Results
Considerations of the board regarding the PLLA-LASYNPRO™ rationale, identification and assessment of candidate subjects, and communication (Section 1 of the survey).
All board experts agreed with the first proposed statement (cf. Supplementary File S1, Statement 1):
“Since the introduction of the first conventional poly-l-lactic acid formulation, a variably
severe inflammatory foreign-body reaction has been the cornerstone of
exogenously induced neocollagenesis by injectable collagen stimulators. The
new goal is to abandon the reactive inflammation-ignited neocollagenesis
paradigm for a novel non-inflammatory paradigm based on a physiological
cascade of events.”
Before the Milan meeting, ten of twelve experts had agreed with the second proposed statement in its original form (cf. Supplementary File S1, Statement 2). After a brief discussion at the board meeting, all twelve voting experts accepted the statement revised as follows:
The “PLLA-LASYNPRO™ microspheres can be considered an
innovative treatment in regenerative aesthetic medicine. After injection,
they act as unique deep bio-regenerators that should not be confused with
traditional fillers whose purpose is to fill hollow spaces transitorily.”
Eleven voting experts had concurred before the meeting on the third proposed statement in its original wording (cf. Supplementary File S1, Statement 3). All experts reached a consensus that the ideal candidate for the new PLLA technology is a subject who complains of degraded skin quality and seeks a rejuvenated appearance:
“It is essential to evaluate whether the patient
requires immediate or gradual results. A patient pursuing instant
gratification may not be an ideal candidate. Patients desiring a rejuvenated
appearance with lasting results are more appropriate candidates for
PLLA-LASYNPRO™ treatment with the JULÄINE medical device.”
Before the meeting, only seven voting experts agreed to the fourth proposed statement in its original wording—35 to 65 years is the ideal age range for candidates to the new PLLA technology (cf. Supplementary File S1, Statement 4). Four experts agreed partially. After discussing the issue at the board meeting, the twelve voting experts stated that subjects of all ages report benefits based on their experiences with the ongoing clinical studies they coordinate. Consequently, the twelve voting experts concurred with the statement reformulated as follows:
“Since adult subjects of all ages report benefits from
PLLA-LASYNPRO™ and the JULÄINE medical device, the appropriate age for
treatment is at least eighteen.”
The fifth survey item addressing the ideal candidates for treatment with the novel PLLA microspheres, or the likely “best responders,” was not articulated in a single statement (
cf. Supplementary File S1, Statement 5, which outlines multiple profiles of candidate subjects and proposals across two slides). All board members, except for one, agreed with all proposed statements. For the profiles of candidate subjects, a severity score of two or three on a four-point scale indicated a potential candidate, reflecting moderate to advanced severity of skin aging and laxity and impairment of skin quality [
3], along with mild to moderate loss of skin thickness and firmness (
Table 1). The amended fourth statement had already addressed the issue of age.
There was only one exception to universal agreement: the experts agreed to replace all references to “volume loss” with “mild to moderate loss of thickness and firmness.” This change aimed to prevent confusion between the new-technology PLLA microspheres and volume-repleting fillers. For instance, the sixth statement(cf. Supplementary File S1, Statement 5, first slide) was revised to read: “Faces objectively demonstrate measurable mild to moderate firmness and thickness loss of the skin over a selected area or the entire face (pinch test), slight to moderate laxity of soft tissues, and poor skin quality.”
All board experts concurred with Supplementary File S1, Statement 6 about the importance of ensuring that the subject has reasonable expectations:
“Effective communication between physicians and patients
is crucial for setting realistic treatment goals. The gradual effects of the
PLLA-LASYNPRO™ microspheres should be clearly explained to patients, who need
to be informed about the aging process and how responses to treatment vary
according to individual profiles.”
Along with supporting the statement in Supplementary File S1, Statement 7, the experts noted that the skin pinch test may be particularly valuable due to its ease of performance and ability to offer a qualitative assessment of skin firmness recovery.
“Some procedures can help demonstrate objective and
quantitative changes during the PLLA-LASYNPRO™ treatment. Patient assessments
may include standard photography (considering background, posture, lighting,
and facial expression), skin analysis imaging systems (e.g., VISIA and
Antera3D®, among others), and the skin pinch test.”
Providing comprehensive information about the new-technology PLLA ingredient and JULÄINE is essential. It is equally important to ensure that the candidate subject understands all details (cf. Supplementary File S1, Statement 8):
“After a preliminary investigation of the medical
history (including skin condition, medications, allergies, prior procedures,
etc.), have the subject sign a thorough informed consent form and provide
complete information about the PLLA-LASYNPRO™ microspheres of the JULÄINE
medical device.”
Considerations of the board regarding the anticipated results with PLLA-LASYNPRO™, contra-indications, and reconstitution (Section 2 of the survey)
Drawing from their preliminary experience with the clinical studies they coordinate, the twelve voting experts observe early benefits in all four dimensions contributing to skin quality—skin firmness, skin tone evenness, skin surface evenness, and skin glow [
3]. However, given the early stage of the new-technology PLLA microspheres clinical research, they prefer to wait for more long-term follow-up data before expressing their views on how long the early benefits may persist or evolve (
cf. Supplementary File S1, Statements 9 and 10). Despite this caution, the experts agreed that a third injection session may not be required, particularly for younger subjects. They recommended revising the proposed Statement 9 into a confident yet less conclusive version:
“Improvement in skin quality is typically noticeable
within one to three months.”
In contrast, there were no changes to Supplementary File S1, Statement 11:
“The PLLA-LASYNPRO™ contraindications and precautions
stated in the approved Instructions For Use internal leaflet are similar to
those of any aesthetic treatment and include acute or chronic skin conditions
(such as infections or inflammations), a history of allergies to ingredients,
age under 18 years, pregnancy or breastfeeding, a history of bleeding
disorders or current anticoagulant therapy, a known tendency to keloids or
hypertrophic scarring, and a history of immune deficiencies.”
The experts achieved unanimous agreement on the procedures for product reconstitution as stated in Supplementary File S1, Statement 12:
“Remove the flip-off cap from the vial and clean the
butyl stopper with an antiseptic. Dissolve the lyophilized powder with 5 mL
of sodium chloride solution (0.9%, Ph. Eur.) using a sterile, single-use 5 mL
syringe with a sterile 18-gauge needle inserted through the butyl stopper.
Rotate the vial for one minute until the microsphere suspension appears
translucent and homogeneous. Reconstitution is generally complete within one
minute without agglomeration. The vial can be used immediately after reconstitution.
Reconstituted JULÄINE can be stored at room temperature (18-25°C) for up to
72 hours. Do not freeze.”
Considerations of the board regarding the injection device and technique, treatment plan, and safety (Section 3 of the survey)
After discussing it at the Milan meeting, the board modified the proposed statements 13, 14, and 15, emphasizing the standard first option of using a 25-gauge, 38-mm, or 50-mm cannula with a retrograde fanning technique. Although a less precise 22-gauge cannula may help decolletage of tissue layers and reduce trauma, it requires advanced manual skills with fluid suspensions (cf. Supplementary File S1, Statements 13 to 15).
“Use a fresh, sterile 18-gauge needle to draw an
adequate volume of the PLLA-LASYNPRO™ suspension into a one mL or three mL
sterile syringe, then switch to a sterile 25-gauge cannula (38 mm or 50 mm,
at the operator’s discretion) as the preferred injection device using a
retrograde fanning technique. A 22-gauge cannula may be suitable in expert
hands to achieve tissue layer separation with minimal trauma. A linear
injection technique with a sterile 13 mm, 26-gauge, or 30-gauge needle may
also be considered.”
Although the experts agreed that cannulas are always ideal for injecting the novel next-generation microspheres, injections using 26-gauge or 32-gauge needles are possible (cf. Supplementary File S1, Statements 16). After some discussions at the Milan meeting, all experts agreed to revise the statement as follows:
“While injecting PLLA-LASYNPRO™ through a cannula is the
preferred method in all situations, using 13-mm 26-gauge or 30-gauge needles
with a linear injection technique is also feasible. In every instance, the
injection target should be the superficial subcutaneous layer just beneath
the dermis.”
So far, no subject has experienced significant pain in the ongoing clinical research program. Given these considerations, the board unanimously agreed to the following slightly revised statement of Supplementary File S1, Statement 17:
“Given the mild discomfort associated with the
PLLA-LASYNPRO™ treatment, there is no need to add lidocaine to the suspension
during the first and later sessions.”
All board experts agreed on the proposed considerations regarding the treatment plan detailed in Supplementary File S1, Statement 18:
“A PLLA-LASYNPRO™ treatment plan with the JULÄINE
medical device can entail up to three injection sessions per patient. The
recommended dose of reconstituted JULÄINE is 5 mL (one vial) for each
treatment session per face (2.5 mL for half a face). Injections can be
repeated every 6 to 8 weeks.”
After discussing the issue at the Milan meeting, the experts unanimously identified the superficial subcutaneous layer of the skin as the target for injection, revising the surveyed statement (cf. Supplementary File S1, Statement 19). The microspheres can rapidly diffuse from this superficial subcutaneous layer into the deeper dermis and underlying subcutaneous layers.
“The PLLA-LASYNPRO™ microspheres of the JULÄINE medical
device should be injected into the superficial subcutaneous layer of the skin
immediately below the dermis at a tangential angle to the epidermis.”
Regarding the indications of the novel medical device based on the new-technology PLLA ingredient (see Supplementary File S1, Statement 20), the experts concurred with the proposed modifications, shifting the focus from the initially suggested wording to the effectiveness of PLLA-LASYNPRO™ microspheres in enhancing skin thickness, thereby establishing it as a primary objective:
“The PLLA-LASYNPRO™ medical device for facial treatment
effectively addresses aging concerns. Its main goals are to enhance skin
thickness, provide a tightening effect (reducing skin laxity and boosting
elasticity), and improve overall skin quality in its four articulations: skin
radiance, skin tone, smooth texture, and firmness. PLLA-LASYNPRO™ subdermal
implants are also beneficial for treating skin depressions caused by
imperfections in connective tissue and scarring.”
There was a consensus on the suggestions regarding the safety and side effects of the novel medical device (cf. Supplementary File S1, Statement 21), only adding “erythema” to the original wording. The experts noted that no serious adverse events had been reported during the current clinical study program. The minor side effects observed are common and expected for all micro-invasive procedures. Some experts suggested using bromelain to prevent frequent and transient post-injection edema, and all experts agreed that there are no contraindications.
“Treatment with the PLLA-LASYNPRO™ medical device is
safe, with only occasional, expected, mild, and transient side effects.
Common immediate reactions to PLLA-LASYNPRO™ treatment include short-term
mild pain, erythema, swelling, and slight bleeding at the injection site,
typically resolving spontaneously within a few hours and no longer than 24 to
48 hours. Current evidence shows late inflammatory side effects, such as
nodules and granulomas, have a negligible or nil incidence.”
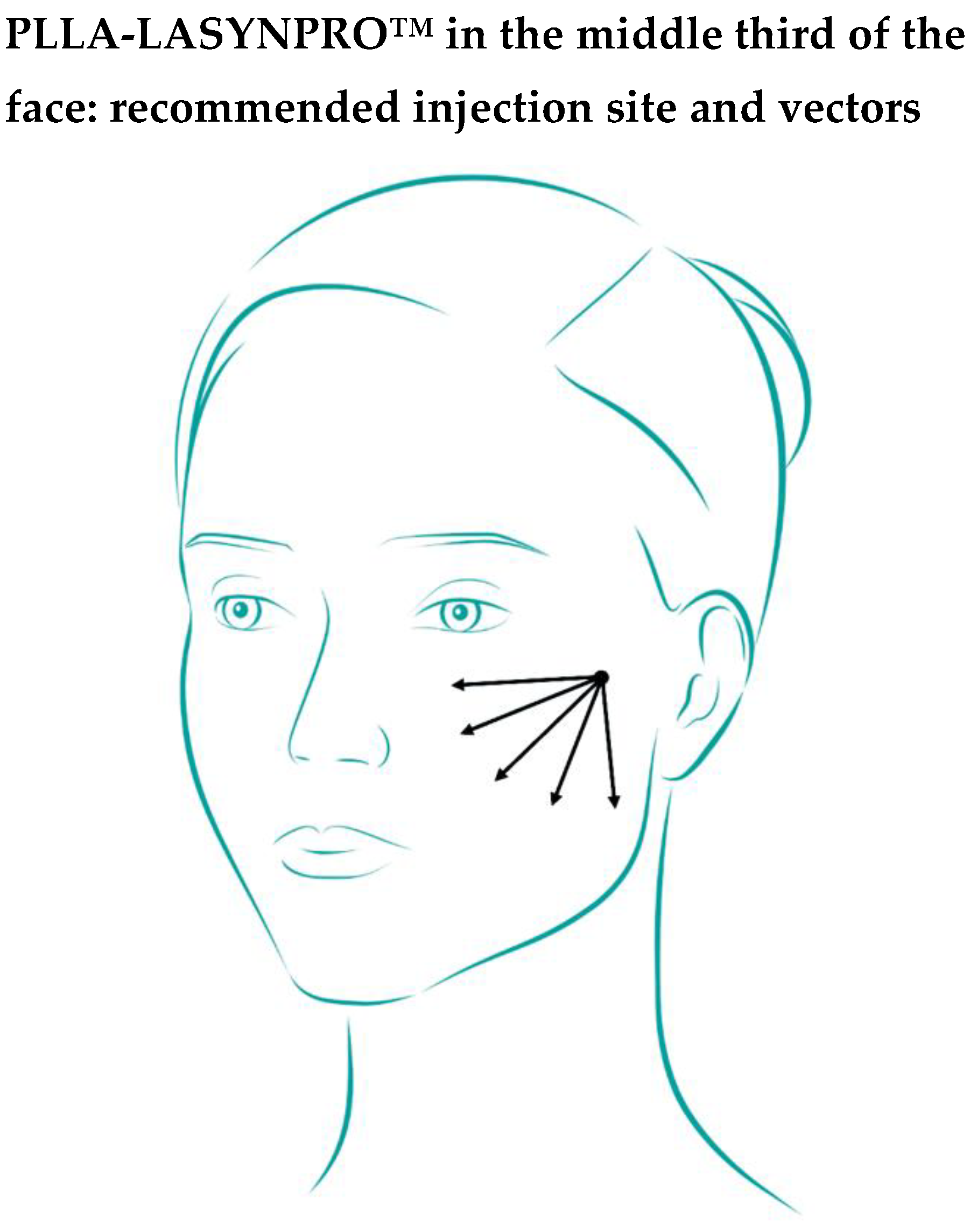
As illustrated in
Figure 1, the recommended injection point for the next-generation PLLA microspheres in the middle-third area is lateral malar, positioned just below the zygomatic arch (
cf. the several surveyed suggestions in Supplementary File S1, Statement 22).
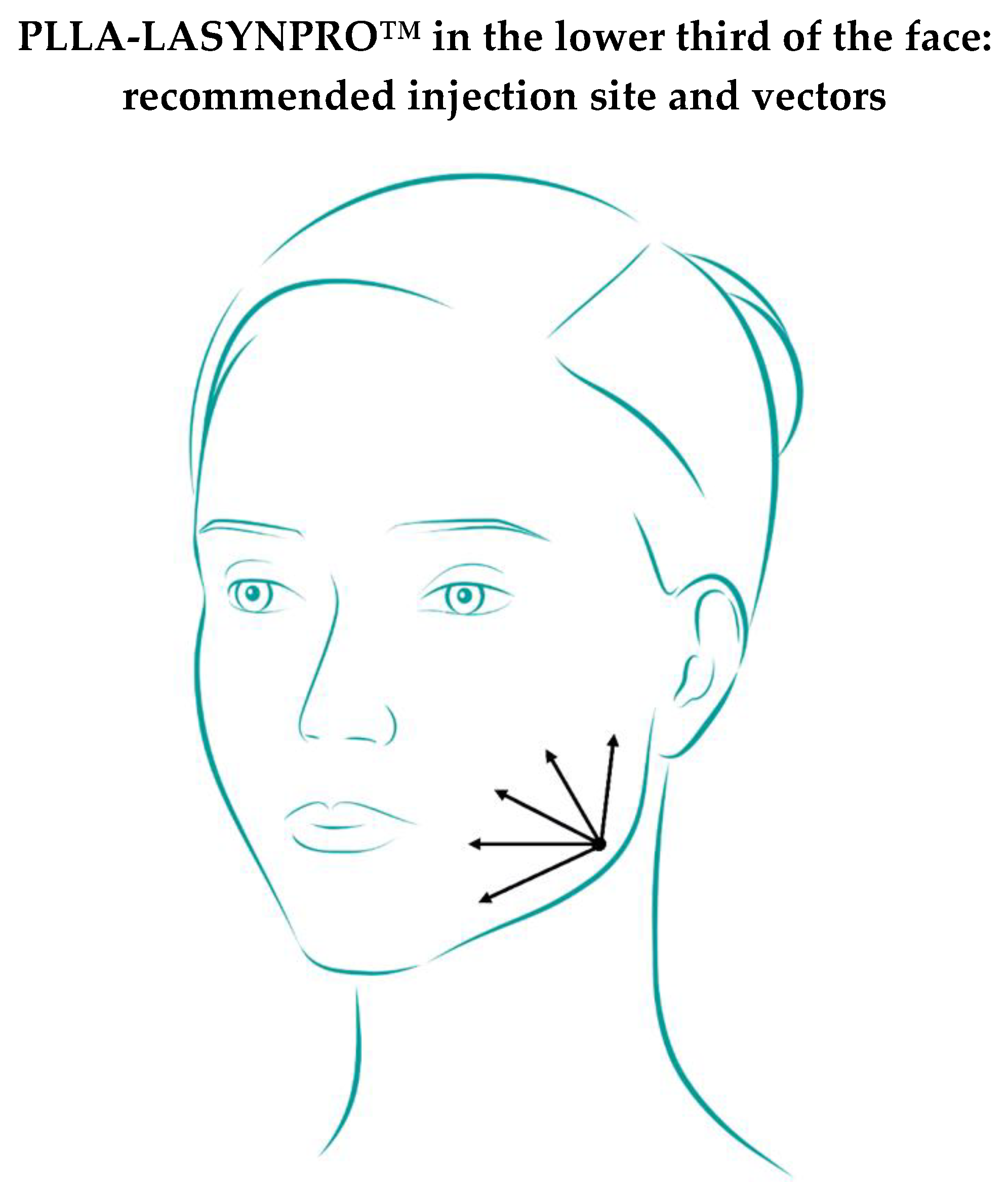
The medial and lateral mandibular and submalar points (
Figure 2) are recommended for injecting the PLLA-LASYNPRO™ microspheres in the lower-third region (
cf. the two proposed options in File S1, Statement 23).
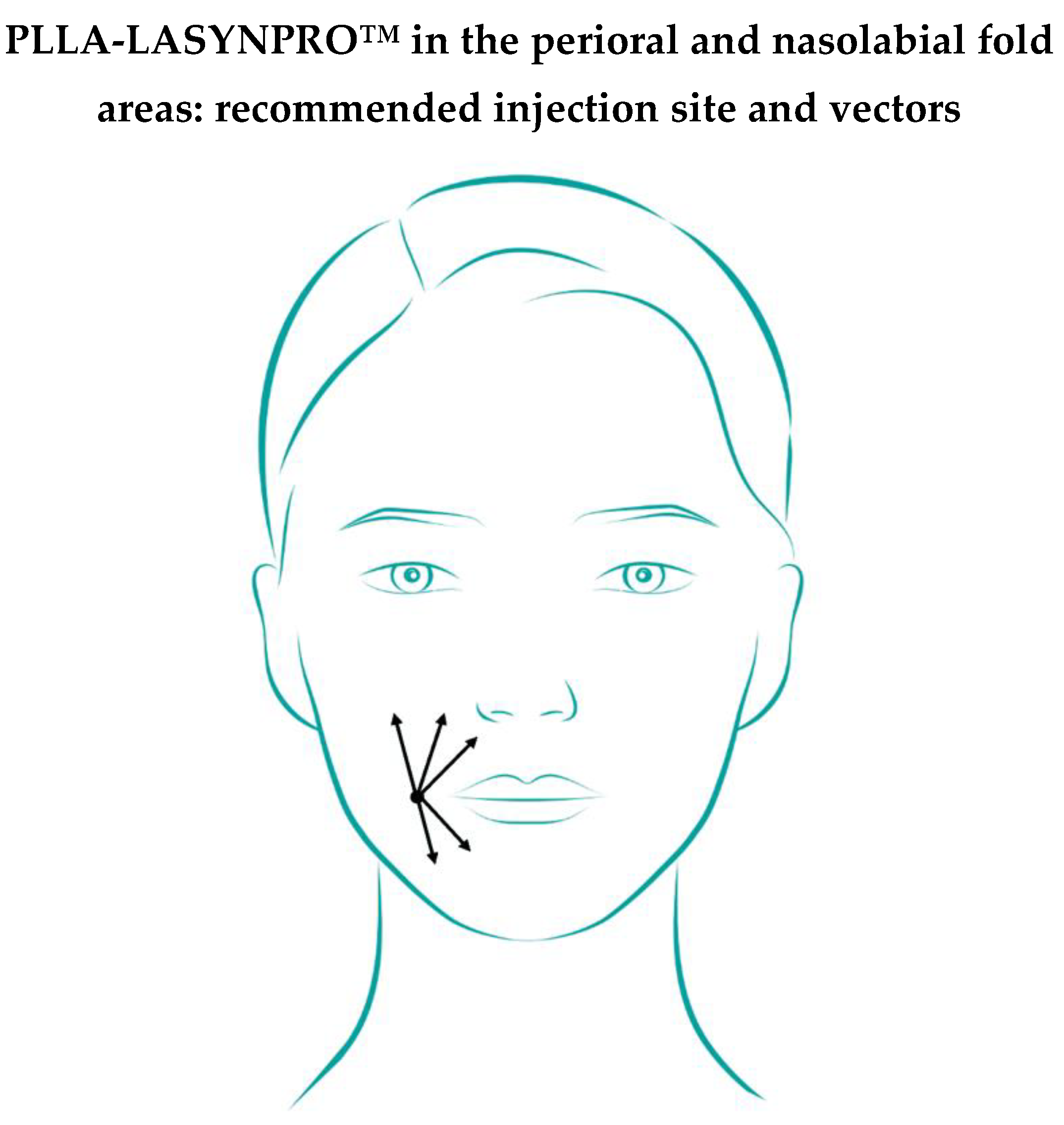
The board unanimously agreed on “One centimeter laterally from the oral commissure” as the recommended injection point in the perioral area. The “Lower part (base) of the nasolabial fold” might also be considered as an injection point in the area affected by nasolabial folds (
cf. the several proposed options in Supplementary File S1, Statements 24 and 25). A recent interim prospective multicenter analysis on 36 subjects confirmed the safety and rejuvenating efficacy of the PLLA-LASYNPRO™ microsphere implants on mild to severe nasolabial folds [
4]. Adverse effects, such as occasional edema, erythema, and infrequent local irritation, were mild, transient, and expected as typical of all micro-invasive procedures. Regarding efficacy, 44.4% and 63.9% reported highly significant improvements compared to baseline on both the five-grade WSRS (Wrinkle Severity Rating Scale) and the six-point MFVDS (Midface Volume Deficit Scale) photo-numeric assessment tools after, respectively, one and two months after the first microsphere injection [
4].
Figure 3.
The injection site and vectors suggested in the diagram are not mandatory. The operator’s assessments
and the subject’s specific needs may suggest other or alternative injection vectors. Diagram drawn and owned
by the authors.
Figure 3.
The injection site and vectors suggested in the diagram are not mandatory. The operator’s assessments
and the subject’s specific needs may suggest other or alternative injection vectors. Diagram drawn and owned
by the authors.
Likewise, the board unanimously agreed that the temple and neck are additional facial areas that could benefit from PLLA-LASYNPRO™ treatment (cf. Supplementary File S1, Statement 26).
The final statement proposed for discussion centered on the need for post-treatment massages (
cf. Supplementary File S1, Statement 27). Based on available preclinical evidence [
1] and their experiences in the ongoing clinical research program, the board agreed that the tiny, regular, and smooth-surfaced new-technology PLLA microspheres diffuse rapidly in the treated area from the superficial subcutaneous layer with no early or late tendency to clump. Consequently, the board unanimously concluded that immediate post-injection massages may be beneficial and are recommended, although they are not strictly essential. Self-massage at home is not required.
“Post-treatment massage is recommended but not
mandatory: the purpose is verifying the even distribution of PLLA-LASYNPRO™
microspheres. Thanks to the optimal tissue integration of the microspheres,
no self-massage at home is warranted.”