Submitted:
02 April 2025
Posted:
03 April 2025
You are already at the latest version
Abstract
Keywords:
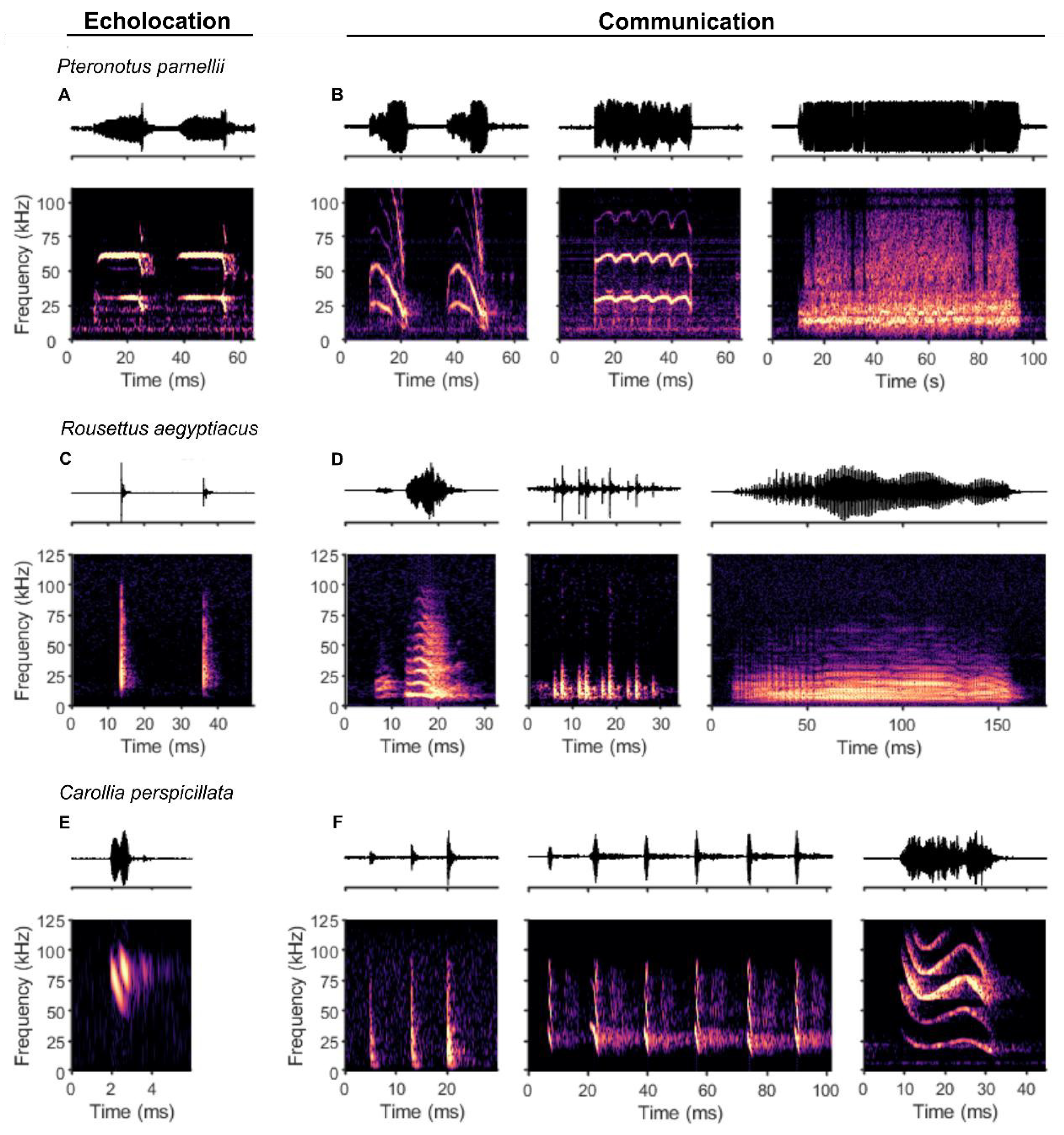
Introduction
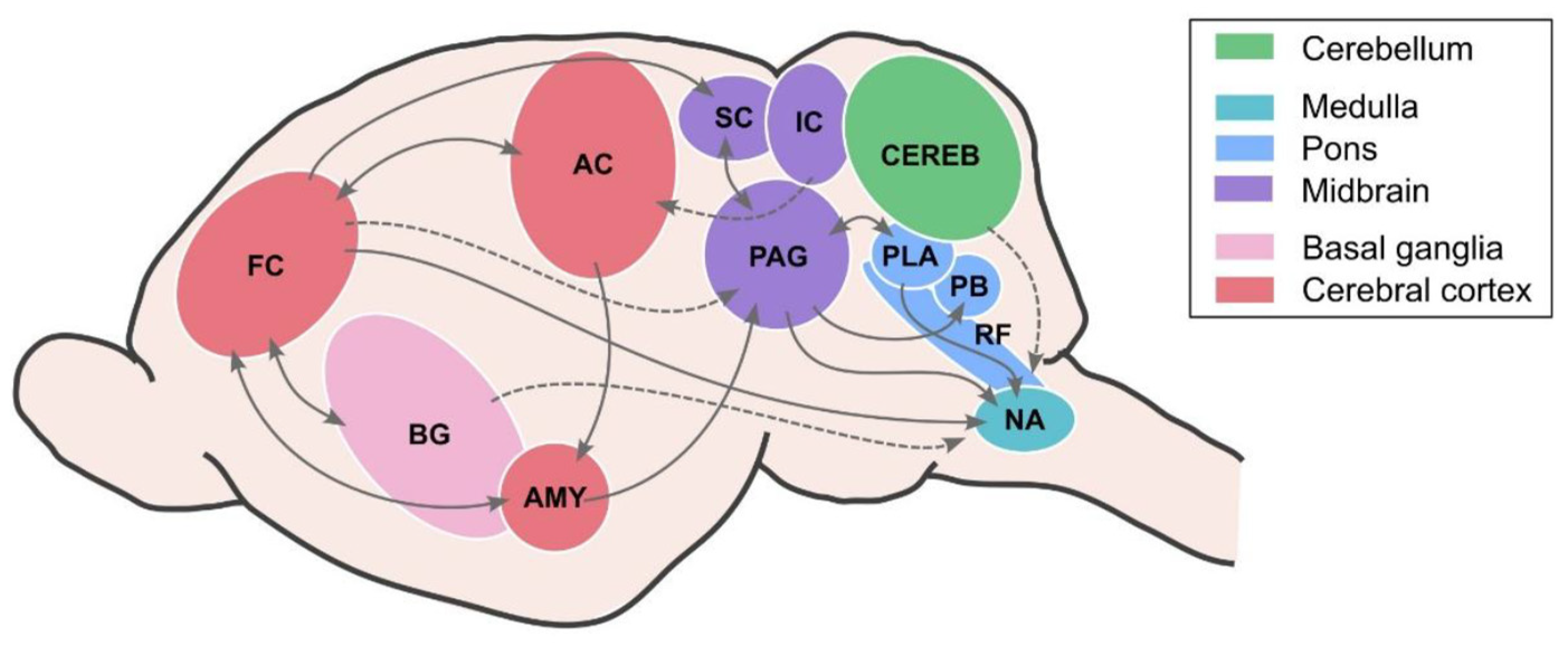
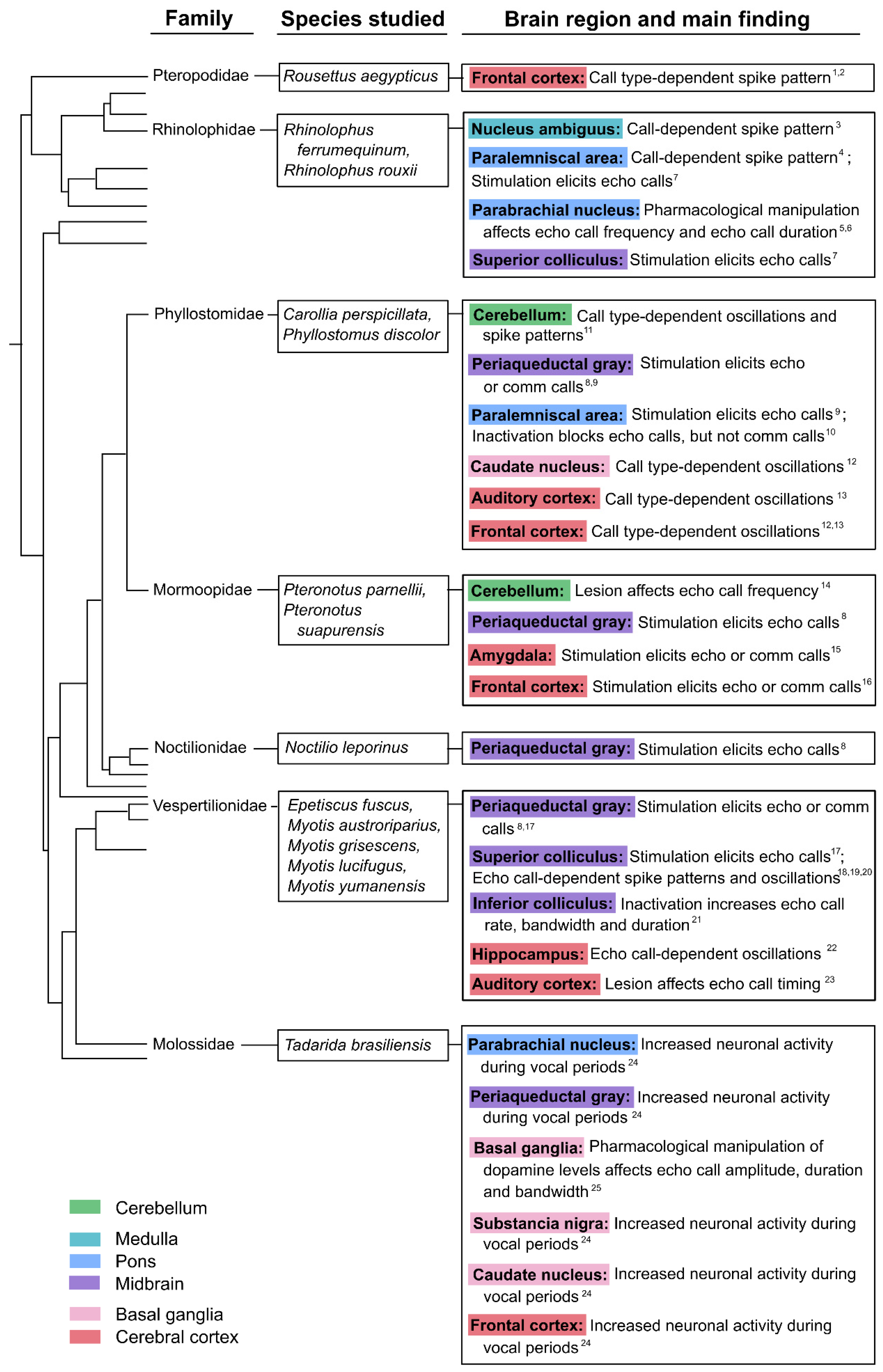
Vocal Control in Brainstem and Midbrain Circuits
I. Laryngeal Output and Its Control in the Medulla
II. The Periaqueductal Gray and Pontine Nuclei
Cerebellar Contributions to Vocal Production
II. Potential Contributions of the Inferior Colliculus
III. Processing of Spatial and Social Information in the Hippocampus
Forebrain Networks in Vocalization
I. Basal Ganglia and Dopaminergic Neurons
II. Production of Emotive Vocalizations via the Amygdala
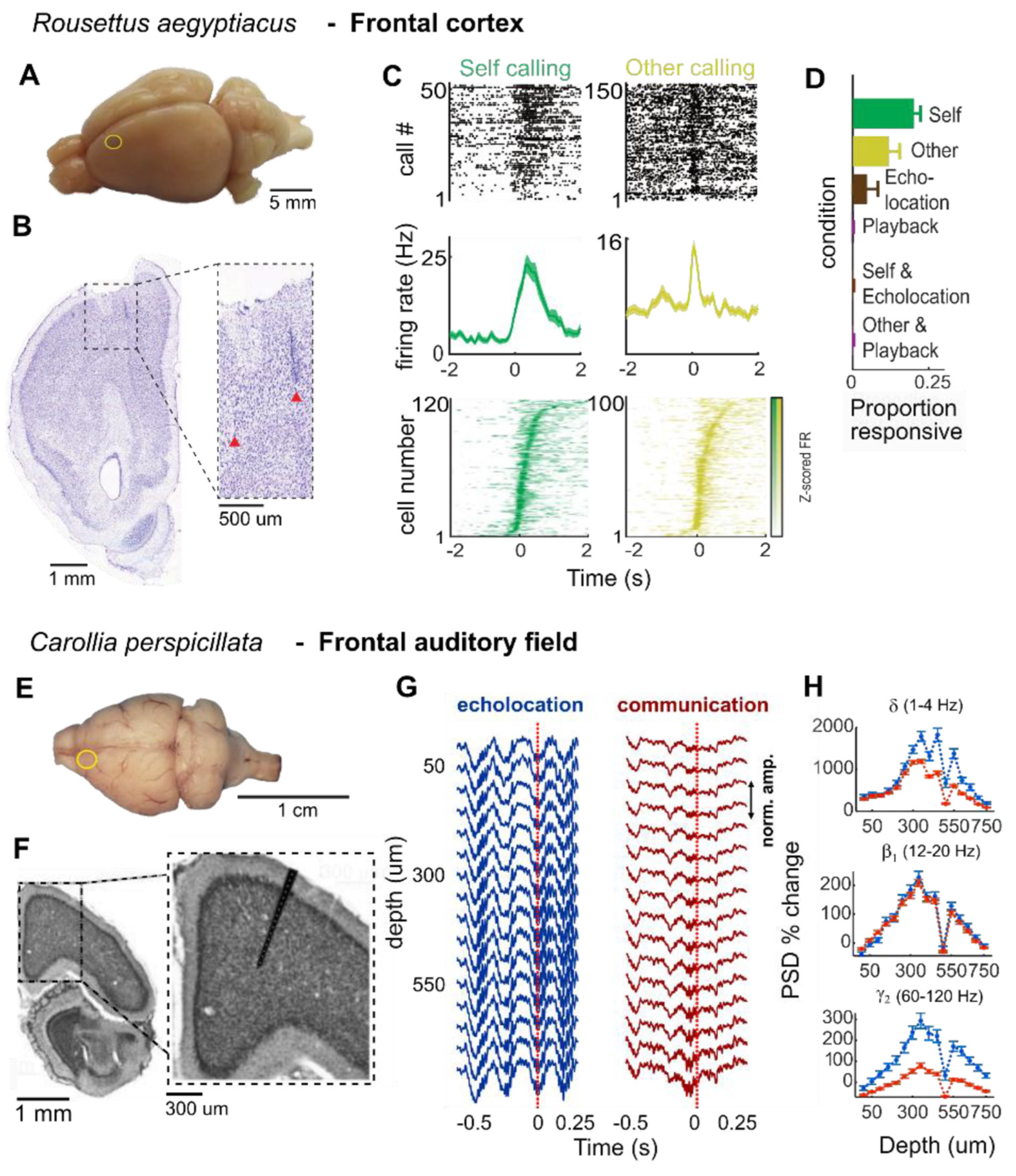
III. Neuronal Activity Preceding Vocal Onset in Frontal Cortical Areas
Concluding Remarks
I. Are echolocation and Communication Calls Controlled Through the Same Vocal Pathways?
II. Limitations and Future Directions
Glossary
Supplementary Materials
Author Contributions
Acknowledgements
References
- Ackermann, H., Mathiak, K., & Riecker, A. (2007). The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum (London, England), 6(3), 202–213. [CrossRef]
- Adhikari, A., Lerner, T. N., Finkelstein, J., Pak, S., Jennings, J. H., Davidson, T. J., Ferenczi, E., Gunaydin, L. A., Mirzabekov, J. J., Ye, L., Kim, S.-Y., Lei, A., & Deisseroth, K. (2015). Basomedial amygdala mediates top-down control of anxiety and fear. Nature, 527(7577), 179–185. [CrossRef]
- Aitken, P. G. (1981). Cortical control of conditioned and spontaneous vocal behavior in rhesus monkeys. Brain and Language, 13(1), 171–184. [CrossRef]
- Beetz, M. J., Hechavarría, J. C., & Kössl, M. (2016). Temporal tuning in the bat auditory cortex is sharper when studied with natural echolocation sequences. Scientific Reports, 6, 29102. [CrossRef]
- Bruns, V., & Schmieszek, E. (1980). Cochlear innervation in the greater horseshoe bat: demonstration of an acoustic fovea. Hearing Research, 3(1), 27–43. [CrossRef]
- Carter, G. G., Fenton, M. B., & Faure, P. A. (2009). White-winged vampire bats (Diaemus youngi) exchange contact calls. Canadian Journal of Zoology, 87(7), 604–608. [CrossRef]
- Carter, R. T. (2020). Reinforcement of the larynx and trachea in echolocating and non-echolocating bats. Journal of Anatomy, 237(3), 495–503. [CrossRef]
- Casseday, J. H., Kobler, J. B., Isbey, S. F., & Covey, E. (1989). Central acoustic tract in an echolocating bat: an extralemniscal auditory pathway to the thalamus. The Journal of Comparative Neurology, 287(2), 247–259. [CrossRef]
- Chamberlin, N. L. (2004). Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respiratory Physiology & Neurobiology, 143(2-3), 115–125. [CrossRef]
- Crapse, T. B., & Sommer, M. A. (2008). Corollary discharge across the animal kingdom. Nature Reviews. Neuroscience, 9(8), 587–600. [CrossRef]
- Dalley, J. W., Cardinal, R. N., & Robbins, T. W. (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews, 28(7), 771–784. [CrossRef]
- Dear, S. P., & Suga, N. (1995). Delay-tuned neurons in the midbrain of the big brown bat. Journal of Neurophysiology, 73(3), 1084–1100. [CrossRef]
- Denzinger, A., Kalko, E. K. V., Tschapka, M., Grinnell, A. D., & Schnitzler, H.-U. (2016). Guild Structure and Niche Differentiation in Echolocating Bats. In Bat Bioacoustics (pp. 141–166). Springer New York. [CrossRef]
- Diebold, C. A., Lawlor, J., Allen, K., Capshaw, G., Humphrey, M. G., Cintron-De Leon, D., Kuchibhotla, K. V., & Moss, C. F. (2024). Rapid sensorimotor adaptation to auditory midbrain silencing in free-flying bats. Current Biology: CB. (21). [CrossRef]
- Eiermann, A., & Esser, K. (2000). Auditory responses from the frontal cortex in the short-tailed fruit bat Carollia perspicillata. Neuroreport, 11(2), 421–425. [CrossRef]
- Ekstrom, A. D., Kahana, M. J., Caplan, J. B., Fields, T. A., Isham, E. A., Newman, E. L., & Fried, I. (2003). Cellular networks underlying human spatial navigation. Nature, 425(6954), 184–187. [CrossRef]
- Elemans, C. P. H., Mead, A. F., Jakobsen, L., & Ratcliffe, J. M. (2011). Superfast muscles set maximum call rate in echolocating bats. Science, 333(6051), 1885–1888. [CrossRef]
- Elie, J. E., Muroy, S. E., Genzel, D., Na, T., Beyer, L. A., Swiderski, D. L., Raphael, Y., & Yartsev, M. M. (2024). Role of auditory feedback for vocal production learning in the Egyptian fruit bat. Current Biology: CB, 34(17), 4062–4070.e7. [CrossRef]
- Esser, K.-H., & Schubert, J. (1998). Vocal Dialects in the Lesser Spear-Nosed Bat Phyllostomus discolor. The Science of Nature, 85(7), 347–349. [CrossRef]
- Euston, D. R., Gruber, A. J., & Mcnaughton, B. L. (2013). The role of medial prefrontal cortex in memory and decision making. Neuron, 76(6), 1057–1070. [CrossRef]
- Fenzl, T., & Schuller, G. (2002). Periaqueductal gray and the region of the paralemniscal area have different functions in the control of vocalization in the neotropical bat, Phyllostomus discolor. The European Journal of Neuroscience, 16(10), 1974–1986. (9). [CrossRef]
- Fenzl, T., & Schuller, G. (2005). Echolocation calls and communication calls are controlled differentially in the brainstem of the bat Phyllostomus discolor. BMC Biology, 3, 17. (1). [CrossRef]
- Fenzl, T., & Schuller, G. (2007). Dissimilarities in the vocal control over communication and echolocation calls in bats. Behavioural Brain Research, 182(2), 173–179. [CrossRef]
- Fernandez, A. A., Burchardt, L. S., Nagy, M., & Knörnschild, M. (2021). Babbling in a vocal learning bat resembles human infant babbling. Science, 373(6557), 923–926. [CrossRef]
- Fernandez, A. A., & Knörnschild, M. (2020). Pup Directed Vocalizations of Adult Females and Males in a Vocal Learning Bat. Frontiers in Ecology and Evolution, 8. [CrossRef]
- Fillinger, C., Yalcin, I., Barrot, M., & Veinante, P. (2017). Afferents to anterior cingulate areas 24a and 24b and midcingulate areas 24a’ and 24b' in the mouse. Brain Structure & Function, 222(3), 1509–1532. [CrossRef]
- Fillinger, C., Yalcin, I., Barrot, M., & Veinante, P. (2018). Efferents of anterior cingulate areas 24a and 24b and midcingulate areas 24a’ and 24b' in the mouse. Brain Structure & Function, 223(4), 1747–1778. [CrossRef]
- Fitzpatrick, D. C., Olsen, J. F., & Suga, N. (1998). Connections among functional areas in the mustached bat auditory cortex. Journal of Comparative Neurology, 391(3), 366–396. [CrossRef]
- Forli, A., & Yartsev, M. M. (2023). Hippocampal representation during collective spatial behaviour in bats. Nature, 621(7980), 796–803. [CrossRef]
- Fries, P. (2009). Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual Review of Neuroscience, 32(1), 209–224. [CrossRef]
- Fuster, J. M. (2015). The Prefrontal Cortex (Fifth Edit, pp. 1–444). Academic Press. [CrossRef]
- Gadziola, M. A., Shanbhag, S. J., & Wenstrup, J. J. (2016). Two distinct representations of social vocalizations in the basolateral amygdala. Journal of Neurophysiology, 115(2), 868–886. [CrossRef]
- Gaioni, S. J., Riquimaroux, H., & Suga, N. (1990). Biosonar behavior of mustached bats swung on a pendulum prior to cortical ablation. Journal of Neurophysiology, 64(6), 1801–1817. [CrossRef]
- García-Rosales, F., López-Jury, L., González-Palomares, E., Wetekam, J., Cabral-Calderín, Y., Kiai, A., Kössl, M., & Hechavarría, J. C. (2022). Echolocation-related reversal of information flow in a cortical vocalization network. Nature Communications, 13(1), 3642. (13). [CrossRef]
- Gemba, H., Miki, N., & Sasaki, K. (1995). Cortical field potentials preceding vocalization and influences of cerebellar hemispherectomy upon them in monkeys. Brain Research, 697(1-2), 143–151. [CrossRef]
- Gooler, D. M., & O’Neill, W. E. (1987). Topographic representation of vocal frequency demonstrated by microstimulation of anterior cingulate cortex in the echolocating bat, Pteronotus parnelli parnelli. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 161(2), 283–294. (16). [CrossRef]
- Hagemann, C., Esser, K.-H., & Kössl, M. (2010). Chronotopically organized target-distance map in the auditory cortex of the short-tailed fruit bat. Journal of Neurophysiology, 103(1), 322–333. [CrossRef]
- Hage, S. R. (2010). Neuronal networks involved in the generation of vocalization. In S. M. Brudzynski (Ed.), Handbook of Behavioral Neuroscience (Vol. 19, pp. 339–349). Elsevier. [CrossRef]
- Håkansson, J., Mikkelsen, C., Jakobsen, L., & Elemans, C. P. H. (2022). Bats expand their vocal range by recruiting different laryngeal structures for echolocation and social communication. PLoS Biology, 20(11), e3001881. [CrossRef]
- Halley, A. C., Baldwin, M. K. L., Cooke, D. F., Englund, M., Pineda, C. R., Schmid, T., Yartsev, M. M., & Krubitzer, L. (2022). Coevolution of motor cortex and behavioral specializations associated with flight and echolocation in bats. Current Biology: CB, 32(13), 2935–2941.e3. [CrossRef]
- Hariharan, S., Palomares, E. G., Babl, S. S., López-Jury, L., & Hechavarria, J. C. (2024). Cerebellar activity predicts vocalization in fruit bats. Current Biology: CB, 0(0). (11). [CrossRef]
- Hartridge, H. (1920). The avoidance of objects by bats in their flight. The Journal of Physiology, 54(1-2), 54–57. [CrossRef]
- Hasselmo, M. E. (2005). What is the function of hippocampal theta rhythm? - Linking bahavioral data to phasic properties of field potential and unit recording data. Hippocampus, 15(7), 936–949. [CrossRef]
- Hechavarría, J. C., & Kössl, M. (2014). Footprints of inhibition in the response of cortical delay-tuned neurons of bats. Journal of Neurophysiology, 111(8), 1703–1716. [CrossRef]
- Hechavarría, J. C., Macías, S., Vater, M., Voss, C., Mora, E. C., & Kössl, M. (2013). Blurry topography for precise target-distance computations in the auditory cortex of echolocating bats. Nature Communications, 4(1), 2587. [CrossRef]
- Hiryu, S., Mora, E. C., & Riquimaroux, H. (2016). Behavioral and physiological bases for Doppler shift compensation by echolocating bats. In Bat Bioacoustics (pp. 239–263). Springer New York. [CrossRef]
- Hitti, F. L., & Siegelbaum, S. A. (2014). The hippocampal CA2 region is essential for social memory. Nature, 508(7494), 88–92. [CrossRef]
- Hoffmann, S., Vega-Zuniga, T., Greiter, W., Krabichler, Q., Bley, A., Matthes, M., Zimmer, C., Firzlaff, U., & Luksch, H. (2016). Congruent representation of visual and acoustic space in the superior colliculus of the echolocating bat Phyllostomus discolor. The European Journal of Neuroscience, 44(9), 2685–2697. [CrossRef]
- Hong, W., Kim, D.-W., & Anderson, D. J. (2014). Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell, 158(6), 1348–1361. [CrossRef]
- Horikawa, J., & Suga, N. (1986). Biosonar signals and cerebellar auditory neurons of the mustached bat. Journal of Neurophysiology, 55(6), 1247–1267. (14). [CrossRef]
- Hoy, J. L., Bishop, H. I., & Niell, C. M. (2019). Defined Cell Types in Superior Colliculus Make Distinct Contributions to Prey Capture Behavior in the Mouse. Current Biology: CB, 29(23), 4130–4138.e5. [CrossRef]
- Jahelková, H., Horáček, I., & Bartonička, T. (2008). The advertisement song of Pipistrellus nathusii (Chiroptera, Vespertilionidae): a complex message containing acoustic signatures of individuals. [CrossRef]
- Janak, P. H., & Tye, K. M. (2015). From circuits to behaviour in the amygdala. Nature, 517(7534), 284–292. [CrossRef]
- Jones, G., & Teeling, E. C. (2006). The evolution of echolocation in bats. Trends in Ecology & Evolution, 21(3), 149–156. [CrossRef]
- Jürgens, U. (2002). Neural pathways underlying vocal control. Neuroscience and Biobehavioral Reviews, 26(2), 235–258. [CrossRef]
- Jürgens, U. (2009). The neural control of vocalization in mammals: a review. Journal of Voice: Official Journal of the Voice Foundation, 23(1), 1–10. [CrossRef]
- Kanwal, J. S., Gordon, M., Peng, J. P., & Heinz-Esser, K. (2000). Auditory responses from the frontal cortex in the mustached bat, Pteronotus parnellii. Neuroreport, 11(2), 367–372. [CrossRef]
- Knörnschild, M. (2014). Vocal production learning in bats. Current Opinion in Neurobiology, 28, 80–85. [CrossRef]
- Knörnschild, M., Feifel, M., & Kalko, E. K. V. (2014). Male courtship displays and vocal communication in the polygynous bat Carollia perspicillata. Behaviour, 151(6), 781–798. [CrossRef]
- Knörnschild, M., Nagy, M., Metz, M., Mayer, F., & von Helversen, O. (2010). Complex vocal imitation during ontogeny in a bat. Biology Letters, 6(2), 156–159. [CrossRef]
- Knörnschild, M., Nagy, M., Metz, M., Mayer, F., & von Helversen, O. (2012). Learned vocal group signatures in the polygynous bat Saccopteryx bilineata. Animal Behaviour, 84(4), 761–769. [CrossRef]
- Kobler, J. B., Isbey, S. F., & Casseday, J. H. (1987). Auditory pathways to the frontal cortex of the mustache bat, Pteronotus parnellii. Science (New York, N.Y.), 236(4803), 824–826. [CrossRef]
- Kohles, J. E., Carter, G. G., Page, R. A., & Dechmann, D. K. N. (2020). Socially foraging bats discriminate between group members based on search-phase echolocation calls. Behavioral Ecology: Official Journal of the International Society for Behavioral Ecology. [CrossRef]
- Kössl, M., Hechavarria, J. C., Voss, C., Macias, S., Mora, E. C., & Vater, M. (2014). Neural maps for target range in the auditory cortex of echolocating bats. Current Opinion in Neurobiology, 24(1), 68–75. [CrossRef]
- Kössl, M., & Vater, M. (1985). The cochlear frequency map of the mustache bat, Pteronotus parnellii. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 157(5), 687–697. [CrossRef]
- Kössl, M., Voss, C., Mora, E. C., Macias, S., Foeller, E., & Vater, M. (2012). Auditory cortex of newborn bats is prewired for echolocation. Nature Communications, 3(1), 773. [CrossRef]
- Kothari, N. B., Wohlgemuth, M. J., & Moss, C. F. (2018). Dynamic representation of 3D auditory space in the midbrain of the free-flying echolocating bat. eLife, 7. (20). [CrossRef]
- Krauzlis, R. J., Lovejoy, L. P., & Zénon, A. (2013). Superior colliculus and visual spatial attention. Annual Review of Neuroscience, 36(Volume 36, 2013), 165–182. [CrossRef]
- Lattenkamp, E. Z., & Vernes, S. C. (2018). Vocal learning: a language-relevant trait in need of a broad cross-species approach. Current Opinion in Behavioral Sciences, 21, 209–215. [CrossRef]
- Liu, Z., Chen, P., Xu, D.-M., Qi, F.-Y., Guo, Y.-T., Liu, Q., Bai, J., Zhou, X., & Shi, P. (2022). Molecular convergence and transgenic evidence suggest a single origin of laryngeal echolocation in bats. iScience, 25(4), 104114. [CrossRef]
- López-Jury, L., Mannel, A., García-Rosales, F., & Hechavarria, J. C. (2019). Modified synaptic dynamics predict neural activity patterns in an auditory field within the frontal cortex. The European Journal of Neuroscience, 51(4), 1011–1025. [CrossRef]
- Macias, S., Bakshi, K., Troyer, T., & Smotherman, M. (2022). The prefrontal cortex of the Mexican free-tailed bat is more selective to communication calls than primary auditory cortex. Journal of Neurophysiology, 128(3), 634–648. [CrossRef]
- Macías, S., Luo, J., & Moss, C. F. (2018). Natural echolocation sequences evoke echo-delay selectivity in the auditory midbrain of the FM bat, Eptesicus fuscus. Journal of Neurophysiology, 120(3), 1323–1339. [CrossRef]
- Macías, S., Mora, E. C., Hechavarría, J. C., & Kössl, M. (2012). Properties of echo delay-tuning receptive fields in the inferior colliculus of the mustached bat. Hearing Research, 286(1-2), 1–8. [CrossRef]
- Ma, J., & Kanwal, J. S. (2014). Stimulation of the basal and central amygdala in the mustached bat triggers echolocation and agonistic vocalizations within multimodal output. Frontiers in Physiology, 5, 55. (15). [CrossRef]
- Metzner, W. (1989). A possible neuronal basis for Doppler-shift compensation in echo-locating horseshoe bats. Nature, 341(6242), 529–532. [CrossRef]
- Metzner, W. (1993). An audio-vocal interface in echolocating horseshoe bats. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 13(5), 1899–1915. (4). [CrossRef]
- Monsen, R. B., Engebretson, A. M., & Vemula, N. R. (1979). Some effects of deafness on the generation of voice. The Journal of the Acoustical Society of America, 66(6), 1680–1690. [CrossRef]
- Moss, C. F., Bohn, K., Gilkenson, H., & Surlykke, A. (2006). Active listening for spatial orientation in a complex auditory scene. PLoS Biology, 4(4), e79. [CrossRef]
- Moss, C. F., & Surlykke, A. (2001). Auditory scene analysis by echolocation in bats. The Journal of the Acoustical Society of America, 110(4), 2207–2226. [CrossRef]
- Naumann, R. T., & Kanwal, J. S. (2011). Basolateral amygdala responds robustly to social calls: spiking characteristics of single unit activity. Journal of Neurophysiology, 105(5), 2389–2404. [CrossRef]
- Neuweiler, G. (1980). Auditory processing of echoes: Peripheral processing. In Animal Sonar Systems (pp. 519–548). Springer US. [CrossRef]
- Nevue, A. A., Mello, C. V., & Portfors, C. V. (2023). Bats possess the anatomical substrate for a laryngeal motor cortex. bioRxiv.org: The Preprint Server for Biology. [CrossRef]
- Nieder, A., & Mooney, R. (2020). The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 375(1789), 20190054. [CrossRef]
- O’Keefe, J., & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34, 171–175. https://www.ncbi.nlm.nih.gov/pubmed/5124915.
- Okobi, D. E., Jr, Banerjee, A., Matheson, A. M. M., Phelps, S. M., & Long, M. A. (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science (New York, N.Y.), 363(6430), 983–988. [CrossRef]
- Oliva, A., Fernández-Ruiz, A., Leroy, F., & Siegelbaum, S. A. (2020). Hippocampal CA2 sharp-wave ripples reactivate and promote social memory. Nature, 587(7833), 264–269. [CrossRef]
- Omer, D. B., Maimon, S. R., Las, L., & Ulanovsky, N. (2018). Social place-cells in the bat hippocampus. Science (New York, N.Y.), 359(6372), 218–224. [CrossRef]
- O’Neill, W. E. (1995). The Bat Auditory Cortex. In Hearing by Bats (pp. 416–480). Springer New York. [CrossRef]
- O’Neill, W. E., & Suga, N. (1979). Target range-sensitive neurons in the auditory cortex of the mustache bat. Science (New York, N.Y.), 203(4375), 69–73. [CrossRef]
- Ono, T., Nakamura, K., Nishijo, H., & Eifuku, S. (1993). Monkey hippocampal neurons related to spatial and nonspatial functions. Journal of Neurophysiology, 70(4), 1516–1529. [CrossRef]
- Payne, H. L., Lynch, G. F., & Aronov, D. (2021). Neural representations of space in the hippocampus of a food-caching bird. Science, 373(6552), 343–348. [CrossRef]
- Poulet, J. F. A., & Hedwig, B. (2002). A corollary discharge maintains auditory sensitivity during sound production. Nature, 418(6900), 872–876. [CrossRef]
- Prat, Y., Taub, M., Pratt, E., & Yovel, Y. (2017). An annotated dataset of Egyptian fruit bat vocalizations across varying contexts and during vocal ontogeny. Figshare. [CrossRef]
- Rodenas-Cuadrado, P. M., Mengede, J., Baas, L., Devanna, P., Schmid, T. A., Yartsev, M., Firzlaff, U., & Vernes, S. C. (2017). Mapping the distribution of language related genes FoxP1, FoxP2, and CntnaP2 in the brains of vocal learning bat species. The Journal of Comparative Neurology, 526(8), 1235–1266. [CrossRef]
- Rolls, E. T., & O’Mara, S. M. (1995). View-responsive neurons in the primate hippocampal complex. Hippocampus, 5(5), 409–424. [CrossRef]
- Rose, J. E., & Woolsey, C. N. (1949). Organization of the mammalian thalamus and its relationships to the cerebral cortex. Electroencephalography and Clinical Neurophysiology, 1(1-4), 391–404. [CrossRef]
- Rose, M. C., Styr, B., Schmid, T. A., Elie, J. E., & Yartsev, M. M. (2021). Cortical representation of group social communication in bats. Science (New York, N.Y.), 374(6566), eaba9584. (1). [CrossRef]
- Rübsamen, R., & Betz, M. (1986). Control of echolocation pulses by neurons of the nucleus ambiguus in the rufous horseshoe bat, Rhinolophus rouxi. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology, 159(5), 675–687. (3). [CrossRef]
- Rübsamen, R., Neuweiler, G., & Sripathi, K. (1988). Comparative collicular tonotopy in two bat species adapted to movement detection,Hipposideros speoris andMegaderma lyra. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 163(2), 271–285. [CrossRef]
- Salles, A., Loscalzo, E., Montoya, J., Mendoza, R., Boergens, K. M., & Moss, C. F. (2024). Auditory processing of communication calls in interacting bats. iScience, 27(6), 109872. [CrossRef]
- Schnitzler, H.-U. (1968). Die Ultraschall-Ortungslaute der Hufeisen-Fledermäuse (Chiroptera-Rhinolophidae) in verschiedenen Orientierungssituationen. Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology, 57(4), 376–408. [CrossRef]
- Schnitzler, H.-U., & Denzinger, A. (2011). Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 197(5), 541–559. [CrossRef]
- Schuller, G., Fischer, S., & Schweizer, H. (1997). Significance of the paralemniscal tegmental area for audio-motor control in the moustached bat,Pteronotus p. Parnellii: The afferent and efferent connections of the paralemniscal area. The European Journal of Neuroscience, 9(2), 342–355. [CrossRef]
- Schuller, G., & Pollak, G. (1979). Disproportionate frequency representation in the inferior colliculus of doppler-compensating Greater Horseshoe bats: Evidence for an acoustic fovea. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 132(1), 47–54. [CrossRef]
- Schuller, G., & Radtke-Schuller, S. (1990). Neural control of vocalization in bats: mapping of brainstem areas with electrical microstimulation eliciting species-specific echolocation calls in the rufous horseshoe bat. Experimental Brain Research, 79(1), 192–206. (7). [CrossRef]
- Schwartz, C. P., & Smotherman, M. S. (2011). Mapping vocalization-related immediate early gene expression in echolocating bats. Behavioural Brain Research, 224(2), 358–368. (24). [CrossRef]
- Sinha, S. R., & Moss, C. F. (2007). Vocal premotor activity in the superior colliculus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(1), 98–110. (18). [CrossRef]
- Smotherman, M., Knörnschild, M., Smarsh, G., & Bohn, K. (2016). The origins and diversity of bat songs. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 202(8), 535–554. [CrossRef]
- Smotherman, M., Kobayasi, K., Ma, J., Zhang, S., & Metzner, W. (2006). A mechanism for vocal-respiratory coupling in the mammalian parabrachial nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 26(18), 4860–4869. (6). [CrossRef]
- Smotherman, M., & Metzner, W. (2003). Fine control of call frequency by horseshoe bats. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 189(6), 435–446. (5). [CrossRef]
- Smotherman, M., Zhang, S., & Metzner, W. (2003). A neural basis for auditory feedback control of vocal pitch. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(4), 1464–1477. [CrossRef]
- Snyder, M. C., Qi, K. K., & Yartsev, M. M. (2024). Neural representation of human experimenters in the bat hippocampus. Nature Neuroscience, 1–5. [CrossRef]
- Suga, N. (1969a). Echo-location and evoked potentials of bats after ablation of inferior colliculus. The Journal of Physiology, 203(3), 707–728. [CrossRef]
- Suga, N. (1969b). Echo-location of bats after ablation of auditory cortex. The Journal of Physiology, 203(3), 729–739. (23). [CrossRef]
- Suga, N., Neuweiler, G., & Müller, J. (1976). Peripheral auditory tuning for fine frequency analysis by the CF-FM bat,Rhinolophus ferrumequinum: IV. Properties of peripheral auditory neurons. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 106(1), 111–125. [CrossRef]
- Suga, N., & O’Neill, W. E. (1979). Neural axis representing target range in the auditory cortex of the mustache bat. Science (New York, N.Y.), 206(4416), 351–353. [CrossRef]
- Suga, N., & Schlegel, P. (1972). Neural attenuation of responses to emitted sounds in echolocating bats. Science (New York, N.Y.), 177(4043), 82–84. [CrossRef]
- Suga, N., Schlegel, P., Shimozawa, T., & Simmons, J. (1973). Orientation sounds evoked from echolocating bats by electrical stimulation of the brain. The Journal of the Acoustical Society of America, 54(3), 793–797. (8). [CrossRef]
- Suga, N., & Shimozawa, T. (1974). Site of neural attenuation of responses to self-vocalized sounds in echolocating bats. Science (New York, N.Y.), 183(130), 1211–1213. [CrossRef]
- Suthers, R. A., Thomas, S. P., & Suthers, B. J. (1972). Respiration, wing-beat and ultrasonic pulse emission in an echo-locating bat. The Journal of Experimental Biology, 56(1), 37–48. [CrossRef]
- Tanaka, M., Kunimatsu, J., Suzuki, T. W., Kameda, M., Ohmae, S., Uematsu, A., & Takeya, R. (2021). Roles of the cerebellum in motor preparation and prediction of timing. Neuroscience, 462, 220–234. [CrossRef]
- Teeling, E. C., Dool, S., & Springer, M. S. (2012). Phylogenies, fossils and functional genes: the evolution of echolocation in bats. In G. F. Gunnell & N. B. Simmons (Eds.), Evolutionary History of Bats (pp. 1–22). Cambridge University Press. [CrossRef]
- Tressler, J., Schwartz, C., Wellman, P., Hughes, S., & Smotherman, M. (2011). Regulation of bat echolocation pulse acoustics by striatal dopamine. The Journal of Experimental Biology, 214(Pt 19), 3238–3247. (25). [CrossRef]
- Tschida, K., Michael, V., Takatoh, J., Han, B.-X., Zhao, S., Sakurai, K., Mooney, R., & Wang, F. (2019). A specialized neural circuit gates social vocalizations in the mouse. Neuron, 103(3), 459–472.e4. [CrossRef]
- Ulanovsky, N., & Moss, C. F. (2007). Hippocampal cellular and network activity in freely moving echolocating bats. Nature Neuroscience, 10(2), 224–233. (22). [CrossRef]
- Ulanovsky, N., & Moss, C. F. (2011). Dynamics of hippocampal spatial representation in echolocating bats. Hippocampus, 21(2), 150–161. [CrossRef]
- Uylings, H. B. M., Groenewegen, H. J., & Kolb, B. (2003). Do rats have a prefrontal cortex? Behavioural Brain Research, 146(1-2), 3–17. [CrossRef]
- Valentine, D. E., & Moss, C. F. (1997). Spatially selective auditory responses in the superior colliculus of the echolocating bat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 17(5), 1720–1733. [CrossRef]
- Valentine, D. E., Sinha, S. R., & Moss, C. F. (2002). Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology, 188(2), 89–108. (17). [CrossRef]
- van Tussenbroek, I. A., Knörnschild, M., Nagy, M., ten Cate, C. J., & Vernes, S. C. (2024). Morphological diversity in the brains of 12 neotropical bat species. Acta Chiropterologica, 25(2). [CrossRef]
- Vernes, S. C., Devanna, P., Hörpel, S. G., Alvarez van Tussenbroek, I., Firzlaff, U., Hagoort, P., Hiller, M., Hoeksema, N., Hughes, G. M., Lavrichenko, K., Mengede, J., Morales, A. E., & Wiesmann, M. (2022). The pale spear-nosed bat: A neuromolecular and transgenic model for vocal learning. Annals of the New York Academy of Sciences, 1517(1), 125–142. [CrossRef]
- Vernes, S. C., & Wilkinson, G. S. (2019). Behaviour, biology and evolution of vocal learning in bats. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 375(1789), 20190061. [CrossRef]
- Wang, Z., Zhu, T., Xue, H., Fang, N., Zhang, J., Zhang, L., Pang, J., Teeling, E. C., & Zhang, S. (2017). Prenatal development supports a single origin of laryngeal echolocation in bats. Nature Ecology & Evolution, 1(2), 21. [CrossRef]
- Weineck, K., García-Rosales, F., & Hechavarría, J. C. (2020). Neural oscillations in the fronto-striatal network predict vocal output in bats. PLoS Biology, 18(3), e3000658. (12). [CrossRef]
- Wirthlin, M. E., Schmid, T. A., Elie, J. E., Zhang, X., Kowalczyk, A., Redlich, R., Shvareva, V. A., Rakuljic, A., Ji, M. B., Bhat, N. S., Kaplow, I. M., Schäffer, D. E., Lawler, A. J., Wang, A. Z., Phan, B. N., Annaldasula, S., Brown, A. R., Lu, T., Lim, B. K., … Zhang, X. (2024). Vocal learning-associated convergent evolution in mammalian proteins and regulatory elements. Science, 383(6690), eabn3263. (2). [CrossRef]
- Wohlgemuth, M. J., Kothari, N. B., & Moss, C. F. (2018). Functional organization and dynamic activity in the superior colliculus of the echolocating bat, Eptesicus fuscus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 38(1), 245–256. (19). [CrossRef]
- Wohlgemuth, M. J., Yu, C., & Moss, C. F. (2018). 3D hippocampal place field dynamics in free-flying echolocating bats. Frontiers in Cellular Neuroscience, 12, 270. [CrossRef]
- Yartsev, M. M., Witter, M. P., & Ulanovsky, N. (2011). Grid cells without theta oscillations in the entorhinal cortex of bats. Nature, 479(7371), 103–107. [CrossRef]
- Yin, J.-X., Ruan, Y.-N., Liu, J.-L., Zhang, S.-Y., & Racey, P. (2017). FoxP2 expression in an echolocating bat (Rhinolophus ferrumequinum): Functional implications. Zeitschrift Für Saugetierkunde [Mammalian Biology], 85(1), 24–29. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




