Submitted:
27 March 2025
Posted:
31 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
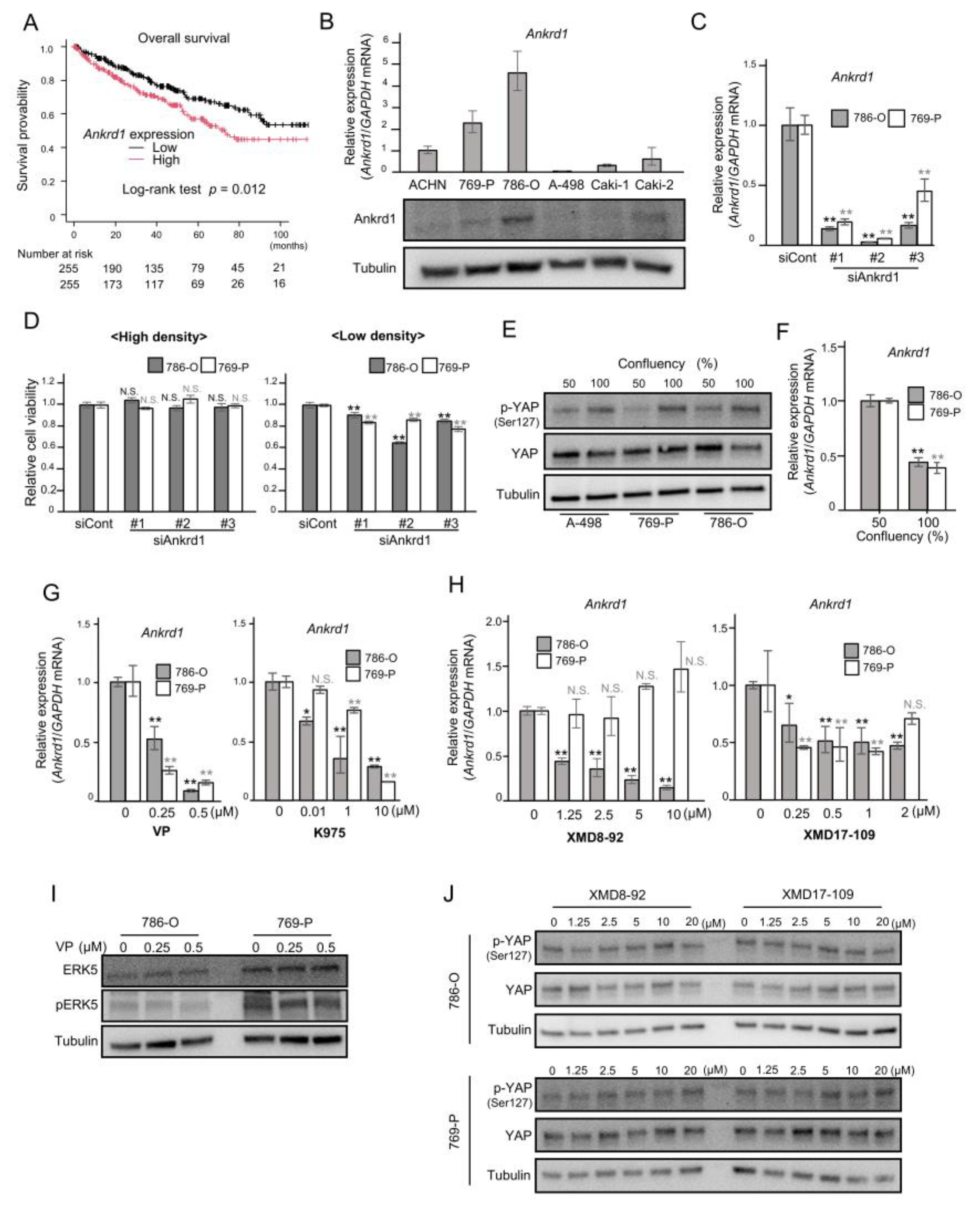
2.1. Subsection Prognostic Relevance of Ankrd1 Expression in TCGA-ccRCC Data Sets
2.2. Expression of Ankrd1 Is Regulated by YAP1 and ERK5
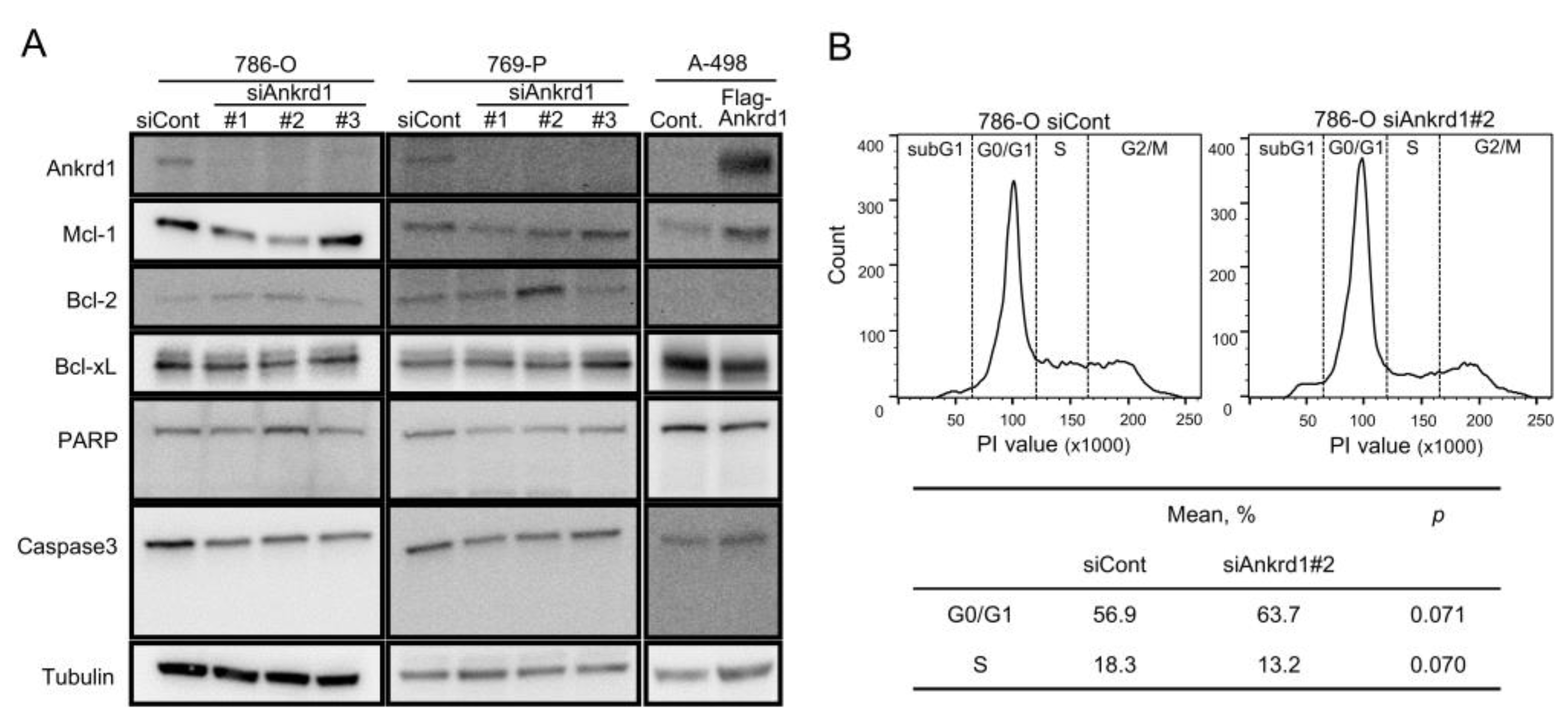
2.3. Knockdown of Ankrd1 Decreases Cell Proliferation
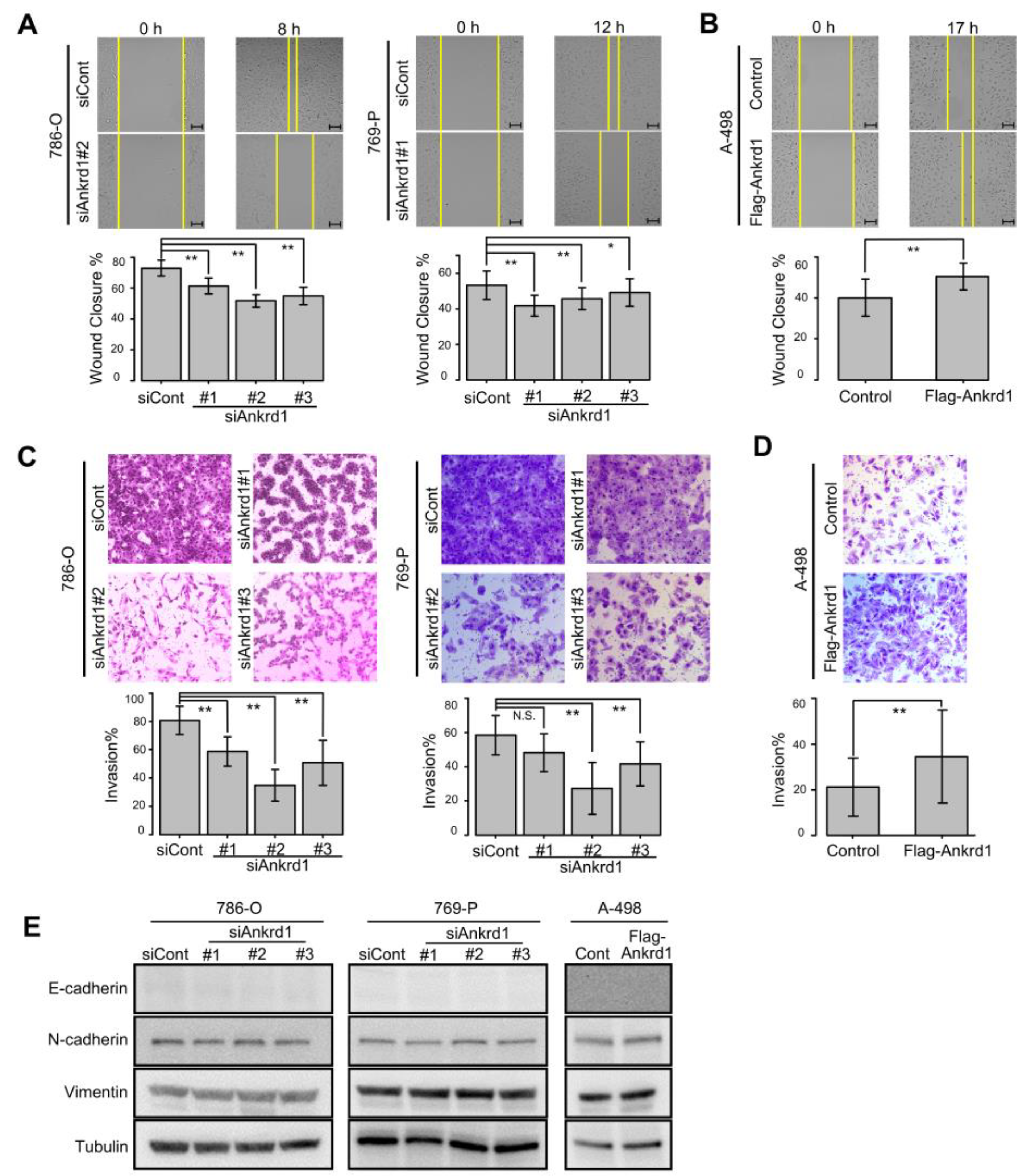
2.4. Ankrd1 Modulates the Migration and Invasion of RCC Cells
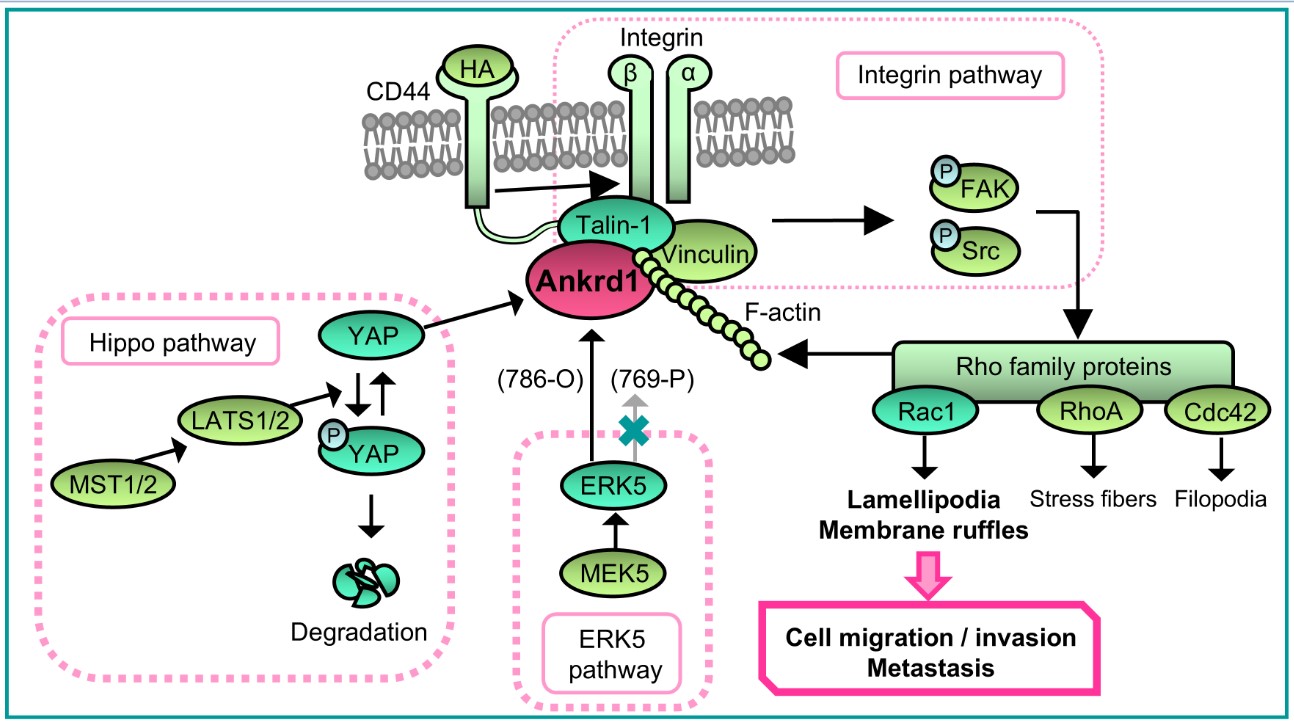
2.5. Interaction of Ankrd1 and talin-1 is Involved in the Formation of Lamellipodia
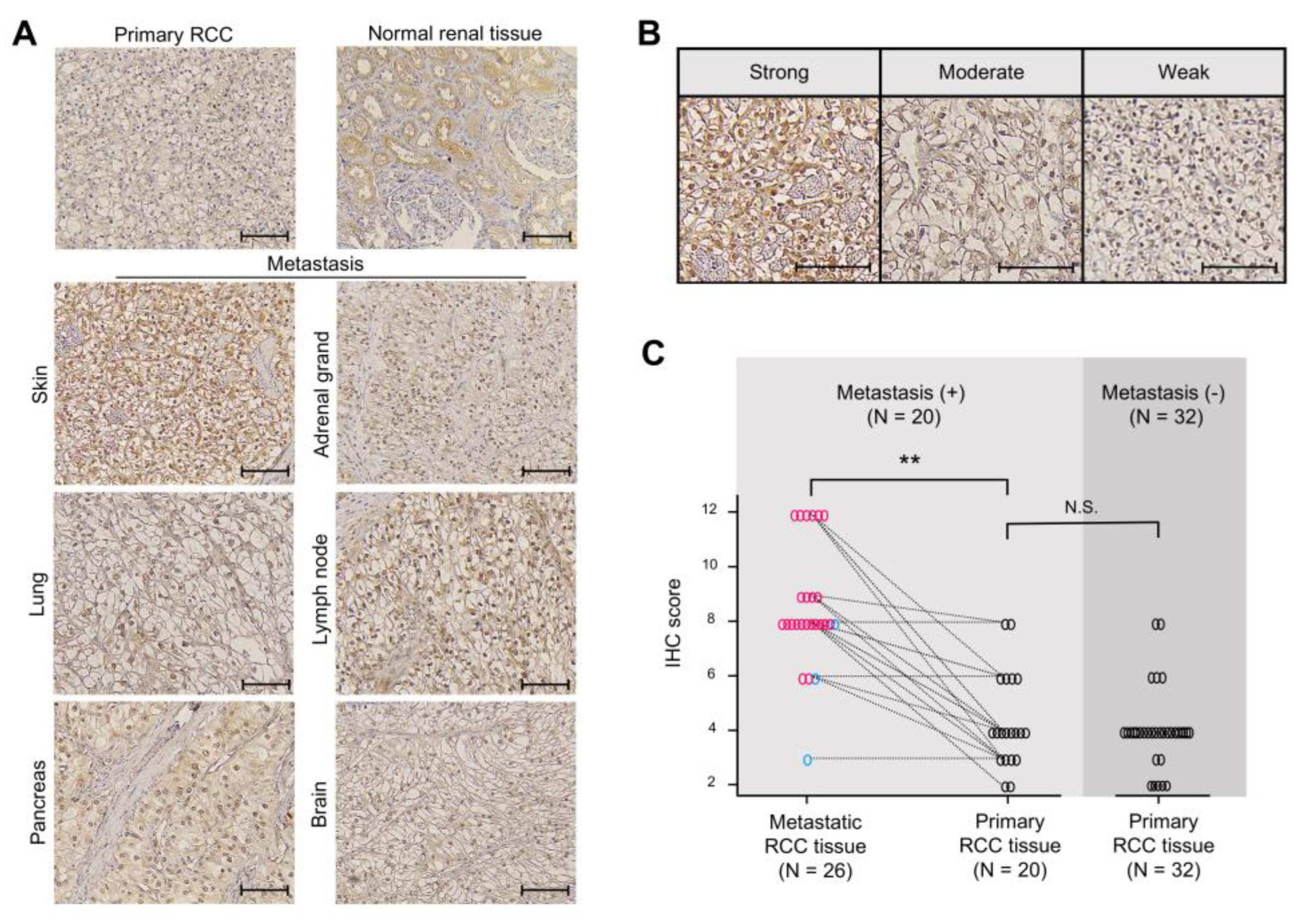
2.6. Ankrd1 Is Highly Expressed in Metastatic RCC Tissues
3. Discussion
4. Materials and Methods
4.1. Prognostic Analysis Using The Cancer Genome Atlas Database
4.2. Cell Culture and Reagents
4.3. RNA Extraction and Real-Time Reverse Transcriptase-PCR
4.4. siRNA Transfection
4.5. Overexpression of Plasmid DNA
4.6. Cell Proliferation Assay
4.7. Immunoblotting
4.8. Cell Cycle Analysis
4.9. Immunoprecipitation Assay
4.10. Wound-Healing Assay
4.11. Invasion Assay
4.12. Immunofluorescence Analysis
4.13. Proximity Ligation Assay (PLA)
4.14. Immunohistochemistry (IHC)
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Abbreviations
| Ankrd1 | Ankyrin repeat domain1 |
| YAP | Yes-associated protein |
| ccRCC | Clear cell renal cell carcinoma |
| EMT | Epithelial–mesenchymal transition |
| ECM | Extracellular matrix |
| TPA | 12-O-Tetradecanoylphorbol 13-acetate |
| HA | Hyaluronic acid |
| FA | Focal adhesion |
References
- Fischer, K. R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S. T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; Schwabe, R. F.; Vahdat, L. T.; Altorki, N. K.; Mittal, V.; Gao, D. , Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J. L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C. C.; LeBleu, V. S.; Kalluri, R. , Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Baer, P. C.; Bereiter-Hahn, J.; Schubert, R.; Geiger, H. , Differentiation status of human renal proximal and distal tubular epithelial cells in vitro: Differential expression of characteristic markers. Cells Tissues Organs 2006, 184, 16–22. [Google Scholar] [CrossRef]
- Prozialeck, W. C.; Lamar, P. C.; Appelt, D. M. , Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol 2004, 4, 10. [Google Scholar] [CrossRef]
- Tan, T. Z.; Miow, Q. H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R. Y.; Thiery, J. P. , Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med 2014, 6, 1279–93. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E. , Mechanisms of cancer cell invasion. Curr Opin Genet Dev 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Chhabra, E. S.; Higgs, H. N. , The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol 2007, 9, 1110–21. [Google Scholar] [CrossRef]
- Ladwein, M.; Rottner, K. , On the Rho'd: the regulation of membrane protrusions by Rho-GTPases. FEBS Lett 2008, 582, 2066–74. [Google Scholar] [CrossRef]
- Mikhailov, A. T.; Torrado, M. , The enigmatic role of the ankyrin repeat domain 1 gene in heart development and disease. Int J Dev Biol 2008, 52, 811–21. [Google Scholar] [CrossRef]
- Miller, M. K.; Bang, M. L.; Witt, C. C.; Labeit, D.; Trombitas, C.; Watanabe, K.; Granzier, H.; McElhinny, A. S.; Gregorio, C. C.; Labeit, S. , The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol 2003, 333, 951–64. [Google Scholar] [CrossRef]
- Ling, S. S. M.; Chen, Y. T.; Wang, J.; Richards, A. M.; Liew, O. W. , Ankyrin Repeat Domain 1 Protein: A Functionally Pleiotropic Protein with Cardiac Biomarker Potential. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Seike, M.; Chiba, M.; Takahashi, S.; Nakamichi, S.; Matsumoto, M.; Takeuchi, S.; Minegishi, Y.; Noro, R.; Kunugi, S.; Kubota, K.; Gemma, A. , Ankyrin Repeat Domain 1 Overexpression is Associated with Common Resistance to Afatinib and Osimertinib in EGFR-mutant Lung Cancer. Sci Rep 2018, 8, 14896. [Google Scholar] [CrossRef]
- Singh, D.; Deshmukh, R. K.; Das, A. , SNAI1-mediated transcriptional regulation of epithelial-to-mesenchymal transition genes in breast cancer stem cells. Cell Signal 2021, 87, 110151. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. , The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014, 94, 1287–312. [Google Scholar] [CrossRef]
- Obara, Y.; Nagasawa, R.; Nemoto, W.; Pellegrino, M. J.; Takahashi, M.; Habecker, B. A.; Stork, P. J. S.; Ichiyanagi, O.; Ito, H.; Tomita, Y.; Ishii, K.; Nakahata, N. , ERK5 induces ankrd1 for catecholamine biosynthesis and homeostasis in adrenal medullary cells. Cell Signal 2016, 28, 177–189. [Google Scholar] [CrossRef]
- Suenaga, S.; Ichiyanagi, O.; Ito, H.; Naito, S.; Kato, T.; Nagaoka, A.; Kato, T.; Yamakawa, M.; Obara, Y.; Tsuchiya, N. , Expression of Extracellular Signal-regulated Kinase 5 and Ankyrin Repeat Domain 1 in Composite Pheochromocytoma and Ganglioneuroblastoma Detected Incidentally in the Adult Adrenal Gland. Intern Med 2016, 55, 3611–3621. [Google Scholar] [CrossRef]
- Kanno, H.; Naito, S.; Obara, Y.; Ito, H.; Ichiyanagi, O.; Narisawa, T.; Kato, T.; Nagaoka, A.; Tsuchiya, N. , Effect of Extracellular Signal-Regulated Protein Kinase 5 Inhibition in Clear Cell Renal Cell Carcinoma. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Chen, T. H.; Chen, C. Y.; Wen, H. C.; Chang, C. C.; Wang, H. D.; Chuu, C. P.; Chang, C. H. , YAP promotes myogenic differentiation via the MEK5-ERK5 pathway. Faseb j 2017, 31, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, F.; Consalvi, V.; Noce, V.; Battistelli, C.; Cicchini, C.; Tripodi, M.; Amicone, L.; Marchetti, A. , Extracellular signal-Regulated Kinase 5 (ERK5) is required for the Yes-associated protein (YAP) co-transcriptional activity. Cell Death Dis 2023, 14, 32. [Google Scholar] [CrossRef]
- Brodaczewska, K. K.; Szczylik, C.; Fiedorowicz, M.; Porta, C.; Czarnecka, A. M. , Choosing the right cell line for renal cell cancer research. Mol Cancer 2016, 15, 83. [Google Scholar] [CrossRef]
- Tatin, F.; Varon, C.; Génot, E.; Moreau, V. , A signalling cascade involving PKC, Src and Cdc42 regulates podosome assembly in cultured endothelial cells in response to phorbol ester. J Cell Sci 2006, 119, 769–81. [Google Scholar] [CrossRef] [PubMed]
- Salmela, M.; Rappu, P.; Lilja, J.; Niskanen, H.; Taipalus, E.; Jokinen, J.; Heino, J. , Tumor promoter PMA enhances kindlin-2 and decreases vimentin recruitment into cell adhesion sites. Int J Biochem Cell Biol 2016, 78, 22–30. [Google Scholar] [CrossRef]
- Goicoechea, S. M.; García-Mata, R.; Staub, J.; Valdivia, A.; Sharek, L.; McCulloch, C. G.; Hwang, R. F.; Urrutia, R.; Yeh, J. J.; Kim, H. J.; Otey, C. A. , Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene 2014, 33, 1265–73. [Google Scholar] [CrossRef] [PubMed]
- Moulik, M.; Vatta, M.; Witt, S. H.; Arola, A. M.; Murphy, R. T.; McKenna, W. J.; Boriek, A. M.; Oka, K.; Labeit, S.; Bowles, N. E.; Arimura, T.; Kimura, A.; Towbin, J. A. , ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol 2009, 54, 325–33. [Google Scholar] [CrossRef]
- Chopra, A.; Lin, V.; McCollough, A.; Atzet, S.; Prestwich, G. D.; Wechsler, A. S.; Murray, M. E.; Oake, S. A.; Kresh, J. Y.; Janmey, P. A. , Reprogramming cardiomyocyte mechanosensing by crosstalk between integrins and hyaluronic acid receptors. J Biomech 2012, 45, 824–31. [Google Scholar] [CrossRef]
- Lee, J. L.; Wang, M. J.; Sudhir, P. R.; Chen, J. Y. , CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol 2008, 28, 5710–23. [Google Scholar] [CrossRef] [PubMed]
- Goult, B. T.; Yan, J.; Schwartz, M. A. , Talin as a mechanosensitive signaling hub. J Cell Biol 2018, 217, 3776–3784. [Google Scholar] [CrossRef]
- Samaras, S. E.; Almodóvar-García, K.; Wu, N.; Yu, F.; Davidson, J. M. , Global deletion of Ankrd1 results in a wound-healing phenotype associated with dermal fibroblast dysfunction. Am J Pathol 2015, 185, 96–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Li, H.; Ai, L.; Ma, Q.; Qiao, X.; Yang, J.; Zhang, H.; Ou, X.; Wang, Y.; Chen, G.; Xue, J.; Zhu, X.; Zhao, Y.; Yang, Y.; Liu, C. , Binding blockade between TLN1 and integrin β1 represses triple-negative breast cancer. Elife 2022, 11. [Google Scholar]
- Jin, J. K.; Tien, P. C.; Cheng, C. J.; Song, J. H.; Huang, C.; Lin, S. H.; Gallick, G. E. , Talin1 phosphorylation activates β1 integrins: a novel mechanism to promote prostate cancer bone metastasis. Oncogene 2015, 34, 1811–21. [Google Scholar] [CrossRef]
- Bostanci, O.; Kemik, O.; Kemik, A.; Battal, M.; Demir, U.; Purisa, S.; Mihmanli, M. , A novel screening test for colon cancer: Talin-1. Eur Rev Med Pharmacol Sci 2014, 18, 2533–7. [Google Scholar] [PubMed]
- Lai, M. T.; Hua, C. H.; Tsai, M. H.; Wan, L.; Lin, Y. J.; Chen, C. M.; Chiu, I. W.; Chan, C.; Tsai, F. J.; Jinn-Chyuan Sheu, J. , Talin-1 overexpression defines high risk for aggressive oral squamous cell carcinoma and promotes cancer metastasis. J Pathol 2011, 224, 367–76. [Google Scholar] [CrossRef]
- Zanjani, L. S.; Vafaei, S.; Abolhasani, M.; Fattahi, F.; Madjd, Z. , Prognostic value of Talin-1 in renal cell carcinoma and its association with B7-H3. Cancer Biomark 2022. [Google Scholar]
- Carisey, A.; Ballestrem, C. , Vinculin, an adapter protein in control of cell adhesion signalling. Eur J Cell Biol 2011, 90, 157–63. [Google Scholar] [CrossRef] [PubMed]
- Nayal, A.; Webb, D. J.; Horwitz, A. F. , Talin: an emerging focal point of adhesion dynamics. Curr Opin Cell Biol 2004, 16, 94–8. [Google Scholar] [CrossRef]
- Koch, A. W.; Farooq, A.; Shan, W.; Zeng, L.; Colman, D. R.; Zhou, M. M. , Structure of the neural (N-) cadherin prodomain reveals a cadherin extracellular domain-like fold without adhesive characteristics. Structure 2004, 12, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Dalgliesh, G. L.; Furge, K.; Greenman, C.; Chen, L.; Bignell, G.; Butler, A.; Davies, H.; Edkins, S.; Hardy, C.; Latimer, C.; Teague, J.; Andrews, J.; Barthorpe, S.; Beare, D.; Buck, G.; Campbell, P. J.; Forbes, S.; Jia, M.; Jones, D.; Knott, H.; Kok, C. Y.; Lau, K. W.; Leroy, C.; Lin, M. L.; McBride, D. J.; Maddison, M.; Maguire, S.; McLay, K.; Menzies, A.; Mironenko, T.; Mulderrig, L.; Mudie, L.; O'Meara, S.; Pleasance, E.; Rajasingham, A.; Shepherd, R.; Smith, R.; Stebbings, L.; Stephens, P.; Tang, G.; Tarpey, P. S.; Turrell, K.; Dykema, K. J.; Khoo, S. K.; Petillo, D.; Wondergem, B.; Anema, J.; Kahnoski, R. J.; Teh, B. T.; Stratton, M. R.; Futreal, P. A. , Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010, 463, 360–3. [Google Scholar] [CrossRef]
- Kim, N. G.; Gumbiner, B. M. , Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol 2015, 210, 503–15. [Google Scholar] [CrossRef]
- Scurr, L. L.; Guminski, A. D.; Chiew, Y. E.; Balleine, R. L.; Sharma, R.; Lei, Y.; Pryor, K.; Wain, G. V.; Brand, A.; Byth, K.; Kennedy, C.; Rizos, H.; Harnett, P. R.; deFazio, A. , Ankyrin repeat domain 1, ANKRD1, a novel determinant of cisplatin sensitivity expressed in ovarian cancer. Clin Cancer Res 2008, 14, 6924–32. [Google Scholar] [CrossRef]
- Lei, Y.; Henderson, B. R.; Emmanuel, C.; Harnett, P. R.; deFazio, A. , Inhibition of ANKRD1 sensitizes human ovarian cancer cells to endoplasmic reticulum stress-induced apoptosis. Oncogene 2015, 34, 485–95. [Google Scholar] [CrossRef]
- Hui, B.; Ji, H.; Xu, Y.; Wang, J.; Ma, Z.; Zhang, C.; Wang, K.; Zhou, Y. , RREB1-induced upregulation of the lncRNA AGAP2-AS1 regulates the proliferation and migration of pancreatic cancer partly through suppressing ANKRD1 and ANGPTL4. Cell Death Dis 2019, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ichiyanagi, O.; Naito, S.; Bilim, V. N.; Tomita, Y.; Kato, T.; Nagaoka, A.; Tsuchiya, N. , GSK-3 directly regulates phospho-4EBP1 in renal cell carcinoma cell-line: an intrinsic subcellular mechanism for resistance to mTORC1 inhibition. BMC Cancer 2016, 16, 393. [Google Scholar] [CrossRef] [PubMed]
- Badi, I.; Cinquetti, R.; Frascoli, M.; Parolini, C.; Chiesa, G.; Taramelli, R.; Acquati, F. , Intracellular ANKRD1 protein levels are regulated by 26S proteasome-mediated degradation. FEBS Lett 2009, 583, 2486–92. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, O.; Ito, H.; Naito, S.; Kabasawa, T.; Kanno, H.; Narisawa, T.; Ushijima, M.; Kurota, Y.; Ozawa, M.; Sakurai, T.; Nishida, H.; Kato, T.; Yamakawa, M.; Tsuchiya, N. , Impact of eIF4E phosphorylation at Ser209 via MNK2a on tumour recurrence after curative surgery in localized clear cell renal cell carcinoma. Oncotarget 2019, 10, 4053–4068. [Google Scholar] [CrossRef]
- Ichiyanagi, O.; Naito, S.; Ito, H.; Kabasawa, T.; Narisawa, T.; Kanno, H.; Kurota, Y.; Kurokawa, M.; Fukuhara, H.; Sakurai, T.; Nishida, H.; Kato, T.; Yamakawa, M.; Tsuchiya, N. , Levels of 4EBP1/eIF4E Activation in Renal Cell Carcinoma Could Differentially Predict Its Early and Late Recurrence. Clin Genitourin Cancer 2018, 16, e1029–e1058. [Google Scholar] [CrossRef]
- Xie, B.; Lin, W.; Ye, J.; Wang, X.; Zhang, B.; Xiong, S.; Li, H.; Tan, G. , DDR2 facilitates hepatocellular carcinoma invasion and metastasis via activating ERK signaling and stabilizing SNAIL1. J Exp Clin Cancer Res 2015, 34, 101. [Google Scholar] [CrossRef]

| Clinical variable | Metastasis (+) (N = 20) |
Metastasis (-) (N = 32) |
p |
|---|---|---|---|
| Age at surgery of primary lesion (year), mean (range) | 60.7 (44 - 76) | 68.4 (34 - 91) | |
| Sex, n (%) | 1 | ||
| Men | 17 (85.0) | 27 (84.4) | |
| Women | 3 (15.0) | 5 (15.6) | |
| Subtype, n (%) | 1 | ||
| Clear | 20 (100) | 32 (100) | |
| pT stage, n (%) | < 0.01 | ||
| 1a | 2 (10.0) | 26 (81.3) | |
| 1b | 7 (35.0) | 5 (15.6) | |
| 2a | 1 (5.0) | 1 (3.1) | |
| 2b | 2 (10.0) | 0 (0) | |
| 3a | 8 (40.0) | 0 (0) | |
| Fuhrman grade, n (%) | 0.658 | ||
| 1 | 2 (10.0) | 8 (25.0) | |
| 2 | 12 (60.0) | 15 (46.9) | |
| 3 | 5 (25.0) | 7 (21.9) | |
| Grade, n (%) | 1(5.0) | 2 (6.2) | |
| Metastasis, n (%) | |||
| Lung | 11 (42.3) | ||
| Adrenal grand | 4 (15.4) | ||
| Skin | 3 (11.5) | ||
| Pancreas | 3 (11.5) | ||
| Lymph node | 3 (11.5) | ||
| Brain | 1 (3.8) | ||
| Vein | 1 (3.8) |
| Pathological variables | IHC score (mean) | p |
|---|---|---|
| Fuhrman grade | 0.414 | |
| 1, 2 (n = 37) | 4.35 | |
| 3, 4 (n = 15) | 3.80 | |
| pT stage | 0.493 | |
| 1a-2a (n = 42) | 4.12 | |
| 2b, 3a (n = 10) | 4.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





