1. Introduction
Podoplanin (PDPN) (also known as Aggrus, T1α, E11 antigen, and PA2.26 antigen) is a type I transmembrane protein that has a highly glycosylated extracellular domain, a transmembrane domain, and a short intracellular domain [
1,
2]. PDPN is expressed in various normal tissues and cells, including lymphatic endothelial cells [
3,
4], kidney podocytes [
5], skin epidermis [
6], and lung type I alveolar epithelial cells [
7,
8].
PDPN is expressed on the apical membrane of lung type I alveolar epithelial cells [
7,
8]. These cells cover over 95% of the alveolar surface and play a crucial role in gas exchange. During lung development, PDPN expression transitions from being widespread in the embryonic lung epithelium to being specifically localized in type I alveolar cells of the distal epithelium [
9]. PDPN-knockout mice showed the lethal phenotype after birth due to respiratory failure. Their lungs fail to properly inflate with air, displaying increased cellular density in the distal lung, abnormal terminal respiratory units, and only a few attenuated type I cells [
10,
11]. These findings suggest that PDPN is essential for the proliferation and differentiation of lung type I alveolar epithelial cells.
In mammalian skin, PDPN is expressed in lymphatic endothelial cells, the outer root sheath cells of hair follicle keratinocytes, and the basal cell layer of sebaceous glands, but it is absent in the interfollicular epidermis [
12]. The keratinocyte-specific PDPN deletion (K5-Cre; PDPN
flox/flox) in mice exhibited a thicker hair bulb during the mid-anagen to catagen phase, suggesting that the PDPN depletion promotes anagen hair growth [
13]. Additionally, hair follicle stem cells isolated from these mice showed reduced focal adhesion and weaker interactions with the extracellular matrix than wild-type mice. This indicates that PDPN loss enhances the migration of hair follicle stem cells toward the bulb area, further supporting its role in stimulating anagen hair growth [
13].
There are two species of hippopotamuses: the larger, commonly known as the common hippopotamus (
Hippopotamus amphibius), and its smaller counterpart, the pygmy hippopotamus (
Choeropsis liberiensis). They are well-adapted to semi-aquatic environments, enabling them to move efficiently both in water and on land [
14]. Molecular data and morphological analyses support the exclusive clade grouping hippopotamuses with cetaceans (whales, dolphins, and porpoises) [
14,
15,
16,
17,
18]. However, the evolutionary pathway from the hippo-cetacean common ancestor to modern hippopotamuses remains unclear due to the lack of fossil evidence.
The Cell-Based Immunization and Screening (CBIS) method contains the immunization of target antigen-overexpressed cells and high-throughput screening using flow cytometry. Using the CBIS method, various monoclonal antibodies (mAbs) that recognize structural epitope [
19], linear epitope [
20], and glycosylated epitope [
21] of membrane protein have been established. Anti-PDPN mAbs against more than 20 species have been established mainly by the CBIS method (
http://www.med-tohoku-antibody.com/topics/001_paper_antibody_PDIS.htm#PDPN). These mAbs contribute not only to the research of each animal, such as a SARS-CoV-2 study [
22], but also to diagnosis [
23] and drug development [
24]. This study aimed to develop anti-hippopotamus PDPN (hipPDPN) mAbs using the CBIS method.
2. Materials and Methods
2.1. Cell Lines and Plasmids
Synthesized DNA encoding hipPDPN (XM_057709258.1, Eurofins Genomics KK, Tokyo, Japan) plus an N-terminal PA16 tag (GLEGGVAMPGAEDDVV) [
25] and an N-terminal MAP16 tag (PGTGDGMVPPGIEDKI) [
26], which are recognized by an anti-PA16 tag mAb (NZ-1) [
27] and an anti-MAP16 tag mAb (PMab-1) [
28], were subcloned into a pCAGzeo vector (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan).
The plasmids were transfected into CHO-K1 [American Type Culture Collection (ATCC), Manassas, VA, USA] cells as described previously [
29], and stable transfectants (CHO/MAP16-hipPDPN and CHO/PA16-hipPDPN) were established. The information of twenty-four species-PDPN expressed CHO-K1 cells, and the clones of anti-PDPN mAbs are available at the WEB site “Antibody bank” (
http://www.med-tohoku-antibody.com/topics/001_paper_antibody_PDIS.htm#PDPN).
2.2. Production of Hybridomas
The female BALB/cAJcl mice were purchased from CLEA Japan (Tokyo, Japan). Animal experiments were approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) and were carried out following the NIH (National Research Council) Guide for the Care and Use of Laboratory Animals. Mice were immunized intraperitoneally with 1 × 10
8 cells/mouse of CHO/MAP16-hipPDPN with Alhydrogel adjuvant 2% (InvivoGen). After four times additional injections, the splenocytes were fused to P3X63Ag8U.1 (P3U1, ATCC) as described previously [
30]. The hybridoma supernatants were screened by flow cytometric analysis using CHO/PA16-hipPDPN and CHO-K1 cells.
2.3. Flow Cytometric Analysis
Cells were rinsed with a blocking buffer of 0.1% bovine serum albumin (BSA) in PBS and incubated with primary mAbs for 30 minutes at 4°C. Subsequently, the cells were exposed to Alexa Fluor 488-conjugated secondary antibodies (1:2,000, Cell Signaling Technology, Inc., Danvers, MA, USA). Fluorescence measurements were then obtained using the SA3800 Cell Analyzer (Sony Corp., Tokyo, Japan).
2.4. Determination of Dissociation Constant (KD) by Flow Cytometry
CHO/PA16-hipPDPN cells were incubated in a series of diluted PMab-322 solutions for 30 minutes at 4°C. Then, the cells were treated with Alexa Fluor 488-conjugated anti-mouse IgG at a dilution of 1:200. Fluorescence data were obtained and the KD was determined using GraphPad PRISM 6 software (GraphPad Software, Inc., La Jolla, CA, USA).
2.5. Immunoblotting
Immunoblotting was conducted as described previously [
30] using 1 μg/mL of PMab-322, 1 μg/mL of NZ-1, or 1 μg/mL of an anti-isocitrate dehydrogenase 1 (IDH1) mAb (RcMab-1) [
31] as primary mAbs.
2.6. Immunohistochemical Analysis
CHO/PA16-hipPDPN and CHO-K1 cell blocks were made using iPGell (Genostaff Co., Ltd., Tokyo, Japan). The paraffin-embedded cell sections were stained with PMab-322 (0.1 μg/mL) using BenchMark ULTRA PLUS with the ultraView Universal DAB Detection Kit (Roche Diagnostics, Indianapolis, IN, USA).
3. Results
3.1. Development of Anti-hipPDPN mAbs Using the CBIS Method
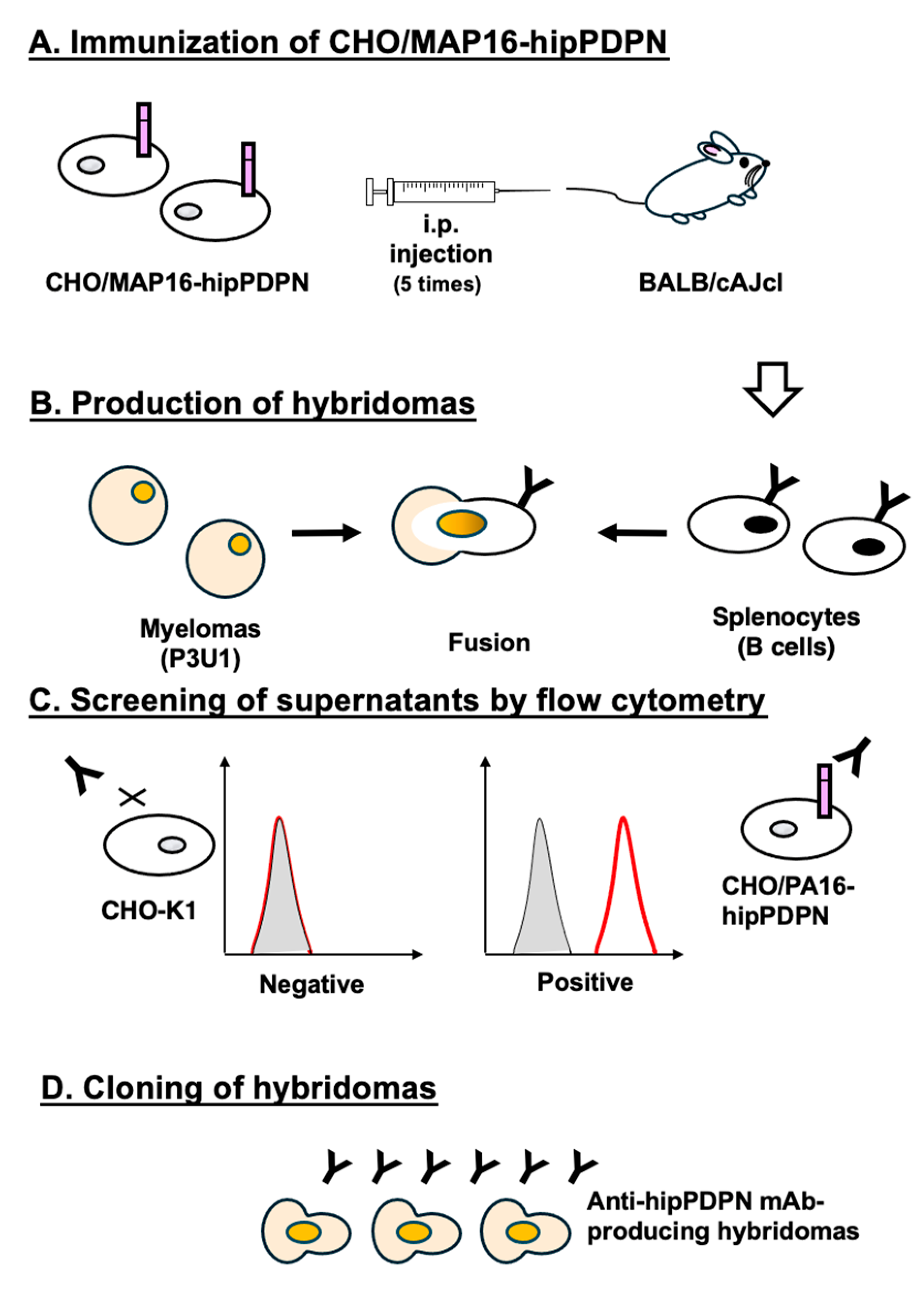
To produce anti-hipPDPN mAbs, two mice were immunized with CHO/MAP16-hipPDPN (
Figure 1A). After immunization, their spleens were harvested, and the splenocytes were fused with myeloma P3U1 cells (
Figure 1B). The hybridomas were then seeded into ten 96-well plates and cultured for six days. Subsequently, supernatants that exhibited reactivity with CHO/PA16-hipPDPN but not with CHO-K1 were identified from 958 wells using flow cytometry (
Figure 1C). Following limiting dilution and multiple screening steps, a mAb clone, PMab-322 (mouse IgG
2a, kappa), was successfully generated (
Figure 1D).
3.2. Flow Cytometry Using PMab-322
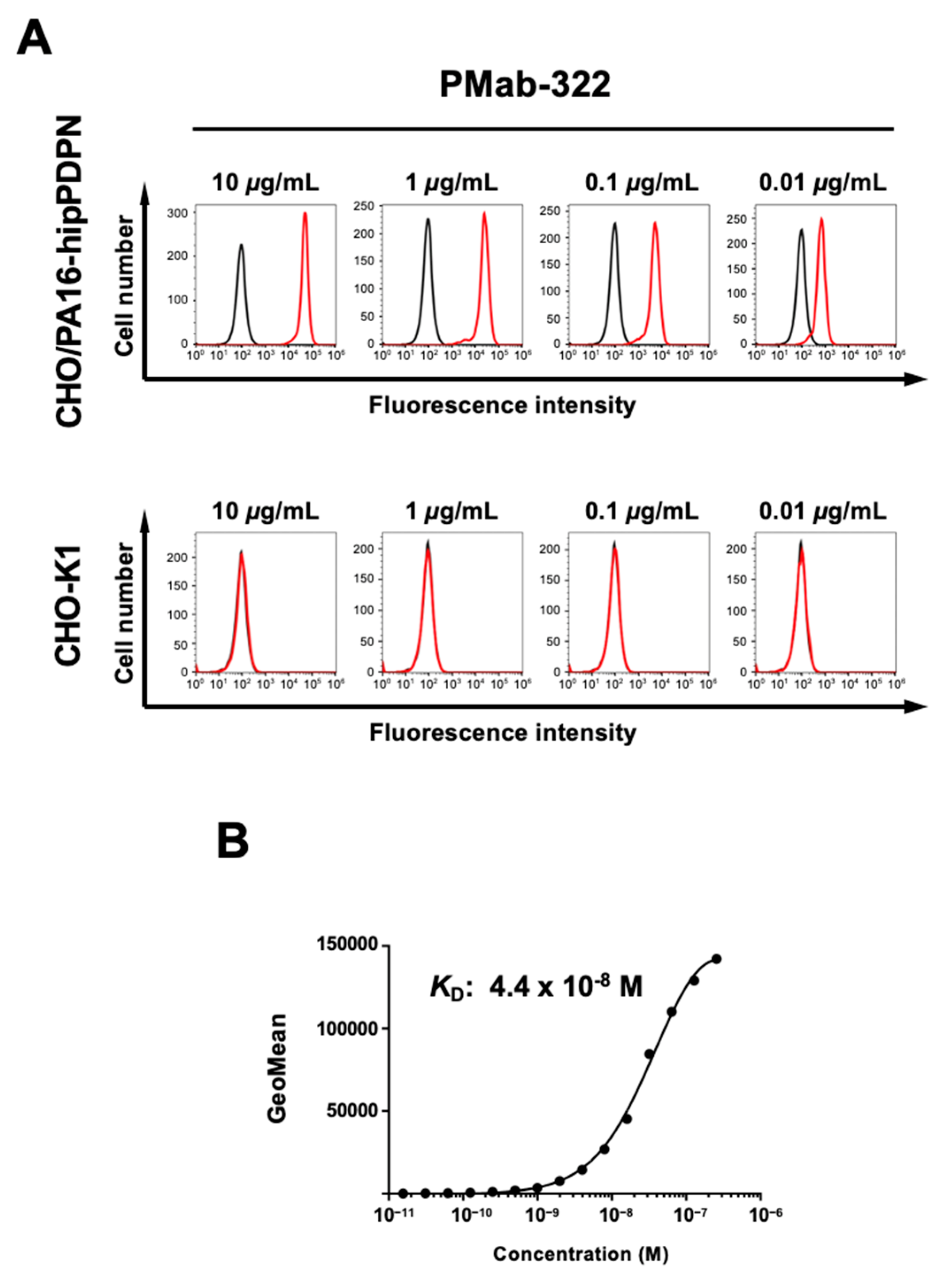
Flow cytometry was conducted using PMab-322 against CHO/PA16-hipPDPN and parental CHO-K1 cells. PMab-322 showed reactivity to CHO/PA16-hipPDPN cells (
Figure 2A) from 10 to 0.01 μg/mL. However, PMab-322 did not recognize CHO-K1 cells even at 10 μg/mL (
Figure 2A). We next conducted flow cytometry to determine the
KD value of PMab-322 against CHO/PA16-hipPDPN. PMab-322 exhibited a moderate affinity (
KD: 4.4 × 10
−8 M) to CHO/PA16-hipPDPN (
Figure 2B).
3.3. Specificity of PMab-322 Against 24 Species-PDPN Expressed CHO-K1
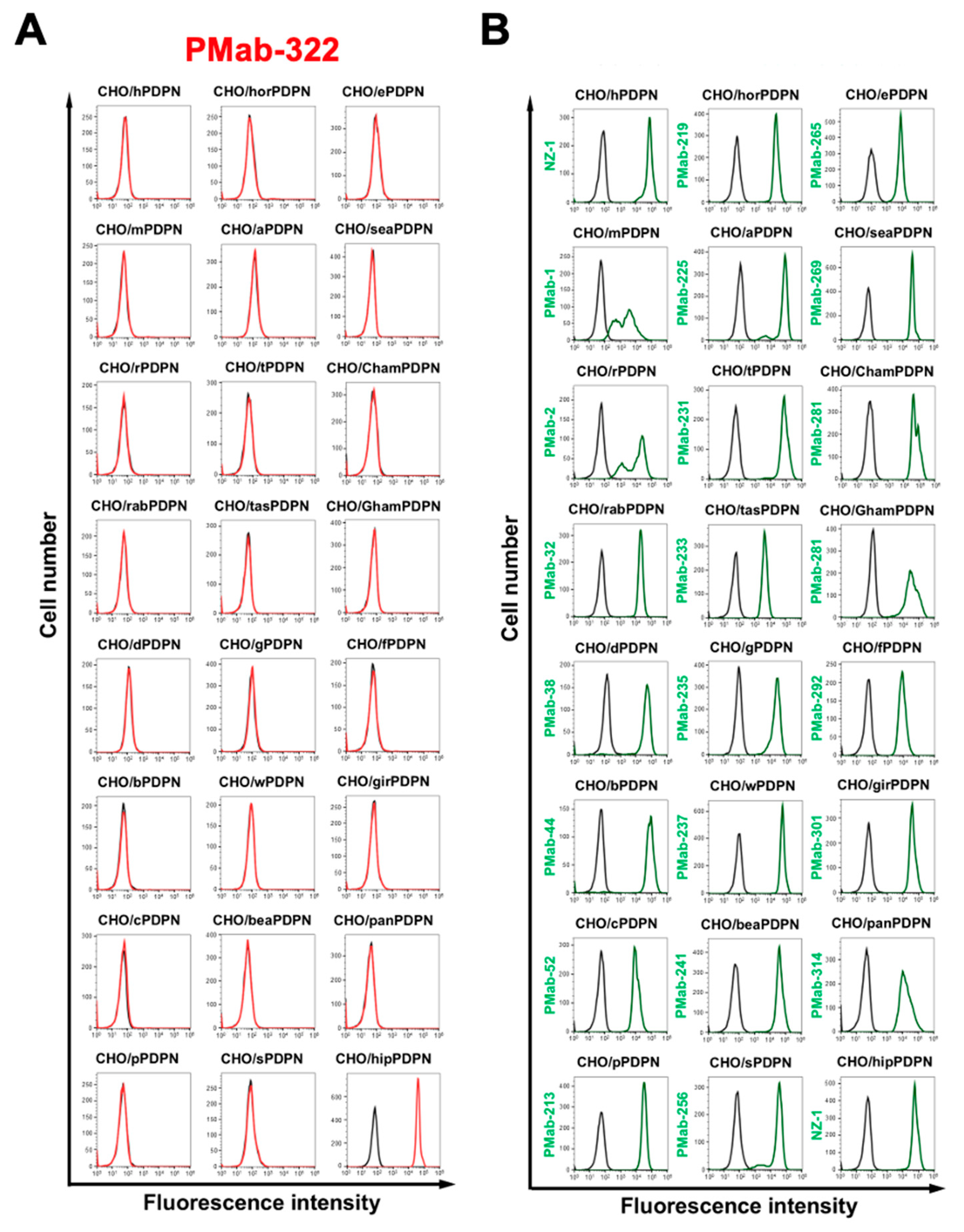
We previously generated anti-PDPN mAbs against human PDPN (hPDPN, clone NZ-1), mouse PDPN (mPDPN, clone PMab-1), rat PDPN (rPDPN, clone PMab-2), rabbit PDPN (rabPDPN, clone PMab-32), dog PDPN (dPDPN, clone PMab-38), bovine PDPN (bPDPN, clone PMab-44), cat PDPN (cPDPN, clone PMab-52), pig PDPN (pPDPN, clone PMab-213), horse PDPN (horPDPN, clone PMab-219), alpaca PDPN (aPDPN, clone PMab-225), tiger PDPN (tPDPN, clone PMab-231), Tasmanian devil PDPN (tasPDPN, clone PMab-233), goat PDPN (gPDPN, clone PMab-235), whale PDPN (wPDPN, clone PMab-237), bear PDPN (beaPDPN, clone PMab-241), sheep PDPN (sPDPN, clone PMab-256), elephant PDPN (ePDPN, clone PMab-265), sealion PDPN (seaPDPN, clone PMab-269), Chinese hamster PDPN (ChamPDPN, clone PMab-281), Golden hamster PDPN (GhamPDPN, clone PMab-281), ferret PDPN (fPDPN, clone PMab-292), giraffe PDPN (girPDPN, clone PMab-301), panda PDPN (panPDPN, clone PMab-314). We next investigated the reactivity of PMab-322 to each PDPN-expressed CHO-K1. As shown in
Figure 3A, PMab-322 solely reacted hipPDPN, but not others. Cell surface expression was confirmed by mAbs mentioned above (
Figure 3B). The PA16-tagged hipPDPN expression was also confirmed by an anti-PA16 tag mAb, NZ-1 (
Figure 3B). These results indicate that PMab-322 specifically recognizes hipPDPN.
3.4. Immunoblotting Using PMab-322
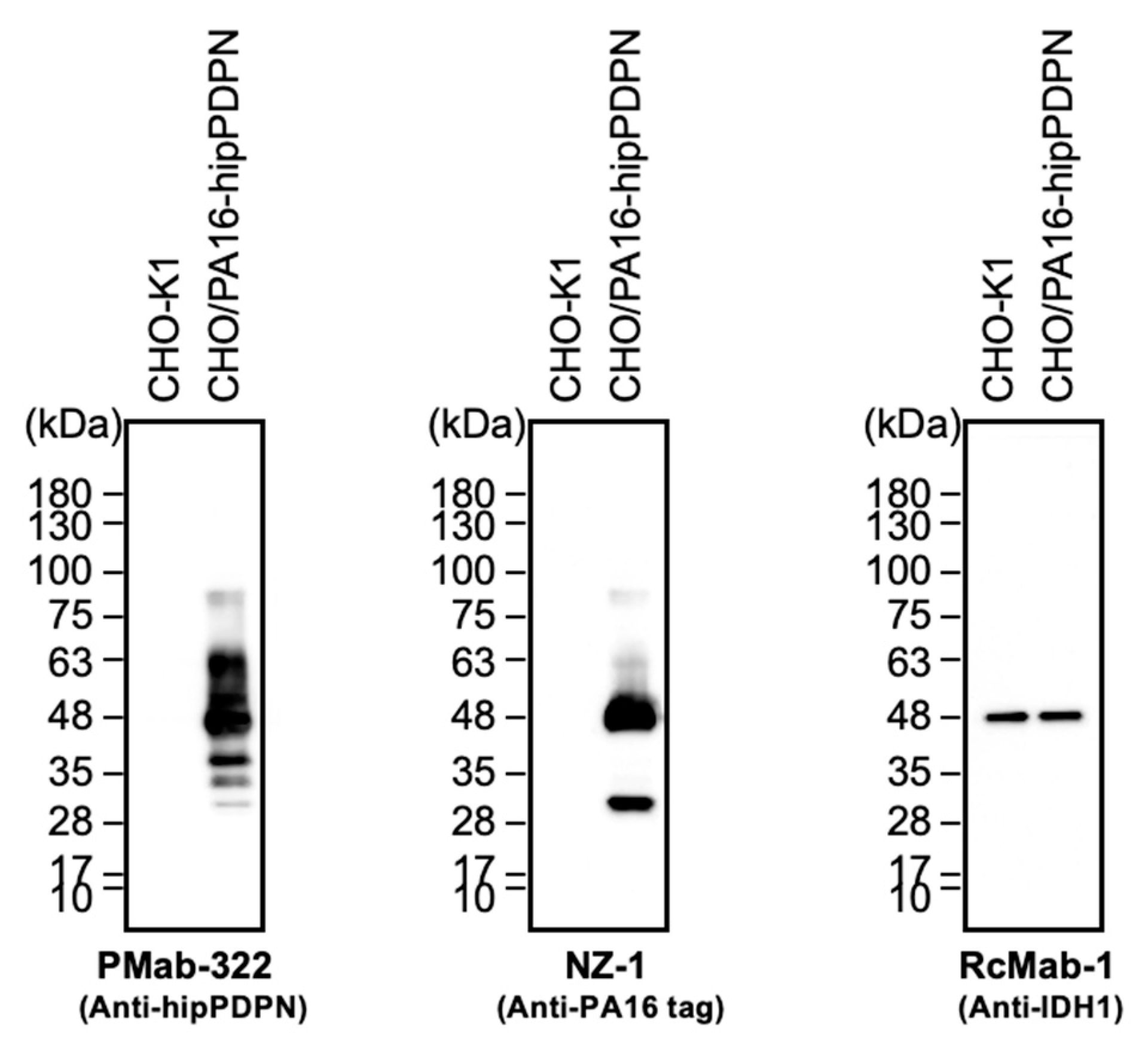
We investigated whether PMab-322 can be used for immunoblotting using CHO/PA16-hipPDPN and CHO-K1 cell lysates. As shown in
Figure 4, PMab-322 could detect hipPDPN as the significant bands around 48 to 63 kDa in CHO/PA16-hipPDPN cell lysates, while no band was detected in CHO-K1 cells. An anti-PA16 tag mAb, NZ-1, could detect PA16-hipPDPN as the main band around 48 kDa in CHO/PA16-hipPDPN cell lysates. An anti-IDH1 mAb (clone RcMab-1) was used for internal control. These results indicate that PMab-322 can detect hipPDPN in immunoblotting.
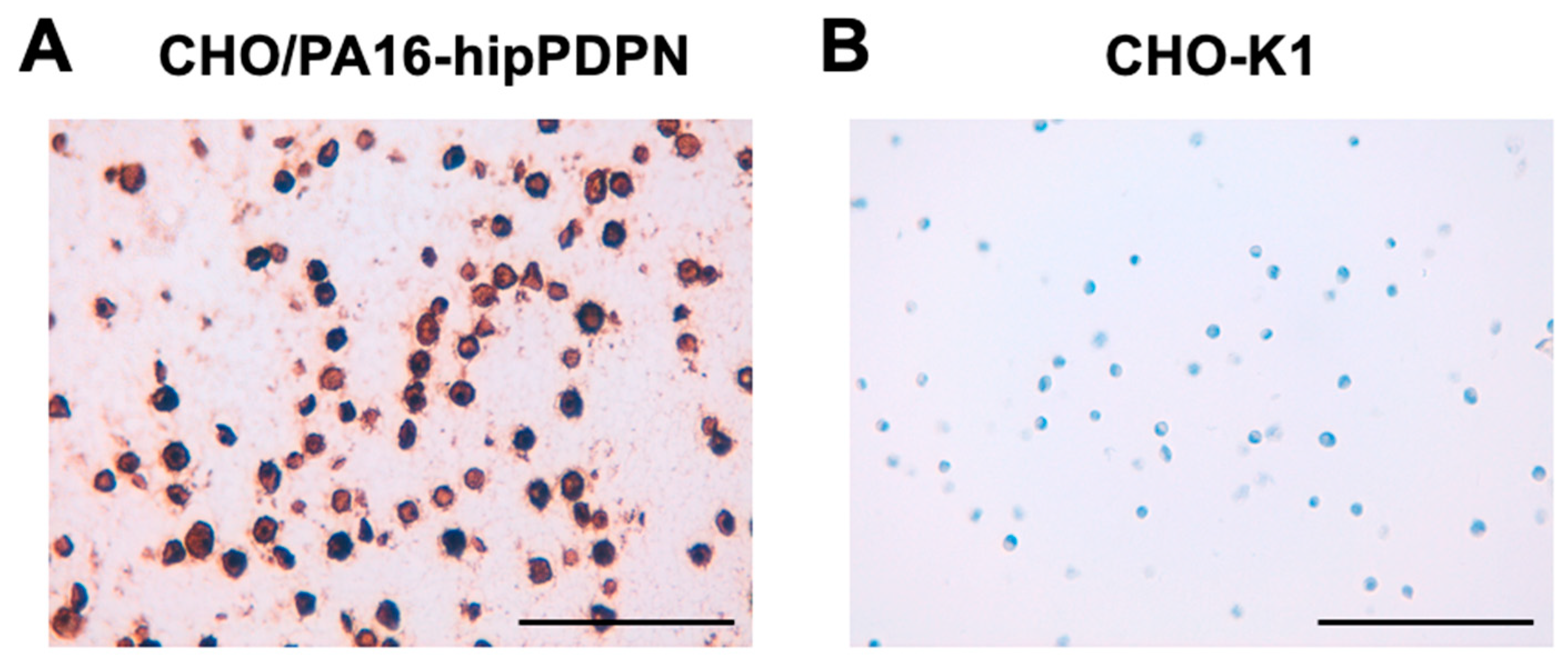
3.5. Immunohistochemistry Using PMab-322
To investigate whether PMab-322 can be used for immunohistochemistry, paraffin-embedded CHO-K1 and CHO/PA16-hipPDPN sections were stained with PMab-322. A membranous staining was observed in CHO/PA16-hipPDPN (
Figure 5A) but not in CHO-K1 (
Figure 5B). These results indicate that PMab-322 is suitable for immunohistochemistry for detecting hipPDPN-positive cells in paraffin-embedded cell samples.
4. Discussion
In this study, a novel anti-hipPDPN mAb, PMab-322 was established using the CBIS method (
Figure 1). PMab-322 was shown to be useful for flow cytometry and exhibited moderate binding affinity (4.4 × 10
−8 M) against CHO/PA16-hipPDPN (
Figure 2). PMab-322 specifically recognized hipPDPN but did not react with PDPNs from other species, including whales, the closest living relatives of hippopotamuses (
Figure 3). Furthermore, PMab-322 successfully detected hipPDPN in immunoblotting (
Figure 4) and immunohistochemistry using paraffin-embedded cell blocks (
Figure 5). Therefore, PMab-322 is expected to facilitate the identification of endogenous hipPDPN-positive cells in tissues, such as the kidney, skin, and lung.
Mammals have fully adapted to life in the ocean only twice in history: in Cetacea and Sirenia (manatees and dugongs) [
15,
32]. In the case of Cetacea, genetic studies clearly show that fully aquatic cetaceans and semiaquatic hippopotamus are the closest living relatives [
16,
17,
18]. Studies suggest that particular water-related adaptations may have evolved in their common ancestor (Cetancodonta: Cetacea + Hippopotamidae) [
33]. Still, another possibility is that these traits developed separately in each group [
14]. The integumentary systems of cetaceans and hippopotamus were analyzed by integrating comprehensive genomic and histological data. Both cetaceans and hippopotamus have lost function in eight skin-related genes involved in epidermal, hair follicles, and sebaceous glands differentiation [
34]. However, none of these genes are shared by cetaceans and hippopotamus. These results support the hypothesis that aquatic skin adaptations evolved independently in cetaceans and hippopotamus [
34]. Since PDPN is expressed in skin epidermis and the lymphatic endothelial cells [
6], PMab-322 (this study) and PMab-237 (an anti-whale PDPN mAb [
29]) would contribute to the molecular and pathological analysis of the skin.
Cetaceans and hippopotamus adapt to the aquatic and semi-aquatic environments, respectively. The lungs of cetaceans have anatomical and physiological adaptations that enable them to hold their breath for extended periods while diving [
14]. The immunohistochemical analysis revealed the presence of smooth muscle in the terminal bronchioles, alveolar ducts, and alveolar septa, which is thought to play a key role in the alveolar collapse reflex and prolonged breath-holding during diving [
35]. However, the pathological analysis of the lung of hippopotamus has not been reported. PMab-322 and PMab-237 could be valuable tools for comparative histological analyses of the lung by staining lung type I alveolar epithelial cells.
Author Contributions
Haruto Yamamoto: Investigation. Hiroyuki Suzuki: Writing – original draft, Investigation. Tomohiro Tanaka: Investigation, Funding acquisition. Mika K. Kaneko: Conceptualization. Yukinari Kato: Conceptualization, Funding acquisition, Project administration, Writing – review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP25am0521010 (to Y.K.), JP25ama121008 (to Y.K.), JP25ama221339 (to Y.K.), and JP25bm1123027 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 24K18268 (to T.T.) and 25K10553 (to Y.K.).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Tohoku University (Permit number: 2022MdA-001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest involving this article.
References
- Kato, Y.; Fujita, N.; Kunita, A.; et al. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem 2003, 278, 51599–51605. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kaneko, M.K.; Kato, Y. Roles of Podoplanin in Malignant Progression of Tumor. Cells 2022;11(3).
- Hirakawa, S.; Hong, Y.K.; Harvey, N.; et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol 2003, 162, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Petrova, T.V.; Mäkinen, T.; Mäkelä, T.P.; et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. Embo j 2002, 21, 4593–4599. [Google Scholar] [CrossRef]
- Breiteneder-Geleff, S.; Matsui, K.; Soleiman, A.; et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 1997, 151, 1141–1152. [Google Scholar]
- Asai, J. The Role of Podoplanin in Skin Diseases. Int J Mol Sci 2022;23(3).
- Dobbs, L.G.; Williams, M.C.; Gonzalez, R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta 1988, 970, 146–156. [Google Scholar] [CrossRef]
- Rishi, A.K.; Joyce-Brady, M.; Fisher, J.; et al. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol 1995, 167, 294–306. [Google Scholar] [CrossRef]
- Williams, M.C.; Cao, Y.; Hinds, A.; Rishi, A.K.; Wetterwald, A. T1 alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am J Respir Cell Mol Biol 1996, 14, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Schacht, V.; Ramirez, M.I.; Hong, Y.K.; et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. Embo j 2003, 22, 3546–3556. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Millien, G.; Hinds, A.; et al. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol 2003, 256, 61–72. [Google Scholar] [CrossRef]
- Honma, M.; Minami-Hori, M.; Takahashi, H.; Iizuka, H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF-β and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J Dermatol Sci 2012, 65, 134–140. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Dieterich, L.C.; Tacconi, C.; et al. An important role of podoplanin in hair follicle growth. PLoS One 2019, 14, e0219938. [Google Scholar] [CrossRef] [PubMed]
- Boisserie, J.R.; Fisher, R.E.; Lihoreau, F.; Weston, E.M. Evolving between land and water: key questions on the emergence and history of the Hippopotamidae (Hippopotamoidea, Cetancodonta, Cetartiodactyla). Biol Rev Camb Philos Soc 2011, 86, 601–625. [Google Scholar] [CrossRef]
- Gatesy, J.; Geisler, J.H.; Chang, J.; et al. A phylogenetic blueprint for a modern whale. Mol Phylogenet Evol 2013, 66, 479–506. [Google Scholar] [CrossRef]
- Shimamura, M.; Yasue, H.; Ohshima, K.; et al. Molecular evidence from retroposons that whales form a clade within even-toed ungulates. Nature 1997, 388, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Gatesy, J. More DNA support for a Cetacea/Hippopotamidae clade: the blood-clotting protein gene gamma-fibrinogen. Mol Biol Evol 1997, 14, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Gatesy, J.; Hayashi, C.; Cronin, M.A.; Arctander, P. Evidence from milk casein genes that cetaceans are close relatives of hippopotamid artiodactyls. Mol Biol Evol 1996, 13, 954–963. [Google Scholar] [CrossRef]
- Okada, Y.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of a Sensitive Anti-Mouse CD39 Monoclonal Antibody (C(39)Mab-1) for Flow Cytometry and Western Blot Analyses. Monoclon Antib Immunodiagn Immunother 2024, 43, 24–31. [Google Scholar] [CrossRef]
- Kudo, Y.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Variant 5 Monoclonal Antibody C(44)Mab-3 for Multiple Applications against Pancreatic Carcinomas. Antibodies (Basel) 2023;12(2).
- Kato, Y.; Kaneko, M.K. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep 2014, 4, 5924. [Google Scholar] [CrossRef]
- Heydemann, L.; Ciurkiewicz, M.; Störk, T.; et al. Respiratory long COVID in aged hamsters features impaired lung function post-exercise with bronchiolization and fibrosis. Nat Commun 2025, 16, 2080. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Honma, R.; Ogasawara, S.; et al. PMab-38 Recognizes Canine Podoplanin of Squamous Cell Carcinomas. Monoclon Antib Immunodiagn Immunother 2016, 35, 263–266. [Google Scholar] [CrossRef]
- Kato, Y.; Ito, Y.; Ohishi, T.; et al. Antibody-Drug Conjugates Using Mouse-Canine Chimeric Anti-Dog Podoplanin Antibody Exerts Antitumor Activity in a Mouse Xenograft Model. Monoclon Antib Immunodiagn Immunother 2020, 39, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; et al. PA tag: a versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr Purif 2014, 95, 240–247. [Google Scholar] [CrossRef]
- Fujii, Y.; Kaneko, M.K.; Kato, Y. MAP Tag: A Novel Tagging System for Protein Purification and Detection. Monoclon Antib Immunodiagn Immunother 2016, 35, 293–299. [Google Scholar] [CrossRef]
- Kato, Y.; Kaneko, M.K.; Kuno, A.; et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem Biophys Res Commun 2006, 349, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Kaji, C.; Tsujimoto, Y.; Kato Kaneko, M.; Kato, Y.; Sawa, Y. Immunohistochemical Examination of Novel Rat Monoclonal Antibodies against Mouse and Human Podoplanin. Acta. Histochem. Cytochem. 2012, 45, 227–237. [Google Scholar] [CrossRef]
- Kato, Y.; Furusawa, Y.; Itai, S.; et al. Establishment of an Anticetacean Podoplanin Monoclonal Antibody PMab-237 for Immunohistochemical Analysis. Monoclon Antib Immunodiagn Immunother 2019, 38, 108–113. [Google Scholar] [CrossRef]
- Satofuka, H.; Suzuki, H.; Tanaka, T.; et al. Development of an anti-human EphA2 monoclonal antibody Ea2Mab-7 for multiple applications. Biochemistry and Biophysics Reports 2025, 42, 101998. [Google Scholar] [CrossRef] [PubMed]
- Ikota, H.; Nobusawa, S.; Arai, H.; et al. Evaluation of IDH1 status in diffusely infiltrating gliomas by immunohistochemistry using anti-mutant and wild type IDH1 antibodies. Brain Tumor Pathol 2015, 32, 237–244. [Google Scholar] [CrossRef]
- Springer, M.S.; Signore, A.V.; Paijmans, J.L.; et al. Interordinal gene capture, the phylogenetic position of Steller’s sea cow based on molecular and morphological data, and the macroevolutionary history of Sirenia. Mol Phylogenet Evol 2015, 91, 178–193. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Gatesy, J. Impact of increased character sampling on the phylogeny of Cetartiodactyla (Mammalia): combined analysis including fossils. Cladistics 2008, 24, 397–442. [Google Scholar] [CrossRef]
- Springer, M.S.; Guerrero-Juarez, C.F.; Huelsmann, M.; et al. Genomic and anatomical comparisons of skin support independent adaptation to life in water by cetaceans and hippos. Curr Biol 2021, 31, 2124–2139e2123. [Google Scholar] [CrossRef] [PubMed]
- Otero-Sabio, C.; Centelleghe, C.; Corain, L.; et al. Microscopic anatomical, immunohistochemical, and morphometric characterization of the terminal airways of the lung in cetaceans. J Morphol 2021, 282, 291–308. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).