Submitted:
20 March 2025
Posted:
21 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Preparation of MoRu-NG Composite
2.2. Preparation of Mo-NG and Ru-NG Composites
2.3. Preparation of A-MR、B-MR and C-MR Composites
2.4. Materials Characterization
3. Result
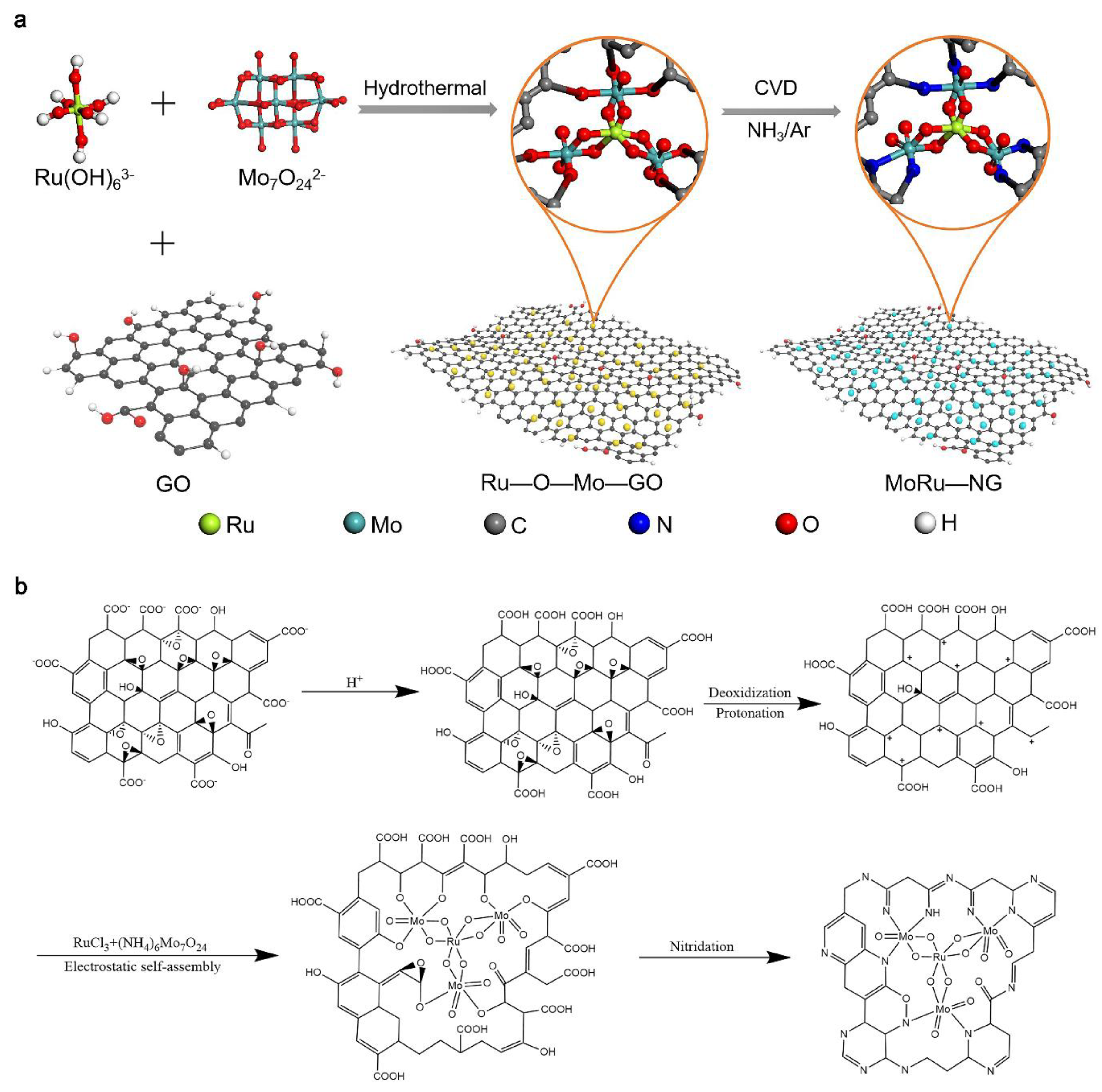
3.1. Synthesis of MoRu-NG
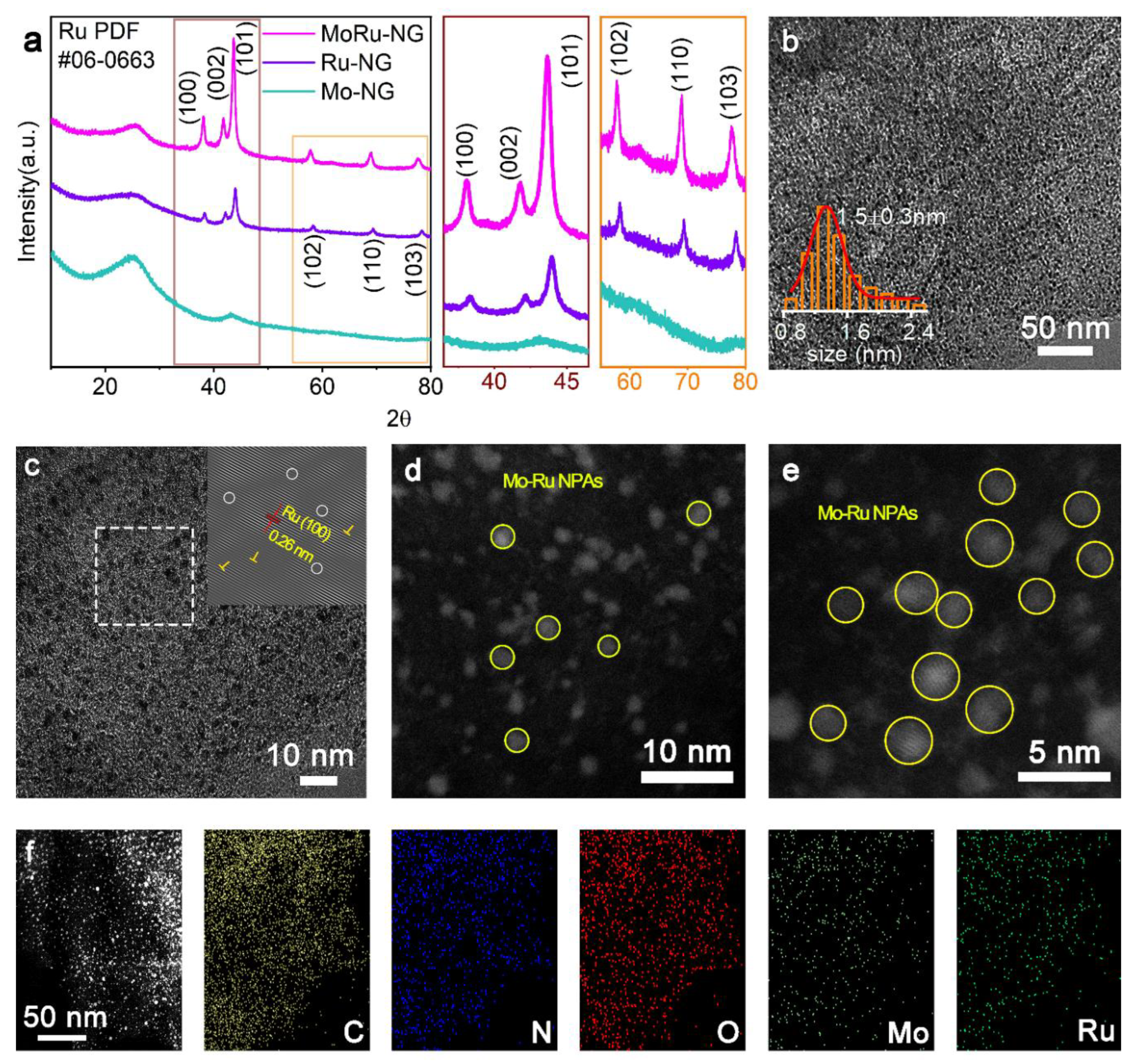
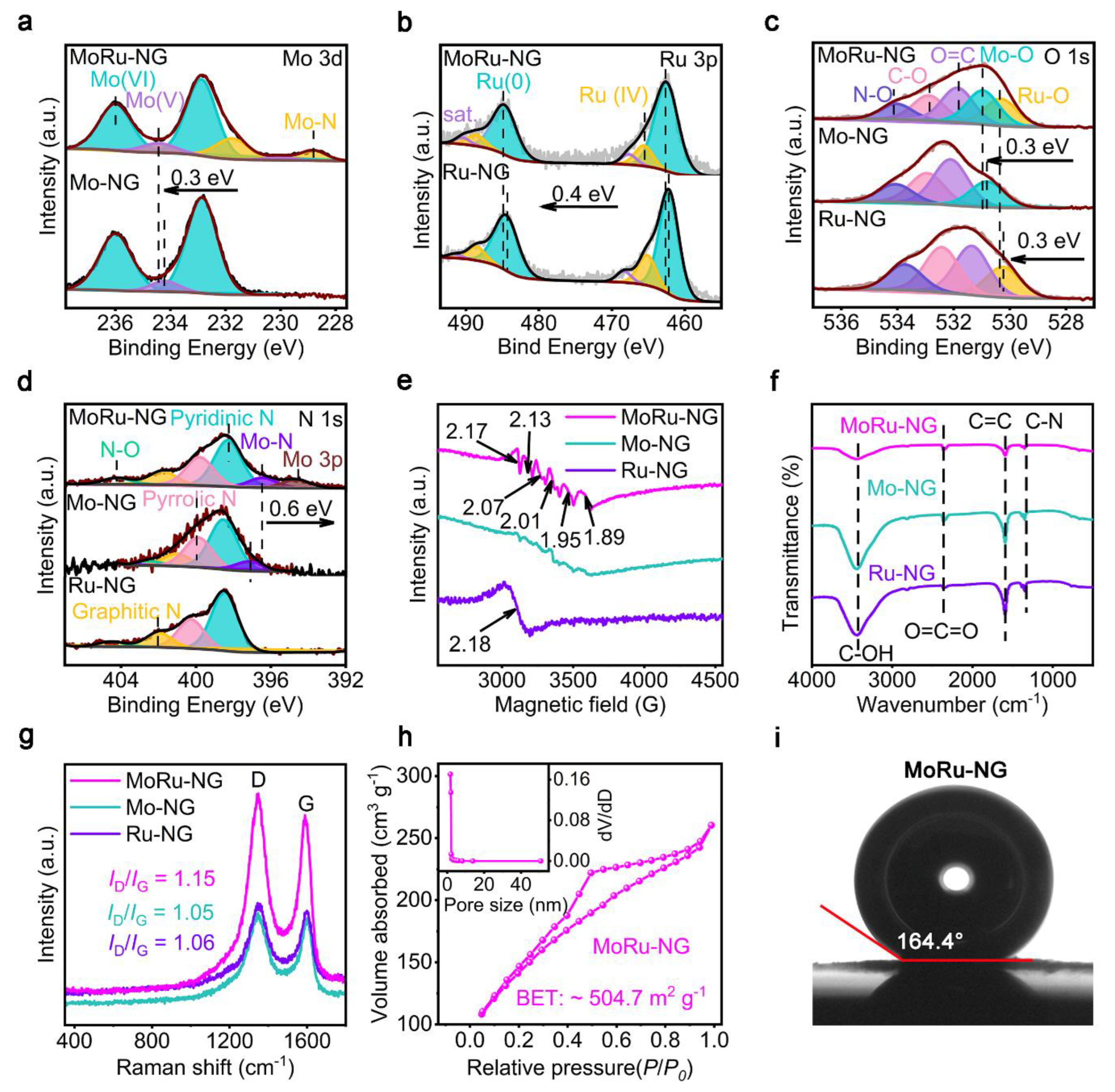
3.2. Structural Characterization of MoRu-NG
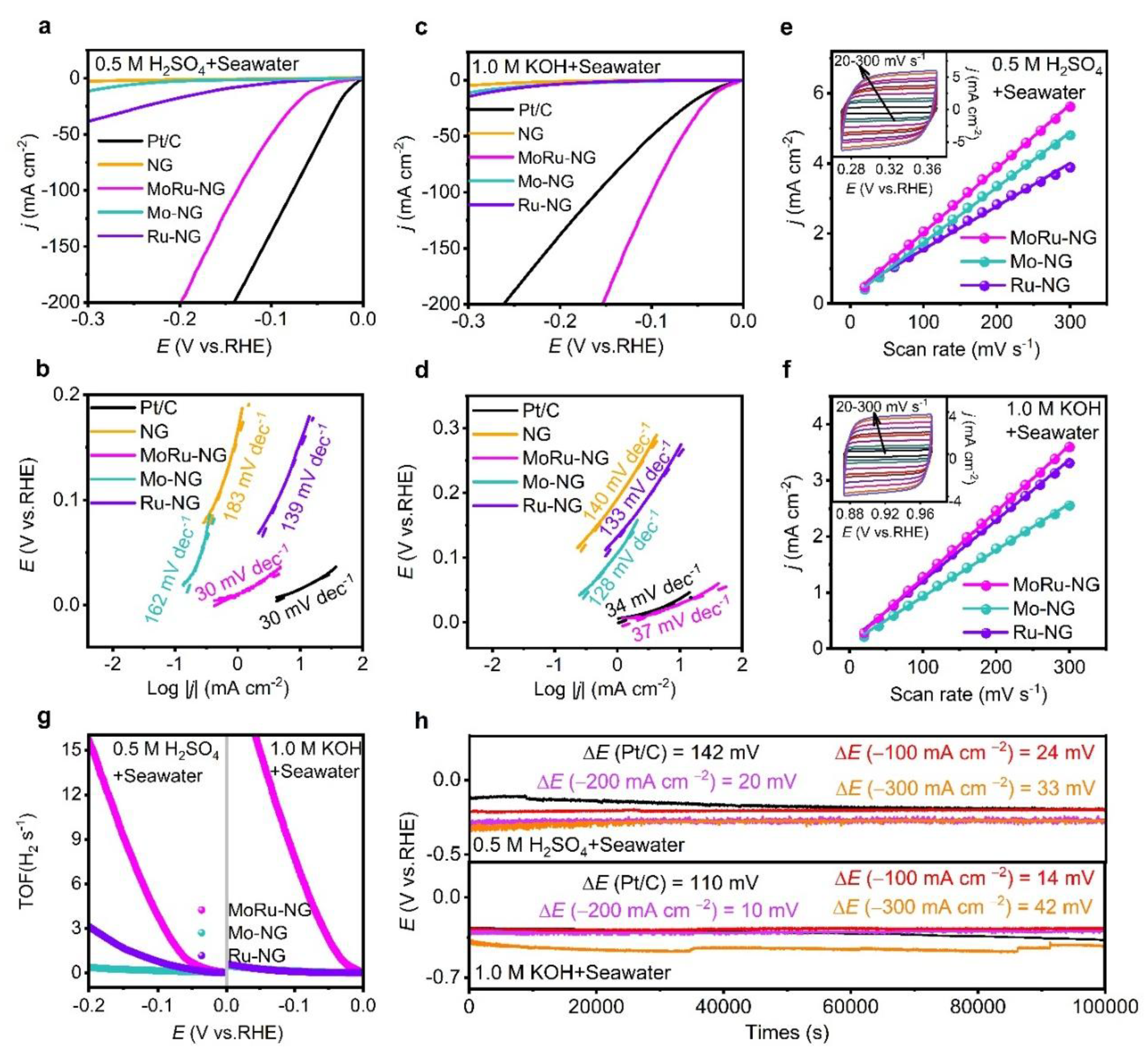
3.3. Electrocatalytic HER Performance
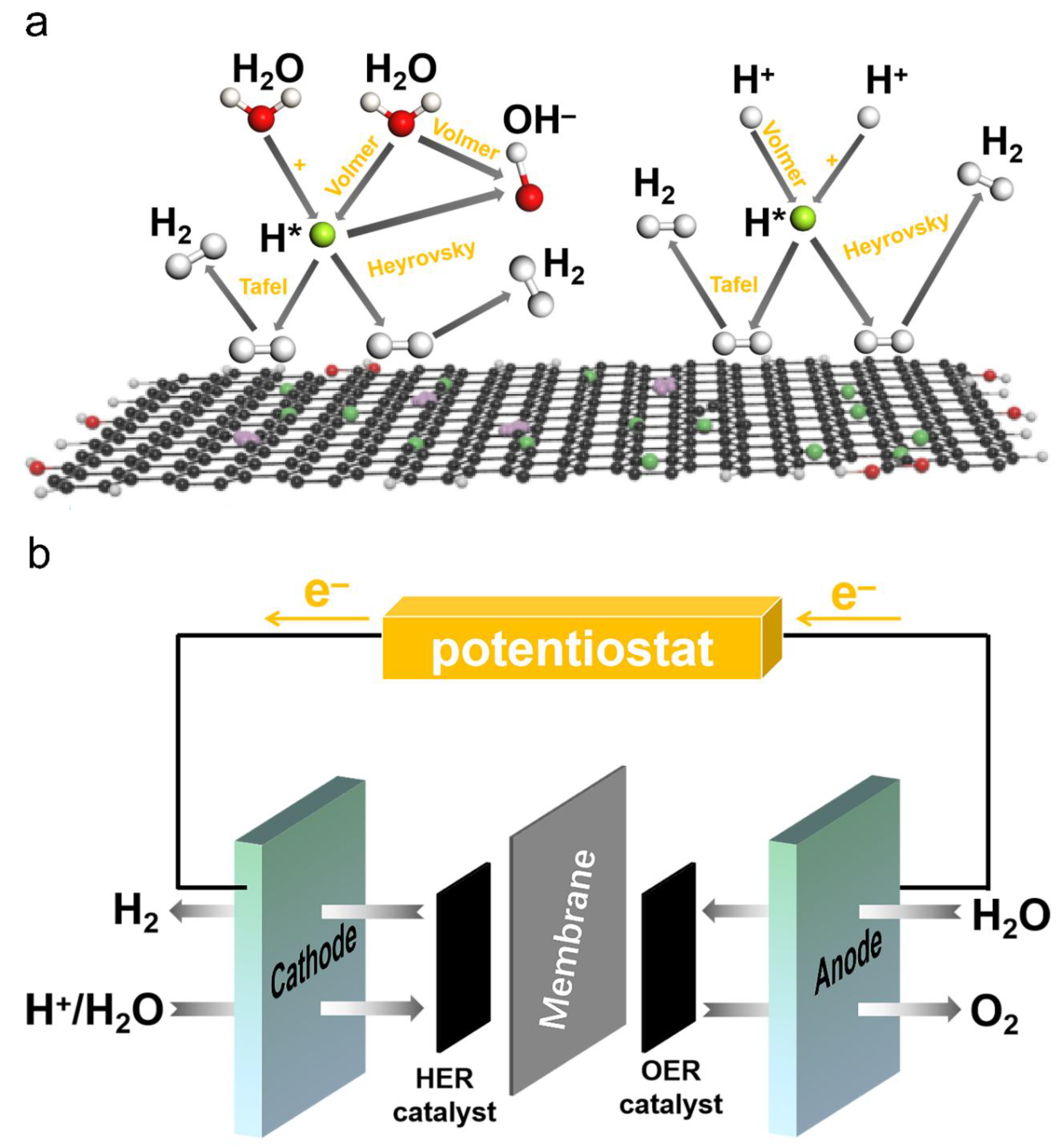
3.4. Mechanism of HER and Schematic Representation of Seawater Electrocatalysis.
Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cai, Z.; Liang, J.; Li, Z.; Yan, T.; Yang, C.; Sun, S.; Yue, M.; Liu, X.; Xie, T.; Wang, Y.; et al. Stabilizing NiFe sites by high-dispersity of nanosized and anionic Cr species toward durable seawater oxidation. Nat. Commun. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Li, L.; Bu, L.; Huang, B.; Wang, P.; Shen, C.; Bai, S.; Chan, T.; Shao, Q.; Hu, Z.; Huang, X. Compensating Electronic Effect Enables Fast Site-to-Site Electron Transfer over Ultrathin RuMn Nanosheet Branches toward Highly Electroactive and Stable Water Splitting. Adv. Mater. 2021, 33, 2105308. [Google Scholar] [CrossRef]
- Xue, Y.; Shi, L.; Liu, X.; Fang, J.; Wang, X.; Setzler, B.P.; Zhu, W.; Yan, Y.; Zhuang, Z. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Cheng, G.; Luo, W. Phosphorus-Induced Activation of Ruthenium for Boosting Hydrogen Oxidation and Evolution Electrocatalysis. ACS Catal. 2020, 10, 11751–11757. [Google Scholar] [CrossRef]
- Zhao, T.; Xiao, D.; Chen, Y.; Tang, X.; Gong, M.; Deng, S.; Liu, X.; Ma, J.; Zhao, X.; Wang, D. Boosting alkaline hydrogen electrooxidation on an unconventional fcc-Ru polycrystal. J. Energy Chem. 2021, 61, 15–22. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Z.; Jiang, J.; Wang, J.; Song, X.; He, Q.; Ding, W.; Wei, Z. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 2020, 3, 454–462. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Cheng, C. Ru-based electrocatalysts for hydrogen evolution reaction:Recent research advances and perspectives. Mater. Today Phys. 2020, 15. [Google Scholar] [CrossRef]
- F. Dionigi, T. Reier, Z. Pawolek, M. Gliech, P. Strasser, Design Criteria, Operating Conditions, and Nickel-Iron Hydroxide Catalyst Materials for Selective Seawater Electrolysis, ChemSusChem, 9 (2016) 962-972.
- Li, J.; Jurasz, J.; Li, H.; Tao, W.-Q.; Duan, Y.; Yan, J. A new indicator for a fair comparison on the energy performance of data centers. Appl. Energy 2020, 276, 115497. [Google Scholar] [CrossRef]
- Haq, T.U.; Haik, Y. Strategies of Anode Design for Seawater Electrolysis: Recent Development and Future Perspective. Small Sci. 2022, 2. [Google Scholar] [CrossRef]
- H.J. Song, H. Yoon, B. Ju, D.-Y. Lee, D.-W. Kim, Electrocatalytic Selective Oxygen Evolution of Carbon-Coated Na2Co1–xFexP2O7 Nanoparticles for Alkaline Seawater Electrolysis, ACS Catalysis, 10 (2020) 702-709.
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.-Q.; Ding, R.; Zeng, Z.-W.; Liu, B.-W.; Zeng, F.-R.; Wang, Y.-Z.; Zhao, H.-B. Satellite-like shielding for dual single-atom catalysis, boosting ampere-level alkaline seawater splitting. Matter 2024, 7, 3189–3204. [Google Scholar] [CrossRef]
- Kang, X.; Yang, F.; Zhang, Z.; Liu, H.; Ge, S.; Hu, S.; Li, S.; Luo, Y.; Yu, Q.; Liu, Z.; et al. A corrosion-resistant RuMoNi catalyst for efficient and long-lasting seawater oxidation and anion exchange membrane electrolyzer. Nat. Commun. 2023, 14, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, Y.; Zheng, X.; Aoki, T.; Pattengale, B.; Huang, J.; He, X.; Bian, W.; Younan, S.; Williams, N.; et al. Atomically engineering activation sites onto metallic 1T-MoS2 catalysts for enhanced electrochemical hydrogen evolution. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.; Urrego-Ortiz, R.; Liao, N.; Calle-Vallejo, F.; Luo, J. Rationally designed Ru catalysts supported on TiN for highly efficient and stable hydrogen evolution in alkaline conditions. Nat. Commun. 2024, 15, 1–14. [Google Scholar] [CrossRef]
- Radovic, L.R.; Bockrath, B. On the Chemical Nature of Graphene Edges: Origin of Stability and Potential for Magnetism in Carbon Materials. J. Am. Chem. Soc. 2005, 127, 5917–5927. [Google Scholar] [CrossRef]
- Zhang, G.; Pei, J.; Wang, Y.; Wang, G.; Wang, Y.; Liu, W.; Xu, J.; An, P.; Huang, H.; Zheng, L.; et al. Selective Activation of Lattice Oxygen Site Through Coordination Engineering to Boost the Activity and Stability of Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2024, 63, e202407509. [Google Scholar] [CrossRef]
- Müller, A.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very Large ClustersNanoscale Magnets. Chem. Rev. 1998, 98, 239–272. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Li, L.; Sakthivel, T.; Guo, Z.; Dai, Z. Intensifying the Supported Ruthenium Metallic Bond to Boost the Interfacial Hydrogen Spillover Toward pH-Universal Hydrogen Evolution Catalysis. Adv. Funct. Mater. 2024, 34. [Google Scholar] [CrossRef]
- Maji, R.; Mallojjala, S.C.; Wheeler, S.E. Electrostatic Interactions in Asymmetric Organocatalysis. Accounts Chem. Res. 2023, 56, 1990–2000. [Google Scholar] [CrossRef]
- Yi, J.; Zhan, S.; Chen, L.; Tian, Q.; Wang, N.; Li, J.; Xu, W.; Zhang, B.; Ahlquist, M.S.G. Electrostatic Interactions Accelerating Water Oxidation Catalysis via Intercatalyst O–O Coupling. J. Am. Chem. Soc. 2021, 143, 2484–2490. [Google Scholar] [CrossRef]
- Xu, X.-L.; Wang, N.-N.; Zou, Y.-H.; Qin, X.; Wang, P.; Lu, X.-Y.; Zhang, X.-Y.; Sun, W.-Y.; Lu, Y. N, N’-bidentate ligand anchored palladium catalysts on MOFs for efficient Heck reaction. Nat. Commun. 2024, 15, 1–10. [Google Scholar] [CrossRef]
- Yun, Y.; Zeng, H.; Li, L.; Li, H.; Cheng, S.; Sun, N.; Li, M.; Sheng, H.; Hu, S.; Yao, T.; et al. Matching Bidentate Ligand Anchoring: an Accurate Control Strategy for Stable Single-Atom/ZIF Nanocatalysts. Adv. Mater. 2022, 35, e2209561. [Google Scholar] [CrossRef]
- Sun, W.; Kuang, T.; Wei, G.; Li, Y.; Liu, Y.; Lyu, S.; Zhang, Y.; Li, J.; Wang, L. Design and construction of size-controlled CoO/CS catalysts for Fischer-Tropsch synthesis. Nano Res. 2023, 17, 2520–2527. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Meng, F.; Peng, H.; Lyu, X.; Zhu, M.; Wang, Y.; Lu, C.; Liu, J.; Lin, T.; et al. Unveiling Strong Ion–Electron–Lattice Coupling and Electronic Antidoping in Hydrogenated Perovskite Nickelate. Adv. Mater. 2023, 35, e2300617. [Google Scholar] [CrossRef] [PubMed]
- H.X. Liu, J.Y. Li, X. Qin, C. Ma, W.W. Wang, K. Xu, H. Yan, D. Xiao, C.J. Jia, Q. Fu, D. Ma, Pt(n)-O(v) synergistic sites on MoO(x)/γ-Mo(2)N heterostructure for low-temperature reverse water-gas shift reaction, Nature communications, 13 (2022) 5800.
- Zhao, Z.; Sun, J.; Li, X.; Qin, S.; Li, C.; Zhang, Z.; Li, Z.; Meng, X. Engineering active and robust alloy-based electrocatalyst by rapid Joule-heating toward ampere-level hydrogen evolution. Nat. Commun. 2024, 15, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Tan, G.; Duan, X.; Yuan, B.; Sendeku, M.G.; Liu, H.; Li, T.; Wang, F.; Kuang, Y.; et al. Single atomic Ru in TiO2 boost efficient electrocatalytic water oxidation to hydrogen peroxide. Sci. Bull. 2023, 68, 613–621. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.; Cheng, Y.; Chen, X.; Zhuansun, M.; Wang, T.; Li, J.; Meira, D.; Sun, H.; Wei, J.; et al. Enriched electrophilic oxygen species facilitate acidic oxygen evolution on Ru-Mo binary oxide catalysts. Nano Res. 2023, 17, 1165–1172. [Google Scholar] [CrossRef]
- Shi, H.; Yang, Y.; Meng, P.; Yang, J.; Zheng, W.; Wang, P.; Zhang, Y.; Chen, X.; Cheng, Z.; Zong, C.; et al. Local Charge Transfer Unveils Antideactivation of Ru at High Potentials for the Alkaline Hydrogen Oxidation Reaction. J. Am. Chem. Soc. 2024, 146, 16619–16629. [Google Scholar] [CrossRef] [PubMed]
- Taratanov, N.A.; Yurkov, G.Y.; Fionov, A.S.; Koksharov, Y.A.; Popkov, O.V.; Kolesov, V.V. Creation and physical properties of the molybdenum-containing polyethylene-based nanomaterials. J. Commun. Technol. Electron. 2009, 54, 937–946. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Qu, J.; Liu, H. Tungsten-Assisted Phase Tuning of Molybdenum Carbide for Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 2451–2459. [Google Scholar] [CrossRef]
- Ma, D.; Shu, Y.; Bao, X.; Xu, Y. Methane Dehydro-aromatization under Nonoxidative Conditions over Mo/HZSM-5 Catalysts: EPR Study of the Mo Species on/in the HZSM-5 Zeolite. J. Catal. 2000, 189, 314–325. [Google Scholar] [CrossRef]
- Liang, H.-W.; Wei, W.; Wu, Z.-S.; Feng, X.; Müllen, K. Mesoporous Metal–Nitrogen-Doped Carbon Electrocatalysts for Highly Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef]
- K. Ye, M. Hu, Q.-K. Li, Y. Han, Y. Luo, J. Jiang, G. Zhang, Cooperative Nitrogen Activation and Ammonia Synthesis on Densely Monodispersed Mo–N–C Sites, The Journal of Physical Chemistry Letters, 11 (2020) 3962-3968.
- Li, L.; Xuan, H.; Wang, J.; Liang, X.; Li, Y.; Han, Z.; Cheng, L. Nanoporous nonprecious multi-metal alloys as multisite electrocatalysts for efficient overall water splitting. Int. J. Hydrogen Energy 2024, 97, 38–45. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Tan, W.; Ma, K.; Zou, W.; Tang, C.; Dong, L. Enhanced low-temperature NH3-SCR performance of CeTiO catalyst via surface Mo modification. Chin. J. Catal. 2020, 41, 364–373. [Google Scholar] [CrossRef]
- S. Cao, Z. Guan, Y. Ma, B. Xu, J. Ma, W. Chu, R. Zhang, G. Giambastiani, Y. Liu, Synergizing Mon Clusters and Mo2C Nanoparticles on Oxidized Carbon Nanotubes Boosting the CO2 Reduction Activity, ACS Catalysis, 14 (2024) 10939-10950.
- Li, H.; Zhang, Z.; Deng, Y.; Xu, F.; Hu, J.; Zhu, D.; Yu, Q.; Shi, C. Geopolymer composites for marine application: Structural properties and durability. Cem. Concr. Compos. 2024, 152. [Google Scholar] [CrossRef]
- J. Duan, S. Chen, C.A. Ortíz-Ledón, M. Jaroniec, S.Z.J.A.C.I.E. Qiao, Phosphorus Vacancies that Boost Electrocatalytic Hydrogen Evolution by Two Orders of Magnitude, (2020).
- Zhang, L.; Xu, Q.; Wen, S.; Zhang, H.; Chen, L.; Jiang, H.; Li, C. Recycling Spent Ternary Cathodes to Oxygen Evolution Catalysts for Pure Water Anion-Exchange Membrane Electrolysis. ACS Nano 2024, 18, 22454–22464. [Google Scholar] [CrossRef]
- M. Kim, M. Anjum, M. Lee, B.-J. Lee, J. Lee, Activating MoS2 Basal Plane with Ni2P Nanoparticles for Pt-Like Hydrogen Evolution Reaction in Acidic Media, Advanced Functional Materials, 29 (2019).
- Jiao, J.; Zhang, N.; Zhang, C.; Sun, N.; Pan, Y.; Chen, C.; Li, J.; Tan, M.; Cui, R.; Shi, Z.; et al. Doping Ruthenium into Metal Matrix for Promoted pH-Universal Hydrogen Evolution. Adv. Sci. 2022, 9, e2200010. [Google Scholar] [CrossRef]
- Xiao, P.; Alam Sk, M.; Thia, L.; Ge, X.; Lim, R.J.; Wang, J.-Y.; Lim, K.H.; Wang, X. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 2624–2629. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, R.; Li, X.; Liu, X.; Huang, W. A nanohybrid consisting of NiPS3 nanoparticles coupled with defective graphene as a pH-universal electrocatalyst for efficient hydrogen evolution. J. Mater. Chem. A 2017, 5, 23536–23542. [Google Scholar] [CrossRef]
- Geng, X.; Sun, W.; Wu, W.; Chen, B.; Al-Hilo, A.; Benamara, M.; Zhu, H.; Watanabe, F.; Cui, J.; Chen, T.-P. Pure and stable metallic phase molybdenum disulfide nanosheets for hydrogen evolution reaction. Nat. Commun. 2016, 7, 10672. [Google Scholar] [CrossRef]
- Li, D.J.; Maiti, U.N.; Lim, J.; Choi, D.S.; Lee, W.J.; Oh, Y.; Lee, G.Y.; So, K. Molybdenum Sulfide/N-Doped CNT Forest Hybrid Catalysts for High-Performance Hydrogen Evolution Reaction. Nano Lett. 2014, 14, 1228–1233. [Google Scholar]
- Bai, L.; Duan, Z.; Wen, X.; Si, R.; Zhang, Q.; Guan, J. Highly Dispersed Ruthenium-Based Multifunctional Electrocatalyst. ACS Catal. 2019, 9, 9897–9904. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, X.; Zhang, J.; Gao, D. Nitrogen-doped RuS2 nanoparticles containing in situ reduced Ru as an efficient electrocatalyst for hydrogen evolution. RSC Adv. 2020, 10, 17862–17868. [Google Scholar] [CrossRef] [PubMed]
- Q. Chen, K. Wang, J. Qin, S. Wang, W. Wei, J. Wang, Q. Shen, P. Qu, D. Liu, Ru (x) Se@MoS(2) hybrid as a highly efficient electrocatalyst toward hydrogen evolution reaction, RSC advances, 9 (2019) 13486-13493.
- H. Lin, H. Li, Y. Li, J. Liu, X. Wang, L.J.J.o.M.C. Wang, Hierarchical CoS/MoS2 and Co3S4/MoS2/Ni2P nanotubes for efficient electrocatalytic hydrogen evolution in alkaline media, 5 (2017) 25410-25419.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang, X. Feng, Interface Engineering of MoS2 /Ni3 S2 Heterostructures for Highly Enhanced Electrochemical Overall-Water-Splitting Activity, Angewandte Chemie (International ed. in English), 55 (2016) 6702-6707.
- Das, D.; Santra, S.; Nanda, K.K. In Situ Fabrication of a Nickel/Molybdenum Carbide-Anchored N-Doped Graphene/CNT Hybrid: An Efficient (Pre)catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wu, M.; Tang, Z.; Tse, J.S.; Yang, B.; Lu, S. Single Atom Ruthenium-Doped CoP/CDs Nanosheets via Splicing of Carbon-Dots for Robust Hydrogen Production. Angew. Chem. Int. Ed. Engl. 2021, 60, 7234–7244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Wang, Z.; Li, Q.; Dong, M. An efficient ruthenium-based dual-electrocatalyst towards hydrogen evolution and oxygen reduction reactions. Mater. Today Phys. 2021, 16. [Google Scholar] [CrossRef]
- Li, G.; Zheng, K.; Li, W.; He, Y.; Xu, C. Ultralow Ru-Induced Bimetal Electrocatalysts with a Ru-Enriched and Mixed-Valence Surface Anchored on a Hollow Carbon Matrix for Oxygen Reduction and Water Splitting. ACS Appl. Mater. Interfaces 2020, 12, 51437–51447. [Google Scholar] [CrossRef]
- Li, W.; Wei, Z.; Wang, B.; Liu, Y.; Song, H.; Tang, Z.; Yang, B.; Lu, S. Carbon quantum dots enhanced the activity for the hydrogen evolution reaction in ruthenium-based electrocatalysts. Mater. Chem. Front. 2019, 4, 277–284. [Google Scholar] [CrossRef]
- Shi, H.; Liu, L.; Shi, Y.; Liao, F.; Li, Y.; Shao, M. Silicon monoxide assisted synthesis of Ru modified carbon nanocomposites as high mass activity electrocatalysts for hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 11817–11823. [Google Scholar] [CrossRef]
- Wang, W.; Shao, Y.; Wang, Z.; Yang, Z.; Zhen, Z.; Zhang, Z.; Mao, C.; Guo, X.; Li, G. Synthesis of Ru-Doped VN by a Soft-Urea Pathway as an Efficient Catalyst for Hydrogen Evolution. ChemElectroChem 2020, 7, 1201–1206. [Google Scholar] [CrossRef]
- M. Anjum, H.Y. Jeong, M. Lee, H. Shin, J.S. Lee, Efficient Hydrogen Evolution Reaction Catalysis in Alkaline Media by All-in-One MoS2 with Multifunctional Active Sites, Advanced Materials, 30 (2017).
- Li, M.; Yang, P.; Lv, W.; Liu, Q.; Wu, Y.; Du, S.; Huang, G.; Jiang, Z.; Wang, J.; Xu, Y.; et al. Ultrastable Ruthenium-Based Electrocatalysts for Hydrogen Oxidation Reaction in High-Temperature Polymer Electrolyte Membrane Fuel Cells. CCS Chem. 2024, 1–9. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, R.; Cao, J.; Zhang, D.; Bai, J.; Han, C.; Xiao, L.; Zhuang, L.; Song, P.; Xu, W. Ru-MnO Heterostructure Clusters Toward Efficient and CO-Tolerant Alkaline Hydrogen Oxidation Reaction. Adv. Energy Mater. 2024, 15. [Google Scholar] [CrossRef]
- S. Lu, Z. Zhang, C. Chuanqi, B. Zhang, Y. Shi, Unveiling the Aggregation of M−N−C Single Atoms into Highly Efficient MOOH Nanoclusters during Alkaline Water Oxidation, Angewandte Chemie, (2024).
- Wang, L.; Wei, Z.; Sun, Z.; Zhu, L.; Gao, Y.; Chen, Z.; Li, S.-H.; Chen, W. Carbon-based double-metal-site catalysts: advances in synthesis and energy applications. J. Mater. Chem. A 2024, 12, 11749–11770. [Google Scholar] [CrossRef]
- H. Wang, R. Niu, J. Liu, S. Guo, Y. Yang, Z. Liu, J. Li, Electrostatic self-assembly of 2D/2D CoWO4/g-C3N4 p–n heterojunction for improved photocatalytic hydrogen evolution: Built-in electric field modulated charge separation and mechanism unveiling, Nano Research, 15 (2022) 6987-6998.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




