Submitted:
03 April 2025
Posted:
04 April 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dynamin-Dependent CIE Pathways
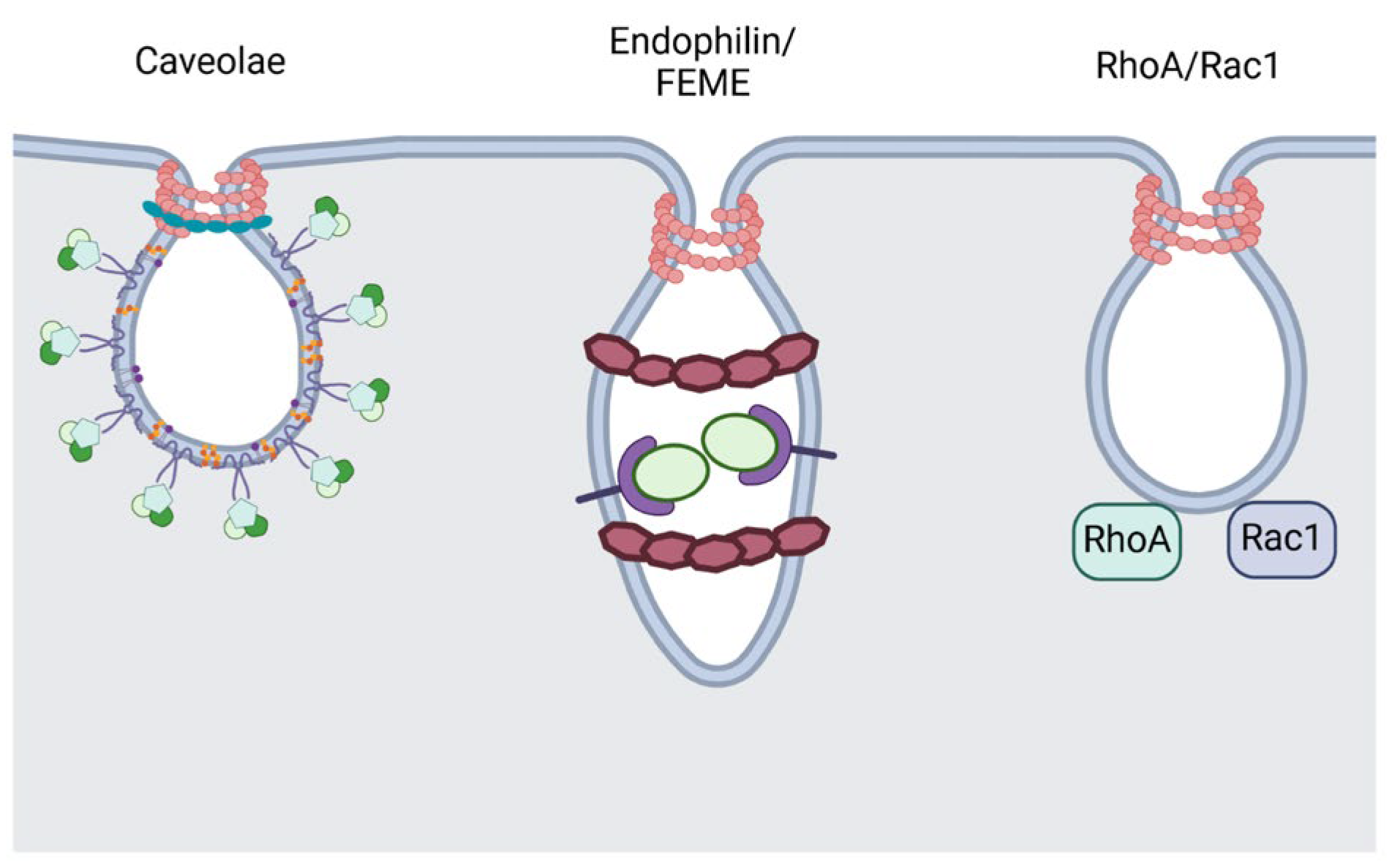
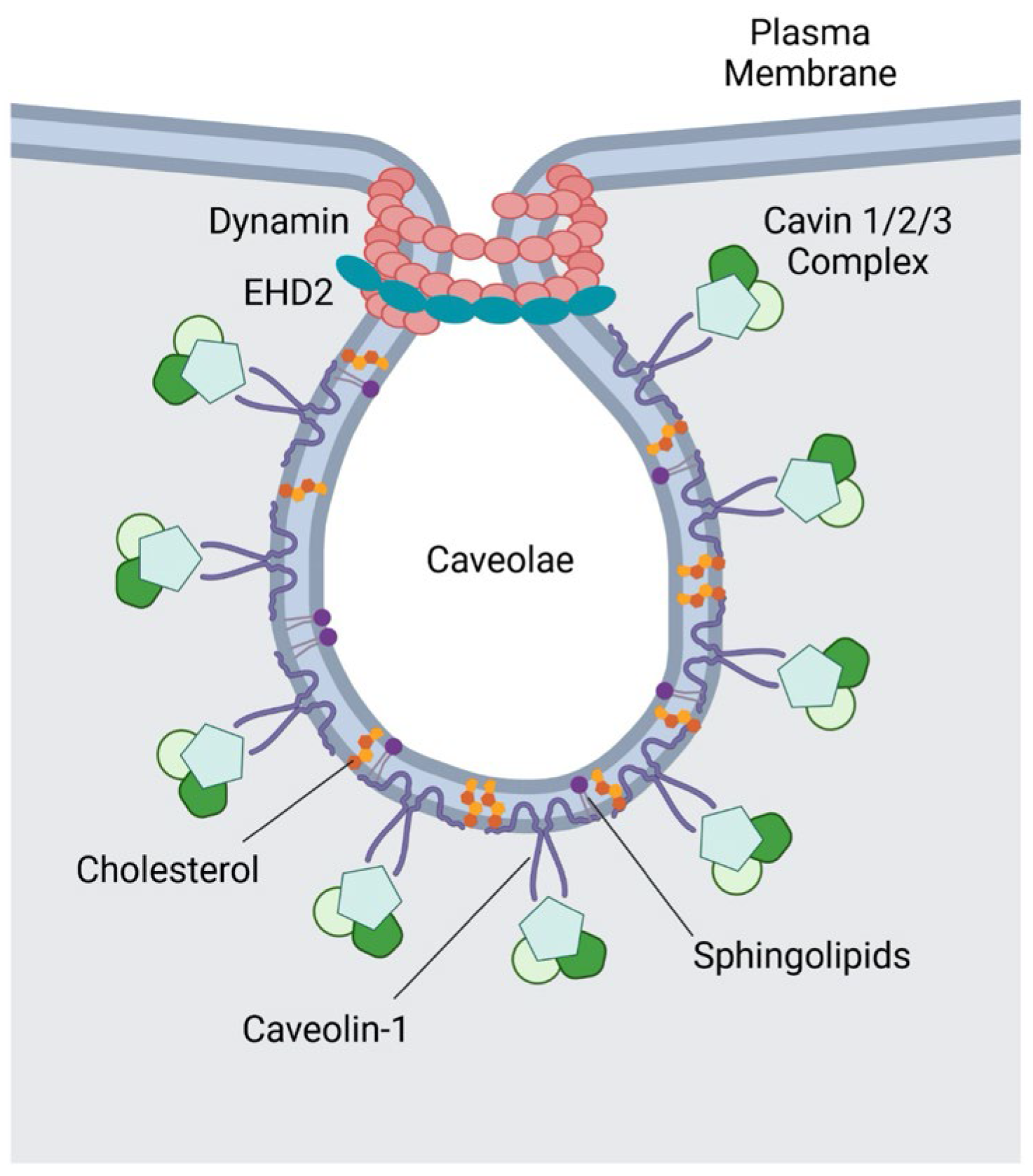
2.1. Caveolae-Mediated Endocytosis
2.2. Small GTPases-Regulated Endocytosis
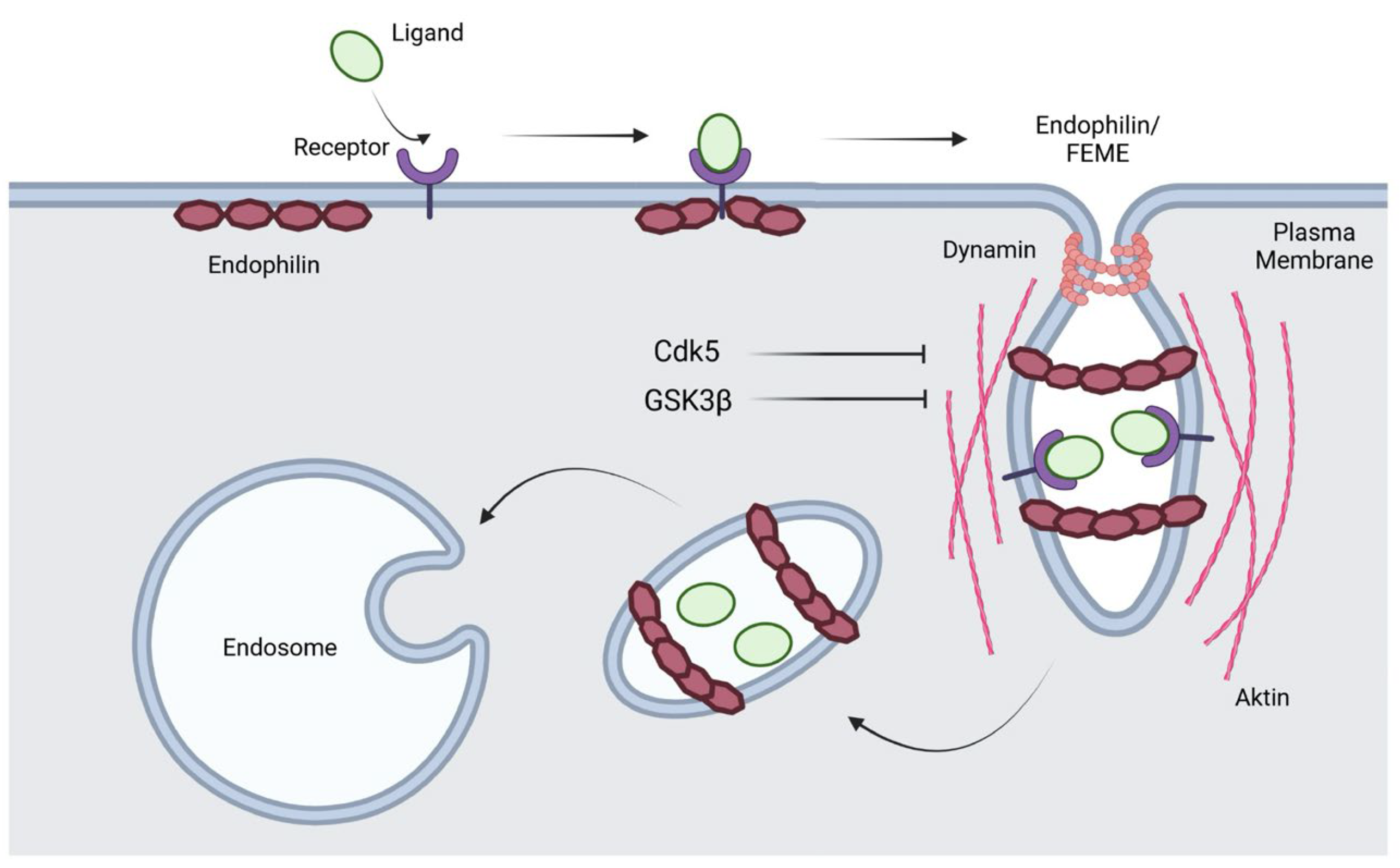
2.3. Fast Endophilin-Mediated Endocytosis (FEME)
3. Dynamin-Independent CIE Pathways
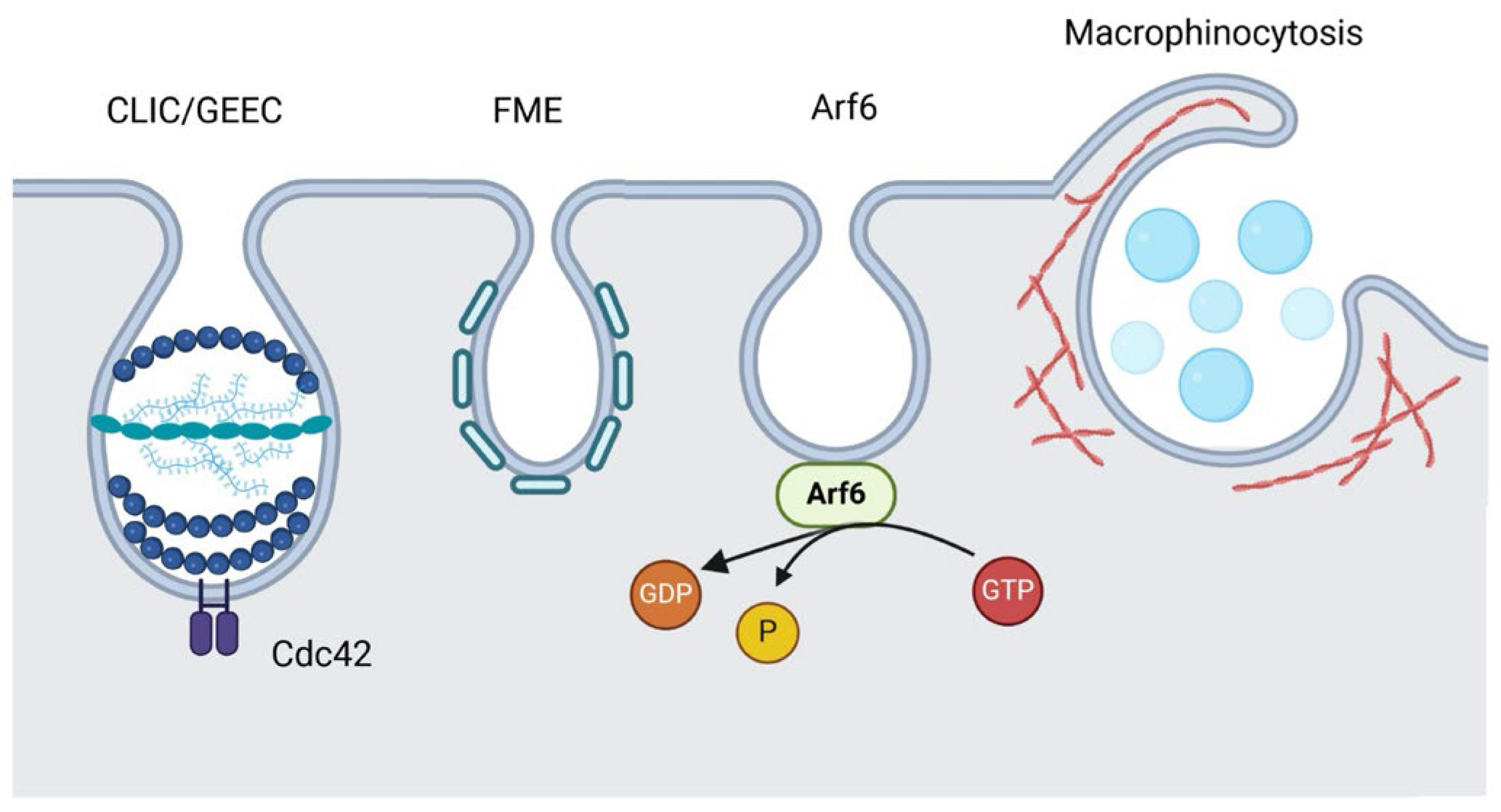
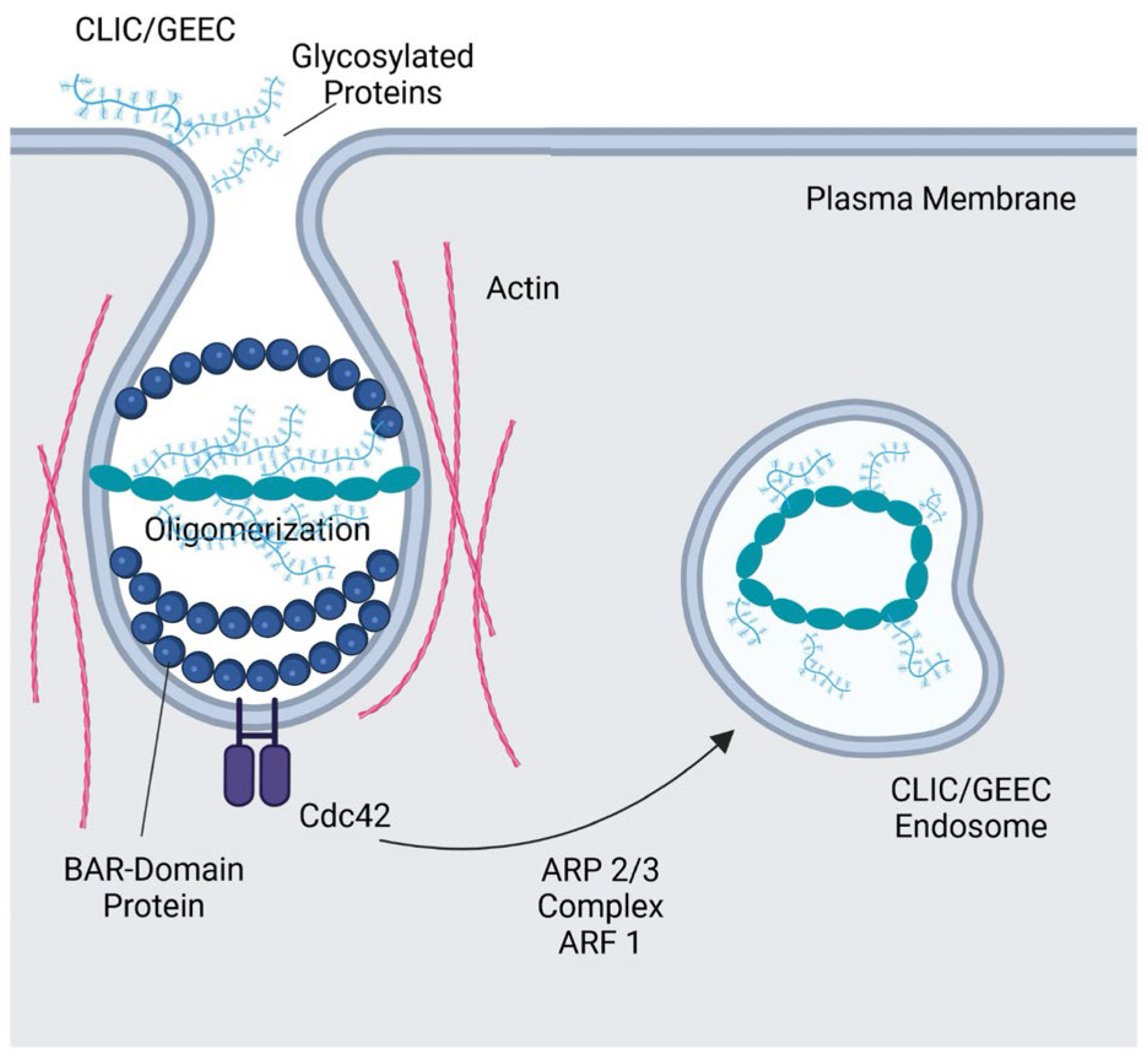
3.1. CLIC/GEEC Pathway
3.2. Arf6-Dependent Endocytosis
3.3. Flotillin-Mediated Endocytosis (FME)
3.4. Macropinocytosis
4. Exploitation of CIE Pathways by Pathogens for Host Cell Entry and Infection
4.1. Exploitation of CIE by Bacterial Pathogens
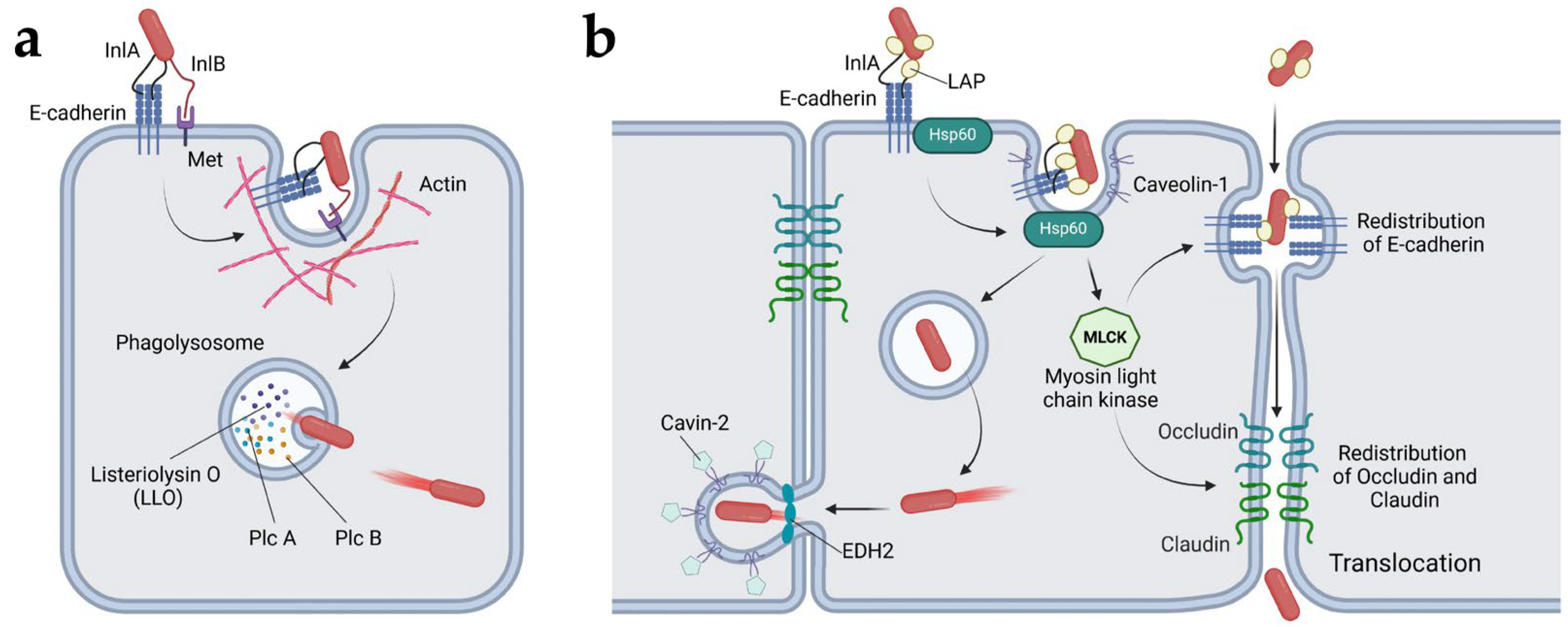
4.1.1. Listeria Monocytogenes
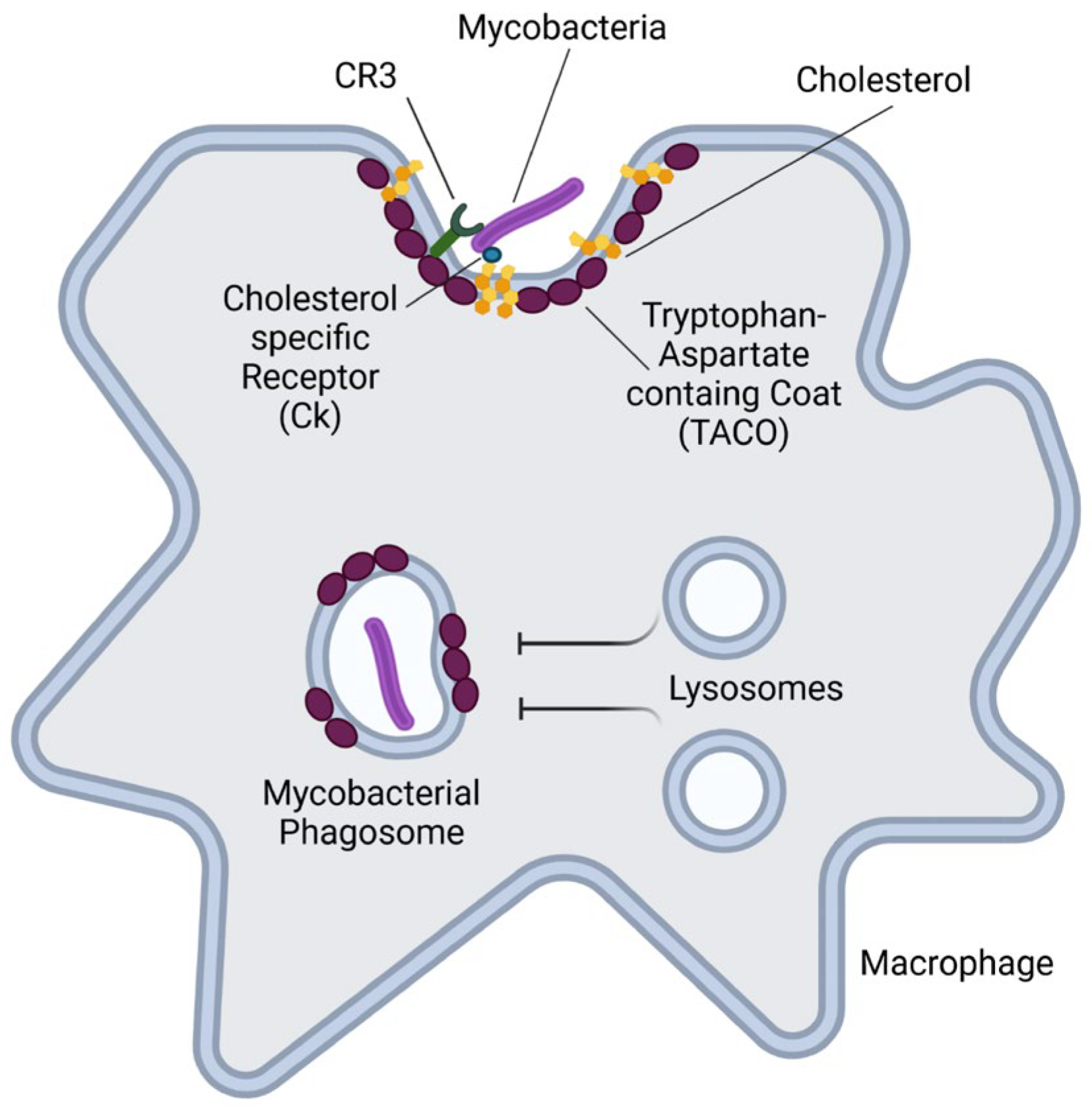
4.1.2. Mycobacterium Tuberculosis
4.1.3. Streptococcus Pyogenes
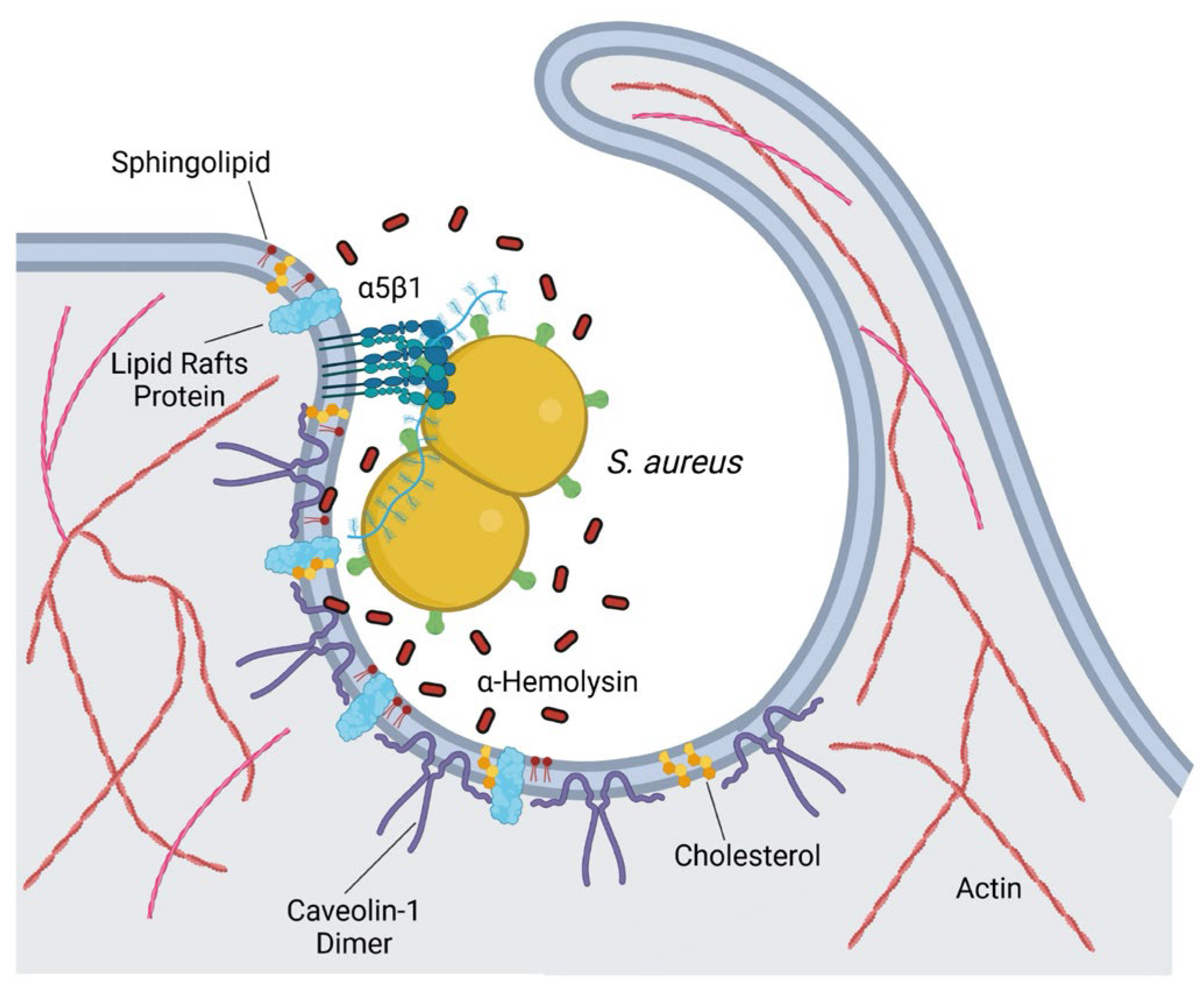
4.1.4. Staphylococcus aureus
4.1.5. Escherichia coli
4.1.6. Salmonella typhimurium
4.1.7. Chlamydia
4.1.8. Other Bacterial Pathogens
4.2. Exploitation of CIE by Viral Pathogens
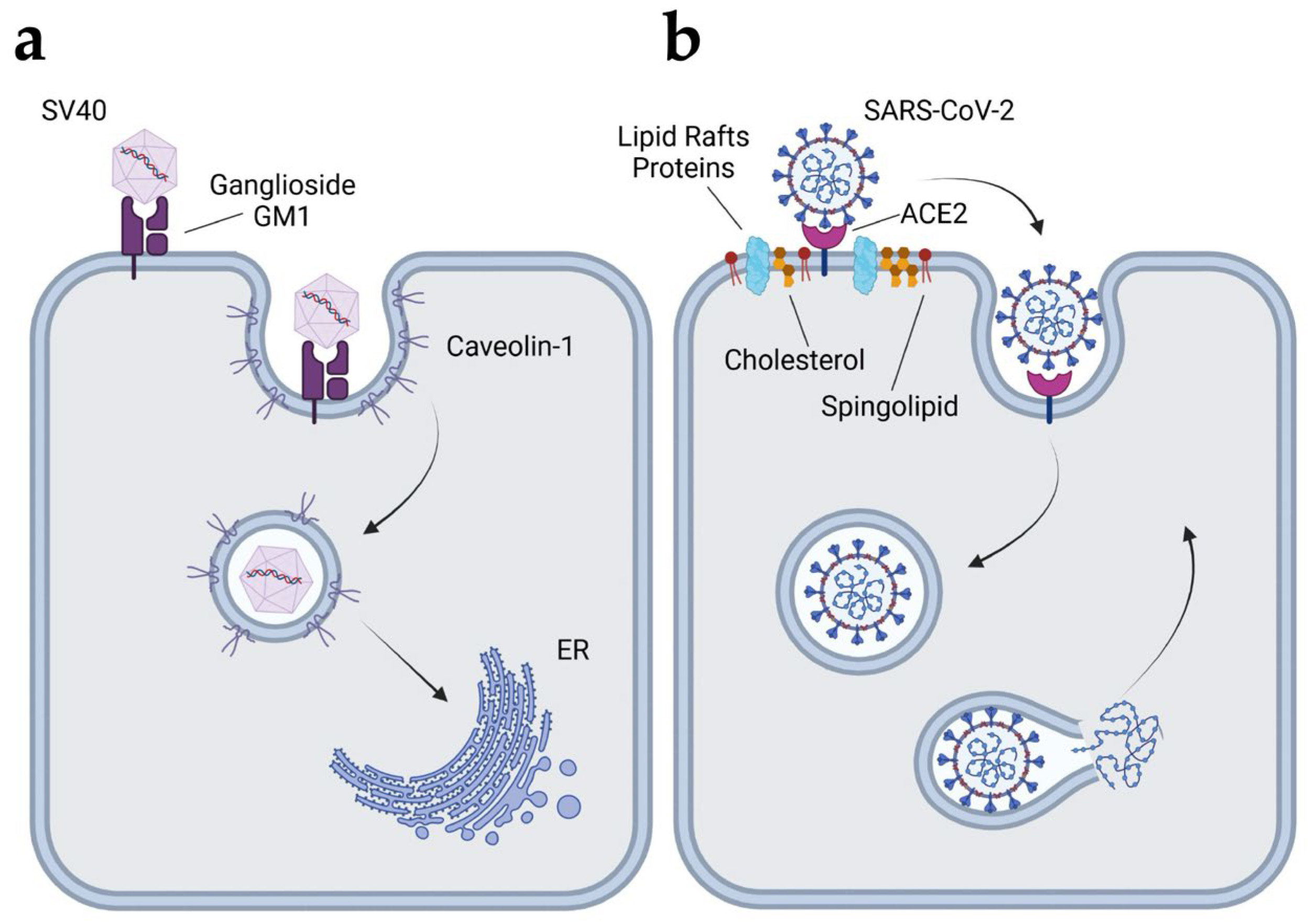
4.2.1. Simian Virus 40 (SV40)
4.2.2. Echoviruses
4.2.3. Coronavirus
4.2.4. Human Immunodeficiency Virus 1 (HIV-1)
4.2.5. Japanese Encephalitis Virus (JEV)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CME | Clathrin-dependent endocytosis |
| CIE | Clathrin-independent endocytosis |
| Arf6 | ADP-ribosylation factor 6 |
| ER | Endoplasmic reticulum |
| RhoA | Ras homolog family member A |
| Rac1 | Rac Family Small GTPase 1 |
| Cdc42 | Cell division control protein 42 |
| RhoG | Ras homolog family member G |
| IL-2R-β | Interleukin-2 receptor subunit beta |
| GPI-Aps | Glycosylphosphatidylinositol-anchored proteins |
| FEME | Fast endophilin-mediated endocytosis |
| SH3 | Src homology 3 |
| BAR | Bin-Amphiphysin-Rvs domain |
| CIP4 | Cdc42-interacting protein 4 |
| FBP17 | formin-binding protein 17 GTPase-activating proteins |
| SHP2 | SH2-containing inositol phosphatase 2 |
| Cdk5 | Cyclin-dependent kinase 5 |
| GSK3β | Glycogen synthase kinase-3 beta |
| CLIC/GEEC | Clathrin-independent carrier (CLIC)/GPI-anchored protein-enriched early endocytic compartments (GEEC) |
| GPI-Aps | GPI-anchored proteins |
| PI3K | Phosphoinositide 3-kinases |
| GTP | Guanosine triphosphate |
| GDP | Guanosine diphosphate |
| GBF1 | Golgi Brefeldin A Resistant Guanine Nucleotide Exchange Factor 1 |
| GEF | Guanine nucleotide exchange factors |
| Arf1 | ADP-ribosylation factor 1 |
| ARHGAP10 | Rho GTPase Activating Protein 10 |
| GRAF1 | GTPase Regulator Associated with Focal Adhesion Kinase 1 |
| GAPs | GTPase-activating proteins |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| FME | Flotillin-mediated endocytosis |
| SPFH | stomatin/prohibitin/flotillin/HflK/C |
| InlA | Internalin A |
| InlB | Internalin B |
| PlcA | Phosphatidylinositol-Specific Phospholipase C |
| PlcB | Broad-Range Phospholipase C |
| LIPI-1 | Listeria pathogenicity island 1 |
| EHD2 | EH Domain Containing 2 |
| LAP | Listeria adhesion protein |
| Hsp60 | Heat shock protein 60 |
| CR3 | Complement receptor 3 |
| TACO | Tryptophan-aspartate-containing coat |
| BCG | Mycobacterium bovis Bacillus Calmette-Guérin |
| SfbI | Streptococcal fibronectin-binding protein I |
| OCRL | Oculocerebrorenal Syndrome of Lowe |
| EPEC | Enteropathogenic E. coli |
| EHEC | Enterohaemorrhagic E. coli |
| UPEC | Uropathogenic E. coli |
| UTIs | Urinary tract infections |
| CNF1 | Cyroroxic necrotizing factor 1 |
| UP1a | Glycosylated uroplakin Ia |
| CEACAM | Carcinoembryonic antigen-related cell adhesion molecule |
| IBCs | Intracellular bacterial communities |
| DAEC | Afa/Dr diffusely adhering E. coli |
| PKCa | Protein kinase C alpha |
| T3SS | Type III secretion system |
| SPI-1 | Salmonella Pathogenicity Island 1 |
| SCVs | Salmonella-containing vacuoles |
| SPI-2 | Salmonella Pathogenicity Island 2 |
| Ebs | Elementary bodies |
| SV40 | Simian virus 40 |
| SARS | Severe acute respiratory syndrome |
| ACE2 | Angiotensin-converting enzyme 2 |
| HIV-1 | Human immunodeficiency virus 1 |
| JEV | Japanese Encephalitis Virus |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
References
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857-902. [CrossRef] [PubMed]
- Cossart P, Helenius A. Endocytosis of viruses and bacteria. Cold Spring Harb Perspect Biol. 2014;6(8). Epub 20140801. [CrossRef] [PubMed]
- Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell Res. 2010;20(3):256-75. Epub 20100202. [CrossRef] [PubMed]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517-33. Epub 20110722. [CrossRef] [PubMed]
- Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313-26. Epub 20180207. [CrossRef] [PubMed]
- Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL. Regulation of Clathrin-Mediated Endocytosis. Annu Rev Biochem. 2018;87:871-96. Epub 20180416. [CrossRef] [PubMed]
- Reider A, Wendland B. Endocytic adaptors--social networking at the plasma membrane. J Cell Sci. 2011;124(Pt 10):1613-22. [CrossRef] [PubMed]
- Hansen CG, Nichols BJ. Molecular mechanisms of clathrin-independent endocytosis. J Cell Sci. 2009;122(Pt 11):1713-21. [CrossRef] [PubMed]
- Sandvig K, Kavaliauskiene S, Skotland T. Clathrin-independent endocytosis: an increasing degree of complexity. Histochem Cell Biol. 2018;150(2):107-18. Epub 20180517. [CrossRef] [PubMed]
- Sandvig K, Pust S, Skotland T, van Deurs B. Clathrin-independent endocytosis: mechanisms and function. Curr Opin Cell Biol. 2011;23(4):413-20. Epub 20110403. [CrossRef] [PubMed]
- Howes MT, Mayor S, Parton RG. Molecules, mechanisms, and cellular roles of clathrin-independent endocytosis. Curr Opin Cell Biol. 2010;22(4):519-27. Epub 20100501. [CrossRef] [PubMed]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603-12. [CrossRef] [PubMed]
- Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135-63. Epub 20100618. [CrossRef] [PubMed]
- Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161(4):673-7. [CrossRef] [PubMed]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569-72. [CrossRef] [PubMed]
- Pike, LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44(4):655-67. Epub 20030201. [CrossRef] [PubMed]
- Sezgin E, Levental I, Mayor S, Eggeling C. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat Rev Mol Cell Biol. 2017;18(6):361-74. Epub 20170330. [CrossRef] [PubMed]
- Rennick JJ, Johnston APR, Parton RG. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat Nanotechnol. 2021;16(3):266-76. Epub 20210312. [CrossRef] [PubMed]
- Hinshaw, JE. Dynamin and its role in membrane fission. Annu Rev Cell Dev Biol. 2000;16:483-519. [CrossRef] [PubMed]
- Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3(5):311-20. [CrossRef] [PubMed]
- Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med. 2008;12(3):796-809. Epub 20080227. [CrossRef] [PubMed]
- Parton, RG. Caveolae--from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4(2):162-7. [CrossRef] [PubMed]
- Parton RG, McMahon KA, Wu Y. Caveolae: Formation, dynamics, and function. Curr Opin Cell Biol. 2020;65:8-16. Epub 20200306. [CrossRef] [PubMed]
- Pilch PF, Liu L. Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol Metab. 2011;22(8):318-24. Epub 20110517. [CrossRef] [PubMed]
- Chow BW, Nunez V, Kaplan L, Granger AJ, Bistrong K, Zucker HL, et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579(7797):106-10. Epub 20200219. [CrossRef] [PubMed]
- Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335(1):41-7. Epub 20080808. [CrossRef] [PubMed]
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14(2):98-112. [CrossRef] [PubMed]
- Lamaze C, Tardif N, Dewulf M, Vassilopoulos S, Blouin CM. The caveolae dress code: structure and signaling. Curr Opin Cell Biol. 2017;47:117-25. Epub 20170620. [CrossRef] [PubMed]
- Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM. Cavin family proteins and the assembly of caveolae. J Cell Sci. 2015;128(7):1269-78. [CrossRef] [PubMed]
- Ludwig A, Nichols BJ, Sandin S. Architecture of the caveolar coat complex. J Cell Sci. 2016;129(16):3077-83. Epub 20160701. [CrossRef] [PubMed]
- Stoeber M, Stoeck IK, Hanni C, Bleck CK, Balistreri G, Helenius A. Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin. EMBO J. 2012;31(10):2350-64. Epub 20120413. [CrossRef] [PubMed]
- Stoeber M, Schellenberger P, Siebert CA, Leyrat C, Helenius A, Grunewald K. Model for the architecture of caveolae based on a flexible, net-like assembly of Cavin1 and Caveolin discs. Proc Natl Acad Sci U S A. 2016;113(50):E8069-E78. Epub 20161110. [CrossRef] [PubMed]
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293(5539):2449-52. Epub 20010809. [CrossRef] [PubMed]
- Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132(1):113-24. [CrossRef] [PubMed]
- Tillu VA, Lim YW, Kovtun O, Mureev S, Ferguson C, Bastiani M, et al. A variable undecad repeat domain in cavin1 regulates caveola formation and stability. EMBO Rep. 2018;19(9). Epub 20180718. [CrossRef] [PubMed]
- Tillu VA, Rae J, Gao Y, Ariotti N, Floetenmeyer M, Kovtun O, et al. Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. Nat Commun. 2021;12(1):931. Epub 20210210. [CrossRef] [PubMed]
- Hansen CG, Bright NA, Howard G, Nichols BJ. SDPR induces membrane curvature and functions in the formation of caveolae. Nat Cell Biol. 2009;11(7):807-14. Epub 20090614. [CrossRef] [PubMed]
- McMahon KA, Zajicek H, Li WP, Peyton MJ, Minna JD, Hernandez VJ, et al. SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function. EMBO J. 2009;28(8):1001-15. Epub 20090305. [CrossRef] [PubMed]
- Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, Lo HP, et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185(7):1259-73. Epub 20090622. [CrossRef] [PubMed]
- Hansen CG, Howard G, Nichols BJ. Pacsin 2 is recruited to caveolae and functions in caveolar biogenesis. J Cell Sci. 2011;124(Pt 16):2777-85. [CrossRef] [PubMed]
- Senju Y, Itoh Y, Takano K, Hamada S, Suetsugu S. Essential role of PACSIN2/syndapin-II in caveolae membrane sculpting. J Cell Sci. 2011;124(Pt 12):2032-40. Epub 20110524. [CrossRef] [PubMed]
- Moren B, Shah C, Howes MT, Schieber NL, McMahon HT, Parton RG, et al. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol Biol Cell. 2012;23(7):1316-29. Epub 20120209. [CrossRef] [PubMed]
- Hoernke M, Mohan J, Larsson E, Blomberg J, Kahra D, Westenhoff S, et al. EHD2 restrains dynamics of caveolae by an ATP-dependent, membrane-bound, open conformation. Proc Natl Acad Sci U S A. 2017;114(22):E4360-E9. Epub 20170221. [CrossRef] [PubMed]
- Matthaeus C, Lahmann I, Kunz S, Jonas W, Melo AA, Lehmann M, et al. EHD2-mediated restriction of caveolar dynamics regulates cellular fatty acid uptake. Proc Natl Acad Sci U S A. 2020;117(13):7471-81. Epub 20200313. [CrossRef] [PubMed]
- Sonnino S, Prinetti A. Sphingolipids and membrane environments for caveolin. FEBS Lett. 2009;583(4):597-606. Epub 20090122. [CrossRef] [PubMed]
- Stan, RV. Structure of caveolae. Biochim Biophys Acta. 2005;1746(3):334-48. Epub 20050916. [CrossRef] [PubMed]
- Fielding CJ, Fielding PE. Cholesterol and caveolae: structural and functional relationships. Biochim Biophys Acta. 2000;1529(1-3):210-22. [CrossRef] [PubMed]
- Ikonen E, Heino S, Lusa S. Caveolins and membrane cholesterol. Biochem Soc Trans. 2004;32(Pt 1):121-3. [CrossRef] [PubMed]
- Zimnicka AM, Husain YS, Shajahan AN, Sverdlov M, Chaga O, Chen Z, et al. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol Biol Cell. 2016;27(13):2090-106. Epub 20160511. [CrossRef] [PubMed]
- Sverdlov M, Shajahan AN, Minshall RD. Tyrosine phosphorylation-dependence of caveolae-mediated endocytosis. J Cell Mol Med. 2007;11(6):1239-50. [CrossRef] [PubMed]
- Cao H, Courchesne WE, Mastick CC. A phosphotyrosine-dependent protein interaction screen reveals a role for phosphorylation of caveolin-1 on tyrosine 14: recruitment of C-terminal Src kinase. J Biol Chem. 2002;277(11):8771-4. Epub 20020122. [CrossRef] [PubMed]
- Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141(1):101-14. [CrossRef] [PubMed]
- Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141(1):85-99. [CrossRef] [PubMed]
- Larsson E, Moren B, McMahon KA, Parton RG, Lundmark R. Dynamin2 functions as an accessory protein to reduce the rate of caveola internalization. J Cell Biol. 2023;222(4). Epub 20230202. [CrossRef] [PubMed]
- Matthaeus C, Sochacki KA, Dickey AM, Puchkov D, Haucke V, Lehmann M, et al. The molecular organization of differentially curved caveolae indicates bendable structural units at the plasma membrane. Nat Commun. 2022;13(1):7234. Epub 20221124. [CrossRef] [PubMed]
- Preta G, Cronin JG, Sheldon IM. Dynasore - not just a dynamin inhibitor. Cell Commun Signal. 2015;13:24. Epub 20150410. [CrossRef] [PubMed]
- Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci. 2002;115(Pt 22):4327-39. [CrossRef] [PubMed]
- Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296(5567):535-9. [CrossRef] [PubMed]
- Matthaeus C, Taraska JW. Energy and Dynamics of Caveolae Trafficking. Front Cell Dev Biol. 2020;8:614472. Epub 20210121. [CrossRef] [PubMed]
- Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, Helenius A. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191(3):615-29. [CrossRef] [PubMed]
- Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118(6):767-80. [CrossRef] [PubMed]
- Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3(5):473-83. [CrossRef] [PubMed]
- Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116(Pt 6):1059-71. [CrossRef] [PubMed]
- Garcia-Nino WR, Correa F, Rodriguez-Barrena JI, Leon-Contreras JC, Buelna-Chontal M, Soria-Castro E, et al. Cardioprotective kinase signaling to subsarcolemmal and interfibrillar mitochondria is mediated by caveolar structures. Basic Res Cardiol. 2017;112(2):15. Epub 20170203. [CrossRef] [PubMed]
- Correa F, Enriquez-Cortina C, Silva-Palacios A, Roman-Anguiano N, Gil-Hernandez A, Ostolga-Chavarria M, et al. Actin-Cytoskeleton Drives Caveolae Signaling to Mitochondria during Postconditioning. Cells. 2023;12(3). Epub 20230202. [CrossRef] [PubMed]
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2(4):411-23. [CrossRef] [PubMed]
- Chadda R, Howes MT, Plowman SJ, Hancock JF, Parton RG, Mayor S. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic. 2007;8(6):702-17. Epub 20070425. [CrossRef] [PubMed]
- Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10(1):30-41. Epub 20071216. [CrossRef] [PubMed]
- Prieto-Sanchez RM, Berenjeno IM, Bustelo XR. Involvement of the Rho/Rac family member RhoG in caveolar endocytosis. Oncogene. 2006;25(21):2961-73. [CrossRef] [PubMed]
- Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7(3):661-71. [CrossRef] [PubMed]
- Grassart A, Dujeancourt A, Lazarow PB, Dautry-Varsat A, Sauvonnet N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep. 2008;9(4):356-62. Epub 20080314. [CrossRef] [PubMed]
- Casamento A, Boucrot E. Molecular mechanism of Fast Endophilin-Mediated Endocytosis. Biochem J. 2020;477(12):2327-45. [CrossRef] [PubMed]
- Boucrot E, Ferreira AP, Almeida-Souza L, Debard S, Vallis Y, Howard G, et al. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517(7535):460-5. Epub 20141217. [CrossRef] [PubMed]
- Yang LQ, Huang AF, Xu WD. Biology of endophilin and it’s role in disease. Front Immunol. 2023;14:1297506. Epub 20231205. [CrossRef] [PubMed]
- Kjaerulff O, Brodin L, Jung A. The structure and function of endophilin proteins. Cell Biochem Biophys. 2011;60(3):137-54. [CrossRef] [PubMed]
- Renard HF, Simunovic M, Lemiere J, Boucrot E, Garcia-Castillo MD, Arumugam S, et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517(7535):493-6. Epub 20141217. [CrossRef] [PubMed]
- Simunovic M, Manneville JB, Renard HF, Evergren E, Raghunathan K, Bhatia D, et al. Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell. 2017;170(1):172-84 e11. Epub 20170622. [CrossRef] [PubMed]
- Chan Wah Hak L, Khan S, Di Meglio I, Law AL, Lucken-Ardjomande Hasler S, Quintaneiro LM, et al. FBP17 and CIP4 recruit SHIP2 and lamellipodin to prime the plasma membrane for fast endophilin-mediated endocytosis. Nat Cell Biol. 2018;20(9):1023-31. Epub 20180730. [CrossRef] [PubMed]
- Ferreira APA, Casamento A, Carrillo Roas S, Halff EF, Panambalana J, Subramaniam S, et al. Cdk5 and GSK3beta inhibit fast endophilin-mediated endocytosis. Nat Commun. 2021;12(1):2424. Epub 20210423. [CrossRef] [PubMed]
- Gundu C, Arruri VK, Yadav P, Navik U, Kumar A, Amalkar VS, et al. Dynamin-Independent Mechanisms of Endocytosis and Receptor Trafficking. Cells. 2022;11(16). Epub 20220817. [CrossRef] [PubMed]
- Ojha R, Jiang A, Mantyla E, Quirin T, Modhira N, Witte R, et al. Dynamin independent endocytosis is an alternative cell entry mechanism for multiple animal viruses. PLoS Pathog. 2024;20(11):e1012690. Epub 20241114. [CrossRef] [PubMed]
- Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3’-kinase-dependent machinery. Mol Biol Cell. 2006;17(8):3689-704. Epub 20060607. [CrossRef] [PubMed]
- Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, Simpson F, et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol. 2010;190(4):675-91. Epub 20100816. [CrossRef] [PubMed]
- Goswami D, Gowrishankar K, Bilgrami S, Ghosh S, Raghupathy R, Chadda R, et al. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135(6):1085-97. [CrossRef] [PubMed]
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, et al. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008;18(22):1802-8. [CrossRef] [PubMed]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7(5):347-58. [CrossRef] [PubMed]
- Schweitzer JK, Sedgwick AE, D’Souza-Schorey C. ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis. Semin Cell Dev Biol. 2011;22(1):39-47. Epub 20100915. [CrossRef] [PubMed]
- Van Acker T, Tavernier J, Peelman F. The Small GTPase Arf6: An Overview of Its Mechanisms of Action and of Its Role in Host(-)Pathogen Interactions and Innate Immunity. Int J Mol Sci. 2019;20(9). Epub 20190505. [CrossRef] [PubMed]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597-608. [CrossRef] [PubMed]
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell. 2004;15(8):3542-52. Epub 20040514. [CrossRef] [PubMed]
- Schafer DA, D’Souza-Schorey C, Cooper JA. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1(11):892-903. [CrossRef] [PubMed]
- Hongu T, Kanaho Y. Activation machinery of the small GTPase Arf6. Adv Biol Regul. 2014;54:59-66. Epub 20131008. [CrossRef] [PubMed]
- Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell. 2003;14(2):417-31. [CrossRef] [PubMed]
- Chesneau L, Dambournet D, Machicoane M, Kouranti I, Fukuda M, Goud B, et al. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol. 2012;22(2):147-53. Epub 20120105. [CrossRef] [PubMed]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154(5):1007-17. [CrossRef] [PubMed]
- Cauvin C, Rosendale M, Gupta-Rossi N, Rocancourt M, Larraufie P, Salomon R, et al. Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes. Curr Biol. 2016;26(1):120-8. Epub 20151224. [CrossRef] [PubMed]
- Dutta D, Donaldson JG. Sorting of Clathrin-Independent Cargo Proteins Depends on Rab35 Delivered by Clathrin-Mediated Endocytosis. Traffic. 2015;16(9):994-1009. Epub 20150604. [CrossRef] [PubMed]
- Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8(1):46-54. Epub 20051211. [CrossRef] [PubMed]
- Otto GP, Nichols BJ. The roles of flotillin microdomains--endocytosis and beyond. J Cell Sci. 2011;124(Pt 23):3933-40. [CrossRef] [PubMed]
- Riento K, Frick M, Schafer I, Nichols BJ. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J Cell Sci. 2009;122(Pt 7):912-8. Epub 20090303. [CrossRef] [PubMed]
- Langhorst MF, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62(19-20):2228-40. [CrossRef] [PubMed]
- Rivera-Milla E, Stuermer CA, Malaga-Trillo E. Ancient origin of reggie (flotillin), reggie-like, and other lipid-raft proteins: convergent evolution of the SPFH domain. Cell Mol Life Sci. 2006;63(3):343-57. [CrossRef] [PubMed]
- Babuke T, Ruonala M, Meister M, Amaddii M, Genzler C, Esposito A, et al. Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis. Cell Signal. 2009;21(8):1287-97. Epub 20090324. [CrossRef] [PubMed]
- Solis GP, Hoegg M, Munderloh C, Schrock Y, Malaga-Trillo E, Rivera-Milla E, et al. Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains. Biochem J. 2007;403(2):313-22. [CrossRef] [PubMed]
- Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007;17(13):1151-6. [CrossRef] [PubMed]
- Babuke T, Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007;86(9):525-32. Epub 20070504. [CrossRef] [PubMed]
- Ait-Slimane T, Galmes R, Trugnan G, Maurice M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol Biol Cell. 2009;20(17):3792-800. Epub 20090715. [CrossRef] [PubMed]
- Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, et al. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28(11):2874-82. [CrossRef] [PubMed]
- Liu J, Deyoung SM, Zhang M, Dold LH, Saltiel AR. The stomatin/prohibitin/flotillin/HflK/C domain of flotillin-1 contains distinct sequences that direct plasma membrane localization and protein interactions in 3T3-L1 adipocytes. J Biol Chem. 2005;280(16):16125-34. Epub 20050214. [CrossRef] [PubMed]
- Neumann-Giesen C, Fernow I, Amaddii M, Tikkanen R. Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci. 2007;120(Pt 3):395-406. Epub 20070109. [CrossRef] [PubMed]
- Stow JL, Hung Y, Wall AA. Macropinocytosis: Insights from immunology and cancer. Curr Opin Cell Biol. 2020;65:131-40. Epub 20200731. [CrossRef] [PubMed]
- Kay, RR. Macropinocytosis: Biology and mechanisms. Cells Dev. 2021;168:203713. Epub 20210624. [CrossRef] [PubMed]
- Salloum G, Bresnick AR, Backer JM. Macropinocytosis: mechanisms and regulation. Biochem J. 2023;480(5):335-62. [CrossRef] [PubMed]
- Wu Y, Hu X, Wei Z, Lin Q. Cellular Regulation of Macropinocytosis. Int J Mol Sci. 2024;25(13). Epub 20240626. [CrossRef] [PubMed]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135(5):1249-60. [CrossRef] [PubMed]
- Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11(10):3453-67. [CrossRef] [PubMed]
- Quinn SE, Huang L, Kerkvliet JG, Swanson JA, Smith S, Hoppe AD, et al. The structural dynamics of macropinosome formation and PI3-kinase-mediated sealing revealed by lattice light sheet microscopy. Nat Commun. 2021;12(1):4838. Epub 20210810. [CrossRef] [PubMed]
- Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89(8):836-43. Epub 20110322. [CrossRef] [PubMed]
- Buckley CM, King JS. Drinking problems: mechanisms of macropinosome formation and maturation. FEBS J. 2017;284(22):3778-90. Epub 20170605. [CrossRef] [PubMed]
- Garcia-del Portillo F, Finlay BB. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect Immun. 1994;62(10):4641-5. [CrossRef] [PubMed]
- Weiner A, Mellouk N, Lopez-Montero N, Chang YY, Souque C, Schmitt C, et al. Macropinosomes are Key Players in Early Shigella Invasion and Vacuolar Escape in Epithelial Cells. PLoS Pathog. 2016;12(5):e1005602. Epub 20160516. [CrossRef] [PubMed]
- Ford C, Nans A, Boucrot E, Hayward RD. Chlamydia exploits filopodial capture and a macropinocytosis-like pathway for host cell entry. PLoS Pathog. 2018;14(5):e1007051. Epub 20180504. [CrossRef] [PubMed]
- Watarai M, Makino S, Fujii Y, Okamoto K, Shirahata T. Modulation of Brucella-induced macropinocytosis by lipid rafts mediates intracellular replication. Cell Microbiol. 2002;4(6):341-55. [CrossRef] [PubMed]
- Garcia-Perez BE, Mondragon-Flores R, Luna-Herrera J. Internalization of Mycobacterium tuberculosis by macropinocytosis in non-phagocytic cells. Microb Pathog. 2003;35(2):49-55. [CrossRef] [PubMed]
- Garcia-Perez BE, Hernandez-Gonzalez JC, Garcia-Nieto S, Luna-Herrera J. Internalization of a non-pathogenic mycobacteria by macropinocytosis in human alveolar epithelial A549 cells. Microb Pathog. 2008;45(1):1-6. Epub 20080403. [CrossRef] [PubMed]
- Watarai M, Derre I, Kirby J, Growney JD, Dietrich WF, Isberg RR. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus. J Exp Med. 2001;194(8):1081-96. [CrossRef] [PubMed]
- Loh LN, McCarthy EMC, Narang P, Khan NA, Ward TH. Escherichia coli K1 utilizes host macropinocytic pathways for invasion of brain microvascular endothelial cells. Traffic. 2017;18(11):733-46. Epub 20170920. [CrossRef] [PubMed]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320(5875):531-5. [CrossRef] [PubMed]
- Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75(22):11166-77. [CrossRef] [PubMed]
- Barman D, Drolia R. Caveolin-Mediated Endocytosis: Bacterial Pathogen Exploitation and Host-Pathogen Interaction. Cells. 2024;14(1). Epub 20241224. [CrossRef] [PubMed]
- Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304(5668):242-8. [CrossRef] [PubMed]
- Kiss AL, Botos E. Endocytosis via caveolae: alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J Cell Mol Med. 2009;13(7):1228-37. Epub 20090327. [CrossRef] [PubMed]
- Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55(3):476-511. [CrossRef] [PubMed]
- Melton-Witt JA, McKay SL, Portnoy DA. Development of a single-gene, signature-tag-based approach in combination with alanine mutagenesis to identify listeriolysin O residues critical for the in vivo survival of Listeria monocytogenes. Infect Immun. 2012;80(6):2221-30. Epub 20120326. [CrossRef] [PubMed]
- Zhang T, Abel S, Abel Zur Wiesch P, Sasabe J, Davis BM, Higgins DE, et al. Deciphering the landscape of host barriers to Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 2017;114(24):6334-9. Epub 20170530. [CrossRef] [PubMed]
- Pizarro-Cerda J, Cossart P. Listeria monocytogenes: cell biology of invasion and intracellular growth. Microbiol Spectr. 2018;6(6). [CrossRef] [PubMed]
- Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16(1):32-46. Epub 20171127. [CrossRef] [PubMed]
- Gaillard JL, Berche P, Frehel C, Gouin E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65(7):1127-41. [CrossRef] [PubMed]
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 1999;18(14):3956-63. [CrossRef] [PubMed]
- Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell. 2000;103(3):501-10. [CrossRef] [PubMed]
- Ruan Y, Rezelj S, Bedina Zavec A, Anderluh G, Scheuring S. Listeriolysin O Membrane Damaging Activity Involves Arc Formation and Lineaction -- Implication for Listeria monocytogenes Escape from Phagocytic Vacuole. PLoS Pathog. 2016;12(4):e1005597. Epub 20160422. [CrossRef] [PubMed]
- Petrisic N, Adamek M, Kezar A, Hocevar SB, Zagar E, Anderluh G, et al. Structural basis for the unique molecular properties of broad-range phospholipase C from Listeria monocytogenes. Nat Commun. 2023;14(1):6474. Epub 20231014. [CrossRef] [PubMed]
- Lambrechts A, Gevaert K, Cossart P, Vandekerckhove J, Van Troys M. Listeria comet tails: the actin-based motility machinery at work. Trends Cell Biol. 2008;18(5):220-7. Epub 20080407. [CrossRef] [PubMed]
- Bonazzi M, Vasudevan L, Mallet A, Sachse M, Sartori A, Prevost MC, et al. Clathrin phosphorylation is required for actin recruitment at sites of bacterial adhesion and internalization. J Cell Biol. 2011;195(3):525-36. [CrossRef] [PubMed]
- Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol. 2005;7(9):894-900. Epub 20050821. [CrossRef] [PubMed]
- Dhanda AS, Yu C, Lulic KT, Vogl AW, Rausch V, Yang D, et al. Listeria monocytogenes Exploits Host Caveolin for Cell-to-Cell Spreading. mBio. 2020;11(1). Epub 20200121. [CrossRef] [PubMed]
- Drolia R, Bryant DB, Tenguria S, Jules-Culver ZA, Thind J, Amelunke B, et al. Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for Listeria monocytogenes translocation. mBio. 2024;15(3):e0282123. Epub 20240220. [CrossRef] [PubMed]
- Drolia R, Tenguria S, Durkes AC, Turner JR, Bhunia AK. Listeria Adhesion Protein Induces Intestinal Epithelial Barrier Dysfunction for Bacterial Translocation. Cell Host Microbe. 2018;23(4):470-84 e7. Epub 20180405. [CrossRef] [PubMed]
- Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13(10):e1002152. Epub 20161025. [CrossRef] [PubMed]
- Gagneux, S. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2018;16(4):202-13. Epub 20180219. [CrossRef] [PubMed]
- Guirado E, Schlesinger LS, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol. 2013;35(5):563-83. Epub 20130718. [CrossRef] [PubMed]
- Pieters J, Gatfield J. Hijacking the host: survival of pathogenic mycobacteria inside macrophages. Trends Microbiol. 2002;10(3):142-6. [CrossRef] [PubMed]
- Hmama Z, Pena-Diaz S, Joseph S, Av-Gay Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol Rev. 2015;264(1):220-32. [CrossRef] [PubMed]
- Chai Q, Wang L, Liu CH, Ge B. New insights into the evasion of host innate immunity by Mycobacterium tuberculosis. Cell Mol Immunol. 2020;17(9):901-13. Epub 20200729. [CrossRef] [PubMed]
- Hasan Z, Schlax C, Kuhn L, Lefkovits I, Young D, Thole J, et al. Isolation and characterization of the mycobacterial phagosome: segregation from the endosomal/lysosomal pathway. Mol Microbiol. 1997;24(3):545-53. [CrossRef] [PubMed]
- Houben EN, Nguyen L, Pieters J. Interaction of pathogenic mycobacteria with the host immune system. Curr Opin Microbiol. 2006;9(1):76-85. Epub 20060109. [CrossRef] [PubMed]
- Schorey JS, Carroll MC, Brown EJ. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 1997;277(5329):1091-3. [CrossRef] [PubMed]
- Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288(5471):1647-50. [CrossRef] [PubMed]
- Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97(4):435-47. [CrossRef] [PubMed]
- Kaul D, Anand PK, Verma I. Cholesterol-sensor initiates M. tuberculosis entry into human macrophages. Mol Cell Biochem. 2004;258(1-2):219-22. [CrossRef] [PubMed]
- Wu Y, Riehle A, Pollmeier B, Kadow S, Schumacher F, Drab M, et al. Caveolin-1 affects early mycobacterial infection and apoptosis in macrophages and mice. Tuberculosis (Edinb). 2024;147:102493. Epub 20240212. [CrossRef] [PubMed]
- Chung HY, Claus RA. Keep Your Friends Close, but Your Enemies Closer: Role of Acid Sphingomyelinase During Infection and Host Response. Front Med (Lausanne). 2020;7:616500. Epub 20210121. [CrossRef] [PubMed]
- Munoz S, Hernandez-Pando R, Abraham SN, Enciso JA. Mast cell activation by Mycobacterium tuberculosis: mediator release and role of CD48. J Immunol. 2003;170(11):5590-6. [CrossRef] [PubMed]
- Dudeck A, Koberle M, Goldmann O, Meyer N, Dudeck J, Lemmens S, et al. Mast cells as protectors of health. J Allergy Clin Immunol. 2019;144(4S):S4-S18. Epub 20181120. [CrossRef] [PubMed]
- Munoz S, Rivas-Santiago B, Enciso JA. Mycobacterium tuberculosis entry into mast cells through cholesterol-rich membrane microdomains. Scand J Immunol. 2009;70(3):256-63. [CrossRef] [PubMed]
- Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, et al. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clin Microbiol Rev. 2014;27(2):264-301. [CrossRef] [PubMed]
- Amelung S, Nerlich A, Rohde M, Spellerberg B, Cole JN, Nizet V, et al. The FbaB-type fibronectin-binding protein of Streptococcus pyogenes promotes specific invasion into endothelial cells. Cell Microbiol. 2011;13(8):1200-11. Epub 20110525. [CrossRef] [PubMed]
- Molinari G, Rohde M, Guzman CA, Chhatwal GS. Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell Microbiol. 2000;2(2):145-54. [CrossRef] [PubMed]
- Siemens N, Patenge N, Otto J, Fiedler T, Kreikemeyer B. Streptococcus pyogenes M49 plasminogen/plasmin binding facilitates keratinocyte invasion via integrin-integrin-linked kinase (ILK) pathways and protects from macrophage killing. J Biol Chem. 2011;286(24):21612-22. Epub 20110426. [CrossRef] [PubMed]
- LaPenta D, Rubens C, Chi E, Cleary PP. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci U S A. 1994;91(25):12115-9. [CrossRef] [PubMed]
- Kaplan EL, Chhatwal GS, Rohde M. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin Infect Dis. 2006;43(11):1398-406. Epub 20061030. [CrossRef] [PubMed]
- Wang B, Cleary PP. Intracellular Invasion by Streptococcus pyogenes: Invasins, Host Receptors, and Relevance to Human Disease. Microbiol Spectr. 2019;7(4). [CrossRef] [PubMed]
- Medina E, Goldmann O, Toppel AW, Chhatwal GS. Survival of Streptococcus pyogenes within host phagocytic cells: a pathogenic mechanism for persistence and systemic invasion. J Infect Dis. 2003;187(4):597-603. Epub 20030207. [CrossRef] [PubMed]
- Medina E, Rohde M, Chhatwal GS. Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect Immun. 2003;71(9):5376-80. [CrossRef] [PubMed]
- Rohde M, Muller E, Chhatwal GS, Talay SR. Host cell caveolae act as an entry-port for group A streptococci. Cell Microbiol. 2003;5(5):323-42. [CrossRef] [PubMed]
- Talay SR, Zock A, Rohde M, Molinari G, Oggioni M, Pozzi G, et al. Co-operative binding of human fibronectin to Sfbl protein triggers streptococcal invasion into respiratory epithelial cells. Cell Microbiol. 2000;2(6):521-35. [CrossRef] [PubMed]
- Lim JY, Barnett TC, Bastiani M, McMahon KA, Ferguson C, Webb RI, et al. Caveolin 1 restricts Group A Streptococcus invasion of nonphagocytic host cells. Cell Microbiol. 2017;19(12). Epub 20170906. [CrossRef] [PubMed]
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG, Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603-61. [CrossRef] [PubMed]
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7(9):629-41. [CrossRef] [PubMed]
- Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. 2015;13(9):529-43. [CrossRef] [PubMed]
- Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164(7):3713-22. [CrossRef] [PubMed]
- Horn J, Stelzner K, Rudel T, Fraunholz M. Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol. 2018;308(6):607-24. Epub 20171126. [CrossRef] [PubMed]
- Hommes JW, Surewaard BGJ. Intracellular Habitation of Staphylococcus aureus: Molecular Mechanisms and Prospects for Antimicrobial Therapy. Biomedicines. 2022;10(8). Epub 20220727. [CrossRef] [PubMed]
- Goldmann O, Lang JC, Rohde M, May T, Molinari G, Medina E. Alpha-hemolysin promotes internalization of Staphylococcus aureus into human lung epithelial cells via caveolin-1- and cholesterol-rich lipid rafts. Cell Mol Life Sci. 2024;81(1):435. Epub 20241016. [CrossRef] [PubMed]
- Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel). 2013;5(6):1140-66. [CrossRef] [PubMed]
- Vijayvargia R, Kaur S, Krishnasastry MV. alpha-Hemolysin-induced dephosphorylation of EGF receptor of A431 cells is carried out by rPTPsigma. Biochem Biophys Res Commun. 2004;325(1):344-52. [CrossRef] [PubMed]
- Vijayvargia R, Suresh CG, Krishnasastry MV. Functional form of Caveolin-1 is necessary for the assembly of alpha-hemolysin. Biochem Biophys Res Commun. 2004;324(3):1130-6. [CrossRef] [PubMed]
- Vijayvargia R, Kaur S, Sangha N, Sahasrabuddhe AA, Surolia I, Shouche Y, et al. Assembly of alpha-hemolysin on A431 cells leads to clustering of Caveolin-1. Biochem Biophys Res Commun. 2004;324(3):1124-9. [CrossRef] [PubMed]
- Pany S, Vijayvargia R, Krishnasastry MV. Caveolin-1 binding motif of alpha-hemolysin: its role in stability and pore formation. Biochem Biophys Res Commun. 2004;322(1):29-36. [CrossRef] [PubMed]
- Pany S, Krishnasastry MV. Aromatic residues of Caveolin-1 binding motif of alpha-hemolysin are essential for membrane penetration. Biochem Biophys Res Commun. 2007;363(1):197-202. Epub 20070830. [CrossRef] [PubMed]
- Hoffmann C, Berking A, Agerer F, Buntru A, Neske F, Chhatwal GS, et al. Caveolin limits membrane microdomain mobility and integrin-mediated uptake of fibronectin-binding pathogens. J Cell Sci. 2010;123(Pt 24):4280-91. Epub 20101123. [CrossRef] [PubMed]
- Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8(1):26-38. [CrossRef] [PubMed]
- Whelan S, Lucey B, Finn K. Uropathogenic Escherichia coli (UPEC)-Associated Urinary Tract Infections: The Molecular Basis for Challenges to Effective Treatment. Microorganisms. 2023;11(9). Epub 20230828. [CrossRef] [PubMed]
- Yang X, Chen H, Zheng Y, Qu S, Wang H, Yi F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front Public Health. 2022;10:888205. Epub 20220727. [CrossRef] [PubMed]
- Sihra N, Goodman A, Zakri R, Sahai A, Malde S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat Rev Urol. 2018;15(12):750-76. [CrossRef] [PubMed]
- Servin, AL. Pathogenesis of Afa/Dr diffusely adhering Escherichia coli. Clin Microbiol Rev. 2005;18(2):264-92. [CrossRef] [PubMed]
- Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000;19(12):2803-12. [CrossRef] [PubMed]
- Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282(5393):1494-7. [CrossRef] [PubMed]
- Mulvey, MA. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002;4(5):257-71. [CrossRef] [PubMed]
- Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69(7):4572-9. [CrossRef] [PubMed]
- Doye A, Mettouchi A, Bossis G, Clement R, Buisson-Touati C, Flatau G, et al. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell. 2002;111(4):553-64. [CrossRef] [PubMed]
- Springall T, Sheerin NS, Abe K, Holers VM, Wan H, Sacks SH. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med. 2001;7(7):801-6. [CrossRef] [PubMed]
- Poole NM, Green SI, Rajan A, Vela LE, Zeng XL, Estes MK, et al. Role for FimH in Extraintestinal Pathogenic Escherichia coli Invasion and Translocation through the Intestinal Epithelium. Infect Immun. 2017;85(11). Epub 20171018. [CrossRef] [PubMed]
- Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, et al. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114(Pt 22):4095-103. [CrossRef] [PubMed]
- Sheikh A, Fleckenstein JM. Interactions of pathogenic Escherichia coli with CEACAMs. Front Immunol. 2023;14:1120331. Epub 20230214. [CrossRef] [PubMed]
- Baorto DM, Gao Z, Malaviya R, Dustin ML, van der Merwe A, Lublin DM, et al. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature. 1997;389(6651):636-9. [CrossRef] [PubMed]
- Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 2007;3(7):e100. [CrossRef] [PubMed]
- Martinez JJ, Hultgren SJ. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell Microbiol. 2002;4(1):19-28. [CrossRef] [PubMed]
- Duncan MJ, Li G, Shin JS, Carson JL, Abraham SN. Bacterial penetration of bladder epithelium through lipid rafts. J Biol Chem. 2004;279(18):18944-51. Epub 20040219. [CrossRef] [PubMed]
- Anderson GG, Palermo JJ, Schilling JD, Roth R, Heuser J, Hultgren SJ. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105-7. [CrossRef] [PubMed]
- Servin, AL. Pathogenesis of human diffusely adhering Escherichia coli expressing Afa/Dr adhesins (Afa/Dr DAEC): current insights and future challenges. Clin Microbiol Rev. 2014;27(4):823-69. [CrossRef] [PubMed]
- Guignot J, Bernet-Camard MF, Pous C, Plancon L, Le Bouguenec C, Servin AL. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for alpha5beta1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect Immun. 2001;69(3):1856-68. [CrossRef] [PubMed]
- Shin JS, Gao Z, Abraham SN. Involvement of cellular caveolae in bacterial entry into mast cells. Science. 2000;289(5480):785-8. [CrossRef] [PubMed]
- Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert AA, McDonald J, et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics. 2017;140(1). Epub 20170609. [CrossRef] [PubMed]
- Kim, KS. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat Rev Neurosci. 2003;4(5):376-85. [CrossRef] [PubMed]
- Sukumaran SK, Quon MJ, Prasadarao NV. Escherichia coli K1 internalization via caveolae requires caveolin-1 and protein kinase Calpha interaction in human brain microvascular endothelial cells. J Biol Chem. 2002;277(52):50716-24. Epub 20021016. [CrossRef] [PubMed]
- Fabrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26(2):308-41. [CrossRef] [PubMed]
- de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. Host-pathogen interaction in invasive Salmonellosis. PLoS Pathog. 2012;8(10):e1002933. Epub 20121004. [CrossRef] [PubMed]
- Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180(1):15-23. [CrossRef] [PubMed]
- Lou L, Zhang P, Piao R, Wang Y. Salmonella Pathogenicity Island 1 (SPI-1) and Its Complex Regulatory Network. Front Cell Infect Microbiol. 2019;9:270. Epub 20190731. [CrossRef] [PubMed]
- Ly KT, Casanova JE. Mechanisms of Salmonella entry into host cells. Cell Microbiol. 2007;9(9):2103-11. Epub 20070625. [CrossRef] [PubMed]
- Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364(6438):639-42. [CrossRef] [PubMed]
- Bakowski MA, Braun V, Brumell JH. Salmonella-containing vacuoles: directing traffic and nesting to grow. Traffic. 2008;9(12):2022-31. Epub 20081008. [CrossRef] [PubMed]
- Lim JS, Shin M, Kim HJ, Kim KS, Choy HE, Cho KA. Caveolin-1 mediates Salmonella invasion via the regulation of SopE-dependent Rac1 activation and actin reorganization. J Infect Dis. 2014;210(5):793-802. Epub 20140312. [CrossRef] [PubMed]
- Lim JS, Choy HE, Park SC, Han JM, Jang IS, Cho KA. Caveolae-mediated entry of Salmonella typhimurium into senescent nonphagocytotic host cells. Aging Cell. 2010;9(2):243-51. Epub 20100120. [CrossRef] [PubMed]
- Lim JS, Na HS, Lee HC, Choy HE, Park SC, Han JM, et al. Caveolae-mediated entry of Salmonella typhimurium in a human M-cell model. Biochem Biophys Res Commun. 2009;390(4):1322-7. Epub 20091029. [CrossRef] [PubMed]
- Cheong HC, Lee CYQ, Cheok YY, Tan GMY, Looi CY, Wong WF. Chlamydiaceae: Diseases in Primary Hosts and Zoonosis. Microorganisms. 2019;7(5). Epub 20190524. [CrossRef] [PubMed]
- Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14(6):385-400. Epub 20160425. [CrossRef] [PubMed]
- Cocchiaro JL, Valdivia RH. New insights into Chlamydia intracellular survival mechanisms. Cell Microbiol. 2009;11(11):1571-8. Epub 20090805. [CrossRef] [PubMed]
- Caven L, Carabeo RA. Pathogenic Puppetry: Manipulation of the Host Actin Cytoskeleton by Chlamydia trachomatis. Int J Mol Sci. 2019;21(1). Epub 20191221. [CrossRef] [PubMed]
- Zhong, G. Chlamydia Spreading from the Genital Tract to the Gastrointestinal Tract - A Two-Hit Hypothesis. Trends Microbiol. 2018;26(7):611-23. Epub 20171227. [CrossRef] [PubMed]
- Bebear C, de Barbeyrac B. Genital Chlamydia trachomatis infections. Clin Microbiol Infect. 2009;15(1):4-10. [CrossRef] [PubMed]
- Taylor HR, Burton MJ, Haddad D, West S, Wright H. Trachoma. Lancet. 2014;384(9960):2142-52. Epub 20140717. [CrossRef] [PubMed]
- Carabeo RA, Grieshaber SS, Fischer E, Hackstadt T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect Immun. 2002;70(7):3793-803. [CrossRef] [PubMed]
- Korhonen JT, Puolakkainen M, Haveri A, Tammiruusu A, Sarvas M, Lahesmaa R. Chlamydia pneumoniae entry into epithelial cells by clathrin-independent endocytosis. Microb Pathog. 2012;52(3):157-64. Epub 20111221. [CrossRef] [PubMed]
- Boleti H, Benmerah A, Ojcius DM, Cerf-Bensussan N, Dautry-Varsat A. Chlamydia infection of epithelial cells expressing dynamin and Eps15 mutants: clathrin-independent entry into cells and dynamin-dependent productive growth. J Cell Sci. 1999;112 ( Pt 10):1487-96. [CrossRef] [PubMed]
- Norkin LC, Wolfrom SA, Stuart ES. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection. Exp Cell Res. 2001;266(2):229-38. [CrossRef] [PubMed]
- Stuart ES, Webley WC, Norkin LC. Lipid rafts, caveolae, caveolin-1, and entry by Chlamydiae into host cells. Exp Cell Res. 2003;287(1):67-78. [CrossRef] [PubMed]
- Jutras I, Abrami L, Dautry-Varsat A. Entry of the lymphogranuloma venereum strain of Chlamydia trachomatis into host cells involves cholesterol-rich membrane domains. Infect Immun. 2003;71(1):260-6. [CrossRef] [PubMed]
- Olofsson A, Nygard Skalman L, Obi I, Lundmark R, Arnqvist A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. mBio. 2014;5(3):e00979-14. Epub 20140520. [CrossRef] [PubMed]
- Naroeni A, Porte F. Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect Immun. 2002;70(3):1640-4. [CrossRef] [PubMed]
- Watarai M, Makino S, Michikawa M, Yanagisawa K, Murakami S, Shirahata T. Macrophage plasma membrane cholesterol contributes to Brucella abortus infection of mice. Infect Immun. 2002;70(9):4818-25. [CrossRef] [PubMed]
- Watson RO, Galan JE. Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog. 2008;4(1):e14. [CrossRef] [PubMed]
- Tamilselvam B, Daefler S. Francisella targets cholesterol-rich host cell membrane domains for entry into macrophages. J Immunol. 2008;180(12):8262-71. [CrossRef] [PubMed]
- Pietiainen VM, Marjomaki V, Heino J, Hyypia T. Viral entry, lipid rafts and caveosomes. Ann Med. 2005;37(6):394-403. [CrossRef] [PubMed]
- Pelkmans, L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim Biophys Acta. 2005;1746(3):295-304. Epub 20050705. [CrossRef] [PubMed]
- Campanero-Rhodes MA, Smith A, Chai W, Sonnino S, Mauri L, Childs RA, et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J Virol. 2007;81(23):12846-58. Epub 20070912. [CrossRef] [PubMed]
- Tsai B, Gilbert JM, Stehle T, Lencer W, Benjamin TL, Rapoport TA. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 2003;22(17):4346-55. [CrossRef] [PubMed]
- Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7(11):1825-34. [CrossRef] [PubMed]
- Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, et al. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131(3):516-29. [CrossRef] [PubMed]
- Milavetz BI, Balakrishnan L. Simian virus 40 (SV40) - Fresh perspectives on a historic virus. Virology. 2025;604:110427. Epub 20250201. [CrossRef] [PubMed]
- Breau WC, Atwood WJ, Norkin LC. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992;66(4):2037-45. [CrossRef] [PubMed]
- Peters AH, O’Grady JE, Milanovich RA. Aseptic meningitis associated with Echovirus type 3 in very young children. Am J Dis Child. 1972;123(5):452-6. [CrossRef] [PubMed]
- Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803-33. [CrossRef] [PubMed]
- Marjomaki V, Pietiainen V, Matilainen H, Upla P, Ivaska J, Nissinen L, et al. Internalization of echovirus 1 in caveolae. J Virol. 2002;76(4):1856-65. [CrossRef] [PubMed]
- Pietiainen V, Marjomaki V, Upla P, Pelkmans L, Helenius A, Hyypia T. Echovirus 1 endocytosis into caveosomes requires lipid rafts, dynamin II, and signaling events. Mol Biol Cell. 2004;15(11):4911-25. Epub 20040908. [CrossRef] [PubMed]
- Tang G, Liu Z, Chen D. Human coronaviruses: Origin, host and receptor. J Clin Virol. 2022;155:105246. Epub 20220721. [CrossRef] [PubMed]
- V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155-70. Epub 20201028. [CrossRef] [PubMed]
- Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290-301. [CrossRef] [PubMed]
- Li GM, Li YG, Yamate M, Li SM, Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9(1):96-102. Epub 20061208. [CrossRef] [PubMed]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444-8. Epub 20200304. [CrossRef] [PubMed]
- Tortorici MA, Walls AC, Lang Y, Wang C, Li Z, Koerhuis D, et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26(6):481-9. Epub 20190603. [CrossRef] [PubMed]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-80 e8. Epub 20200305. [CrossRef] [PubMed]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450-4. [CrossRef] [PubMed]
- Lu Y, Liu DX, Tam JP. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun. 2008;369(2):344-9. Epub 20080213. [CrossRef] [PubMed]
- Harrison CM, Doster JM, Landwehr EH, Kumar NP, White EJ, Beachboard DC, et al. Evaluating the Virology and Evolution of Seasonal Human Coronaviruses Associated with the Common Cold in the COVID-19 Era. Microorganisms. 2023;11(2). Epub 20230210. [CrossRef] [PubMed]
- Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, et al. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357(6377):420-2. [CrossRef] [PubMed]
- Nomura R, Kiyota A, Suzaki E, Kataoka K, Ohe Y, Miyamoto K, et al. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J Virol. 2004;78(16):8701-8. [CrossRef] [PubMed]
- Murray RS, Brown B, Brian D, Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. 1992;31(5):525-33. [CrossRef] [PubMed]
- Lau SKP, Li KSM, Li X, Tsang KY, Sridhar S, Woo PCY. Fatal Pneumonia Associated With a Novel Genotype of Human Coronavirus OC43. Front Microbiol. 2021;12:795449. Epub 20220114. [CrossRef] [PubMed]
- Collins, AR. HLA class I antigen serves as a receptor for human coronavirus OC43. Immunol Invest. 1993;22(2):95-103. [CrossRef] [PubMed]
- Owczarek K, Szczepanski A, Milewska A, Baster Z, Rajfur Z, Sarna M, et al. Early events during human coronavirus OC43 entry to the cell. Sci Rep. 2018;8(1):7124. Epub 20180508. [CrossRef] [PubMed]
- Manes S, del Real G, Lacalle RA, Lucas P, Gomez-Mouton C, Sanchez-Palomino S, et al. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 2000;1(2):190-6. [CrossRef] [PubMed]
- Nguyen DH, Hildreth JE. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74(7):3264-72. [CrossRef] [PubMed]
- Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98(24):13925-30. [CrossRef] [PubMed]
- Turtle L, Solomon T. Japanese encephalitis - the prospects for new treatments. Nat Rev Neurol. 2018;14(5):298-313. [CrossRef] [PubMed]
- Zhu YZ, Xu QQ, Wu DG, Ren H, Zhao P, Lao WG, et al. Japanese encephalitis virus enters rat neuroblastoma cells via a pH-dependent, dynamin and caveola-mediated endocytosis pathway. J Virol. 2012;86(24):13407-22. Epub 20120926. [CrossRef] [PubMed]
- Xu Q, Cao M, Song H, Chen S, Qian X, Zhao P, et al. Caveolin-1-mediated Japanese encephalitis virus entry requires a two-step regulation of actin reorganization. Future Microbiol. 2016;11:1227-48. Epub 20160317. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




