Submitted:
30 March 2025
Posted:
31 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background and Motivation
1.2. Advances in AI-Driven Medical Image Segmentation
- U-Net and Variants: Initially introduced for biomedical image segmentation, U-Net has been widely adopted due to its encoder-decoder structure, which effectively captures spatial and contextual information. Variants like Attention U-Net and 3D U-Net have further improved segmentation accuracy for volumetric imaging [8].
- Transformers in Segmentation: Vision Transformers (ViTs) and Swin Transformers have recently demonstrated superior performance in segmenting medical images by leveraging self-attention mechanisms to capture long-range dependencies [9].
- Generative Adversarial Networks (GANs): GAN-based segmentation models enhance the precision of medical image delineation by generating realistic synthetic data and refining segmentation boundaries [10].
- EEG and MEG: While traditionally used for functional brain mapping, AI-assisted segmentation techniques now improve spatial resolution by segmenting source-localized brain activity. DL enhances artifact removal and signal interpretation [11].
- fNIRS: AI models segment hemodynamic responses from fNIRS data, distinguishing oxygenated and deoxygenated hemoglobin concentrations to map cortical activity with higher precision.
- EMG: AI-driven segmentation aids in the precise identification of muscle activity patterns, improving applications in neuromuscular disorder diagnosis and prosthetic control.
- ECoG and High-Density Arrays: AI models segment cortical activity recorded from ECoG and high-density electrode arrays, enabling more refined brain mapping for epilepsy monitoring and BCI applications [14].
1.3. Challenges and Future Directions
1.4. Importance of Precision Neurosurgery
2. Advanced Neuroimaging Modalities for Precision Neurosurgery
2.1. Role of Neuroimaging in Precision Neurosurgery
- Magnetic Resonance Imaging (MRI) and Computed Tomography (CT): MRI and CT scans serve as foundational tools for visualizing anatomical structures, aiding in tumor resection, and identifying vascular abnormalities [22].
- Fluorescence-Guided Surgery (FGS): The use of fluorescence agents such as 5-ALA enhances real-time intraoperative tumor visualization, thereby improving the accuracy of surgical resection [28].
2.2. Clinical Relevance in Neurosurgical Practice
- Applications of AI in Neurosurgery:
- Brain Tumor Resection: AI-enhanced segmentation assists in accurately distinguishing tumor margins from healthy tissue, thereby reducing the risk of postoperative neurological deficits [40]. Studies have demonstrated that DL models such as CNNs and transformers outperform traditional segmentation methods in identifying tumor boundaries, leading to improved surgical planning [41].
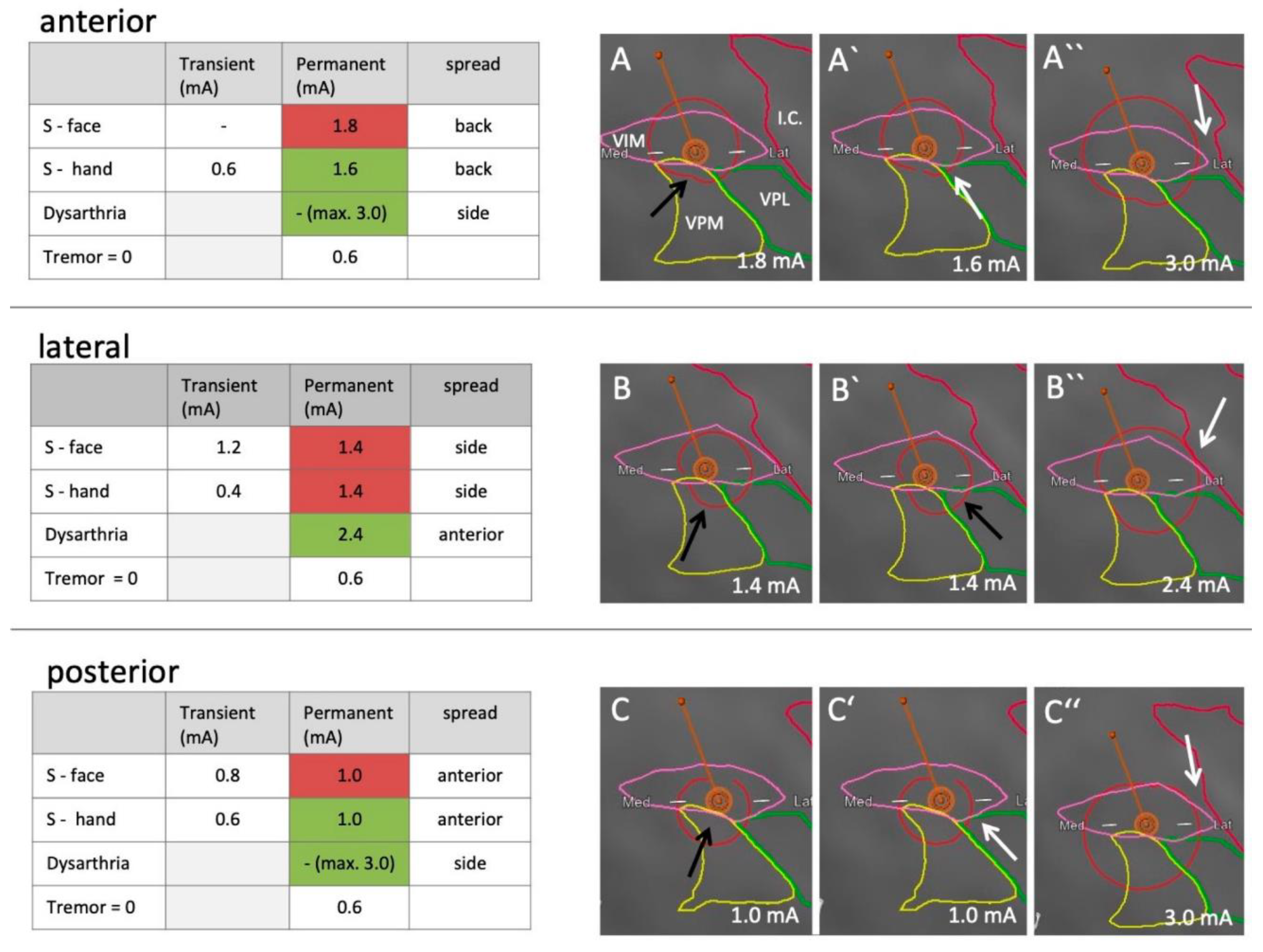
- Deep Brain Stimulation (DBS) Planning: Accurate segmentation of subcortical structures is crucial for optimal electrode placement in DBS procedures used to treat movement disorders such as Parkinson's disease [43]. AI-based volumetric segmentation has been shown to enhance the precision of target selection in DBS, thereby improving therapeutic outcomes [44].
- Epilepsy Surgery: AI-based identification of seizure foci enhances the precision of both resective and neuromodulatory treatments for epilepsy [46]. Machine learning algorithms, particularly support vector machines (SVMs) and recurrent neural networks (RNNs), have been employed to analyze intracranial EEG (iEEG) signals and detect epileptogenic zones with high accuracy [47].
- Challenges and Considerations:
- Interpretability: The "black box" nature of many AI models remains a significant barrier to clinical adoption. To improve transparency, XAI approaches such as attention mechanisms and saliency maps are being explored to provide visual interpretability of AI-generated segmentations [49]. These techniques enhance clinician trust and facilitate regulatory approval [50].
- Regulatory Approvals: AI-driven medical imaging tools require rigorous validation and approval from regulatory bodies such as the U.S. Food and Drug Administration (USFDA) and the European Conformité Européenne (ECE) certification before they can be deployed in clinical settings [51]. Regulatory frameworks are continually evolving to address concerns related to data privacy, bias, and reliability.
- Intraoperative Validation: Real-time validation of AI-generated segmentations during surgery remains a challenge. AI must seamlessly integrate with intraoperative imaging systems, such as neuronavigation platforms, to ensure reliable guidance during neurosurgical procedures [52,53]. Additionally, AR and AI-assisted robotics are emerging as potential solutions for improving intraoperative accuracy [54].
3. Brain-Computer Interfaces: Principles and Applications
3.1. Fundamentals of BCIs
- Signal Acquisition
- Signal Processing and Feature Extraction
- Control and Feedback Mechanisms
3.2. BCI Paradigms
- Motor Imagery (MI)
- P300 Event-Related Potential (ERP)
- Steady-State Visual Evoked Potentials (SSVEPs)
3.3. Neuroimaging Modalities for BCI
- Electrophysiological Modalities
- Electroencephalography (EEG)
- Electrocorticography (ECoG)
- Local Field Potentials (LFPs)
- Single-Unit and Multi-Unit Recordings
- Hemodynamic and Metabolic Modalities
- Functional Near-Infrared Spectroscopy (fNIRS)
- Functional Magnetic Resonance Imaging (fMRI)
- Magnetoencephalography (MEG)
- Emerging and Hybrid Modalities
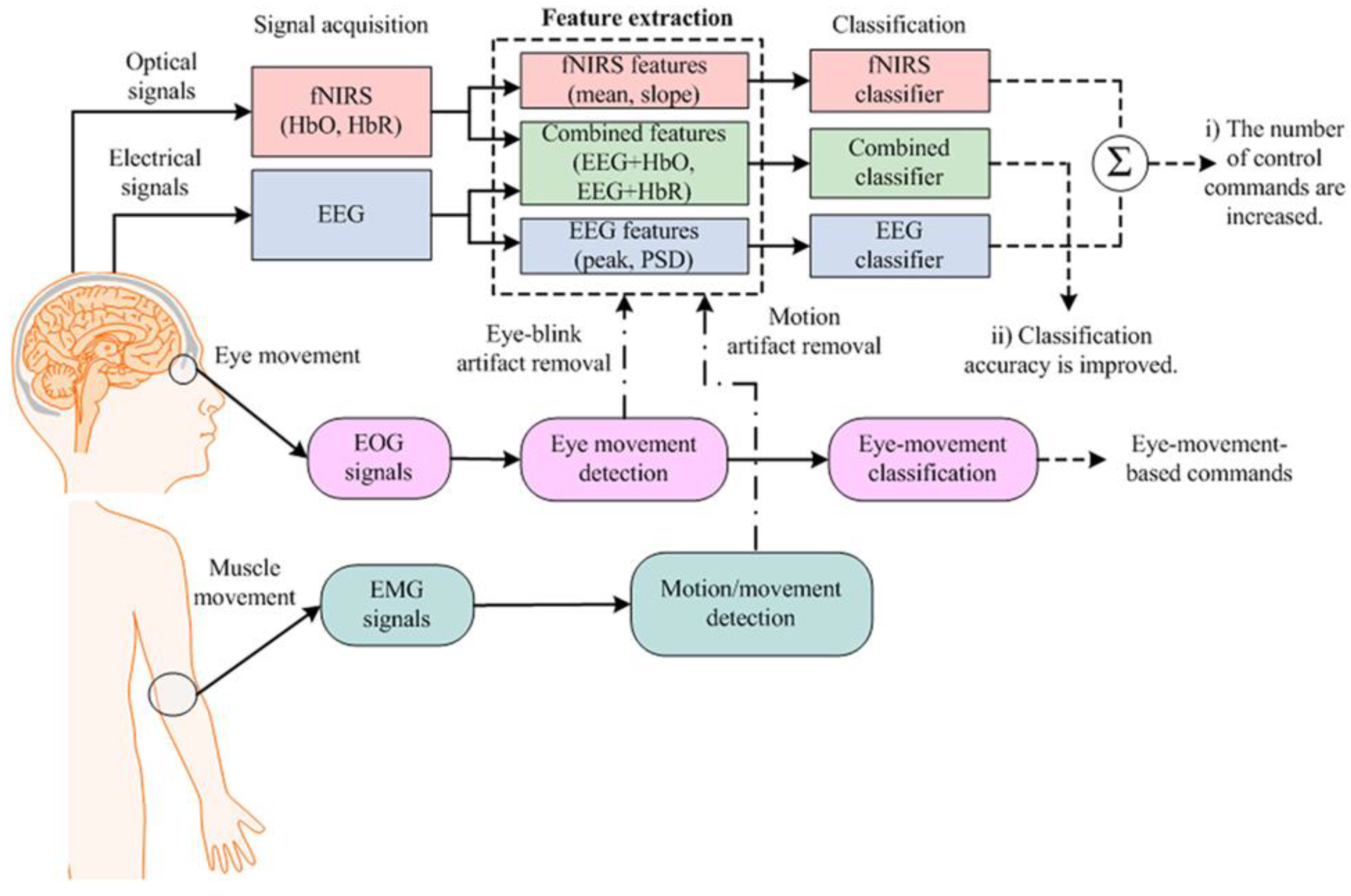
- EEG-fNIRS Hybrid Systems
- EEG-fMRI Hybrid Systems
- Invasive Hybrid BCIs
3.4. Latest Developments
4. AI-Driven Brain Image Segmentation: State-of-the-Art
4.1. Machine Learning and Deep Learning in Image Segmentation
4.2. Mathematical Formulation of CNN-Based Segmentation
- Cross-entropy loss, used for pixel-wise classification:
- Dice loss, which quantifies the degree of overlap between the predicted and true segmentation masks:
- Focal loss, designed to mitigate class imbalance by down-weighting easily classified examples:
4.3. Comparison of Traditional vs. AI-Based Segmentation Methods
- Manual Segmentation
- AI-Based Segmentation
- Speed: AI models can process and segment brain images within seconds, significantly reducing analysis time compared to manual segmentation, which can take hours.
- Accuracy: DL models often achieve Dice similarity coefficients exceeding 0.90, demonstrating expert-level performance.
- Reproducibility: AI models eliminate inter-observer variability, ensuring consistent segmentation results across different datasets and clinical environments.
- Dataset Bias: Models trained on limited datasets may exhibit reduced generalizability to unseen patient populations.
- Interpretability: The inherent "black box" nature of DL models complicates clinical validation and trust in automated segmentation results.
- Regulatory Constraints: AI-driven segmentation tools require extensive validation and regulatory approvals before integration into standard clinical workflows.
5. Hybrid BCI and Image Segmentation Model for Precision Neurosurgery
5.1. System Architecture and Workflow
- Neural Signal Acquisition: EEG and ECoG signals are collected using high-resolution sensors to capture real-time brain activity. Recent advances in non-invasive and minimally invasive BCI techniques improve the spatial resolution and signal fidelity, enabling finer neuro-modulatory applications [79,91,125].
- Preprocessing Pipeline: Raw neural signals undergo artifact removal, band-pass filtering, and feature extraction to ensure noise-free input for classification. State-of-the-art signal processing frameworks integrate ICA and wavelet decomposition to enhance the robustness of feature extraction [126].
- DL-Based Image Segmentation: MRI and CT images are processed using transformer-based segmentation models, such as Swin UNETR, for precise delineation of brain structures. The combination of CNNs and self-attention mechanisms significantly improves segmentation accuracy in glioma detection and tumor boundary definition [38].
- Decision Support System (DSS): The integration of BCI-derived cognitive feedback and AI-based image analysis aids neurosurgeons in optimizing surgical interventions. Multimodal data fusion techniques enhance real-time surgical decision-making, reducing intraoperative errors and improving patient outcomes [127].
- Cloud Integration: A cloud-based AI/ML framework ensures scalability and real-time computational efficiency. Federated learning models deployed in cloud-based medical AI systems facilitate secure, distributed model training while maintaining patient data privacy [128].
5.2. Signal Processing for Real-Time Neurosurgical Assistance
- Fourier and Wavelet Transforms: Fourier and wavelet transforms are essential mathematical tools for analyzing EEG signals in the frequency domain. The Fourier Transform (FT) decomposes EEG waveforms into constituent frequency components, allowing researchers to identify specific oscillatory patterns associated with cognitive processes and motor intentions. However, the FT assumes stationarity in the signal, which is not always applicable to dynamic brain activity [129].
- Independent Component Analysis (ICA): Neural signal recordings, especially EEG, often contain artifacts from non-neural sources such as eye blinks, muscle movements, and external electrical noise. ICA is a powerful statistical technique used to separate and remove these unwanted artifacts while preserving relevant neural information.
-
Deep Neural Networks (DNNs): Recent advancements in DL have significantly improved EEG-based neural decoding. CNNs and RNNs are particularly effective in extracting spatial and temporal features from EEG data, enabling the classification of brain states with high precision [131].
- ○
- CNNs: These networks process EEG signals as spatially structured data, identifying patterns related to motor imagery, cognitive load, and surgical stress responses. CNNs efficiently learn hierarchical representations, making them robust against variations in electrode placement and signal noise.
- ○
- RNNs and Long Short-Term Memory (LSTM) Networks: Unlike CNNs, RNNs capture temporal dependencies in EEG signals. LSTM networks, a variant of RNNs, are particularly effective in modeling sequential EEG data, predicting user intent, and tracking dynamic changes in brain activity over time.
-
Kalman Filters (KFs) and Hidden Markov Models (HMMs): Decoding neural signals in real time involves inherent uncertainty due to noise, signal fluctuations, and measurement errors. KFs and HMMs are probabilistic frameworks designed to address these challenges by smoothing and predicting neural signal patterns.
- ○
- Kalman Filters: These are widely used in brain-computer interfaces to estimate dynamic brain states based on noisy EEG measurements. In neurosurgical applications, Kalman filters improve the real-time tracking of neural activity, making it possible to predict intended movements with greater precision.
- ○
- Hidden Markov Models: HMMs are particularly effective for modeling sequential neural events, such as transitions between different mental states or MI patterns. HMMs assign probabilistic states to EEG sequences, enhancing the accuracy of neurofeedback and BCI-driven assistive technologies.
5.3. Automated Brain Image Analysis Using DL
-
Transformer-Based Segmentation: Traditional convolutional networks often struggle to maintain spatial consistency in brain MRI segmentation. Transformer-based models such as Swin UNETR and TransUNet address this limitation by incorporating self-attention mechanisms that improve feature representation across long-range spatial dependencies.
- ○
- Swin UNETR: A hierarchical vision transformer that refines feature extraction while preserving high-resolution structural details in brain MRI scans.
- ○
- TransUNet: A hybrid model that combines CNN feature extraction with transformer-based contextual modeling, leading to superior segmentation accuracy in neurosurgical planning and brain tumor delineation [133].
- Hybrid Attention Mechanisms: DL-based brain segmentation benefits from hybrid attention models, which combine self-attention (global feature learning) and spatial attention (local feature refinement). This approach enhances the precision of region delineation, crucial for neurosurgical decision-making [134].
- Self-Supervised Learning (SSL): One major limitation of DL in medical imaging is the reliance on large manually labeled datasets. mitigates this issue by leveraging contrastive learning techniques to pre-train models using unlabeled data. This method significantly reduces annotation requirements while maintaining high segmentation accuracy [135].
- Multi-Modal Fusion: Combining data from multiple imaging modalities, including MRI, CT, and fMRI, enhances diagnostic accuracy by integrating complementary information. DL models perform multi-modal fusion using attention mechanisms, improving robustness against modality-specific noise and artifacts [136].
5.4. Integration with Cloud-Based AI/ML Platforms
- Edge Computing for Low-Latency Processing: To ensure real-time inference in surgical settings, edge computing is employed, enabling on-device processing with minimal latency. This is critical for applications requiring immediate neural signal decoding and feedback mechanisms [137].
- AutoML for Continuous Model Optimization: AutoML techniques automate model selection, hyperparameter tuning, and retraining, allowing continuous improvement of neurosurgical AI models [140].
- Blockchain for Data Integrity: Blockchain technology ensures tamper-proof medical records through smart contracts, enhancing transparency and security in neurosurgical data management. Smart contracts ensure unbiased and tamper-proof record-keeping of surgical decisions and patient data [141].
6. Performance Evaluation and Statistical Analysis
6.1. Performance Metrics for BCI Systems
6.2. Evaluating Segmentation Accuracy
6.3. Statistical Significance Testing
- Paired t-test: Used when comparing the performance of two models on the same dataset, evaluating whether the mean difference between paired observations is statistically significant.
- Wilcoxon Signed-Rank Test: A non-parametric alternative to the paired t-test, suitable when the data does not follow a normal distribution.
- Analysis of Variance (ANOVA): Applied when comparing multiple models or experimental conditions to determine whether significant differences exist among them.
- Permutation Testing: A robust statistical method used to assess the significance of performance differences by randomly shuffling labels and recalculating metrics to generate a null distribution.
7. Challenges, Ethical Considerations, and Future Directions
7.1. Challenges in Real-World Implementation
7.2. Ethical Considerations in AI-Driven Neurosurgical Systems
7.3. Future Research Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mudgal, S.K.; Sharma, S.K.; Chaturvedi, J.; Sharma, A. Brain Computer Interface Advancement in Neurosciences: Applications and Issues. Interdiscip Neurosurg 2020, 20. [Google Scholar] [CrossRef]
- Vidal, J.J. Toward Direct Brain-Computer Communication. Annu Rev Biophys Bioeng 1973, 2, 157–180. [Google Scholar] [CrossRef] [PubMed]
- Maiseli, B.; Abdalla, A.T.; Massawe, L. V.; Mbise, M.; Mkocha, K.; Nassor, N.A.; Ismail, M.; Michael, J.; Kimambo, S. Brain–Computer Interface: Trend, Challenges, and Threats. Brain Inform 2023, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Gao, X.; Hong, B.; Gao, S. A Practical Vep-Based Brain-Computer Interface. IEEE Trans Neural Syst Rehabil Eng 2006, 14, 234–240. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhou, L.; Tang, K.Y.; Ephraim Joseph, G.J.; Keong Kuah, C.W.; Geok Chua, K.S. Brain-Computer Interface-Based Robotic End Effector System for Wrist and Hand Rehabilitation: Results of a Three-Armed Randomized Controlled Trial for Chronic Stroke. Front Neuroeng 2014, 7. [Google Scholar] [CrossRef]
- Yuste, R.; Goering, S.; Agüeray Arcas, B.; Bi, G.; Carmena, J.M.; Carter, A.; Fins, J.J.; Friesen, P.; Gallant, J.; Huggins, J.E.; et al. Four Ethical Priorities for Neurotechnologies and AI. Nature 2017 551:7679 2017, 551, 159–163. [Google Scholar] [CrossRef]
- Brocal, F. Brain-Computer Interfaces in Safety and Security Fields: Risks and Applications. Saf Sci 2023, 160. [Google Scholar] [CrossRef]
- Xu, Y.; Quan, R.; Xu, W.; Huang, Y.; Chen, X.; Liu, F. Advances in Medical Image Segmentation: A Comprehensive Review of Traditional, Deep Learning and Hybrid Approaches. Bioengineering 2024, Vol. 11, Page 1034 2024, 11, 1034. [Google Scholar] [CrossRef]
- Khan, R.F.; Lee, B.D.; Lee, M.S. Transformers in Medical Image Segmentation: A Narrative Review. Quant Imaging Med Surg 2023, 13, 8747–8767. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. 3rd International Conference on Learning Representations, ICLR 2015 - Conference Track Proceedings 2015.
- Liu, Z.; Ding, L.; He, B. Integration of EEG/MEG with MRI and FMRI in Functional Neuroimaging. IEEE Eng Med Biol Mag 2006, 25, 46. [Google Scholar] [CrossRef]
- Christ, P.F.; Ettlinger, F.; Grün, F.; Elshaera, M.E.A.; Lipkova, J.; Schlecht, S.; Ahmaddy, F.; Tatavarty, S.; Bickel, M.; Bilic, P.; et al. Automatic Liver and Tumor Segmentation of CT and MRI Volumes Using Cascaded Fully Convolutional Neural Networks. 2017.
- Panayides, A.S.; Amini, A.; Filipovic, N.D.; Sharma, A.; Tsaftaris, S.A.; Young, A.; Foran, D.; Do, N.; Golemati, S.; Kurc, T.; et al. AI in Medical Imaging Informatics: Current Challenges and Future Directions. IEEE J Biomed Health Inform 2020, 24, 1837–1857. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.S.; Chang, D.L.; Lee, M.B.; Chang, E.F. Semi-Automated Anatomical Labeling and Inter-Subject Warping of High-Density Intracranial Recording Electrodes in Electrocorticography. Front Neuroinform 2017, 11, 272432. [Google Scholar] [CrossRef]
- Ruberto, C. Di; Stefano, A.; Comelli, A.; Putzu, L.; Loddo, A.; Kebaili, A.; Lapuyade-Lahorgue, J.; Ruan, S. Deep Learning Approaches for Data Augmentation in Medical Imaging: A Review. Journal of Imaging 2023, Vol. 9, Page 81 2023, 9, 81. [Google Scholar] [CrossRef]
- Chen, X.; Konukoglu, E. Unsupervised Detection of Lesions in Brain MRI Using Constrained Adversarial Auto-Encoders. 2018.
- Pham, D.L.; Xu, C.; Prince, J.L. Current Methods in Medical Image Segmentation. Annu Rev Biomed Eng 2000, 2, 315–337. [Google Scholar] [CrossRef]
- Singh, A.; Sengupta, S.; Lakshminarayanan, V. Explainable Deep Learning Models in Medical Image Analysis. J Imaging 2020, 6, 52. [Google Scholar] [CrossRef]
- Chen, H.; Gomez, C.; Huang, C.M.; Unberath, M. Explainable Medical Imaging AI Needs Human-Centered Design: Guidelines and Evidence from a Systematic Review. npj Digital Medicine 2022 5:1 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. 3rd International Conference on Learning Representations, ICLR 2015 - Conference Track Proceedings 2015.
- Mahmood, A.; Patille, R.; Lam, E.; Mora, D.J.; Gurung, S.; Bookmyer, G.; Weldrick, R.; Chaudhury, H.; Canham, S.L. Correction: Mahmood et al. Aging in the Right Place for Older Adults Experiencing Housing Insecurity: An Environmental Assessment of Temporary Housing Program. Int. J. Environ. Res. Public Health 2022, 19, 14857. International Journal of Environmental Research and Public Health 2023, Vol. 20, Page 6260 2023, 20, 6260. [Google Scholar] [CrossRef] [PubMed]
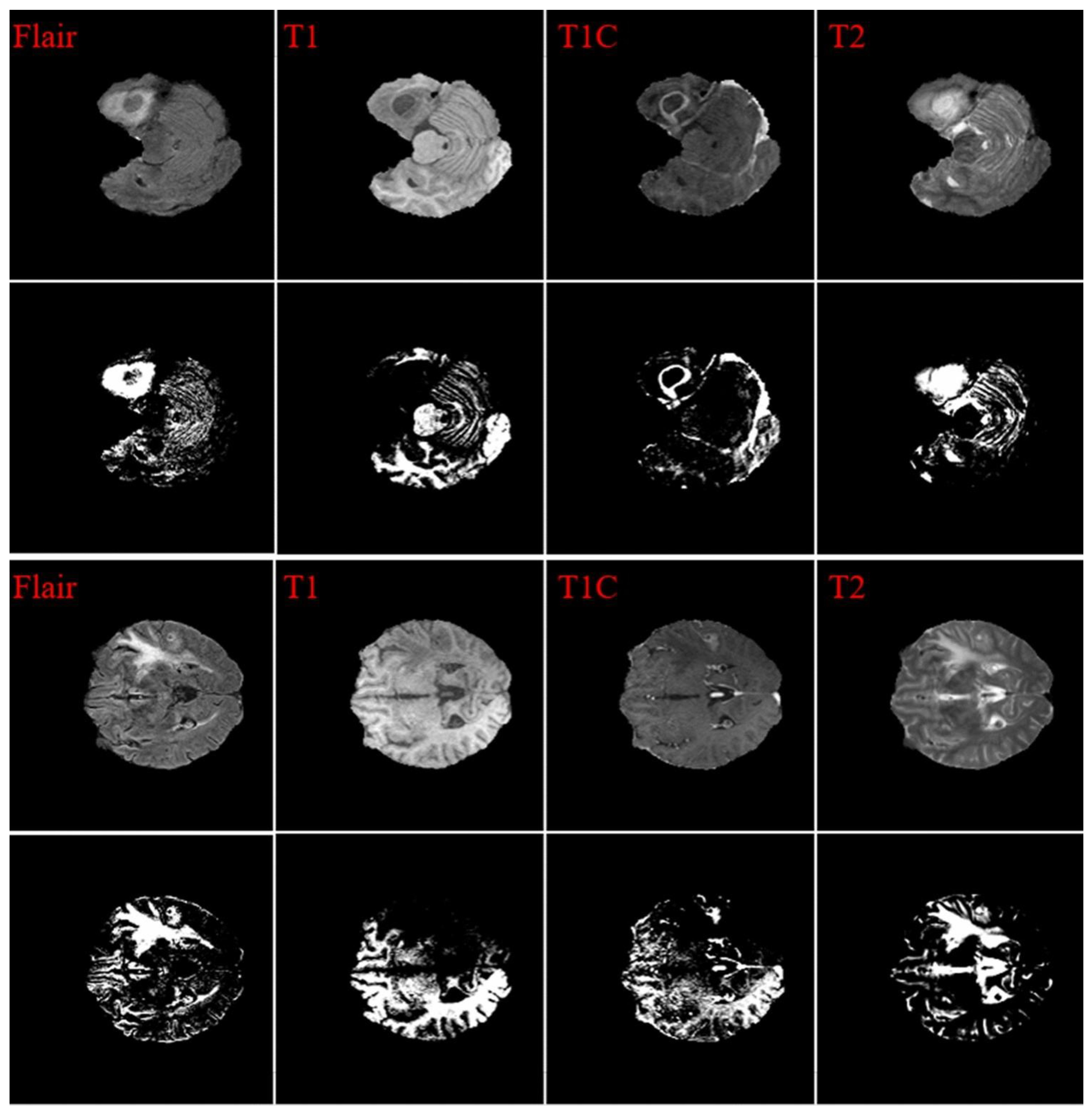
- Fathi Kazerooni, A.; Arif, S.; Madhogarhia, R.; Khalili, N.; Haldar, D.; Bagheri, S.; Familiar, A.M.; Anderson, H.; Haldar, S.; Tu, W.; et al. Automated Tumor Segmentation and Brain Tissue Extraction from Multiparametric MRI of Pediatric Brain Tumors: A Multi-Institutional Study. Neurooncol Adv 2023, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, J.W.; Kennedy, D.N.; McKinstry, R.C.; Buchbinder, B.R.; Weisskoff, R.M.; Cohen, M.S.; Vevea, J.M.; Brady, T.J.; Rosen, B.R. Functional Mapping of the Human Visual Cortex by Magnetic Resonance Imaging. Science (1979) 1991, 254, 716–719. [Google Scholar] [CrossRef]
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic Signal Changes Accompanying Sensory Stimulation: Functional Brain Mapping with Magnetic Resonance Imaging. Proc Natl Acad Sci U S A 1992, 89, 5951–5955. [Google Scholar] [CrossRef]
- Sterman, M.B.; Friar, L. Suppression of Seizures in Epileptic Following on Sensorimotor EEG Feedback Training. Electroencephalogr. Clin. Neurophysiol. 1972, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, G.; Garcia Seoane, J.J.; Binnie, C.D.; Martin Miguel, M.C.; Juler, J.; Polkey, C.E.; Elwes, R.D.C.; Ortiz Blasco, J.M. Origin and Propagation of Interictal Discharges in the Acute Electrocorticogram. Implications for Pathophysiology and Surgical Treatment of Temporal Lobe Epilepsy. Brain 1997, 120, 2259–2282. [Google Scholar] [CrossRef]
- Pan, R.; Yang, C.; Li, Z.; Ren, J.; Duan, Y. Magnetoencephalography-Based Approaches to Epilepsy Classification. Front Neurosci 2023, 17, 1183391. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front Neurol 2021, 12, 682151. [Google Scholar] [CrossRef]
- Hassan, A.M.; Rajesh, A.; Asaad, M.; Nelson, J.A.; Coert, J.H.; Mehrara, B.J.; Butler, C.E. Artificial Intelligence and Machine Learning in Prediction of Surgical Complications: Current State, Applications, and Implications. Am Surg 2022, 89, 25. [Google Scholar] [CrossRef] [PubMed]
- Spyrantis, A.; Woebbecke, T.; Rueß, D.; Constantinescu, A.; Gierich, A.; Luyken, K.; Visser-Vandewalle, V.; Herrmann, E.; Gessler, F.; Czabanka, M.; et al. Accuracy of Robotic and Frame-Based Stereotactic Neurosurgery in a Phantom Model. Front Neurorobot 2022, 16, 762317. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Kumatoriya, K.; Tando, M.; Kometani, T.; Shinohara, M. Polyphenols from Persimmon Fruit Attenuate Acetaldehyde-Induced DNA Double-Strand Breaks by Scavenging Acetaldehyde. Scientific Reports 2022 12:1 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Belkacem, A.N.; Jamil, N.; Khalid, S.; Alnajjar, F. On Closed-Loop Brain Stimulation Systems for Improving the Quality of Life of Patients with Neurological Disorders. Front Hum Neurosci 2023, 17, 1085173. [Google Scholar] [CrossRef]
- Mokienko, O.A. Brain–Computer Interfaces with Intracortical Implants for Motor and Communication Functions Compensation: Review of Recent Developments. Modern Technologies in Medicine 2024, 16, 78. [Google Scholar] [CrossRef]
- Vadhavekar, N.H.; Sabzvari, T.; Laguardia, S.; Sheik, T.; Prakash, V.; Gupta, A.; Umesh, I.D.; Singla, A.; Koradia, I.; Patiño, B.B.R.; et al. Advancements in Imaging and Neurosurgical Techniques for Brain Tumor Resection: A Comprehensive Review. Cureus 2024, 16, e72745. [Google Scholar] [CrossRef]
- Livanis, E.; Voultsos, P.; Vadikolias, K.; Pantazakos, P.; Tsaroucha, A. Understanding the Ethical Issues of Brain-Computer Interfaces (BCIs): A Blessing or the Beginning of a Dystopian Future? Cureus 2024, 16, e58243. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, M.; Saqib, M.; Zareen, M.; Mumtaz, H. Artificial Intelligence: Revolutionizing Robotic Surgery: Review. Annals of Medicine and Surgery 2024, 86, 5401. [Google Scholar] [CrossRef]
- Abu Mhanna, H.Y.; Omar, A.F.; Radzi, Y.M.; Oglat, A.A.; Akhdar, H.F.; Al Ewaidat, H.; Almahmoud, A.; Bani Yaseen, A.B.; Al Badarneh, L.; Alhamad, O.; et al. Systematic Review of Functional Magnetic Resonance Imaging (FMRI) Applications in the Preoperative Planning and Treatment Assessment of Brain Tumors. Heliyon 2025, 11, e42464. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Zhang, H.; Zhou, J.; Li, G.; Tang, Z.; Sun, Z.; Cai, J.; Tian, N.; Gao, S.; Dong, J.; et al. Deep Learning-Based Automatic Segmentation for Size and Volumetric Measurement of Breast Cancer on Magnetic Resonance Imaging. Front Oncol 2022, 12, 984626. [Google Scholar] [CrossRef] [PubMed]
- Manakitsa, N.; Maraslidis, G.S.; Moysis, L.; Fragulis, G.F. A Review of Machine Learning and Deep Learning for Object Detection, Semantic Segmentation, and Human Action Recognition in Machine and Robotic Vision. Technologies 2024, Vol. 12, Page 15 2024, 12, 15. [Google Scholar] [CrossRef]
- Agadi, K.; Dominari, A.; Tebha, S.S.; Mohammadi, A.; Zahid, S. Neurosurgical Management of Cerebrospinal Tumors in the Era of Artificial Intelligence : A Scoping Review. J Korean Neurosurg Soc 2022, 66, 632. [Google Scholar] [CrossRef]
- Rayed, M.E.; Islam, S.M.S.; Niha, S.I.; Jim, J.R.; Kabir, M.M.; Mridha, M.F. Deep Learning for Medical Image Segmentation: State-of-the-Art Advancements and Challenges. Inform Med Unlocked 2024, 47, 101504. [Google Scholar] [CrossRef]
- Ranjbarzadeh, R.; Bagherian Kasgari, A.; Jafarzadeh Ghoushchi, S.; Anari, S.; Naseri, M.; Bendechache, M. Brain Tumor Segmentation Based on Deep Learning and an Attention Mechanism Using MRI Multi-Modalities Brain Images. Scientific Reports 2021 11:1 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Fariba, K.A.; Gupta, V. Deep Brain Stimulation. Encyclopedia of Movement Disorders, Three-Volume Set. [CrossRef]
- Chandra, V.; Hilliard, J.D.; Foote, K.D. Deep Brain Stimulation for the Treatment of Tremor. J Neurol Sci 2022, 435, 120190. [Google Scholar] [CrossRef]
- Krüger, M.T.; Kurtev-Rittstieg, R.; Kägi, G.; Naseri, Y.; Hägele-Link, S.; Brugger, F. Evaluation of Automatic Segmentation of Thalamic Nuclei through Clinical Effects Using Directional Deep Brain Stimulation Leads: A Technical Note. Brain Sciences 2020, Vol. 10, Page 642 2020, 10, 642. [Google Scholar] [CrossRef]
- Miller, K.J.; Fine, A.L. Decision Making in Stereotactic Epilepsy Surgery. Epilepsia 2022, 63, 2782. [Google Scholar] [CrossRef] [PubMed]
- Mirchi, N.; Warsi, N.M.; Zhang, F.; Wong, S.M.; Suresh, H.; Mithani, K.; Erdman, L.; Ibrahim, G.M. Decoding Intracranial EEG With Machine Learning: A Systematic Review. Front Hum Neurosci 2022, 16, 913777. [Google Scholar] [CrossRef] [PubMed]
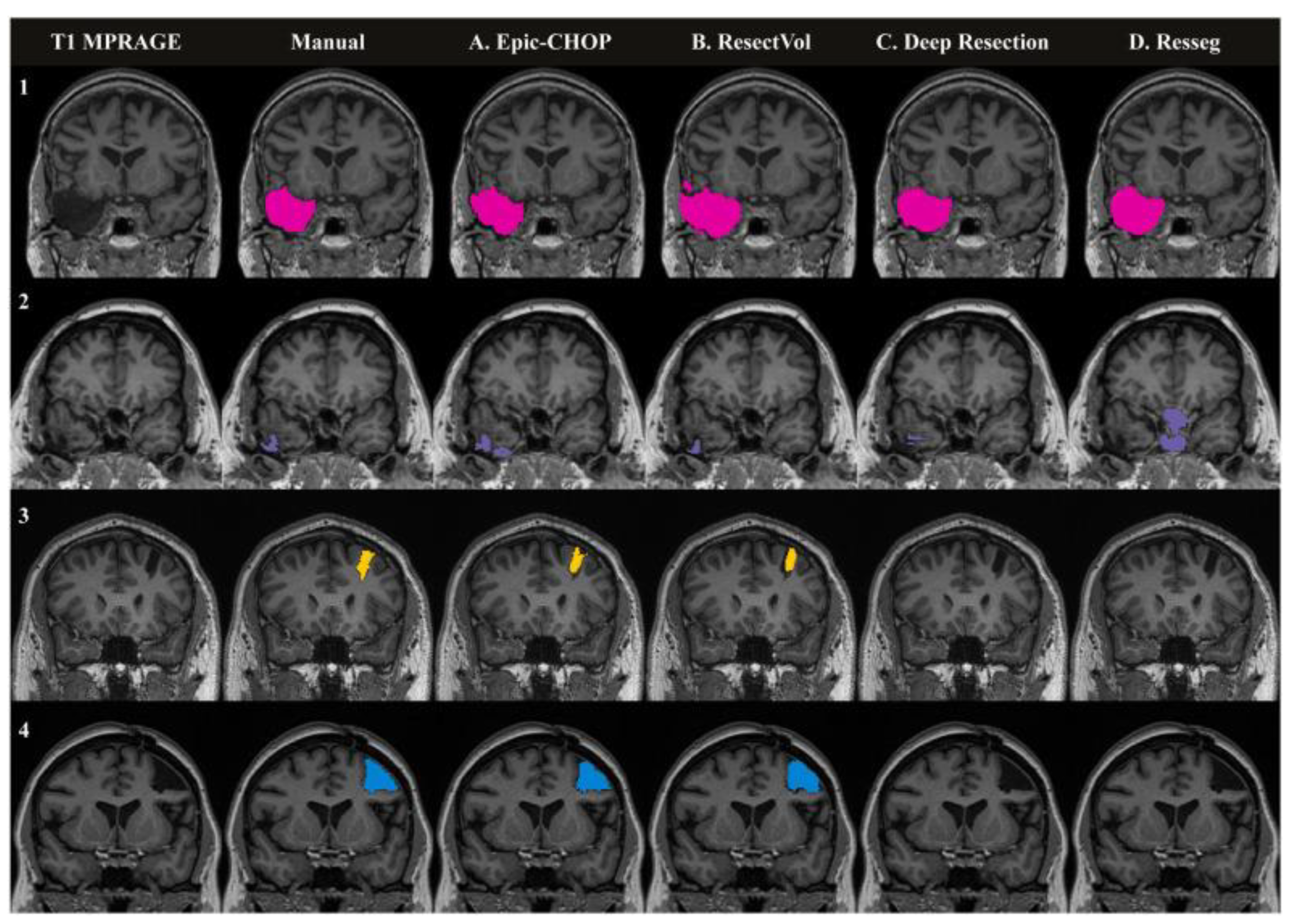
- Courtney, M.R.; Sinclair, B.; Neal, A.; Nicolo, J.P.; Kwan, P.; Law, M.; O’Brien, T.J.; Vivash, L. Automated Segmentation of Epilepsy Surgical Resection Cavities: Comparison of Four Methods to Manual Segmentation. Neuroimage 2024, 296, 120682. [Google Scholar] [CrossRef]
- Hassija, V.; Chamola, V.; Mahapatra, A.; Singal, A.; Goel, D.; Huang, K.; Scardapane, S.; Spinelli, I.; Mahmud, M.; Hussain, A. Interpreting Black-Box Models: A Review on Explainable Artificial Intelligence. Cognit Comput 2024, 16, 45–74. [Google Scholar] [CrossRef]
- Mienye, I.D.; Obaido, G.; Jere, N.; Mienye, E.; Aruleba, K.; Emmanuel, I.D.; Ogbuokiri, B. A Survey of Explainable Artificial Intelligence in Healthcare: Concepts, Applications, and Challenges. Inform Med Unlocked 2024, 51, 101587. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, W.; Dillon, T. Regulatory Responses and Approval Status of Artificial Intelligence Medical Devices with a Focus on China. NPJ Digit Med 2024, 7, 255. [Google Scholar] [CrossRef]
- Khan, D.Z.; Valetopoulou, A.; Das, A.; Hanrahan, J.G.; Williams, S.C.; Bano, S.; Borg, A.; Dorward, N.L.; Barbarisi, S.; Culshaw, L.; et al. Artificial Intelligence Assisted Operative Anatomy Recognition in Endoscopic Pituitary Surgery. NPJ Digit Med 2024, 7, 314. [Google Scholar] [CrossRef]
- Nam, S.M.; Byun, Y.H.; Dho, Y.-S.; Park, C.-K. Envisioning the Future of the Neurosurgical Operating Room with the Concept of the Medical Metaverse. J Korean Neurosurg Soc 2025, 68, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Brockmeyer, P.; Wiechens, B.; Schliephake, H. The Role of Augmented Reality in the Advancement of Minimally Invasive Surgery Procedures: A Scoping Review. Bioengineering 2023, 10, 501. [Google Scholar] [CrossRef]
- Tangsrivimol, J.A.; Schonfeld, E.; Zhang, M.; Veeravagu, A.; Smith, T.R.; Härtl, R.; Lawton, M.T.; El-Sherbini, A.H.; Prevedello, D.M.; Glicksberg, B.S.; et al. Artificial Intelligence in Neurosurgery: A State-of-the-Art Review from Past to Future. Diagnostics 2023, 13, 2429. [Google Scholar] [CrossRef]
- Mudgal, S.K.; Sharma, S.K.; Chaturvedi, J.; Sharma, A. Brain Computer Interface Advancement in Neurosciences: Applications and Issues. Interdisciplinary Neurosurgery 2020, 20, 100694. [Google Scholar] [CrossRef]
- Caiado, F.; Ukolov, A. The History, Current State and Future Possibilities of the Non-Invasive Brain Computer Interfaces. Med Nov Technol Devices 2025, 25, 100353. [Google Scholar] [CrossRef]
- Brookes, M.J.; Leggett, J.; Rea, M.; Hill, R.M.; Holmes, N.; Boto, E.; Bowtell, R. Magnetoencephalography with Optically Pumped Magnetometers (OPM-MEG): The next Generation of Functional Neuroimaging. Trends Neurosci 2022, 45, 621–634. [Google Scholar] [CrossRef]
- Acuña, K.; Sapahia, R.; Jiménez, I.N.; Antonietti, M.; Anzola, I.; Cruz, M.; García, M.T.; Krishnan, V.; Leveille, L.A.; Resch, M.D.; et al. Functional Near-Infrared Spectrometry as a Useful Diagnostic Tool for Understanding the Visual System: A Review. J Clin Med 2024, 13, 282. [Google Scholar] [CrossRef]
- Coles, L.; Ventrella, D.; Carnicer-Lombarte, A.; Elmi, A.; Troughton, J.G.; Mariello, M.; El Hadwe, S.; Woodington, B.J.; Bacci, M.L.; Malliaras, G.G.; et al. Origami-Inspired Soft Fluidic Actuation for Minimally Invasive Large-Area Electrocorticography. Nat Commun 2024, 15, 6290. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Yoon, C.; Jo, K.; Won, J.H.; Park, S. Recent Advances in Recording and Modulation Technologies for Next-Generation Neural Interfaces. iScience 2021, 24, 103550. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Rastegarnia, A.; Sanei, S. Signal Artifacts and Techniques for Artifacts and Noise Removal. Intelligent Systems Reference Library 2021, 192, 23–79. [Google Scholar] [CrossRef]
- Barnova, K.; Mikolasova, M.; Kahankova, R.V.; Jaros, R.; Kawala-Sterniuk, A.; Snasel, V.; Mirjalili, S.; Pelc, M.; Martinek, R. Implementation of Artificial Intelligence and Machine Learning-Based Methods in Brain–Computer Interaction. Comput Biol Med 2023, 163, 107135. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Sekula, P.; Ding, L. Machine Learning in Construction: From Shallow to Deep Learning. Developments in the Built Environment 2021, 6, 100045. [Google Scholar] [CrossRef]
- Chaudhary, U. Machine Learning with Brain Data. Expanding Senses using Neurotechnology 2025, 179–223. [Google Scholar] [CrossRef]
- Si-Mohammed, H.; Petit, J.; Jeunet, C.; Argelaguet, F.; Spindler, F.; Evain, A.; Roussel, N.; Casiez, G.; Lecuyer, A. Towards BCI-Based Interfaces for Augmented Reality: Feasibility, Design and Evaluation. IEEE Trans Vis Comput Graph 2020, 26, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.; Kang, H.; Kim, S.; Ahn, M. P300 Brain–Computer Interface-Based Drone Control in Virtual and Augmented Reality. Sensors 2021, Vol. 21, Page 5765 2021, 21, 5765. [Google Scholar] [CrossRef]
- Farwell, L.A.; Donchin, E. Talking off the Top of Your Head: Toward a Mental Prosthesis Utilizing Event-Related Brain Potentials. Electroencephalogr Clin Neurophysiol 1988, 70, 510–523. [Google Scholar] [CrossRef]
- McFarland, D.J.; Wolpaw, J.R. EEG-Based Brain-Computer Interfaces. Curr Opin Biomed Eng 2017, 4, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Awuah, W.A.; Ahluwalia, A.; Darko, K.; Sanker, V.; Tan, J.K.; Tenkorang, P.O.; Ben-Jaafar, A.; Ranganathan, S.; Aderinto, N.; Mehta, A.; et al. Bridging Minds and Machines: The Recent Advances of Brain-Computer Interfaces in Neurological and Neurosurgical Applications. World Neurosurg 2024, 189, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C. Motor Imagery Activates Primary Sensorimotor Area in Humans. Neurosci Lett 1997, 239, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Saibene, A.; Caglioni, M.; Corchs, S.; Gasparini, F. EEG-Based BCIs on Motor Imagery Paradigm Using Wearable Technologies: A Systematic Review. Sensors 2023, 23, 2798. [Google Scholar] [CrossRef]
- Pan, J.; Chen, X.N.; Ban, N.; He, J.S.; Chen, J.; Huang, H. Advances in P300 Brain–Computer Interface Spellers: Toward Paradigm Design and Performance Evaluation. Front Hum Neurosci 2022, 16, 1077717. [Google Scholar] [CrossRef]
- Norcia, A.M.; Gregory Appelbaum, L.; Ales, J.M.; Cottereau, B.R.; Rossion, B. The Steady-State Visual Evoked Potential in Vision Research: A Review. J Vis 2015, 15, 4. [Google Scholar] [CrossRef]
- Mnih, V.; Kavukcuoglu, K.; Silver, D.; Graves, A.; Antonoglou, I.; Wierstra, D.; Riedmiller, M. Playing Atari with Deep Reinforcement Learning. 2013.
- Haslacher, D.; Akmazoglu, T.B.; van Beinum, A.; Starke, G.; Buthut, M.; Soekadar, S.R. AI for Brain-Computer Interfaces. 2024, 7, 3–28. [CrossRef]
- Siribunyaphat, N.; Punsawad, Y. Steady-State Visual Evoked Potential-Based Brain–Computer Interface Using a Novel Visual Stimulus with Quick Response (QR) Code Pattern. Sensors (Basel) 2022, 22, 1439. [Google Scholar] [CrossRef]
- Neuper, C.; Müller-Putz, G.R.; Scherer, R.; Pfurtscheller, G. Motor Imagery and EEG-Based Control of Spelling Devices and Neuroprostheses. Prog Brain Res 2006, 159, 393–409. [Google Scholar] [CrossRef]
- Pan, J.; Chen, X.N.; Ban, N.; He, J.S.; Chen, J.; Huang, H. Advances in P300 Brain–Computer Interface Spellers: Toward Paradigm Design and Performance Evaluation. Front Hum Neurosci 2022, 16, 1077717. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.O.; Serruya, M.D.; Harris, J.P.; Burrell, J.C.; Petrov, D.; Chen, H.I.; Wolf, J.A.; Cullen, D.K. The Evolution of Neuroprosthetic Interfaces. Crit Rev Biomed Eng 2016, 44, 123. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.P.; Pels, E.G.M.; Sars, R.H.; Aarnoutse, E.J.; Ramsey, N.F.; Vansteensel, M.J.; Nijboer, F. Brain-Computer Interfaces for Communication: Preferences of Individuals With Locked-in Syndrome. Neurorehabil Neural Repair 2021, 35, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Collinger, J.L.; Gaunt, R.A.; Schwartz, A.B. Progress towards Restoring Upper Limb Movement and Sensation through Intracortical Brain-Computer Interfaces. Curr Opin Biomed Eng 2018, 8, 84–92. [Google Scholar] [CrossRef]
- Collinger, J.L.; Wodlinger, B.; Downey, J.E.; Wang, W.; Tyler-Kabara, E.C.; Weber, D.J.; McMorland, A.J.C.; Velliste, M.; Boninger, M.L.; Schwartz, A.B. High-Performance Neuroprosthetic Control by an Individual with Tetraplegia. The Lancet 2013, 381, 557–564. [Google Scholar] [CrossRef]
- Hu, X.; Assaad, R.H. The Use of Unmanned Ground Vehicles (Mobile Robots) and Unmanned Aerial Vehicles (Drones) in the Civil Infrastructure Asset Management Sector: Applications, Robotic Platforms, Sensors, and Algorithms. Expert Syst Appl 2023, 232. [Google Scholar] [CrossRef]
- Flesher, S.N.; Downey, J.E.; Weiss, J.M.; Hughes, C.L.; Herrera, A.J.; Tyler-Kabara, E.C.; Boninger, M.L.; Collinger, J.L.; Gaunt, R.A. A Brain-Computer Interface That Evokes Tactile Sensations Improves Robotic Arm Control. Science (1979) 2021, 372, 831–836. [Google Scholar] [CrossRef]
- Karmakar, S.; Kamilya, S.; Dey, P.; Guhathakurta, P.K.; Dalui, M.; Bera, T.K.; Halder, S.; Koley, C.; Pal, T.; Basu, A. Real Time Detection of Cognitive Load Using FNIRS: A Deep Learning Approach. Biomed Signal Process Control 2023, 80, 104227. [Google Scholar] [CrossRef]
- Mughal, N.E.; Khan, M.J.; Khalil, K.; Javed, K.; Sajid, H.; Naseer, N.; Ghafoor, U.; Hong, K.S. EEG-FNIRS-Based Hybrid Image Construction and Classification Using CNN-LSTM. Front Neurorobot 2022, 16, 873239. [Google Scholar] [CrossRef]
- Murphy, E.; Poudel, G.; Ganesan, S.; Suo, C.; Manning, V.; Beyer, E.; Clemente, A.; Moffat, B.A.; Zalesky, A.; Lorenzetti, V. Real-Time FMRI-Based Neurofeedback to Restore Brain Function in Substance Use Disorders: A Systematic Review of the Literature. Neurosci Biobehav Rev 2024, 165, 105865. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lande, G.J.M.; Casas-Torremocha, D.; Manasanch, A.; Dalla Porta, L.; Gosseries, O.; Alnagger, N.; Barra, A.; Mejías, J.F.; Panda, R.; Riefolo, F.; et al. Brain State Identification and Neuromodulation to Promote Recovery of Consciousness. Brain Commun 2024, 6, fcae362. [Google Scholar] [CrossRef]
- Papadopoulos, S.; Bonaiuto, J.; Mattout, J. An Impending Paradigm Shift in Motor Imagery Based Brain-Computer Interfaces. Front Neurosci 2022, 15, 824759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yagi, K.; Shibahara, Y.; Tate, L.; Tamura, H. A Study on Analysis Method for a Real-Time Neurofeedback System Using Non-Invasive Magnetoencephalography. Electronics 2022, Vol. 11, Page 2473 2022, 11, 2473. [Google Scholar] [CrossRef]
- Aghajani, H.; Omurtag, A. Assessment of Mental Workload by EEG+FNIRS. Annu Int Conf IEEE Eng Med Biol Soc 2016, 2016, 3773–3776. [Google Scholar] [CrossRef]
- Warbrick, T. Simultaneous EEG-FMRI: What Have We Learned and What Does the Future Hold? Sensors (Basel) 2022, 22, 2262. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, P.; Nedumaran, A.M.; Mishra, S.; Pandarinathan, G.; Archunan, G.; Gulyás, B. The Advents of Hybrid Imaging Modalities: A New Era in Neuroimaging Applications. Adv Biosyst 2017, 1. [Google Scholar] [CrossRef]
- Freudenburg, Z. V.; Branco, M.P.; Leinders, S.; Vijgh, B.H. van der; Pels, E.G.M.; Denison, T.; Berg, L.H. van den; Miller, K.J.; Aarnoutse, E.J.; Ramsey, N.F.; et al. Sensorimotor ECoG Signal Features for BCI Control: A Comparison Between People With Locked-In Syndrome and Able-Bodied Controls. Front Neurosci 2019, 13, 457334. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Nie, C.; Jiang, C.T.; Cao, S.H.; Tian, K.X.; Yu, S.; Gu, J.W. Modulating Brain Activity with Invasive Brain–Computer Interface: A Narrative Review. Brain Sci 2023, 13, 134. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Liu, Y.; Xu, Z.; Wang, H.; Wu, T.; Mukhopadhyay, S.C. Recent Advancement of Electrocorticography (ECoG) Electrodes for Chronic Neural Recording/Stimulation. Mater Today Commun 2021, 29, 102853. [Google Scholar] [CrossRef]
- Merk, T.; Peterson, V.; Köhler, R.; Haufe, S.; Richardson, R.M.; Neumann, W.J. Machine Learning Based Brain Signal Decoding for Intelligent Adaptive Deep Brain Stimulation. Exp Neurol 2022, 351, 113993. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T. Decoding Thoughts, Encoding Ethics: A Narrative Review of the BCI-AI Revolution. Brain Res 2025, 1850, 149423. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Mamun, K.A.; Ahmed, K.; Mostafa, R.; Naik, G.R.; Darvishi, S.; Khandoker, A.H.; Baumert, M. Progress in Brain Computer Interface: Challenges and Opportunities. Front Syst Neurosci 2021, 15, 578875. [Google Scholar] [CrossRef]
- Zhang, H.; Jiao, L.; Yang, S.; Li, H.; Jiang, X.; Feng, J.; Zou, S.; Xu, Q.; Gu, J.; Wang, X.; et al. Brain–Computer Interfaces: The Innovative Key to Unlocking Neurological Conditions. Int J Surg 2024, 110, 5745. [Google Scholar] [CrossRef] [PubMed]
- Merk, T.; Peterson, V.; Lipski, W.J.; Blankertz, B.; Turner, R.S.; Li, N.; Horn, A.; Richardson, R.M.; Neumann, W.J. Electrocorticography Is Superior to Subthalamic Local Field Potentials for Movement Decoding in Parkinson’s Disease. Elife 2022, 11, e75126. [Google Scholar] [CrossRef]
- Cao, T.D.; Truong-Huu, T.; Tran, H.; Tran, K. A Federated Deep Learning Framework for Privacy Preservation and Communication Efficiency. Journal of Systems Architecture 2022, 124. [Google Scholar] [CrossRef]
- Lebedev, M.A.; Nicolelis, M.A.L. Brain-Machine Interfaces: From Basic Science to Neuroprostheses and Neurorehabilitation. Physiol Rev 2017, 97, 767–837. [Google Scholar] [CrossRef]
- Fick, T.; Van Doormaal, J.A.M.; Tosic, L.; Van Zoest, R.J.; Meulstee, J.W.; Hoving, E.W.; Van Doormaal, T.P.C. Fully Automatic Brain Tumor Segmentation for 3D Evaluation in Augmented Reality. Neurosurg Focus 2021, 51, E14. [Google Scholar] [CrossRef]
- Kazemzadeh, K.; Akhlaghdoust, M.; Zali, A. Advances in Artificial Intelligence, Robotics, Augmented and Virtual Reality in Neurosurgery. Front Surg 2023, 10, 1241923. [Google Scholar] [CrossRef]
- Zhou, T.; Yu, T.; Li, Z.; Zhou, X.; Wen, J.; Li, X. Functional Mapping of Language-Related Areas from Natural, Narrative Speech during Awake Craniotomy Surgery. Neuroimage 2021, 245, 118720. [Google Scholar] [CrossRef]
- Sarubbo, S.; Annicchiarico, L.; Corsini, F.; Zigiotto, L.; Herbet, G.; Moritz-Gasser, S.; Dalpiaz, C.; Vitali, L.; Tate, M.; De Benedictis, A.; et al. Planning Brain Tumor Resection Using a Probabilistic Atlas of Cortical and Subcortical Structures Critical for Functional Processing: A Proof of Concept. Oper Neurosurg (Hagerstown) 2021, 20, E175–E183. [Google Scholar] [CrossRef] [PubMed]
- Lachance, B.; Wang, Z.; Badjatia, N.; Jia, X. Somatosensory Evoked Potentials (SSEP) and Neuroprognostication after Cardiac Arrest. Neurocrit Care 2020, 32, 847. [Google Scholar] [CrossRef]
- Nikolov, P.; Heil, V.; Hartmann, C.J.; Ivanov, N.; Slotty, P.J.; Vesper, J.; Schnitzler, A.; Groiss, S.J. Motor Evoked Potentials Improve Targeting in Deep Brain Stimulation Surgery. Neuromodulation 2022, 25, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, H.; Troxler, P.; Hodel, S.; Suter, D.; Farshad, M.; Cavalcanti, N.; Wetzel, O.; Mania, S.; Cornaz, F.; Selman, F.; et al. Introducing a Brain-Computer Interface to Facilitate Intraoperative Medical Imaging Control – a Feasibility Study. BMC Musculoskelet Disord 2022, 23, 1–10. [Google Scholar] [CrossRef]
- Mridha, M.F.; Das, S.C.; Kabir, M.M.; Lima, A.A.; Islam, M.R.; Watanobe, Y. Brain-Computer Interface: Advancement and Challenges. Sensors 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Park, H.; Kwon, I.; An, K.O.; Kim, H.; Park, G.; Hyung, W.; Im, C.H.; Shin, J.H. Efficacy of Brain-Computer Interface Training with Motor Imagery-Contingent Feedback in Improving Upper Limb Function and Neuroplasticity among Persons with Chronic Stroke: A Double-Blinded, Parallel-Group, Randomized Controlled Trial. J Neuroeng Rehabil 2025, 22, 1–13. [Google Scholar] [CrossRef]
- Pignolo, L.; Servidio, R.; Basta, G.; Carozzo, S.; Tonin, P.; Calabrò, R.S.; Cerasa, A. The Route of Motor Recovery in Stroke Patients Driven by Exoskeleton-Robot-Assisted Therapy: A Path-Analysis. Medical Sciences 2021, 9, 64. [Google Scholar] [CrossRef]
- Yang, S.; Li, R.; Li, H.; Xu, K.; Shi, Y.; Wang, Q.; Yang, T.; Sun, X. Exploring the Use of Brain-Computer Interfaces in Stroke Neurorehabilitation. Biomed Res Int 2021, 2021, 9967348. [Google Scholar] [CrossRef]
- Jin, W.; Zhu, X.X.; Qian, L.; Wu, C.; Yang, F.; Zhan, D.; Kang, Z.; Luo, K.; Meng, D.; Xu, G. Electroencephalogram-Based Adaptive Closed-Loop Brain-Computer Interface in Neurorehabilitation: A Review. Front Comput Neurosci 2024, 18, 1431815. [Google Scholar] [CrossRef]
- Mane, R.; Wu, Z.; Wang, D. Poststroke Motor, Cognitive and Speech Rehabilitation with Brain–Computer Interface: A Perspective Review. Stroke Vasc Neurol 2022, 7, 541–549. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Zheng, H.; Li, T.; Chen, K.; Wang, X.; Liu, C.; Xu, L.; Wu, X.; Lin, D.; et al. The Combination of Brain-Computer Interfaces and Artificial Intelligence: Applications and Challenges. Ann Transl Med 2020, 8, 712. [Google Scholar] [CrossRef] [PubMed]
- Menze, B.H.; Jakab, A.; Bauer, S.; Kalpathy-Cramer, J.; Farahani, K.; Kirby, J.; Burren, Y.; Porz, N.; Slotboom, J.; Wiest, R.; et al. The Multimodal Brain Tumor Image Segmentation Benchmark (BRATS). IEEE Trans Med Imaging 2015, 34, 1993–2024. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 2015, 9351, 234–241. [Google Scholar] [CrossRef]
- Chen, L.C.; Papandreou, G.; Kokkinos, I.; Murphy, K.; Yuille, A.L. DeepLab: Semantic Image Segmentation with Deep Convolutional Nets, Atrous Convolution, and Fully Connected CRFs. IEEE Trans Pattern Anal Mach Intell 2018, 40, 834–848. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. An Image Is Worth 16*16 Words: Transformers for Image Recognition at Scale. Adv Neural Inf Process Syst 2017. 2017-Decem. [Google Scholar]
- Hatamizadeh, A.; Nath, V.; Tang, Y.; Yang, D.; Roth, H.R.; Xu, D. Swin UNETR: Swin Transformers for Semantic Segmentation of Brain Tumors in MRI Images. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 2022, 12962 LNCS, 272–284. [Google Scholar] [CrossRef]
- Hong, K.S.; Khan, M.J. Hybrid Brain-Computer Interface Techniques for Improved Classification Accuracy and Increased Number of Commands: A Review. Front Neurorobot 2017, 11, 275683. [Google Scholar] [CrossRef]
- Peng, C.J.; Chen, Y.C.; Chen, C.C.; Chen, S.J.; Cagneau, B.; Chassagne, L. An EEG-Based Attentiveness Recognition System Using Hilbert–Huang Transform and Support Vector Machine. J Med Biol Eng 2020, 40, 230–238. [Google Scholar] [CrossRef]
- Rakhmatulin, I.; Dao, M.S.; Nassibi, A.; Mandic, D. Exploring Convolutional Neural Network Architectures for EEG Feature Extraction. Sensors 2024, Vol. 24, Page 877 2024, 24, 877. [Google Scholar] [CrossRef]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, Vol. 23, Page 6001 2023, 23, 6001. [Google Scholar] [CrossRef]
- Mungoli, N. Scalable, Distributed AI Frameworks: Leveraging Cloud Computing for Enhanced Deep Learning Performance and Efficiency. 2023.
- Subasi, A. Practical Guide for Biomedical Signals Analysis Using Machine Learning Techniques: A MATLAB Based Approach. Practical Guide for Biomedical Signals Analysis Using Machine Learning Techniques: A MATLAB Based Approach 2019, 1–443. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J Neurosci Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Schirrmeister, R.T.; Springenberg, J.T.; Fiederer, L.D.J.; Glasstetter, M.; Eggensperger, K.; Tangermann, M.; Hutter, F.; Burgard, W.; Ball, T. Deep Learning with Convolutional Neural Networks for EEG Decoding and Visualization. Hum Brain Mapp 2017, 38, 5391–5420. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Hao, S.; Peng, X.; Hu, L. Deep Learning for Sensor-Based Activity Recognition: A Survey. Pattern Recognit Lett 2019, 119, 3–11. [Google Scholar] [CrossRef]
- Hatamizadeh, A.; Nath, V.; Tang, Y.; Yang, D.; Roth, H.R.; Xu, D. Swin UNETR: Swin Transformers for Semantic Segmentation of Brain Tumors in MRI Images. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 2022, 12962 LNCS, 272–284. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Yu, Q.; Luo, X.; Adeli, E.; Wang, Y.; Lu, L.; Yuille, A.L.; Zhou, Y. TransUNet: Transformers Make Strong Encoders for Medical Image Segmentation. 2021.
- He, K.; Fan, H.; Wu, Y.; Xie, S.; Girshick, R. Momentum Contrast for Unsupervised Visual Representation Learning. Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition 2019, 9726–9735. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med Image Anal 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Cao, J.; Zhang, Q.; Li, Y.; Xu, L. Edge Computing: Vision and Challenges. IEEE Internet Things J 2016, 3, 637–646. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Chen, T.; Tong, Y. Federated Machine Learning: Concept and Applications. ACM Trans Intell Syst Technol 2019, 10, 19. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Chen, T.; Tong, Y. Federated Machine Learning. ACM Transactions on Intelligent Systems and Technology (TIST) 2019, 10. [Google Scholar] [CrossRef]
- Zoph, B.; Le, Q. V. Neural Architecture Search with Reinforcement Learning. 5th International Conference on Learning Representations, ICLR 2017 - Conference Track Proceedings 2016.
- Chen, Z.; Jing, L.; Li, Y.; Li, B. Bridging the Domain Gap: Self-Supervised 3D Scene Understanding with Foundation Models. Adv Neural Inf Process Syst 2023, 36. [Google Scholar]
- Edelman, B.J.; Zhang, S.; Schalk, G.; Brunner, P.; Muller-Putz, G.; Guan, C.; He, B. Non-Invasive Brain-Computer Interfaces: State of the Art and Trends. IEEE Rev Biomed Eng 2025, 18. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Bolton, D.A.E.; Kennedy, N.C.; Soekadar, S.R.; Ruddy, K.L. Challenges and Opportunities for the Future of Brain-Computer Interface in Neurorehabilitation. Front Neurosci 2021, 15, 699428. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhang, F.; Fan, H.; Zhang, C. Brief Review of Image Denoising Techniques. Vis Comput Ind Biomed Art 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Li, T.; Zhao, L.; Gong, A.; Nan, W.; Ding, P.; Fu, Y. Considerations and Discussions on the Clear Definition and Definite Scope of Brain-Computer Interfaces. Front Neurosci 2024, 18, 1449208. [Google Scholar] [CrossRef]
- Mridha, M.F.; Das, S.C.; Kabir, M.M.; Lima, A.A.; Islam, M.R.; Watanobe, Y. Brain-Computer Interface: Advancement and Challenges. Sensors (Basel) 2021, 21, 5746. [Google Scholar] [CrossRef]
- Rajpura, P.; Cecotti, H.; Kumar Meena, Y. Explainable Artificial Intelligence Approaches for Brain-Computer Interfaces: A Review and Design Space. J Neural Eng 2023, 21. [Google Scholar] [CrossRef]
- Maiseli, B.; Abdalla, A.T.; Massawe, L. V.; Mbise, M.; Mkocha, K.; Nassor, N.A.; Ismail, M.; Michael, J.; Kimambo, S. Brain–Computer Interface: Trend, Challenges, and Threats. Brain Inform 2023, 10. [Google Scholar] [CrossRef]
- Salles, A.; Farisco, M. Neuroethics and AI Ethics: A Proposal for Collaboration. BMC Neurosci 2024, 25, 1–10. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain-Computer Interfaces for Communication and Rehabilitation. Nat Rev Neurol 2016, 12, 513–525. [Google Scholar] [CrossRef]
- Sun, X. yu; Ye, B. The Functional Differentiation of Brain–Computer Interfaces (BCIs) and Its Ethical Implications. Humanities and Social Sciences Communications 2023 10:1 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Keskinbora, K.H.; Keskinbora, K. Ethical Considerations on Novel Neuronal Interfaces. Neurol Sci 2018, 39, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Vlek, R.J.; Steines, D.; Szibbo, D.; Kübler, A.; Schneider, M.J.; Haselager, P.; Nijboer, F. Ethical Issues in Brain-Computer Interface Research, Development, and Dissemination. J Neurol Phys Ther 2012, 36, 94–99. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.C.; Hahn, P.J. Network Perspectives on the Mechanisms of Deep Brain Stimulation. Neurobiol Dis 2010, 38, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Borton, D.A.; Yin, M.; Aceros, J.; Nurmikko, A. An Implantable Wireless Neural Interface for Recording Cortical Circuit Dynamics in Moving Primates. J Neural Eng 2013, 10. [Google Scholar] [CrossRef]
- Fernandez-Leon, J.A.; Parajuli, A.; Franklin, R.; Sorenson, M.; Felleman, D.J.; Hansen, B.J.; Hu, M.; Dragoi, V. A Wireless Transmission Neural Interface System for Unconstrained Non-Human Primates. J Neural Eng 2015, 12. [Google Scholar] [CrossRef]
- Yin, M.; Borton, D.A.; Aceros, J.; Patterson, W.R.; Nurmikko, A. V. A 100-Channel Hermetically Sealed Implantable Device for Chronic Wireless Neurosensing Applications. IEEE Trans Biomed Circuits Syst 2013, 7, 115–128. [Google Scholar] [CrossRef]
- Tibor Schirrmeister, R.; Springenberg, J.T.; Fiederer, L.D.J.; Glasstetter, M.; Eggensperger, K.; Tangermann, M.; Hutter, F.; Burgard, W.; Ball, T. Deep Learning with Convolutional Neural Networks for EEG Decoding and Visualization. ArXiv 2017, arXiv:1703.05051. [Google Scholar] [CrossRef]
- Ienca, M.; Andorno, R. Towards New Human Rights in the Age of Neuroscience and Neurotechnology. Life Sci Soc Policy 2017, 13, 1–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




