1. Introduction
Antibiotic resistance is a significant global health crisis with far-reaching implications. The overuse and misuse of antibiotics have accelerated the emergence of resistant bacterial strains, making it increasingly difficult to treat infections that were once easily managed [
1]. This challenge is particularly acute in the case of pneumonia, where the rapid and accurate detection of antibiotic resistance is crucial for effective treatment. The rise of multidrug-resistant pathogens has led to longer hospital stays, increased medical costs, and higher mortality rates, underscoring the urgent need for early and precise diagnostic methods to identify resistant strains and guide appropriate therapy [
2].
Pneumonia remains one of the leading causes of morbidity and mortality worldwide, affecting millions of people each year. In Jordan, the burden of pneumonia is significant, particularly among vulnerable populations such as the elderly and those with underlying health conditions. Timely and effective treatment is essential to prevent complications and reduce mortality. However, the increasing prevalence of antibiotic-resistant bacteria complicates treatment efforts, making it more challenging to achieve positive patient outcomes [
2].
Various diagnostic techniques are employed to identify the causative pathogens in pneumonia, ranging from traditional microbiological cultures to advanced molecular methods. While culture methods are considered the gold standard, they are time-consuming and can delay the initiation of appropriate therapy [
3]. In contrast, molecular techniques like polymerase chain reaction (PCR) offer rapid and highly sensitive detection of pathogens and antibiotic resistance genes, enabling quicker clinical decision-making. PCR has emerged as a powerful tool in the diagnosis of pneumonia, especially in cases where rapid identification of resistant strains is crucial [
4].
The growing threat of antibiotic resistance in pneumonia-causing pathogens presents a significant challenge to public health. With the rise of multidrug-resistant strains, there is a pressing need for diagnostic methods that can quickly and accurately identify these resistant pathogens [
5].
The primary objective of this study is to detect antibiotic resistance genes in sputum and bronchoalveolar lavage (BAL) samples from pneumonia patients using PCR. Additionally, the study aims to evaluate the prevalence of specific resistance genes, and assess the clinical and demographic factors associated with antibiotic resistance in pneumonia patients. The findings are expected to provide valuable insights into the prevalence of antibiotic resistance in Jordan and help refine diagnostic practices to enhance patient outcomes in the treatment of pneumonia.
This research aims to address this need by developing and validating PCR-based diagnostic techniques that can be integrated into routine clinical practice. By focusing on the detection of antibiotic resistance genes in sputum and BAL samples from pneumonia patients, this study seeks to improve the management of pneumonia in Jordan and contribute to the global effort to combat antibiotic resistance.
3. Results
The findings of this study, encompassing demographic and clinical features, hospitalization characteristics, clinical outcomes, microbial pathogenesis, clinical features, risk factors, and pneumonia severity, are summarized below. A comprehensive overview of these results is provided in
Table 11, which consolidates key data points for clarity and ease of reference.
3.1. Demographic and Clinical Features
A total of 114 patients with CAP were included in this study. The study had a median age of 73 years (IQR: 47–79), with a mean age of 64.114 years (±20.331), reflecting a predominantly elderly population. The youngest patient was 16 years old, while the oldest was 95 years old. Male patients constituted 55.263% (n=63), while females accounted for 44.737% (n=51). This gender distribution aligns with global data on pneumonia prevalence, where males are typically more affected due to higher risk factors such as smoking and chronic lung diseases.
3.1.1. Length of Stay and Hospitalization Characteristics
The median hospital length of stay (LOS) was 7 days (IQR: 3–10), with a mean of 11.272 days (±15.091). Hospital stays ranged from a minimum of 1 day to a maximum of 83 days, reflecting significant variability in disease severity. Among the patients requiring ICU admission, the median ICU length of stay was 1 day (IQR: 0–5), with a mean of 4.763 days (±11.882). The median number of hospitalizations was 2 (IQR: 1–3), with a mean of 2.772 (±3.005), and the median number of pneumonia episodes was 0.5 (IQR: 0–1), with a mean of 0.895 (±1.366).
3.1.2. Clinical Outcomes
Of the 114 patients, 23.684% (n=27) succumbed to their illness, indicating a high mortality rate consistent with severe pneumonia cases in older populations. Conversely, 76.316% (n=87) showed clinical improvement and were successfully discharged. This mortality rate highlights the burden of CAP in a vulnerable population, especially in the presence of comorbidities or antibiotic resistance.
3.1.3. Microbial Pathogenesis
The microbiological analysis revealed diverse etiologies of CAP, with bacterial pathogens being the most frequently identified. Bacterial infections accounted for 28.947% (n=33) of cases, followed by bacterial-viral co-infections at 24.561% (n=28). Viral infections alone constituted 20.175% (n=23), and polymicrobial infections were common, including polybacterial (10.526%, n=12) and polymicrobial viral infections (13.158%, n=15). Fungal-bacterial co-infections were the least frequent, comprising 1.754% (n=2). These findings emphasize the complexity of CAP pathogenesis, with a significant proportion of cases involving mixed infections, which may complicate treatment strategies. The data were taken from another study that performed PCR tests on these samples.
3.1.4. Clinical Features and Risk Factors
The most common clinical features in this study included shortness of breath (SOB), which was observed in 54.369% (n=56) of patients, and cough, which occurred in 13.158% (n=15). Fever was noted in 13.592% (n=14) of cases, while sputum production was recorded in 6.140% (n=7). Less frequent symptoms included pleuritic chest pain (8.738%, n=9). Hemoptysis or rust-colored sputum was absent in 103 patients (100%) due to 11 missing values, suggesting that no patients with available data exhibited these symptoms.
Regarding comorbidities, COPD was present in 3.509% (n=4) of patients, and asthma was documented in 5.263% (n=6). A significant majority, 91.228% (n=104), of patients had not received immunosuppressive treatment, indicating that most individuals in this study did not have this specific risk factor.
In terms of respiratory distress, 7.018% (n=8) of patients demonstrated signs of respiratory distress, while 84.211% (n=96) did not exhibit these signs. The prevalence of aspiration pneumonia was 15.358% (n=16), and 5.769% (n=6) of patients required intubation with mechanical ventilation, reflecting the severity of pneumonia in these cases.
Regarding prior antibiotic use, 91.228% (n=104) of patients did not have any antibiotic exposure within the 30 days preceding their current illness. This high proportion suggests that recent antibiotic use may not be a significant factor influencing the selection of antibiotic-resistant strains in this population.
3.1.5. Severity and Guideline Adherence
The CURB-65 score, used to assess pneumonia severity, showed that 24.561% (n=28) of patients had a score of 0, and 57.895% (n=66) had a score of 1, indicating low-risk CAP. A detailed summary of the pneumonia severity, is provided in
Table 11 for a comprehensive overview.
The PSI, also known as the PORT score, was used to assess the severity of pneumonia and predict patient outcomes. Based on the PSI scores, patients were categorized into four risk classes, with each class corresponding to specific mortality rates and clinical management strategies.
59.649% (n=68) of patients had a PSI score ≤50, indicating low mortality risk (0.1%) and typically managed with outpatient care. This class represents the largest proportion of the study.
24.561% (n=28) of patients had PSI scores between 51 and 70, placing them at low to moderate risk, with a mortality rate of 0.6%. These patients may require either outpatient care or observation admission, depending on clinical judgment.
8.772% (n=10) of patients had PSI scores between 71 and 90, indicating moderate risk with a mortality rate of 0.9%. Inpatient admission is generally recommended for these patients due to the potential for complications and the need for closer monitoring.
7.018% (n=8) of patients had PSI scores >130, classifying them as high risk with a mortality rate of 9.3%. These patients typically require inpatient admission and more intensive management due to the significantly elevated risk of adverse outcomes.
Guideline-concordant antibiotic use was recorded in only 35.106% (n=33) of cases, underscoring suboptimal adherence to treatment protocols. Completion of prescribed antibiotic therapy was achieved in 63.158% (n=72) of patients, while 36.842% (n=42) either discontinued treatment prematurely or received incomplete regimens.
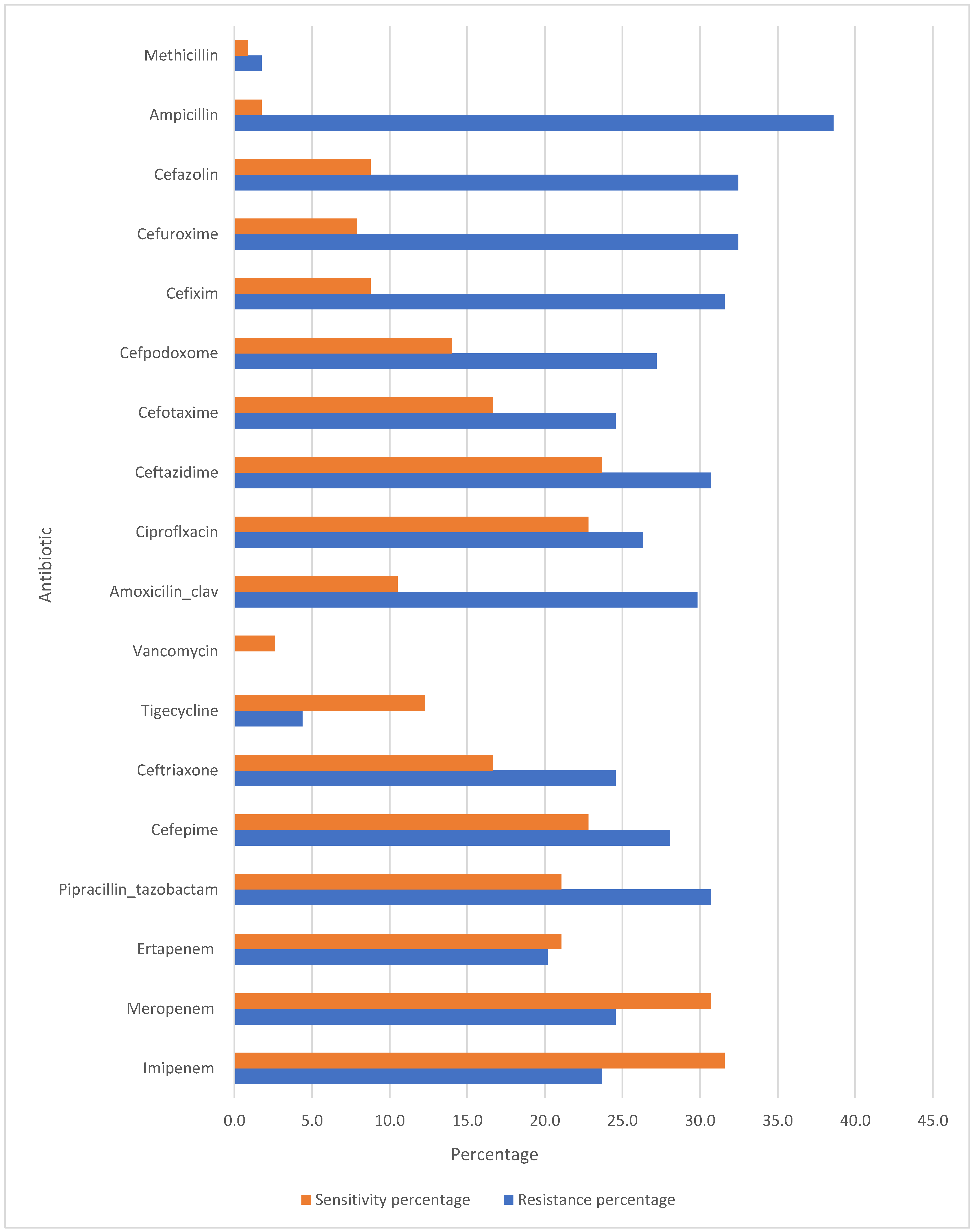
3.2. Antibiotic Sensitivity Test
Community-Acquired Pneumonia (CAP) management is increasingly challenged by the rising incidence of antibiotic resistance. The data provided in
Figure 1 highlights the resistance and sensitivity patterns for various antibiotics used. Understanding these patterns is crucial for guiding effective treatment strategies.
Imipenem and Meropenem: Both carbapenems, Imipenem and Meropenem, exhibit resistance rates of 23.7% and 24.6%, respectively. Their corresponding sensitivity percentages are 31.6% and 30.7%.
Ertapenem: This carbapenem shows a slightly lower resistance rate of 20.2% with a sensitivity of 21.1%.
Piperacillin-Tazobactam: With a resistance rate of 30.7% and a sensitivity of 21.1%, this combination antibiotic is frequently used for its synergistic effect against a wide range of pathogens.
Cefepime, Ceftriaxone, and Ceftazidime: These cephalosporins illustrate varied resistance rates (28.1%, 24.6%, and 30.7%, respectively) and sensitivities (22.8%, 16.7%, and 23.7%, respectively). Ceftriaxone and Ceftazidime are often used as first-line agents for CAP due to their efficacy against Streptococcus pneumoniae and Haemophilus influenzae. Cefepime is typically reserved for more severe cases or suspected gram-negative infections because of its extended spectrum.
Tigecycline: This antibiotic has a notably low resistance rate of 4.4% and a sensitivity of 12.3%.
Amoxicillin-Clavulanate and Ciprofloxacin: Amoxicillin-Clavulanate shows a resistance rate of 29.8% and a sensitivity of 10.5%, whereas Ciprofloxacin has a resistance rate of 26.3% and a sensitivity of 22.8%. Both antibiotics are commonly used for outpatient CAP treatment.
Cefotaxime, Cefpodoxime, Cefixime, Cefuroxime, and Cefazolin: These cephalosporins exhibit significant resistance rates ranging from 24.6% to 32.5%, with sensitivities between 7.9% and 16.7%. Their use in CAP treatment is often guided by susceptibility results.
Ampicillin and Methicillin: Ampicillin shows a high resistance rate of 38.6% with a minimal sensitivity of 1.8%, reflecting limited efficacy against common CAP pathogens. Methicillin’s exceptionally low resistance (1.8%) and sensitivity (0.9%) confirm its limited role, being primarily of historical interest.
3.3. Antibiotic Utilization Patterns
The antibiotic utilization patterns in this study reflect the use of empiric and subsequent therapies. A summary of the findings is summarized in
Table 13, which provides a detailed breakdown of empiric therapy, next-line therapy, and initial continuation therapy.
3.4. Empiric and Subsequent Antibiotic Use
3.4.1. Empiric Therapy
Empiric therapy was prescribed for most patients (n=114), with meropenem being the most frequently used antibiotic, accounting for 31.60% (n=36) of cases. Piperacillin-tazobactam was the second most common empiric antibiotic, utilized in 26.30% (n=30) of cases. Other antibiotics included amoxicillin-clavulanate (7.90%, n=9), which was favored for moderate infections, and ceftriaxone (6.10%, n=7), often prescribed for community-acquired infections. Less frequently used antibiotics included levofloxacin (3.50%, n=4) for respiratory pathogens, azithromycin (4.40%, n=5) for atypical coverage, and ampicillin (3.50%, n=4) for Gram-positive infections. Vancomycin was prescribed in 23.70% (n=27) of cases, reflecting its role in managing suspected MRSA infections. Targeted therapies like doxycycline (0.90%, n=1) and amikacin (1.80%, n=2) were used sparingly for atypical and Gram-negative pathogens, respectively.
3.4.2. Next-Line Therapy
Next-line therapies were initiated in patients who did not respond to empiric treatment or required escalation due to multidrug-resistant organisms. Amoxicillin-clavulanate was the most commonly used next-line antibiotic, prescribed in 14.04% (n=16) of cases to narrow therapy when pathogens were susceptible. Azithromycin and colistin were each used in 9.64% (n=11) of cases, with colistin targeting multidrug-resistant Gram-negative pathogens and azithromycin providing coverage for respiratory and atypical pathogens.
3.4.3. Initial Continuation Therapy
Empiric antibiotics were continued as initial therapy in some cases when clinical progress or culture results supported their use. Colistin was the most frequently continued antibiotic, used in 11.40% (n=13) of cases, primarily for resistant Gram-negative organisms. Doxycycline was continued in 7.02% (n=8) of cases for atypical pathogens, while azithromycin was continued in 6.14% (n=7) for respiratory or atypical infections. Other antibiotics continued included amikacin (4.39%, n=5), metronidazole (1.75%, n=2) for anaerobic infections, levofloxacin (2.63%, n=3), and amoxicillin-clavulanate (0.88%, n=1). Ceftriaxone was continued in 0.88% (n=1) of cases when susceptibility testing confirmed its efficacy.
The hospital opted for carbapenems as empiric therapy for CAP patients due to their broad-spectrum activity against a wide range of pathogens, including resistant strains. Carbapenems, particularly meropenem, are effective against Gram-negative bacteria that are commonly involved in severe CAP cases, such as those caused by multidrug-resistant organisms. Their strong effectiveness against organisms like Escherichia coli and Klebsiella pneumoniae, which frequently exhibit resistance to other antibiotic classes, makes them a reliable choice in critical settings.
Additionally, the rapid deterioration of CAP patients and the potential for severe complications necessitate the use of powerful antibiotics that can provide cover for a variety of possible pathogens during the initial treatment phase. The increasing prevalence of resistant bacteria calls for aggressive empirical therapy, and carbapenems serve this need, bridging the gap until susceptibility patterns can be confirmed through microbiological testing. This approach aims not only to ensure immediate treatment efficacy but also to reduce the risk of treatment failure and the associated morbidity and mortality in these patients.
Table 12.
Antibiotic prescription patterns.
Table 12.
Antibiotic prescription patterns.
| Category |
Antibiotic |
Number of Patients (n) |
Percentage (%) |
Details |
| Empiric Therapy |
Meropenem |
36 |
31.60% |
Most frequently used for severe infections. |
| |
Piperacillin-tazobactam |
30 |
26.30% |
Broad-spectrum agent for Gram-negative and polymicrobial infections. |
| |
Levofloxacin |
4 |
3.50% |
Targets respiratory pathogens. |
| |
Amoxicillin-clavulanate |
9 |
7.90% |
Used for moderate infections, particularly when ESBL-producing organisms are less likely. |
| |
Ceftriaxone |
7 |
6.10% |
Commonly prescribed for community-acquired infections. |
| |
Doxycycline |
1 |
0.90% |
Targeted use for atypical pathogens. |
| |
Amikacin |
2 |
1.80% |
Used for Gram-negative organisms, typically in severe infections. |
| |
Ampicillin |
4 |
3.50% |
Commonly prescribed for less severe Gram-positive infections. |
| |
Azithromycin |
5 |
4.40% |
Often used for atypical respiratory pathogens. |
| |
Vancomycin |
27 |
23.70% |
Used for suspected MRSA infections. |
| Next-Line Therapy |
Amoxicillin-clavulanate |
16 |
14.04% |
Continued when pathogens are susceptible, representing a shift to narrow-spectrum therapy. |
| |
Azithromycin |
11 |
9.64% |
Often used for respiratory infections or atypical coverage. |
| |
Colistin |
11 |
9.64% |
Escalated for multidrug-resistant Gram-negative pathogens. |
| Initial Continuation Therapy |
Ceftriaxone |
1 |
0.88% |
Continued when culture results support its efficacy. |
| |
Amoxicillin-clavulanate |
1 |
0.88% |
Continued when pathogens are susceptible to narrow-spectrum therapy. |
| |
Colistin |
13 |
11.40% |
Used for multidrug-resistant Gram-negative organisms. |
| |
Amikacin |
5 |
4.39% |
Continued for Gram-negative coverage in severe cases. |
| |
Metronidazole |
2 |
1.75% |
Used for anaerobic infections, particularly in intra-abdominal or polymicrobial infections |
| |
Levofloxacin |
3 |
2.63% |
Targets respiratory pathogens. |
| |
Doxycycline |
8 |
7.02% |
Continued for atypical pathogen coverage. |
| |
Azithromycin |
7 |
6.14% |
Often used for respiratory infections or atypical coverage. |
3.5. Antibiotic Resistance Gene Detection
The study investigated the prevalence of antibiotic resistance genes in pneumonia patients, with a focus on several classes of antibiotics. The data revealed variable levels of resistance and sensitivity to different antibiotics, as well as significant variation in the detection of specific resistance genes. A detailed overview of all detected resistance genes and their associations is provided in
Table 14.
3.5.1. Imipenem Resistance
Oxa-40-like gene was detected in 7% of samples from patients resistant to Imipenem, and 93% of samples did not exhibit this gene. The p-value for this association was 0.915, indicating no significant correlation between Oxa-40-like gene presence and Imipenem resistance.
Oxa-48-like gene was found in 13.2% of patients resistant to Imipenem, with 86.8% of samples undetected. The p-value of 0.200 suggests that there is no statistically significant relationship between the presence of this gene and Imipenem resistance.
Oxa-51-like gene detection was higher, with 74% of Imipenem-resistant samples showing the gene and 64.9% undetected. The p-value for this gene was 0.062, which approaches statistical significance, but the association was not strong enough to conclude a definitive relationship.
Imp gene had a detection rate of 4.4% in Imipenem-resistant samples, with a p-value of 0.209, showing no significant association.
Kpc gene was detected in only 1.8% of resistant samples, with a p-value of 0.535, indicating that Kpc does not significantly contribute to Imipenem resistance in the study population.
Ndm gene was present in 33.3% of Imipenem-resistant samples, and the p-value of 0.601 suggests no significant impact on resistance patterns.
Vim gene was detected in 8.8% of resistant samples, with a p-value of 0.074, indicating a potential, but non-significant, association.
3.5.2. Meropenem Resistance
Similar to Imipenem, resistance to Meropenem showed no significant association with the tested genes (p-values for Oxa-40-like, Oxa-48-like, Imp, and Kpc ranged from 0.200 to 0.930). The Oxa-51-like gene exhibited a trend towards significance, with a p-value of 0.093, but the association was not strong enough to conclude a definitive relationship. Vim gene was found in 8.8% of Meropenem-resistant samples, with 91.2% of samples undetected. The p-value of 0.08 shows a potential, but non-significant, association with Meropenem resistance.
3.5.3. Ertapenem Resistance
The Oxa-51-like gene showed the only significant association with Etrapenem resistance, with a p-value of 0.046, indicating that it may contribute to resistance in a portion of the population. The other genes tested, including Oxa40-like, Oxa-48-like, and Imp, showed no significant association with Ertapenem resistance, indicating that they may not play a major role in resistance in this population.
3.5.4. Methicillin Resistance
The Mec A gene was detected in 76.3% of samples resistant to methicillin, with a highly significant p-value of 0.000, indicating a strong association between the Mec A gene and methicillin resistance.
3.5.5. Resistance to Other Antibiotics
Pipracillin-Tazobactam resistance was linked to the Tem gene (70.2% of resistance samples), but no statistically significant association was found (p = 0.904).
Cefepime resistance also showed a strong association with the Tem gene (70.2% of resistance), but this was not statistically significant (p = 0.848). The Ctx-M-1 and shv genes were detected at varying frequencies in different antibiotic resistance profiles, though p-values were consistently higher than 0.05, indicating no significant correlation.
Table 12.
Association between antibiotic resistance genes and antibiotic susceptibility.
Table 12.
Association between antibiotic resistance genes and antibiotic susceptibility.
| Antibiotic |
Gene name |
Resistance gene (%) |
Suciptability (frequency) |
P-value(chi-square ) |
| Resistance |
Sensitive |
| Imipenem |
Oxa-40-like |
7 |
2 |
2 |
0.915 |
| |
Oxa-48-like |
13.2 |
6 |
5 |
0.200 |
| |
Oxa-23-like |
53.5 |
17 |
19 |
0.499 |
| |
Oxa-51-like |
64.9 |
22 |
24 |
0.062 |
| |
Imp |
4.4 |
1 |
0 |
0.209 |
| |
Kpc |
1.8 |
1 |
0 |
0.535 |
| |
Ndm |
33.3 |
11 |
12 |
0.601 |
| |
Vim |
8.8 |
3 |
0 |
0.074 |
| Meropenem |
Oxa-40-like |
6 |
2 |
2 |
0.930 |
| |
Oxa-48-like |
13.2 |
6 |
5 |
0.226 |
| |
Oxa-23-like |
53.5 |
18 |
18 |
0.410 |
| |
Oxa-51-like |
64.9 |
22 |
24 |
0.093 |
| |
Imp |
4.4 |
1 |
0 |
0.212 |
| |
Kpc |
1.8 |
1 |
0 |
0.556 |
| |
Ndm |
33.3 |
11 |
12 |
0.666 |
| |
Vim |
8.8 |
3 |
0 |
0.080 |
| Etrapenem |
Oxa-40-like |
7 |
2 |
1 |
0.811 |
| |
Oxa-48-like |
13.2 |
5 |
2 |
0.357 |
| |
Oxa-23-like |
53.5 |
16 |
10 |
0.151 |
| |
Oxa-51-like |
64.9 |
20 |
14 |
0.046 |
| |
Imp |
4.4 |
1 |
0 |
0.472 |
| |
Kpc |
1.8 |
1 |
0 |
0.508 |
| |
Ndm |
33.3 |
9 |
6 |
0.569 |
| |
Vim |
8.8 |
3 |
0 |
0.216 |
| Pipracillin-Tazobactam |
Tem |
70.2 |
23 |
17 |
0.904 |
| |
Ctx-M-1 |
33.3 |
14 |
6 |
0.692 |
| |
Shv |
35.1 |
13 |
11 |
0.249 |
| Cefepime |
Tem |
70.2 |
12 |
11 |
0.848 |
| |
Ctx-M-1 |
33.3 |
13 |
6 |
0.553 |
| |
shv |
35.1 |
12 |
12 |
0.417 |
| Ceftriaxone |
Tem |
70.2 |
14 |
15 |
0.656 |
| |
Ctx-M-1 |
33.3 |
12 |
5 |
0.431 |
| |
Shv |
35.1 |
12 |
8 |
0.375 |
| Amoxicillin-Clavulanic Acid |
Tem |
70.2 |
24 |
9 |
0.888 |
| |
Ctx-M-1 |
33.3 |
13 |
3 |
0.413 |
| |
shv |
35.1 |
14 |
5 |
0.329 |
| Ceftazidime |
Tem |
70.2 |
23 |
19 |
0.762 |
| |
Ctx-M-1 |
33.3 |
15 |
6 |
0.230 |
| |
shv |
35.1 |
14 |
12 |
0.231 |
| Cefotaxime |
Tem |
70.2 |
19 |
15 |
0.656 |
| |
Ctx-M-1 |
33.3 |
12 |
5 |
0.431 |
| |
shv |
35.1 |
12 |
8 |
0.375 |
| Cefpodoxime |
Tem |
70.2 |
21 |
13 |
0.577 |
| |
Ctx-M-1 |
33.3 |
12 |
5 |
0.758 |
| |
shv |
35.1 |
12 |
8 |
0.280 |
| Cefixime |
Tem |
70.2 |
25 |
8 |
0.776 |
| |
Ctx-M-1 |
33.3 |
14 |
3 |
0.693 |
| |
shv |
35.1 |
16 |
4 |
0.294 |
| Cefuroxime |
Tem |
70.2 |
26 |
7 |
0.867 |
| |
Ctx-M-1 |
33.3 |
14 |
3 |
0.770 |
| |
shv |
35.1 |
17 |
3 |
0.236 |
| Cefazolin |
Tem |
70.2 |
26 |
8 |
0.765 |
| |
Ctx-M-1 |
33.3 |
14 |
3 |
0.770 |
| |
shv |
35.1 |
17 |
3 |
0.242 |
| Ampicillin |
Tem |
70.2 |
31 |
2 |
0.641 |
| |
Ctx-M-1 |
33.3 |
17 |
0 |
0.419 |
| |
shv |
35.1 |
20 |
0 |
0.128 |
| Methicillin |
Mec A |
76.3 |
1 |
0 |
<0.001 |
| Teicoplanin |
VanA/B |
25.4 |
0 |
0 |
0.405 |
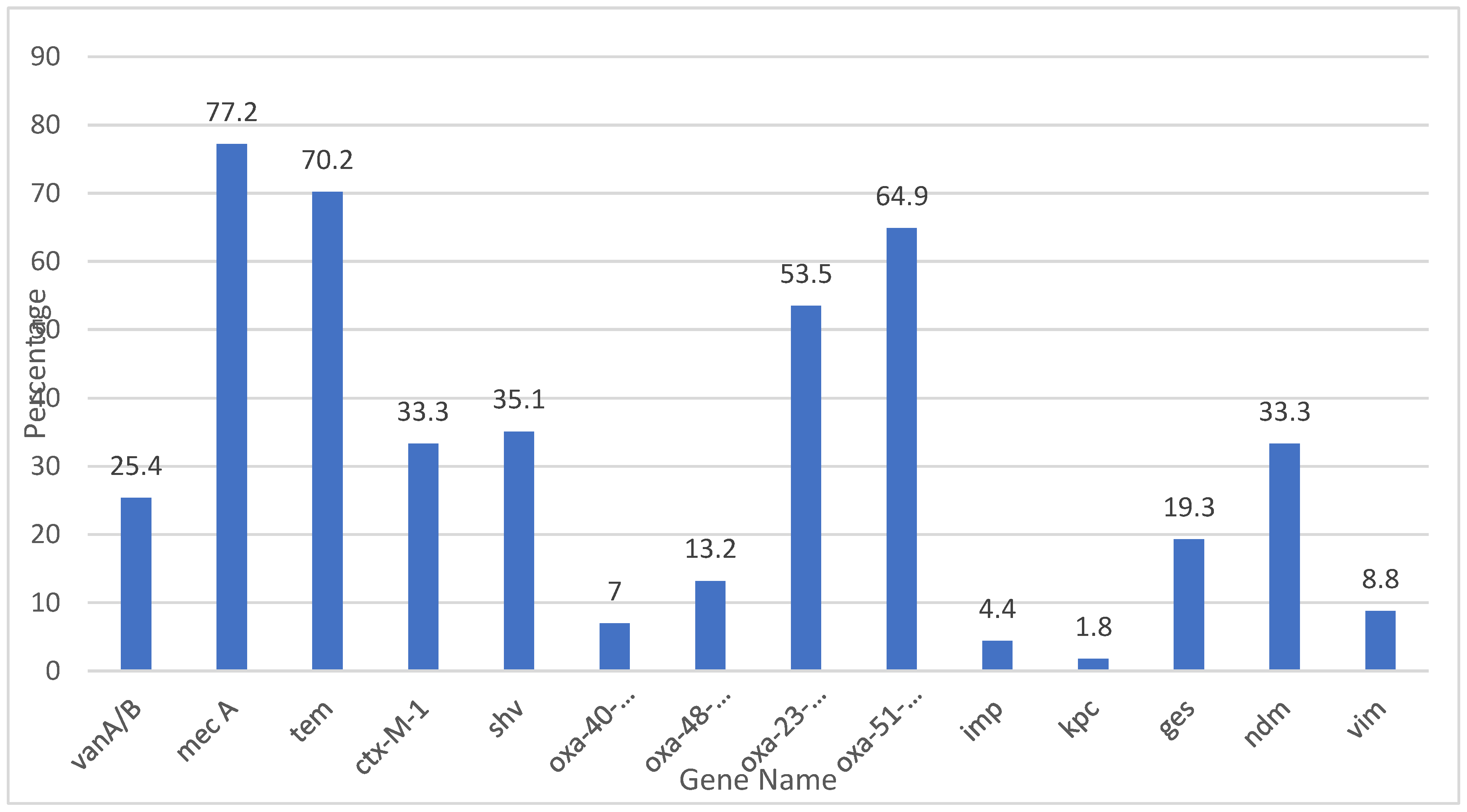
3.6. Antibiotic Resistance Gene Prevalence
In this study, 14 antibiotic resistance genes were evaluated for their prevalence in the tested samples.
Table 14 The gene Mec A was the most commonly detected, identified in 87 samples, corresponding to 77.2% of the total samples analyzed. This gene, known for conferring resistance to methicillin and other beta-lactam antibiotics, reflects a significant presence of methicillin-resistant
Staphylococcus aureus (MRSA) or other resistant strains. Similarly, the
Tem gene, which is associated with extended-spectrum beta-lactamases (ESBLs), was detected in 80 samples (70.2%), indicating beta-lactam resistance.
Other notable findings include the Oxa-51-like and Oxa-23-like genes, detected in 71 (64.9%) and 61 (53.5%) samples, respectively. These genes are typically linked to carbapenem resistance in Acinetobacter baumannii and other Gram-negative pathogens. Their high prevalence signals the significant threat posed by carbapenem-resistant organisms in the tested population. Furthermore, the Ctx-M-1 gene, another key marker for ESBL production, was identified in 38 samples (33.3%), underscoring the continued prevalence of beta-lactamase-producing organisms.
Genes associated with other forms of resistance, such as shv and Ndm, were also frequently detected. The shv gene, which codes for beta-lactamases, was identified in 40 samples (35.1%), while the Ndm gene, responsible for carbapenemase production, was present in 38 samples (33.3%). These findings highlight the growing challenge of multidrug-resistant pathogens, particularly those producing carbapenemases and ESBLs.
The detection of VanA/B (29 samples, 25.4%), ges (22 samples, 19.3%), and Oxa-48-like (15 samples, 13.2%) further emphasizes the presence of resistance mechanisms in the population.. The ges gene, associated with resistance to aminoglycosides, and Oxa-48-like, a carbapenemase gene, further highlight the diverse range of resistance mechanisms present in the tested samples.
Lower frequencies of detection were observed for the Oxa-40-like (7%), Vim (8.8%), and Imp (4.4%) genes. The Oxa-40-like and Vim genes, both related to carbapenem resistance, were detected in a smaller proportion of samples, but their presence still indicates potential concerns in the resistance landscape. The imp gene, found in only 5 samples (4.4%), also contributes to carbapenem resistance, though it appears to be less prevalent in this study. The Kpc gene, associated with carbapenemase production, was the least commonly detected, appearing in just 2 samples (1.8%).
The distribution of these resistance genes demonstrates a complex and multifaceted resistance profile within the population. The high prevalence of Mec A, Tem, and shv suggests a substantial burden of beta-lactam and methicillin resistance. Meanwhile, the presence of carbapenemase genes such as Oxa-51-like, Oxa-23-like, Ndm, and Kpc emphasizes the growing threat of multidrug-resistant Gram-negative bacteria. Additionally, the detection of VanA/B and ges highlights the ongoing issue of resistance to glycopeptides and aminoglycosides.
Figure 2.
Antibiotic resistance genes percentage.
Figure 2.
Antibiotic resistance genes percentage.
Table 13.
Antibiotic resistance genes frequency.
Table 13.
Antibiotic resistance genes frequency.
| Gene |
Frequency |
| VanA/B |
29 |
| Mec A |
87 |
| Tem |
80 |
| Ctx-M-1 |
38 |
| shv |
40 |
| Oxa-40-like |
8 |
| Oxa-48-like |
15 |
| Oxa-23-like |
61 |
| Oxa-51-like |
71 |
| Imp |
5 |
| Kpc |
2 |
| ges |
22 |
| Ndm |
38 |
| Vim |
10 |
5.6. Comparison between Culture and qPCR
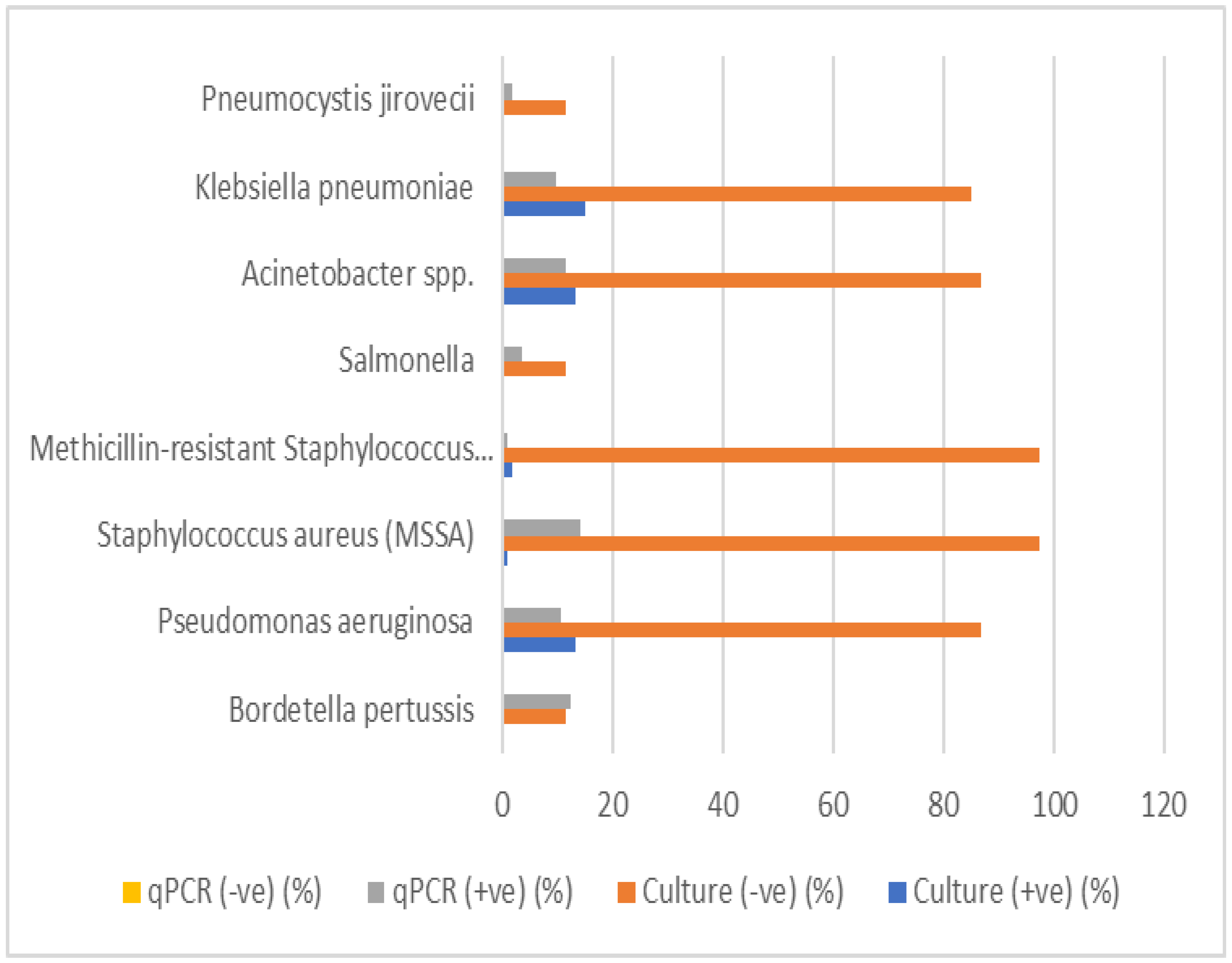
The comparison of culture and qPCR detection for various respiratory pathogens was conducted to assess the diagnostic performance of both methods. The results for each pathogen are summarized in Table 16. The table includes the valid percentages for positive and negative culture results, qPCR results, as well as the p-values for statistical significance.
Bordetella pertussis: A low percentage (12.3%) of positive results were detected by qPCR, with no culture positive samples. The p-value (0.152) indicates no statistically significant difference between culture and qPCR results.
Streptococcus pneumoniae, Mycoplasma pneumoniae, Non-typeable Haemophilus influenzae, Moraxella catarrhalis, Chlamydia pneumoniae, and Legionella pneumophila: These pathogens showed a 100% negative culture rate, but no positive qPCR results were found for most of them. The p-values were marked as "a," indicating no available statistical test was performed or these pathogens did not meet criteria for testing.
Pseudomonas aeruginosa: This pathogen showed 13.2% positive culture results and 10.5% positive qPCR results, with a p-value of 0, suggesting a highly significant difference between culture and qPCR detection methods.
Meticillin-sensitive Staphylococcus aureus (MSSA) and MRSA: While MSSA and MRSA showed a low percentage of positive culture results (0.9% and 1.8%, respectively), both had significantly higher qPCR positivity (14% for MSSA, 0.9% for MRSA). The p-values were 0.778 for MSSA and 0 for MRSA, indicating no significant difference for MSSA but a significant difference for MRSA.
Salmonella, Acinetobacter spp., Klebsiella pneumoniae: Salmonella had a culture positivity of 0%, but the qPCR detected 3.5% positive samples, with a p-value of 0.465, suggesting no significant difference between the two methods. Acinetobacter spp. and Klebsiella pneumoniae had similar findings, with culture positivity percentages of 13.2% and 14.9%, respectively, and qPCR positivity of 11.4% and 9.6%. Both had p-values of 0, indicating no significant difference.
Legionella longbeachae: This pathogen showed no percentage of positive culture (0%) and no qPCR positivity (0%), with all results marked as "not available" for qPCR. The p-value is marked as "a," indicating no comparison.
Pneumocystis jirovecii: This pathogen showed a culture positivity of 0% and qPCR positivity of 1.8%, with a p-value of 0.609, suggesting no significant difference.
Table 14.
Comparison of culture and qPCR results for respiratory pathogens.
Table 14.
Comparison of culture and qPCR results for respiratory pathogens.
| Bacteria Name |
Culture (+ve) (%) |
Culture (-ve) (%) |
Culture Not Available (%) |
qPCR (+ve) (%) |
qPCR (-ve) (%) |
Not Available qPCR (%) |
P-value |
| Bordetella pertussis |
0 |
11.4 |
88.6 |
12.3 |
0 |
87.7 |
0.152 |
| Streptococcus pneumoniae |
0 |
100 |
0 |
0 |
0 |
100 |
a |
| Mycoplasma pneumoniae |
0 |
100 |
0 |
0.9 |
0 |
99.1 |
a |
| Non-typeable Haemophilus influenzae |
0 |
100 |
0 |
2.6 |
0 |
97.4 |
a |
| Moraxella catarrhalis |
0 |
100 |
0 |
0 |
0 |
100 |
a |
| Chlamydia pneumoniae |
0 |
100 |
0 |
0 |
0 |
100 |
a |
| Legionella pneumophila |
0 |
100 |
0 |
0 |
0 |
100 |
a |
| Pseudomonas aeruginosa |
13.2 |
86.8 |
0 |
10.5 |
0 |
89.5 |
<0.001 |
| Staphylococcus aureus (MSSA) |
0.9 |
97.4 |
1.8 |
14 |
0 |
86 |
0.778 |
| Methicillin-resistant Staphylococcus aureus (MRSA) |
1.8 |
97.4 |
0.9 |
0.9 |
0 |
99.1 |
<0.001 |
| Salmonella |
0 |
11.4 |
88.6 |
3.5 |
0 |
96.5 |
0.465 |
| Acinetobacter spp. |
13.2 |
86.8 |
0 |
11.4 |
0 |
88.6 |
<0.001 |
| Klebsiella pneumoniae |
14.9 |
85.1 |
0 |
9.6 |
0 |
90.4 |
<0.001 |
| Legionella longbeachae |
0 |
7.9 |
92.1 |
0 |
0 |
100 |
a |
| Pneumocystis jirovecii |
0 |
11.4 |
88.6 |
1.8 |
0 |
98.2 |
0.609 |
Figure 3.
Comparison between culture and qPCR detection of respiratory pathogens.
Figure 3.
Comparison between culture and qPCR detection of respiratory pathogens.
4. Discussion
This study utilized polymerase chain reaction (PCR) to detect antibiotic resistance genes in pneumonia pathogens, providing rapid and precise identification of genetic resistance mechanisms. The findings shed light on the genetic basis of antibiotic resistance in pneumonia patients, highlighting current management challenges. Empirical antibiotic treatment poses significant challenges, particularly in low- and middle-income countries with widespread multidrug-resistant bacteria [
6]. Rapid molecular PCR-based diagnostics are urgently needed to guide targeted antibiotic therapy, mitigating delayed treatment, mortality, and antimicrobial resistance [
7].
This study used a multiplex PCR assay for rapid detection of 14 antibiotic resistance genes directly from respiratory samples, facilitating timely diagnosis and targeted therapy. Our study identified the
Mec A gene in 77.2% of samples, making it the most prevalent resistance gene detected. This finding corroborates global evidence underscoring
Mec A’s critical role in methicillin resistance, particularly among
Staphylococcus aureus isolates [
8]. The strong association between Mec A and methicillin resistance (p < 0.001) emphasizes its clinical significance in managing
S. aureus-related infections, including pneumonia [
9].
Carbapenem resistance genes (
Oxa-48-like, Ndm, Kpc) exhibited varying prevalence rates, with
Oxa-48-like detected in 13.1% of samples. Although statistical significance was not achieved (p > 0.05), these genes highlight the emerging threat of carbapenemase-producing pathogens in multidrug-resistant pneumonia [
10]
Extended-spectrum beta-lactamase (ESBL) genes, specifically
Ctx-M-1 and
Shv, confirmed the prevalence of beta-lactam resistance. Notably,
Ctx-M-1 (33.3%) contributes significantly to global cephalosporin resistance, emphasizing the importance of considering ESBL-producing bacteria in empirical pneumonia treatment [
11,
12]
The
Oxa-51-like gene demonstrated a borderline significant association with ertapenem resistance (p = 0.046), suggesting its potential role in conferring resistance. Typically intrinsic to
Acinetobacter baumannii [
10], this finding underscores the need for targeted surveillance of
A. baumannii infections, particularly in mechanically ventilated patients.
The hospital selected antibiotics such as meropenem and piperacillin-tazobactam primarily for their broad-spectrum efficacy against various pathogens, particularly those causing severe infections. Meropenem emerged as the most frequently used empiric antibiotic due to its effectiveness against resistant Gram-negative bacteria. In contrast, piperacillin-tazobactam was chosen for its synergistic effects and capability to cover both Gram-positive and Gram-negative organisms.
However, the widespread use of these antibiotics poses a risk of developing resistance. Notably, the prevalence of resistance genes, like
Oxa-51-like and
Oxa-23-like, signifies a considerable threat from carbapenem-resistant organisms. Resistance rates for carbapenems are concerning, with 23.7% for imipenem and 24.6% for meropenem, and similar resistance is observed with piperacillin-tazobactam at 30.7%. (Sharma et al. [
13])
When comparing sensitivity tests to antibiotic utilization, discrepancies arise. For example, despite meropenem’s sensitivity being at 30.7%, it is used in 31.6% of cases, indicating prescriptions often occur despite a significant portion of pathogens exhibiting resistance. Similarly, although piperacillin-tazobactam has a low sensitivity of 21.1%, it is frequently utilized. This gap stresses the importance of aligning empirical treatment decisions with actual resistance patterns and highlights the necessity for ongoing surveillance and antibiotic stewardship programs to combat resistance development effectively.
The results of this study underscore the significant diagnostic advantages of qPCR over traditional culture-based methods for detecting various respiratory pathogens, for example, was identified by qPCR in 12.3% of cases, while no positive results were obtained through culture, although the p-value (0.152) did not indicate statistical significance [
14]
. Pseudomonas aeruginosa displayed a significant detection difference between culture (13.2%) and qPCR (10.5%), with a highly significant p-value (<0.001). This emphasizes qPCR’s capability to complement or even surpass culture methods in detecting certain pathogens [
15]. Similarly, both MSSA and MRSA showed low culture positivity (0.9% and 1.8%, respectively) but much higher qPCR detection rates (14% for MSSA and 0.9% for MRSA). The p-value for MSSA (0.778) indicated no significant difference, while MRSA (p < 0.001) highlighted qPCR’s superiority in detecting resistant strains. Other pathogens such as
Salmonella,
Acinetobacter spp., and
Klebsiella pneumoniae presented with low culture positivity but comparable qPCR detection, with p-values suggesting no significant difference for Salmonella (0.465) but highly significant differences for
Acinetobacter spp. and
Klebsiella pneumoniae (both <0.001).
Pneumocystis jirovecii, which had 0% culture positivity but 1.8% qPCR positivity, indicates that qPCR can detect pathogens missed by culture, though the difference was not statistically significant (p-value: 0.609). Overall, this study’s findings strongly advocate for the integration of qPCR into routine clinical diagnostics. [
16]
The comparison of culture and PCR methods for detecting bacteria reveals that, in some cases, culture proved more effective than PCR, which is typically expected to be more sensitive. However, errors in performing PCR—such as sample handling, DNA extraction, personnel errors, and contamination—can lead to false-negative results. For example, Pseudomonas aeruginosa showed 13.2% culture positivity compared to 10.5% with qPCR. Similarly, for Staphylococcus aureus (MRSA), culture positivity was 0.9%, while qPCR positivity was notably higher at 14%. In the case of Acinetobacter spp., there was 13.2% culture positivity and 11.4% qPCR positivity, while Klebsiella pneumoniae showed 14.9% culture positivity and 9.6% qPCR positivity.
PCR offers several advantages over traditional culture-based methods, making it a superior diagnostic tool in many clinical settings. One of the key benefits of PCR is its high sensitivity, which allows for the detection of low levels of pathogens that may go undetected with culture methods [
15]. Additionally, PCR boasts high specificity, enabling the precise identification of particular DNA sequences from complex mixtures [
17]. This method is also extremely fast compared to traditional techniques, providing rapid results that can significantly expedite clinical decision-making and patient management [
18]. Another advantage is that PCR can be performed easily and can use various fluid samples, including blood, without being influenced by prior antimicrobial therapy. This minimizes the risk of multidrug-resistant pathogen emergence and reduces the need for unnecessarily administered drugs [
19]. Furthermore, PCR minimizes the risk of cross-contamination and allows for the simultaneous identification of multiple microorganisms and resistance genes, enhancing diagnostic throughput and accuracy .However, it is important to note that PCR also has some limitations, such as higher costs compared to traditional methods, the inability to assess susceptibility to all antibiotics, and challenges in diagnosing infections due to non-viable organism detection. Despite these drawbacks, the significant advantages of PCR make it an invaluable tool in modern diagnostics, especially in the fight against antibiotic resistance [
20].
5. Conclusions
This study comprehensively investigated the prevalence of antibiotic resistance genes in pneumonia pathogens using PCR, with a focus on several key antibiotic classes, including beta-lactams, carbapenems, and others. The findings highlighted the complex and dynamic nature of antibiotic resistance in Pneumonia pathogens and underscore the critical need for robust molecular surveillance to guide treatment decisions and combat the growing threat of resistant infections.
The study also highlighted the advantages of using PCR as a diagnostic tool to detect antibiotic resistance genes. PCR provided a rapid, sensitive, and specific method for detecting resistance genes directly from clinical samples, including those from patients with negative or slow-growing cultures. The increased sensitivity of PCR over traditional culture techniques was particularly evident in the detection of pathogens such as Staphylococcus aureus (MSSA and MRSA) and Pseudomonas aeruginosa, where PCR detected resistance in cases that culture failed to identify. This underscores the potential role of PCR in improving the diagnostic accuracy of respiratory infections, enabling more targeted and timely therapeutic interventions.
5.1. Clinical Implications and Recommendations
5.1.1. Patient Sample Collection
Collect respiratory samples (e.g., sputum, nasopharyngeal swabs, or bronchoalveolar lavage) and blood cultures from CAP patients. Ensure sterile collection, labeling, and rapid transport to the lab.
5.1.2. qPCR Analysis
Perform qPCR to detect specific pathogens (e.g., Streptococcus pneumoniae, Haemophilus influenzae, atypical bacteria) and resistance genes (e.g., Mec A, Tem, Ctx-M-1, or carbapenemase genes like Oxa-48-like).
5.1.3. Initial Empiric Therapy for CAP
Begin treatment immediately, tailoring the choice of antibiotics to the severity of the illness and local resistance patterns.
Low-Severity CAP (CURB-65 Score 0–1):
Atypical coverage: Add Azithromycin or Doxycycline.
Combination therapy with Ceftriaxone (or Cefotaxime) and Azithromycin.
Broad-spectrum: Piperacillin-Tazobactam or Meropenem, plus Azithromycin for atypical coverage. Suspected MRSA: Add Vancomycin or Linezolid.
5.2. Tailored Therapy Based on qPCR Results
Adjust therapy once qPCR results identify the pathogen and resistance genes:
Methicillin-Resistant Staphylococcus aureus (MRSA): Use Vancomycin or Linezolid.
Beta-Lactamase Producers (e.g., Tem, shv, or Ctx-M-1): Use Meropenem or Ertapenem.
Carbapenemase-Producing Pathogens (e.g., Oxa-51-like, Ndm genes): Use Colistin plus Tigecycline.
Multidrug-Resistant Pseudomonas or Acinetobacter spp.: Add Amikacin or Polymyxins.
Atypical Pathogens (e.g., Mycoplasma pneumoniae, Legionella spp.): Use Azithromycin or Levofloxacin.
Typical Pathogens Without Resistance Genes: Narrow therapy to Ceftriaxone, Amoxicillin-Clavulanate, or Doxycycline, depending on pathogen susceptibility.
5.3. Limitations of the Study
Despite the valuable insights provided by this study, several limitations must be considered when interpreting the results. First, the sample size, while adequate for a preliminary analysis, may not fully represent the broader population of pneumonia patients. A larger cohort across multiple centers would be needed to confirm the generalizability of these findings, particularly given the variation in resistance patterns that may exist across different geographical regions.
Second, the study focused primarily on a targeted set of resistance genes, which may not capture the full spectrum of resistance mechanisms present in pneumonia pathogens. It is possible that other uncharacterized genes or mechanisms, such as efflux pump systems or alterations in antibiotic targets, contribute to resistance but were not included in the analysis. Additionally, the study did not assess the phenotypic resistance of the pathogens, which would have provided complementary data on the clinical significance of the detected genes.
Furthermore, while PCR is a powerful tool for identifying resistance genes, it does not provide information about the functional expression of these genes in bacterial populations. In some cases, resistance genes may be present but not actively contributing to the resistance phenotype. Future studies using whole-genome sequencing or transcriptomic approaches could provide a more comprehensive understanding of the genetic and phenotypic relationships between resistance genes and resistance phenotypes.
Another limitation was the use of a single diagnostic kit (Bacresista GLA kit), which may not have detected all possible resistance genes, particularly those that are rare or specific to certain pathogens. Future work could involve using broader or more customized panels for PCR detection to ensure the inclusion of a wider variety of resistance determinants.
5.4. Future Work
Future studies should aim to expand on the findings of this research by addressing the limitations outlined above. Key directions for future work include:
Larger, Multicenter Studies: A larger and more geographically diverse sample would allow for more robust data on the prevalence and distribution of antibiotic resistance genes across different populations. Multicenter studies could provide a clearer picture of regional differences in resistance patterns, which is crucial for tailoring local and national antibiotic stewardship policies.
Broadening the Range of Resistance Genes Studied: Future work should include a broader range of resistance genes, particularly those related to newer antibiotics and emerging resistance mechanisms. Whole-genome sequencing or next-generation sequencing (NGS) could provide a more comprehensive assessment of both known and novel resistance determinants, including those associated with plasmids, integrons, or other mobile genetic elements. This would offer a more complete understanding of the genetic basis of resistance in pneumonia pathogens.
Phenotypic Resistance Testing: Complementing molecular techniques like PCR with phenotypic resistance testing could help establish a clearer link between gene presence and antibiotic resistance. This would be especially useful for genes that are not fully expressed or may have reduced functional significance in clinical isolates.
Functional Studies: Investigating the functional role of resistance genes, such as Ndm, Oxa-48-like, and Ctx-M-1, in clinical isolates would provide valuable insights into how these genes contribute to resistance in a dynamic clinical setting. Transcriptomic analyses or protein assays could help determine the expression levels and activity of these genes under different conditions, thus further clarifying their role in infection outcomes.
Development of PCR-based Diagnostic Tools for Routine Clinical Use: As the study demonstrates the value of PCR in detecting resistance genes, future research could focus on optimizing and validating PCR-based diagnostic kits for routine clinical use in pneumonia diagnostics. Such kits could be used to rapidly identify both the pathogens and their resistance profiles, allowing for faster, more targeted treatment strategies.
Author Contributions
Conceptualization, E.A.A., M.A.Z., and A.R.A.; methodology, A.R.A.; software, A.R.A.; validation, A.R.A., M.S.Z., and L.Z.H.; formal analysis, A.R.A. and E.A.A.; investigation, A.R.A. and E.A.A; resources, A.R.A.; data curation, A.R.A. and M.A.Z.; writing—original draft preparation, A.R.A. and E.A.A.; writing—review and editing, M.A.M., H.A.K., M.A.S., and A.R.A.; visualization, E.A.A.; supervision, A.R.A. and M.S.Z.,; project administration, A.R.A.; funding acquisition, A.R.A. and M.S.Z., All authors have read and agreed to the published version of the manuscript.