1. Introduction
Breast cancer (BC) may be associated with neurological paraneoplastic syndromes (NPS) in about 1% of cases [
1,
2,
3]. NPS may include subacute cerebellar degeneration, brainstem encephalitis, encephalomyelitis, myelopathy, Stiff Person syndrome, paraneoplastic retinopathy, lower motor neuron diseases, and opsoclonus–myoclonus syndrome (OMS). OMS consists of large-amplitude conjugate saccades, known as opsoclonus, occurring in all directions, which are associated with spontaneous myoclonus of the head, trunk, or limbs, along with ataxia, strabismus, aphasia, or loss of speech [
1,
2,
3]. Symptom onset is acute and peaks within one month, potentially progressing to stupor and coma. Ectopic expression of neural antigens by the tumor in patients with PNS triggers a potent immune response that finally targets the neurons expressing the shared antigen (onconeural antigen) with cancer [
1]. It is an autoimmune disorder associated with onco-neural autoantibodies, specifically the type II anti-Ri nuclear antibody (ANNA-2), which is directed against the intracellular Ri antigen (Ri-PNS) that cross-reacts with two neuron-specific antigens, Nova-1 and Nova-2 [
4,
5,
6,
7]. These are neuron-specific KH-type RNA-binding proteins found in patients with BC. However, the development of OMS in patients with different types of cancer, and sometimes different autoantibodies, such as the anti-Yo and anti-Purkinje cell antibodies, along with the multifaceted symptoms, suggests that it may result from the involvement of several structures in the central nervous system. This syndrome may precede the diagnosis of BC and tends to recur and persist after cancer treatment. The diagnosis of suspicious OMS should prompt physicians to search for an undetected BC, although it may also be associated with lung cancer and other malignancies, such as ovarian teratoma. Patients with paraneoplastic OMS are generally older, with a worse prognosis and higher relapse rate than those with the idiopathic form. Outcomes also vary depending on disease duration, treatments employed, and the responsiveness of the underlying cancer. In this paper, we report a case of a woman presenting with a complex neurological picture found to harbor breast cancer and treated after a multidisciplinary team evaluation where neurologists played a pivotal role. Clinical cases in the medical literature with almost complete neurological and oncological data are also reviewed.
2. Case Presentation
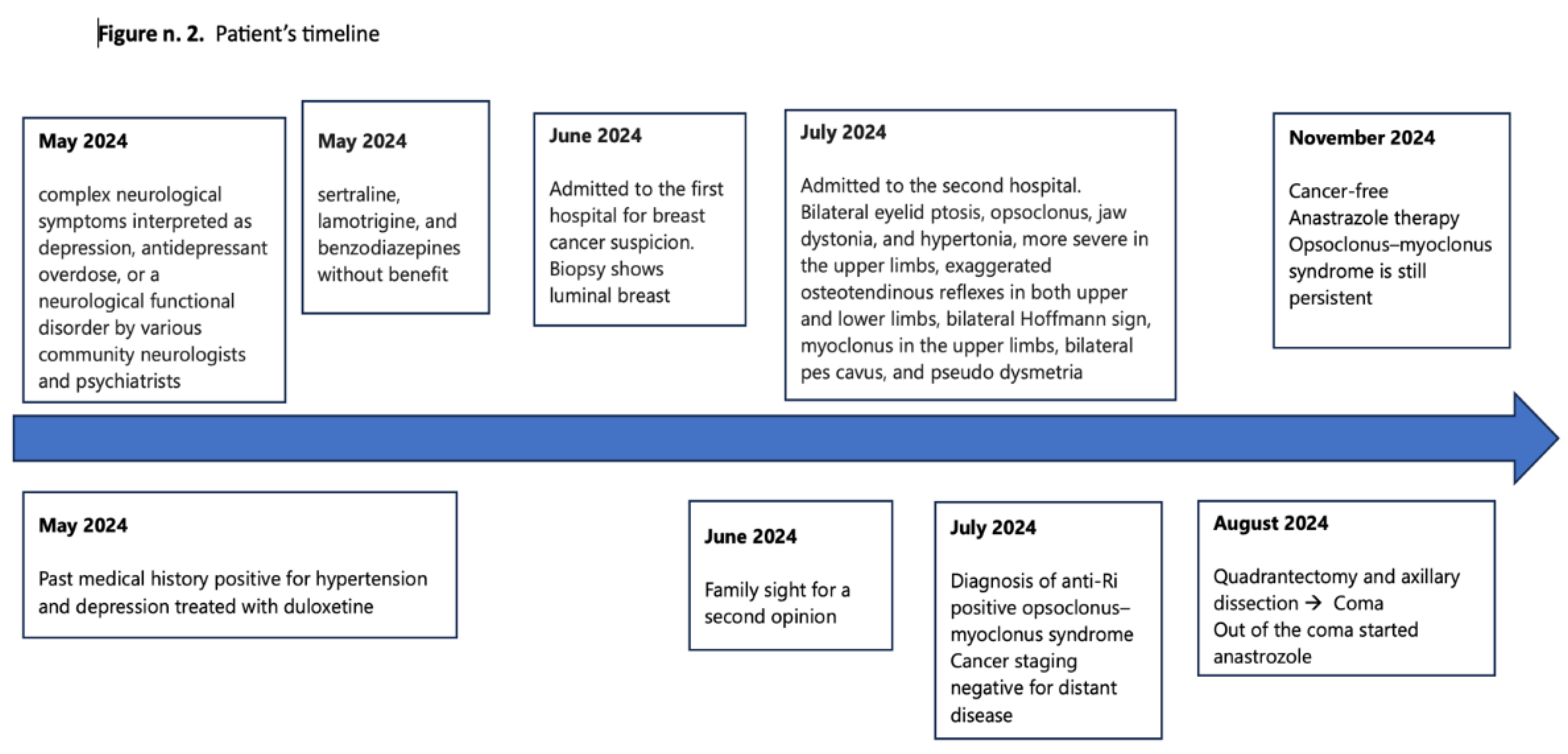
On June 1, 2024, a 58-year-old Caucasian woman was admitted to a secondary oncology center for suspicious breast cancer and complex neurological symptoms, which were initially interpreted as depression, antidepressant overdose, or a neurological functional disorder by various community neurologists and psychiatrists.
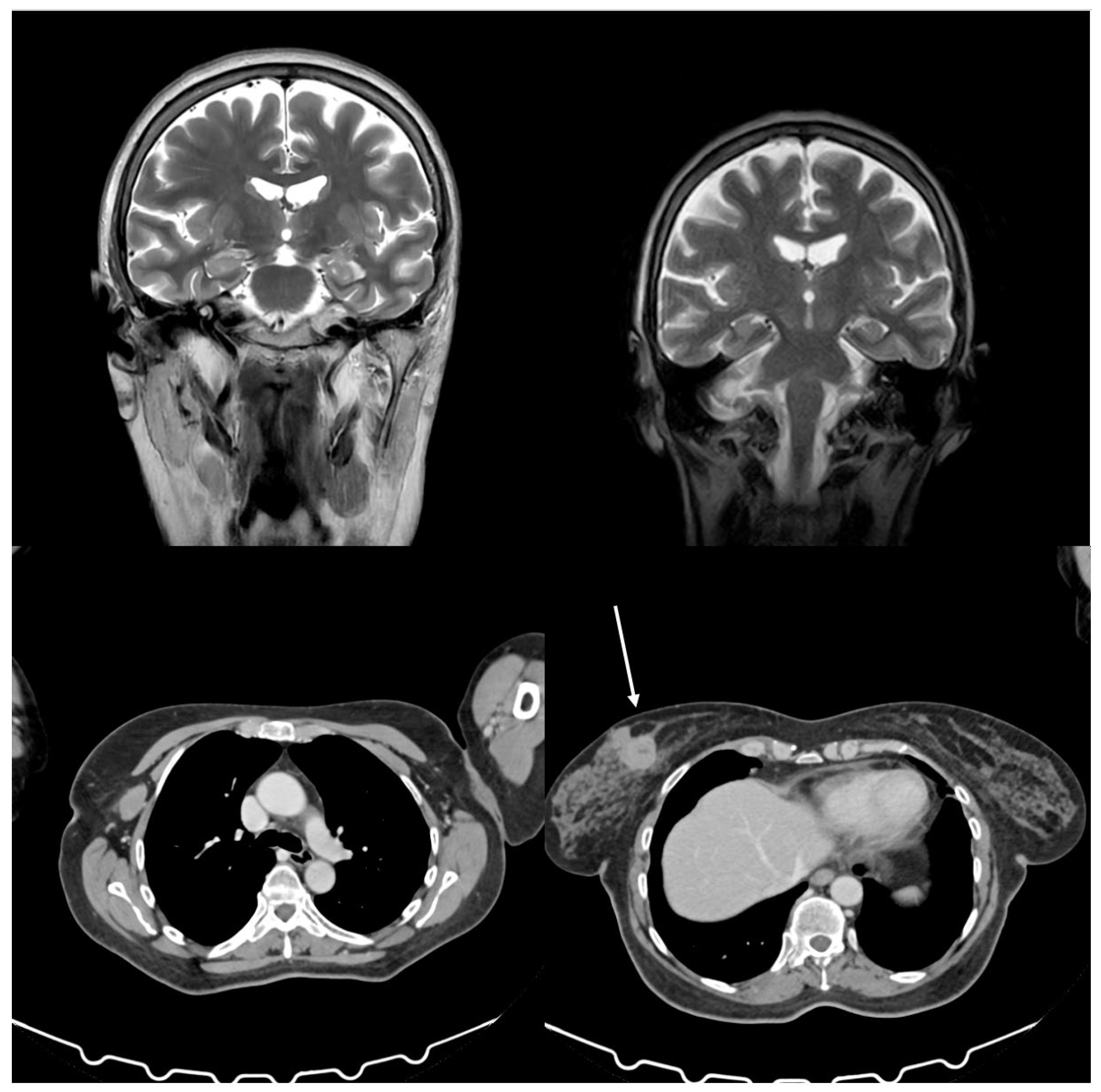
Figure 1 shows patient’s history timeline. She was treated with sertraline, lamotrigine, and benzodiazepines without any clinical benefit. Her medical history was positive for hypertension and depression, treated with duloxetine, and negative for diabetes, allergies, and any other significant comorbidities. Mammography and breast sonogram revealed a spicular mass measuring 3.3 x 2.6 cm located in the internal upper quadrant of the left breast. A total body CT scan confirmed the mass in the left breast, the presence of several lymph nodes in the homolateral axilla measuring 2.7 cm without distant metastases, and a mass in the right kidney consistent with an angiomyolipoma (
Figure 2). An MRI confirmed the findings in the breast and kidney (
Figure 2). A biopsy of the breast lesion and axillary nodes showed poorly differentiated ductal infiltrating carcinoma of the left breast, with 95% positive estrogen receptors, 12% positive progesterone receptors, HER-2 2+, FISH not amplified, Ki67 at 50%, E-cadherin positive, CK5 positive, and no vascular or perineural invasion. The cardiology function evaluation was normal. The breast unit multidisciplinary team concluded that the patient was unfit for neoadjuvant chemotherapy due to neurological symptoms and referred her back to the surgeon for upfront surgery. The family then sought a second opinion at the University Hospital of Palermo, Italy, where the patient was admitted in the same month with a suspected paraneoplastic neurological syndrome. Upon admission, the patient complained of vertigo, nausea, vomiting, and rigidity in all four limbs that had occurred ten months prior and progressively worsened. During the neurological evaluation, the patient presented with bilateral eyelid ptosis, opsoclonus, jaw dystonia, and hypertonia, more severe in the upper limbs, as well as exaggerated osteotendinous reflexes in both upper and lower limbs, bilateral Hoffmann sign, myoclonus in the upper limbs, bilateral pes cavus, and pseudo dysmetria. Only the hypertonia in the limbs and axis improved after administering 10 mg of intramuscular diazepam. Repetitive nerve stimulation, brain scan with DAT-Scan, electrocardiogram, and supra-aortic doppler were unremarkable. Nerve conduction studies and needle electromyography revealed bilateral carpal tunnel syndrome and radiculopathy at C8-T1. In contrast, brain and cervical cord resonance imaging (NMR) highlighted bilateral mild atrophy in the temporal and frontal lobes (
Figure 2). Antibodies against acetylcholine receptor (AChR), muscle-specific kinase (MuSK), and GABA decarboxylase (GAD) were within the normal range. Blood tests and a lumbar puncture were performed, revealing serum CA 15-3 at 45 U/mL, neuron-specific enolase at 59.2 μg/L, ANA 1:160 (speckled pattern), low vitamin B12, high heavy chain neurofilaments, and 80 cells in the cerebrospinal fluid. The final diagnosis was invasive ductal carcinoma and OMS with positivity for anti-Ri antibodies. According to the ECOG scale, medical oncologists classified the patients with a performance status of 2 due to the neurological picture rather than cancer. The patient became increasingly drowsy with an evident walking deficit. On August 19, 2024, a second CT scan showed no vascular damage or metastasis in the CNS and stable disease in the breast. The second oncologist at the University Hospital prescribed anastrozole at 1 mg/day based on histology, positive hormonal receptors status, and post-menopausal status reserving the use of chemotherapy in case of improvement of the general conditions. The multisciplinary team approved therapeutic strategy and scheduled surgery.
On August 24, 2024, the patient underwent surgery and was transferred to the postoperative recovery unit after a rapid deterioration that led to a coma. For this reason, she was treated with a 5-day cycle of intravenous immunoglobulins and three cycles of plasmapheresis, which showed slight clinical benefit, restoring her consciousness and improving her hypertonia. Clonazepam and Baclofen provided no clinical benefits. Over two weeks, her neurological status slowly but progressively improved. Three months after surgery, the patient’s performance status has slightly improved but remains persistent. Therfore chemotherapy was not started. She is cancer-free and regularly takes anastrozole.
3. Discussion
Diagnosing a paraneoplastic syndrome can be challenging to distinguish from true PNS and other neurological syndromes related to cancer [
8,
9]. Nevertheless, early identification of both the paraneoplastic syndrome and the underlying cancer can lead to a better prognosis. In 2019, an international panel of neurologists specializing in PNS reviewed existing diagnostic criteria and developed the PNS-care scoring system [
10]. The panel proposed using the term “high-risk phenotypes” instead of “classical syndromes” to describe the connection to cancer and introduced the concept of “intermediate-risk phenotypes.” “High risk” (>70% associated with cancer) and “intermediate risk” (30%-70% associated with cancer) antibodies have replaced the term “onconeural antibody.” The panel classified three levels of PNS evidence: definite, probable, and potential. The PNS-Care Score, which incorporates clinical phenotype, antibody type, malignancy status, and follow-up duration, can help determine each level. High- or intermediate-risk antibodies are essential for diagnosing definite PNS, except for opsoclonus-myoclonus. Furthermore, guidelines are provided for similar symptoms induced by immune checkpoint inhibitors.
A French series involving 36 patients with anti-Ri antibodies-positive neurologic syndromes showed that 90% had cancer, particularly BC [
11]. Almost one-quarter of the cases were misdiagnosed as functional neurologic disorders, encephalitis, neuritis, or atypical parkinsonism. Opsoclonus-myoclonus was less frequent than expected, often mimicking neurodegenerative diseases. Additionally, opsoclonus-myoclonus syndrome is not pathognomonic for the associated anti-Ri paraneoplastic neurological syndromes.
Screening for the presence of cancer is crucial in PNS, as cancer directly affects prognosis and treatment. The search for malignancy should be performed as soon as possible. The European Federation of Neurological Societies’ recommendations report that the nature of the antibody, and to a lesser extent, the clinical syndrome, determine the risk and type of any underlying malignancy [
12]. Therefore, a CT scan of the thorax, mammography, breast MRI, pelvic ultrasound, and CT scan are necessary to identify the underlying malignancy.
The case presented in this paper reports a woman presenting with a complex neurological picture in which the diagnosis of a suspected BC was readily evident from clinical and radiological exams. The neurological picture was rapidly worsening and challenging for oncologists. In such a case, a multidisciplinary approach is mandatory to correctly frame the neurological symptoms, diagnose OMS, and reach an optimal therapeutic decision. There are a few reports of PNS associated with HER2-positive BC in the medical literature, of which most are anti-Yo antibodies-associated [
13]. The case of HER2 3+ presented here was not related to such autoantibodies but was positive for anti-Ri ones. The patient was treated with upfront surgery since the rapid neurological deterioration contraindicated a neoadjuvant approach with chemotherapy and anti-HER agents. Hormonal therapy was, therefore, preferred even if a benefit was lacking.
OMS cases in BC patients have been published in the medical literature for decades. However, few cases provide relatively complete data on both neurological and oncological evaluations.
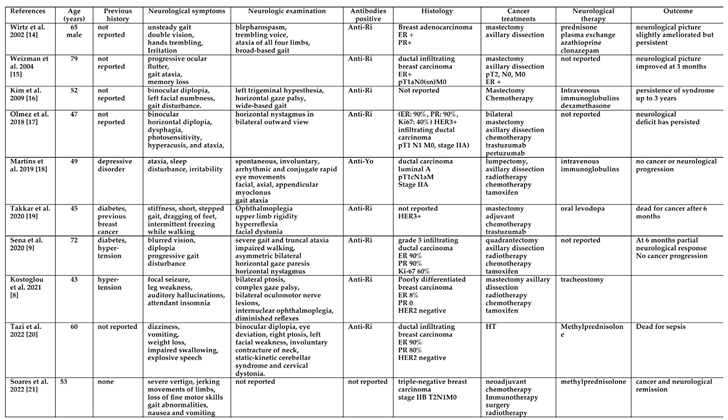
Table 1 shows most case reports with nearly complete data [
8,
9,
14,
15,
16,
17,
18,
19,
20,
21,
22].
Other patients with OMS and BC are mentioned in the medical literature, but their data is largely incomplete or part of a larger series with no individual data. Rarely, OMS may coexist with other peripheral nervous system conditions, such as Stiff-person syndrome in patients with BC [
23]. The observations reported above represent a limitation of this paper.
4. Conclusions
OMS is a rare syndrome associated with BC. The cancer therapeutic strategy varies considerably among cases depending on the neurological condition’s initial severity, the evolution’s rapidity, and the characteristics of BC. Most patients are treated with upfront surgery followed by complete adjuvant therapy, with only occasional reports of neoadjuvant therapy generally reserved for triple-negative BC or high-risk ones. Treatment of primary cancer may result in neurological amelioration, but in many cases, the neurological symptomatology persists beyond cancer control, and there is no fully established treatment. In conclusion, patients with suspected OMS should be checked for occult cancer and managed by a multidisciplinary team in which neurologists play a crucial role.
Author Contributions
Conceptualization, C.C. and M.R.V.; methodology. V.D.S., C.M., V.G., F.B., C.M.; M.G., A.C.; validation, V.G., M.R.V.; formal analysis, V.D.S., C.M., V.G., M.G., A.C.; M.R.V.; investigation, D.S.; data curation, All Authors; writing – original draft, V.D.S., C.M., D.S., F.B., V.G., C.M., M.G., M.R.V.; writing – review & editing, M.R.V. and V.G.
Funding
This research received funding for APC from the University of Palermo.
Institutional Review Board Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the patient provided written informed consent. IRB approval is not required at our institution for case-report studies.
Informed Consent Statement
Written informed consent was obtained from the participant’s legal guardian or next of kin for the publication of any potentially identifiable images or data included in this article.
Data Availability Statement
All data underlying the findings of this study are available within this publication. Patient data from the West China Hospital in Sichuan University have been anonymized to ensure confidentiality. Due to ethical and legal restrictions related to data protection regulations, raw patient data cannot be shared publicly. Requests for further information may be di-rected to the corresponding author.
Acknowledgments
The authors kindly thank the physicians, nurses, and other staff at the hospital who assisted with the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OMS |
Opsoclonus–myoclonus syndrome |
| NPS |
Neurological paraneoplastic syndromes |
| BC |
Breast cancer |
| |
|
References
- Darnell, R.B.; Posner, J.B. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003, 349(16):1543-1554. [CrossRef]
- Gatti, G.; Simsek, S.; Kurne, A.; Zurrida, S.; Naninato, P.; Veronesi, P.; et al. Paraneoplastic neurological disorders in breast cancer. Breast. 2003; 12(3):203-207. [CrossRef]
- Luque, F.A.; Furneaux, H.M.; Ferziger, R.; Rosenblum, M.K.; Wray, S.H.; Schold, S.C. Jr; et al. Anti-Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann Neurol. 1991; 29(3):241-251. [CrossRef]
- Yang, Y.Y.; Yin, G.L.; Darnell, R.B. The neuronal RNA-binding protein Nova-2 is implicated as the autoantigen targeted in POMA patients with dementia. Proc Natl Acad Sci U S A. 1998; 95(22):13254-13259. [CrossRef]
- Bataller, L.; Graus, F.; Saiz, A.; Vilchez JJ; Spanish Opsoclonus-Myoclonus Study Group. Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus-myoclonus. Brain. 2001; 124:437-443. [CrossRef]
- Fanous, I.; Dillon, P. Paraneoplastic neurological complications of breast cancer. Exp Hematol Oncol. 2016; 24; 5:29. [CrossRef]
- Armangué, T.; Sabater, L.; Torres-Vega, E.; Martínez-Hernández, E.; Ariño, H.; Petit-Pedrol, M.; et al. Clinical and Immunological Features of Opsoclonus-Myoclonus Syndrome in the Era of Neuronal Cell Surface Antibodies. JAMA Neurol. 2016; 73(4):417-424. [CrossRef]
- Kostoglou, A.; Vlastos, D.; Bakalis, A.; Ghosh, D. Breast cancer-associated opsoclonus-myoclonus syndrome: a case report. World J Surg Oncol. 2021; 19(1):328. [CrossRef]
- Sena, G.; Gallo, G.; Vescio, G.; Gambardella, D.; de Franciscis, S.; Renne, M. Anti-Ri-associated paraneoplastic ophthalmoplegia-ataxia syndrome in a woman with breast cancer: a case report and review of the literature. J Med Case Rep. 2020; 14(1):67. [CrossRef]
- Graus, F.; Vogrig, A.; Muñiz-Castrillo, S.; Antoine, J.G.; Desestret, V.; Dubey, D.; et al. Updated Diagnostic Criteria for Paraneoplastic Neurologic Syndromes. Neurol Neuroimmunol Neuroinflamm. 2021; 8(4): e1014. [CrossRef]
- Simard, C.; Vogrig, A.; Joubert, B.; Muñiz-Castrillo, S.; Picard, G.; Rogemond, V.; et al. Clinical spectrum and diagnostic pitfalls of neurologic syndromes with Ri antibodies. Neurol Neuroimmunol Neuroinflamm. 2020; 7(3):e699. [CrossRef]
- Titulaer, M.J.; Soffietti, R.; Dalmau, J.; Gilhus, N.E.; Giometto, B.; Graus, F.; et al. European Federation of Neurological Societies. Screening for tumours in paraneoplastic syndromes: report of an EFNS task force. Eur J Neurol. 2011, 18(1):19-e3. [CrossRef]
- Rojas-Marcos, I.; Picard, G.; Chinchón, D.; Gelpi, E.; Psimaras, D.; Giometto, B.; et al. Human epidermal growth factor receptor 2 overexpression in breast cancer of patients with anti-Yo--associated paraneoplastic cerebellar degeneration. Neuro Oncol. 2012; 14(4):506-510. [CrossRef]
- Wirtz, P.W.; Sillevis Smitt, P.A.; Hoff, J.I.; de Leeuw, B.; Lammers, G.J.; van Duinen, S.G.; et al. Anti-Ri antibody positive opsoclonus-myoclonus in a male patient with breast carcinoma. J Neurol. 2002; 249(12):1710-2. [CrossRef]
- Weizman, D.A.; Leong, W.L. Anti-Ri antibody opsoclonus-myoclonus syndrome and breast cancer: a case report and a review of the literature. J Surg Oncol. 2004; 87(3):143-145. [CrossRef]
- Kim, H.; Lim,Y.; Kim, K.K. Anti-ri-antibody-associated paraneoplastic syndrome in a man with breast cancer showing a reversible pontine lesion on MRI. J Clin Neurol. 2009; 5(3):151-152. [CrossRef]
- Olmez, O.F.; Kinikoglu, O.; Yilmaz, N.H.; Bilici, A.; Cubukcu, E.; Seker, M.; et al. Anti-Ri-associated paraneoplastic neurological syndrome: Initial symptom of breast cancer with HER2 overexpression and treatment by dual HER2 blockade. J Oncol Pharm Pract. 2019; 25(6):1526-1530.. [CrossRef]
- Martins, L.; Galvão, D.; Silva, A.; Vieira, B.; Reis, Ó.; Vitorino, R.; Pires, P. Paraneoplastic opsoclonus-myoclonus syndrome as a rare presentation of breast cancer. J Surg Case Rep. 2019; (2): rjy365. [CrossRef]
- Takkar, A.; Mehta, S.; Gupta, N.; Bansal, S.; Lal, V. Anti- RI antibody associated progressive supranuclear palsy like presentation in a patient with breast carcinoma. J Neuroimmunol. 2020; 347:577345.. [CrossRef]
- Tazi, R.; Salimi, Z.; Fadili, H.; Aasfara, J.; Hazim, A. Anti-Ri-Associated Paraneoplastic Neurological Syndrome Revealing Breast Cancer: A Case Report. Cureus. 2022; 14(1): e21106. [CrossRef]
- Soares, R.; Mittapalli, A.; Ramakrishnan, M.; Farooq, U. Breast Cancer Presenting as Onconeural Antibody Negative Opsoclonus-Myoclonus Syndrome. Cureus. 2022; 14(8): e28417. [CrossRef]
- Samah, Y.; Sahar, B.; Mebrouk, Y. Opsoclonus-Myoclonus with Anti-YO Antibodies Revealing Breast Cancer: A Case Report. Cureus. 2024; 16(5): e60452. [CrossRef]
- Thümen, A.; Moser, A. An uncommon paraneoplastic Ri-positive opsoclonus-myoclonus-like syndrome and stiff-person syndrome with elevated glutamate/GABA ratio in the cerebrospinal fluid after breast cancer. J Neurol. 2010; 257(7):1215-1217. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).