Submitted:
26 March 2025
Posted:
27 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
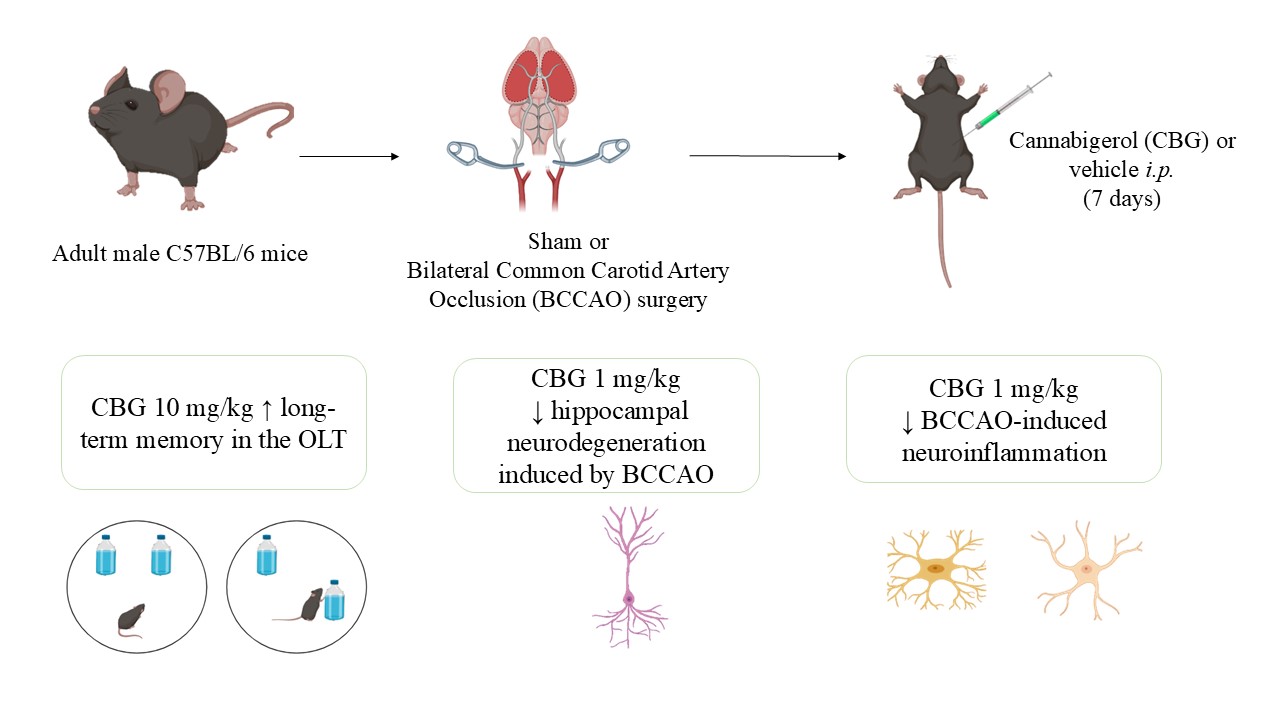
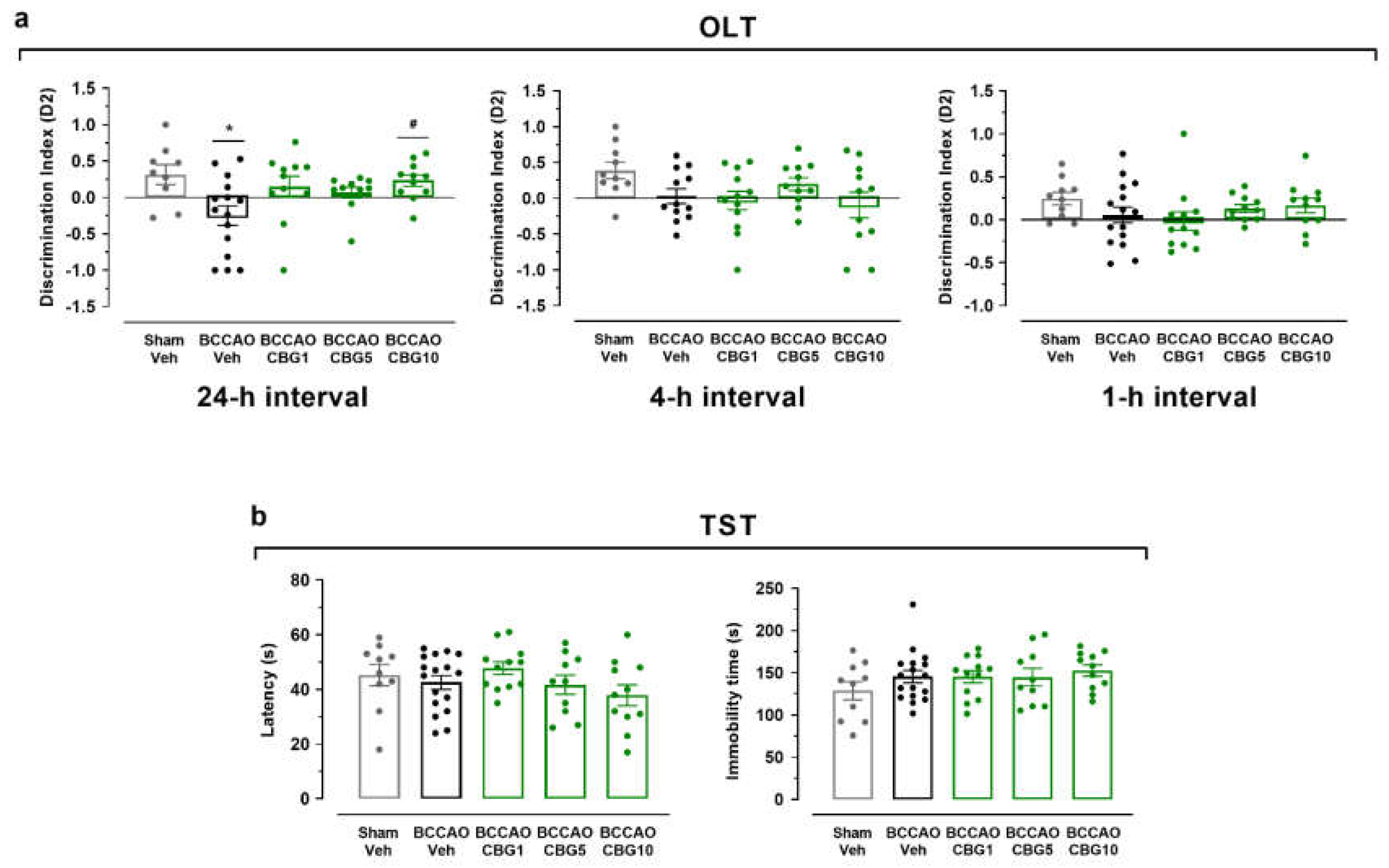
2.1. CBG Improves Memory Impairment Induced by BCCAO in Mice
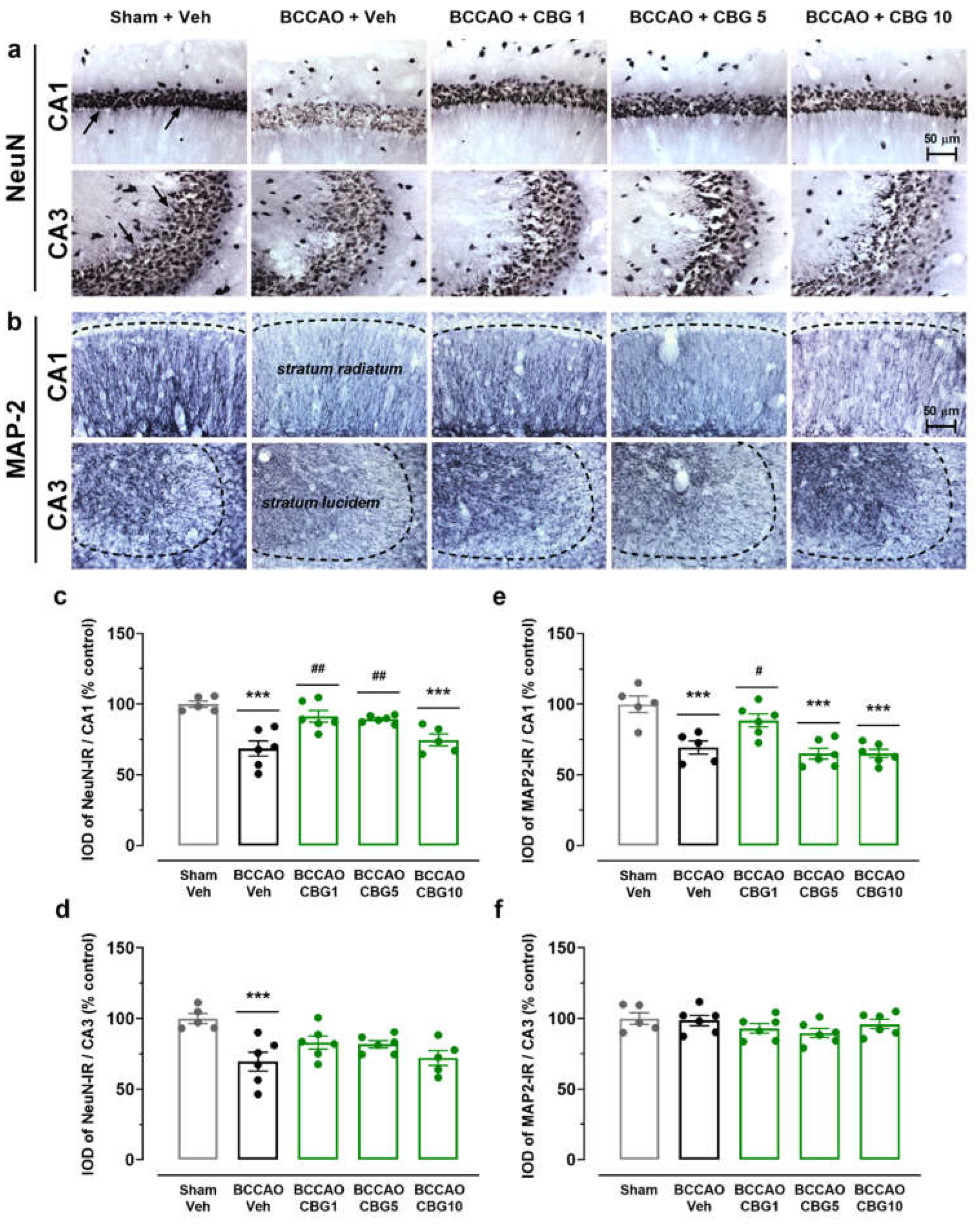
2.2. CBG Decreases Hippocampal Neurodegeneration Induced by BCCAO
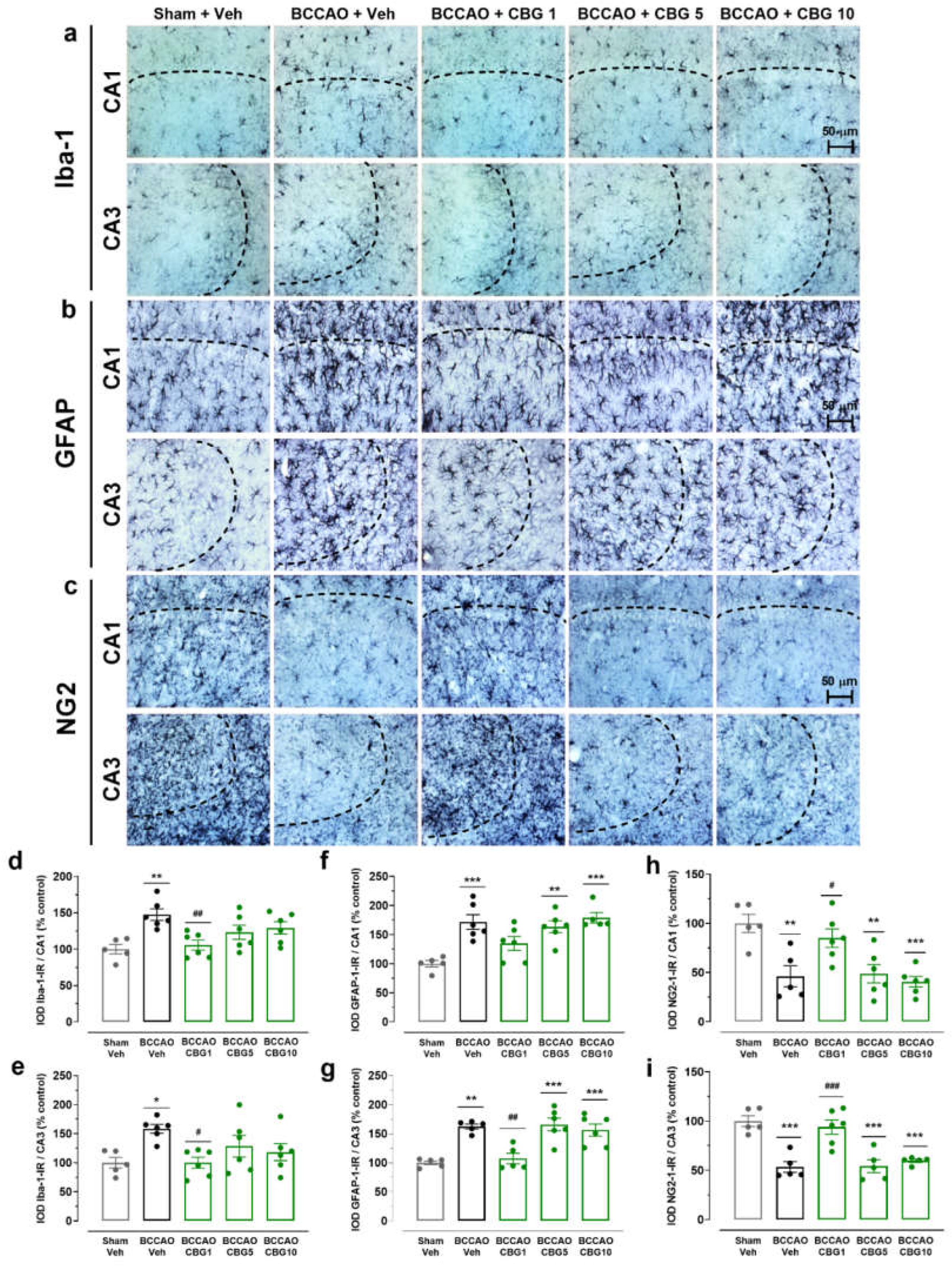
2.3. CBG Reduces BCCAO-Induced Neuroinflammation
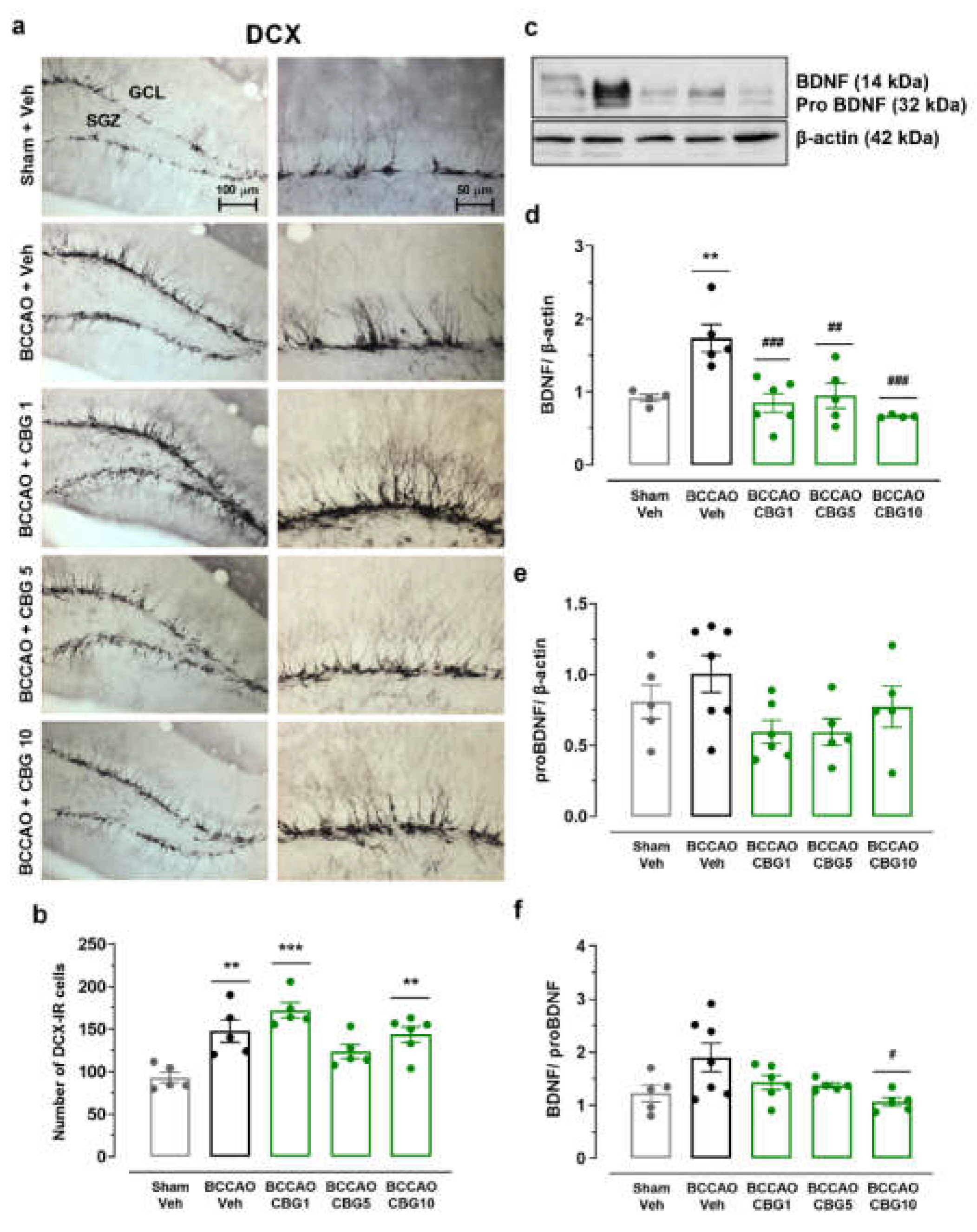
2.4. The Impact of CBG on Ischemia-Induced Neuroplasticity
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Bilateral Common Carotid Arteries Occlusion (BCCAO)
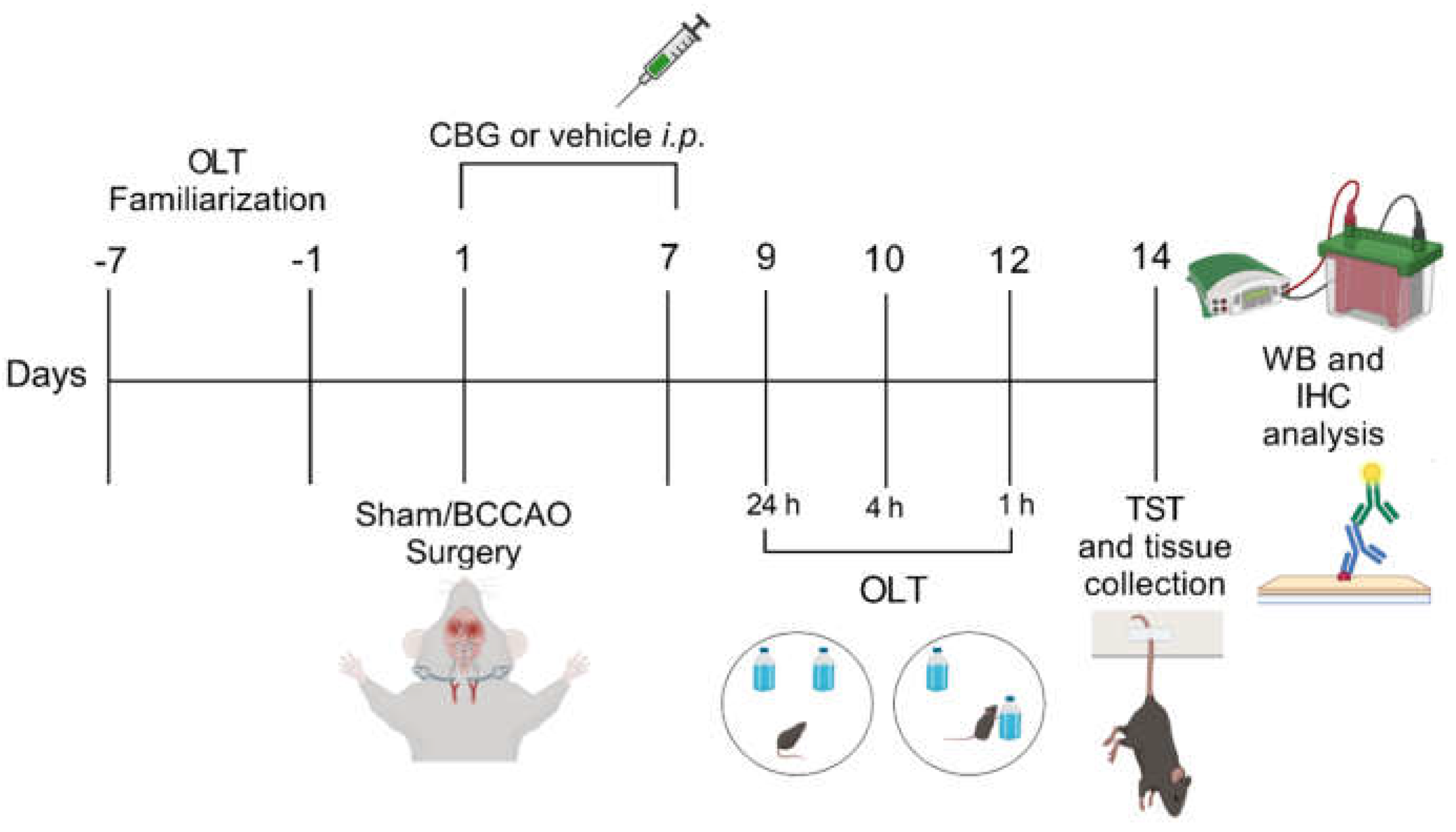
4.4. Experimental Design
4.5. Behavioral Testing
4.5.1. Object Location Test (OLT)
4.5.2. Tail Suspension Test (TST)
4.6. Western Blot
4.7. Immunohistochemistry
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT1A ANOVA BBB BDNF BCCAO BSA CNS CBG CBG1 CBG5 CBG10 |
Serotonin 1A receptor Analysis of variance Blood brain barrier Brain-derived neurotrophic factor Common carotid artery occlusion Bovine serum albumin Central nervous system Cannabigerol CBG at a dose of 1 mg/Kg CBG at a dose of 5 mg/Kg CBG at a dose of 10 mg/Kg |
| DAB DCX DMSO GCL GFAP IFN IL Iba-1 iNOS IOD i.p. IR H2O2 HRP PPAR PBS PBST PB PFA MAP-2 MAPK NaCl NeuN NiCl2 NF-κB NG2 NSC OLT SGZ SOD-1 TBST-T TGCI |
3,3'-diaminobenzidine Doublecortin Dimethyl sulfoxide Granular cell layer Glial fibrillary acidic protein Interferon Interleukin Ionized calcium-binding adaptor molecule 1 Inducible nitric oxide synthase Integrated optical density Intraperitoneal Immunoreactive Hydrogen peroxide Horseradish peroxidase Peroxisome proliferator-activated receptor Phosphate-buffered saline PBS with 0.3% Triton X-100 Phosphate buffer Paraformaldehyde Microtubule-associated protein 2 Mitogen-activated protein kinase Sodium chloride Neuronal nuclear protein Nickel chloride Nuclear factor kappa B Neural/glial antigen 2 Neuroblastoma spinal cord Object location test Subgranular zone Superoxide dismutase 1 Tris buffer saline-tween Transient global cerebral ischemia |
| TNF | Tumor necrosis factor |
| TRP TST Veh WM |
Transient receptor potential Tail suspension test Vehicle White matter |
References
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Alternat. Med. 2022, 3336516. [CrossRef]
- Stone, N.L.; England, T.J.; O'Sullivan, S.E. Protective Effects of Cannabidivarin and Cannabigerol on Cells of the Blood-Brain Barrier Under Ischemic Conditions. Cannabis Cannabinoid Res. 2021, 6(4), 315-326. [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; Platt, B.; Riedel, G. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (CBD), Cannabidivarine (CBDV), Δ⁹-Tetrahydrocannabivarin (THCV) and Cannabigerol (CBG) in Rats and Mice Following Oral and Intraperitoneal Administration and CBD Action on Obsessive-Compulsive Behaviour. Psychopharmacology (Berl.) 2012, 219, 859–873. https://10.1007/s00213-011-2415-0.
- Gugliandolo, A.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. In Vitro Model of Neuroinflammation: Efficacy of Cannabigerol, a Non-Psychoactive Cannabinoid. Int. J. Mol. Sci. 2018, 19, 1992. https://10.3390/ijms19071992.
- di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L.; Menghini, L.; Zengin, G.; Ak, G.; Abdallah, H. H.; Ferrante, C. Antioxidant and Neuroprotective Effects Induced by Cannabidiol and Cannabigerol in Rat CTX-TNA2 Astrocytes and Isolated Cortexes. Int. J. Mol. Sci. 2020, 21, 3575. https://10.3390/ijms21103575.
- Valdeolivas, S.; Navarrete, C.; Cantarero, I.; Bellido, M.; Muñoz, E.; Sagredo, O. Neuroprotective Properties of Cannabigerol in Huntington’s Disease: Studies in R6/2 Mice and 3-Nitropropionate-Lesioned Mice. Neurotherapeutics 2015, 12, 185-199. [CrossRef]
- Anchesi, I.; Betto, F.; Chiricosta, L.; Gugliandolo, A.; Pollastro, F.; Salamone, S.; Mazzon, E. Cannabigerol Activates Cytoskeletal Remodeling via Wnt/PCP in NSC-34: An In Vitro Transcriptional Study. Plants 2023, 12(1), 193. [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol Reduces Neuroinflammation and Promotes Neuroplasticity and Functional Recovery After Brain Ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94-105. [CrossRef]
- Washida, K.; Hattori, Y.; Ihara, M. Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate. Int. J. Mol. Sci. 2019, 20(24), 6176. [CrossRef]
- León-Moreno, L.C.; Castañeda-Arellano, R.; Rivas-Carrillo, J.D.; Dueñas-Jiménez, S.H. Challenges and Improvements of Developing an Ischemia Mouse Model Through Bilateral Common Carotid Artery Occlusion. J. Stroke Cerebrovasc. Dis. 2020, 29(5), 104773. [CrossRef]
- Rahmati, H.; Momenabadi, S.; Vafaei, A.A.; Bandegi, A.Z.; Mazaheri, Z.; Vakili, A. Probiotic Supplementation Attenuates Hippocampus Injury and Spatial Learning and Memory Impairments in a Cerebral Hypoperfusion Mouse Model. Mol. Biol. Rep. 2019, 46, 4985–4995. [CrossRef]
- Soares, L.M.; Meyer, E.; Milani, H.; Steinbusch, H.W.; Prickaerts, J.; de Oliveira, R.M. The Phosphodiesterase Type 2 Inhibitor BAY 60-7550 Reverses Functional Impairments Induced by Brain Ischemia by Decreasing Hippocampal Neurodegeneration and Enhancing Hippocampal Neuronal Plasticity. Eur. J. Neurosci. 2016a, 45, 510–520. http://dx.doi.org/10.1111/EJN.13461.
- Dirnagl, U. Pathobiology of Injury After Stroke: The Neurovascular Unit and Beyond. Ann. N.Y. Acad. Sci. 2012, 1268, 21-25. [CrossRef]
- Jiwa, N.S.; Garrard, P.; Hainsworth, A.H. Experimental Models of Vascular Dementia and Vascular Cognitive Impairment: A Systematic Review. J. Neurochem. 2010, 115(4), 814-828. [CrossRef]
- Soares, L.M.; Schiavon, A.P.; Milani, H.; de Oliveira, R.M. Cognitive Impairment and Persistent Anxiety-Related Responses Following Bilateral Common Carotid Artery Occlusion in Mice. Behav. Brain Res. 2013, 249, 28–37. [CrossRef]
- Aguiar, R.P.; Soares, L.M.; Meyer, E.; da Silveira, F.C.; Milani, H.; Newman-Tancredi, A.; Varney, M.; Prickaerts, J.; Oliveira, R.M.W. Activation of 5-HT1A Postsynaptic Receptors by NLX-101 Results in Functional Recovery and an Increase in Neuroplasticity in Mice with Brain Ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109832. [CrossRef]
- Soares, L.M.; De Vry, J.; Steinbusch, H.W.; Milani, H.; Prickaerts, J.; Weffort de Oliveira, R.M. Rolipram Improves Cognition, Reduces Anxiety- and Despair-Like Behaviors and Impacts Hippocampal Neuroplasticity After Transient Global Cerebral Ischemia. Neuroscience 2016b, 326, 69-83. http://dx.doi.org/10.1016/j.neuroscience.2016.03.062.
- Fleisher-Berkovich, S.; Ventura, Y.; Amoyal, M.; Dahan, A.; Feinshtein, V.; Alfahel, L.; Israelson, A.; Bernstein, N.; Gorelick, J.; Ben-Shabat, S. Therapeutic Potential of Phytocannabinoid Cannabigerol for Multiple Sclerosis: Modulation of Microglial Activation In Vitro and In Vivo. Biomolecules 2023, 13, 376. [CrossRef]
- Hu, G.; Zhou, C.; Wang, J.; Ma, X.; Ma, H.; Yu, H.; Peng, Z.; Huang, J.; Cai, M. Electroacupuncture Treatment Ameliorates Depressive-Like Behavior and Cognitive Dysfunction via CB1R Dependent Mitochondria Biogenesis After Experimental Global Cerebral Ischemic Stroke. Front. Cell Neurosci. 2023, 17, 1135227. http://dx.doi.org/10.3389/fncel.2023.1135227.
- Bueters, T.; von Euler, M.; Bendel, O.; von Euler, G. Degeneration of Newly Formed CA1 Neurons Following Global Ischemia in the Rat. Exp. Neurol. 2008, 209(1), 114-124. [CrossRef]
- Bachevalier, J.; Meunier, M. Cerebral Ischemia: Are the Memory Deficits Associated with Hippocampal Cell Loss? Hippocampus 1996, 6, 553–560. https://10.1002/(SICI)1098-1063(1996)6:5<553::AID-HIPO8>3.0.CO;2-J.
- Aronowski, J.; Samways, E.; Strong, R.; Rhoades, H.M.; Grotta, J.C. An Alternative Method for the Quantitation of Neuronal Damage After Experimental Middle Cerebral Artery Occlusion in Rats: Analysis of Behavioral Deficit. J. Cereb. Blood Flow Metab. 1996, 16, 705–713. https://10.1097/00004647-199607000-00022.
- Gehrmann, J.; Bonnekoh, P.; Miyazawa, T.; Hossmann, K.A.; Kreutzberg, G.W. Immunocytochemical Study of an Early Microglial Activation in Ischemia. J. Cereb. Blood Flow Metab. 1992, 12, 257–269. [CrossRef]
- Stoll, M.; Capper, D.; Dietz, K.; Warth, A.; Schleich, A.; Schlaszus, H.; Meyermann, R.; Mittelbronn, M. Differential Microglial Regulation in the Human Spinal Cord under Normal and Pathological Conditions. Neuropathol. Appl. Neurobiol. 2006, 32, 650–661. https://10.1111/j.1365-2990.2006.00774.x.
- Yasuda, Y.; Shimoda, T.; Uno, K.; Tateishi, N.; Furuya, S.; Tsuchihashi, Y.; et al. Temporal and Sequential Changes of Glial Cells and Cytokine Expression During Neuronal Degeneration After Transient Global Ischemia in Rats. J. Neuroinflammation 2011, 8, 70. [CrossRef]
- Collino, M.; Aragno, M.; Mastrocola, R.; Benetti, E.; Gallicchio, M.; Dianzani, C.; Danni, O.; Thiemermann, C.; Fantozzi, R. Oxidative Stress and Inflammatory Response Evoked by Transient Cerebral Ischemia/Reperfusion: Effects of the PPAR-Alpha Agonist WY14643. Free Radic. Biol. Med. 2006, 41, 579–589. https:// 10.1016/j.freeradbiomed.2006.04.030.
- Chehaibi, K.; Trabelsi, I.; Mahdouani, K.; Slimane, M.N. Correlation of Oxidative Stress Parameters and Inflammatory Markers in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 2585–2593. [CrossRef]
- Giacoppo, S.; Gugliandolo, A.; Trubiani, O.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Cannabinoid CB₂ Receptors Are Involved in the Protection of RAW264.7 Macrophages Against Oxidative Stress: An In Vitro Study. Eur. J. Histochem. 2017, 61, 2749. https://10.4081/ejh.2017.2749.
- Hill, R.A.; Nishiyama, A. NG2 Cells (Polydendrocytes): Listeners to the Neural Network with Diverse Properties. Glia 2014, 62(8), 1195–1210. [CrossRef]
- Vigano, F.; Dimou, L. The Heterogeneous Nature of NG2-Glia. Brain Res. 2016, 1638, 129–137. [CrossRef]
- Kirdajova, D.; Anderova, M. NG2 Cells and Their Neurogenic Potential. Curr. Opin. Pharmacol. 2020, 50, 53–60. [CrossRef]
- Sugimoto, K.; Nishioka, R.; Ikeda, A.; Mise, A.; Takahashi, H.; Yano, H.; Kumon, Y.; Ohnishi, T.; Tanaka, J. Activated Microglia in a Rat Stroke Model Express NG2 Proteoglycan in Peri-Infarct Tissue Through the Involvement of TGF-β1. Glia 2014, 62, 185–198. https://10.1002/glia.22598.
- Jin, X.; Riew, T.; Kim, S.; Kim, H.L.; Lee, M. Spatiotemporal Profile and Morphological Changes of NG2 Glia in the CA1 Region of the Rat Hippocampus After Transient Forebrain Ischemia. Exp. Neurobiol. 2020, 29, 50–69. [CrossRef]
- Steliga, A.; Lietzau, G.; Wójcik, S.; Kowiański, P. Transient Cerebral Ischemia Induces the Neuroglial Proliferative Activity and the Potential to Redirect Neuroglial Differentiation. J. Chem. Neuroanat. 2023, 127, 102192. [CrossRef]
- Kirdajova, D.; Valihrach, L.; Valny, M.; Kriska, J.; Krocianova, D.; Benesova, S.; Abaffy, P.; Zucha, D.; Klassen, R.; Kolenicova, D.; Honsa, P.; Kubista, M.; Anderova, M. Transient Astrocyte-Like NG2 Glia Subpopulation Emerges Solely Following Permanent Brain Ischemia. Glia 2021, 69(11), 2658–2681. [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [CrossRef]
- Feng, B.; Jia, S.; Li, L.; Wang, J.; Zhou, F.; Gou, X.; Wang, Q.; Xiong, L.; Zeng, Y.; Zhong, H. TAT-LBD-Ngn2-Improved Cognitive Functions After Global Cerebral Ischemia by Enhancing Neurogenesis. Brain Behav. 2023, 13(1), e2847. [CrossRef]
- Conde, C.; Caceres, A. Microtubule Assembly, Organization and Dynamics in Axons and Dendrites. Nat. Rev. Neurosci. 2009, 10, 319–332. [CrossRef]
- DeGiosio, R.A.; Grubisha, M.J.; MacDonald, M.L.; McKinney, B.C.; Camacho, C.J.; Sweet, R.A. More Than a Marker: Potential Pathogenic Functions of MAP2. Front. Mol. Neurosci. 2022, 15, 974890. [CrossRef]
- Matesic, D.; Lin, R. Microtubule-Associated Protein 2 as an Early Indicator of Ischemia-Induced Neurodegeneration in the Gerbil Forebrain. J. Neurochem. 1994, 63. [CrossRef]
- Bacarin, C.C.; Godinho, J.; de Oliveira, R.M.W.; Matsushita, M.; Gohara, A.K.; Cardozo Filho, L.; Lima, J.C.; Previdelli, I.S.; Melo, S.R.; Ribeiro, M.H.M.; Milani, H. Postischemic Fish Oil Treatment Restores Long-Term Retrograde Memory and Dendritic Density: An Analysis of the Time Window of Efficacy. Behav. Brain Res. 2016, 31, 425–439. [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E. I.; Borea, P. A.; Nadal, X.; Franco, R. Cannabigerol Action at Cannabinoid CB₁ and CB₂ Receptors and at CB₁-CB₂ Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. https://10.3389/fphar.2018.00632.
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [CrossRef]
- Müller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [CrossRef]
- Cascio, M.G.; Gauson, L.A.; Stevenson, L.A.; Ross, R.A.; Pertwee, R.G. Evidence that the Plant Cannabinoid Cannabigerol is a Highly Potent Alpha2-Adrenoceptor Agonist and Moderately Potent 5HT1A Receptor Antagonist. Br. J. Pharmacol. 2010, 159(1), 129-141. [CrossRef]
- Seif el Nasr, M.; Nuglisch, J.; Krieglstein, J. Prevention of Ischemia-Induced Cerebral Hypothermia by Controlling the Environmental Temperature. J. Pharmacol. Toxicol. Methods 1992, 27, 23–26. [CrossRef]
- Denninger, J.K.; Smith, B.M.; Kirby, E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018, 141, 10.3791/58593. [CrossRef]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [CrossRef]
- Anuncibay-Soto, B.; Pérez-Rodríguez, D.; Santos-Galdiano, M.; Font-Belmonte, E.; Ugidos, I. F.; Gonzalez-Rodriguez, P.; Regueiro-Purriños, M.; Fernández-López, A. Salubrinal and Robenacoxib Treatment After Global Cerebral Ischemia: Exploring the Interactions Between ER Stress and Inflammation. Biochemical Pharmacology 2018, 151, 26-37. [CrossRef]
- Paxinos, G., & Franklin, K. B. J. (2004). The Mouse Brain in Stereotaxic Coordinates, (2nd ed.); Elsevier Academic Press.
- Haley, M. J.; Lawrence, C. B. The Blood-Brain Barrier After Stroke: Structural Studies and the Role of Transcytotic Vesicles. J. Cereb. Blood Flow Metab. 2017, 37 (2), 456–470. [CrossRef]
- Kho, A. R.; Choi, B. Y.; Lee, S. H.; Hong, D. K.; Lee, S. H.; Jeong, J. H.; Park, K. H.; Song, H. K.; Choi, H. C.; Suh, S. W. Effects of Protocatechuic Acid (PCA) on Global Cerebral Ischemia-Induced Hippocampal Neuronal Death. Int. J. Mol. Sci. 2018, 19 (5), 1420. [CrossRef]
| Antibodies (dilution) | Company | Code |
|---|---|---|
| Rabbit anti-Iba-1 (1:1500) | Wako Chemicals | 019-19741 |
| Rabbit anti-GFAP (1:2000) Rabbit anti-NG2 (1:200) Rabbit anti-NeuN (1:500) Rabbit anti-DCX (1:1000) Mouse anti-pBDNF (1:300) Rabbit anti-MAP-2 (1:500) Rabbit anti-β-actin (1:5000) |
Abcam Merck Millipore Abcam Cell Signaling Technology Santa Cruz Biotechnology Sigma-Aldrich Abcam |
Ab7260 AB5320 Ab177487 4604S Sc65514 M3696 Ab227387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





