Neosynthesis of Collagen and Extracellular Matrix: The Long-Dominant Foreign Body Reaction Paradigm
Since the introduction of the first sterile, water-reconstituted poly-L-lactic acid (PLLA) formulation around the turn of the century, the notion of an inflammatory foreign-body reaction (FBR), whether acknowledged or downplayed, has been central to understanding how injectable resorbable collagen stimulators enhance the synthesis of collagen and other components of the extracellular matrix in connective tissues [
1,
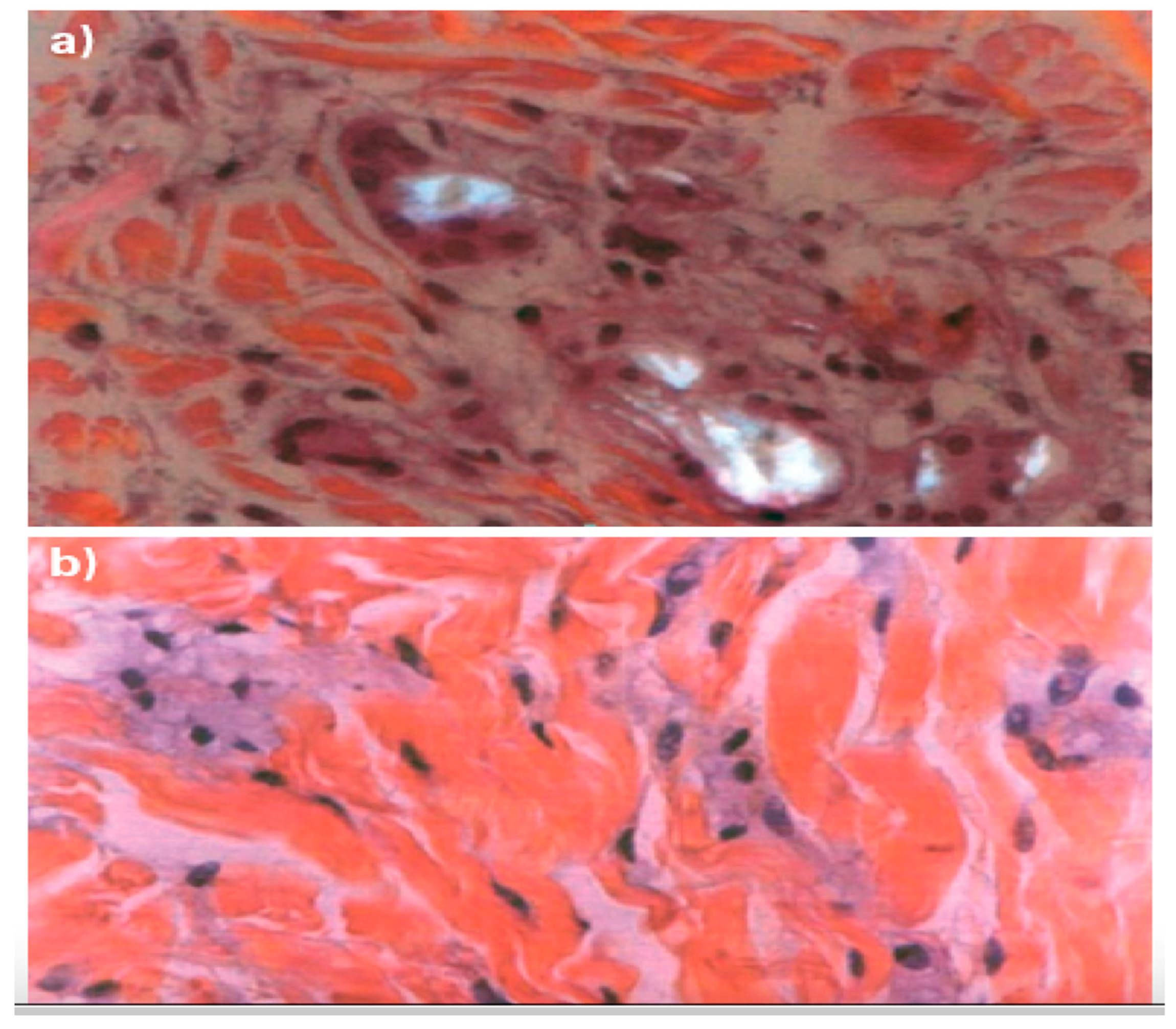
2]. The FBR is a spontaneous response that gradually encases and sequesters subdermal implants, such as traditional injectable collagen stimulators, in a fibrous shell. Unfortunately, inflammation also contributes to the most severe late side effects, including nodules and hardened skin indurations, which can occasionally progress into persistent granulomas lasting for months or even years (
Figure 1) [
1].
A 2021 multicenter, retrospective chart review of U.S. medical records involving 4,483 treatments across the midface, temple, and jawline in 1,002 subjects revealed a persistently concerning long-term incidence of nodules at 0.4% for early-generation PLLA despite reconstituting the dry powder to 8-10 mL [
3]. Other recent reviews indicate that the long-term incidence of late adverse effects—including nonvisible but palpable subcutaneous nodules, visible nodules, and chronic granuloma—ranges from a non-negligible 0.2% to 1.2% [
4]. The burden of inflammatory late adverse effects appears unrelated to geographical latitude and ethnicity. Moreover, it is likely underestimated due to diagnostic challenges and the late occurrence, which masks the cause-and-effect relationship [
5].
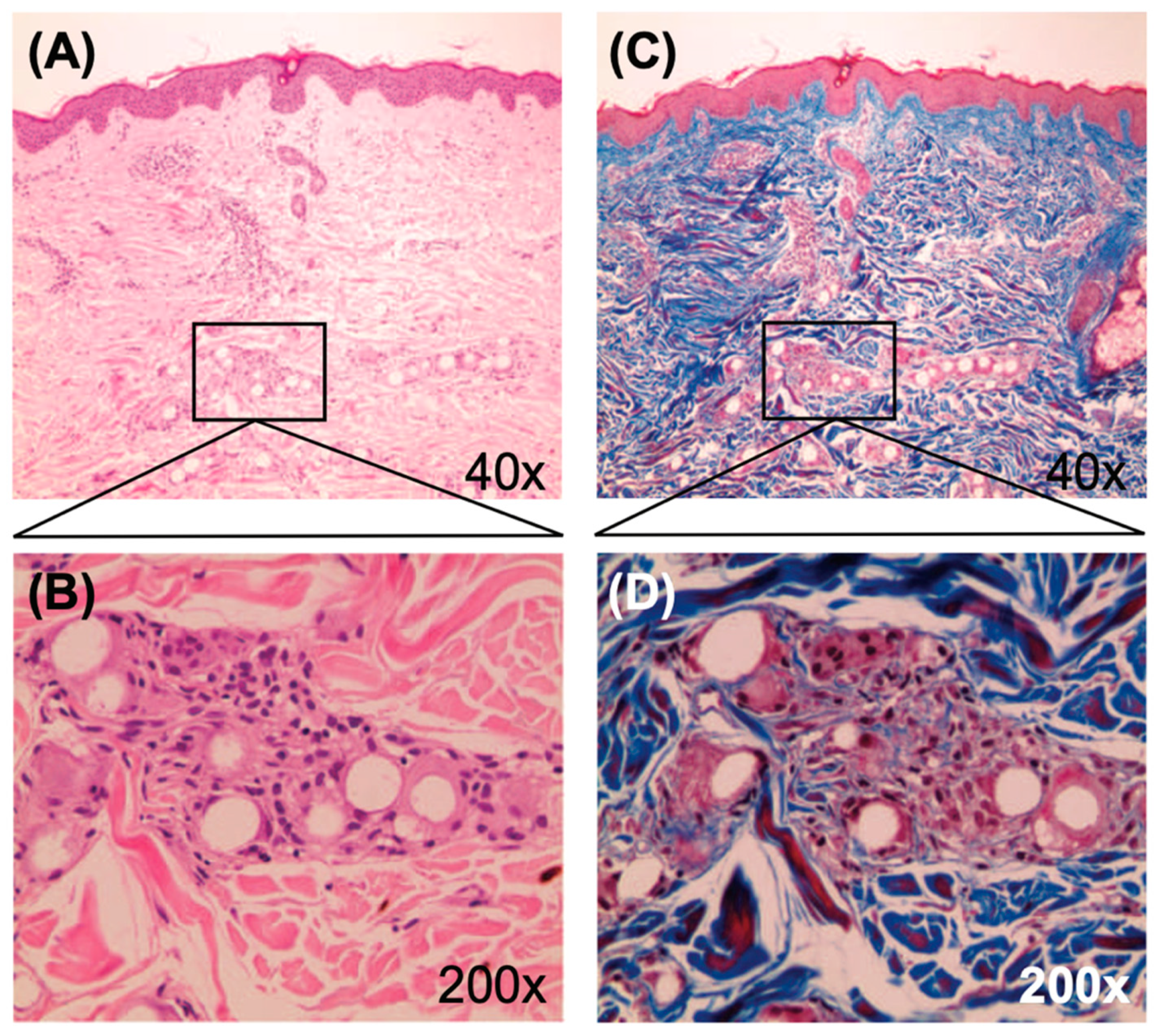
Inflammatory side effects do not exempt the injectable collagen bio-stimulator poly(ε-caprolactone) or PCL; however, they occur only occasionally and are significantly milder, as confirmed by the U.S. FDA Center for Devices and Radiological Health (
Figure 2) [
2,
6]. According to a 2020 review of PCL,
“The host response includes protein coating of the material, macrophage migration, and encapsulation at around three weeks. Inflammatory reactions and wound healing pathways participate in this stepwise repair process” [
7].
Invoking the inflammatory FBR postulate seems less convincing for resorbable ceramic-derivative calcium hydroxylapatite. Unlike conventional PLLA microparticles, the macrophage expression of several pro-inflammatory cytokines, including IL-1α, IL-1β, IL-8ß, and Chemokine (C-X-C motif) Ligand 6 (CXCL6), appears significantly downregulated after exposure to the calcium hydroxylapatite microspheres [
8,
9]. However, other evidence seems to contradict these findings. Inflammatory biomarkers such as Chemokine (C-X-C motif) Ligand 8/IL-8 (CXCL8/IL-8), IL-6, and prostaglandin-endoperoxide synthase 2 (PTGS2) increase with calcium hydroxylapatite implants in over half of a panel of subjects treated for nasolabial folds [
10]. Furthermore, compared with conventional PLLA in the opposing arms of the five female subjects following superficial subcutaneous injection, histology revealed a similar new production of collagen and elastic fibers and a comparable moderate to intense inflammatory reaction involving lymphocytic and giant cell infiltrate [
11]. More generally, the most recent literature does not exclude the evidence of focal accumulations and nodules with calcium hydroxylapatite [
12].
Is It Conceivable to Progress Beyond the Foreign Body Response in Skin Connective tissue Regeneration?
The FBR postulate has never been denied or refused, although international literature has occasionally downplayed the inflammatory nature of induced extracellular matrix regeneration. A shift from the inflammatory FBR paradigm to a novel non-inflammatory collagen/ECM regeneration that mitigates the risks of delayed inflammatory side effects and improves skin physiology may have emerged with the next-generation PLLA-LASYNPRO™ microspheres. These highly uniform microspheres, produced using advanced patented freeze-drying technologies, are negligibly prone to disrupting phagosome membranes and causing the pro-inflammatory leakage of cathepsin into the cytosol. Furthermore, the new-technology, smooth-surfaced, rounded poly-L-lactic acid microparticles preferentially activate the subpopulation of M2-polarized macrophages that secrete anti-inflammatory cytokines such as interleukin-4 (IL-4), IL-10, and IL-13 [
13,
14].
Two decades after injectable poly-L-lactic acid received approval in Europe (1999) and the United States (2004) [
15], the PLLA-LASYNPRO™ subdermal implants and the JULÄINE™ medical device aim to signify a significant turnaround. 2are currently underway in Europe to validate the non-inflammatory rationale behind the new-technology PLLA ingredient and its anticipated efficacy and safety benefits through methodologically sound, high-quality studies. A recent Spanish interim multicenter analysis on 36 adult subjects confirmed the safety and rejuvenating efficacy of the PLLA-LASYNPRO™ microsphere implants on mild to severe nasolabial folds [
16]. Assessed with two photo-numeric tools, the five-grade WSRS (Wrinkle Severity Rating Scale) and the six-point MFVDS (Midface Volume Deficit Scale focused on middle third facial volume loss), 44.4% and 63.9% of subjects reported highly significant reductions, uniform on the right and left facial sides, of at least one point compared to baseline one and two months after the first injection. Procollagen type I Carboxy-terminal Propeptide (P1CP) circulating levels, a marker of type-1 collagen neosynthesis, rapidly showed significant increases one month after the first dose. Adverse effects, such as occasional edema, erythema, and infrequent local irritation, were mild, transient, and expected, typical with all micro-invasive procedures [
16].
A board of thirteen experts in aesthetic and regenerative medicine, dermatology, and aesthetic plastic surgery convened to discuss and share their insights with their European colleagues about the rationale and role of PLLA-LASYNPRO™ subdermal implants drawing from the available evidence and their direct clinical experience in the clinical research program [
17]. While research is progressing, the board experts believe that the results of their collaborative efforts deserve a broader audience, including a set of preliminary suggestions for integrating the novel CE-approved JULÄINE™ medical device based on PLLA-LASYNPRO™ subdermal implants into everyday regenerative medicine practice [
17].
The PLLA-LASYNPRO™ Rationale Beyond the FBR Paradigm
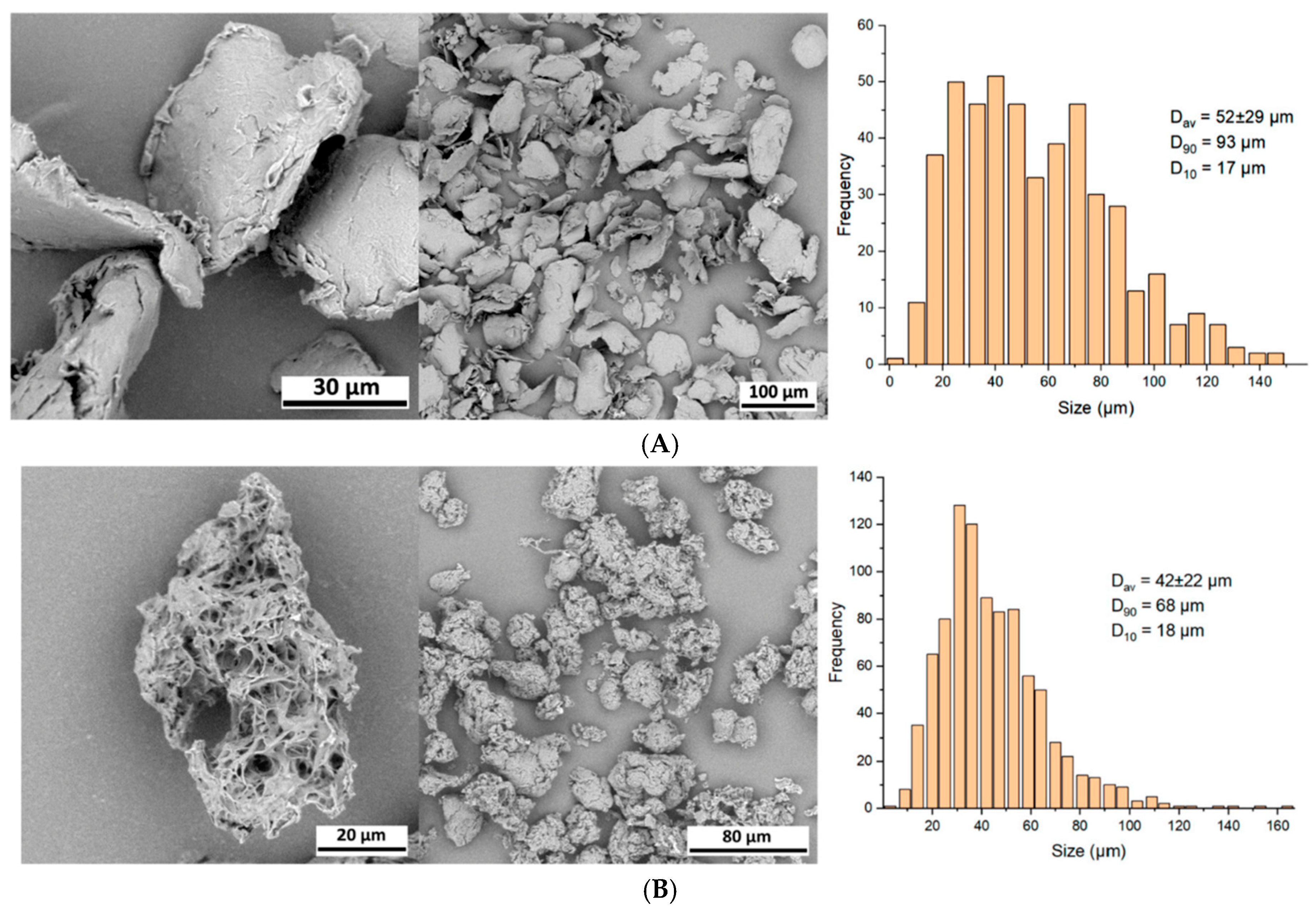
On average, early-generation PLLA microparticles appear oblong, irregular, and heterogeneous in size and shape. They resemble irregular, spiky micro-flakes ranging from 2 to 150 µm along their longer axis, with nearly half of the microparticles measuring less than 20 µm in diameter (
Figure 3A,B) [
18,
19,
20]. This characteristic makes early-generation PLLA microparticles susceptible to an inflammatory response and phagocytosis by macrophages, which can ultimately lead to the development of granulomas and delayed-onset nodules. Additionally, the microparticles in some formulations of early-generation PLLA are porous, further influencing inflammatory responses [
18,
19,
20]. Furthermore, a sizable fraction of earlier PLLA microparticles that are at least 100 µm risks becoming trapped in the standard 26-gauge needle (internal diameter: 100 µm) used for injection [
18].
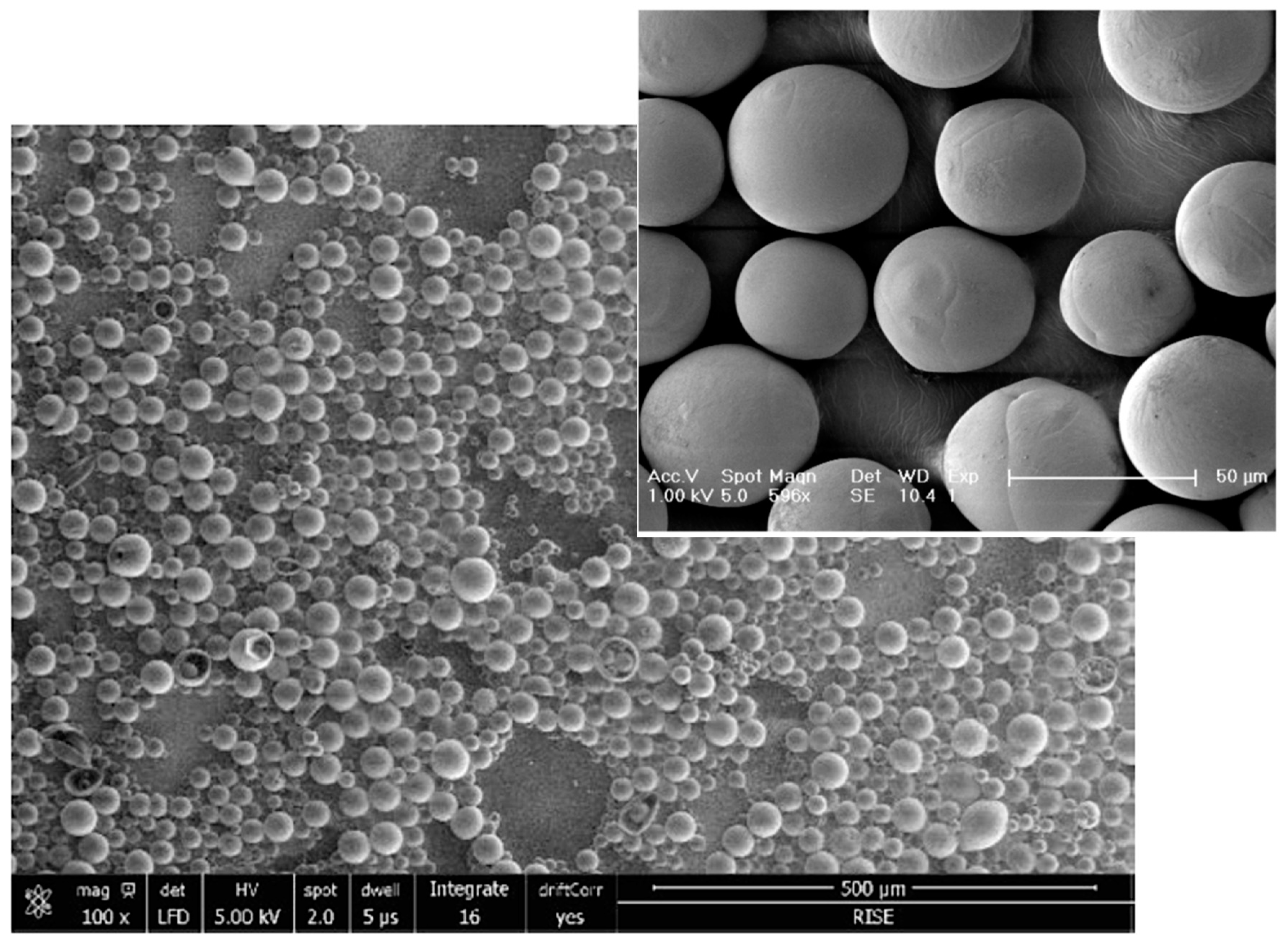
Conversely, the highly pure and readily dispersible microspheres of the innovative Class III JULÄINE™ medical device (Nordberg Medical AB, Huddinge, Sweden), produced using proprietary patented freeze-drying technologies, exhibit precise spherical shapes and smooth surfaces with a non-porous structure. They possess a uniform diameter ranging from 20 to 50 µm (average: 33.3 µm) and exhibit a consistent
in vivo degradation rate over two years, along with long-term stability and shelf life (
Figure 4) [
21]. Each JULÄINE™ vial of dry powder contains 150 mg of PLLA-LASYNPRO™ microspheres; additional components include 45 mg of sodium carboxymethyl cellulose and 145 mg of non-pyrogenic mannitol [
21]. Moisture and pro-inflammatory heavy metal and tin residues are below 0.5%, 0.001%, and 6.0 µg/mL (ppm), respectively, significantly lower than the levels found in earlier-generation PLLA derivatives [
21].
The Non-Inflammatory Action of the New-Technology Microspheres
Even with earlier-generation PLLA formulations, the FBR paradigm does not fully explain collagen and extracellular matrix neosynthesis. For instance, the purely inflammatory FBR model does not clarify why a noticeable facial tightening effect often occurs just one month after earlier-generation PLLA injections, as the temporal framework seems too short [
22].
In vitro evidence may provide a basis for elucidating the early tightening effects observed in vivo from earlier-generation PLLA formulations. For example, exposing fibroblasts to these conventional PLLA formulations for 48 hours activates the p38, Akt (protein kinase B), and JNK (c-Jun N-terminal kinase) signaling proteins, which are key regulators in signal transduction related to cell growth, differentiation, and apoptosis. This exposure also elevates the expression of the Type-I collagen gene [
23]. The Akt signaling pathway contributes to fibroblast migration, differentiation into myofibroblasts, collagen synthesis, and cutaneous wound contraction. Gene transcription increases rapidly within 48 hours and is likely independent of any FBR-like effects. Following the upregulation of Type-I collagen gene expression, procollagen concentrations also rise quickly in the incubation medium [
23].
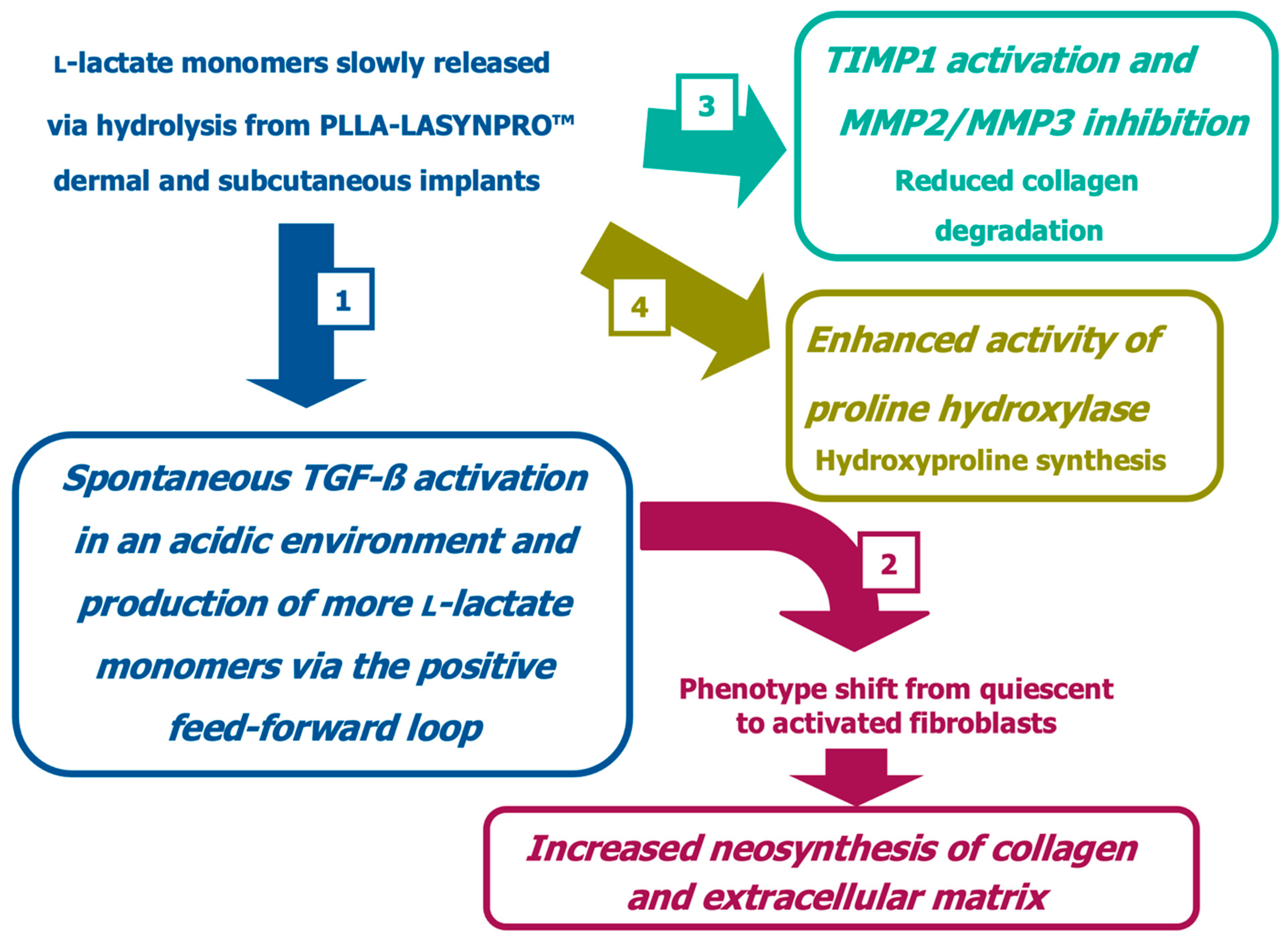
Another well-known FBR-independent regenerative sequence of events that leads to collagen and ECM neosynthesis involves the pH-dependent activation of latent TGF-ß (Transforming Growth Factor-ß). Acidic lactate, produced by the gradual degradation of PLLA, triggers the regenerative signal [
24,
25]. Furthermore, active TGF-β directs fibroblasts to adopt the contractile myocyte phenotype, differentiate into myofibroblasts, and enhance the production of extracellular collagen and matrix. Additionally, active TGF-β prompts fibroblasts and myofibroblasts to upregulate the lactate-generating enzyme LDHA (lactate dehydrogenase-A), resulting in persistently high lactate concentrations and continuous local TGF-ß activation [
24,
25,
26,
27].
In vitro, upregulation of the TIMP1 (Tissue Inhibitor of MetalloProteinase 1) signaling pathway by lactic acid represents a third TGF-β-triggered event, ultimately leading to sustained inhibition of collagen catabolism [
28,
29].
All such non-inflammatory events appear strongly activated by PLLA-LASYNPRO™ subdermal implants [
22]. Furthermore, the gradual degradation of the new-technology PLLA microspheres into lactate monomers supports cellular energy production through the tricarboxylic acid cycle and the electron transport chain [
30].
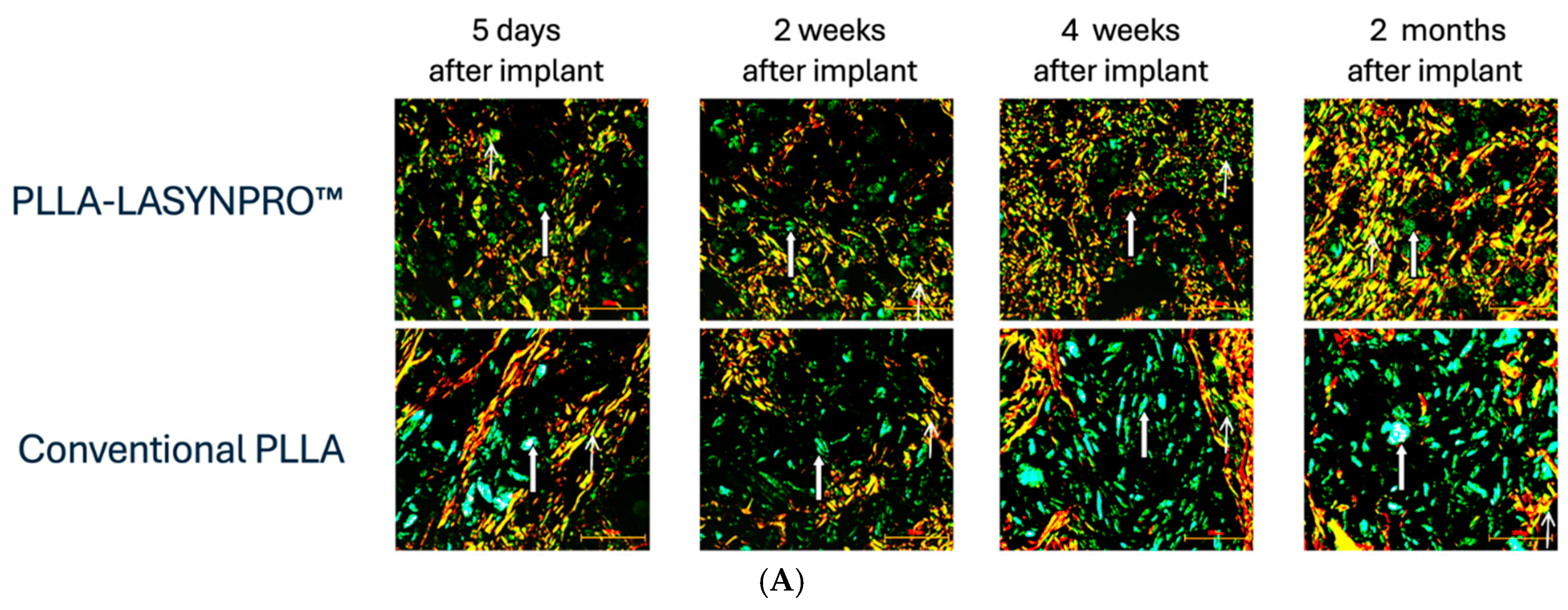
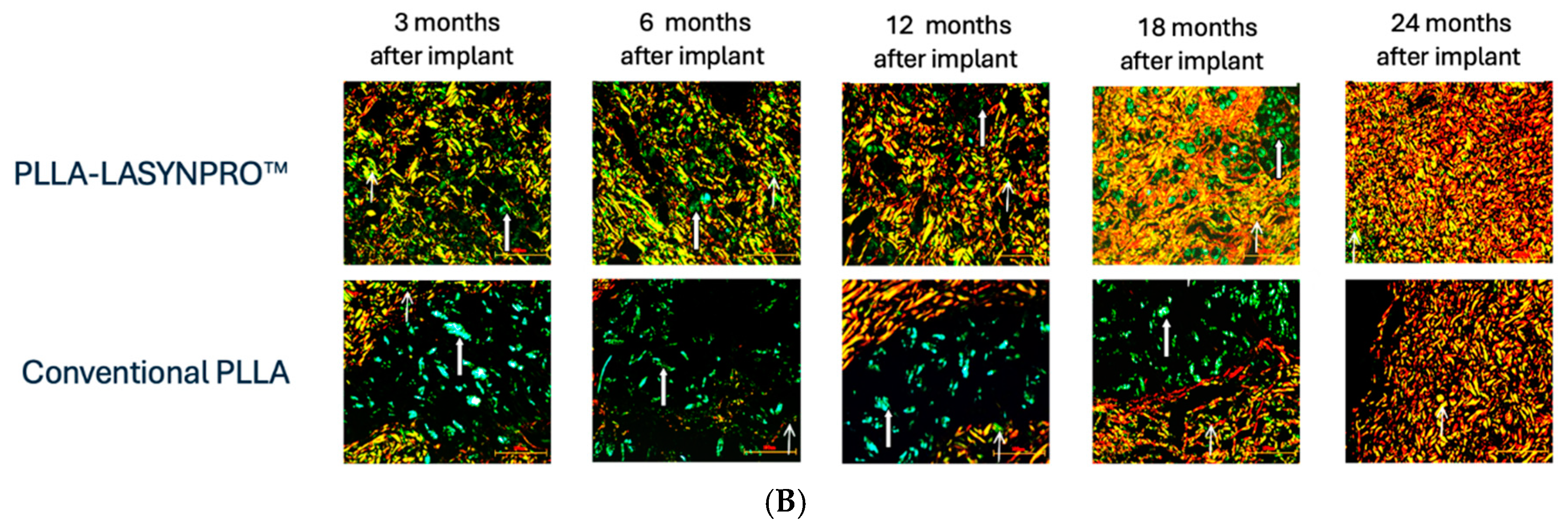
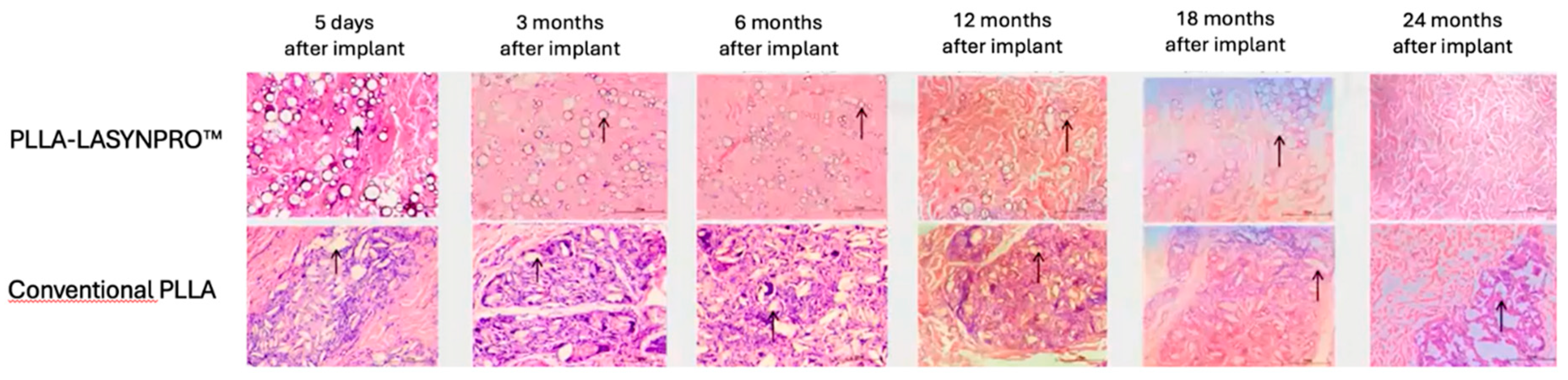
Figure 5 summarizes the likely in vivo effects on fibroblasts in dermal and subcutaneous connective tissues following exposure to the lactic acid monomers gradually released from the PLLA-LASYNPRO™ microspheres over several months. The activation of TGF-β by the acidic microenvironment induced by the lactate residues released from the microspheres promotes myofibroblast differentiation, collagen synthesis, and the formation of the extracellular matrix while reducing collagen degradation with minimal inflammatory responses (
Figure 6A,B) [
20,
32,
33,
34]. The negligible inflammatory response, with no scar-like tissues or nodules and even dispersion without focal aggregations of the steadily and slowly degrading new-technology PLLA microspheres, persists for 24 months. There are no tissue compressions or deformities. Some microspheres remain detectable at month 18, with complete degradation occurring by month 24, leaving no residue or tissue gaps after degradation (
Figure 7) [
13,
21]. The positive feedback loop would wane with the resorption of the microspheres, thus eliminating any long-term risk of fibrosis. The stimulating role of lactate on fibroblast collagen proline hydroxylase may also promote self-sustaining neocollagenesis [
34,
35]. The increased levels of IL-4 and IL-13 led to macrophage polarization toward the M2 subtype and tissue remodeling, further enhancing TGF-β secretion [
31].
Moreover, recent
in vitro studies with cultured adipocytes suggest that PLLA monomers may help stimulate adipogenesis in subcutaneous adipose tissues, potentially countering the loss of subcutaneous fat due to aging and photoaging, possibly contributing to deep wrinkles [
37]. In general, PLLA monomers are more and more emerging as crucial signaling factors in the cell machinery. For instance, in skin fibroblasts, L-lactate protects mitochondria from aging-related dysfunction. Mito-hormesis, the name of the modulation process, is a persistent cellular adaptive response of mitochondria and mitochondria-associated membranes to mild stressors whereby skin fibroblasts enhance their survival and stress resistance, possibly by inducing the release of stress-triggered mitokines Fibroblast Growth Factor 21 (FGF21) and Growth and Differentiation Factor 15 (GDF15) [
38].
5. Conclusions
Technological innovations have led to the novel PLLA-LASYNPRO™ subdermal implants. Are these implants a genuine breakthrough in addressing the inflammatory foreign-body response paradigm? We know from the past that some degree of inflammation has consistently accompanied the action of resorbable collagen inducers before the new-technology PLLA derivative [
1].
As discussed in Part 2 of this introduction to PLLA-LASYNPRO™ non-inflammatory collagen and extracellular matrix regeneration, the Next-Generation PLLA-LASYNPRO™ Regenerative Medicine Expert Board cautiously endorsed the non-inflammatory rationale behind the new medical device [
17]. They found the initial findings from preclinical and microscopic investigations [
10,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36] persuasive and aligned with their clinical experience as leaders in the ongoing clinical research program of the new PLLA technology. Their initial impression is that the CE-approved JULÄINE medical device, based on novel PLLA technology, may effectively address the challenge of inflammatory side effects [
17]. In this context, the Next-Generation PLLA-LASYNPRO™ Regenerative Medicine Expert Board expressed the opinion that the new PLLA technology might indeed emerge as a breakthrough in skin regeneration without no more than mild and transitory inflammation. Caution and further research efforts remain essential in this initial phase to substantiate the first favorable results.
Author Contributions
All authors contributed to designing the survey and engaging in subsequent discussions, reviewed the manuscript drafts, and consented to its submission. The authors are responsible for the clinical and editorial accuracy and integrity of the manuscript submitted to the Journal of Clinical Medicine. They confirm that they have followed the journal’s ethical policies as outlined in the guidelines for authors.
Funding
All authors actively participate in the JULÄINE clinical research program. However, no author has received funding or benefits for their board activities or manuscript submission.
Institutional Review Board Statement
No board activities involved interactions with human subjects, thus waiving the need for formal preliminary Institutional Review Board approval.
Informed Consent Statement
Not relevant.
Data Availability Statement
Minutes from the discussion at the final board meeting are available upon reasonable request.
Acknowledgments
The board would like to thank Mauro Raichi for his contributions to discussions about the manuscript structure and his assistance with medical writing.
Conflicts of Interest
Over the past three years, all board members have received grants and fees from companies involved in aesthetic medicine and surgery, serving as consultants in research and development programs, investigators in national and international clinical studies, and lecturers or tutors in Continuous Medical Education activities and sponsored educational meetings. However, the authors declare no conflicts of interest related to the manuscript submission. Nordberg Medical AG, the holder of the international patents for PLLA-LASYNPRO™ and the manufacturer and exclusive marketer of the JULÄINE™ medical device, provided support only for the secretarial and logistical expenses of the board members and will also financially assist with publication costs after the manuscript undergoes peer review and acceptance.
References
- Vleggaar, D. Facial volumetric correction with injectable poly-L-lactic acid. Dermatol Surg 2005, 31 (11, Pt 2), 1511–1518. [Google Scholar] [CrossRef]
- Kim, J.A.; Van Abel, D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J Cosm Laser Ther 2015, 17, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.; Mayoral, F.; Rajani, A.; et al. Chart review presenting the safety of injectable PLLA used with alternative reconstitution volume for facial treatments. J Drugs Dermatol 2021, 20, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, W.; Wang, F. Injectable fillers: current status, physicochemical properties, function mechanism, and perspectives. RSC Adv 2023, 13, 23841–23858. [Google Scholar] [CrossRef]
- Ianhez, M.; de Goés E Silva Freire, G.; Sigrist, R.M.S.; Colpas, P.T.; Alves de Faria, I.; Américo Brasil Parada, M.O.; Miot, H.A. Complications of collagen biostimulators in Brazil: Description of products, treatments, and evolution of 55 cases. J Cosmet Dermatol 2024, 23, 2829–2835. [Google Scholar] [CrossRef]
- U.S. FDA Center for Devices and Radiological Health. Polycaprolactone (PCL) Safety Profile. Published by ECRI, Plymouth Meeting, PA, USA. December 2, 2021. Downloaded on September 12, 2024, from www.fda.gov/media/158492/download.
- Christsen, M.-O.; Vercesi, F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin Cosmet Investig Dermatol 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Nowag, B.; Schäfer, D.; Hengl, T.; Corduff, N.; Goldie, K. Biostimulating fillers and induction of inflammatory pathways: A preclinical investigation of macrophage response to calcium hydroxylapatite and poly-L-lactic acid. J Cosmet Dermatol 2024, 23, 99–106. [Google Scholar] [CrossRef]
- McCarthy, A.D.; Hartmann, C.; Durkin, A.; Shahriar, S.; Khalifian, S.; Xie, J. A morphological analysis of calcium hydroxylapatite and poly-L-lactic acid biostimulator particles. Skin Res Technol 2024, 30, e13764. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.; Nguyen, T.Q.; Le, J.H.T.D.; et al. Gene analysis of biostimulators: Poly-L-lactic acid triggers regeneration while calcium hydroxylapatite induces inflammation upon facial injection. J Drugs Dermatol 2025, 24, 34–40. [Google Scholar] [CrossRef]
- Mazzuco, R.; Evangelista, C.; Gobbato, D.O.; de Almeida, L.M. Clinical and histological comparative outcomes after injections of poly-L-lactic acid and calcium hydroxyapatite in arms: A split side study. J Cosmet Dermatol 2022, 21, 6727–6733. [Google Scholar] [CrossRef]
- McCarthy, A.D.; van Loghem, J.; Martinez, K.A.; Aguilera, S.B.; Funt, D. A structured approach for treating calcium hydroxylapatite focal accumulations. Aesthet Surg J 2024, 44, 869–879. [Google Scholar] [CrossRef] [PubMed]
- The effect of size and shape of PLLA particles: usability, technical and clinical perspectives. RISE Research Institutes of Sweden AB, Gothenburg, 2023.
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of immune responses by particle size and shape. Front Immunol 2021, 11, 607945. [Google Scholar] [CrossRef]
- Ao, Y.J.; Yi, Y.; Wu, G.H. Application of PLLA (Poly-L-Lactic acid) for rejuvenation and reproduction of facial cutaneous tissue in aesthetics. A review. Medicine (Baltimore) 2024, 103, e37506. [Google Scholar] [CrossRef]
- Urdiales-Gálvez, F.; Benítez, P.A.; Díaz, I. Facial rejuvenation with an innovative poly-L-lactic acid (Juläine) for nasolabial folds: interim data analysis of a prospective, non-randomized, multicenter, open-label Spanish study. J Cosm Dermatol 2025, 24, e70137. [Google Scholar] [CrossRef] [PubMed]
- Dario Bertossi, Maurizio Cavallini, Alessandra Camporese on behalf of The Next-Generation PLLA-LASYNPRO™ Regenerative Medicine Expert Board. First insights on the upcoming role of next-generation PLLA-LASYNPRO™ in aesthetic and regenerative medicine. A survey of experts — Practical Suggestions. This accompanying manuscript is under contemporary review by J Clin Med.
- Sedush, N.G.; Kalinin, K.T.; Azarkevich, P.N.; Gorskaya, A.A. Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: A comparative study. Cosmetics 2023, 10, 110. [Google Scholar] [CrossRef]
- McCarthy, A.D.; Hartmann, C.; Durkin, A.; Shahriar, S.; Khalifian, S.; Xie, J. A morphological analysis of calcium hydroxylapatite and poly-L-lactic acid biostimulator particles. Skin Res Technol 2024, 30, e13764. [Google Scholar] [CrossRef]
- Lemperle, G.; Neugebauer, P.; Kernke, R.; Lerche, K.-H.; Lemperle, S. Microspheres for cosmetic and medical injections must be free of phagocytosable microparticles under 20 microns. Biomed J Sci & Tech Res 2017, 1, 1682–1686. [Google Scholar]
- Nordberg Medical R&D internal reports available upon request.
- Sung-Ae, K.; Hyo-Seon, K.; Jin-Woong, J.; Sung-Il, S.; Young-Wook, R. Poly-L-Lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the p38 MAPK pathway. Ann Dermatol 2019, 31, 97–100. [Google Scholar]
- Li, G.; Li, Y.-Y.; Sun, J.-E.; Lin, W.-H.; Zhou, R.-X. ILK-PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab Invest 2016, 96, 741–51. [Google Scholar] [CrossRef]
- Kottmann, R.M.; Kulkarni, A.A.; Smolnycki, K.A.; et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med 2012, 186, 740–751. [Google Scholar] [CrossRef]
- Judge, J.L.; Owens, K.M.; Pollock, S.J.; et al. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol 2015, 309, L879–L887. [Google Scholar] [CrossRef]
- Meng, K.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017, 127, 3770–3783. [Google Scholar] [CrossRef]
- Stein, P.; Vitavska, O.; Kind, P.; Hoppe, W.; Wieczorek, H.; Schürer, N.Y. The biological basis for poly-L-lactic acid-induced augmentation. J Dermatol Sci 2015, 78, 26–33. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, C. Poly-L-Lactic acid increases collagen gene expression and synthesis in cultured dermal fibroblast (Hs68) through the TGF-beta/Smad pathway. J Cosmet Dermatol 2023, 22, 1213–1219. [Google Scholar] [CrossRef]
- Vavřička, J.; Brož, P.; Follprecht, D.; Novák, J.; Kroužecký, A. Modern perspective of lactate metabolism. Physiol Res 2024, 73, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, J.H.; Kim, H.M.; et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells 2023, 12, 1320. [Google Scholar] [CrossRef] [PubMed]
- Kottmann, R.M.; Kulkarni, A.A.; Smolnycki, K.A.; Lyda, E.; Dahanayake, T.; Salibi, R.; Honnons, S.; et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am J Respir Crit Care Med 2012, 186, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Judge, J.L.; Owens, K.M.; Pollock, S.J.; et al. Ionizing radiation induces myofibroblast differentiation via lactate dehydrogenase. Am J Physiol Lung Cell Mol Physiol 2015, 309, L879–L887. [Google Scholar] [CrossRef]
- Meng, K.-M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; et al. Fibroblast-specific TGF-beta-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Comstock, J.P.; Udenfriend, S. Effect of lactate on collagen proline hydroxylase activity in cultured L-929 fibroblasts. Proc Natl Acad Sci U S A. 1970, 66, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Jung, Y.A.; Yun, J.M.; Kim, Y.; Kim, S.A.; Suh, S.I.; Ryoo, Y.W. Effects of poly-L-lactic acid on adipogenesis and collagen gene expression in cultured adipocytes irradiated with ultraviolet B rays. Ann Dermatol 2023, 35, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S.; · Bertossi, D.; Magistretti, P. Insights on the role of l-lactate as a signaling molecule in skin aging. Biogerontology 2023, 24, 709–726. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).