Submitted:
21 March 2025
Posted:
23 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Data Collection: Clinicopathological Data
2.3. Tissue Sample Preparation
2.4. Antioxidant Assays
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Antioxidant Markers and Their Relationships with Clinicopathologic Features
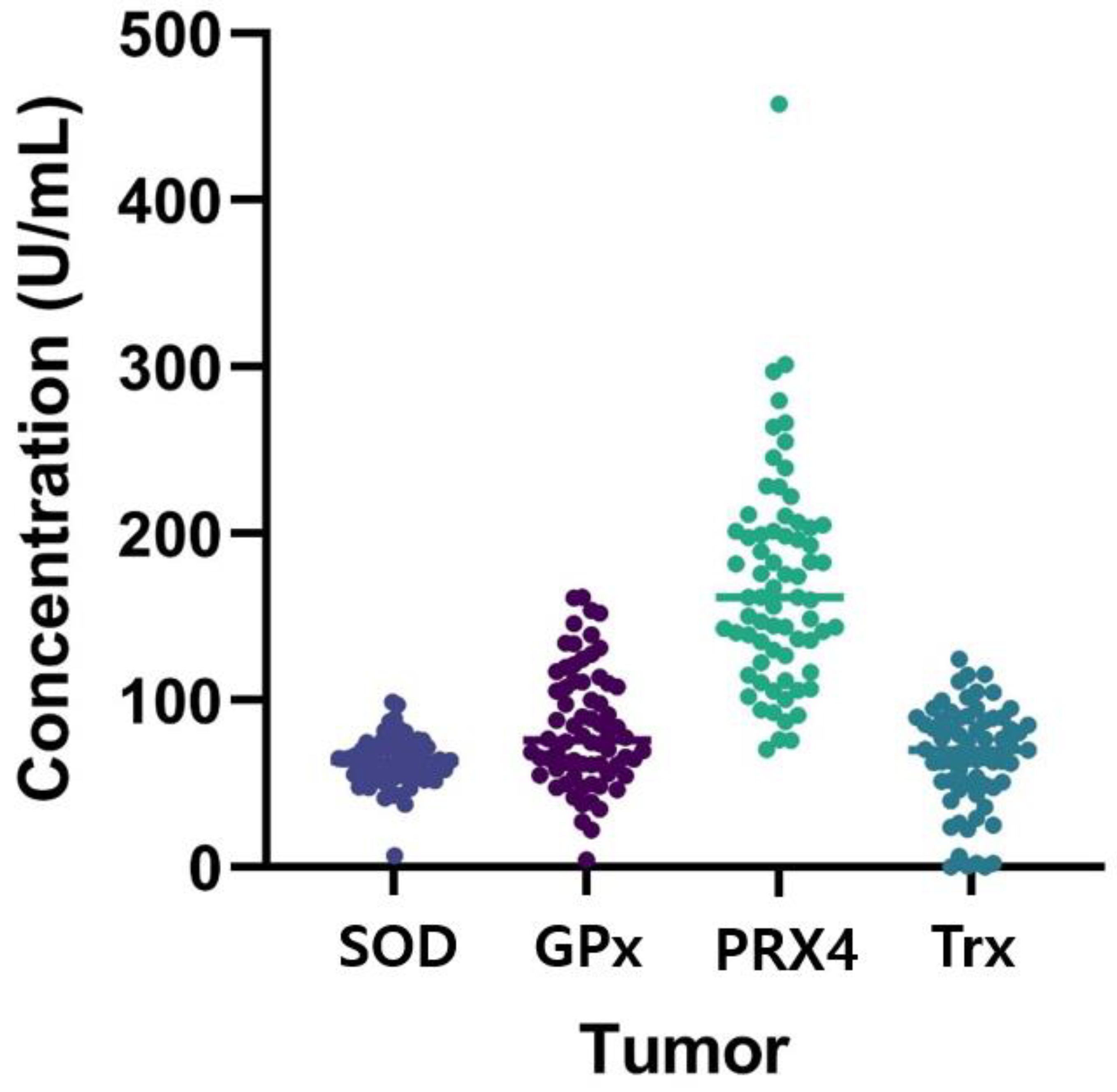
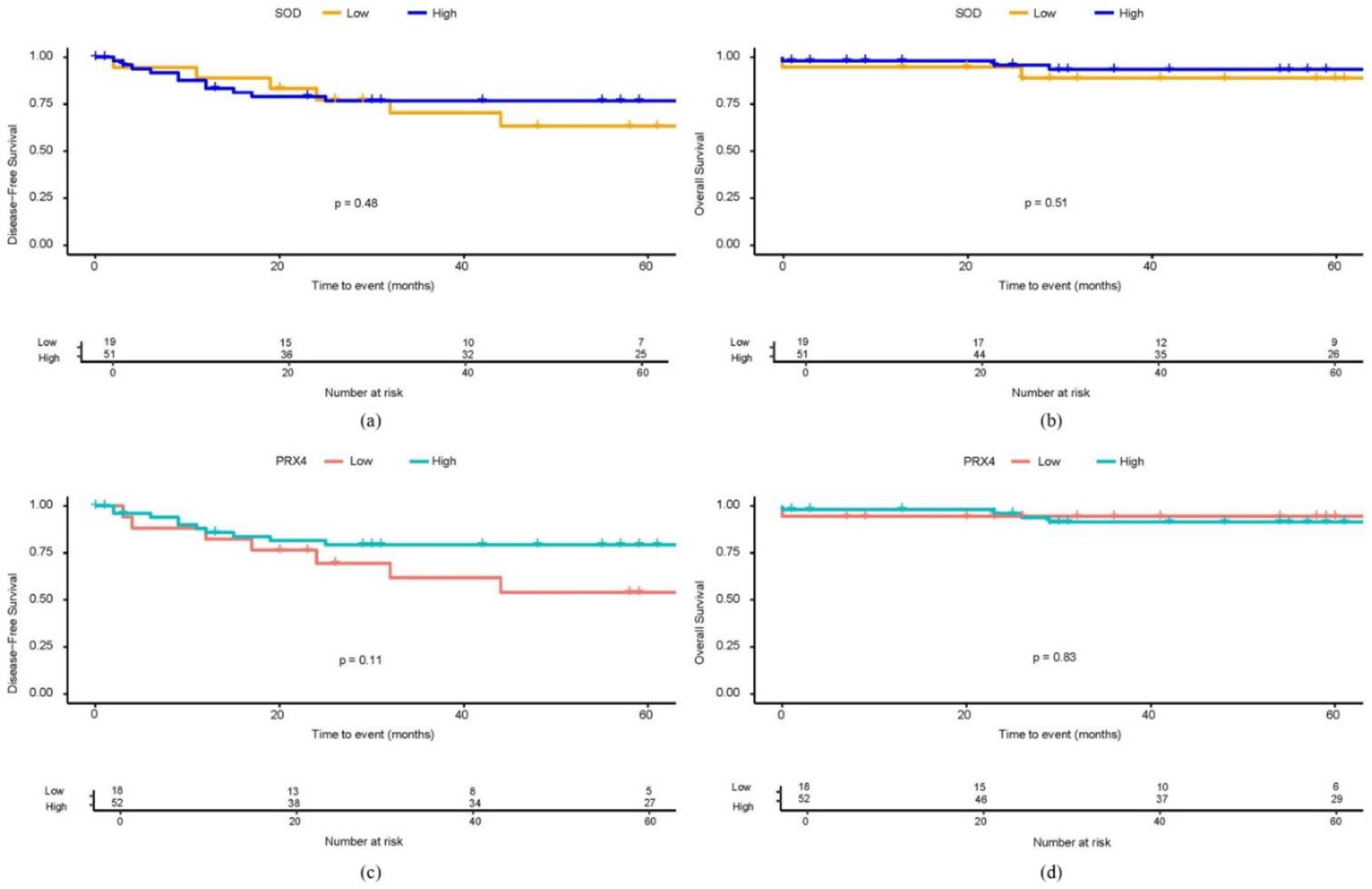
3.2.1. Superoxide Dismutase
3.2.2. Glutathione Peroxidase
3.2.3. Peroxiredoxin 4
3.2.4. Thioredoxin
3.3. Antioxidant Markers and Laboratory Findings
3.4. The Relationship Between Antioxidant Markers and Long-Term Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Kunst, N.; Alarid-Escudero, F.; Aas, E.; Coupe, V.M.H.; Schrag, D.; Kuntz, K.M. Estimating population-based recurrence rates of colorectal cancer over time in the united states. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 2710–2718. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant therapy in cancer: Rationale and progress. Antioxidants (Basel) 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.P.; Huddlestun, S.E.; Zhao, Z.Y.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra215. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Diplock, A.T.; Charleux, J.L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid, M.; Stahl, W.; Vina-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80 Suppl 1, S77–112. [Google Scholar] [CrossRef]
- Che, M.; Wang, R.; Li, X.; Wang, H.Y.; Zheng, X.F.S. Expanding roles of superoxide dismutases in cell regulation and cancer. Drug Discov. Today 2016, 21, 143–149. [Google Scholar] [CrossRef]
- Condello, M.; Meschini, S. Role of natural antioxidant products in colorectal cancer disease: A focus on a natural compound derived from prunus spinosa, trigno ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef]
- Stone, W.L.; Krishnan, K.; Campbell, S.E.; Palau, V.E. The role of antioxidants and pro-oxidants in colon cancer. World J. Gastrointest. Oncol. 2014, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yin, C.; Li, X.X.; Yang, X.Z.; Yang, Y.; Zhang, M.Y.; Wang, H.Y.; Zheng, X.F.S. Reduced sod2 expression is associated with mortality of hepatocellular carcinoma patients in a mutant p53-dependent manner. Aging (Albany NY) 2016, 8, 1184–1200. [Google Scholar] [CrossRef]
- Warsinggih; Irawan, B.; Labeda, I.; Lusikooy, R.E.; Sampetoding, S.; Kusuma, M.I.; Uwuratuw, J.A.; Syarifuddin, E.; Prihantono; Faruk, M. Association of superoxide dismutase enzyme with staging and grade of differentiation colorectal cancer: A cross-sectional study. Ann. Med. Surg. (Lond) 2020, 58, 194–199. [Google Scholar] [CrossRef]
- Thapa, P.; Ding, N.; Hao, Y.; Alshahrani, A.; Jiang, H.; Wei, Q. Essential roles of peroxiredoxin iv in inflammation and cancer. Molecules 2022, 27, 6513. [Google Scholar] [CrossRef]
- Ding, N.; Jiang, H.; Thapa, P.; Hao, Y.; Alshahrani, A.; Allison, D.; Izumi, T.; Rangnekar, V.M.; Liu, X.; Wei, Q. Peroxiredoxin iv plays a critical role in cancer cell growth and radioresistance through the activation of the akt/gsk3 signaling pathways. J. Biol. Chem. 2022, 298, 102123. [Google Scholar] [CrossRef]
- Yi, N.; Xiao, M.B.; Ni, W.K.; Jiang, F.; Lu, C.H.; Ni, R.Z. High expression of peroxiredoxin 4 affects the survival time of colorectal cancer patients, but is not an independent unfavorable prognostic factor. Mol. Clin. Oncol. 2014, 2, 767–772. [Google Scholar] [CrossRef]
- Isohookana, J.; Haapasaari, K.M.; Soini, Y.; Karihtala, P. Loss of peroxiredoxin expression is associated with an aggressive phenotype in pancreatic adenocarcinoma. Anticancer Res. 2016, 36, 427–433. [Google Scholar]
- Guo, X.; Noguchi, H.; Ishii, N.; Homma, T.; Hamada, T.; Hiraki, T.; Zhang, J.; Matsuo, K.; Yokoyama, S.; Ishibashi, H.; et al. The association of peroxiredoxin 4 with the initiation and progression of hepatocellular carcinoma. Antioxid. Redox Signal. 2019, 30, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual role of reactive oxygen species and their application in cancer therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Aboul-Enein, H.Y. Reactive oxygen and nitrogen species in carcinogenesis: Implications of oxidative stress on the progression and development of several cancer types. Mini Rev. Med. Chem. 2017, 17, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gupta Vallur, P.; Phaeton, R.; Mythreye, K.; Hempel, N. Insights into the dichotomous regulation of sod2 in cancer. Antioxidants (Basel) 2017, 6, 86. [Google Scholar] [CrossRef]
- Walton, E.L. The dual role of ros, antioxidants and autophagy in cancer. Biomed. J. 2016, 39, 89–92. [Google Scholar] [CrossRef]
- Gu, X.; Mu, C.; Zheng, R.; Zhang, Z.; Zhang, Q.; Liang, T. The cancer antioxidant regulation system in therapeutic resistance. Antioxidants (Basel) 2024, 13, 778. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The complex interplay between antioxidants and ros in cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef]
- Montero, A.J.; Diaz-Montero, C.M.; Deutsch, Y.E.; Hurley, J.; Koniaris, L.G.; Rumboldt, T.; Yasir, S.; Jorda, M.; Garret-Mayer, E.; Avisar, E.; et al. Phase 2 study of neoadjuvant treatment with nov-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with her-2 negative clinical stage ii-iiic breast cancer. Breast Cancer Res. Treat. 2012, 132, 215–223. [Google Scholar] [CrossRef]
- Bachet, J.B.; Blons, H.; Hammel, P.; Hariry, I.E.; Portales, F.; Mineur, L.; Metges, J.P.; Mulot, C.; Bourreau, C.; Cain, J.; et al. Circulating tumor DNA is prognostic and potentially predictive of eryaspase efficacy in second-line in patients with advanced pancreatic adenocarcinoma. Clin. Cancer Res. 2020, 26, 5208–5216. [Google Scholar] [CrossRef]
- Myung, S.K.; Kim, Y.; Ju, W.; Choi, H.J.; Bae, W.K. Effects of antioxidant supplements on cancer prevention: Meta-analysis of randomized controlled trials. Ann. Oncol. 2010, 21, 166–179. [Google Scholar] [CrossRef]
|
Number of patients (n=70) |
Percentage (%) |
|
| Age, mean ± SD | 69.6 ± 10.8 | |
| Gender | ||
| Male | 38 | 54.3 |
| Female | 32 | 45.7 |
| Body mass index, mean ± SD | 23.4 ± 3.5 | |
| ASA score | ||
| II | 35 | 50.0 |
| III | 35 | 50.0 |
| Medical history | ||
| None | 19 | 27.1 |
| One | 18 | 25.7 |
| Two or more | 33 | 47.1 |
| Tumor location | ||
| Right | 19 | 27.1 |
| Left | 27 | 38.6 |
| Rectum | 24 | 34.3 |
| CEA, median [IQR] | 3.1 [2.0; 9.1] | |
| Operation method | ||
| Open | 15 | 21.4 |
| MIS | 55 | 78.6 |
| T stage | ||
| Tis | 1 | 1.4 |
| 3 | 53 | 75.7 |
| 4 | 16 | 22.9 |
| N stage | ||
| 0 | 28 | 40.0 |
| 1 | 28 | 40.0 |
| 2 | 14 | 20.0 |
| M stage | ||
| 0 | 57 | 81.4 |
| 1 | 13 | 18.6 |
| TNM stage | ||
| 0 | 1 | 1.4 |
| 2 | 25 | 35.7 |
| 3 | 31 | 44.3 |
| 4 | 13 | 18.6 |
| Metastatic lymph node, mean ± SD | 2.2 ± 3.6 | |
| Harvested lymph node, mean ± SD | 24.8 ± 11.1 | |
| Tumor differentiation | ||
| Well-differentiated | 13 | 18.8 |
| Moderately differentiated | 53 | 76.8 |
| Poorly differentiated | 1 | 1.4 |
| Mucinous adenocarcinoma | 2 | 2.9 |
| Tumor size (cm), median [IQR] | 4.5 [3.5; 6.0] | |
| Microsatellite status | ||
| MSS | 63 | 94.0 |
| MSI-H | 4 | 6.0 |
| Chemotherapy | ||
| No | 24 | 34.3 |
| Yes | 46 | 65.7 |
| Radiotherapy | ||
| No | 69 | 98.6 |
| Yes | 1 | 1.4 |
| Recurrence | ||
| No | 42 | 60.0 |
| Yes | 17 | 24.3 |
| Death | ||
| No | 45 | 64.3 |
| Yes | 5 | 7.1 |
| SOD | GPx | PRX4 | Trx | |||||||||
| Low (19) | High (51) | p | Low (n=18) | High (52) | p | Low (18) | High (52) | p | Low (17) | High (50) | p | |
| Age | 66.1 ± 13.3 | 71.0 ± 9.5 | 0.09 | 69.8 ± 11.6 | 69.6 ± 10.6 | 0.95 | 65.6 ± 14.6 | 71.1 ± 8.8 | 0.15 | 69.5 ± 13.0 | 69.5 ± 10.2 | 1.00 |
| Gender | 0.24 | 0.49 | 1.00 | 0.53 | ||||||||
| F | 6 (31.6%) | 26 (51.0%) | 10 (55.6%) | 22 (42.3%) | 8 (44.4%) | 24 (46.2%) | 6 (35.3%) | 24 (48.0%) | ||||
| M | 13 (68.4%) | 25 (49.0%) | 8 (44.4%) | 30 (57.7%) | 10 (55.6%) | 28 (53.8%) | 11 (64.7%) | 26 (52.0%) | ||||
| BMI | 22.5 ± 4.0 | 23.7 ± 3.3 | 0.19 | 23.6 ± 3.6 | 23.3 ± 3.5 | 0.74 | 22.8 ± 4.2 | 23.6 ± 3.3 | 0.39 | 24.0 ± 3.8 | 23.1 ± 3.5 | 0.37 |
| ASA classification | 0.59 | 0.41 | 0.41 | 0.94 | ||||||||
| II | 8 (42.1%) | 27 (52.9%) | 11 (61.1%) | 24 (46.2%) | 7 (38.9%) | 28 (53.8%) | 9 (52.9%) | 24 (48.0%) | ||||
| III | 11 (57.9%) | 24 (47.1%) | 7 (38.9%) | 28 (53.8%) | 11 (61.1%) | 24 (46.2%) | 8 (47.1%) | 26 (52.0%) | ||||
| Medical History | 0.57 | 0.50 | 0.32 | 0.57 | ||||||||
| none | 6 (31.6%) | 13 (25.5%) | 3 (16.7%) | 16 (30.8%) | 7 (38.9%) | 12 (23.1%) | 6 (35.3%) | 13 (26.0%) | ||||
| one | 6 (31.6%) | 12 (23.5%) | 5 (27.8%) | 13 (25.0%) | 5 (27.8%) | 13 (25.0%) | 3 (17.6%) | 15 (30.0%) | ||||
| ≥2 | 7 (36.8%) | 26 (51.0%) | 10 (55.6%) | 23 (44.2%) | 6 (33.3%) | 27 (51.9%) | 8 (47.1%) | 22 (44.0%) | ||||
| Tumor Location | 0.27 | 1.00 | 0.77 | 0.35 | ||||||||
| Rt. | 3 (15.8%) | 16 (31.4%) | 5 (27.8%) | 14 (26.9%) | 6 (33.3%) | 13 (25.0%) | 4 (23.5%) | 15 (30.0%) | ||||
| Lt. | 10 (52.6%) | 17 (33.3%) | 7 (38.9%) | 20 (38.5%) | 6 (33.3%) | 21 (40.4%) | 5 (29.4%) | 21 (42.0%) | ||||
| Rectum | 6 (31.6%) | 18 (35.3%) | 6 (33.3%) | 18 (34.6%) | 6 (33.3%) | 18 (34.6%) | 8 (47.1%) | 14 (28.0%) | ||||
| CEA | 3.8 [ 2.0;19.6] | 2.7 [ 2.0; 7.5] | 0.52 | 2.3 [ 2.0; 4.5] | 3.8 [ 2.0;12.9] | 0.13 | 3.4 [ 2.0;16.6] | 2.9 [ 2.0; 7.1] | 0.68 | 2.3 [ 2.0; 4.2] | 3.9 [ 2.0; 9.1] | 0.33 |
| T stage | 0.49 | 0.31 | 0.01 | 0.05 | ||||||||
| Tis | 0 (0%) | 1 (2%) | 0 (0%) | 1 (1.9%) | 0 (0%) | 1 (1.9%) | 1 (5.9%) | 0 (0%) | ||||
| 3 | 13 (68.4%) | 41 (78.4%) | 16 (88.9%) | 37 (71.2%) | 9 (50.0%) | 45 (86.5%) | 16 (88.2%) | 36 (72.0%) | ||||
| 4 | 6 (31.6%) | 10 (19.6%) | 2 (11.1%) | 14 (26.9%) | 9 (50.0%) | 7 (13.5%) | 1 (5.9%) | 14 (28.0%) | ||||
| N stage | 0.95 | 0.20 | 0.60 | 0.54 | ||||||||
| 0 | 7 (36.8%) | 21 (41.2%) | 8 (44.4%) | 20 (38.5%) | 6 (33.3%) | 22 (42.3%) | 9 (52.9%) | 19 (38.0%) | ||||
| 1 | 8 (42.1%) | 20 (39.2%) | 9 (50.0%) | 19 (36.5%) | 7 (38.9%) | 21 (40.4%) | 5 (29.4%) | 21 (42.0%) | ||||
| 2 | 4 (21.1%) | 10 (19.6%) | 1 (5.6%) | 13 (25.0%) | 5 (27.8%) | 9 (17.3%) | 3 (17.6%) | 10 (20.0%) | ||||
| M stage | 0.04 | 1.00 | 0.03 | 1.00 | ||||||||
| 0 | 12 (63.2%) | 45 (88.2%) | 15 (83.3%) | 42 (80.8%) | 11 (61.1%) | 46 (88.5%) | 14 (82.4%) | 40 (80.0%) | ||||
| 1 | 7 (36.8%) | 6 (11.8%) | 3 (16.7%) | 10 (19.2%) | 7 (38.9%) | 6 (11.5%) | 3 (17.6%) | 10 (20.0%) | ||||
| TNM Stage | 0.11 | 0.89 | 0.08 | 0.35 | ||||||||
| 0 | 0 (0.0%) | 1 (2.0%) | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 1 (1.9%) | 1 (5.9%) | 0 (0.0%) | ||||
| 2 | 6 (31.6%) | 19 (37.3%) | 6 (33.3%) | 19 (36.5%) | 5 (27.8%) | 20 (38.5%) | 7 (41.2%) | 18 (36.0%) | ||||
| 3 | 6 (31.6%) | 25 (49.0%) | 9 (50.0%) | 22 (42.3%) | 6 (33.3%) | 25 (48.1%) | 6 (35.3%) | 22 (44.0%) | ||||
| 4 | 7 (36.8%) | 6 (11.8%) | 3 (16.7%) | 10 (19.2%) | 7 (38.9%) | 6 (11.5%) | 3 (17.6%) | 10 (20.0%) | ||||
| Metastatic LNs | 1.0 [ 0.0; 3.0] | 1.0 [ 0.0; 3.0] | 0.8 | 0.5 [ 0.0; 2.0] | 1.0 [ 0.0; 3.5] | 0.41 | 1.5 [ 0.0; 4.0] | 1.0 [ 0.0; 3.0] | 0.44 | 0.0 [ 0.0; 3.0] | 1.0 [ 0.0; 3.0] | 0.56 |
| Harvested LNs | 24.0 [19.0;29.0] | 24.0 [16.5;30.0] | 0.71 | 24.0 [19.0;32.0] | 24.0 [16.5;30.0] | 0.53 | 23.5 [19.0;26.0] | 25.0 [17.0;31.0] | 0.24 | 21.0 [14.0;26.0] | 24.5 [18.0;33.0] | 0.09 |
| Differentiation | 1.00 | 1.00 | 0.02 | 0.75 | ||||||||
| Good (wd/md) | 18 (94.7%) | 48 (96.0%) | 17 (94.4%) | 49 (96.1%) | 15 (83.3%) | 51 (100.0%) | 16 (100.0%) | 47 (94.0%) | ||||
| Poor (pd/mucinous) | 1 (5.3%) | 2 (4.0%) | 1 (5.6%) | 2 (3.9%) | 3 (16.7%) | 0 (0.0%) | 0 (0.0%) | 3 (6.0%) | ||||
| Tumor size | 5.3 [ 4.5; 6.1] | 4.5 [ 3.2; 5.6] | 0.14 | 4.5 [ 3.2; 6.5] | 4.5 [ 4.0; 6.0] | 0.85 | 4.8 [ 4.5; 6.2] | 4.5 [ 3.1; 6.0] | 0.40 | 4.6 [ 4.5; 5.5] | 4.8 [ 3.2; 6.0] | 0.80 |
| Lymphatic invasion | 0.24 | 0.34 | 0.21 | 0.72 | ||||||||
| 0 | 13 (68.4%) | 25 (49.0%) | 12 (66.7%) | 26 (50.0%) | 7 (38.9%) | 31 (59.6%) | 8 (47.1%) | 28 (56.0%) | ||||
| 1 | 6 (31.6%) | 26 (51.0%) | 6 (33.3%) | 26 (50.0%) | 11 (61.1%) | 21 (40.4%) | 9 (52.9%) | 22 (44.0%) | ||||
| Venous invasion | 1 | 0.78 | 0.12 | 0.24 | ||||||||
| 0 | 17 (89.5%) | 46 (90.2%) | 17 (94.4%) | 46 (88.5%) | 14 (77.8%) | 49 (94.2%) | 17 (100.0%) | 43 (86.0%) | ||||
| 1 | 2 (10.5%) | 5 (9.8%) | 1 (5.6%) | 6 (11.5%) | 4 (22.2%) | 3 (5.8%) | 0 (0.0%) | 7 (14.0%) | ||||
| Perineural invasion | 0.52 | 1.00 | 0.83 | 1.00 | ||||||||
| 0 | 12 (63.2%) | 38 (74.5%) | 13 (72.2%) | 37 (71.2%) | 12 (66.7%) | 38 (73.1%) | 12 (70.6%) | 37 (74.0%) | ||||
| 1 | 7 (36.8%) | 13 (25.5%) | 5 (27.8%) | 15 (28.8%) | 6 (33.3%) | 14 (26.9%) | 5 (29.4%) | 13 (26.0%) | ||||
| Microsatellite status | 0.5 | 1.00 | 0.08 | 1.00 | ||||||||
| MSS | 18 (100.0%) | 45 (91.8%) | 16 (94.1%) | 47 (94.0%) | 14 (82.4%) | 49 (98.0%) | 15 (93.8%) | 45 (93.8%) | ||||
| MSI-H | 0 (0.0%) | 4 (8.2%) | 1 (5.9%) | 3 (6.0%) | 3 (17.6%) | 1 (2.0%) | 1 (6.2%) | 3 (6.2%) | ||||
| KRAS | 0.26 | 0.04 | 0.49 | 0.73 | ||||||||
| Wild | 13 (72.2%) | 26 (53.1%) | 14 (82.4%) | 25 (50.0%) | 11 (68.8%) | 28 (54.9%) | 9 (52.9%) | 29 (61.7%) | ||||
| Mutant | 5 (27.8%) | 23 (46.9%) | 3 (17.6%) | 25 (50.0%) | 5 (31.2%) | 23 (45.1%) | 8 (47.1%) | 18 (38.3%) | ||||
| NRAS | 1 | 1.00 | 1.00 | 1.00 | ||||||||
| Wild | 12 (100.0%) | 35 (94.6%) | 10 (100.0%) | 37 (94.9%) | 9 (100.0%) | 38 (95.0%) | 11 (100.0%) | 34 (94.4%) | ||||
| Mutant | 0 (0.0%) | 2 (5.4%) | 0 (0.0%) | 2 (5.1%) | 0 (0.0%) | 2 (5.0%) | 0 (0.0%) | 2 (5.6%) | ||||
| BRAF | 0.66 | 1.00 | 1.00 | 1.00 | ||||||||
| Wild | 18 (100.0%) | 44 (93.6%) | 16 (94.1%) | 46 (95.8%) | 15 (93.8%) | 47 (95.9%) | 16 (94.1%) | 44 (97.8%) | ||||
| Mutant | 0 (0.0%) | 3 (6.4%) | 1 (5.9%) | 2 (4.2%) | 1 (6.2%) | 2 (4.1%) | 1 (5.9%) | 1 (2.2%) | ||||
| Chemotherapy | 0.58 | 0.70 | 1.00 | 0.16 | ||||||||
| None | 8 (42.1%) | 16 (31.4%) | 5 (27.8%) | 19 (36.5%) | 6 (33.3%) | 18 (34.6%) | 9 (52.9%) | 15 (30.0%) | ||||
| Done | 11 (57.9%) | 35 (68.6%) | 13 (72.2%) | 33 (63.5%) | 12 (66.7%) | 34 (65.4%) | 8 (47.1%) | 35 (70.0%) | ||||
| Radiotherapy | 1 | 0.58 | 1.00 | 1.00 | ||||||||
| None | 19 (100.0%) | 50 (98.0%) | 17 (94.4%) | 52 (100.0%) | 18 (100.0%) | 51 (98.1%) | 17 (100.0%) | 49 (98.0%) | ||||
| Done | 0 (0.0%) | 1 (2.0%) | 1 (5.6%) | 0 (0.0%) | 0 (0.0%) | 1 (1.9%) | 0 (0.0%) | 1 (2.0%) | ||||
| SOD | GPx | PRX4 | Trx | |||||||||
| Low (19) | High (51) | p | Low (n=18) | High (52) | p | Low (18) | High (52) | p | Low (17) | High (50) | p | |
| WBC | 7.5 [ 6.0; 9.4] | 6.6 [ 5.7; 8.7] | 0.229 | 7.1 [ 5.9;10.8] | 6.9 [ 5.6; 9.1] | 0.506 | 7.5 [ 6.4; 9.4] | 6.6 [ 5.2; 9.1] | 0.134 | 7.6 [ 6.9;10.1] | 6.6 [ 5.5; 9.1] | 0.073 |
| Hb | 11.9 [10.1;13.5] | 12.4 [10.2;13.6] | 0.88 | 12.6 [10.0;13.3] | 12.1 [10.2;13.8] | 0.925 | 11.8 [10.6;12.7] | 12.4 [9.9;13.9] | 0.662 | 12.6 [9.7;14.3] | 11.8 [10.2;13.3] | 0.67 |
| PLT | 294.0 [235.0;331.5] | 259.0 [209.5;328.0] | 0.219 | 269.5 [204.0;315.0] | 259.0 [222.5;331.5] | 0.6 | 253.5 [204.0;318.0] | 275.5 [219.0;331.0] | 0.432 | 259.0 [237.0;326.0] | 269.5 [215.0;330.0] | 0.908 |
| Neutrophil | 5.0 [ 3.9; 7.7] | 4.7 [ 3.1; 6.4] | 0.2 | 5.5 [ 3.1; 8.1] | 4.7 [ 3.4; 6.0] | 0.432 | 5.6 [ 4.8; 7.6] | 4.3 [ 3.1; 6.4] | 0.027 | 5.6 [ 4.6; 7.8] | 4.8 [ 3.3; 6.8] | 0.115 |
| Lymphocyte | 1.4 [1.2;1.7] | 1.6 [1.1;1.9] | 0.5 | 1.5 [1.0;1.9] | 1.5 [1.2;1.9] | 0.536 | 1.3 [1.0;1.8] | 1.6 [1.3;1.9] | 0.173 | 1.7 [1.3;1.9] | 1.4 [1.1;1.8] | 0.503 |
| NLR | 3.6 [ 2.5; 5.6] | 2.8 [ 2.2; 4.4] | 0.348 | 3.7 [ 2.1; 6.2] | 2.9 [ 2.3; 4.3] | 0.268 | 4.3 [ 3.6; 5.8] | 2.6 [ 2.0; 4.1] | 0.003 | 3.8 [ 2.6; 5.4] | 3.0 [ 2.2; 4.5] | 0.29 |
| PLR | 228.3 [153.2;268.9] | 160.8 [132.7;269.7] | 0.413 | 166.7 [131.3;335.4] | 200.0 [132.7;269.1] | 0.804 | 209.0 [149.4;270.2] | 162.7 [128.9;273.6] | 0.532 | 172.5 [140.5;263.8] | 209.0 [134.7;278.0] | 0.724 |
| CRP | 1.2 [ 0.5; 1.9] | 0.4 [ 0.3; 1.2] | 0.095 | 0.9 [ 0.3; 1.8] | 0.4 [ 0.3; 1.5] | 0.423 | 0.6 [ 0.3; 1.7] | 0.7 [ 0.3; 1.7] | 0.727 | 0.6 [ 0.3; 1.1] | 0.7 [ 0.3; 1.8] | 0.842 |
| Albumin | 3.9 [3.5;4.2] | 3.8 [3.5;4.2] | 0.89 | 3.8 [3.2;4.3] | 3.9 [3.5;4.2] | 0.691 | 3.8 [3.7;4.2] | 3.9 [3.3;4.3] | 0.366 | 4.2 [3.6;4.3] | 3.8 [3.4;4.3] | 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





