1. Introduction
The number of procedures performed with filler materials in the field of aesthetic medicine has increased by 40% over the last 5 years. In parallel, the number of procedures aimed at removing them has increased by 46% over the same period [
1].
There are increasingly more new filler materials on the market that differ in terms of composition, origin, density, mechanism of action, biodurability, and longevity. However, knowledge regarding the morphological characterization of these materials remains limited. When we seek information in product technical data sheets, we also encounter missing data, which hinders our ability to understand these materials and select those that offer better characteristics according to the indications for which they will be used.
Morphological study using optical microscopy can be a valuable tool for understanding the structure of different filler materials. This approach could provide better insight into how each type of filler interacts with human tissues, as well as their behavior over time and biological compatibility.
It is important to understand that all filler materials act primarily through space occupation, which varies depending on particle size, concentration, and quantity. Secondarily, they act through foreign body reaction, which produces an inflammatory response and triggers other biological reactions [
2]. The structural morphological image of each material and its size is determinant in this process, so understanding these characteristics can help predict the integration of the filler material into dermal tissue.
Furthermore, all filler materials can present adverse effects after injection. The lack of knowledge about the type of material previously injected complicates the repair and healing process of the damage caused. Morphological characterization of different filler materials allows for identification of the injected material, thereby enabling better control over the treatment of adverse effects.
This work aims to highlight the relevance of morphological study through microscopy of different filler materials to better understand their behavior and tissue integration, as well as their structural identification for proper management of adverse effects.
2. Materials and Methods
A total of 10 gels commonly used as filler materials in routine clinical practice in aesthetic medicine were selected. A drop of each material was placed onto a glass slide and photographed. Subsequently, the material was spread and stained using Diff-Quik. Each sample was then examined under optical microscopy.
Table 1.
Composition and HA Characteristics of Injectable Gels.
Table 1.
Composition and HA Characteristics of Injectable Gels.
| Gel/Product Name |
Composition |
HA Characteristics |
| Gel 1 (Belotero Lips Contour®) |
Cross-linked sodium hyaluronate: 22.5 mg/mL
Lidocaine hydrochloride: 3 mg/mL
Phosphate buffer pH 7: 0.6 mL |
Cross-linked |
Gel 2 (Harmonie® 1.5%, IT Pharma)
|
Cross-linked sodium hyaluronate: 15 mg/mL
Disodium hydrogen phosphate: 0.6 mg/mL
Sodium dihydrogen phosphate: 0.05 mg/mL
Sodium chloride: 8 mg/mL
Water for injection
|
Cross-linked
|
Gel 3 (Regenovue®)
|
Cross-linked sodium hyaluronate: 24 mg/mL
Lidocaine hydrochloride: 0.3%
Phosphate saline buffer
|
Cross-linked
|
Gel 4 (Restylane Kysse®)
|
Cross-linked sodium hyaluronate: 20 mg/ml
Lidocaine hydrochloride: 3 mg/mL
Phosphate buffer pH 7
|
Cross-linked with BDDE1 (1,4-butanediol diglycidyl ether)
|
Gel 5 (Genefill Contour®)
|
Non-cross-linked sodium hyaluronate: 2.0 mg
Cross-linked sodium hyaluronate: 20.0 mg
Sodium chloride: 6.9 mg
Water for injection: 1mL
|
Cross-linked with BDDE <0.001 mg/mL
|
Gel 6 (Karisma®)
|
Polypeptide chain R α1 (Rh collagen)
Carboxymethyl cellulose (CMC)
High molecular weight hyaluronic acid (HMW-HA / 400 mg)
|
Non-cross-linked
|
Gel 7 (Croma Philart Eye®)
|
Polynucleotides; Sodium hyaluronate; Mannitol
Water; Sodium chloride
Sodium dihydrogen phosphate dihydrate
Disodium phosphate dodecahydrate
|
Non-cross-linked
|
Gel 8 (Haymonyca®,2)
|
Calcium hydroxyapatite microspheres (25–45 μm diameter, 55.7%)
Cross-linked sodium hyaluronate: 20 mg/mL
Phosphate buffer
Lidocaine hydrochloride: 3 mg/mL
|
Cross-linked
|
Gel 9 (Algeness 1,5% Agarose®)
|
Agarose 1%; Water 88.7%
Phosphate buffer 9.8%
|
Does not contain HA
|
| Gel 10 (Inbiotec Amber®) |
2 mL with 1.1% (22 mg) hyaluronic acid and 1.6% succinic acid |
Non-cross-linked
|
3. Results
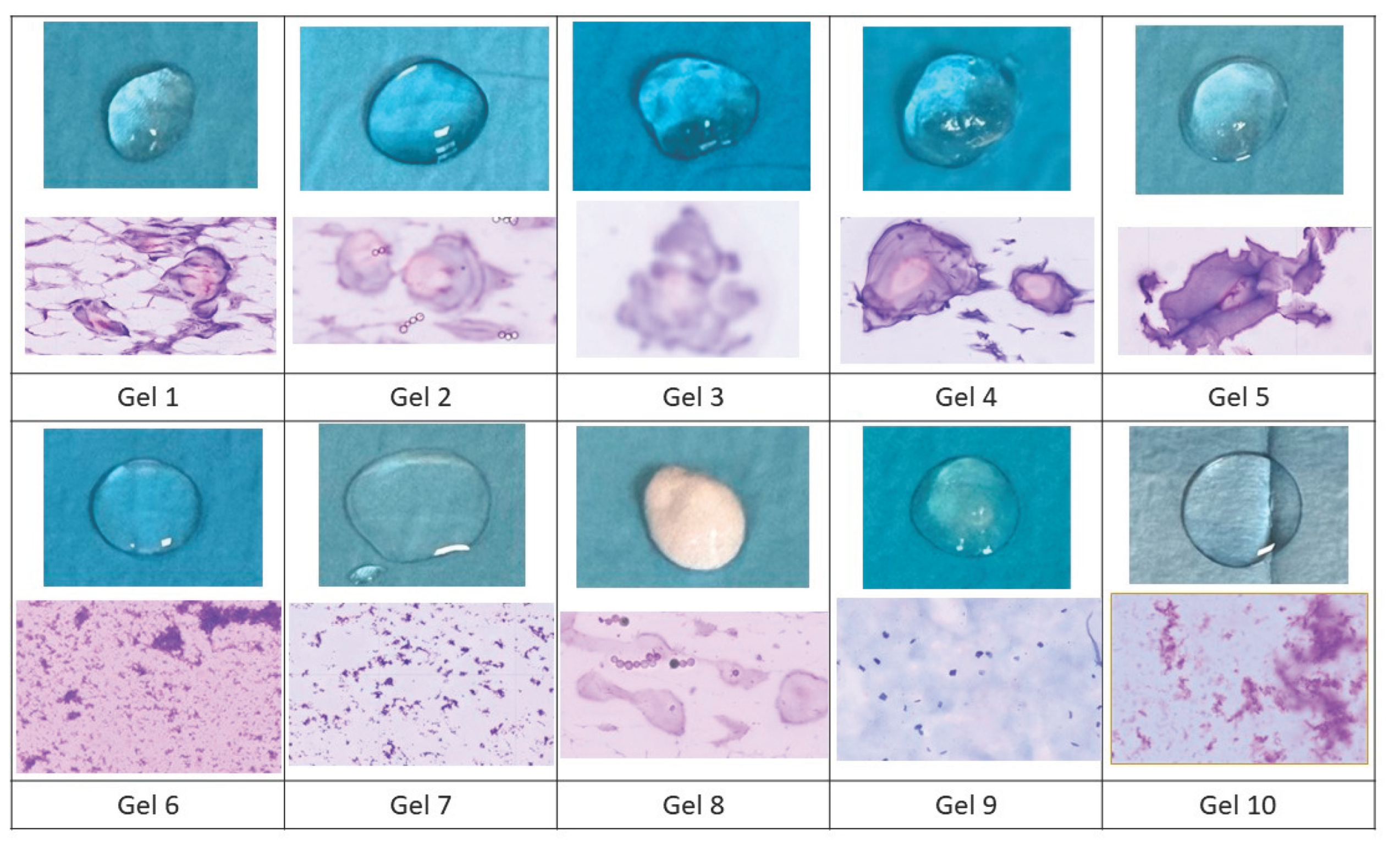
Macroscopically, all gels except gel 8 exhibit transparent and translucent coloration on the microscope slides (
Figure 1). Gels 1, 2, 3, 4, and 5 are denser. Gels 6, 7, 9, and 10 spread more easily on the microscope slides.
At the microscopic level, all studied gels contain a background of gelified texture. However, in gels 1, 2, 3, 4, 5, and 8, particles resembling “pearls” are additionally identified, with spherical and ovoidal morphology, constituted by a pinkish-colored nucleus (acidic) with a peripheral concentric layer of purple coloration (more basic). These particles appear to correspond to the crosslinked HA component contained in the different gels. Regarding the particle or pearl size, it does not vary significantly among the studied gels.
In gels 6, 7, 9, and 10, no recognizable particles are identified. They behave as a bodiless material, which redundantly corresponds to a gel-textured material reminiscent of “dust motes on glass”. Gels 6 and 7 show an overlapping morphology with a basic purple-colored gel texture. In contrast, gel 9 showed a gel texture in this case with polygonal-shaped motes of bluish coloration, more basic. Gel 10 differs from the other three in that the motes are denser.
4. Discussion
In the field of aesthetic medicine, various types of gels are available as injectable filler materials, which must be thoroughly understood to avoid adverse effects. Although significant advances have been made in the formulation and application of fillers [
3], morphological research often receives less attention compared to imaging techniques such as ultrasound.
Despite its importance, the macro and microscopic morphological study of fillers is relatively less known and less explored in the scientific literature. Most studies focus on safety and efficacy from a clinical perspective [
4] but are not always accompanied by detailed morphological analyses. This creates a gap in knowledge regarding particle structure, distribution, and the nature of the surrounding matrix.
Nicola Zerbinati et al. [
5] selected seven injectable HA for optical microscopic study and evaluated their cohesivity properties. They demonstrated that the six fillers cross-linked with PEGDE showed a matrix structure resembling a “spider web”. The same concept could not be demonstrated for the non-cross-linked hydrogel (18 mg/mL).
Patrick Micheels et al. [
6] analyzed Belotero Balance
® and Juvéderm Volbella
® under microscopy, whose composition is based on cross-linked HA. The difference between both lay in the gel technology. No particles were observed in Belotero’s CPM gel
®, whereas they were observed in Juvéderm Volbella’s Vycross technology gel
®. They make no reference to the morphology of HA in vitro.
This study aims to demonstrate that gels containing cross-linked HA (gels 1,2,3,4,5 and 8) have a characteristic common structure in the form of “pearls” with similar size, despite differences in manufacturer. In contrast, gels 6,7 and 10 contain non-cross-linked HA, which is not identifiable at the microscopic level. When immersing the preparation in fixative, and subsequently in stains, the non-cross-linked HA can be easily detached, suggesting that these are not heavy materials, as they are easily eliminated in an aqueous medium.
Gel 9 is a purified agarose gel, which, as we can corroborate with microscopic study, resembles gels with non-cross-linked HA, so its effect when injected into tissue could be similar to that of gels 6,7 and 10.
Regarding tissue integration of HAs, it would be expected that non-cross-linked HAs, being easily eliminated in aqueous media, would diffuse and be eliminated easily in tissue. In contrast, the “pearls” of cross-linked HA are particles with body, which remain on the slide despite washing, suggesting that their permanence in tissue will be greater.
Gels containing cross-linked HA contain “solid” particles with structure and body (1,2,3,4,5 and 8). In contrast, those with gel texture (6,7,9 and 10) are more “liquid”. When injected into tissue, it would be expected that gels with these “solid” particles will provide more localized volume, greater water attraction, combined with a more lasting effect. Gels with “liquid” texture when injected into tissue will dissipate, which would correspond to volume increase with more homogeneous distribution, causing a more subtle effect, and presumably less durable over time.
In the field of adverse effects, something similar could be expected: gels with cross-linked HA may cause more adverse effects than non-cross-linked types, due to their longer time occupying volume and their greater capacity to produce foreign body reaction.
5. Conclusions
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
The morphological study of gels used as filler materials in aesthetic medicine is a field that deserves greater attention in scientific research. Different filler materials exhibit distinct microscopic morphology depending on the type of material from which they are composed.
The type of visible morphological structure could help predict the integration of filler material into dermal tissue and could be correlated with possible adverse effects. It is expected that gels containing non-cross-linked HA will harmonize with the injected tissue, causing fewer adverse effects than cross-linked types. Purified agarose gel resembles gels with non-cross-linked HA at the morphological level, so their effects in tissue could be similar.
In our study, we have been able to establish an in vitro morphological label for some of the gels available on the market. In the event of an adverse effect, puncture-aspiration of the filler material and its subsequent microscopic study can be a tool for identifying injectable gels not recorded in the clinical history.
Further studies are required to advance understanding of the microstructural properties of fillers. Multidisciplinary research that includes morphological study of materials could contribute significantly to the safety and efficacy of aesthetic treatments, improving the experience and outcomes for patients.
Author Contributions
Conceptualization, M.F., M.R., P.T., S.M. and M.O.; methodology, M.F. and M.R.; validation, M.F. and M.R.; formal analysis, M.F. and M.R.; investigation, M.F. and M.R., P.T., S.M. and M.O.; resources, M.R., P.T., S.M. and M.O.; data curation, M.F. and M.R.; writing—original draft preparation, M.F., M.R., P.T., S.M. and M.O.; writing—review and editing, M.F., M.R., P.T., S.M. and M.O.; visualization, M.F., M.R., P.T., S.M., M.O. and S.C.; supervision, M.F., M.R. and S.C.; project administration, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BDDE |
1,4-butanediol diglycidyl ether |
| CMC |
Carboxymethyl cellulose |
| CPM |
Cohesive Polydensified Matrix |
| HA |
Hyaluronic acid |
| HMW-HA |
High molecular weight hyaluronic acid |
| |
|
References
- Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Correction: Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS. Aesthetic Plast Surg. 2024; 48(21):4601. [CrossRef]
- Coca S. Mecanismo acción fillers. In: Tejero P, Bordegaray S, eds. Efectos adversos de los materiales de relleno inyectables. 2nd ed. Madrid: Didot; 2024 Feb 25:37-45.
- Salwowska NM, Bebenek KA, Żądło DA, Wcisło-Dziadecka DL. Physiochemical properties and application of hyaluronic acid: a systematic review. J Cosmet Dermatol. 2016;15(4):520-526. [CrossRef]
- Sánchez-Carpintero I, Candelas D, Ruiz-Rodríguez R. Materiales de relleno: tipos, indicaciones y complicaciones [Dermal fillers: types, indications, and complications]. Actas Dermosifiliogr. 2010;101(5):381-393. [CrossRef]
- Zerbinati N, Sommatis S, Maccario C, et al. Toward Physicochemical and Rheological Characterization of Different Injectable Hyaluronic Acid Dermal Fillers Cross-Linked with Polyethylene Glycol Diglycidyl Ether. Polymers (Basel). 2021;13(6):948. Published 2021 Mar 19. [CrossRef]
- Micheels P, Besse S, Sarazin D. Two Crosslinking Technologies for Superficial Reticular Dermis Injection: A Comparative Ultrasound and Histologic Study. J Clin Aesthet Dermatol. 2017;10(1):29-36.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).