HEART DEVELOPMENT. Postępy Biol. Kom. 2015, 42, 107–126.
- Jonas, S.; Izaurralde, E. Towards a Molecular Understanding of MicroRNA-Mediated Gene Silencing. Nat. Rev. Genet. 2015, 16, 421–433. [CrossRef]
- Pratt, A.J.; MacRae, I.J. The RNA-Induced Silencing Complex: A Versatile Gene-Silencing Machine. J. Biol. Chem. 2009, 284, 17897–17901. [CrossRef]
- Wakiyama, M.; Takimoto, K.; Ohara, O.; Yokoyama, S. Let-7 MicroRNA-Mediated MRNA Deadenylation and Translational Repression in a Mammalian Cell-Free System. Genes. Dev. 2007, 21, 1857–1862. [CrossRef]
- Liu, J.; Valencia-Sanchez, M.A.; Hannon, G.J.; Parker, R. MicroRNA-Dependent Localization of Targeted MRNAs to Mammalian P-Bodies. Nat. Cell. Biol. 2005, 7, 719–723. [CrossRef]
- Weeden, C.E.; Hill, W.; Lim, E.L.; Grönroos, E.; Swanton, C. Impact of Risk Factors on Early Cancer Evolution. Cell 2023, 186, 1541–1563. [CrossRef]
- Lewandowska, A.M.; Rudzki, M.; Rudzki, S.; Lewandowski, T.; Laskowska, B. Environmental Risk Factors for Cancer - Review Paper. Ann. Agric. Environ. Med. 2019, 26, 1–7. [CrossRef]
- Garutti, M.; Foffano, L.; Mazzeo, R.; Michelotti, A.; Da Ros, L.; Viel, A.; Miolo, G.; Zambelli, A.; Puglisi, F. Hereditary Cancer Syndromes: A Comprehensive Review with a Visual Tool. Genes (Basel) 2023, 14. [CrossRef]
- Giatagana, E.M.; Berdiaki, A.; Tsatsakis, A.; Tzanakakis, G.N.; Nikitovic, D. Lumican in Carcinogenesis-Revisited. Biomolecules 2021, 11. [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat. Commun. 2020, 11. [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [CrossRef]
- Willmott, L.J.; Monk, B.J. Cervical Cancer Therapy: Current, Future and Anti-Angiogensis Targeted Treatment. Expert. Rev. Anticancer Ther. 2009, 9, 895–903. [CrossRef]
- Kroemer, G.; Pouyssegur, J. Tumor Cell Metabolism: Cancer’s Achilles’ Heel. Cancer Cell 2008, 13, 472–482. [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. MiRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23. [CrossRef]
- Pekarek, L.; Torres-Carranza, D.; Fraile-Martinez, O.; García-Montero, C.; Pekarek, T.; Saez, M.A.; Rueda-Correa, F.; Pimentel-Martinez, C.; Guijarro, L.G.; Diaz-Pedrero, R.; et al. An Overview of the Role of MicroRNAs on Carcinogenesis: A Focus on Cell Cycle, Angiogenesis and Metastasis. Int. J. Mol. Sci. 2023, 24. [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. MicroRNAs as Oncogenes and Tumor Suppressors. Dev. Biol. 2007, 302, 1–12. [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target Ther. 2016, 1, 15004. [CrossRef]
- Yan, H.; Bu, P. Non-Coding RNA in Cancer. Essays Biochem. 2021, 65, 625. [CrossRef]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Non-Coding RNA (LncRNA) and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965. [CrossRef]
- Skrzypek, K.; Majka, M. Interplay among SNAIL Transcription Factor, MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Regulation of Tumor Growth and Metastasis. Cancers (Basel) 2020, 12, 209. [CrossRef]
- Han, Q.; Li, J.; Xiong, J.; Song, Z. Long Noncoding RNA LINC00514 Accelerates Pancreatic Cancer Progression by Acting as a CeRNA of MiR-28-5p to Upregulate Rap1b Expression. J. Exp. Clin. Cancer Res. 2020, 39, 1–13. [CrossRef]
- Liu, Y.; Guo, C.; Li, F.; Wu, L. LncRNA LOXL1-AS1/MiR-28-5p/SEMA7A Axis Facilitates Pancreatic Cancer Progression. Cell Biochem. Funct. 2020, 38, 58–65. [CrossRef]
- Han, C.; Tang, F.; Chen, J.; Xu, D.; Li, X.; Xu, Y.; Wang, S.; Zhou, J. Knockdown of LncRNA UCA1 Inhibits the Proliferation and Migration of Melanoma Cells through Modulating the MiR 28 5p/HOXB3 Axis. Exp. Ther. Med. 2019, 17, 4294–4302. [CrossRef]
- Xu, J.H.; Zhao, W.Y.; Fang, Q.Q.; Wang, X.F.; Zhang, D.D.; Hu, Y.Y.; Zheng, B.; Tan, W.Q. Long Noncoding RNA LUADT1 Is Upregulated in Melanoma and May Sponge MiR-28-5p to Upregulate RAP1B. Cancer Biother. Radiopharm. 2020, 35, 307–312. [CrossRef]
- Hosseini, S.F.; Javanshir-giv, S.; Soleimani, H.; Mollaei, H.; Sadri, F.; Rezaei, Z. The Importance of Hsa-MiR-28 in Human Malignancies. Biomed. Pharmacother. 2023, 161. [CrossRef]
- Chen, Q.; Xu, H.; Zhu, J.; Feng, K.; Hu, C. LncRNA MCM3AP-AS1 Promotes Breast Cancer Progression via Modulating MiR-28-5p/CENPF Axis. Biomed. Pharmacother. 2020, 128, 110289. [CrossRef]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, Functions and Interactions with Proteins. Mol. Cancer 2020, 19, 172. [CrossRef]
- Zan, X.; Li, W.; Wang, G.; Yuan, J.; Ai, Y.; Huang, J.; Li, Z. Circ-CSNK1G1 Promotes Cell Proliferation, Migration, Invasion and Glycolysis Metabolism during Triple-Negative Breast Cancer Progression by Modulating the MiR-28-5p/LDHA Pathway. Reprod. Biol. Endocrinol. 2022, 20, 138. [CrossRef]
- Wang, J.F.; Zhao, X.H.; Wang, Y.B.; Ren, F.H.; Sun, D.W.; Yan, Y.B.; Kong, X.L.; Bu, J.L.; Liu, M.F.; Xu, S.D. CircRNA-002178 Act as a CeRNA to Promote PDL1/PD1 Expression in Lung Adenocarcinoma. Cell Death Dis. 2020, 11, 32. [CrossRef]
- He, S.L.; Zhao, X.; Yi, S.J. CircAHNAK Upregulates EIF2B5 Expression to Inhibit the Progression of Ovarian Cancer by Modulating the JAK2/STAT3 Signaling Pathway. Carcinogenesis 2022, 43, 941–955. [CrossRef]
- Wang, X.; Yao, Y.; Jin, M. Circ-0001068 Is a Novel Biomarker for Ovarian Cancer and Inducer of PD1 Expression in T Cells. Aging 2020, 12, 19095–19106. [CrossRef]
- Mao, Y.; Wang, C. A Cytoplasm-Enriched CircRNA Circ-MYBL2 Is Downregulated in Non-Small Cell Lung Cancer and Sponges Oncogenic MiR-28 to Regulate Cancer Cell Proliferation and Apoptosis. Cancer Manag. Res. 2021, 13, 6499–6506. [CrossRef]
- Ma, X.; Wang, C.; Chen, J.; Wei, D.; Yu, F.; Sun, J. CircAGFG1 Sponges MiR-28-5p to Promote Non-Small-Cell Lung Cancer Progression through Modulating HIF-1α Level. Open Med. (Poland) 2021, 16, 703–717. [CrossRef]
- Tang, M.; Rong, Y.; Liu, S.; Wu, Z.; Ma, G.; Li, X.; Cai, H. Potential Role of LncRNA LOXL1-AS1 in Human Cancer Development: A Narrative Review. Transl. Cancer. Res. 2024, 13, 1997–2011. [CrossRef]
- Han, C.; Tang, F.; Chen, J.; Xu, D.; Li, X.; Xu, Y.; Wang, S.; Zhou, J. Knockdown of LncRNA UCA1 Inhibits the Proliferation and Migration of Melanoma Cells through Modulating the MiR 28 5p/HOXB3 Axis. Exp. Ther. Med. 2019, 17, 4294–4302. [CrossRef]
- Cui, M.; Chen, M.; Shen, Z.; Wang, R.; Fang, X.; Song, B. LncRNA-UCA1 Modulates Progression of Colon Cancer through Regulating the MiR-28-5p/HOXB3 Axis. J. Cell Biochem. 2019, 120, 6926–6936. [CrossRef]
- You, Z.; Liu, C.; Wang, C.; Ling, Z.; Wang, Y.; Wang, Y.; Zhang, M.; Chen, S.; Xu, B.; Guan, H.; et al. LncRNA CCAT1 Promotes Prostate Cancer Cell Proliferation by Interacting with DDX5 and MIR-28-5P. Mol. Cancer Ther. 2019, 18, 2469–2479. [CrossRef]
- Ma, M.L.; Zhang, H.Y.; Zhang, S.Y.; Yi, X.L. LncRNA CDKN2B-AS1 Sponges MiR-28-5p to Regulate Proliferation and Inhibit Apoptosis in Colorectal Cancer. Oncol. Rep. 2021, 46, 1–11. [CrossRef]
- Mao, W.; Wang, S.; Chen, R.; He, Y.; Lu, R.; Zheng, M. LncRNA NORAD Promotes Lung Cancer Progression by Competitively Binding to MiR-28-3p with E2F2. Open Med. (Poland) 2022, 17, 1538–1549. [CrossRef]
- Yu, J.; He, C.; Peng, Y.; Wen, Y.; Wang, J. LncRNA CASC9 Facilitates Papillary Thyroid Cancer Development and Doxorubicin Resistance via MiR-28-3p/BCL-2 Axis and PI3K/AKT Signaling Pathway. J. Cardiothorac. Surg. 2024, 19, 1–13. [CrossRef]
- Wang, J.; Xu, B.; Liang, L.; Chen, Q. Long Non-Coding RNA 02298 Promotes the Malignancy of HCC by Targeting the MiR-28-5p/CCDC6 Pathway. Biochem. Genet. 2024, 62, 4967–4986. [CrossRef]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal Carcinoma. The Lancet 2019, 394, 64–80. [CrossRef]
- Lv, Y.; Yang, H.; Ma, X.; Wu, G. Strand-Specific MiR-28-3p and MiR-28-5p Have Differential Effects on Nasopharyngeal Cancer Cells Proliferation, Apoptosis, Migration and Invasion. Cancer Cell Int. 2019, 19. [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Invest. 2009, 119, 1420–1428. [CrossRef]
- Stenzel, A.; Żuryń, A.; Grzanka, A.A.; Grzanka, A. Cykliny Jako Markery Chorób Nowotworowych. Nowotwory. J. Oncol. 2012, 62, 115–122.
- Huang, F.L.; Yu, S.J. Esophageal Cancer: Risk Factors, Genetic Association, and Treatment. Asian J. Surg. 2018, 41, 210–215. [CrossRef]
- Zhang, L.; Wang, X.; Liu, X.; Lv, M.; Shen, E.; Zhu, G.; Sun, Z. MiR-28-5p Targets MTSS1 to Regulate Cell Proliferation and Apoptosis in Esophageal Cancer. Acta Biochim. Biophys. Sin. (Shanghai) 2020, 52, 842–852. [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric Cancer: Descriptive Epidemiology, Risk Factors, Screening, and Prevention. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 700. [CrossRef]
- Xiao, F.; Cheng, Z.; Wang, P.; Gong, B.; Huang, H.; Xing, Y.; Liu, F. MicroRNA-28-5p Inhibits the Migration and Invasion of Gastric Cancer Cells by Suppressing AKT Phosphorylation. Oncol. Lett. 2018, 15, 9777–9785. [CrossRef]
- Yuan, D.; Xia, H.; Zhang, Y.; Chen, L.; Leng, W.; Chen, T.; Chen, Q.; Tang, Q.; Mo, X.; Liu, M.; et al. P-Akt/MiR 200 Signaling Regulates Epithelial-Mesenchymal Transition, Migration and Invasion in Circulating Gastric Tumor Cells. Int. J. Oncol. 2014, 45, 2430–2438. [CrossRef]
- Yue, C.F.; Li, L.S.; Ai, L.; Deng, J.K.; Guo, Y.M. SMicroRNA-28-5p Acts as a Metastasis Suppressor in Gastric Cancer by Targeting Nrf2. Exp. Cell Res. 2021, 402. [CrossRef]
- Xu, R.; Guo, Q.; Zhao, P.; Lu, H.; Zhang, B. MiR-28-5p’s Targeting of GAGE12I Inhibits Proliferation, Migration, and Invasion of Gastric Cancer in Vitro. Evid. Based Complement. Alternat. Med. 2022, 2022. [CrossRef]
- Li, L.; Zhu, X.; Shou, T.; Yang, L.; Cheng, X.; Wang, J.; Deng, L.; Zheng, Y. MicroRNA-28 Promotes Cell Proliferation and Invasion in Gastric Cancer via the Pten/Pi3k/Akt Signalling Pathway. Mol. Med. Rep. 2018, 17, 4003–4010. [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. The Lancet 2019, 394, 1467–1480. [CrossRef]
- Jia, S.; Guo, B.; Wang, L.; Peng, L.; Zhang, L. The Current Status of SSRP1 in Cancer: Tribulation and Road Ahead. J. Healthc. Eng. 2022, 2022. [CrossRef]
- Wu, W.; He, K.; Guo, Q.; Chen, J.; Zhang, M.; Huang, K.; Yang, D.; Wu, L.; Deng, Y.; Luo, X.; et al. SSRP1 Promotes Colorectal Cancer Progression and Is Negatively Regulated by MiR-28-5p. J. Cell Mol. Med. 2019, 23, 3118–3129. [CrossRef]
- Luan, X.F.; Wang, L.; Gai, X.F. The MiR-28-5p-CAMTA2 Axis Regulates Colon Cancer Progression via Wnt/β-Catenin Signaling. J. Cell Biochem. 2021, 122, 945–957. [CrossRef]
- Almeida, M.I.; Nicoloso, M.S.; Zeng, L.; Ivan, C.; Spizzo, R.; Gafà, R.; Xiao, L.; Zhang, X.; Vannini, I.; Fanini, F.; et al. Strand-Specific MiR-28-5p and MiR-28-3p Have Distinct Effects in Colorectal Cancer Cells. Gastroenterology 2012, 142, 886. [CrossRef]
- Zhu, S.; Yang, Z.; Zhang, Z.; Zhang, H.; Li, S.; Wu, T.; Chen, X.; Guo, J.; Wang, A.; Tian, H.; et al. HOXB3 Drives WNT-Activation Associated Progression in Castration-Resistant Prostate Cancer. Cell Death Dis. 2023, 14. [CrossRef]
- Mátyási, B.; Farkas, Z.; Kopper, L.; Sebestyén, A.; Boissan, M.; Mehta, A.; Takács-Vellai, K. The Function of NM23-H1/NME1 and Its Homologs in Major Processes Linked to Metastasis. Pathol. Oncol. Res. 2020, 26, 49. [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS Induced Lipid Peroxidation and Their Role in Ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [CrossRef]
- Dixon, S.J. Ferroptosis: Bug or Feature? Immunol. Rev. 2017, 277, 150–157. [CrossRef]
- Hu, J.C.; Zhu, T.P.; Gui, Y.C.; Tan, Z.B.; Wei, R.Q.; Hu, B.L.; Xu, J.W. MiR-28-5p Inhibits Carcinogenesis in Colon Cancer Cells and Is Necessary for Erastin-Induced Ferroptosis. Transl. Cancer Res. 2020, 9, 2931. [CrossRef]
- Taniguchi, H. Liver Cancer 2.0. Int. J. Mol. Sci. 2023, 24, 17275. [CrossRef]
- Xia, Q.; Han, T.; Yang, P.; Wang, R.; Li, H.; Zhang, J.; Zhou, X. MicroRNA-28-5p Regulates Liver Cancer Stem Cell Expansion via IGF-1 Pathway. Stem Cells Int. 2019, 2019. [CrossRef]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168. [CrossRef]
- Chen, T.; Wang, H.; Yan, H. MiR-28-5p Inhibits Cholangiocarcinoma Progression and Predicts Good Prognosis of Patients. Cell Cycle 2022, 21, 2079–2090. [CrossRef]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [CrossRef]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer. J. Clin. 2022, 72, 524–541. [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [CrossRef]
- Ma, L.; Zhang, Y.; Hu, F. MiR-28-5p Inhibits the Migration of Breast Cancer by Regulating WSB2. Int. J. Mol. Med. 2020, 46, 1562–1570. [CrossRef]
- Zan, X.; Li, W.; Wang, G.; Yuan, J.; Ai, Y.; Huang, J.; Li, Z. Circ-CSNK1G1 Promotes Cell Proliferation, Migration, Invasion and Glycolysis Metabolism during Triple-Negative Breast Cancer Progression by Modulating the MiR-28-5p/LDHA Pathway. Reprod. Biol. Endocrinol. 2022, 20, 1–14. [CrossRef]
- Zhang, Y.; Li, Z.; Zhao, W.; Hu, H.; Zhao, L.; Zhu, Y.; Yang, X.; Gao, B.; Yang, H.; Huang, Y.; et al. WD Repeat and SOCS Box Containing Protein 2 in the Proliferation, Cycle Progression, and Migration of Melanoma Cells. Biomed.Pharmacother. 2019, 116, 108974. [CrossRef]
- Yang, M.; Yao, Y.; Eades, G.; Zhang, Y.; Zhou, Q. MiR-28 Regulates Nrf2 Expression through a Keap1-Independent Mechanism. Breast Cancer Res. Treat. 2011, 129, 983. [CrossRef]
- Wu, S.; Lu, H.; Bai, Y. Nrf2 in Cancers: A Double-edged Sword. Cancer Med. 2019, 8, 2252. [CrossRef]
- Liu, J.; Zhang, C.; Zhang, T.; Chang, C.Y.; Wang, J.; Bazile, L.; Zhang, L.; Haffty, B.G.; Hu, W.; Feng, Z. Metabolic Enzyme LDHA Activates Rac1 GTPase as a Noncanonical Mechanism to Promote Cancer. Nat. Metab. 2022, 4, 1830. [CrossRef]
- Xiao, X.; Huang, X.; Ye, F.; Chen, B.; Song, C.; Wen, J.; Zhang, Z.; Zheng, G.; Tang, H.; Xie, X. The MiR-34a-LDHA Axis Regulates Glucose Metabolism and Tumor Growth in Breast Cancer. Scientific Reports 2016 6:1 2016, 6, 1–9. [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [CrossRef]
- Xu, J.; Jiang, N.; Shi, H.; Zhao, S.; Yao, S.; Shen, H. MiR-28-5p Promotes the Development and Progression of Ovarian Cancer through Inhibition of N4BP1. Int. J. Oncol. 2017, 50, 1383–1391. [CrossRef]
- Murillas, R.; Simms, K.S.; Hatakeyama, S.; Weissman, A.M.; Kuehn, M.R. Identification of Developmentally Expressed Proteins That Functionally Interact with Nedd4 Ubiquitin Ligase. J. Biol. Chem. 2002, 277, 2897–2907. [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27. [CrossRef]
- Zhang, J.; Yao, Y.; Li, H.; Ye, S. MiR-28-3p Inhibits Prostate Cancer Cell Proliferation, Migration and Invasion, and Promotes Apoptosis by Targeting ARF6. Exp. Ther. Med. 2021, 22. [CrossRef]
- Rizzo, M.; Berti, G.; Russo, F.; Evangelista, M.; Pellegrini, M.; Rainaldi, G. The MiRNA Pull Out Assay as a Method to Validate the MiR-28-5p Targets Identified in Other Tumor Contexts in Prostate Cancer. Int. J. Genomics 2017, 2017. [CrossRef]
- Fazio, S.; Berti, G.; Russo, F.; Evangelista, M.; D’Aurizio, R.; Mercatanti, A.; Pellegrini, M.; Rizzo, M. The MiR-28-5p Targetome Discovery Identified SREBF2 as One of the Mediators of the MiR-28-5p Tumor Suppressor Activity in Prostate Cancer Cells. Cells 2020, 9. [CrossRef]
- Dobruch, J.; Oszczudłowski, M. Bladder Cancer: Current Challenges and Future Directions. Medicina (Kaunas) 2021, 57. [CrossRef]
- Piao, X.M.; Cha, E.J.; Yun, S.J.; Kim, W.J. Role of Exosomal MiRNA in Bladder Cancer: A Promising Liquid Biopsy Biomarker. Int. J. Mol. Sci. 2021, 22, 1–14. [CrossRef]
- Yasui, T.; Yanagida, T.; Ito, S.; Konakade, Y.; Takeshita, D.; Naganawa, T.; Nagashima, K.; Shimada, T.; Kaji, N.; Nakamura, Y.; et al. Unveiling Massive Numbers of Cancer-Related Urinary-MicroRNA Candidates via Nanowires. Sci. Adv. 2017, 3. [CrossRef]
- Wu, K.L.; Tsai, Y.M.; Lien, C.T.; Kuo, P.L.; Hung, J.Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20. [CrossRef]
- Cui, F.; Zhou, Q.; Xiao, K.; Qian, H. MicroRNA-28 Promotes the Proliferation of Non-Small-Cell Lung Cancer Cells by Targeting PTEN. Mol. Med. Rep. 2020, 21, 2589–2596. [CrossRef]
- Hopkins, B.D.; Parsons, R.E. Molecular Pathways: Intercellular PTEN and the Potential of PTEN Restoration Therapy. Clin. Cancer Res. 2014, 20, 5379. [CrossRef]
- Chen, R.; Smith-Cohn, M.; Cohen, A.L.; Colman, H. Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 2017, 14, 284. [CrossRef]
- Wan, J.; Guo, A.A.; Chowdhury, I.; Guo, S.; Hibbert, J.; Wang, G.; Liu, M. TRPM7 Induces Mechanistic Target of Rap1b Through the Downregulation of MiR-28-5p in Glioma Proliferation and Invasion. Front. Oncol. 2019, 9, 492527. [CrossRef]
- Zhang, L.; Cui, M.; Song, L.; Zhang, M.; Zhangd, J. Function, Significance, and Regulation of Rap1b in Malignancy. Crit Rev Eukaryot. Gene Expr. 2019, 29, 151–160. [CrossRef]
- Zhu, G.; Wang, Z.; Mijiti, M.; Du, G.; Li, Y.; Dangmurenjiafu, G. MiR-28-5p Promotes Human Glioblastoma Cell Growth through Inactivation of FOXO1. Int. J. Clin. Exp. Pathol. 2019, 12, 2972.
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers. 2019, 5. [CrossRef]
- Skrzypek, K.; Nieszporek, A.; Badyra, B.; Lasota, M.; Majka, M. Enhancement of Myogenic Differentiation and Inhibition of Rhabdomyosarcoma Progression by MiR-28-3p and MiR-193a-5p Regulated by SNAIL. Mol. Ther. Nucleic Acids 2021, 24, 888–904. [CrossRef]
- Skrzypek, K.; Kot, M.; Konieczny, P.; Nieszporek, A.; Kusienicka, A.; Lasota, M.; Bobela, W.; Jankowska, U.; Kędracka-Krok, S.; Majka, M. SNAIL Promotes Metastatic Behavior of Rhabdomyosarcoma by Increasing EZRIN and AKT Expression and Regulating MicroRNA Networks. Cancers (Basel) 2020, 12, 1–25. [CrossRef]
- Skrzypek, K.; Kusienicka, A.; Trzyna, E.; Szewczyk, B.; Ulman, A.; Konieczny, P.; Adamus, T.; Badyra, B.; Kortylewski, M.; Majka, M. SNAIL Is a Key Regulator of Alveolar Rhabdomyosarcoma Tumor Growth and Differentiation through Repression of MYF5 and MYOD Function. Cell Death Dis. 2018, 9. [CrossRef]
- Hsieh, M.Y.; Hsu, S.K.; Liu, T.Y.; Wu, C.Y.; Chiu, C.C. Melanoma Biology and Treatment: A Review of Novel Regulated Cell Death-Based Approaches. Cancer Cell Int. 2024 24:1 2024, 24, 1–21. [CrossRef]
- Conforti, C.; Zalaudek, I. Epidemiology and Risk Factors of Melanoma: A Review. Dermatol. Pract. Concept. 2021, 11, e2021161S. [CrossRef]
- Li, Q.; Johnston, N.; Zheng, X.; Wang, H.; Zhang, X.; Gao, D.; Min, W. MiR-28 Modulates Exhaustive Differentiation of T Cells through Silencing Programmed Cell Death-1 and Regulating Cytokine Secretion. Oncotarget 2016, 7, 53735–53750. [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-Cell Exhaustion in the Tumor Microenvironment. Cell Death Dis. 2015 6:6 2015, 6, e1792–e1792. [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on Tumor Cells in the Escape from Host Immune System and Tumor Immunotherapy by PD-L1 Blockade. Proc. Natl. Acad. Sci. U S A 2002, 99, 12293–12297. [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. (Basel) 2021, 9. [CrossRef]
- Schneider, C.; Setty, M.; Holmes, A.B.; Maute, R.L.; Leslie, C.S.; Mussolin, L.; Rosolen, A.; Dalla-Favera, R.; Bassoa, K. MicroRNA 28 Controls Cell Proliferation and Is Down-Regulated in B-Cell Lymphomas. Proc. Natl. Acad. Sci. U S A 2014, 111, 8185–8190. [CrossRef]
- Bartolome-Izquierdo, N.; De Yebenes, V.G.; Alvarez-Prado, A.F.; Mur, S.M.; Del Olmo, J.A.L.; Roa, S.; Vazquez, J.; Ramiro, A.R. MiR-28 Regulates the Germinal Center Reaction and Blocks Tumor Growth in Preclinical Models of Non-Hodgkin Lymphoma. Blood 2017, 129, 2408–2419. [CrossRef]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112. [CrossRef]
- Wang, L.; Zhao, Y.; Xu, M.; Zhou, F.; Yan, J. Serum MiR-1301-3p, MiR-335-5p, MiR-28-5p, and Their Target B7-H3 May Serve as Novel Biomarkers for Colorectal Cancer. J. BUON 2019, 24, 1120–1127.
- Jeddi, F.; Alipour, S.; Najafzadeh, N.; Dadashpour, M.; Pouremamali, F.; Sadeghi, M.R.; Samadi, N.; Soozangar, N.; Khamaneh, A.M. Reduced Levels of MiR–28 and MiR–200a Act as Predictor Biomarkers of Aggressive Clinicopathological Characteristics in Gastric Cancer Patients. Galen Med. J. 2019, 8, e1329. [CrossRef]
- Silva, C.M.S.; Barros-Filho, M.C.; Wong, D.V.T.; Mello, J.B.H.; Nobre, L.M.S.; Wanderley, C.W.S.; Lucetti, L.T.; Muniz, H.A.; Paiva, I.K.D.; Kuasne, H.; et al. Circulating Let-7e-5p, MiR-106a-5p, MiR-28-3p, and MiR-542-5p as a Promising MicroRNA Signature for the Detection of Colorectal Cancer. Cancers (Basel) 2021, 13. [CrossRef]
- Guo, Y.; Cui, X.; Zhang, Y.; Ma, X.; Ren, A.; Huang, H. Diagnostic and Prognostic Value of Serum MiR-296-5p and MiR-28-3p in Human Gastric Cancer. Cancer Biother. Radiopharm. 2023, 38, 95–101. [CrossRef]
- Sun, R.; Zheng, Z.; Wang, L.; Cheng, S.; Shi, Q.; Qu, B.; Fu, D.; Leboeuf, C.; Zhao, Y.; Ye, J.; et al. A Novel Prognostic Model Based on Four Circulating MiRNA in Diffuse Large B-cell Lymphoma: Implications for the Roles of MDSC and Th17 Cells in Lymphoma Progression. Mol. Oncol. 2020, 15, 246. [CrossRef]
- Wang, C.; Hu, J.; Lu, M.; Gu, H.; Zhou, X.; Chen, X.; Zen, K.; Zhang, C.Y.; Zhang, T.; Ge, J.; et al. A Panel of Five Serum MiRNAs as a Potential Diagnostic Tool for Early-Stage Renal Cell Carcinoma. Sci. Rep. 2015, 5, 7610. [CrossRef]
- Fuertes, T.; Álvarez-Corrales, E.; Gómez-Escolar, C.; Ubieto-Capella, P.; Serrano-Navarro, Á.; de Molina, A.; Méndez, J.; Ramiro, A.R.; de Yébenes, V.G. MiR-28-Based Combination Therapy Impairs Aggressive B Cell Lymphoma Growth by Rewiring DNA Replication. Cell Death Dis. 2023, 14. [CrossRef]
- Yan, S.; Shang, Q.; Zhu, H.; Chen, K.; Li, X.; Gao, H.; Liu, B.; Feng, M.; Gao, L. Effects of Hsa-MiR-28-5p on Adriamycin Sensitivity in Diffuse Large B-Cell Lymphoma. Evid. Based Complement. Alternat. Med. 2022, 2022, 4290994. [CrossRef]
- Kang, T.; Sun, W.L.; Lu, X.F.; Wang, X.L.; Jiang, L. MiR-28-5p Mediates the Anti-Proliferative and pro-Apoptotic Effects of Curcumin on Human Diffuse Large B-Cell Lymphoma Cells. J. Int. Med. Res. 2020, 48. [CrossRef]
- Shah, M.Y.; Calin, G.A. MicroRNAs as Therapeutic Targets in Human Cancers. Wiley Interdiscip. Rev. RNA 2014, 5, 537. [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [CrossRef]
- Zhang, S.; Cheng, Z.; Wang, Y.; Han, T. The Risks of MiRNA Therapeutics: In a Drug Target Perspective. Drug Des. Devel. Ther. 2021, 15, 721. [CrossRef]
- Ganju, A.; Khan, S.; Hafeez, B.B.; Behrman, S.W.; Yallapu, M.M.; Chauhan, S.C.; Jaggi, M. MiRNA Nanotherapeutics for Cancer. Drug Discov. Today 2016, 22, 424. [CrossRef]
- Yu, J.; Xu, Q.; Zhang, X.; Zhu, M. Circulating MicroRNA Signatures Serve as Potential Diagnostic Biomarkers for Helicobacter Pylori Infection. J. Cell Biochem. 2019, 120, 1735–1741. [CrossRef]
- Bai, X.T.; Nicot, C. MiR-28-3p Is a Cellular Restriction Factor That Inhibits Human T Cell Leukemia Virus, Type 1 (HTLV-1) Replication and Virus Infection. J. Biol. Chem. 2015, 290, 5381–5390. [CrossRef]
- Huang, J.; Wang, F.; Argyris, E.; Chen, K.; Liang, Z.; Tian, H.; Huang, W.; Squires, K.; Verlinghieri, G.; Zhang, H. Cellular MicroRNAs Contribute to HIV-1 Latency in Resting Primary CD4+ T Lymphocytes. Nat. Med. 2007, 13, 1241–1247. [CrossRef]
- COVID-19 Deaths | WHO COVID-19 Dashboard Available online: https://data.who.int/dashboards/covid19/deaths?n=o (accessed on 26 February 2025).
- COVID-19 Cases | WHO COVID-19 Dashboard Available online: https://data.who.int/dashboards/covid19/cases?n=o (accessed on 26 February 2025).
- Das, R.; Sinnarasan, V.S.P.; Paul, D.; Venkatesan, A. A Machine Learning Approach to Identify Potential MiRNA-Gene Regulatory Network Contributing to the Pathogenesis of SARS-CoV-2 Infection. Biochem. Genet. 2024, 62, 987–1006. [CrossRef]
- XU, Y.; LI, Y. MicroRNA-28-3p Inhibits Angiotensin-Converting Enzyme 2 Ectodomain Shedding in 293T Cells Treated with the Spike Protein of Severe Acute Respiratory Syndrome Coronavirus 2 by Targeting A Disintegrin and Metalloproteinase 17. Int. J. Mol. Med. 2021, 48, 189. [CrossRef]
- Prats-Puig, A.; Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Bonet, N.; Ricart, W.; López-Bermejo, A.; Fernández-Real, J.M. Changes in Circulating MicroRNAs Are Associated With Childhood Obesity. J. Clin. Endocrinol. Metab. 2013, 98, E1655–E1660. [CrossRef]
- Mahjoob, G.; Ahmadi, Y.; Fatima rajani, H.; khanbabaei, N.; Abolhasani, S. Circulating MicroRNAs as Predictive Biomarkers of Coronary Artery Diseases in Type 2 Diabetes Patients. J. Clin. Lab. Anal. 2022, 36, e24380. [CrossRef]
- Milluzzo, A.; Maugeri, A.; Barchitta, M.; Sciacca, L.; Agodi, A. Epigenetic Mechanisms in Type 2 Diabetes Retinopathy: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 10502. [CrossRef]
- Liang, Z.; Gao, K.P.; Wang, Y.X.; Liu, Z.C.; Tian, L.; Yang, X.Z.; Ding, J.Y.; Wu, W.T.; Yang, W.H.; Li, Y.L.; et al. RNA Sequencing Identified Specific Circulating MiRNA Biomarkers for Early Detection of Diabetes Retinopathy. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E374–E385. [CrossRef]
- Chang, S.; Dunaif, A. Diagnosis of Polycystic Ovary Syndrome: Which Criteria to Use When? Endocrinol. Metab. Clin. North Am. 2021, 50, 11. [CrossRef]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of Endometrial, Ovarian and Breast Cancer in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 748–758. [CrossRef]
- Meng, L.; Yang, H.; Jin, C.; Quan, S. MiR-28-5p Suppresses Cell Proliferation and Weakens the Progression of Polycystic Ovary Syndrome by Targeting Prokineticin-1. Mol. Med. Rep. 2019, 20, 2468–2475. [CrossRef]
- Seco-Cervera, M.; Ibáñez-Cabellos, J.S.; Pallardo, F. V.; García-Giménez, J.L.; Aulinas, A.; Martel-Duguech, L.; Webb, S.M.; Valassi, E. Circulating MiR-28-5p Is Overexpressed in Patients with Sarcopenia despite Long-Term Remission of Cushing’s Syndrome: A Pilot Study. Front. Endocrinol. (Lausanne) 2024, 15, 1410080. [CrossRef]
- Schakman, O.; Gilson, H.; Thissen, J.P. Mechanisms of Glucocorticoid-Induced Myopathy. J. Endocrinol. 2008, 197, 1–10. [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385. [CrossRef]
- He, S.; Huang, L.; Shao, C.; Nie, T.; Xia, L.; Cui, B.; Lu, F.; Zhu, L.; Chen, B.; Yang, Q. Several MiRNAs Derived from Serum Extracellular Vesicles Are Potential Biomarkers for Early Diagnosis and Progression of Parkinson’s Disease. Transl. Neurodegener. 2021, 10, 25. [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [CrossRef]
- Bao, Y.; Wang, S.; Xie, Y.; Jin, K.; Bai, Y.; Shan, S. MiR-28-5p Relieves Neuropathic Pain by Targeting Zeb1 in CCI Rat Models. J. Cell Biochem. 2018, 119, 8555–8563. [CrossRef]
- Radu, A.F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [CrossRef]
- Hemmatzadeh, M.; Parvin, E.A.; Ghanavatinejad, A.; Rostami, N.; Hajaliloo, M.; Shomali, N.; Mohammadi, H.; Jadidi-Niaragh, F. MicroRNAs Targeting Programmed Cell Death Protein 1 (PD-1) Promote Natural Killer Cell Exhaustion in Rheumatoid Arthritis. Iran J. Allergy Asthma Immunol. 2022, 21, 646–656. [CrossRef]
- Machanahalli Balakrishna, A.; Reddi, V.; Belford, P.M.; Alvarez, M.; Jaber, W.A.; Zhao, D.X.; Vallabhajosyula, S. Intermediate-Risk Pulmonary Embolism: A Review of Contemporary Diagnosis, Risk Stratification and Management. Medicina (Kaunas) 2022, 58. [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Qian, J.; Li, H.; Jiang, T.; Wang, W.; Cheng, W.; Wang, F.; Qi, L.; et al. MiR-28-3p as a Potential Plasma Marker in Diagnosis of Pulmonary Embolism. Thromb. Res. 2016, 138, 91–95. [CrossRef]
- Wang, M.; Dai, T.; Meng, Q.; Wang, W.; Li, S. Regulatory Effects of MiR-28 on Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Bioengineered 2022, 13, 684. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).