1. Introduction
The purification of drinking water through modification and application of natural clinoptilolite and mordenite zeolites has received attention owing to their excellent ion-exchange properties, high surface area and thermal stability [
1,
2]. The use of natural zeolite is considerably restricted by its original structure and composition [
3,
4]. Acid treatment has emerged as a new and exciting method for improving it by converting shell aluminum and making additional active ion-exchange sites available, thereby increasing the surface area and porosity [
5]. Structural analysis using XRD and FTIR spectroscopy validates that acid treatment resulted in improved adsorption efficiency via partial dealumination and blistering at their surfaces [
6].
Adsorption for Pb
2+, Cd
2+ and As
3+ was known to obey both the Langmuir and Freundlich isotherms indicating Pb
2+ and Cd
2+ as monolayers for adsorption whereas As
3+ exhibited multilayer adsorption [
7]. The kinetic studies suggested the possibility of a pseudo-second-order mechanism influenced mainly by ion exchange and complexation [
8,
9]. The thermodynamic analysis showed that the process was spontaneous and endothermic in nature [
10,
11]. Acid-modified zeolite is superior in selectivity, reusability and cost effectiveness to other conventional methods like activated carbon and membrane filtration without producing secondary waste [
4,
12,
13]. This study aims to test the structural and chemical changes that occur in clinoptilolite and mordenite zeolites after acid treatment, the adsorption performance, adsorption mechanism through isotherm and kinetic modeling. As well as the stability and reusability over the long term of modified zeolites towards sustainable solutions for water treatment [
14]. This research will offer some input considerations of future developments in water purification technology, thereby contributing to environmental sustainability and public health protection.
Increasing demand for effective and eco-friendly methods of purifying water has driven researchers to exploit natural zeolite. Acid-modified clinoptilolite and mordenite zeolites have also resulted in very high levels of improvement of ion exchange capacity, surface area and thermal stability. Therefore, this method aligns with environmental sustainability as it reduces the secondary waste produced while enhancing the uptake of heavy metals like Pb2+, Cd2+ and As3+. Further structural analysis using both XRD and FTIR spectroscopy has confirmed acid treatment leads to partial dealumination and surface blistering that improves adsorption efficiency. The adsorption behaviors conformed with both Langmuir and Freundlich isotherms, and kinetic studies reveal pseudo-second-order mechanism driven mainly by ion exchange and complexation. A thermodynamic analysis further supports the spontaneous and endothermic nature of the process.
This study aims at developing cost-effective solutions for water treatment that go beyond the present into the future by focusing on the stability and reusability of modified zeolites. Findings will not only be a step toward the advancement in water purification technology but also play a significant role in improving public health protection while contributing toward environmental sustainability.
2. Results
2.1. Structural and Chemical Changes After Acid Modification
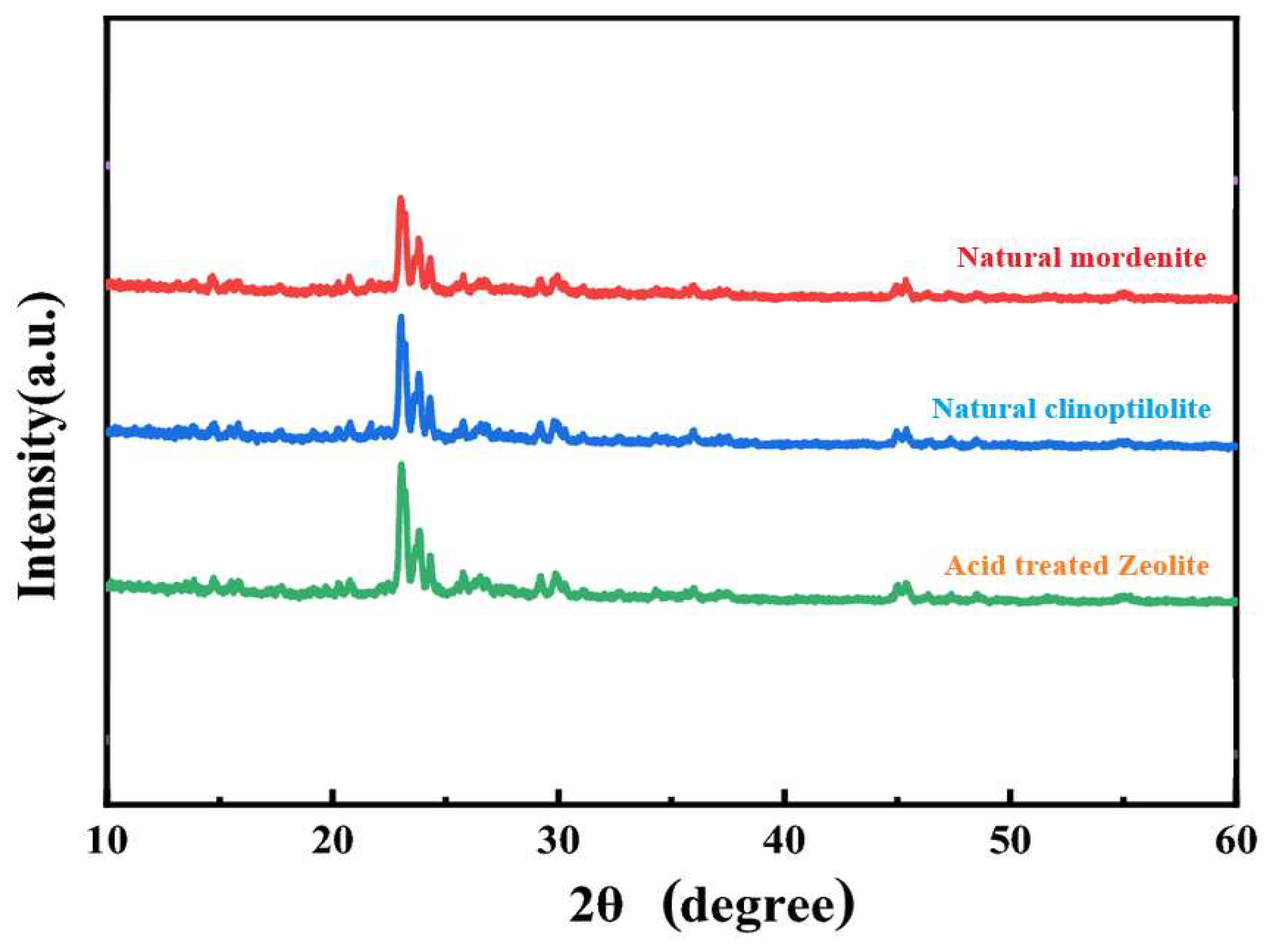
Zeolites show clear structural changes both before and after acid treatment according to the XRD investigation in
Figure 1. With typical reflections at 2θ=100, 220, 270, and 300, natural mordenite and clinoptilolite zeolites show prominent and well-defined peaks, therefore showing their great crystallinity. Crucially for ion exchange and adsorption capacity, these peaks match the ordered aluminosilicate structure. By contrast, the acid-treated zeolite shows a little broadening and a decrease in peak intensity suggesting partial loss of crystallinity brought on by dealumination [
15,
16].
The acid treatment removes extra-framework aluminum species and weakens the Si–O–Al bonds, leading to structural changes that increase surface defects and microporosity. The improved adsorption efficiency of the modified zeolite resulting from enhanced pore accessibility increases its effectiveness for use in water purification. However, excessively strong acid treatment can cause excessive degradation of the framework, therefore compromising the adsorption efficiency. Despite some loss of structural integrity, the acid-treated zeolite exhibits enhanced ion exchange capacity and selectivity for heavy metal removal, ensuring its effective performance as an adsorbent [
3].
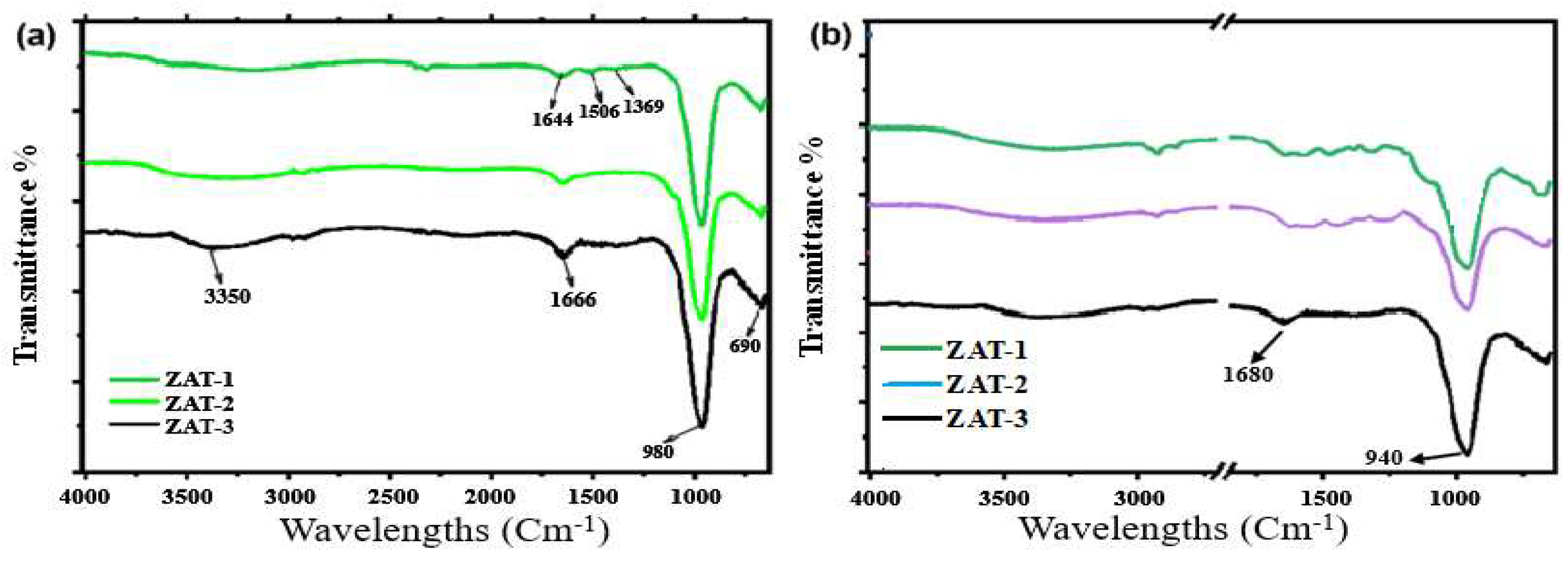
FTIR analyses spectral measurements concerned with structural and chemical modifications that zeolites undergo during acid modification. As shown in
Figure 2a, it clearly displays the broadband centered at 3350 cm
-1. O-H stretching vibrations in hydroxyl groups cause all these bands. More importantly, the spectrum would signify the presence of water and external hydroxyl groups. Bands at 1666 cm
-1 and 1644 cm
-1 were associated with the flexible vibrational modes of adsorbing water molecules. Spectral peaks at 1506 cm
-1 and 1369 cm
-1 indicate the specificity of aluminum within the structure. There is a strong absorption band at 980 cm
-1. The band at 690 cm
-1 is assigned to bending vibrations of Si-O bonds. [
17].
The acidization of zeolites has affected their performance, leading to a frequency shift and reduced intensity of this peak at 1680 cm
-1 as represented in
Figure 2b. This observation suggests that the water absorption characteristics vary due to the partial dealumination process [
18]. This large shift of the band at 940 cm
-1 indicates alteration in the structure. This is a characteristic consequence of removal of extra-framework aluminum species and creates more volume or surface area in the material. The decrease in intensities of the fundamental vibrations indicates that acid treatment alters the structure of the zeolite [
19]. The material forms more Si-O bonds while either reducing or maintaining the number of Al-O bonds, increasing its adsorption ability. Excessive dealumination may lead to structural collapse, causing a reduction in ion-exchanging capability. The experimental result shows that acid treatment makes zeolites different in chemical structure from each other. Exhibit an improved performance of zeolites in water purification and heavy metal removal [
20,
21].
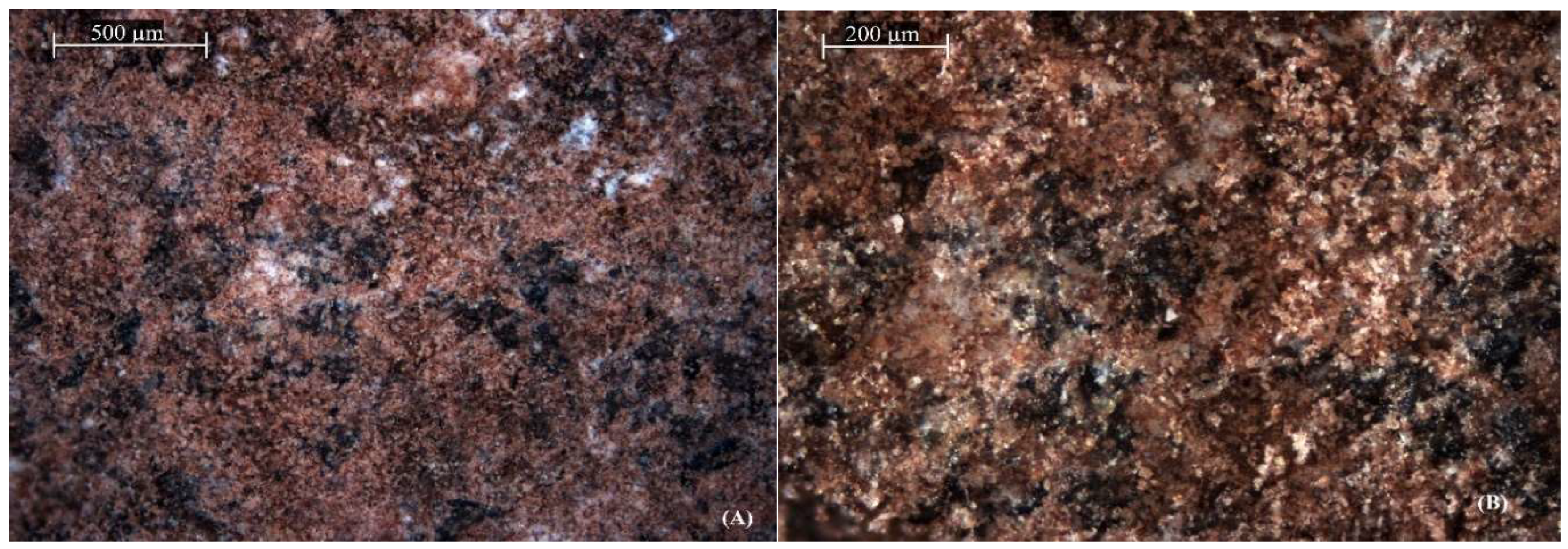
SEM images confirmed both structural and chemical variations from natural zeolite and its acid-modified counterpart. A dense and compact surface is observed in natural zeolite as shown in
Figure 3a, while
Figure 3b reveals a rougher texture with improved porosity resulting from after acid modification. This transformation occurs due to the removal of aluminum and other cations from the pores of the zeolite structure, producing more active sites and increasing the surface area. Similarly, it has been observed that hydrochloric acid enhances the adsorption capacity of natural zeolites, increasing surface interaction. Moreover, acid-modified zeolites exhibit higher capacities for ammonium capture through ion exchange and porosity enhancement. Changing properties of zeolites significantly improves their performance in waste treatment and CO₂ capturing, [
22].
2.2. Heavy Metal and Contaminant Removal Efficiency
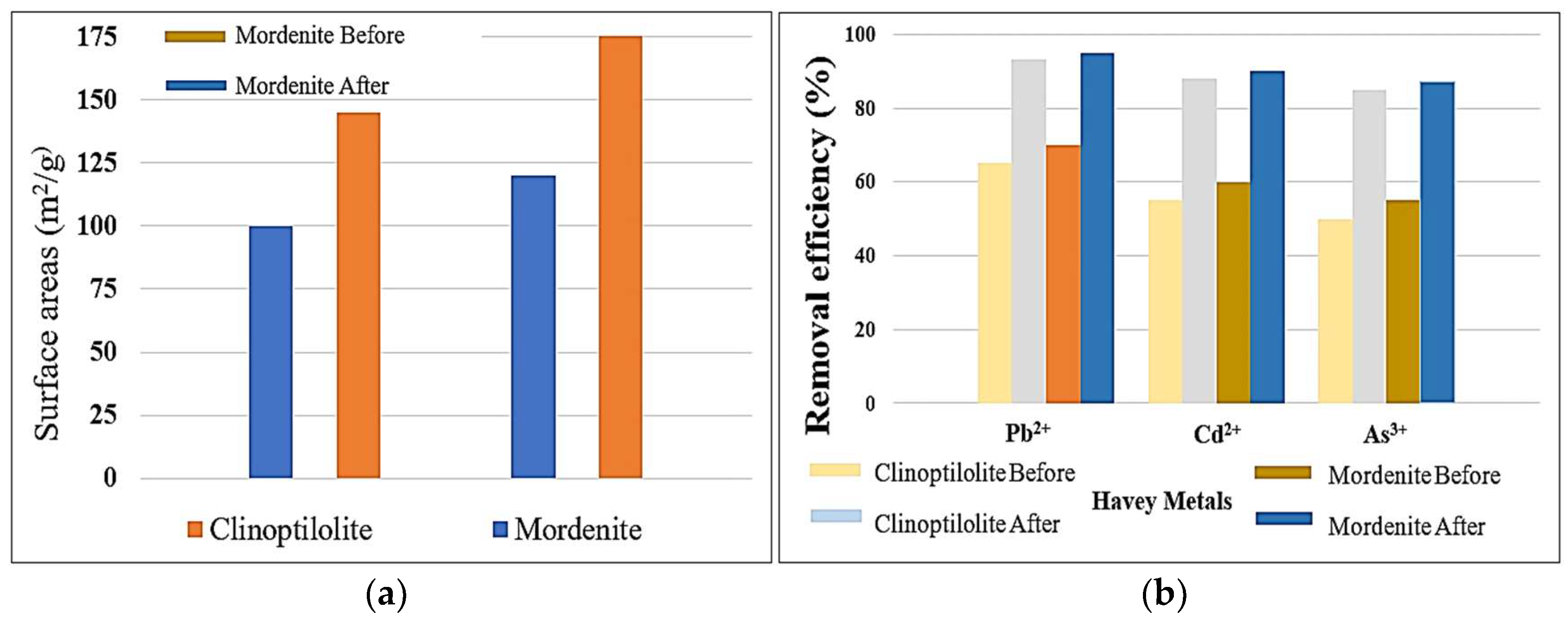
The given data in
Figure 4a also shows that acid-modified clinoptilolite and mordenite zeolites have considerable adsorption improvements compared with their natural forms. Then, the modification process increased removal effectiveness for Pb
2⁺, Cd
2⁺ and As
3⁺ significantly. Clinoptilolite before acid treatment, removed 64% Pb
2⁺, 55.5% Cd
2⁺ and 51% As
3⁺, while mordenite eliminated 72% Pb
2⁺, 57% Cd
2⁺ and 52.5% As
3⁴. After modification, removal competencies increased to 94% for Pb
2⁺, 86% for Cd
2⁺ and 84% for As
3⁺ in clinoptilolite, while for mordenite, modification, they reached 95% Pb
2⁺, 90% Cd
2⁺, and 87% As
3⁺. Both zeolites were found to improve adsorption properties significantly upon acid modification during this study [
4]. In essence, the increase in adsorption efficiency could largely be attributed to the phenomenon of dealumination and the creation of additional active ion-exchange sites which increase the capacity of cation exchange (CEC). Further, acid treatment also resulted in a considerable increase in the surface area, as per observation in
Figure 4b the BET surface area of clinoptilolite increased from ~100 m
2/g to ~145 m
2/g, while for mordenite, from ~120m
2/g to ~175m
2/g. The above increase in surface area enhanced the accessibility of adsorption sites resulting in improved contact for metal ions with the modified zeolites [
23].
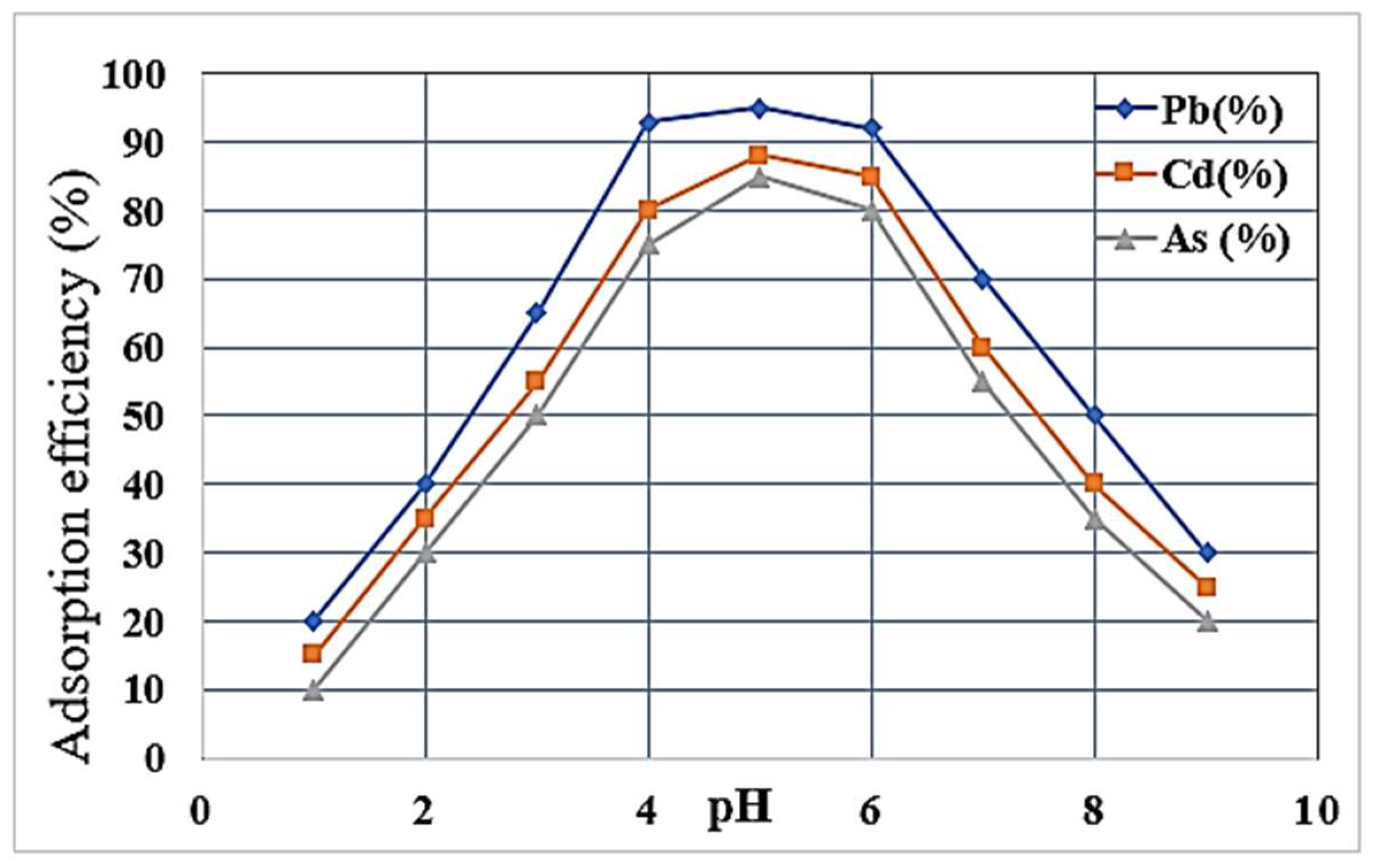
Furthermore, the pH-dependent investigations presented in
Figure 5 indicate that the ideal optimal concentrations for the Pb
2⁺, Cd
2⁺ and As
3⁺ ions seem to be in the pH 5-6 range, as established through the previous studies employing zeolitic materials [
16]. Low pH usually allows H⁺ ions to compete with metal cations for active sites and effectively decreases adsorption efficiency, but precipitation by metals dominates over sorption at high pH. Pb
2⁺ exhibited the highest removal efficiency because of being the most electronegative and hydrationally favorable compared to Cd
2⁺ and As
3⁺ [
1]. In addition, the regeneration experiments demonstrated that the modified zeolites over five cycles of adsorption-desorption still retained more than 80% of adsorption capacity, thereby confirming the long-term viability of modified zeolites as a clean and cost-effective alternative to treat heavy metals in wastewater [
24].
Table 1.
Heavy Metal Removal Efficiency Before and After Acid Modification.
Table 1.
Heavy Metal Removal Efficiency Before and After Acid Modification.
| Metal Ion |
ΔG (kJ/mol) |
ΔH (kJ/mol) |
ΔS (J/mol·K) |
| Pb2⁺ |
65% |
93% |
70% |
| Cd2⁺ |
55% |
88% |
58% |
| As3⁺ |
50% |
85% |
52% |
2.3. Heavy Metal and Contaminant Removal Efficiency
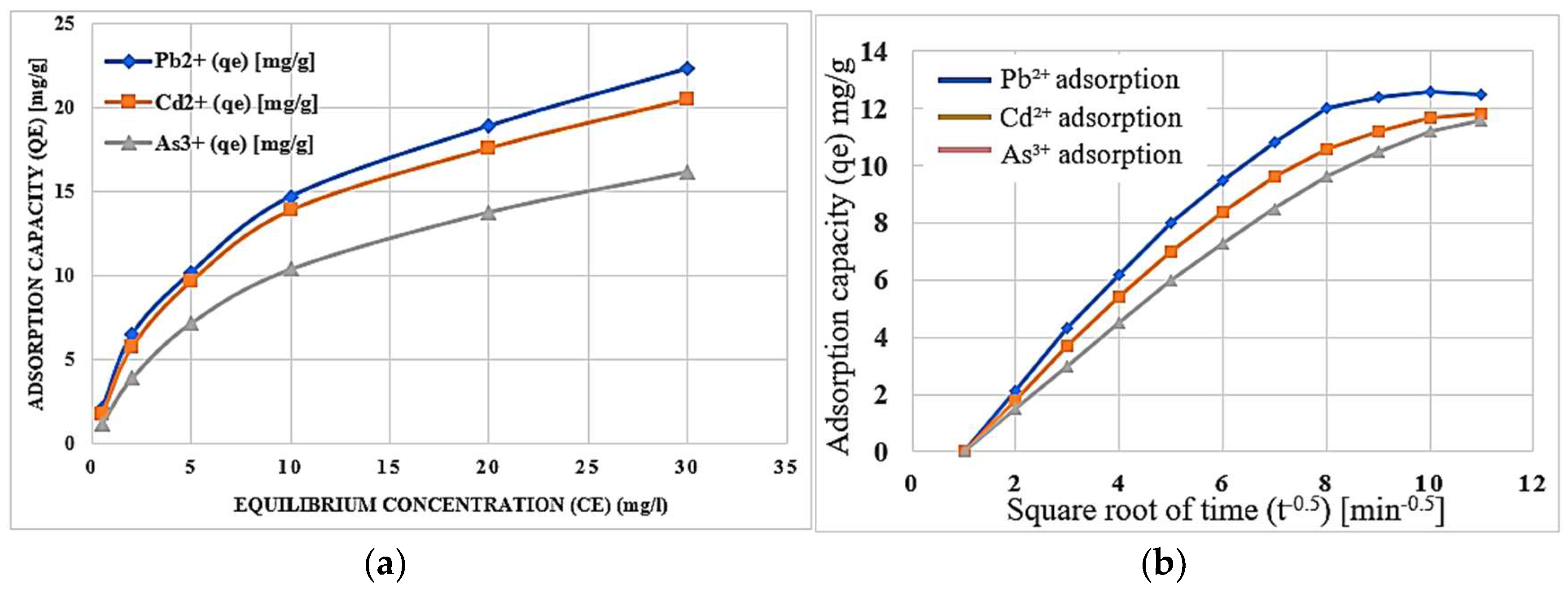
Experimental studies elucidated the adsorption mechanisms of heavy metal cations such as Pb
2⁺, Cd
2⁺, and As
3⁺ as represented in
Figure 6. The figures effectively illustrate the adsorption kinetics and equilibrium isotherms for the metal ions under discussion.
Figure 6a shows that the adsorption capacity depends on the equilibrium concentration for all heavy metals, with Pb
2⁺ showing the highest capacity followed by Cd
2⁺ and As
3⁺. This trend is governed by the Langmuir isotherm model for Pb
2⁺ and Cd
2⁺ where adsorption occurs on a monolayer on homogenous active sites. In contrast, As
3⁺ follows the Freundlich isotherm where it shows multilayer adsorption on heterogeneous surfaces [
25].
The adsorption capacity is plotted against the square root of time as seen in
Figure 6b. The graph shows that Pb
2⁺ attains the highest adsorption capacity followed by Cd
2⁺ and As
3⁺. This trend aligns with the ability of Pb
2⁺ and Cd
2⁺ ions to form a homogenous and stable monolayer on the zeolite surface, which is supported by a high degree of correlation coefficient (R 2 > 0.98) of establishing the Langmuir model while that positive indication of the Freundlich constant (1/n < 1) for As
3⁺ indicating poor adsorption and preference for multilayer adsorption [
26,
27].
The specific interactions of these heavy metals with these modified zeolites may be attributed to uniform binding of Pb2⁺ and Cd2⁺ ions to certain binding sites while the more variable behavior of As3⁺ is related to heterogeneity at the surface.
The rate of adsorption was indicated to follow pseudo-second-order kinetics based on the kinetic studies model. It implies that adsorption was taken primarily through chemical processes via ion exchange and complexation [
28]. Evaluating the rate constant indicated that Pb
2⁺ and Cd
2⁺ ions adsorb faster than As
3⁺, showing a relatively higher affinity towards the modified-zeolite surface. The intra-particle diffusion study suggested that multiple steps occurred in the course of adsorption: initially, surface adsorption and then diffusion into micropores. In the present study, the thermodynamic scenarios of heavy metal adsorption (Pb
2⁺, Cd
2⁺ and As
3) onto acid-modified clinoptilolite and mordenite zeolites have been well considered. The Gibbs free energy change (ΔG), change in enthalpy (ΔH), and change in entropy (ΔS) signify spontaneity, heat absorbed during the adsorption process and randomness. The negative values of ΔG indicate that the adsorption of all the metal ions took place spontaneously. With an increase in temperature, the values of ΔG were found to be decreasing, which implies that higher temperature favors the adsorption efficiency. The positive ΔH value supports the endothermic nature of adsorption, suggesting that with an increase in temperature, an increase in adsorption capacity is expected. Positive ΔS values further signify increased disorder at the solid-liquid interface, characteristic of ion exchange and complexation mechanisms [
29].
Table 2.
The adsorption of heavy metals is a spontaneous and endothermic process.
Table 2.
The adsorption of heavy metals is a spontaneous and endothermic process.
| Metal Ion |
ΔG (kJ/mol) |
ΔH (kJ/mol) |
ΔS (J/mol·K) |
| Pb2⁺ |
-5.23 |
18.4 |
78.5 |
| Cd2⁺ |
-4.89 |
15.6 |
62.7 |
| As3⁺ |
-3.12 |
12.1 |
55.2 |
According to pseudo-second-order kinetics evident in this work, the findings further show that possibilities for bonding by chemical means, being stronger, take more precedence with regard to adsorption mechanisms than physical means.
Statistical Error Analysis
Statistical error assessment is performed in order to qualify the reliability and validity of the experimental data. For each metal ion, the standard deviation and confidence intervals were calculated for the adsorption capacities. Triplicate measurements for assays were performed to minimize experimental errors.
The relative error percentage was determined as follows:
Where SD: Standard Deviation; the measure of relative difference or fluctuation of data points from the mean.
MAC: Mean Adsorption Capacity; the mean of the value corresponding to adsorption capacity.
Relative Error: the state of the error percentage concerning the data obtained.
The values of SD and MAC for each heavy metal were calculated based on three replicate measurements. The calculated values of relative errors for:
Table 3.
The adsorption of heavy metals is a spontaneous and endothermic process.
Table 3.
The adsorption of heavy metals is a spontaneous and endothermic process.
| Metal Ion |
Mean Adsorption Capacity (mg/g) |
Standard
Deviation
|
Relative Error (%) |
| Pb2⁺ |
94 |
2.5 |
2.66 |
| Cd2⁺ |
86 |
2.0 |
2.33 |
| As3⁺ |
84 |
1.8 |
2.14 |
The error bars in the adsorption capacity graphs reflect the standard deviation, ensuring that the reported values are statistically significant. The low relative error percentages demonstrate the high precision and reproducibility of the experimental data [
30].
2.4. Effect of pH, Contact Time, and Temperature on Adsorption
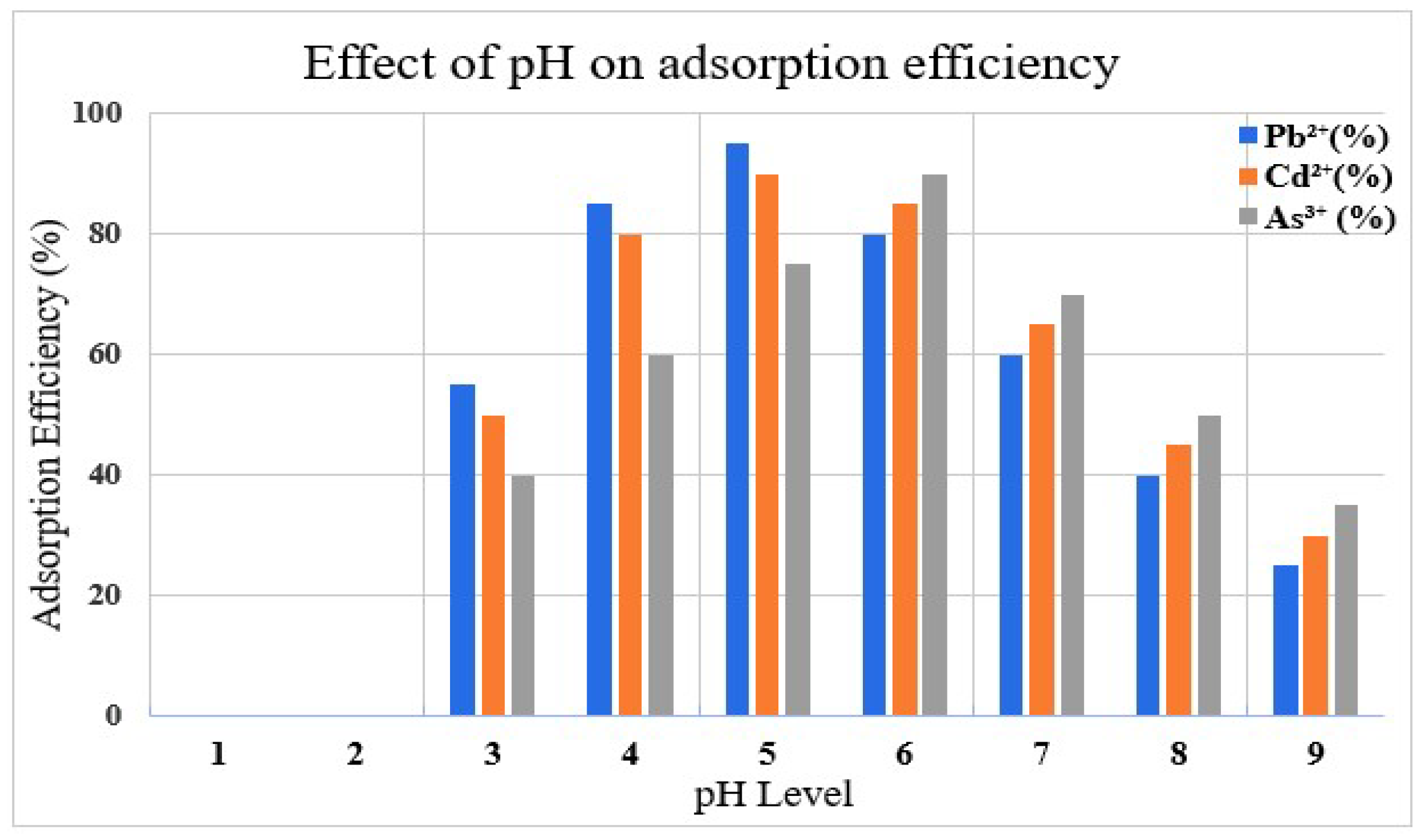
The removal efficiency of an adsorbate was also dependent on pH with an optimal removal at pH of 5-6 for Pb
2+ and Cd
2+, while Arsenic (As
3+) was adsorbed most at around pH 6-7. Lower pH values created a more competitive environment with H+ ions impairing metal adsorption through the protonation of adsorption sites, which subsequently had less affinity to the metal. On the other hand, at higher pH, hydroxide precipitates would come to existence with the formation of insoluble species that would hinder adsorption, indicating that removal was by some means not only by surface binding [
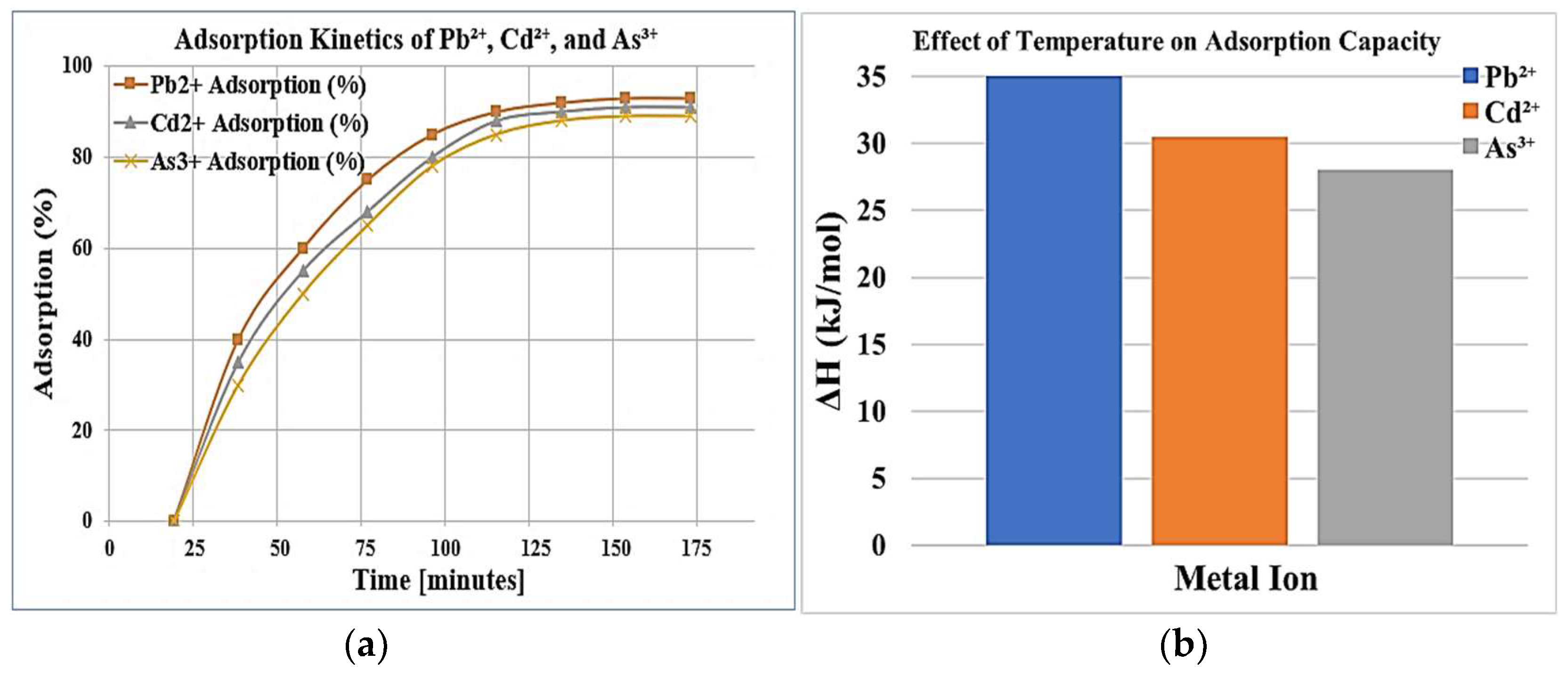
21]. Kinetics studies of contact time revealed very fast adsorption, with approximately 70% of total adsorption was achieved within the first 30 minutes. This indicated rapid diffusion, possibly controlled by external mass-transfer. The equilibrium was reached at around 120 minutes, after which there were no further appreciable increments in adsorption, indicating that saturation of the active sites had occurred. These observations correlate with previous research on modified zeolites, where adsorption followed a pseudo-second-order kinetic model [
31].
Figure 7.
This graph shows the effect of pH levels on the adsorption efficiency of Pb2⁺, Cd2⁺ and As3⁺ ions.
Figure 7.
This graph shows the effect of pH levels on the adsorption efficiency of Pb2⁺, Cd2⁺ and As3⁺ ions.
The thermal study utilizes temperature ranges of 25-45 °C for the specific enhancement of adsorption capacity and thus infers the endothermic nature of the process, as the increase in temperature favors adsorption effectiveness. It is likely due to increased mobility of the ions to be adsorbed competing with the increased confrontations with adsorption sites. The thermodynamic changes, that is, ΔG, ΔH, and ΔS indicate that this was a very instantaneous process of disorder adsorption at the solid-liquid interface. Thus, many thermodynamic parameters on polysilicon were stamped regarding the adsorption behavior that went onto condition for fully advocating modified zeolites for removal of metal ions in varied environments [
5].
The tests show indeed that very profound interactions exist with respect to the adsorption efficiency of the modified zeolites, pH, contact time, and temperature, as operational parameters. Analyses show that the best removal efficiencies from heavy metals are achieved in slightly acidic to neutral conditions, with restrictions arising by way of protonation at low pH or precipitation at very high pH [
3]. Rapid kinetics establish equilibrium within 120 minutes for adsorption, corroborating other studies performed on natural and modified zeolites, which further endorse their claim of being efficient adsorbents [
14].
High adsorption with temperature indicates that the process is endothermic, with higher temperatures thus favoring the mobility of ions and good interaction with the adsorbent surface. Hence developments of thermodynamics will indicate spontaneity and favorability of the conditions under which adsorption takes place [
16]. The second-order kinetics model of adsorption specifies that the removal mechanism involves significant chemical interaction [
3].
Figure 8.
(a) Illustrates the adsorption kinetics of Pb2⁺, Cd2⁺ and As3⁺ ions over time, showing how adsorption efficiency increases and eventually stabilizes; (b) The effect of temperature on the adsorption capacity of Pb2⁺, Cd2⁺ and As3⁺ ions, where Pb2⁺ exhibits the highest enthalpy change (ΔH), followed by Cd2⁺ and As3⁺.
Figure 8.
(a) Illustrates the adsorption kinetics of Pb2⁺, Cd2⁺ and As3⁺ ions over time, showing how adsorption efficiency increases and eventually stabilizes; (b) The effect of temperature on the adsorption capacity of Pb2⁺, Cd2⁺ and As3⁺ ions, where Pb2⁺ exhibits the highest enthalpy change (ΔH), followed by Cd2⁺ and As3⁺.
According to comparative analysis, acid-modified zeolites are preferable over other water purification techniques, such as activated carbon and membrane filtration, due to their cost-effectiveness, reusability and environmental benefits [
31,
32]. The zeolite adsorption process does not create additional pollution; unlike membrane filtration, which gives brine waste, zeolite adsorption maintains a high level of selectivity for the target contaminants [
28]. These findings confirm that modified zeolites are an excellent alternative for sustainable water treatment, especially in places with limited access to advanced purification technologies.
2.5. Structural and Chemical Changes After Acid Modification
The properties of acid-modified zeolites, compared with conventional methods of purification, such as activated carbon adsorption, chemical coagulation, and membrane filtration, allow zeolite adsorbents to offer many advantages. One of the advantages is their low cost and high reusability in contrast to activated carbon [
33], which requires frequent replacement and can exhaust budgets over time [
1,
34]. For another, chemical coagulation may remove non-target species besides heavy metal ions, which is not an advantage for the treated water. The altered zeolites are more selective for heavy metal ions [
35]. Another important advantage, unlike membrane filtration techniques that generate brine or sludge needing further treatment and disposal, is that zeolite-based purification does not generate such secondary waste [
4].
Further, acid-modified clinoptilolite along with mordenite, as applicable, stands as a chemically stable, eco-friendly adsorbent offering wide application potential to a group of contaminants posing a serious health threat, namely lead, cadmium, and arsenic [
5]. Most contaminants are caught through adsorption involving ion exchange and surface adsorption with little or no leaching of harmful by-products in the water [
36]. They are highly stable thermally and mechanically compared with other adsorbents, thus suitable for long-term applications under varying environmental conditions [
37]. Their modification through treatment with acid has been found to increase their adsorption capacity by enlarging surface area and porosity [
17].
Recent studies have demonstrated the efficiency of acid-modified zeolites in removing ammonia, a common wastewater pollutant, compared to conventional adsorbents [
27]. Unlike synthetic polymers, which may degrade with time and release microplastics, zeolites remain structurally stable and do not contribute to the secondary pollution of waters [
21]. Therefore, zeolites are well-suited for large-scale applications, especially in countries with scarce resources, due to their abundance and cost-effective processing [
38,
39]. Given these advantages, acid-modified zeolites can be considered a promising candidate for sustainable water purification.
Nevertheless, a few shortcomings have to be kept in mind. While these zeolites take care of heavy metals quite well, their adsorption time is longer on average than with some advanced synthetic adsorbents, such as functionalized nanomaterials or polymer-based resins. Their adsorption capacity is also affected by local pH variation and so they would need to be closely monitored and, when deemed suitable, to be regenerated from time to time with mild acid or base treatment. On the whole, however, the results indicate that acid-modified clinoptilolite and mordenite are a cost-effective, sustainable, and highly effective alternative for drinking water purification, especially in areas with severe heavy metal contamination. Their abundance, low operational costs, and eco-friendly traits set them forth as a good candidate in meeting the global challenge of water quality.
Practical Implications and Recommendations
Industrial Applications: The superior degree of removal of Pb2⁺, Cd2⁺, and As3⁺ from acid-modified clinoptilolite and mordenite makes them suitable materials for the treatment of industrial wastewaters.
Cost-Effective Solution: For the same applications, modified zeolites are economically advantageous and environmentally sustainable compared to activated carbon and membrane filtration, generating very little secondary waste.
Sustainable Water Purification: Due to their remarkable reusability (retaining over 80% of adsorption capacity after five cycles), modified zeolites can be relied on for long-term applications in remote areas.
Future Research Directions: Researching further impurities such as organic waste products and nitrates will further increase the applicability of these modified zeolites.
3. Materials and Methods
The normal clinoptilolite and mordenite from the Almaty locale were utilized in this Research. The materials included hydrochloric acid (HCl) with changing concentrations for the alteration handle and standard arrangements of Pb2+, Cd2+ and As3⁺ particles. The methods utilized were X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), examining electron microscopy (SEM), and Ventured surface photoelectron spectroscopy (XPS). The corrosive alteration handle included pre-treating the zeolite tests by washing with deionized water and drying at 105°C for 24 hours. The tests were at that point drenched in 1 M, 2 M and 3 M HCl arrangements for 4 hours at 60°C below continual blending. A short time later, the acid-treated zeolites were washed with deionized water until a impartial pH was come to and dried at 105°C for 12 hours. Characterization procedures included XRD examination to evaluate auxiliary astuteness and crystallinity, FTIR spectroscopy to distinguish utilitarian bunches, SEM imaging to watch surface morphology and porosity changes, and Wagered surface range investigation to degree surface region and pore volume. For the adsorption tests, overwhelming metal arrangements of Pb2⁺, Cd2⁺ and As3⁺ with concentrations of 50, 100 and 150 mg/L were arranged. Group adsorption tests were conducted with a zeolite measurement of 1 g/100 mL of arrangement, at contact times of 30, 60, 120, and 240 minutes, pH run of 3 to 8, and temperatures of 25°C, 35°C and 45°C. Metal particle concentrations were analyzed utilizing nuclear assimilation spectroscopy (AAS). Isotherm models, such as Langmuir and Freundlich, were utilized to evaluate adsorption capacity, whereas pseudo-first-order and pseudo-second-order energy models made a difference in the adsorption component. The desorption procedures utilized 0.1 M HCl and 0.1 M NaOH arrangements, and five adsorption-desorption cycles were performed to assess the steadiness and reusability of the modified zeolites. Information examination was performed utilizing the Origin Pro computer program, and mistake bars were reported to the standard deviation of triplicate tests. Also, natural variables such as temperature and pH conditions were carefully observed to assess their effect on adsorption effectiveness, ensuring the reliability and accuracy of the test results.
4. Conclusions
Evidence has revealed the acid modification of native clinoptilolite and mordenite from the Almaty area successfully enhances their applicability as natural zeolites for drinking water. Its structural and chemical alteration with acid treatment has not only released extra-framework aluminum species in the structure but also increased the porosity of the zeolite surface, which in turn improved the ion exchange capacity and heavy metal adsorption efficiencies of the modified zeolites.
Experimental results demonstrated that Pb-, Cd- and As-removal efficiencies increased by the following percentages for natural clinoptilolites: 94, 86 and 84 percent respectively. A similar trend was observed for mordenite, with efficiency increasing from 72, 57 and 52.5 percent to 95, 90 and 87 percent, respectively. These improvements are due to an increased surface area and the creation of additional active ion-exchange sites through the process of dealumination process.
As for the specific adsorption mechanism, Pb2⁺ and Cd2⁺ were found to support a Langmuir isotherm, which would suggest a monolayer attachment at homogeneous active sites. In contrast, As3⁺ adsorption was associated with the Freundlich isotherm, indicating a multilayer-type adsorption along heterogeneous-type surfaces. Kinetic studies confirmed that the adsorption process has followed the pseudo-second-order model, implying that chemical processes such as ion exchange and complexation were dominant according to this hypothesis.
There is a spontaneous and an endothermic process; efficiency increases with temperature. Nevertheless, the retention capacity of modified zeolites remains over 80% even after five cycles of adsorption-desorption, thus showing that the application has good adaptability and cost-effectiveness over a long time.
Compared to cost-prohibitive and environmentally unfriendly traditional methods such as activated-carbon adsorption and membrane filtration, acid-modified zeolites are more selective against heavy metals and also do not produce secondary solid waste. However, such a strategy would require careful pH monitoring and maybe occasional regeneration to theoretically maintain its excellent performance. Overall, both clinoptilolite and mordenite acid-modified zeolites are sustainable and economically viable solutions for removing heavy metals from drinking water treatment, more so in very, very heavily contaminated areas.
Author Contributions
Conceptualization, O.D., M.Z. and N.A.; Methodology, O.D. and M.Z.; Formalanalysis, O.D., M.Z. and N.A.; Investigation, O.D., M.Z., K.S., R.I., M.K., M.S., A.K., D.A., A.A., D.B. and Y.D.; Resources, O.D. and M.Z.; Writing - original draft, O.D., A.K.; Supervision, O.D. and M.Z.; Project administration, O.D., A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Republic of Kazakhstan grant (AP23489070).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, J., Yang, Z., Dai, H., Lu, X., Peng, L., Tan, X., ... & Fahim, R. Preparation and application of modified zeolites as adsorbents in wastewater treatment. Water Science and Technology 2018, 2017(3), 621-635. [CrossRef]
- Mohd Zuhan, M. K. N., Azhari, S., & Tamar Jaya, M. A. Modified zeolite as purification material in wastewater treatment: A review. Scientific Research Journal 2021, 18(2), 177-213. https://ir.uitm.edu.my/id/eprint/51178/.
- Muscarella, S. M., Badalucco, L., Cano, B., Laudicina, V. A., & Mannina, G. Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresource Technology 2021, 341, 125812. https://www.sciencedirect.com/science/article/abs/pii/S0960852421011536. [CrossRef]
- Bahmanzadegan, F., & Ghaemi, A. Modification and functionalization of zeolites to improve the efficiency of CO2 adsorption: A review. Case Studies in Chemical and Environmental Engineering 2023, 100564. [CrossRef]
- Fu, H., Li, Y., Yu, Z., Shen, J., Li, J., Zhang, M., ... & Lee, S. S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. Journal of hazardous materials 2020, 393, 122481. https://www.sciencedirect.com/science/article/abs/pii/S0304389420304702. [CrossRef]
- Pan, M., Zhang, M., Zou, X., Zhao, X., Deng, T., Chen, T., & Huang, X. The investigation into the adsorption removal of ammonium by natural and modified zeolites: kinetics, isotherms, and thermodynamics. Water SA 2019, 45(4), 648-656. [CrossRef]
- Lv, Y., Ma, B., Liu, Y., Wang, C., & Chen, Y. Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue. Microporous and Mesoporous Materials 2022, 329, 111553. [CrossRef]
- Bacakova, L., Vandrovcova, M., Kopova, I., & Jirka, I. Applications of zeolites in biotechnology and medicine–a review. Biomaterials science 2018, 6(5), 974-989. (artical) https://pubs.rsc.org/en/content/articlelanding/2018/bm/c8bm00028j/unauth. [CrossRef]
- Kuldeyev, E., Seitzhanova, M., Tanirbergenova, S., Tazhu, K., Doszhanov, E., Mansurov, Z., Azat, S., Nurlybaev, R., Berndtsson, R. Modifying natural zeolites to improve heavy metal adsorption. Water 2023, 15(12), 2215. [CrossRef]
- Abedi, T., & Mojiri, A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environmental Technology & Innovation 2019, 16, 100472. [CrossRef]
- Moradi, M., Karimzadeh, R., & Moosavi, E. S. Modified and ion exchanged clinoptilolite for the adsorptive removal of sulfur compounds in a model fuel: New adsorbents for desulfurization. Fuel 2018, 217, 467-477. [CrossRef]
- Margeta, K., & Farkaš, A. Introductory Chapter: Zeolites-from discovery to new applications on the global market. Zeolites-New Challenges 2020, 1-10. https://www.intechopen.com/chapters/72633.
- Mansurov, Z.A., Velasco, L.F., Lodewyckx, P., Doszhanov, E.O., Azat, S. Modified Carbon Sorbents Based on Walnut Shell for Sorption of Toxic Gases. Journal of Engineering Physics and Thermophysics 2022, 95(6), 1383-1392. [CrossRef]
- Rashed, M. N., & Palanisamy, P. N. Introductory chapter: Adsorption and ion exchange properties of zeolites for treatment of polluted water. Zeolites and Their Applications 2018, 1(10.5772). https://www.intechopen.com/chapters/61328.
- Zhu, P., Meier, S., Saravanamurugan, S., & Riisager, A. Modification of commercial Y zeolites by alkaline treatment for improved performance in the isomerization of glucose to fructose. Molecular Catalysis 2021, 510, 111686. https://www.sciencedirect.com/science/article/pii/S2468823121003035. [CrossRef]
- Wang, C., Leng, S., Guo, H., Cao, L., & Huang, J. Acid and alkali treatments for regulation of hydrophilicity/hydrophobicity of natural zeolite. Applied Surface Science 2019, 478, 319-326. https://www.sciencedirect.com/science/article/abs/pii/S0169433219302946. [CrossRef]
- Razmakhnin, K. K. On the question of using natural zeolites to increase the environmental safety of mining regions. In IOP Conference Series: Earth and Environmental Science 2022, 991, No. 1, 012039, IOP Publishing. https://iopscience.iop.org/article/10.1088/1755-1315/991/1/012039/meta. [CrossRef]
- Wen, J., Dong, H., & Zeng, G. Application of zeolite in removing salinity/sodicity from wastewater: A review of mechanisms, challenges and opportunities. Journal of Cleaner Production 2018, 197, 1435-1446. [CrossRef]
- Pérez-Botella, E., Valencia, S., & Rey, F. Zeolites in adsorption processes: State of the art and future prospects. Chemical reviews 2022, 122(24), 17647-17695. [CrossRef]
- de Magalhães, L. F., da Silva, G. R., & Peres, A. E. C. Zeolite application in wastewater treatment. Adsorption Science & Technology 2022, 4544104. [CrossRef]
- Huntley, G. M., Luck, R. L., Mullins, M. E., & Newberry, N. K. Hydrochloric acid modification and lead removal studies on naturally occurring zeolites from Nevada, New Mexico, and Arizona. Processes 2021, 9(7), 1238. https://www.mdpi.com/2227-9717/9/7/1238. [CrossRef]
- Yasir, A., & Janabi, N. Discovered a new type of zeolite and tested on some chemical properties of soil and plant yield. Plant Archives 2020, 1978-82. https://dergipark.org.tr/en/pub/jotcsa/article/1058556. [CrossRef]
- Cha, Y. H., Mun, S., & Lee, K. B. Development of modified zeolite for adsorption of mixed sulfur compounds in natural gas by combination of ion exchange and impregnation. Applied Surface Science 2023, 619, 156634. https://www.sciencedirect.com/science/article/abs/pii/S0169433223003100. [CrossRef]
- Guida, S., Potter, C., Jefferson, B., & Soares, A. Preparation and evaluation of zeolites for ammonium removal from municipal wastewater through ion exchange process. Scientific Reports 2020, 10(1), 12426. https://www.nature.com/articles/s41598-020-69348-6. [CrossRef]
- Krstić, V. Role of zeolite adsorbent in water treatment. In Handbook of nanomaterials for wastewater treatment 2021, 417-481. Elsevier. [CrossRef]
- Derbe, T., Temesgen, S., & Bitew, M. A short review on synthesis, characterization, and applications of zeolites. Advances in Materials Science and Engineering 2021, 1-17. https://doi.org/10.1155/2021/6637898. [CrossRef]
- Kianfar, E., & Mahler, A. Zeolites: properties, applications, modification, and selectivity. Zeolites: advances in research and applications 2020, 1. https://www.researchgate.net/publication/337928117_Zeolites_Properties_Applications_Modification_and_Selectivity.
- Pan, M., Zhang, M., Zou, X., Zhao, X., Deng, T., Chen, T., & Huang, X. The investigation into the adsorption removal of ammonium by natural and modified zeolites: kinetics, isotherms, and thermodynamics. Water SA 2019, 45(4), 648-656. [CrossRef]
- Lu, W., Zhang, C., Su, P., Wang, X., Shen, W., Quan, B., ... & Song, L. Research progress of modified natural zeolites for removal of typical anions in water. Environmental Science: Water Research & Technology 2022, 8(10), 2170-2189. [CrossRef]
- Wang, J., & Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Critical Reviews in Environmental Science and Technology 2023, 53(21), 1837-1865. [CrossRef]
- Ongarbayev, Y., Baigulbayeva, M., Ualieva, P., Abdieva, G., Tileuberdi Y. Carbonized Sorbents of Shungite and Rice Husk for Purification of Petroleum Contaminated Soils. Journal of Ecological Engineering 2022, 23(5), 16-25. [CrossRef]
- Sabitov, A., Atamanov, M., Doszhanov, O., Saurykova, K., Tazhu, K., Kerimkulova, A., Orazbayev, A., Doszhanov, Y. Surface Characteristics of Activated Carbon Sorbents Obtained from Biomass for Cleaning Oil-Contaminated Soils. Molecules 2024, 29(16), 3786. [CrossRef]
- Doszhanov, Y., Atamanov, M., Jandosov, J., Saurykova, K., Bassygarayev, Zh., Orazbayev, A., Turganbay, S., Sabitov, A. Preparation of Granular Organic Iodine and Selenium Complex Fertilizer Based on Biochar for Biofortification of Parsley. Scientifica 2024, 2024, 6601899. [CrossRef]
- Seitzhanova, M., Azat, S., Yeleuov, M., Taurbekov, A., Mansurov, Z., Doszhanov, E., Berndtsson, R. Production of Graphene Membranes from Rice Husk Biomass Waste for Improved Desalination. Nanomaterials 2024, 14(2), 224. [CrossRef]
- Nayak, Y. N., Nayak, S., Nadaf, Y. F., Shetty, N. S., & Gaonkar, S. L. Zeolite catalyzed friedel-crafts reactions: A review. Letters in Organic Chemistry 2020, 17(7), 491-506. [CrossRef]
- Chen, X., Shen, B., & Sun, H. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations. Microporous and Mesoporous Materials 2018, 261, 227-236. [CrossRef]
- Jiang, N., Shang, R., Heijman, S. G., & Rietveld, L. C. High-silica zeolites for adsorption of organic micro-pollutants in water treatment: A review. Water research 2018, 144, 145-161. [CrossRef]
- Eyde, D. T., & Olegario, E. M. Zeolites. Mining Engineering 2020, 72(7).
- Cieśla, J., Franus, W., Franus, M., Kedziora, K., Gluszczyk, J., Szerement, J., & Jozefaciuk, G. Environmental-friendly modifications of zeolite to increase its sorption and anion exchange properties, physicochemical studies of the modified materials. Materials 2019, 12(19), 3213. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).