Submitted:
30 March 2025
Posted:
31 March 2025
You are already at the latest version
Abstract
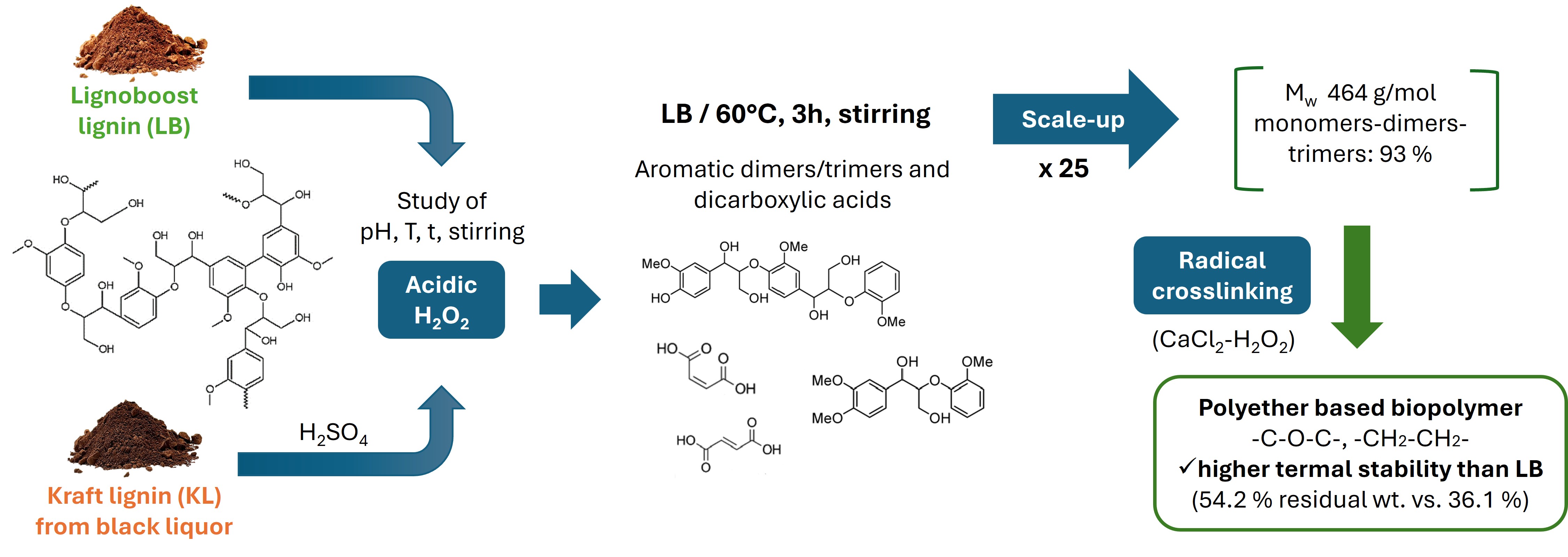
Keywords:
1. Introduction
2. Results and Discussion
2.1. Acidic Oxidative Depolymerization/Functionalization Under Different Temperatures
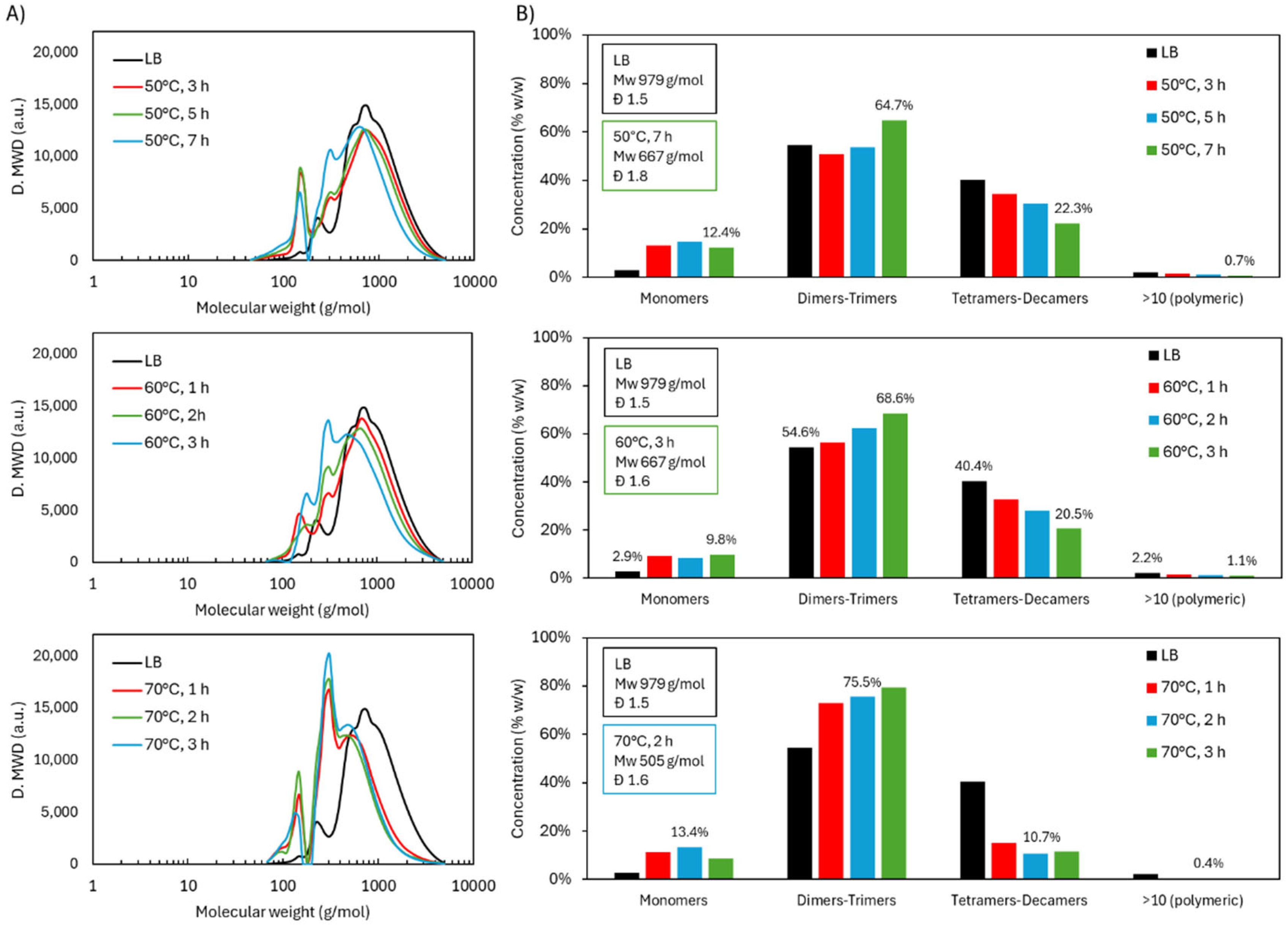
2.1.1. Evolution of the Molecular Weight Distribution
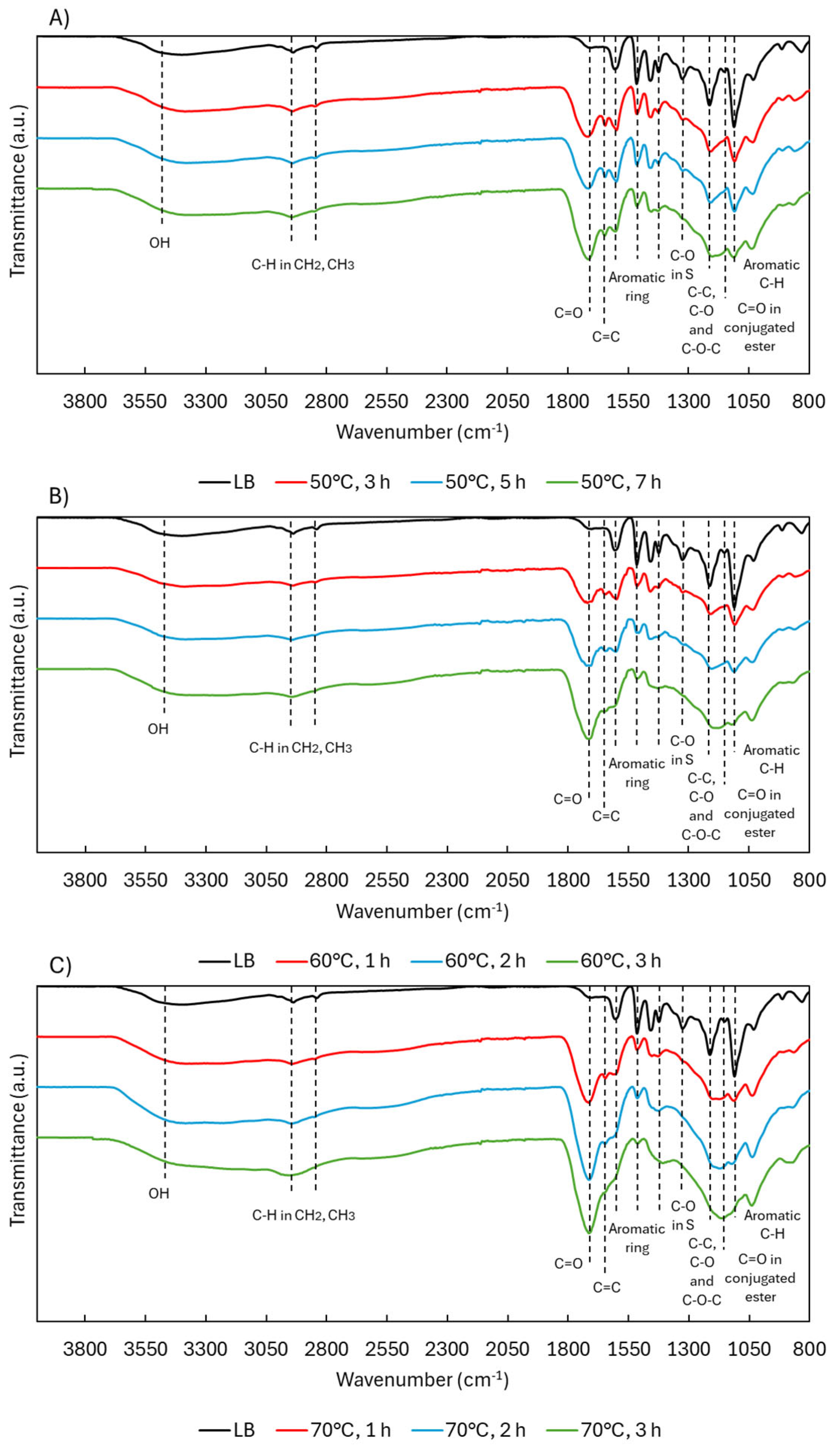
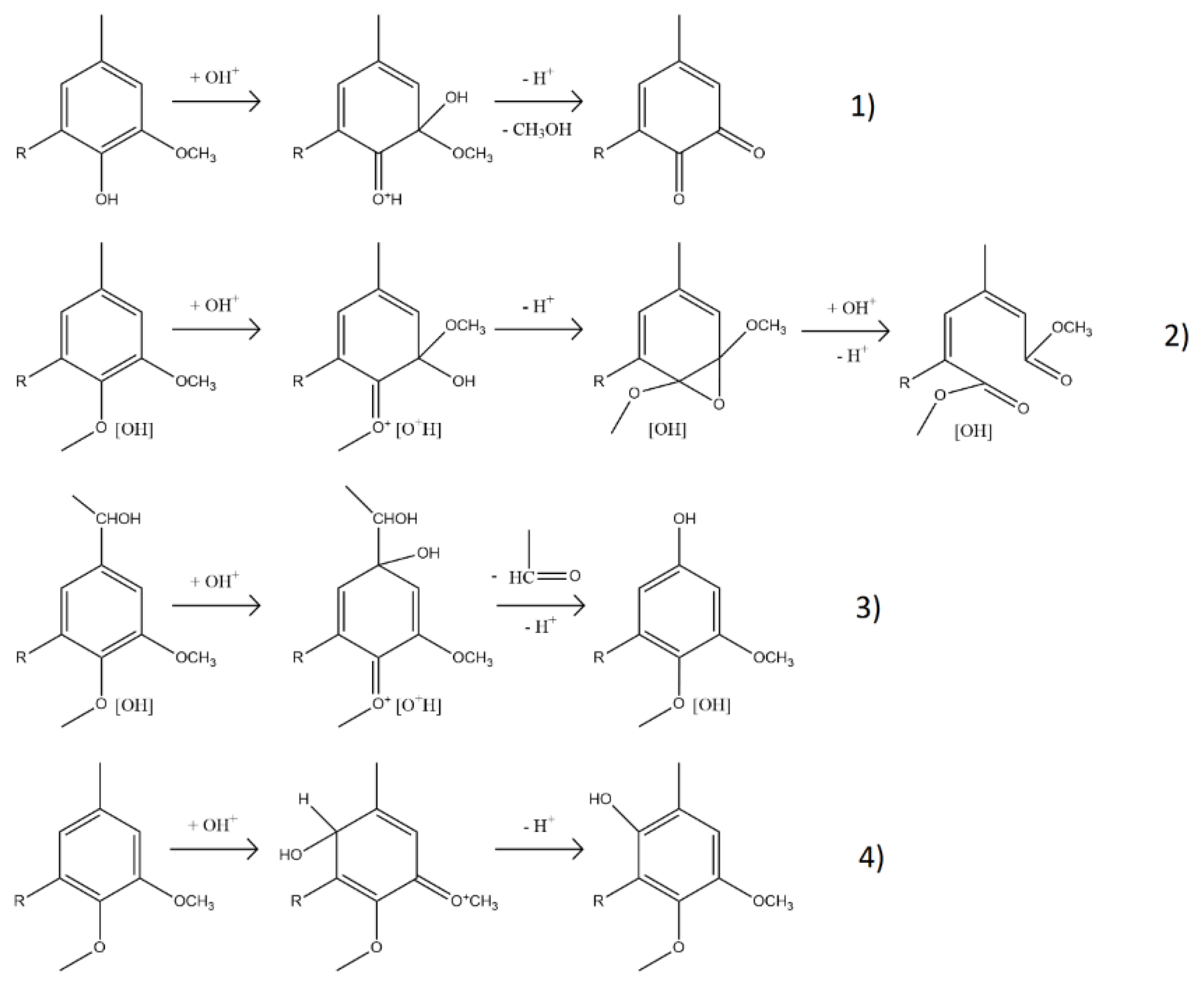
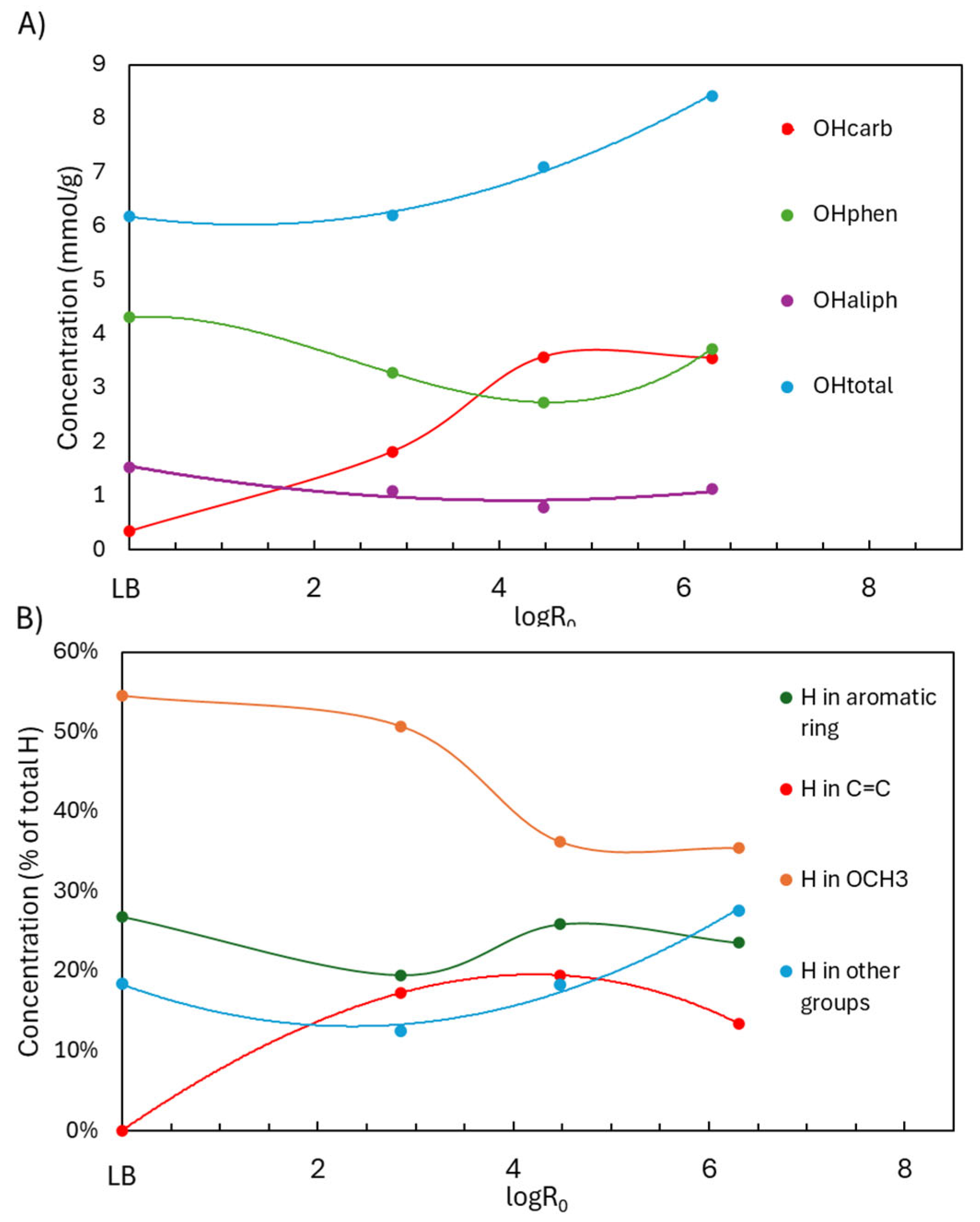
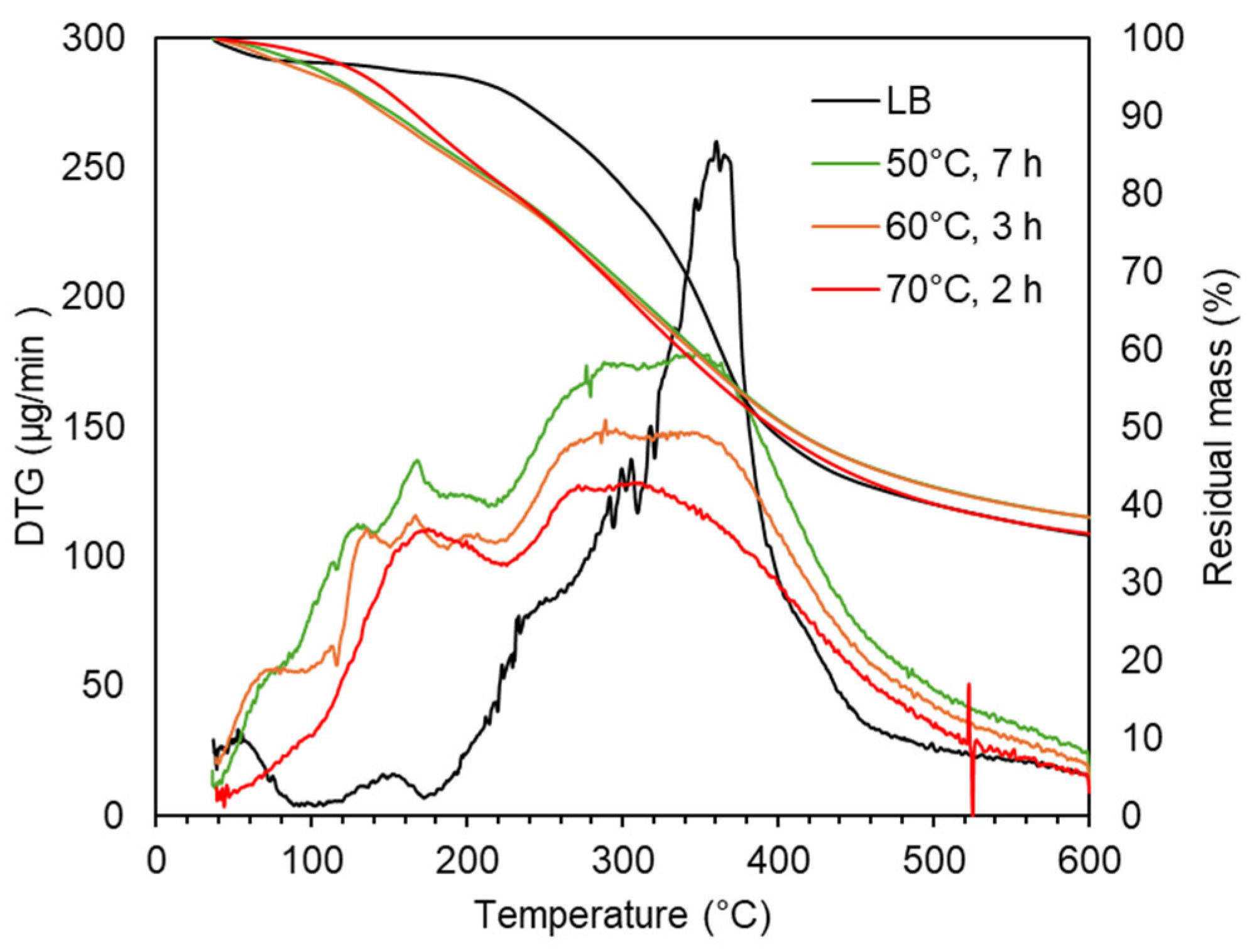
2.1.2. Structural Characterization (ATR-FTIR, 1H NMR, 31P NMR, EA, TGA, GC-FID/(TOF-MS))
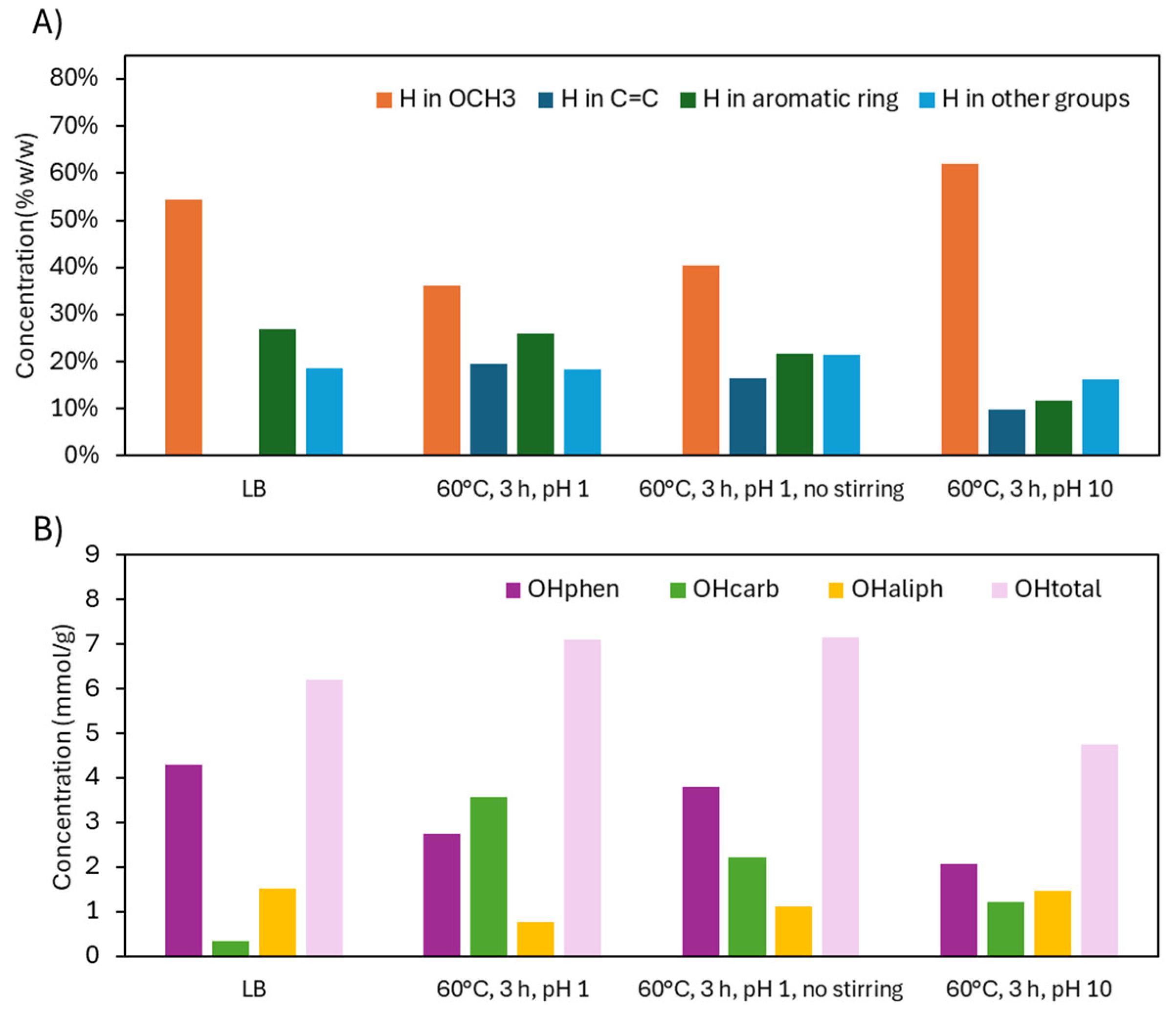
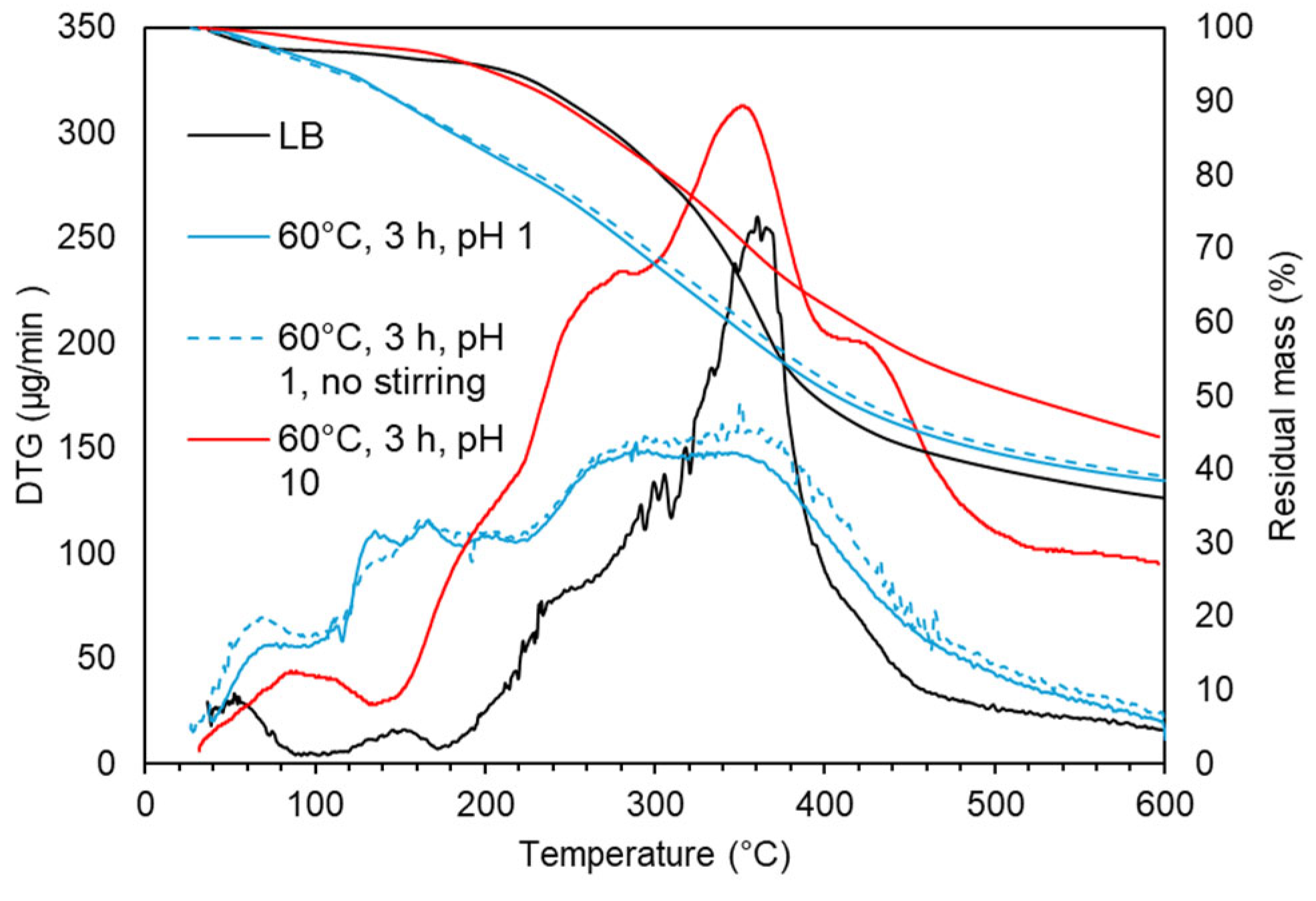
2.2. Oxidative Depolymerization/Functionalization: Effect of Stirring and pH
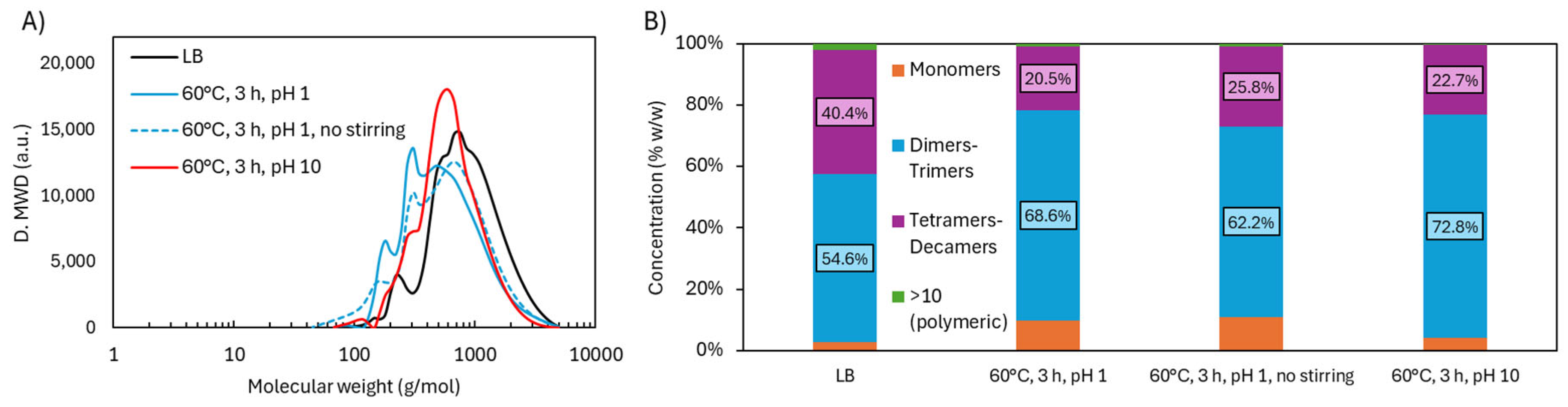
2.2.1. Molecular Weight Distribution
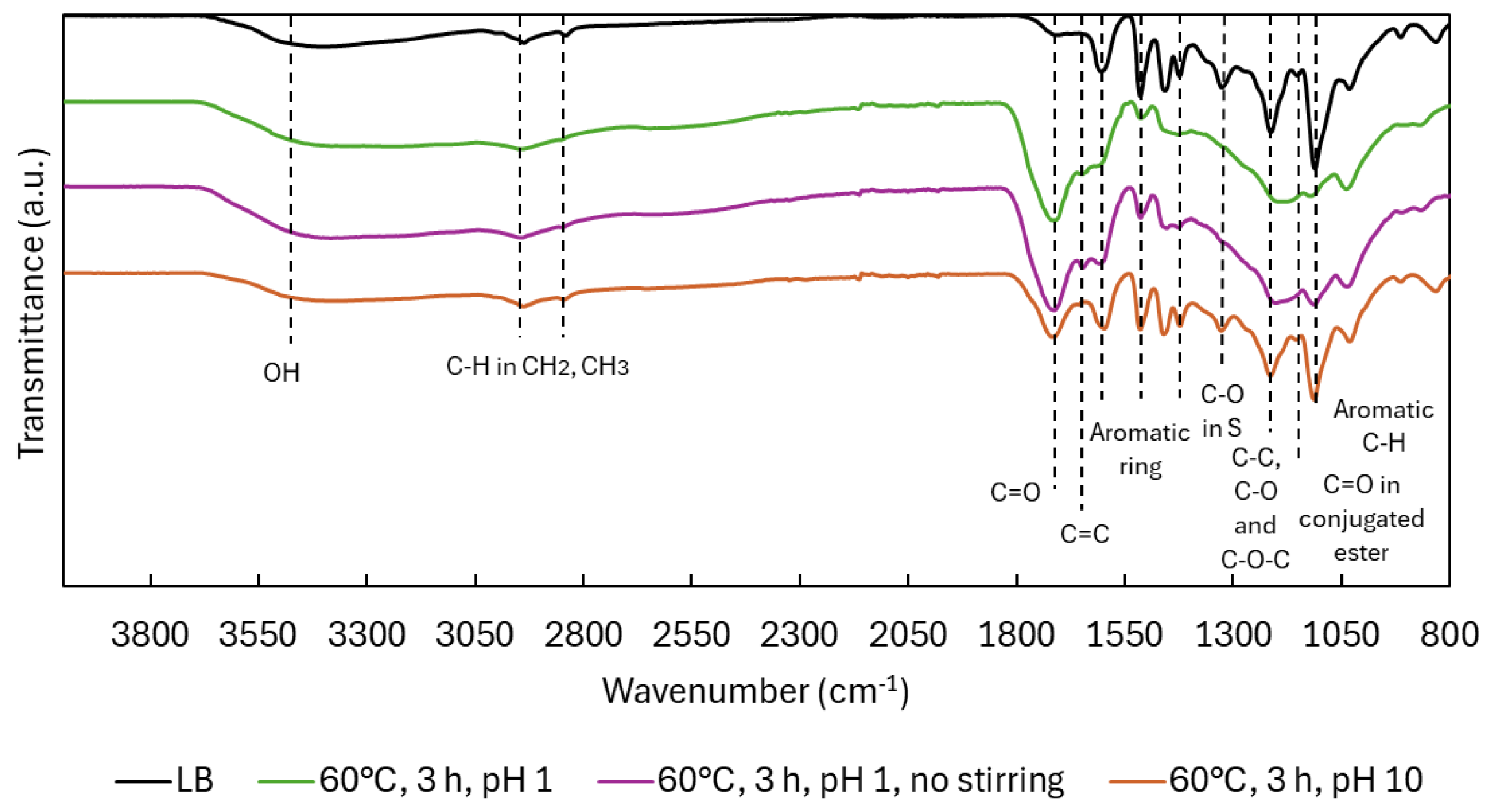
2.2.2. Structural Characterization (ATR-FTIR, 1H NMR, 31P NMR, EA, TGA)
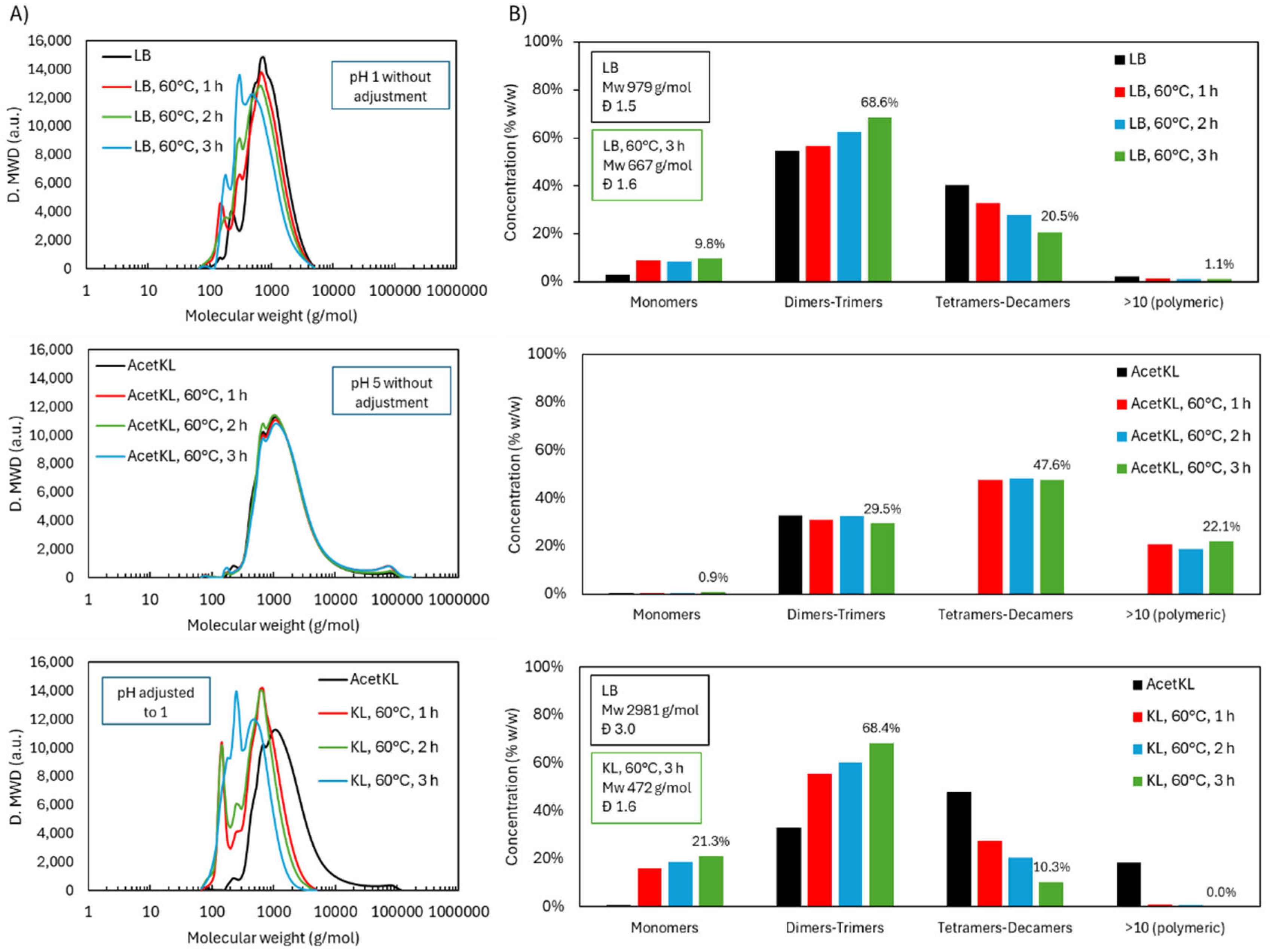
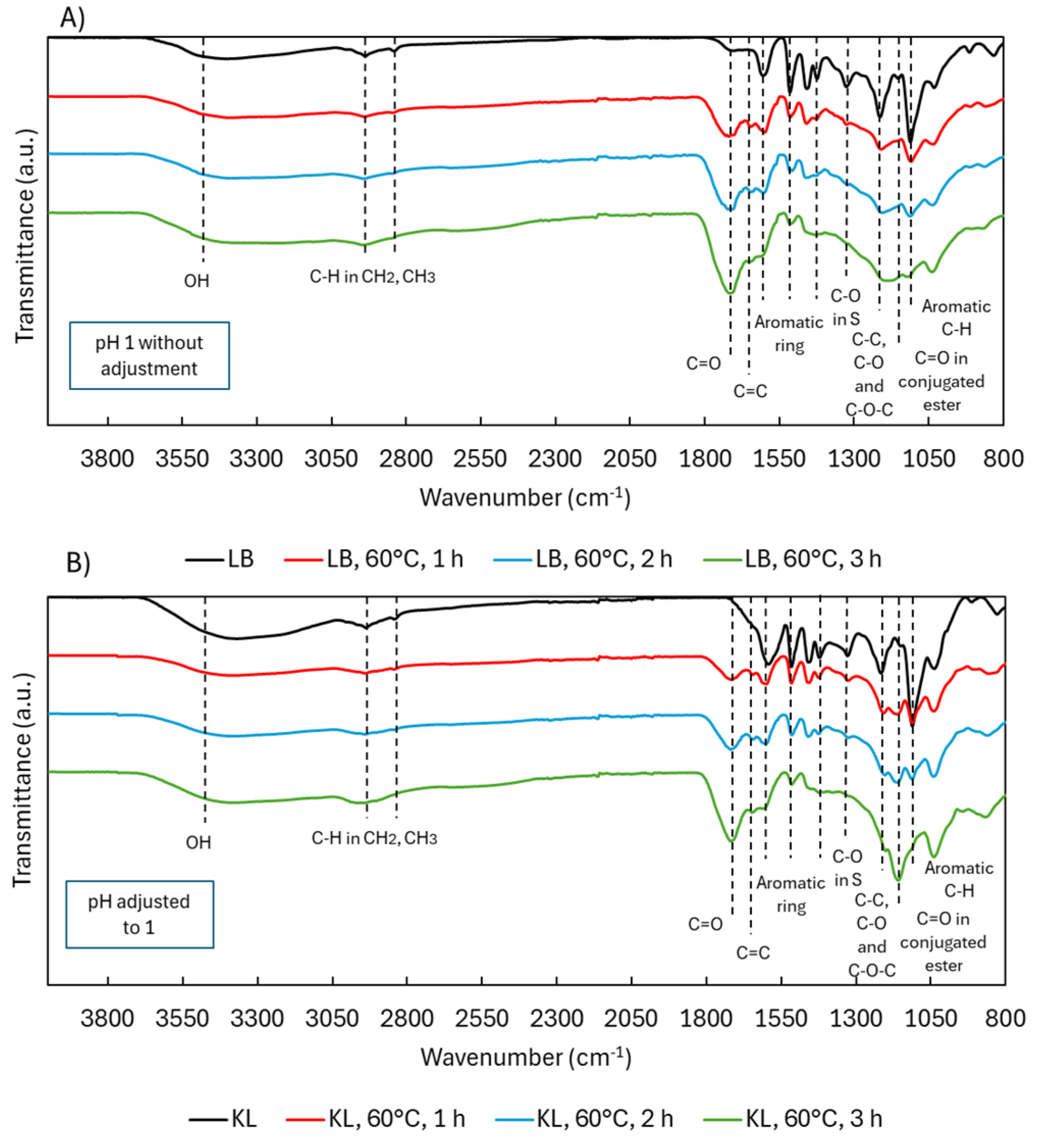
2.3. Oxidative Depolymerization/Functionalization of KL Versus LB
2.3.1. Evolution of the Molecular Weight Distribution
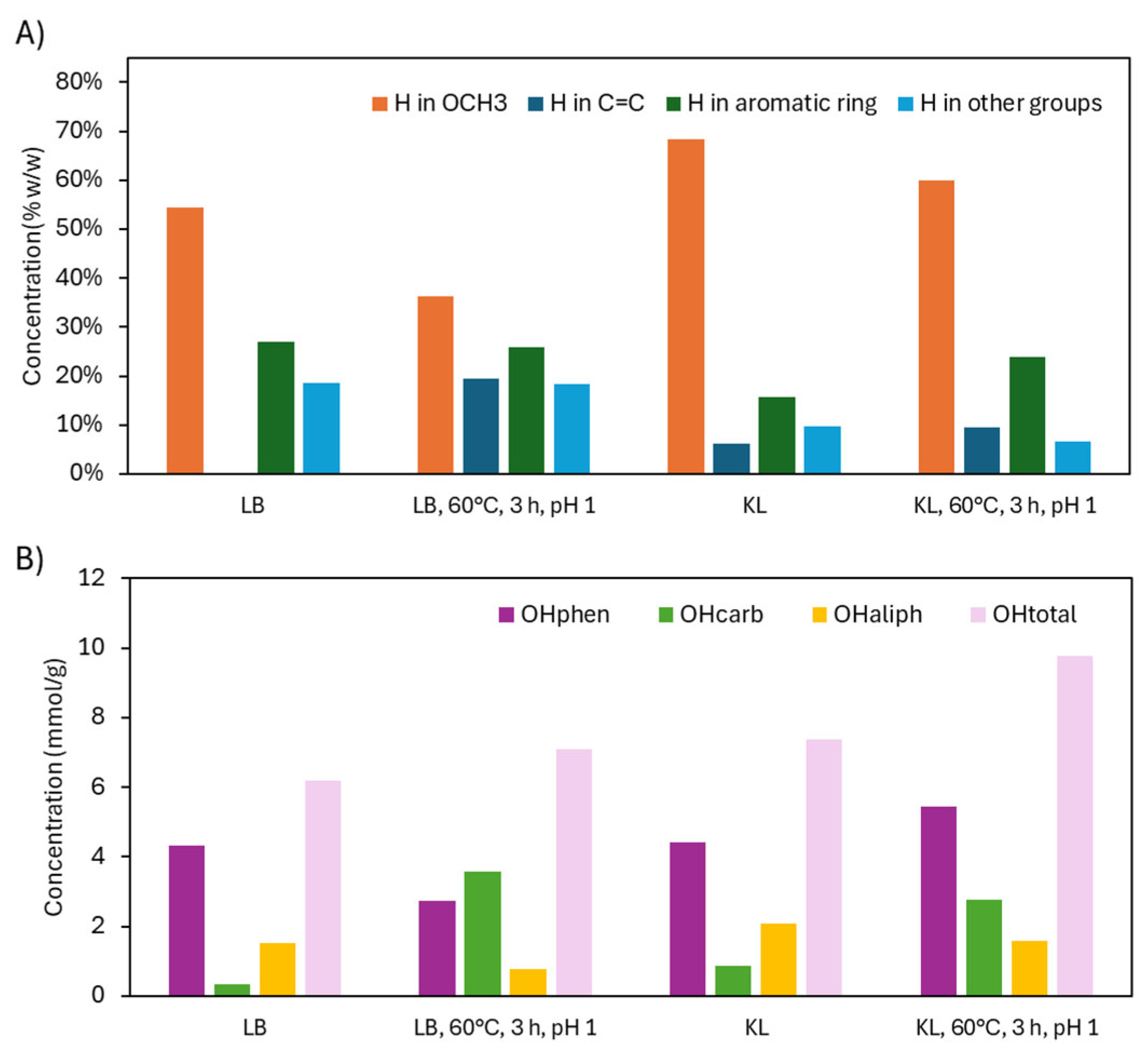
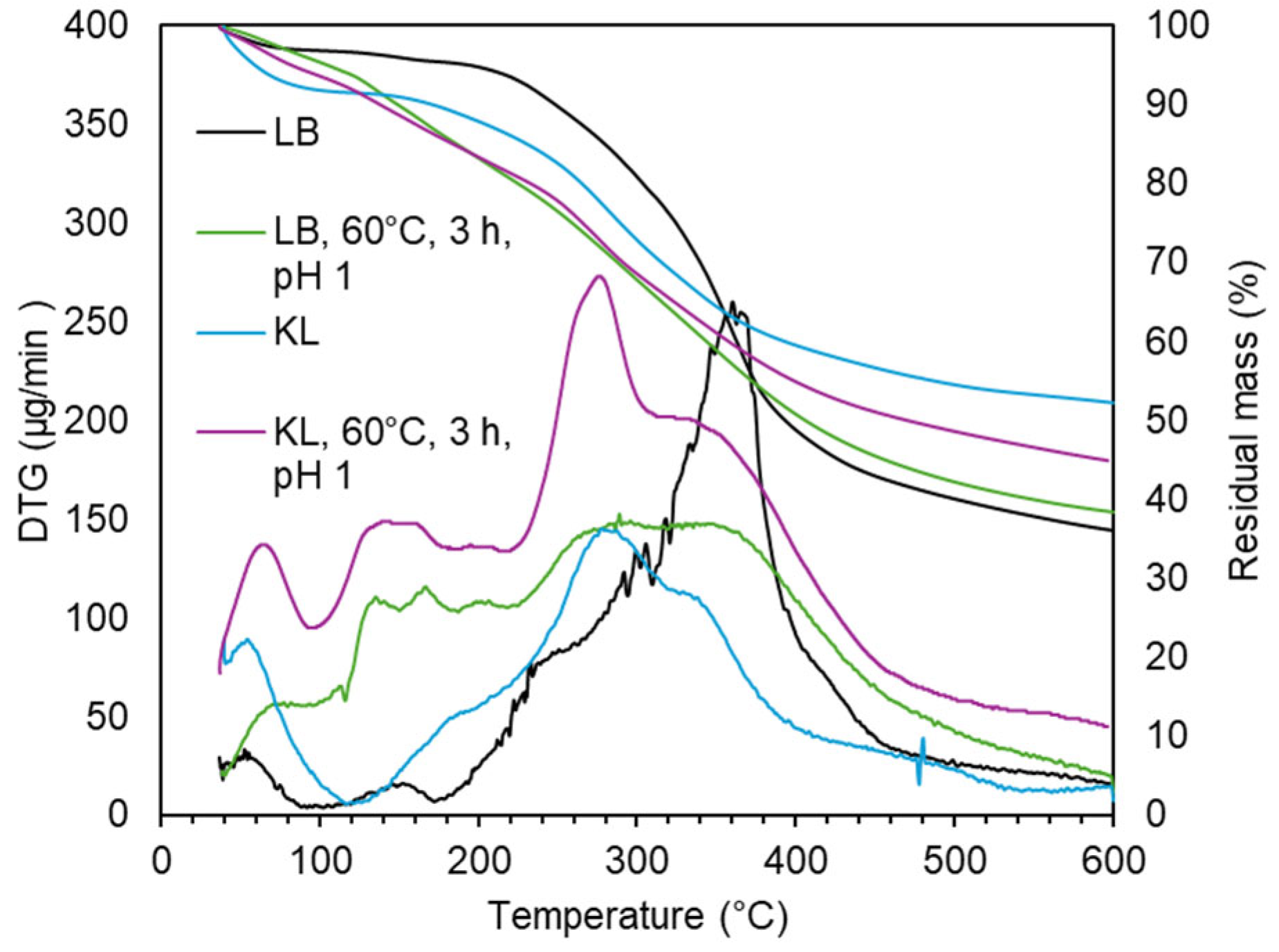
2.3.2. Structural Characterization (ATR-FTIR, 1H NMR, 31P NMR, TGA, and GC-FID/(TOF-MS))
2.4. Scale-Up of Acidic Oxidative Depolymerization of LB
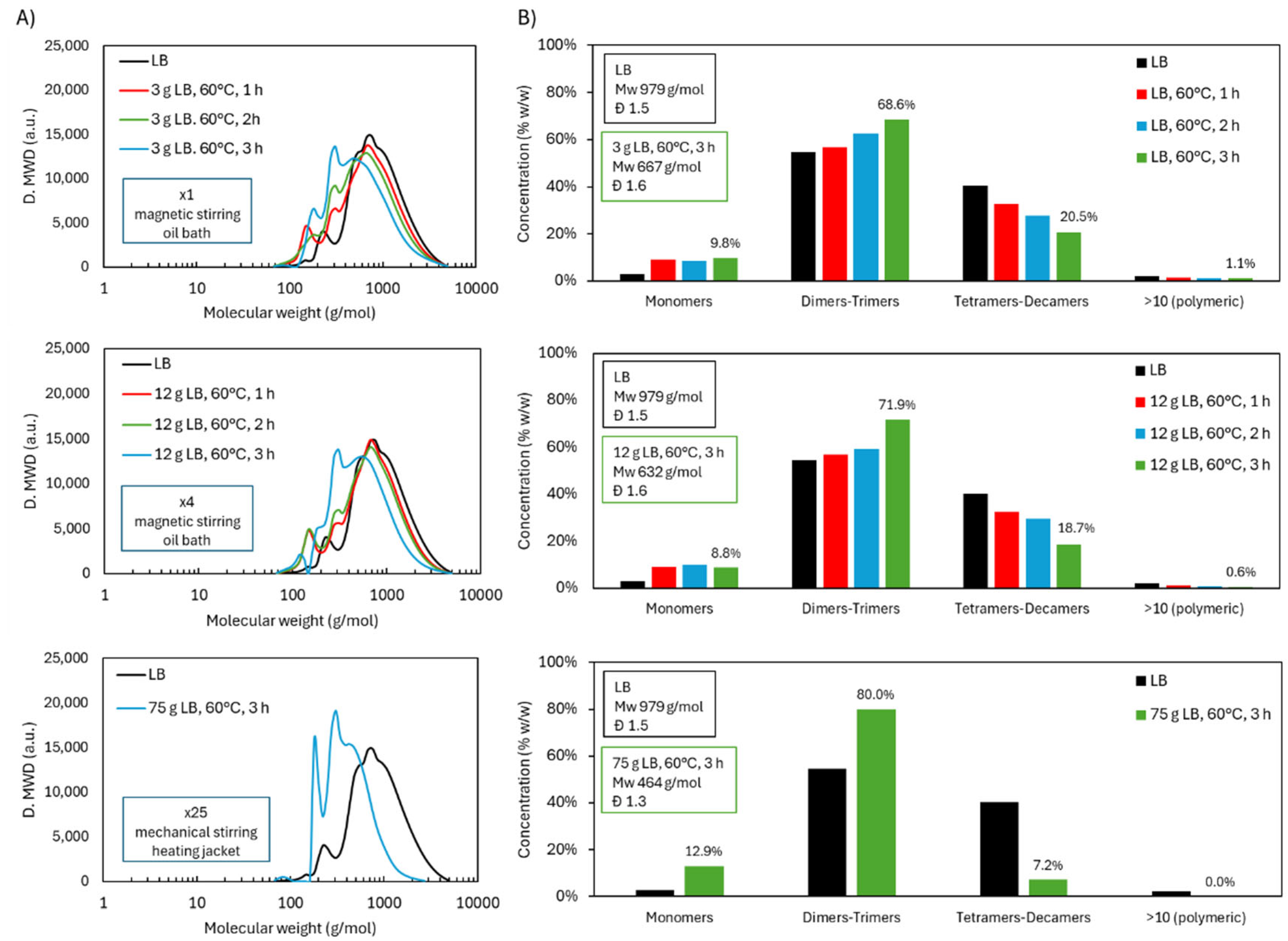
2.4.1. Evolution of the Molecular Weight Distribution
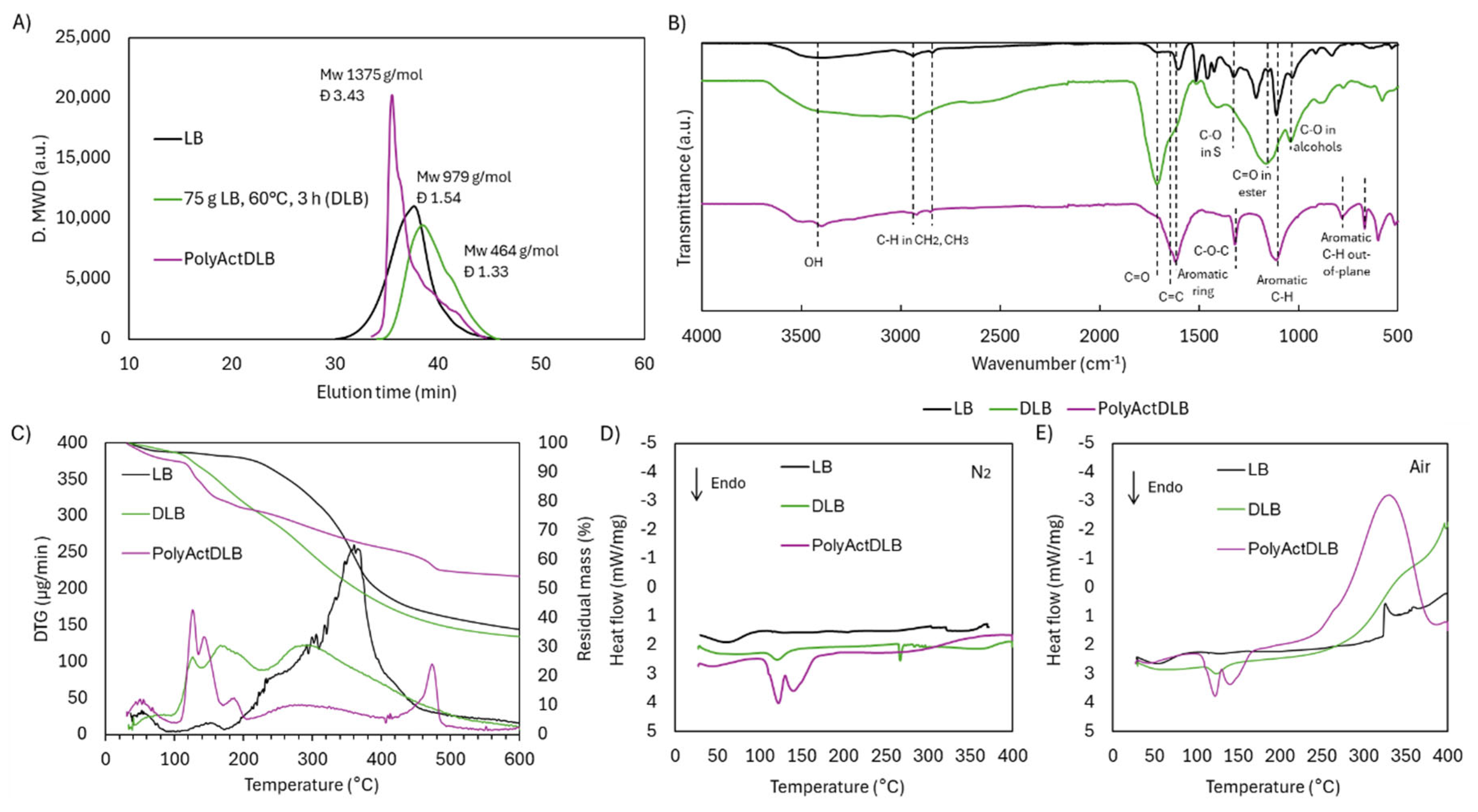
2.4.2. Structural Characterization (ATR-FTIR, 1H NMR, 31P NMR, and TGA)
2.5. Reactivity of Depolymerized/Functionalized LB Through Radical Crosslinking
3. Materials and Methods
3.1. Raw Material
3.2. Oxidative Depolymerization of Lignin
3.3. Radical Crosslinking of the Depolymerized Lignin
3.4. Characterization of Lignoboost Lignin, Kraft Lignin, Depolymerized Lignin Samples, and Lignin-Derived Crosslinked Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LB | Lignoboost lignin |
| KL | Kraft lignin |
| DLB | Depolymerized Lignoboost lignin |
| PolyActDLB | Crosslinked polymer derived from DLB |
| PPU | Phenylpropane unit |
| Mw | Weight-average molecular weight |
| Đ | Dispersity index (Mw/Mn) |
| logR0 | Logarithmic severity factor |
| OHphen | Phenolic hydroxyl group |
| OHaliph | Aliphatic hydroxyl group |
| OHcarb | Carboxylic hydroxyl group |
| OCH3 | Methoxy group |
| C=C / -CH=CH- | Aliphatic carbon-carbon double bond (alkene) |
| C=O | Carbonyl group (in ketones, acids, esters, etc.) |
| C-O-C | Ether linkage |
| THF | Tetrahydrofuran |
| DMSO-d6 | Lignoboost lignin |
| MSTFA | N-Methyl-N-(trimethylsilyl)trifluoroacetamide (derivatizing agent) |
| TMDP | 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (for ³¹P NMR) |
| ¹H NMR | Proton nuclear magnetic resonance spectroscopy |
| ³¹P NMR | Phosphorus-31 nuclear magnetic resonance spectroscopy |
| ATR-FTIR | Attenuated total reflectance Fourier-transform infrared spectroscopy |
| GPC | Gel permeation chromatography |
| RI | Refractive index |
| UV | Ultraviolet |
| EA | Elemental Analysis |
| TGA/DTG | Thermogravimetric analysis/Derivative thermogravimetry |
| DSC | Differential scanning calorimetry |
| GC-FID/(TOF-MS) | Gas chromatography with flame ionization detector and time-of-flight mass spectrometry |
References
- Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun Biomass Based Carbon Nanofibers as High-Performance Supercapacitors. Ind. Crops Prod. 2020, 148, 112181. [Google Scholar] [CrossRef]
- Erfani Jazi, M.; Narayanan, G.; Aghabozorgi, F.; Farajidizaji, B.; Aghaei, A.; Kamyabi, M.A.; Navarathna, C.M.; Mlsna, T.E. Structure, Chemistry and Physicochemistry of Lignin for Material Functionalization. SN Appl. Sci. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Andriani, F.; Lawoko, M. Oxidative Carboxylation of Lignin: Exploring Reactivity of Different Lignin Types. Biomacromolecules 2024, 25, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
- More, A.; Elder, T.; Jiang, Z. Towards a New Understanding of the Retro-Aldol Reaction for Oxidative Conversion of Lignin to Aromatic Aldehydes and Acids. Int. J. Biol. Macromol. 2021, 183, 1505–1513. [Google Scholar] [CrossRef]
- Schorr, D.; Diouf, P.N.; Stevanovic, T. Evaluation of Industrial Lignins for Biocomposites Production. Ind. Crops Prod. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin Recovery from Spent Alkaline Pulping Liquors Using Acidification, Membrane Separation, and Related Processing Steps: A Review. BioResources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Gärtner, A.; Gellerstedt, G.; Tamminen, T. Determination of Phenolic Hydroxyl Groups in Residual Lignin Using a Modified UV-Method Anna. Nord. Pulp Pap. Res. J. 1999. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel Lignin–Chitosan–PVA Composite Hydrogel for Wound Dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef]
- Qu, W.; Huang, Y.; Luo, Y.; Kalluru, S.; Cochran, E.; Forrester, M.; Bai, X. Controlled Radical Polymerization of Crude Lignin Bio-Oil Containing Multihydroxyl Molecules for Methacrylate Polymers and the Potential Applications. ACS Sustain. Chem. Eng. 2019, 7, 9050–9060. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Vega-Aguilar, C.A.; Filomena Barreiro, M. Effect of Methoxy Substituents on Wet Peroxide Oxidation of Lignin and Lignin Model Compounds: Understanding the Pathway to C4 Dicarboxylic Acids. Ind. Eng. Chem. Res. 2021, 60, 3543–3553. [Google Scholar] [CrossRef]
- More, A.; Elder, T.; Jiang, Z. A Review of Lignin Hydrogen Peroxide Oxidation Chemistry with Emphasis on Aromatic Aldehydes and Acids. Holzforschung 2021, 75, 806–823. [Google Scholar] [CrossRef]
- Ahmad, Z.; Dajani, W.W. Al; Paleologou, M.; Xu, C. Sustainable Process for the Depolymerization/Oxidation of Softwood and Hardwood Kraft Lignins Using Hydrogen Peroxide under Ambient Conditions. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Junghans, U.; Bernhardt, J.J.; Wollnik, R.; Triebert, D.; Unkelbach, G.; Pufky-Heinrich, D. Valorization of Lignin via Oxidative Depolymerization with Hydrogen Peroxide: Towards Carboxyl-Rich Oligomeric Lignin Fragments. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Skunde, R.; Tanase-Opedal, M.; Syverud, K. Carboxylation of Lignin by Oxidation with Hydrogen Peroxide and Its Use as Emulsion Stabilizer. Ind. Crops Prod. 2025, 223, 120019. [Google Scholar] [CrossRef]
- Kim, J.-C.; Choi, J.-H.; Kim, J.-H.; Cho, S.-M.; Park, S.-W.; Cho, Y.-M.; Park, S.-Y.; Kwak, H.W.; Choi, I.-G. Development of Lignin-Based Polycarboxylates as a Plasticizer for Cement Paste via Peracetic Acid Oxidation. BioResources 2020, 15, 8133–8145. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y. Metal Catalyst-Free Oxidative C−C Bond Cleavage of a Lignin Model Compound by H2O2 in Formic Acid. ChemSusChem 2020, 13, 1740–1745. [Google Scholar] [CrossRef]
- Vega-Aguilar, C.A.; Barreiro, M.F.; Rodrigues, A.E. Lignin Conversion into C4 Dicarboxylic Acids by Catalytic Wet Peroxide Oxidation Using Titanium Silicalite-1. Ind. Crops Prod. 2021, 173, 114155. [Google Scholar] [CrossRef]
- Sun, S.; Qiu, X.; Hao, S.; Ravichandran, S.; Song, J.; Zhang, W. Electrochemical Conversion of Lignin to Short-Chain Carboxylic Acids. Green Chem. 2023, 25, 3127–3136. [Google Scholar] [CrossRef]
- Bi, Z.; Li, Z.; Yan, L. Catalytic Oxidation of Lignin to Dicarboxylic Acid over the CuFeS2 Nanoparticle Catalyst. Green Process. Synth. 2018, 7, 306–315. [Google Scholar] [CrossRef]
- Ding, R.; Wu, H.; Thunga, M.; Bowler, N.; Kessler, M.R. Processing and Characterization of Low-Cost Electrospun Carbon Fibers from Organosolv Lignin/Polyacrylonitrile Blends. Carbon N. Y. 2016, 100, 126–136. [Google Scholar] [CrossRef]
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin - An Alternative Precursor for Sustainable and Cost-Effective Automotive Carbon Fiber. J. Mater. Res. Technol. 2015, 4, 283–296. [Google Scholar] [CrossRef]
- Ouyang, Q.; Cheng, L.; Wang, H.; Li, K. Mechanism and Kinetics of the Stabilization Reactions of Itaconic Acid-Modified Polyacrylonitrile. Polym. Degrad. Stab. 2008, 93, 1415–1421. [Google Scholar] [CrossRef]
- Schorr, D.; Diouf, P.N.; Stevanovic, T. Evaluation of Industrial Lignins for Biocomposites Production. Ind. Crops Prod. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Gonçalves, S.; Martins, J.; Paiva, N.T.; Paiva, D.; Carvalho, L.H.; Magalhães, F.D. The Potential of Visible Spectroscopy as a Tool for the In-Line Monitoring of Lignin Methylolation. Polymers (Basel). 2023, 15. [Google Scholar] [CrossRef]
- Mainka, H.; Hilfert, L.; Busse, S.; Edelmann, F.; Haak, E.; Herrmann, A.S. Characterization of the Major Reactions during Conversion of Lignin to Carbon Fiber. J. Mater. Res. Technol. 2015, 4, 377–391. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Yu, J.; Li, S.; Wang, J.; Wang, C.; Chu, F. Integration of Lignin and Acrylic Monomers towards Grafted Copolymers by Free Radical Polymerization. Int. J. Biol. Macromol. 2014, 67, 483–489. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Deng, S.; Chen, X.; Essawy, H.; Lee, S.H.; Lum, W.C.; Zhou, X.; Zhang, J. Graft Copolymer of Tannin and Polyvinyl Alcohol with Acrylic Acid for the Preparation of Hydrophobic Biodegradable Film. Prog. Org. Coatings 2024, 186, 108090. [Google Scholar] [CrossRef]
- Falcão, L.; Araújo, M.E.M. Application of ATR-FTIR Spectroscopy to the Analysis of Tannins in Historic Leathers: The Case Study of the Upholstery from the 19th Century Portuguese Royal Train. Vib. Spectrosc. 2014, 74, 98–103. [Google Scholar] [CrossRef]
- Dutra, L.M.; Teles, P.H.V.; de Melo, N.F.; Nagata, N.; Almeida, J.R.G. da S. ATR-FTIR Spectroscopy Combined with Chemometric Tools for Rapid Distinction of Passiflora L. Species. Rev. Bras. Plantas Med. 2023, 25, 54–62. [Google Scholar]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of Lignocellulosics by Steam-Aqueous Pretreatments. Philos. Trans. R. Soc. London. Ser. A, Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Maitz, S.; Schlemmer, W.; Hobisch, M.A.; Hobisch, J.; Kienberger, M. Preparation and Characterization of a Water-Soluble Kraft Lignin. Adv. Sustain. Syst. 2020, 4. [Google Scholar] [CrossRef]
- Ramos-Andrés, M.; Díaz-Cesteros, S.; Majithia, N.; García-Serna, J. Pilot-Scale Biorefinery for the Production of Purified Biopolymers Based on Hydrothermal Treatment in Flow-through Reactor Cycles. Chem. Eng. J. 2022, 437. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Characterization of Lignins Isolated from Industrial Residues and Their Beneficial Uses. BioResources 2016, 11, 8435–8456. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of Hydroxyl Groups in Biorefinery Resources via Quantitative 31P NMR Spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef]

| Sample | logR0 | C | H | O | S | N | PPU empirical formula | MwPPU (g/mol) |
|---|---|---|---|---|---|---|---|---|
| LB | - | 57.9 ± 0.03 |
5.55 ± 0.02 |
32.86 ± 0.06 |
3.10 ± 0.24 |
0.52 ± 0.33 |
C9H5.944O2.461S0.228N0.087(OCH3)2.372 | 235.69 |
| 50°C, 7 h | 2.85 | 50.73 ± 0.19 |
4.71 ± 0.02 |
39.41 ± 0.34 |
4.66 ± 0.28 |
0.51 ± 0.17 |
C9H6.083O4.373S0.382N0.095(OCH3)2.085 | 262.24 |
| 60°C, 3 h | 4.48 | 48.56 ± 0.16 |
4.36 ± 0.02 |
41.90 ± 0.26 |
4.57 ± 0.21 |
0.62 ± 0.11 |
C9H7.102O5.350S0.365N0.113(OCH3)1.343 | 255.60 |
| 70°C, 2 h | 6.30 | 46.48 ± 0.09 |
4.71 ± 0.20 |
43.46 ± 0.12 |
4.84 ± 0.25 |
0.52 ± 0.12 |
C9H8.254O5.860S0.410N0.101(OCH3)1.508 | 271.28 |
| Formula | Mw (g/mol) |
LB 50°C, 7 h logR0 2.85 |
LB 60°C, 3 h logR0 4.48 |
LB 70°C, 2 h logR0 6.30 |
KL 60°C, 3 h logR0 4.48 |
|
|---|---|---|---|---|---|---|
| Alcohols | - | - | 2.01% | 2.04% | 1.44% | 1.33% |
| Ethylene glycol | C₂H₆O₂ | 62.08 | 2.01% | 2.04% | 1.44% | 1.33% |
| Hydroxycarboxylic acids | - | - | 14.73% | 14.73% | 16.93% | 19.21% |
| Lactic acid | C₃H₆O₃ | 90.09 | 0.72% | 0.74% | 0.53% | 4.24% |
| Glycolic acid | C₂H₄O₃ | 76.06 | 9.15% | 9.27% | 11.40% | 8.94% |
| 2-Hydroxybutyric acid | C₄H₈O₃ | 104.12 | 0.43% | 0.44% | 0.22% | 3.10% |
| Glyceric acid | C₃H₆O₄ | 106.09 | 3.03% | 3.07% | 3.15% | 1.95% |
| Malic acid | C₄H₆O₅ | 134.10 | 1.40% | 1.42% | 1.63% | 0.97% |
| Dicarboxylic acids | - | - | 50.28% | 53.40% | 56.21% | 29.36% |
| Oxalic acid | C₂H₂O₄ | 90.04 | 27.14% | 27.52% | 15.72% | 8.88% |
| Propanedioic acid | C₃H₄O₄ | 104.07 | 19.96% | 22.65% | 36.26% | 18.23% |
| Succinic acid | C₄H₆O₄ | 118.10 | 1.76% | 1.79% | 2.19% | 1.26% |
| Ethylmalonic acid | C₅H₈O₄ | 132.13 | 1.42% | 1.44% | 2.03% | 0.99% |
| Esters | - | - | 1.65% | 1.68% | 11.28% | 6.43% |
| Methyl 2-hydroxypropanoate | C₄H₈O₃ | 104.12 | 0.18% | 0.18% | 1.88% | 0.99% |
| Monomethyl succinate | C₅H₈O₄ | 132.13 | n.d. | n.d. | 3.35% | 1.28% |
| Methyl 2-hydroxyethyl malonate | C₆H₁₀O₅ | 162.16 | n.d. | n.d. | 4.88% | 2.71% |
| Butyl 6-methylheptanoate | C₁₂H₂₄O₂ | 200.36 | 1.47% | 1.49% | 1.17% | 1.45% |
| Lactones | - | - | 0.54% | 0.55% | 2.39% | 2.69% |
| 3-Hydroxy-3-hydroxymethyl-dihydro-2(3H)-furanone | C₅H₈O₄ | 132.13 | 0.39% | 0.40% | 2.08% | 0.55% |
| 2,3,4,5-Tetrahydroxypentanoic acid-1,4-lactone | C₅H₈O₆ | 164.11 | n.d. | n.d. | n.d. | 0.42% |
| Erythrono-1,4-lactone | C₄H₆O₄ | 118.10 | 0.15% | 0.15% | 0.31% | 1.32% |
| D-Erythro-Pentonic Acid, γ-Lactone | C₅H₈O₅ | 148.11 | n.d. | n.d. | n.d. | 0.41% |
| Monosaccharides | - | - | 12.16% | 10.87% | 2.10% | 29.91% |
| D-Arabinopyranose | C₅H₁₀O₅ | 150.15 | 0.78% | 0.79% | 0.00% | 1.40% |
| β-Arabinopyranose | C₅H₁₀O₅ | 150.15 | 0.00% | 0.00% | 0.72% | 1.58% |
| D-ribose | C₅H₁₀O₅ | 150.15 | 1.44% | 0.00% | 0.55% | 0.99% |
| D-xylose | C₅H₁₀O₅ | 150.15 | 4.76% | 4.83% | 0.42% | 11.95% |
| β-D(-)-Lyxopyranose | C₅H₁₀O₅ | 150.15 | 5.18% | 5.25% | 0.41% | 11.56% |
| Methyl xylopyranoside | C₆H₁₂O₅ | 164.18 | n.d. | n.d. | n.d. | 2.43% |
| Aromatics | - | - | 12.89% | 12.57% | 2.73% | 2.48% |
| 2,6-Dimethoxyhydroquinone | C₆H₄(OH)(OCH₃)₂ | 170.18 | 0.50% | n.d. | 0.19% | 0.34% |
| Vanillic acid | C₆H₄(OH)(COOH)(OCH₃) | 168.16 | 1.91% | 1.93% | 0.74% | 0.89% |
| Benzoic acid, 4-hydroxy-3,5-dimethoxy- | C₆H₄(OH)(COOH)(OCH₃)₂ | 198.19 | 0.32% | 0.32% | n.d. | n.d. |
| Protocatechuic acid | C₆H₄(OH)(COOH)(OCH₃) | 154.13 | 0.70% | 0.71% | 0.39% | n.d. |
| Syringic acid | C₆H₄(OH)(COOH)(OCH₃)₂ | 198.19 | 3.38% | 3.43% | 0.30% | n.d. |
| Acetyl syringic acid | C₉H₁₀O₅ | 240.23 | 1.16% | 1.17% | 0.40% | 1.26% |
| Phthalic acid, di(2,3-dimethylphenyl) ester | C₆H₄(CO₂R)₂(OCH₃)₂ | 350.44 | 0.40% | 0.40% | 0.25% | n.d. |
| 4,4'-Methylenedi-2,6-xylenol | C₁₄H₁₄O₂ | 214.28 | 3.70% | 3.75% | 0.46% | n.d. |
| 3-Hydroxy-7,8,2',3'-tetramethoxyflavone | C₁₉H₁₈O₇ | 358.37 | 0.83% | 0.84% | n.d. | n.d. |
| Other monomers | - | - | 5.74% | 3.97% | 6.93% | 8.58% |
| Sample | C | H | O | S | N | PPU empirical formula | MwPPU (g/mol) |
|---|---|---|---|---|---|---|---|
| LB | 57.9 ± 0.03 |
5.55 ± 0.02 |
32.86 ± 0.06 |
3.10 ± 0.24 |
0.52 ± 0.33 |
C9H5.944O2.461S0.228N0.087(OCH3)2.372 | 235.69 |
| 60°C, 3 h, pH 1 | 48.56 ± 0.16 |
4.36 ± 0.02 |
41.90 ± 0.26 |
4.57 ± 0.21 |
0.62 ± 0.11 |
C9H7.102O5.350S0.365N0.113(OCH3)1.343 | 255.60 |
| 60°C, 3 h, pH 1, no stirring | 49.74 ± 0.08 |
4.58 ± 0.01 |
40.97 ± 0.14 |
4.23 ± 0.12 |
0.50 ± 0.06 |
C9H6.956O4.959S0.337N0.091(OCH3)1.571 | 255.06 |
| 60°C, 3 h, pH 10 | 56.71 ± 0.22 |
5.17 ± 0.08 |
34.89 ± 0.18 |
2.62 ± 0.15 |
0.61 ± 0.26 |
C9H4.837O2.732S0.202N0.107(OCH3)2.637 | 246.26 |
| Experiment | Type of lignin |
Lignin (g) |
30% (w/v) H2O2 (mL) |
Stirring | pH | T (°C) | Time (h) |
|---|---|---|---|---|---|---|---|
| LB-pH1-50°C | LB | 3 | 10 | Magnetic | 1 1 1 |
50 | 3, 5, 7 |
| LB-pH1-60°C | 60 | 1, 2, 3 | |||||
| LB-pH1-70°C | 70 | 1, 2, 3 | |||||
| LB-pH10-60°C | 10 | 60 | 1, 2, 3 | ||||
| LB-pH1-0rpm-60°C | No | 1 | 60 | 1, 2, 3 | |||
| KL-pH5-60°C | KL | 3 | 10 | Magnetic | 5 | 60 | 1, 2, 3 |
| KL-pH1-60°C | 1 | ||||||
| LB-pH1-60°C-×4 | LB | 12 | 40 | Magnetic | 1 | 60 | 1, 2, 3 |
| LB-pH1-60°C-×25 | 75 | 250 | Mechanical | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





