1. Introduction
Potato is the fourth most important staple crop globally, plays a crucial role in ensuring food security(Aksoy et al., 2021). However, potato production is severely affected by potato common scab (PCS), a disease caused by Streptomyces spp., which is widespread and significantly impacts tuber appearance and economic value (TAKEUCHI et al., 1996). Common scab causes corky lesions on the tuber surface, significantly reducing market value. Its cryptic nature and limited efficacy of current management strategies underscore the urgent need for innovative approaches.
The pathogenicity of common scab is closely associated with the production of the phytotoxin Thaxtomin A, which disrupts plant cell walls and induces disease symptoms. In addition to its role in potato scab, Thaxtomin A exhibits phytotoxicity towards other crops, such as sugar beets and radishes. Current management strategies for common scab include breeding resistant cultivars, agricultural practices, chemical control, and biological control, each of which has limitations. Resistant cultivar development is time-consuming and costly, agricultural measures often yield limited efficacy, chemical treatments may lead to environmental pollution and resistance issues, and biological control methods tend to act slowly. Thus, developing safe and efficient strategies for controlling common scab is of paramount importance (Shortall et al., 2024).
Copper (Cu) and zinc (Zn) exhibit dual roles as essential micronutrients and potent antibacterial agents, providing a novel avenue for integrated disease management. Studies have shown that copper- and zinc-based formulations have significant potential to inhibit plant pathogens, reduce toxin production, and enhance crop resistance. This study systematically evaluates the efficacy and underlying mechanisms of copper sulfate (CuSO4) and zinc sulfate (ZnSO4) in controlling potato common scab through in vitro antimicrobial assays, toxin analysis, pot experiments, and molecular biology techniques. Special emphasis is placed on the suppression of Thaxtomin A production and the regulation of potato resistance. The findings provide scientific insights for developing novel strategies to manage common scab (Hunjan & Sabhikhi, 2020).
2. Materials and Methods
2.1. Materials and Culture Media
The Streptomyces strains used in this study included HRB-1 (S. scabies), HL-12 (S. rochei), JX-15 (S. lavendulae), JMS-3 (S. acidiscabies), and AC-15 (S. bottropensis), which were isolated, identified, and preserved by our research team. The test plant was the potato cultivar “Yujing 885.” The primary reagents included copper sulfate (CuSO₄), zinc sulfate (ZnSO₄), and widely used agricultural antibiotics (e.g., streptomycin sulfate and Zhongshengmycin), ensuring comparability of results. Other chemical reagents such as propidium iodide (PI) stain, Thaxtomin A, and chromatographic-grade acetonitrile were purchased from reputable suppliers and met analytical grade specifications. The culture media included ISP2, modified Gause’s No.1, oat bran liquid medium, and 1% water agar, all of which were sterilized at 120°C for 20 minutes before use.
2.2. Antibacterial Effects of Copper and Zinc
The antibacterial activity of CuSO₄ and ZnSO₄ against Streptomyces spp. was evaluated using the disk diffusion method. The CuSO₄ concentrations (0.04–0.16 g/mL) and ZnSO₄ concentrations (0.10–0.50 g/mL) were selected based on preliminary experiments and relevant literature to ensure optimal pathogen suppression without phytotoxicity. A 100 μL aliquot of bacterial suspension was evenly spread onto ISP2 agar plates, and sterilized filter paper discs (0.6 cm in diameter) were placed at the center, each loaded with 15 μL of the corresponding concentrations of the reagents. After incubation at 28°C for 7 days, inhibition zone diameters were measured, and inhibition rates were calculated. Additionally, the inhibitory effects of agricultural streptomycin sulfate, Zhongshengmycin, Polyoxin, Kasugamycin, and Chunlei·Wangtong at gradient concentrations were tested. Half-maximal inhibitory concentrations (EC50) were calculated using regression analysis to evaluate the toxicity of the reagents. Data were analyzed using SPSS 25.0 with ANOVA and Tukey’s test (p < 0.05).
2.3. Mechanisms of Copper and Zinc Effects on Streptomyces scabies
The impact of CuSO₄ and ZnSO₄ on bacterial cell membrane permeability and nuclear integrity was assessed using electrical conductivity and PI staining assays. HRB-1 suspensions (100 μL) were inoculated into ISP2 liquid media containing different concentrations of CuSO₄ (1.00, 1.50, 2.00 g/L) and ZnSO₄ (2.00, 3.50, 5.00 g/L) and cultured in a shaker. Changes in electrical conductivity of the fermentation broth were measured at 0, 1, 2, 3, and 4 hours to evaluate cell membrane damage. Bacterial cells were collected by centrifugation, stained with PI, and observed under a fluorescence microscope to assess nuclear damage. Additionally, high-performance liquid chromatography (HPLC) was used to analyze the Thaxtomin A production by S. scabies under different treatments.
2.4. Effects of Copper and Zinc on Potato Growth
Healthy, pre-sprouted potato tubers were cut into seed pieces and planted in pots containing equal amounts of soil and compound fertilizer, with a planting depth of 10 cm. After one month, roots were inoculated with a mixed suspension of Streptomyces spp. at a concentration of 1 × 10⁸ cfu/mL, applied every 7 days for three consecutive treatments. Subsequently, different concentrations of CuSO₄ (1.00, 1.50, 2.00 g/L) and ZnSO₄ (2.00, 3.50, 5.00 g/L) were applied, while the control group received sterile water, and the positive control group was treated with 0.35 g/L agricultural streptomycin sulfate. At the end of the experiment, plant growth parameters, including height, stem diameter, fresh weight, and dry weight, were measured. Relative chlorophyll content was determined using a chlorophyll meter to evaluate the growth-promoting effects of copper and zinc treatments.
2.5. Analysis of Resistance Enzyme Activity and Gene Expression
After treatment with CuSO₄ (2.00 g/L) and ZnSO₄ (5.00 g/L), plant samples were collected at 4, 8, 12, 36, and 60 hours to measure the activities of resistance-related enzymes, including superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), polyphenol oxidase (PPO), and malondialdehyde (MDA). Total RNA was extracted from samples ground in liquid nitrogen and reverse-transcribed into cDNA. Quantitative real-time PCR (qPCR) was performed to analyze the expression of resistance-related genes (PR1, PR3, PR9, SOD1, HSF1). Gene expression levels were calculated using the 2^-△△Ct method.
3. Results
3.1. Antibacterial Effects of Copper and Zinc on Streptomyces spp. Causing Potato Common Scab
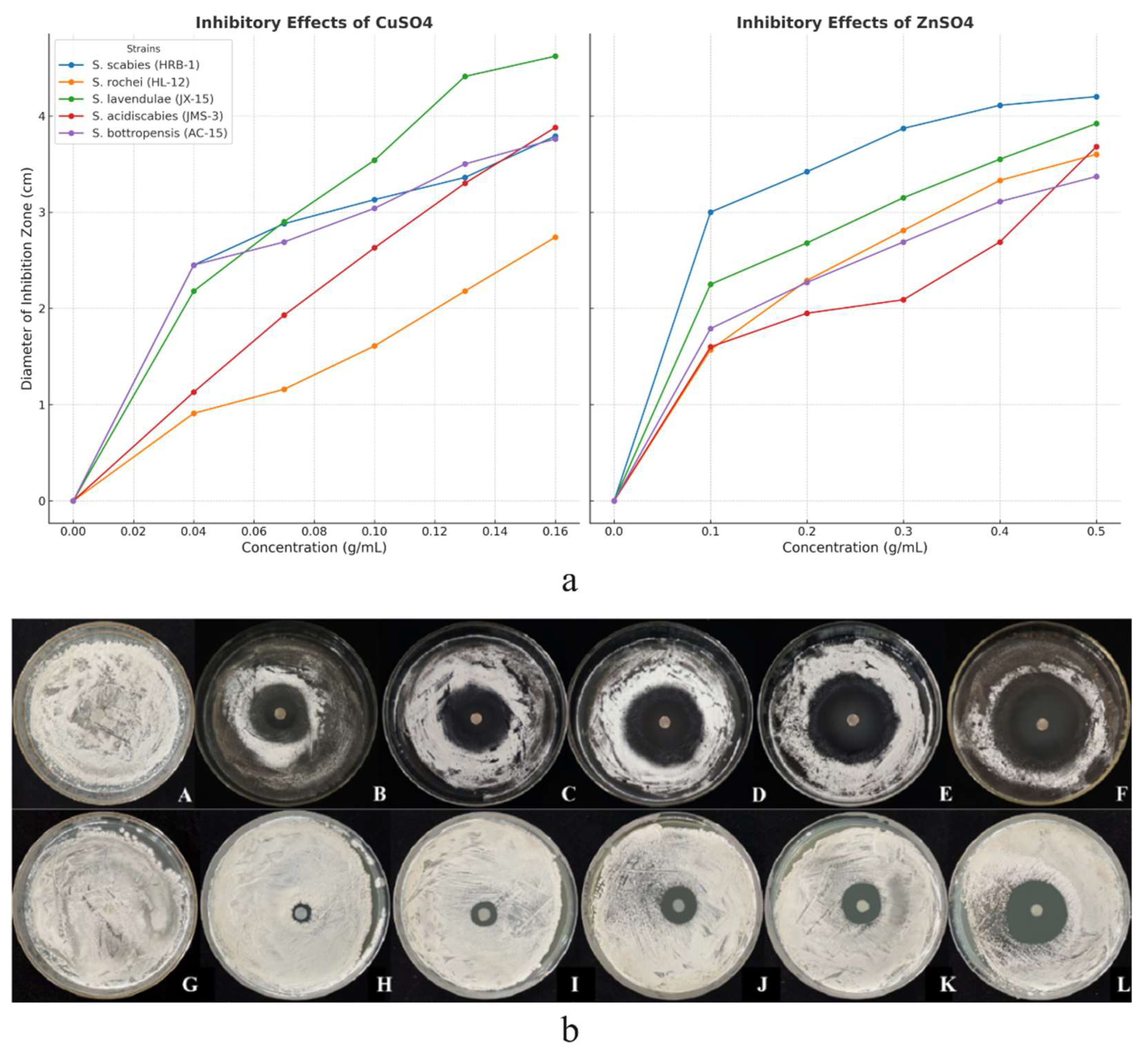
The results showed that copper sulfate (CuSO4) and zinc sulfate (ZnSO4) exhibited significant antibacterial activity against five Streptomyces strains causing potato common scab, with their inhibitory effects increasing in a dose-dependent manner. At a concentration of 0.1 g/mL CuSO₄, the inhibition rates for strains HRB-1 and AC-15 were 22.02% and 19.84%, respectively. ZnSO₄ (0.5 g/mL) demonstrated a 42.86% inhibition rate against strain HRB-1, outperforming other strains. Statistical analysis confirmed the significant differences (p < 0.05) (
Figure 1).
Toxicity assays conducted in vitro indicated that CuSO₄ was most effective against strain JX-15, with an EC50 value of 0.1724 g/mL, while ZnSO₄ demonstrated the highest sensitivity to strain HRB-1, with an EC50 value of 0.9693 g/mL (
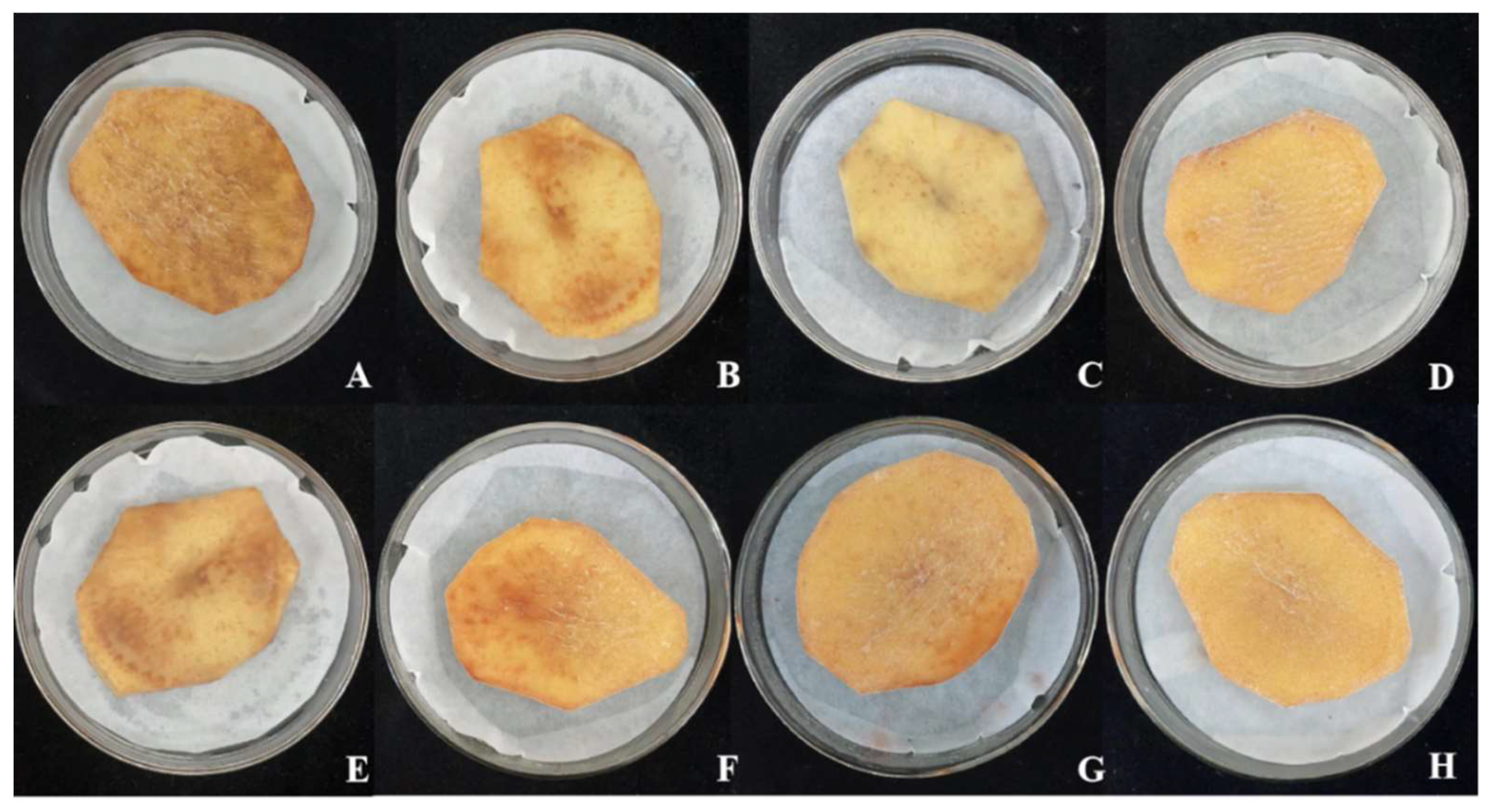
Table 1). Detached tuber assays revealed that both CuSO₄ and ZnSO₄ significantly inhibited browning of potato slices induced by S. scabies (
Figure 2). Potato slices treated with CuSO₄ or ZnSO₄ maintained their shape and showed no signs of decay, whereas those in the untreated control group exhibited progressive browning over time. These findings confirm that CuSO₄ and ZnSO₄ are safe for use on potato tubers at the tested concentrations.
3.2. Disruptive Effects of Copper and Zinc on the Cellular Structure of Pathogens
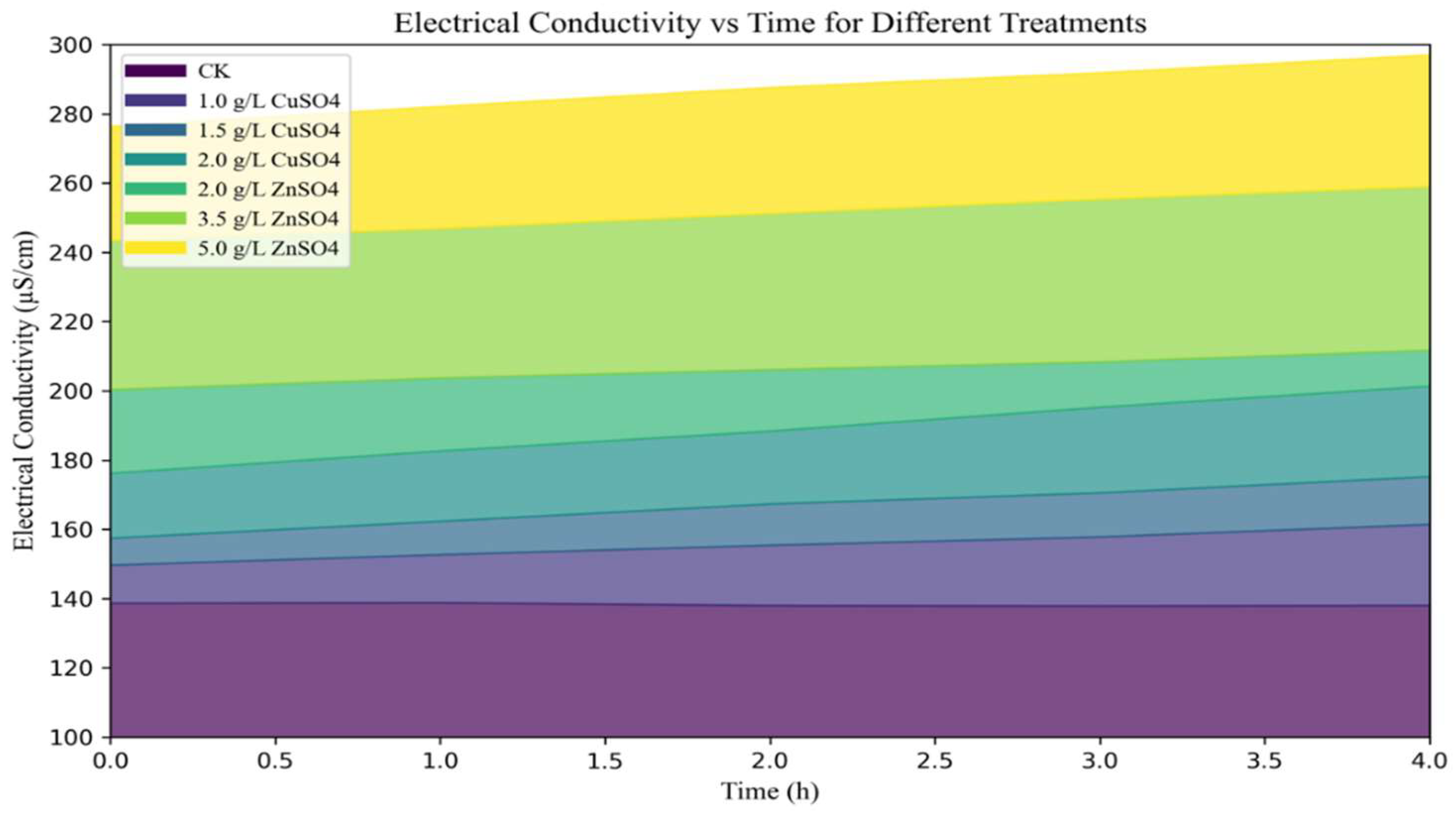
The mechanisms of action of copper and zinc were further elucidated through electrical conductivity and propidium iodide (PI) staining assays. After treatment with copper sulfate (CuSO₄, 2.00 g/L), the electrical conductivity of the solution increased to 201.39 µS/cm, while zinc sulfate (ZnSO₄, 5.00 g/L) treatment resulted in a conductivity incre44ase to 297.05 µS/cm, indicating that high concentrations of these agents significantly disrupted the permeability of the bacterial cell membrane (
Figure 3).
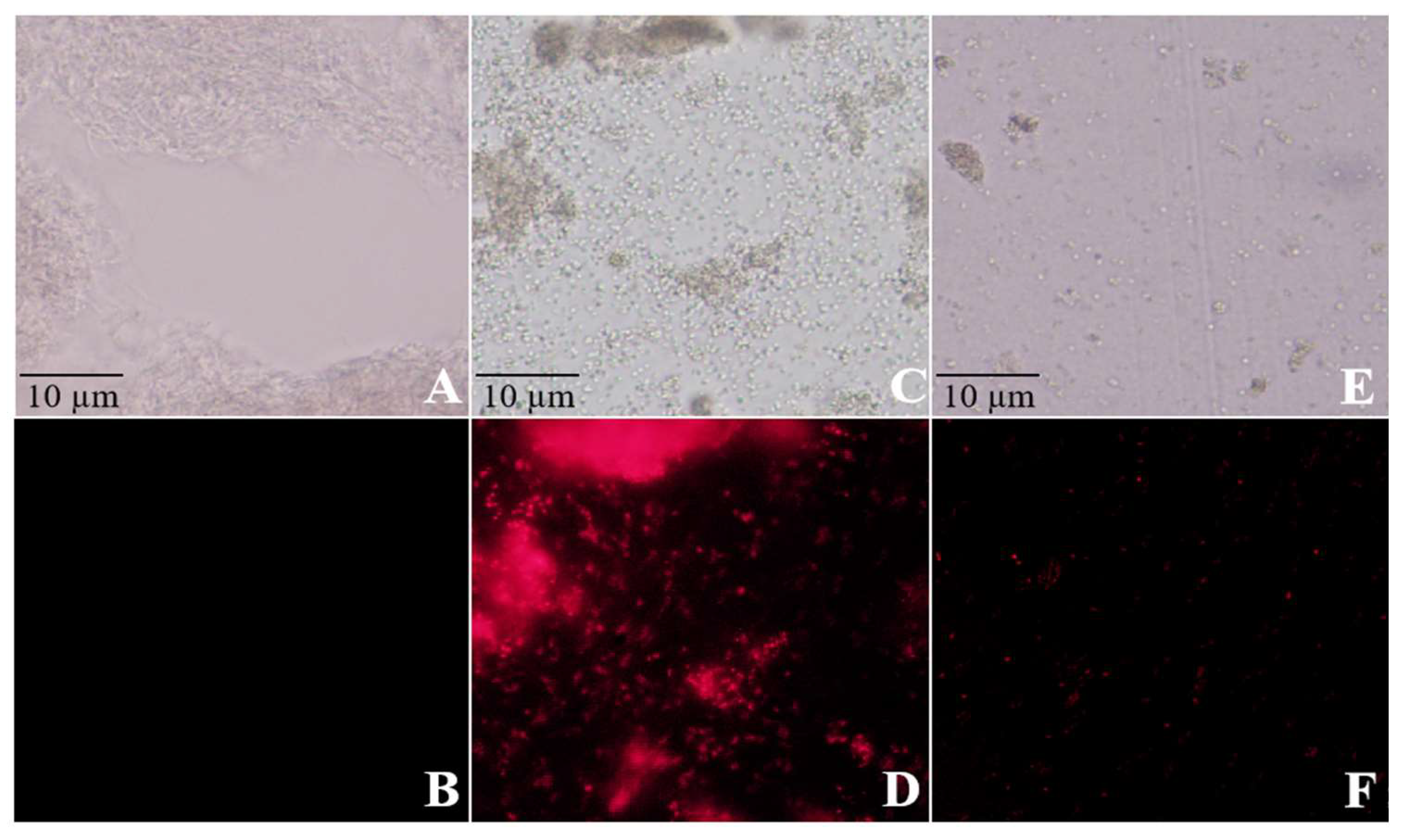
Moreover, PI staining assays revealed intense red fluorescence signals in the nuclei of bacteria treated with CuSO4 or ZnSO4, indicating nucleic acid leakage. These findings further confirm the damaging effects of CuSO₄ and ZnSO₄ on the bacterial cell nucleus (
Figure 4).
3.3. Inhibitory Effects of Copper and Zinc on the Production of the Virulence Toxin Thaxtomin A
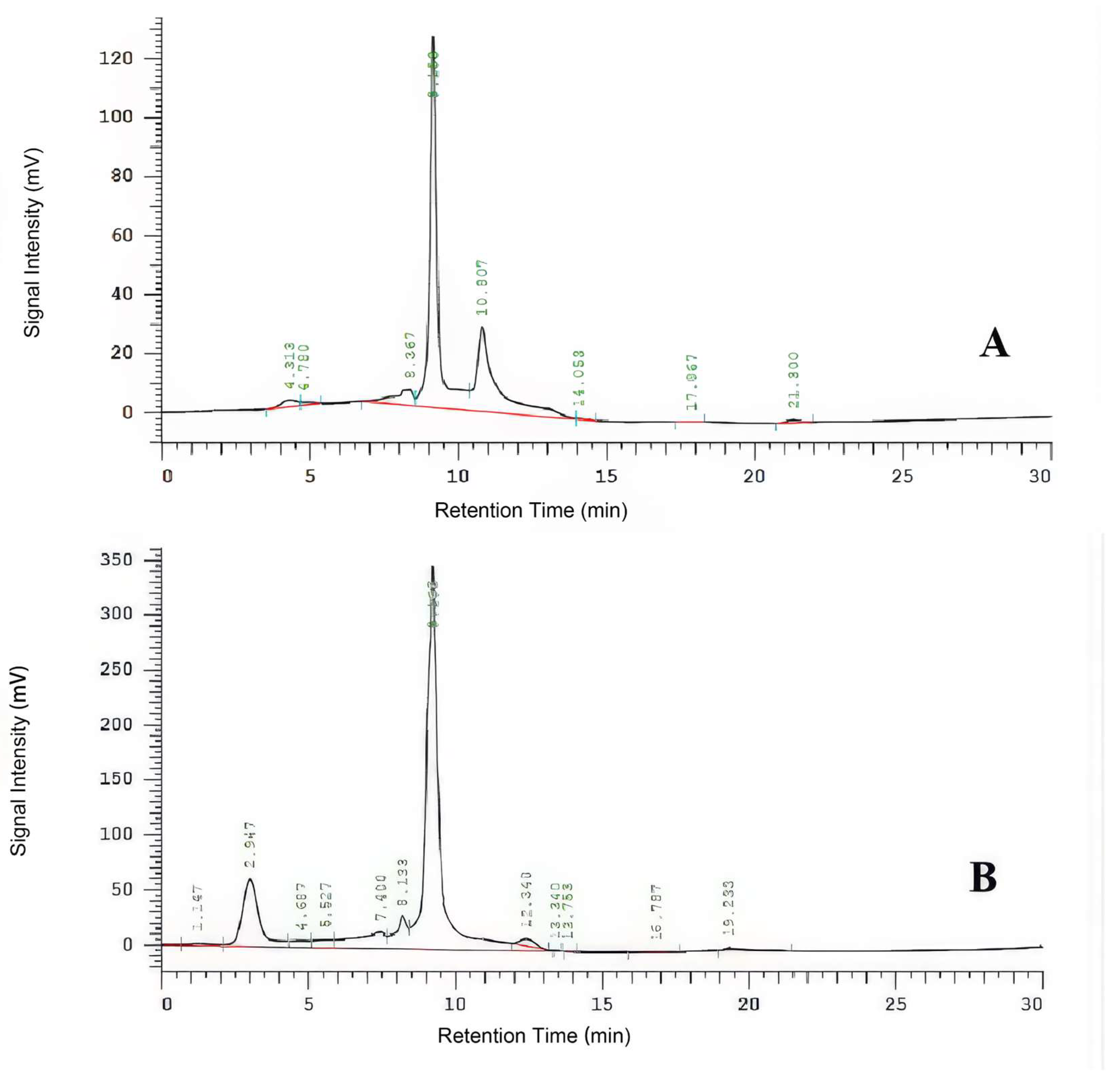
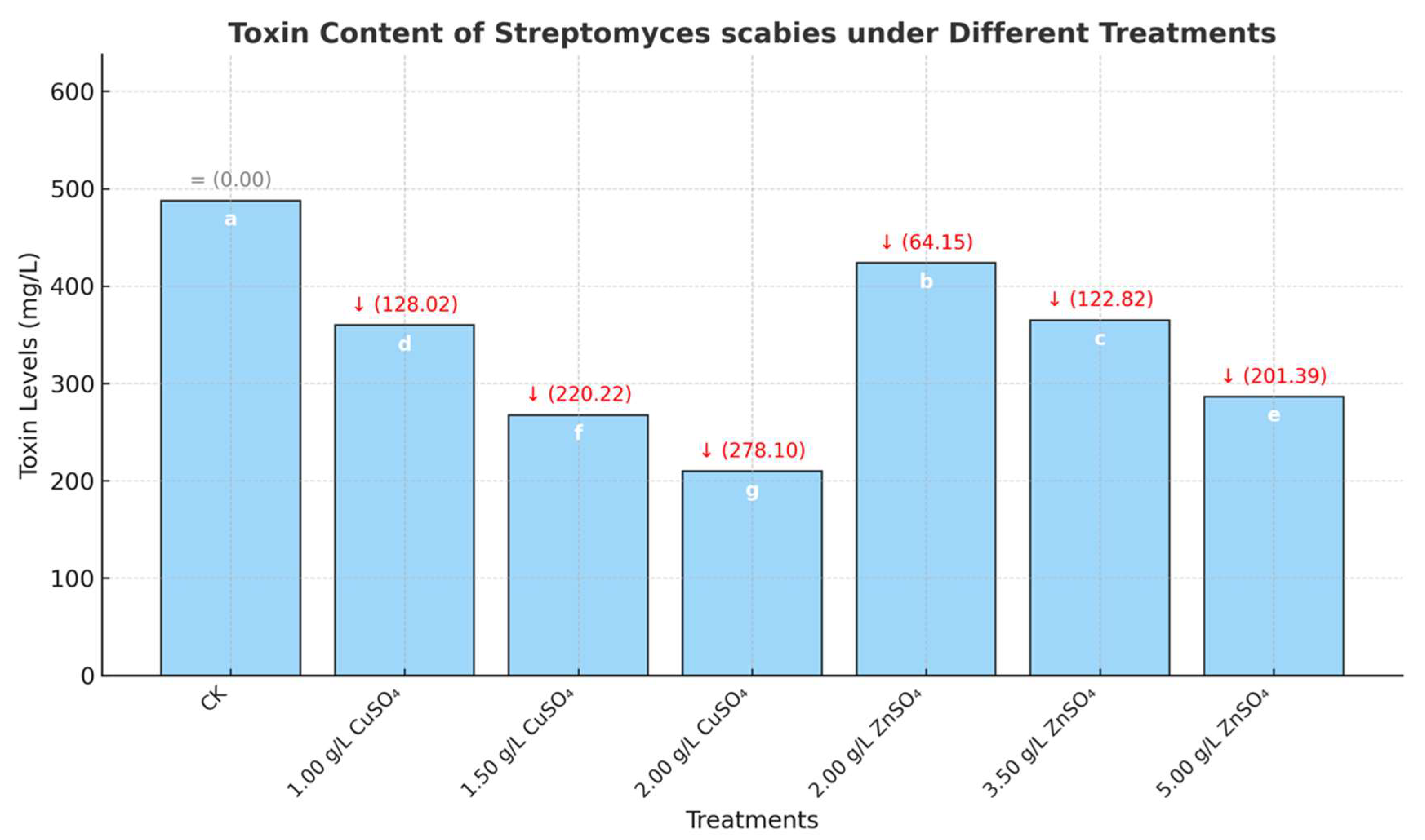
High-performance liquid chromatography (HPLC) analysis demonstrated that copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) significantly suppressed the production of the virulence toxin Thaxtomin A by
Streptomyces spp. (
Figure 5). In the control group, the toxin content was 487.75 mg/L. CuSO₄ treatments (1.00–2.00 g/L) progressively reduced toxin levels, achieving a maximum inhibition rate of 57.02%. ZnSO₄ showed comparable effects, indicating its potential for practical scab management. Similarly, treatment with 5.00 g/L ZnSO₄ decreased the toxin content to 286.36 mg/L (inhibition rate of 41.29%) (
Figure 6). These results indicate that CuSO₄ and ZnSO₄ significantly reduce the pathogenicity of
Streptomyces spp. by inhibiting the production of Thaxtomin A.
3.4. Effects of Copper and Zinc on Potato Growth and Disease Resistance
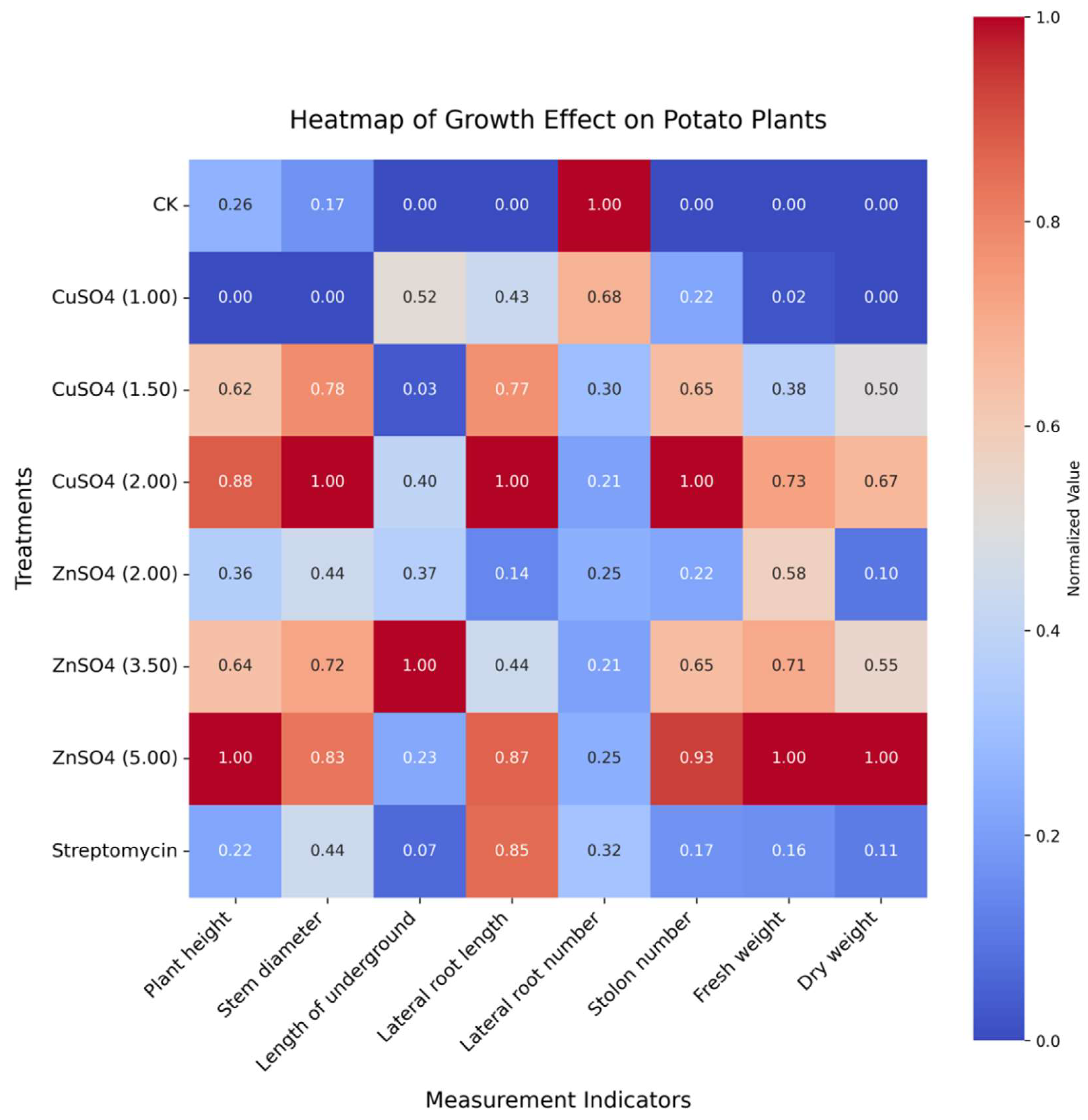
The treatments with copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) significantly improved the growth and development of potato plants. After treatment with 5.00 g/L ZnSO₄, plant height reached 43.48 cm compared to 40.24 cm in the control group, with fresh weight and dry weight increasing to 126.14 g and 42.25 g, respectively. Treatment with 2.00 g/L CuSO₄ increased stem diameter to 1.13 cm, significantly higher than that of the control (
Figure 7).
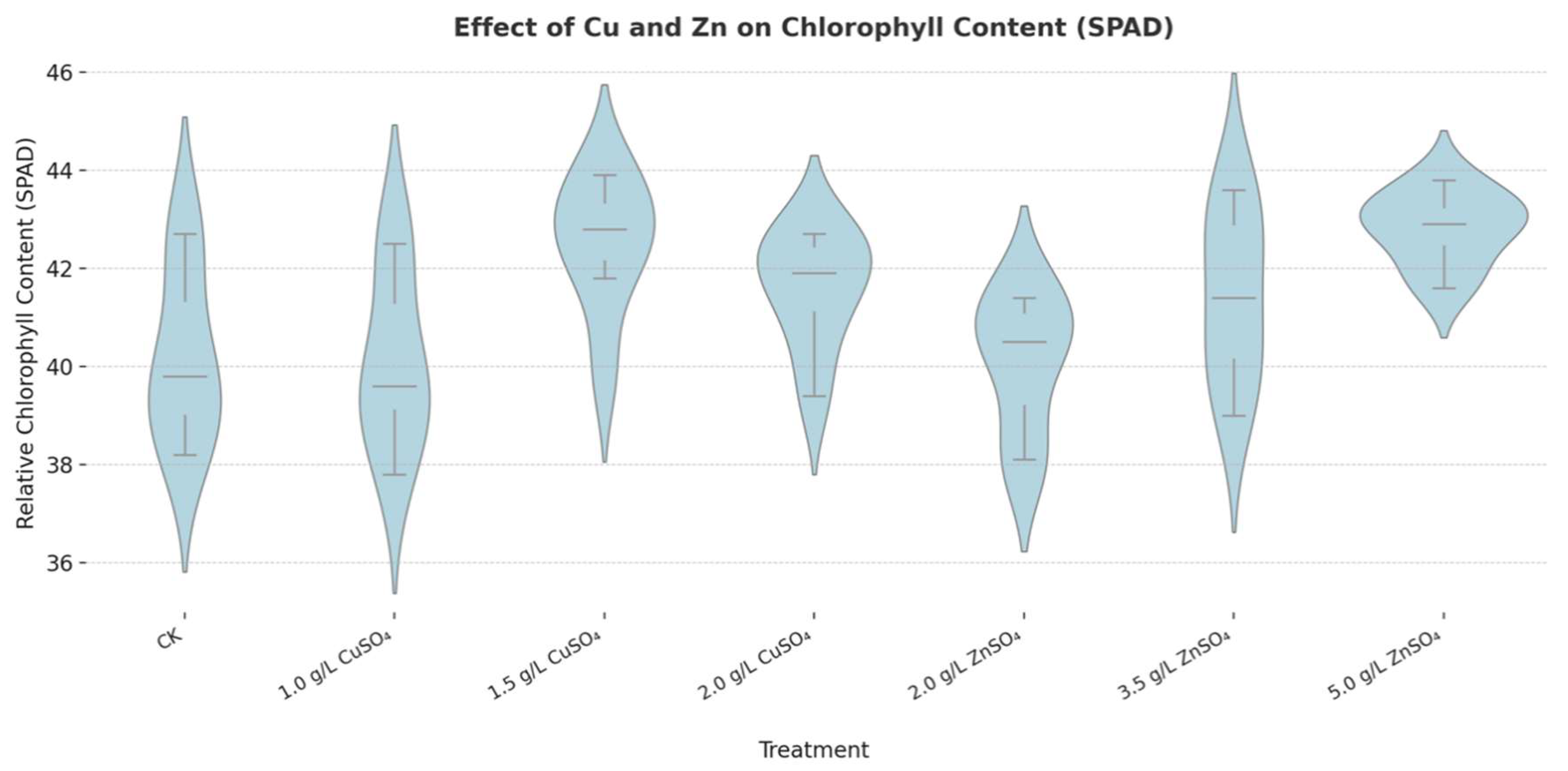
In addition, both CuSO₄ and ZnSO₄ treatments significantly enhanced leaf chlorophyll content, measured as 42.51 SPAD and 42.81 SPAD, respectively, compared to 40.20 SPAD in the control group (
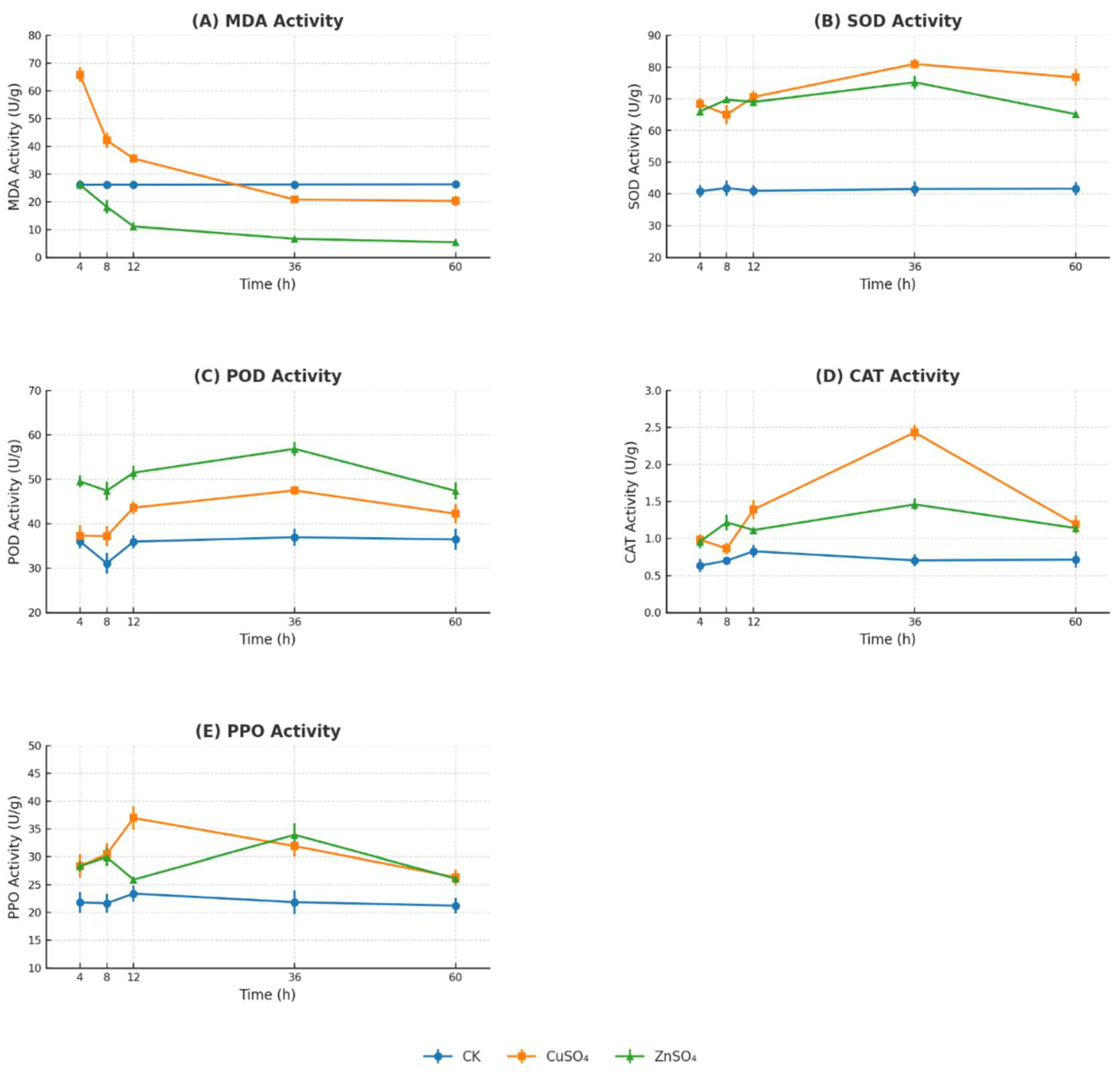
Figure 8). Antioxidant enzyme activity analysis revealed that the treatments markedly increased the activities of SOD, POD, CAT, and PPO, while reducing MDA levels (
Figure 9). These findings indicate that CuSO₄ and ZnSO₄ play a critical role in mitigating oxidative damage and promoting disease resistance in potato plants by enhancing their physiological and biochemical defenses, suggesting their potential application in sustainable agricultural practices.
3.5. Dynamic Changes in the Expression of Disease-Resistance Genes
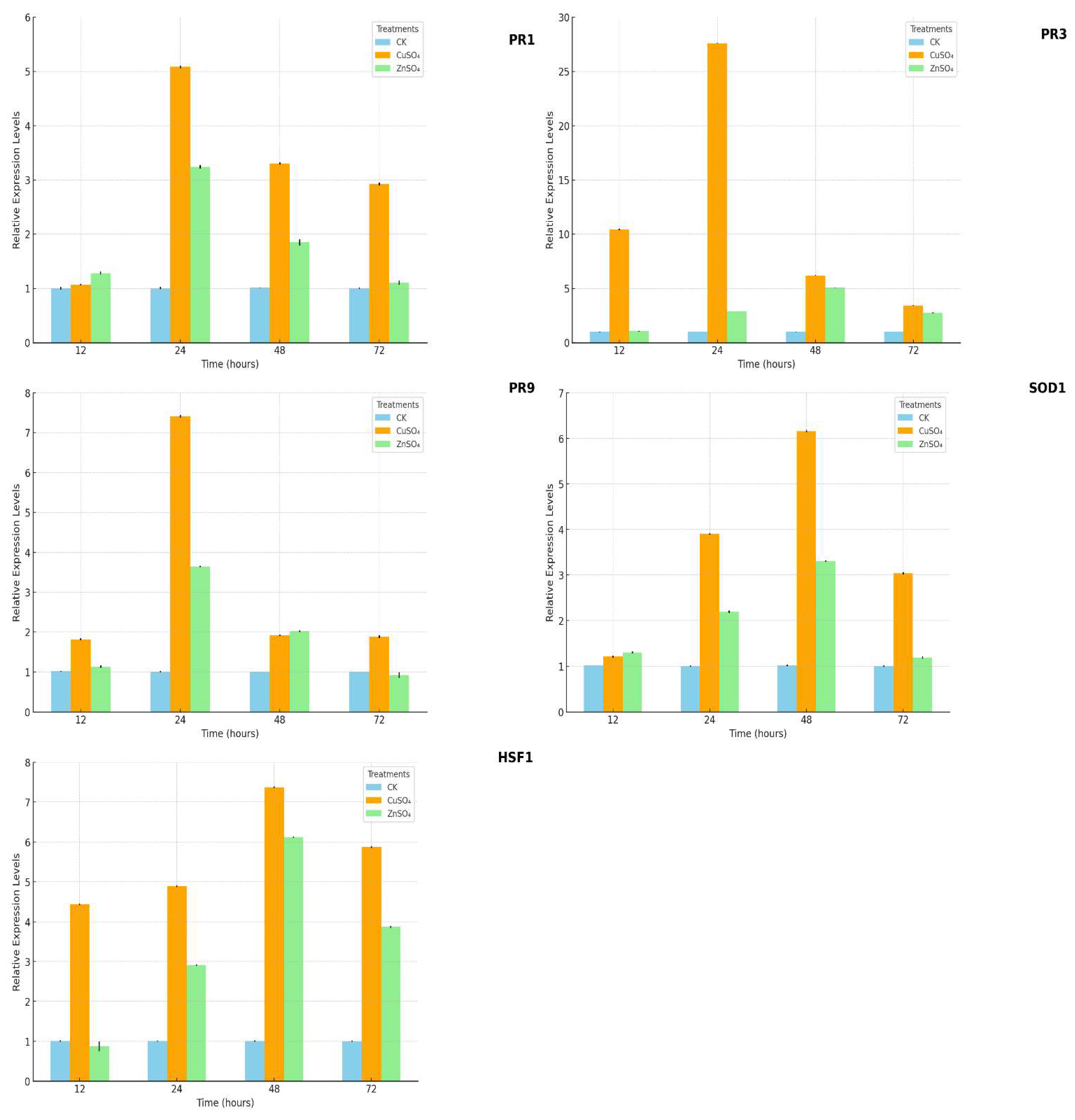
Quantitative real-time PCR (qPCR) analysis revealed that treatments with copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) significantly upregulated the expression of key disease-resistance genes, including PR1, PR3, PR9, SOD1, and HSF1. Notably, the expression level of PR3 peaked at 48 hours after ZnSO₄ treatment, reaching 5.09-fold relative to the control group (
Figure 10).
These results demonstrate that CuSO₄ and ZnSO₄ enhance plant disease resistance through a multifaceted mechanism. This involves the upregulation of defense-related genes, activation of systemic acquired resistance (SAR), and strengthening of antioxidative defense systems. The increased expression of SOD1 highlights a role in mitigating oxidative stress, while the elevated HSF1 levels suggest enhanced stress signaling. Together, these responses synergistically improve the plant’s ability to counteract biotic and abiotic stressors.
Our findings provide a molecular basis for CuSO₄ and ZnSO₄-induced resistance, supporting their potential as effective agrochemical agents for crop protection. Further research should focus on elucidating downstream regulatory networks and assessing the field applicability of these treatments across diverse environmental conditions.
4. Discussion
This study demonstrates that copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) are highly effective in suppressing Streptomyces spp., the causative agents of potato common scab, and in enhancing plant resistance. Both CuSO₄ and ZnSO₄ exhibited dose-dependent antibacterial activity against five pathogenic Streptomyces strains, as evidenced by significant inhibition of lesion expansion in detached tuber assays ((Kopecky et al., 2021; Hassan et al., 2021)). At the tested concentrations, both agents displayed good safety for potato tubers (Abd El-Rahman et al., 2018). Mechanistically, CuSO₄ and ZnSO₄ disrupted bacterial cell structures, evidenced by elevated membrane permeability and nucleic acid leakage, consistent with findings that metal ions can destabilize bacterial membranes through oxidative stress pathways (Quaglia et al., 2021). These effects likely stem from oxidative stress induced by metal ion accumulation, further corroborating previous studies on the antibacterial roles of copper and zinc against soil-borne pathogens (Habib et al., 2022).
The production of Thaxtomin A, a key virulence factor of Streptomyces spp., was significantly inhibited by CuSO₄ and ZnSO₄, with maximum inhibition rates of 57.02% and 41.29%, respectively. Thaxtomin A has been shown to disrupt plant cell walls and induce disease symptoms. Consistent with prior studies(Lawrence et al., 1990), our findings indicate that reducing Thaxtomin A levels can effectively limit pathogen virulence. Previous research has highlighted the potential of metal ions, such as copper and zinc, in modulating toxin production in plant pathogens ((Begg, 2019;Quaglia et al., 2021;Kopecky et al., 2021;Hassan et al., 2021). Our study corroborates these findings and extends their applicability to common scab management by demonstrating that these ions not only suppress toxin production but also disrupt bacterial cell structures, thereby enhancing plant resistance.
At the plant level, CuSO₄ and ZnSO₄ significantly improved growth parameters, including plant height, stem diameter, fresh weight, and dry weight, while also enhancing chlorophyll content. These results suggest a dual role for CuSO₄ and ZnSO₄ in promoting plant growth and boosting disease resistance. Similar growth-promoting effects of zinc have been reported in maize and rice, where it enhanced enzymatic activities and chlorophyll synthesis (Moghadam et al., 2013;Yaghoubian et al., 2021). Furthermore, these metal ions contribute to better physiological traits and antioxidant enzyme activities under stress conditions, as demonstrated in multiple crops(Khan et al., 2022;Yaghoubian et al., 2021).
The treatments also significantly enhanced the activities of antioxidant enzymes, such as SOD, POD, CAT, and PPO, while reducing MDA levels. These changes indicate a reduction in oxidative damage caused by pathogen invasion. Antioxidant defense mechanisms play a critical role in mitigating the effects of reactive oxygen species (ROS) generated during pathogen attack. CuSO₄ and ZnSO₄ treatments likely activate these defense systems by upregulating related pathways. qPCR analysis highlighted the upregulation of key defense-related genes, such as PR3 and SOD1, suggesting activation of systemic acquired resistance pathways mediated by CuSO₄ and ZnSO₄ treatments. These genes are known to mediate plant defense responses, including pathogenesis-related protein production, oxidative stress mitigation, and systemic acquired resistance (Li, 2023;Pal et al., 2023).
Field trials further confirmed the efficacy of CuSO₄ and ZnSO₄ in controlling potato common scab, with maximum control efficiencies of 56.58% and 59.06%, respectively. Notably, ZnSO₄ treatment increased potato yield by 19.29% compared to the control, highlighting its practical application potential. The use of zinc to enhance crop yield and resistance has been previously documented in wheat and soybean, where it was shown to improve nutrient uptake, resistance to diseases, and yield under stress conditions(Kopecky et al., 2021;Alloway, 2008;Khande et al., 2017). This aligns with our findings in potato, further emphasizing the role of micronutrients like zinc sulfate in integrated disease management strategies.
This study provides valuable insights into the potential use of CuSO₄ and ZnSO₄ as effective agents for managing potato common scab. Their dual role in suppressing pathogen growth and enhancing plant resistance underscores their utility in sustainable agricultural practices (Kopecky et al., 2021.;Alloway, 2008). Future research should focus on optimizing application strategies, exploring synergistic effects with other control methods, and evaluating long-term impacts on soil health and microbial ecology (Huber & Graham, 2024). Furthermore, molecular studies are needed to unravel the precise pathways through which copper and zinc mediate their effects on pathogen suppression and plant resistance, particularly in the context of systemic acquired resistance and oxidative stress mechanisms (Bocchini et al., 2021).
Author Contributions
Conceptualization, Nianzhou Chen; Data curation, Nianzhou Chen and Xin Yuan; Formal analysis, Nianzhou Chen; Funding acquisition, Jie Liu and Xuanzhe Zhang; Investigation, Nianzhou Chen and Shuning Zhou; Methodology, Nianzhou Chen; Project administration, Weiqi Jiao and Xuanzhe Zhang; Resources, Nianzhou Chen and Shuo Yan; Software, Nianzhou Chen; Supervision, Xinbo Wang and Xuanzhe Zhang; Validation, Nianzhou Chen, Shuning Zhou and Shuo Yan; Writing – original draft, Nianzhou Chen and Weiqi Jiao; Writing – review & editing, Xuanzhe Zhang.
Funding
This research was funded by Project of the Department of Science, Technology and Education of the Ministry of Agriculture and Rural Affairs (13220135), Horizontal Project on the Control of Diseases of Economic Crops in Heilongjiang Province (2024HXZB010)
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Gene & Accession Numbers
PR1 (Pathogenesis-related protein 1), PR3 (Chitinase), PR9 (Peroxidase), SOD1 (Superoxide dismutase 1), HSF1 (Heat shock factor 1)
Abbreviations
The following abbreviations are used in this manuscript:
| CAT |
Catalase |
| CuSO₄ |
Copper sulfate |
| HPLC |
High-performance liquid chromatography |
| MDA |
Malondialdehyde |
References
- El-Rahman, A.F.A.; El-Kafrawy, A.A.; El-Hafez, O.A.A.; El-Ghany, R.E.A. EVALUATION OF SOME FUNGICIDES EFFECTIVENESS IN CONTROL OF BLACKLEG AND COMMON SCAB OF POTATO. Egypt. J. Agric. Res. 2018, 96, 1307–1323. [Google Scholar] [CrossRef]
- Aksoy, E. , Demirel, U., Bakhsh, A., Zia, M. A. B., Naeem, M., Saeed, F., Çalışkan, S., & Çalışkan, M. E. (2021). Recent Advances in Potato (Solanum tuberosum L.) Breeding. In J. M. Al-Khayri, S. M. Jain, & D. V. Johnson (Eds.), Advances in Plant Breeding Strategies: Vegetable Crops: Volume 8: Bulbs, Roots and Tubers (pp. 409–487). Springer International Publishing.
- Alloway, B. J. (2008). Micronutrients and Crop Production: An Introduction. In B. J. Alloway (Ed.), Micronutrient Deficiencies in Global Crop Production (pp. 1–39). Springer Netherlands.
- Begg, S.L. The role of metal ions in the virulence and viability of bacterial pathogens. Biochem. Soc. Trans. 2019, 47, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.; Islam, M.; Islam, A.T.M.S.; Hassan, S.M.E.; Hasan, M. In vitro and in vivo efficacy of some chemicals against common scab (Streptomyces scabies) of potato. Arch. Phytopathol. Plant Prot. 2022, 55, 886–899. [Google Scholar] [CrossRef]
- Hassan, M.M.; Elfarash, A.E.; Abo-Elyousr, K.A.; Hussein, M.A. Efficacy of Some Biocontrol Agents Against Streptomyces scabiei the Causative of Common Scab Disease in Potatoes. Egypt. J. Phytopathol. 2021, 49, 168–178. [Google Scholar] [CrossRef]
- Hunjan, M.S.; Sabhikhi, H.S. Designing a crop rotation strategy to manage Streptomyces scabies causing potato scab in north India. J. Phytopathol. 2020, 168, 469–477. [Google Scholar] [CrossRef]
- Khan, M.A.; Yasmin, H.; Shah, Z.A.; Rinklebe, J.; Alyemeni, M.N.; Ahmad, P. Co application of biofertilizer and zinc oxide nanoparticles upregulate protective mechanism culminating improved arsenic resistance in maize. Chemosphere 2022, 294, 133796. [Google Scholar] [CrossRef] [PubMed]
- Khande, R.; Sharma, S.K.; Ramesh, A.; Sharma, M.P. Zinc solubilizing Bacillus strains that modulate growth, yield and zinc biofortification of soybean and wheat. Rhizosphere 2017, 4, 126–138. [Google Scholar] [CrossRef]
- Kopecky, J.; Rapoport, D.; Sarikhani, E.; Stovicek, A.; Patrmanova, T.; Sagova-Mareckova, M. Micronutrients and Soil Microorganisms in the Suppression of Potato Common Scab. Agronomy 2021, 11, 383. [Google Scholar] [CrossRef]
- Lawrence, C. H.; Clark, M. C.; King, R. R. Induction of Common Scab Symptoms in Aseptically Cultured Potato Tubers by the Vivotoxin, Thaxtomin. Phytopathology 1990, 80, 606–608. [Google Scholar] [CrossRef]
- Li, F. (2023). Inflammation, vascular biology, endothelium, and oxidative stress. Clinical Chemistry and Laboratory Medicine (CCLM), 61(s1), s1376–s1417.
- Moghadam, H. R. T. , Zahedi, H., & Ashkiani, A. (2013). Effect of zinc foliar application on auxin and gibberellin hormones and catalase and superoxide dismutase enzyme activity of corn (Zea mays L) under water stress.
- Pal, G.; Saxena, S.; Kumar, K.; Verma, A.; Kumar, D.; Shukla, P.; Pandey, A.; Verma, S.K. Seed endophytic bacterium Bacillus velezensis and its lipopeptides acts as elicitors of defense responses against Fusarium verticillioides in maize seedlings. Plant Soil 2023, 492, 109–124. [Google Scholar] [CrossRef]
- Quaglia, M.; Bocchini, M.; Orfei, B.; D’amato, R.; Famiani, F.; Moretti, C.; Buonaurio, R. Zinc phosphate protects tomato plants against Pseudomonas syringae pv. tomato. J. Plant Dis. Prot. 2021, 128, 989–998. [Google Scholar] [CrossRef]
- Shortall, O.; Mahon, N.; Hardy, C.; Kyle, C. “Nobody here is an individual”: Developing a place-based understanding of biosecurity for managing sheep scab on the Western Isles of Scotland. Prev. Veter- Med. 2024, 233, 106332. [Google Scholar] [CrossRef] [PubMed]
- TAKEUCHI, T.; SAWADA, H.; TANAKA, F.; MATSUDA, I. Phylogenetic Analysis of Streptomyces spp. Causing Potato Scab Based on 16S rRNA Sequences. International Journal of Systematic and Evolutionary Microbiology 1996, 46(2), 476–479. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubian, I.; Ghassemi, S.; Nazari, M.; Raei, Y.; Smith, D.L. Response of physiological traits, antioxidant enzymes and nutrient uptake of soybean to Azotobacter Chroococcum and zinc sulfate under salinity. South Afr. J. Bot. 2021, 143, 42–51. [Google Scholar] [CrossRef]
Figure 1.
Inhibitory effects of copper sulfate and zinc sulfate solutions on selected Streptomyces spp. at different concentrations. (a) Inhibitory effects of copper sulfate and zinc sulfate on five Streptomyces spp. at varying concentrations. (b) Specific inhibitory effects on selected strains:. A–F: Inhibitory effects of copper sulfate on strain AC-15 at different concentrations. A: CK (control), B: 0.04 g/mL, C: 0.07 g/mL, D: 0.10 g/mL, E: 0.13 g/mL, F: 0.16 g/mL. G–L: Inhibitory effects of zinc sulfate on strain JMS-3 at different concentrations. G: CK (control), H: 0.10 g/mL, I: 0.20 g/mL, J: 0.30 g/mL, K: 0.40 g/mL, L: 0.50 g/mL.
Figure 1.
Inhibitory effects of copper sulfate and zinc sulfate solutions on selected Streptomyces spp. at different concentrations. (a) Inhibitory effects of copper sulfate and zinc sulfate on five Streptomyces spp. at varying concentrations. (b) Specific inhibitory effects on selected strains:. A–F: Inhibitory effects of copper sulfate on strain AC-15 at different concentrations. A: CK (control), B: 0.04 g/mL, C: 0.07 g/mL, D: 0.10 g/mL, E: 0.13 g/mL, F: 0.16 g/mL. G–L: Inhibitory effects of zinc sulfate on strain JMS-3 at different concentrations. G: CK (control), H: 0.10 g/mL, I: 0.20 g/mL, J: 0.30 g/mL, K: 0.40 g/mL, L: 0.50 g/mL.
Figure 2.
Infection effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on Streptomyces scabies in potato chips in vitro. Note: A、E: CK,B: 1.00 g/L CuSO4,C: 1.50 g/L CuSO4,D: 2.00 g/L CuSO4,F: 2.00 g/L ZnSO4,G: 3.50 g/L ZnSO4,H: 5.00 g/L ZnSO4.
Figure 2.
Infection effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on Streptomyces scabies in potato chips in vitro. Note: A、E: CK,B: 1.00 g/L CuSO4,C: 1.50 g/L CuSO4,D: 2.00 g/L CuSO4,F: 2.00 g/L ZnSO4,G: 3.50 g/L ZnSO4,H: 5.00 g/L ZnSO4.
Figure 3.
Effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on the cell membrane conductivity of Streptomyces scabies.
Figure 3.
Effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on the cell membrane conductivity of Streptomyces scabies.
Figure 4.
Effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on the cell nucleus of Streptomyces scabies. Note: A: CK had no fluorescence, B: CK had fluorescence, C: CuSO4 treatment had no fluorescence, D: CuSO4 treatment had fluorescence, E: ZnSO4 treatment had no fluorescence, F: ZnSO4 treatment had fluorescence.
Figure 4.
Effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on the cell nucleus of Streptomyces scabies. Note: A: CK had no fluorescence, B: CK had fluorescence, C: CuSO4 treatment had no fluorescence, D: CuSO4 treatment had fluorescence, E: ZnSO4 treatment had no fluorescence, F: ZnSO4 treatment had fluorescence.
Figure 5.
HPLC analysis of Thaxtomin A toxin. Note: A: HPLC for standards, B: HPLC for part of samples.
Figure 5.
HPLC analysis of Thaxtomin A toxin. Note: A: HPLC for standards, B: HPLC for part of samples.
Figure 6.
Toxin content produced by Streptomyces scabies under different concentrations of medicament treatments.
Figure 6.
Toxin content produced by Streptomyces scabies under different concentrations of medicament treatments.
Figure 7.
Growth effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on potato plants.
Figure 7.
Growth effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on potato plants.
Figure 8.
Effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on chlorophyll content.
Figure 8.
Effects of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) on chlorophyll content.
Figure 9.
Changes in the activities of SOD, POD, CAT, PPO, and MDA in potato leaves under different concentrations of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) treatments.
Figure 9.
Changes in the activities of SOD, POD, CAT, PPO, and MDA in potato leaves under different concentrations of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) treatments.
Figure 10.
Expression changes of PR1, PR3, PR9, SOD1, and HSF1 genes in potato leaves under different concentrations of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) treatments.
Figure 10.
Expression changes of PR1, PR3, PR9, SOD1, and HSF1 genes in potato leaves under different concentrations of copper sulfate (CuSO₄) and zinc sulfate (ZnSO₄) treatments.
Table 1.
Determination of the in vitro virulence of copper sulfate and zinc sulfate against five Streptomyces spp.
Table 1.
Determination of the in vitro virulence of copper sulfate and zinc sulfate against five Streptomyces spp.
| Medicament |
Strains |
Regression equation |
R2
|
EC50 (g/mL) |
| CuSO4
|
HRB-1 (S. scabies) |
Y=0.7256x+5.2297 |
0.9709 |
0.4824 |
| HL-12 (S. rochei) |
Y=1.8788x+5.7666 |
0.9708 |
0.3908 |
| JX-15 (S. lavendulae) |
Y=1.4410x+6.1002 |
0.9841 |
0.1724 |
| JMS-3 (S. acidiscabies) |
Y=2.0650x+6.3676 |
0.9997 |
0.2176 |
| AC-15 (S. bottropensis) |
Y=0.7739x+5.2670 |
0.9426 |
0.4518 |
| ZnSO4
|
HRB-1 (S. scabies) |
Y=0.5828x+5.0079 |
0.9872 |
0.9693 |
| HL-12 (S. rochei) |
Y=1.2072x+5.0065 |
0.9988 |
0.9877 |
| JX-15 (S. lavendulae) |
Y=0.8400x+4.9510 |
0.9775 |
1.1438 |
| JMS-3 (S. acidiscabies) |
Y=1.0692x+4.8013 |
0.8255 |
1.5341 |
| AC-15 (S. bottropensis) |
Y=0.9122x+4.8199 |
0.9917 |
1.5756 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).