Submitted:
08 April 2025
Posted:
08 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
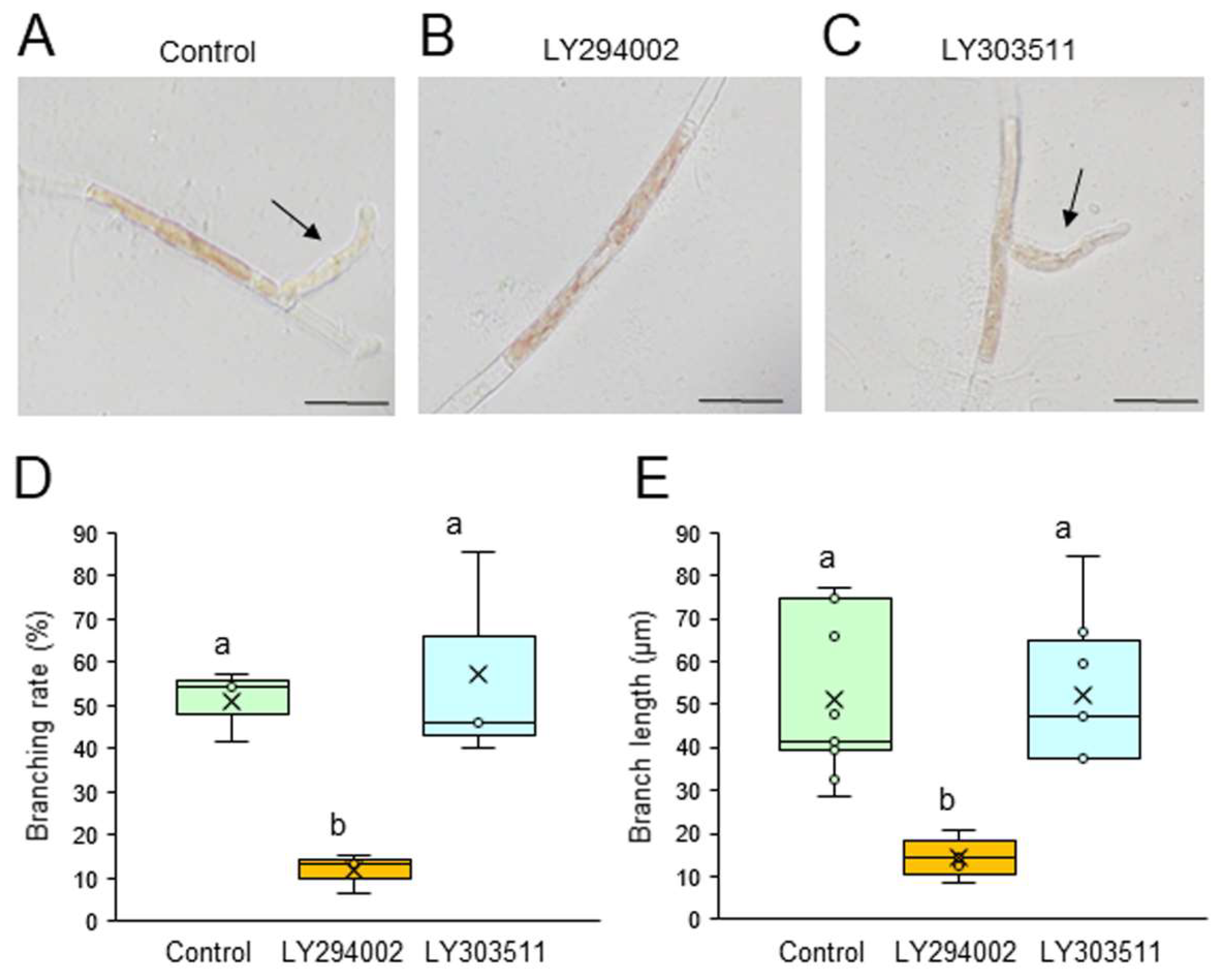
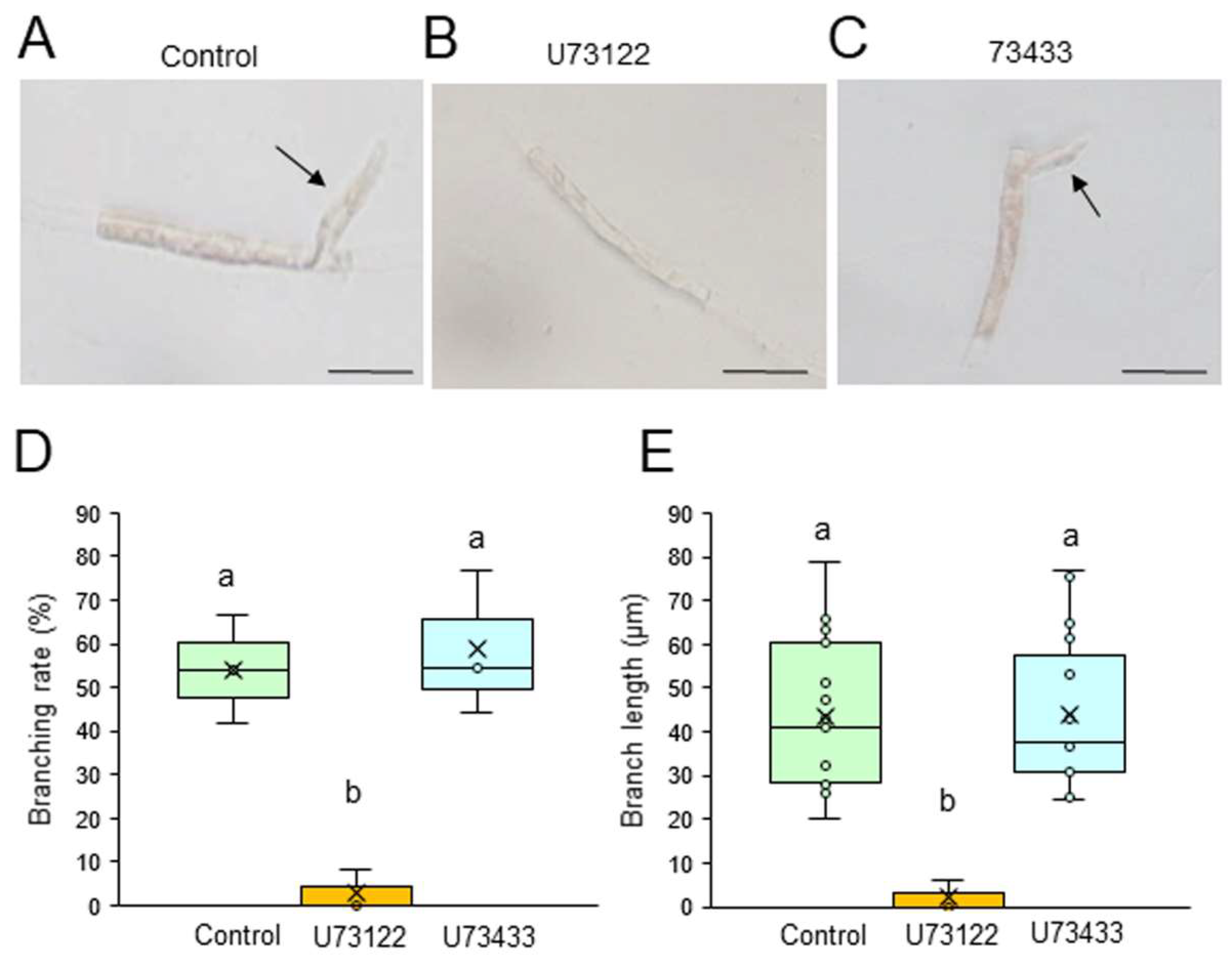
2.1. Effects of PI3K and PLC Inhibitors
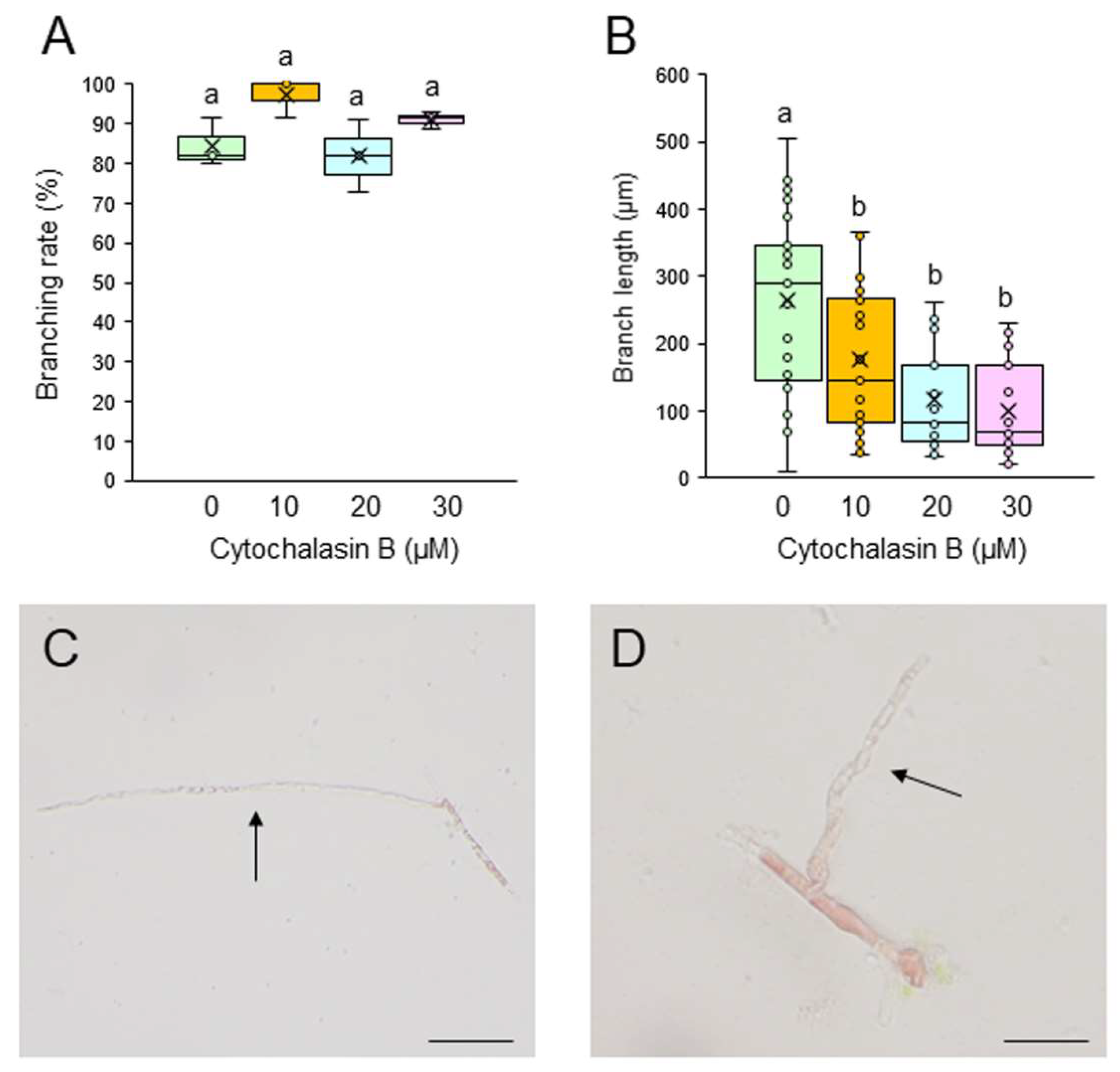
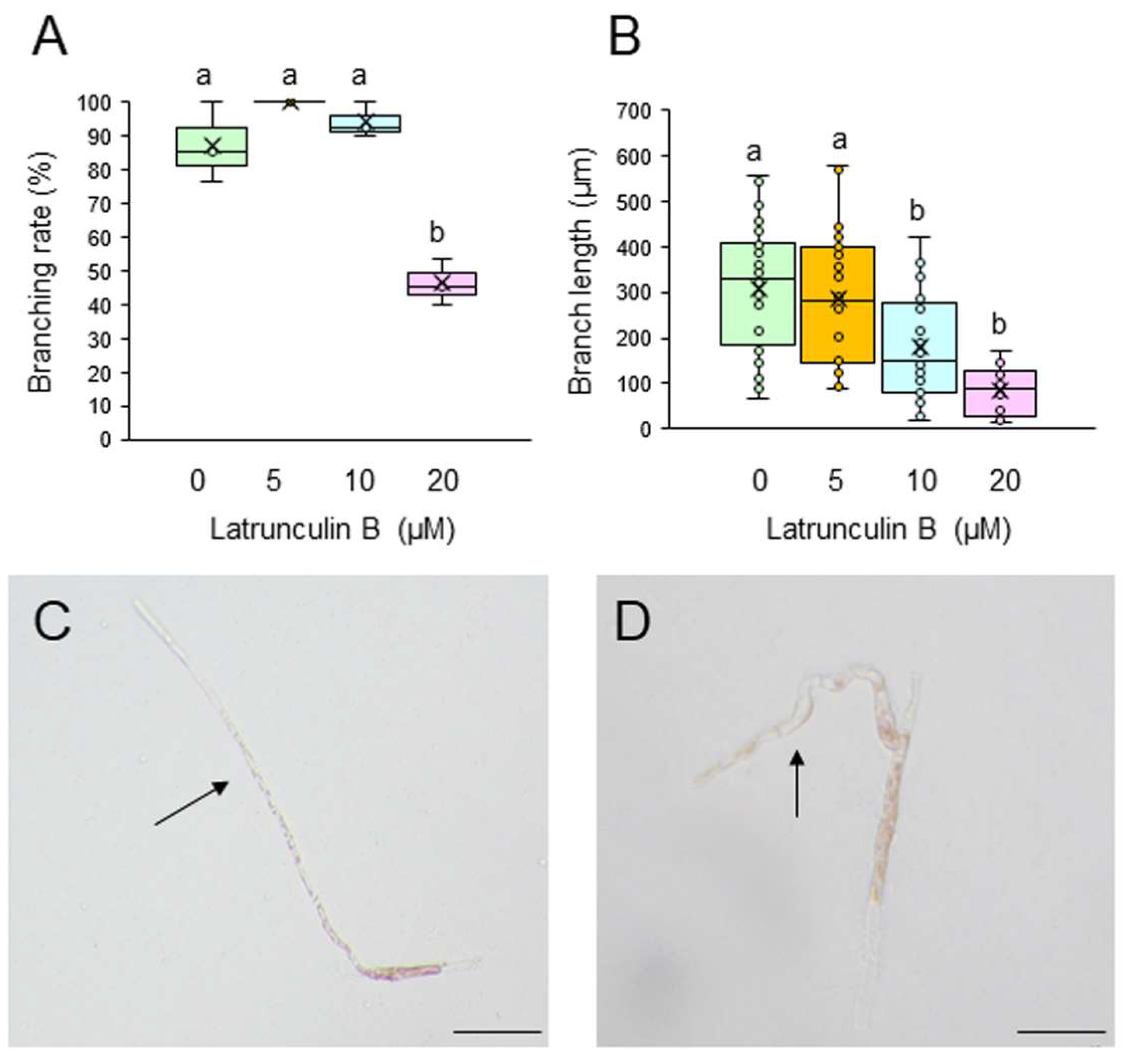
2.2. Effects of Actin Polymerization Inhibitors
3. Discussion
4. Materials and Methods
4.1. Algal Materials and Culture Conditions
4.2. Preparation of Single-Celled Conchocelis
4.3. Treatment of Single-Celled Conchocelis with Inhibitors of PI3K and PLC
4.4. Treatment of Single-Celled Conchocelis with Inhibitors of Actin Polymerization
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rounds, C.M.; Bezanilla, M. Growth mechanisms in tip-growing plant cells. Annu. Rev. Plant Biol. 2013, 64, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; Mouriño-Pérez, R.R.; Takeshita, N.; Fischer, R. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068–17. [Google Scholar] [CrossRef] [PubMed]
- Rabillé, H.; Billoud, B.; Tesson, B.; Le Panse, S.; Rolland, É.; Charrier, B. The brown algal mode of tip growth: Keeping stress under control. PLoS Biol. 2019, 17, e2005258. [Google Scholar] [CrossRef]
- Geitmann, A.; Emons, A.M. The cytoskeleton in plant and fungal cell tip growth. J. Microsc. 2000, 198(Pt 3) Pt 3, 218–245. [Google Scholar] [CrossRef]
- Campanoni, P.; Blatt, M.R. Membrane trafficking and polar growth in root hairs and pollen tubes. J. Exp. Bot. 2007, 58, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, T.; Seiler, S.; Heilmann, I. At the poles across kingdoms: phosphoinositides and polar tip growth. Protoplasma 2010, 240, 13–31. [Google Scholar] [CrossRef]
- Pei, W.; Du, F.; Zhang, Y.; He, T.; Ren, H. Control of the actin cytoskeleton in root hair development. Plant Sci. 2012, 187, 10–18. [Google Scholar] [CrossRef]
- Guan, Y.; Guo, J.; Li, H.; Yang, Z. Signaling in pollen tube growth: crosstalk, feedback, and missing links. Mol. Plant 2013, 6, 1053–1064. [Google Scholar] [CrossRef]
- Malhó, R.; Serrazina, S.; Saavedra, L.; Dias, F.V.; Ul-Rehman, R. Ion and lipid signaling in apical growth-a dynamic machinery responding to extracellular cues. Front. Plant Sci. 2015, 6, 816. [Google Scholar] [CrossRef]
- Velasquez, S.M.; Barbez, E.; Kleine-Vehn, J.; Estevez, J.M. Auxin and cellular elongation. Plant Physiol. 2016, 170, 1206–1215. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, L.; Yan, A.; Liu, Y.; Liu, B.; Yu, C.; Zhang, A.; Schiefelbein, J.; Gan, Y. Multiple phytohormones promote root hair elongation by regulating a similar set of genes in the root epidermis in Arabidopsis. J. Exp. Bot. 2016, 67, 6363–6372. [Google Scholar] [CrossRef] [PubMed]
- Bibeau, J.P.; Galotto, G.; Wu, M.; Tüzel, E.; Vidali, L. Quantitative cell biology of tip growth in moss. Plant Mol Biol. 2021, 107, 227–244. [Google Scholar] [CrossRef]
- Oubohssaine, M.; Hnini, M.; Rabeh, K. Phospholipid signaling in plant growth and development: Insights, biotechnological implications and future directions. J. Plant Physiol. 2025, 307, 154454. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yang, Z. Tip growth: signaling in the apical dome. Curr. Opin. Plant Biol. 2008, 11, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Scholz, P.; Anstatt, J.; Krawczyk, H.E.; Ischebeck, T. Signalling pinpointed to the tip: the complex regulatory network that allows pollen tube growth. Plants 2020, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.A.; Brodie, J.A.; Grossman, A.C.; Xu, P.; Brawley, S.H. Porphyra: a marine crop shaped by stress. Trends Plant Sci. 2011, 16, 29–37. [Google Scholar] [CrossRef]
- Takahashi, M.; Mikami, K. Phototropism in the marine red macroalga Pyropia yezoensis. Am. J. Plant Sci. 2016, 7, 2412–2428. [Google Scholar] [CrossRef]
- Mikami, K.; Li, C.; Irie, R.; Hama, Y. A unique life cycle transition in the red seaweed Pyropia yezoensis depends on apospory. Commun. Biol. 2019, 2, 299. [Google Scholar] [CrossRef]
- Hiwatashi, Y.; Shimada, M.; Mikami, K.; Takada, N. Establishment of a live-imaging analysis for polarized growth of conchocelis in the multicellular red alga Neopyropia yezoensis. Front. Plant Sci. 2022, 12, 716011. [Google Scholar] [CrossRef]
- Taya, K.; Takeuchi, S.; Takahashi, M.; Hayashi, K.I.; Mikami, K. Auxin regulates apical stem cell regeneration and tip growth in the marine red alga Neopyropia yezoensis. Cells 2022, 11, 2652. [Google Scholar] [CrossRef]
- Kofuji, R.; Hasebe, M. Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr. Opin. Plant Biol. 2014, 17, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Saga, N.; Mikami, K. Phosphatidylinositol 3-kinase activity and asymmetrical accumulation of F-actin are necessary for establishment of cell polarity in the early development of monospores from the marine red alga Porphyra yezoensis. J. Exp. Bot. 2008, 59, 3575–3586. [Google Scholar] [CrossRef]
- Li, L.; Saga, N.; Mikami, K. Effects of cell wall synthesis on cell polarity in the red alga Porphyra yezoensis. Plant Signal. Behav. 2008, 3, 1126–1128. [Google Scholar] [CrossRef]
- Li, L.; Saga, N.; Mikami, K. Ca2+ influx and phosphoinositide signalling are essential for the establishment and maintenance of cell polarity in monospores from the red alga Porphyra yezoensis. J. Exp. Bot. 2009, 60, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Li, L.; Takahashi, M.; Saga, N. Photosynthesis-dependent Ca2+ influx and functional diversity between phoss pholipases in the formation of cell polarity in migrating cells of red algae. Plant Signal. Behav. 2009, 4, 911–913. [Google Scholar] [CrossRef]
- Heilmann, I. Plant phosphoinositide signaling - dynamics on demand. Biochim. Biophys. Acta 2016, 1861(Pt B), 1345–1351. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, J.; Ding, H.; Li, X.; Xie, X. Phospholipase C: Diverse functions in plant biotic stress resistance and fungal pathogenicity. Mol. Plant Pathol. 2023, 24, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Helling, D.; Possart, A.; Cottier, S.; Klahre, U.; Kost, B. Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 2006, 18, 3519–3534. [Google Scholar] [CrossRef]
- Lee, Y.; Bak, G.; Choi, Y.; Chuang, W.I.; Cho, H.T.; Lee, Y. Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol. 2008, 147, 624–635. [Google Scholar] [CrossRef]
- Kusano, H.; Testerink, C.; Vermeer, J.E.; Tsuge, T.; Shimada, H.; Oka, A.; Munnik, T.; Aoyama, T. The Arabidopsis phosphatidylinositol phosphate 5-kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 2008, 20, 367–380. [Google Scholar] [CrossRef]
- Stenzel, I.; Ischebeck,T. ; König, S.; Hołubowska, A.; Sporysz, M.; Hause, B.; Heilmann, I. The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell 2008, 20, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, L.; Balbi, V.; Lerche, J.; Mikami, K.; Heilmann, I.; Sommarin, M. PIPKs are essential for rhizoid elongation and caulonemal cell development in the moss Physcomitrella patens. Plant J. 2011, 67, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, L.; Mikami, K.; Malhó, R.; Sommarin, M. : PIP kinases and their role in plant tip growing cells. Plant Signal. Behav. 2012, 7, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.F.; Chang, T.Y.; Chiang, S.F.; Wang, W.D.; Charng, Y.Y.; Chiou, T.J. Arabidopsis inositol pentakisphosphate 2-kinase, AtIPK1, is required for growth and modulates phosphate homeostasis at the transcriptional level. Plant J. 2014, 80, 503–515. [Google Scholar] [CrossRef]
- Wada, Y.; Kusano, H.; Tsuge, T.; Aoyama, T. Phosphatidylinositol phosphate 5-kinase genes respond to phosphate deficiency for root hair elongation in Arabidopsis thaliana. Plant J. 2015, 81, 426–437. [Google Scholar] [CrossRef]
- Zhang, Q.; van Wijk, R.; Zarza, X.; Shahbaz, M.; van Hooren, M.; Guardia, A.; Scuffi, D.; García-Mata, C.; Van den Ende, W.; Hoffmann-Benning, S.; Haring, M.A.; Laxalt, A.M.; Munnik, T. Knock-down of Arabidopsis PLC5 reduces primary root growth and secondary root formation whereas overexpression improves drought tolerance and causes stunted root hair growth. Plant Cell Physiol. 2018, 59, 2004–2019. [Google Scholar] [CrossRef]
- Kost, B.; Lemichez, E.; Spielhofer, P.; Hong, Y.; Tolias, K.; Carpenter, C.; Chua, N.H. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 1999, 145, 317–330. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wong, E.I.; Vidali, L.; Estavillo, A.; Hepler, P.K.; Wu, H.M.; Cheung, A.Y. The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 2002, 14, 2175–2190. [Google Scholar] [CrossRef]
- Dowd, P.E.; Coursol, S.; Skirpan, A.L.; Kao, T.H.; Gilroy, S. Petunia phospholipase c1 is involved in pollen tube growth. Plant Cell 2006, 18, 1438–1453. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Duan, Q.H.; Costa, S.S.; de Graaf, B.H.; Di Stilio, V.S.; Feijo, J.; Wu, H.M. The dynamic pollen tube cytoskeleton: live cell studies using actin-binding and microtubule-binding reporter proteins. Mol. Plant 2008, 1, 686–702. [Google Scholar] [CrossRef]
- Hempel, F.; Stenzel, I.; Heilmann, M.; Krishnamoorthy, P.; Menzel, W.; Golbik, R.; Helm, S.; Dobritzsch, D.; Baginsky, S.; Lee, J.; Hoehenwarter, W.; Heilmann, I. MAPKs influence pollen tube growth by controlling the formation of phosphatidylinositol 4,5-bisphosphate in an apical plasma membrane domain. Plant Cell 2017, 29, 3030–3050. [Google Scholar] [CrossRef]
- Mikami, K.; Hirata, R.; Takahashi, M.; Uji, T.; Saga, N. Transient transformation of red algal cells: Breakthrough toward genetic transformation of marine crop Porphyra species. In Genetic Transformation; Alvarez, M., ed.; InTech Open Access Publisher, Rijeka, Croatia, 2011, pp 241-258.
- Mikami, K. Current advances in seaweed transformation. In An Integrated View of the Molecular Recognition and Toxinology - From Analytical Procedures to Biomedical Applications; Baptista, G.R., ed., InTech Open Access Publisher, Rijeka, Croatia, 2013, pp 323-347.
- Mikami, K. A technical breakthrough close at hand: Feasible approaches toward establishing a gene-targeting genetic transformation system in seaweeds. Front. Plant Sci. 2014, 5, 498. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K. Recent developments in nuclear reverse-genetic manipulations that advance seaweed biology in the genomic era. J. Aquat. Res. Mar. Sci. 2018, 2018, 39–42. [Google Scholar]
- Mikami, K.; Mori, I.C.; Matsuura, T.; Ikeda, Y.; Kojima, M.; Sakakibara, H.; Hirayama, T. Comprehensive quantification and genome survey reveal the presence of novel phytohormone action modes in red seaweeds. J. Appl. Phycol. 2016, 28, 2539–2548. [Google Scholar] [CrossRef]
- Mori, I.C.; Ikeda, Y.; Matsuura, T.; Hirayama, T.; Mikami, K. Phytohormones in red seaweeds: a technical review of methods for analysis and a consideration of genomic data. Bot. Mar. 2017, 60, 153–170. [Google Scholar] [CrossRef]
- Le Bail, A.; Billoud, B.; Kowalczyk, N.; Kowalczyk, M.; Gicquel, M.; Le Panse, S.; Stewart, S.; Scornet, D.; Cock, J.M.; Ljung, K.; Charrier, B. Auxin metabolism and function in the multicellular brown alga Ectocarpus siliculosus. Plant Physiol. 2010, 153, 128–144. [Google Scholar] [CrossRef]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef]
- Shibata, M.; Sugimoto, K. A gene regulatory network for root hair development. J. Plant Res. 2019, 132, 301–309. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Y.; Li, S.; Zhang, W.; Yin, C.; Lin, Y. Regulation of phytohormones on the growth and development of plant root hair. Front. Plant Sci. 2022, 13, 865302. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y.L. : Qin, Z.; Xu, M.; Qiao, Q.; Li, S.; Li, SW.; Zhang, Y. Signaling network controlling ROP-mediated tip growth in Arabidopsis and beyond. Plant Commun. 2023, 4, 100451. [Google Scholar] [CrossRef]
- Bittisnich, D.J.; Williamson, R.E. Tip-localised H+-fluxes and the applicability of the acid-growth hypothesis to tip-growing cells: Control of chloronemal extension in Funaria hygrometrica by auxin and light. Planta 1989, 178, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Lin, Y.; Zhang, X.L.; Pang, D. W; Zha,o J. IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. J. Exp. Bot. 2008, 59, 2529–2543. [Google Scholar] [CrossRef]
- Bhosale, R.; Giri, J.; Pandey, B.K.; Giehl, R.F.H.; Hartmann, A.; Traini, R.; Truskina, J.; Leftley, N.; Hanlon, M.; Swarup, K.; Rashed, A.; Voß, U.; Alonso, J.; Stepanova, A.; Yun, J.; Ljung, K.; Brown, K.M.; Lynch, J.P.; Dolan, L.; Vernoux, T.; Bishopp, A.; Wells, D.; von Wirén, N.; Bennett, M.J.; Swarup, R. A mechanistic framework for auxin dependent Arabidopsis root hair elongation to low external phosphate. Nat Commun. 2018, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Schoenaers, S.; Balcerowicz, D.; Breen, G.; Hill, K.; Zdanio, M.; Mouille, G.; Holman, T.J.; Oh, J.; Wilson, M.H.; Nikonorova, N.; Vu, L.D.; De Smet, I.; Swarup, R.; De Vos, W.H.; Pintelon, I.; Adriaensen, D.; Grierson, C.; Bennett, M.J.; Vissenberg, K. The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr. Biol. 2018, 28, 722–732.e6. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K. The interaction and integration of auxin signaling components. Plant Cell Physiol. 2012, 53, 965–975. [Google Scholar] [CrossRef]
- Fukui, K.; Hayashi, K.I. Manipulation and sensing of auxin metabolism, transport and signaling. Plant Cell Physiol. 2018, 59, 1500–1510. [Google Scholar] [CrossRef]
- Li, C.; Ariga, I.; Mikami, K. Difference in nitrogen starvation-inducible expression patterns among phylogenetically diverse ammonium transporter genes in the red seaweed Pyropia yezoensis. Am. J. Plant Sci. 2019, 10, 1325–1349. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




