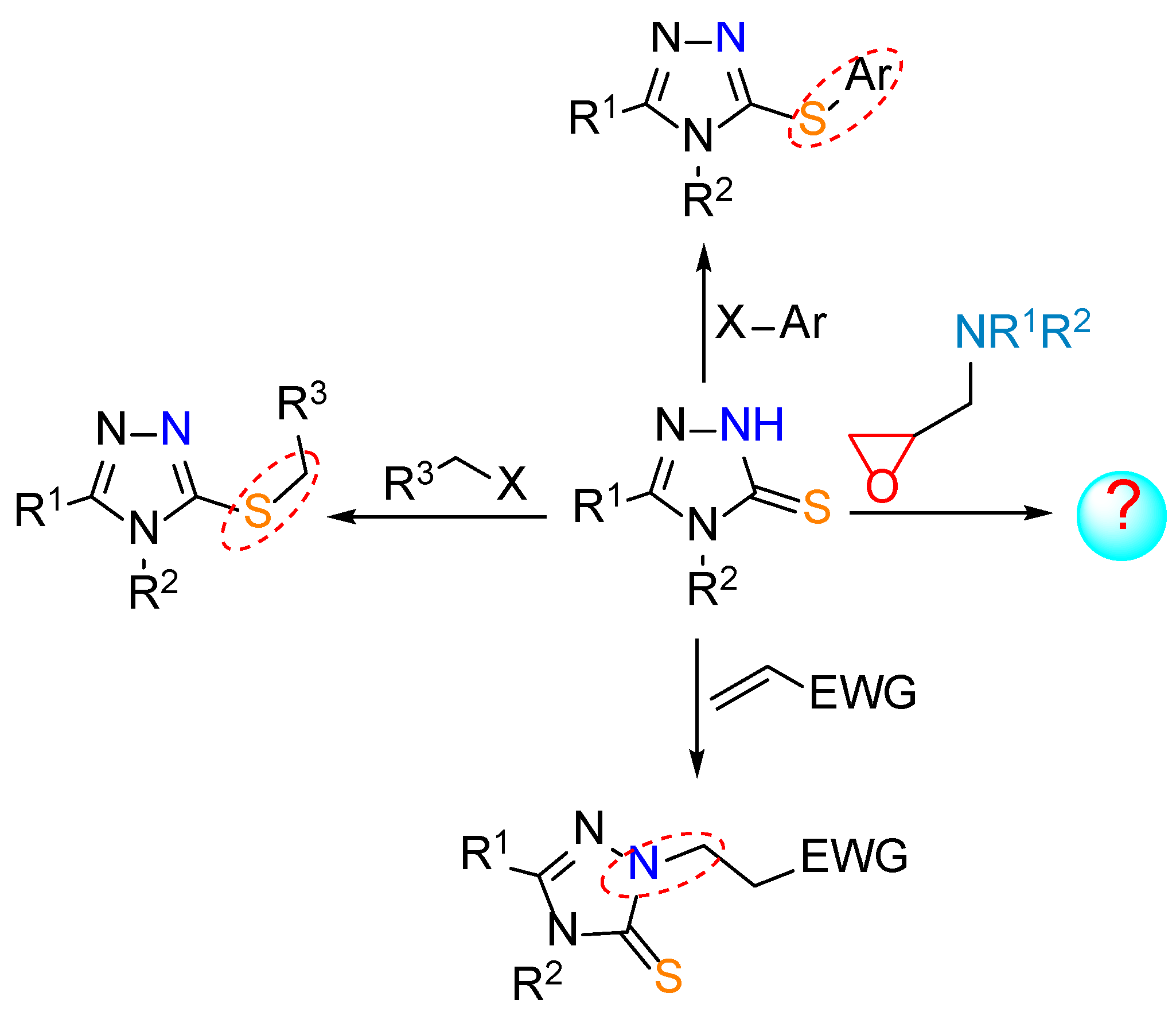
2. Materials and Methods
1H and
13C NMR spectra were recorded on Varian Mercury 300 MHz or on Bruker Avance Neo 400 MHz spectrometer in DMSO/CCl
4 mixture (1:3) or DMSO-
d6. Chemical shifts (δ) in ppm are reported as quoted relative to the residual signals of solvents, for instance, DMSO-d
6 (2.5 for
1H NMR and 39.5 for
13C NMR) as internal references. The coupling constants (J) are given in Hertz. ESI-MS spectra were measured with a Waters Xevo G3 QTof Mass Spectrometer. Spectroscopic measurements were performed on a Nicolet IS 50 FTIR spectrometer (Thermo Scientific Fisher, USA) coupled with an ATR accessory. TLC analysis was performed on “Silufol UV-254” plates. All reagents were of reagent grade and were used as such or distilled prior to use. Starting 1,2,4-triazoles were prepared as previously reported (
1a,
1k,
1m-o,
1r [56];
1f [58];
1g – CAS 21358-12-3;
1j – CAS: 93378-58-6;
1v [52];
1h,
1i [
57];
1e [59];
1q [60];
1u [
61]). Melting points were determined on “SMP-10”.
4-propyl-5-(thiophen-2-yl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1b). Yield 84%, white solid, m.p. 135 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 13.77 (s, 1H, NH), 7.63 (dd, J = 5.1, 1.1 Hz, 1Hthph), 7.52 (dd, J = 3.7, 1.1 Hz, 1Hthph), 7.18 (dd, J = 5.1, 3.7 Hz, 1Hthph), 4.19 – 4.03 (m, 2H, NCH2), 1.84 – 1.67 (m, 2H, CH2CH3), 0.97 (t, J = 7.4 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 167.4 (C=S), 145.2 (C=N), 128.4 (CH=), 127.8 (CH=), 127.5 (CH=), 126.8, 45.1 (NCH2), 21.1 (CH2), 10.6 (CH3). IRνmax = 3094.23; 3083.14; 3062.41; 3006.96; 2959.23; 2927.41; 2911.50; 2869.56; 2819.42; 2793.38; 2757.71; 2723.00; 2683.46; 2583.66; 2573.54; 1792.03; 1719.23; 1648.36; 1623.29; 1571.22; 1544.22; 1515.29; 1480.10; 1458.89; 1443.46; 1432.85; 1400.55; 1372.59; 1352.82; 1323.89; 1299.79; 1282.91; 1253.02; 1230.36; 1217.83; 1204.81; 1145.03; 1102.60; 1085.73; 1070.78; 1060.17; 1035.10; 1014.37; 950.73; 942.06; 895.29; 867.81; 853.83; 846.11; 824.42; 779.58; 758.85; 750.17; 742.46; 727.51; 705.34; 694.73; 668.70; 660.50; 654.71; 577.09; 567.93; 493.21; 434.39; 423.30 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C9H12N3S+ 226.0473; Found 226.0473.
5-(4-bromophenyl)-4-cyclohexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (1c). Yield 89%, white solid, m.p. 198 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 13.72 (s, 1H, NH), 7.74 – 7.58 (m, 2Harom), 7.46 – 7.35 (m, 2Harom), 4.39 – 4.17 (m, 1H, NCH), 2.38 – 1.91 (m, 2H, Cy), 1.86 – 1.67 (m, 4H, Cy), 1.67 – 1.53 (m, 1H, Cy), 1.37 – 1.19 (m, 2H, Cy), 1.16 – 0.99 (m, 1H, Cy). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 166.5 (C=S), 149.8 (C=N), 131.4 (2*CH=), 131.1 (2*CH=), 126.1, 124.3, 56.8 (NCH), 29.5 (2*CH2), 25.4 (2*CH2), 24.4 (CH2). IRνmax = 3090.85; 3068.67; 3058.55; 3025.28; 2979.96; 2931.27; 2894.15; 2863.29; 2853.65; 2824.24; 2778.92; 2746.62; 2688.77; 1600.15; 1574.59; 1544.22; 1500.35; 1473.83; 1450.21; 1442.01; 1408.75; 1382.71; 1340.28; 1307.02; 1289.18; 1281.47; 1255.91; 1241.93; 1188.42; 1150.81; 1140.69; 1116.10; 1096.82; 1084.76; 1066.44; 1057.28; 1009.55; 995.57; 970.50; 942.06; 925.18; 895.77; 854.79; 832.13; 818.63; 793.08; 782.48; 759.33; 728.00; 718.84; 706.30; 670.14; 666.29; 629.16; 604.09; 539.01; 508.63; 484.05; 461.39; 451.74; 432.46; 420.41; 413.66 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C14H1779BrN3S+ 338.0327; Found 338.0327.
5-(4-bromophenyl)-4-phenethyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (1d). Yield 81%, white solid, m.p. 178 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 13.83 (s, 1H, NH), 7.61 – 7.54 (m, 2Harom), 7.31 – 7.25 (m, 2Harom), 7.20 – 7.11 (m, 3Harom), 7.04 – 6.98 (m, 2Harom), 4.27 – 4.17 (m, 2H, NCH2), 3.06 – 2.98 (m, 2H, CH2Ph). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 167.1 (C=S), 149.9 (C=N), 136.9, 131.4 (2*CH=), 129.9 (2*CH), 128.4 (2*CH=), 128.0 (2*CH=), 126.1, 125.0, 124.0, 45.0 (NCH2), 33.1 (CH2). IRνmax = 3119.78; 3084.10; 3041.67; 3029.62; 2983.82; 2953.45; 2936.57; 2908.13; 2867.63; 2840.63; 2770.24; 1604.48; 1558.20; 1503.24; 1473.35; 1442.98; 1404.41; 1392.84; 1372.10; 1352.34; 1332.57; 1321.96; 1274.72; 1199.99; 1188.90; 1177.33; 1139.24; 1104.05; 1077.53; 1061.14; 1031.25; 1023.05; 1010.03; 967.61; 944.47; 925.18; 900.11; 836.47; 828.76; 776.69; 761.74; 754.03; 748.25; 731.37; 719.32; 698.59; 674.96; 658.57; 622.89; 600.72; 555.40; 538.04; 492.72; 454.64; 444.99; 417.03; 403.53 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C16H1579BrN3S+ 360.0170; Found 360.0170.
4-allyl-5-(2-iodophenyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1l). Yield 88%, white solid, m.p. 145 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 13.84 (s, 1H, NH), 7.96 (dd, J = 8.0, 1.1 Hz, 1Harom), 7.50 (td, J = 7.5, 1.1 Hz, 1Harom), 7.41 (dd, J = 7.7, 1.8 Hz, 1Harom), 7.28 (td, J = 7.7, 1.7 Hz, 1Harom), 5.69 (ddt, J = 16.1, 10.3, 5.7 Hz, 1H, CH=), 5.01 (dd, J = 10.3, 1.4 Hz, 1Ha, =CH2), 4.80 (dt, J = 17.1, 1.6 Hz, 1Hb, =CH2), 4.42 (dt, J = 6.0, 1.6 Hz, 2H, NCH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 166.7 (C=S), 151.3 (C=N), 138.8 (CH=), 131.8 (CH=), 131.5 (CH=), 130.5 (CH=), 130.4, 127.8 (CH=), 117.9 (=CH2), 98.9 (=CI), 45.7 (NCH2). IRνmax = 3083.14; 3057.58; 3037.34; 2967.43; 2922.59; 2909.57; 2849.79; 2835.81; 2754.33; 2649.71; 1643.54; 1624.25; 1596.29; 1561.09; 1527.83; 1497.94; 1458.89; 1441.53; 1425.14; 1394.76; 1353.78; 1330.64; 1292.55; 1262.18; 1249.65; 1197.10; 1158.53; 1147.44; 1099.23; 1076.57; 1018.23; 993.16; 981.59; 948.81; 914.09; 870.70; 779.10; 754.03; 728.00; 719.80; 708.71; 664.84; 641.22; 617.59; 602.16; 576.13; 525.99; 484.53; 465.24; 444.03; 420.89; 404.50 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C11H11IN3S+ 343.9718; Found 343.9726.
4-phenyl-5-(m-tolyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (1t). Yield 90%, white solid, m.p. 245 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 13.94 (s, 1H, NH), 7.52 – 7.43 (m, 3Harom), 7.30 – 7.23 (m, 2Harom), 7.18 – 7.07 (m, 3Harom), 6.99 – 6.92 (m, 1Harom), 2.25 (s, 3H, CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 168.5 (C=S), 149.7 (C=N), 137.4, 134.5, 130.3, 128.7 (CH=), 128.7 (2CH=), 128.3 (CH=), 128.2 (2*CH=), 127.7 (CH=), 125.6, 124.7 (CH=), 20.7 (CH3). IRνmax =3107.24; 3084.58; 3057.58; 3031.07; 2995.39; 2927.90; 2823.28; 2770.24; 2752.40; 1591.47; 1550.97; 1496.49; 1488.29; 1459.37; 1437.67; 1402.48; 1382.71; 1334.50; 1313.29; 1285.32; 1273.75; 1243.38; 1208.18; 1174.92; 1129.60; 1098.26; 1083.32; 1073.67; 1037.03; 1003.28; 977.25; 918.91; 885.17; 851.42; 796.94; 772.83; 737.16; 717.39; 700.52; 691.84; 668.70; 660.02; 620.97; 613.73; 545.27; 524.54; 513.94; 481.15; 442.10; 427.16 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C15H14N3S+ 268.0909; Found 268.0931.
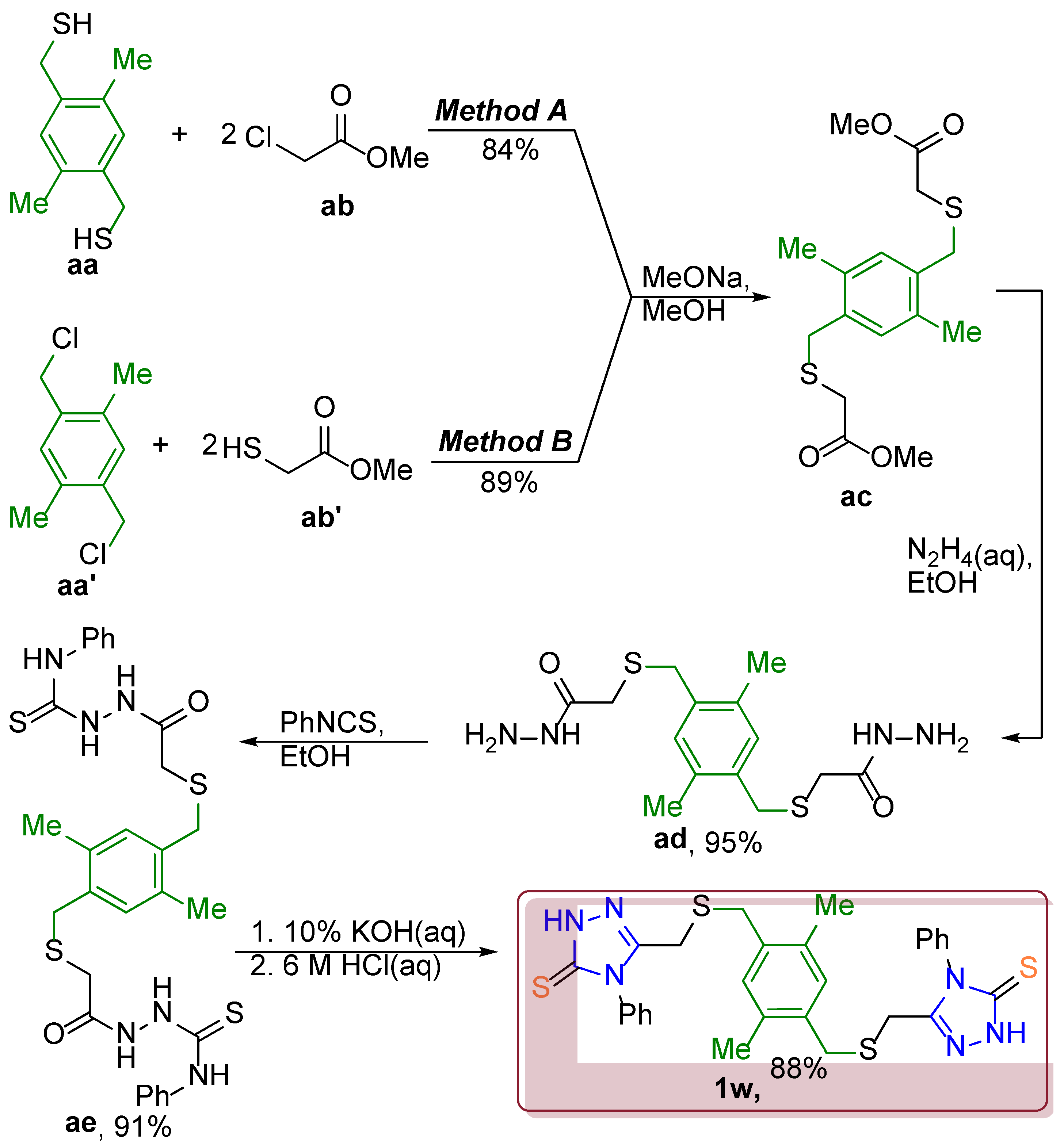
Dimethyl 2,2′-(((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl)) diacetate (ac).
Method A: 0.48 g (21 mmol) of sodium was dissolved in 25 ml of methanol, then 4.16 g (21 mmol) of (2,5-dimethyl-1,4-phenylene)dimethanethiol was added and the mixture was stirred at r.t. for 30min. Then 1.09 g (10 mmol, 0.95 ml) of ethyl chloroformate and 25 ml of methanol were added to the mixture and refluxed for 4h. Resulting mixture was left overnight, and the precipitate was filtered and washed with water and dried. Yield 84%.
Method B: 0.48 g (21 mmol) sodium was dissolved in 25 ml of methanol, then 2.22 g (21 mmol, 1.88 ml) methyl 2-mercaptoacetate was added and the mixture was stirred at r.t. for 30min. Then 2.03 g (10 mmol) of 1,4-bis(chloromethyl)-2,5-dimethylbenzene and 25 ml of methanol were added to the mixture and refluxed for w4h. Resulting mixture was left overnight, and the precipitate was filtered and washed with water and dried. Yield 89%. White solid, m.p. 100 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 6.99 (s, 2Harom), 3.75 (s, 4H, CH2CO), 3.69 (s, 6H, OCH3), 3.11 (s, 4H, SCH2Ar), 2.32 (s, 6H, ArCH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 169.5 (2*C=O), 133.4 (2*C), 133.3 (2*C), 131.8 (2*CH=), 51.4 (2*OCH3), 33.4 (2*SCH2), 31.8 (2*SCH2), 17.9 (2*CH3). IRνmax = 3427.85; 3032.51; 3004.07; 2972.73; 2950.07; 2918.25; 2894.15; 2862.33; 2727.33; 1723.09; 1680.18; 1505.65; 1436.23; 1427.55; 1408.75; 1392.84; 1371.62; 1290.63; 1214.93; 1144.06; 1113.21; 1038.00; 1004.73; 936.75; 907.83; 866.85; 828.28; 795.49; 718.84; 700.03; 595.41; 579.99; 466.21; 424.26 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C16H23O4S2+ 343.1038; Found 343.1048.
2,2′-(((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl))di(acetohydrazide) (ad). The mixture of 3.42 g dimethyl 2,2′-(((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl)) diacetate and 8 ml hydrazine hydrate in 10 ml of ethanol was stirred for 1h at r.t., then refluxed for 3h. Resulting mixture was left overnight, and the precipitate was filtered and washed with ethanol and water and dried. Yield 95%, white solid, m.p. 179 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 9.07 (br.s., 2H, NH), 7.03 (s, 2Harom), 4.11 (br.s., 4H, NH2), 3.77 (s, 4H, CH2CO), 2.96 (s, 4H, SCH2Ar), 2.30 (s, 6H, ArCH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 168.3 (2*C=O), 134.0 (2*C), 133.3 (2*C), 131.7 (2*CH), 33.6 (2*SCH2), 32.1 (2*SCH2), 18.0 (2*CH3). IRνmax = 3329.98; 3307.32; 3255.25; 3206.56; 3140.99; 3015.64; 2991.05; 2963.57; 2953.45; 2933.68; 2914.40; 2847.38; 1654.14; 1622.81; 1501.31; 1458.40; 1433.82; 1396.21; 1376.93; 1325.34; 1296.41; 1240.00; 1217.83; 1178.78; 1127.19; 1117.06; 1034.14; 986.89; 920.36; 889.02; 853.83; 801.76; 780.06; 706.30; 696.18; 659.05; 582.88; 536.60; 463.31 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C14H23N4O4S2+ 343.1263; Found 343.1263.
2,2′-(2,2′-(((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl))bis(acetyl))bis(N-phenylhydrazine-1-carbothioamide) (ae). The mixture of 1.026 g (3 mmol) 2,2′-(((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl))di(acetohydrazide) and 0.81 g (6 mmol) phenyl isothiocyanate in 5 ml ethanol was stirred at r.t for 1h, then was refluxed for 2h. The resulting mixture was left overnight, and the precipitate was filtered and washed with ethanol and dried. Yield 91%, white solid, m.p 210 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 10.03 (br.s., 2H, NH), 9.68 – 9.32 (m, 4H, NH), 7.55 (d, J = 8.0 Hz, 4Harom), 7.29 (t, J = 7.8 Hz, 4Harom), 7.15 – 7.02 (m, 4Harom), 3.83 (s, 4H, CH2CO), 3.14 (s, 4H, SCH2Ar), 2.33 (s, 6H, CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) 180.3(2*C), 138.9 (2*C), 133.8 (2*C), 133.3 (2*CH), 131.8 (2*CH=), 127.6 (4*CH=), 124.1 (br, 6*CH), 33.5 (2*SCH2), 32.3 (2*SCH2), 18.1 (2*CH3). IRνmax = 3303.46; 3222.47; 3165.58; 3066.74; 3004.55; 2970.32; 2923.07; 2861.36; 1680.18; 1654.14; 1623.77; 1604.97; 1560.13; 1535.54; 1499.38; 1476.72; 1445.87; 1419.83; 1363.43; 1327.75; 1314.25; 1294.00; 1254.95; 1238.56; 1222.17; 1204.81; 1194.20; 1164.31; 1156.12; 1141.65; 1081.87; 1032.21; 996.54; 970.02; 928.56; 906.86; 897.22; 876.01; 837.92; 797.42; 752.58; 747.28; 731.37; 710.64; 690.87; 574.20; 502.85; 464.76 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C28H33N6O2S4+ 613.1548; Found 613.1545.
5,5′-((((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl))bis(methylene))bis(4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione) (1w). The mixture of 1.23 g (2 mmol) 2,2′-(2,2′-(((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl))bis(acetyl))bis(N-phenylhydrazine-1-carbothioamide) and 0.35 g (6 mmol) KOH in 5 ml of water was refluxed for 4h. The resulting mixture was acidified with 6M HCl, the precipitate was filtered and washed with water and dried. Yield 88%, white solid, m.p. 270 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 13.72 (s, 2H, NH), 7.57 – 7.46 (m, 6Harom), 7.37 – 7.29 (m, 4Harom), 6.91 (s, 2Harom), 3.62 (s, 4H, CH2CNN), 3.42 (s, 4H, SCH2Ar), 2.21 (s, 6H, CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 168.2 (2*C=S), 148.6 (2*C=N), 133.4 (2*C), 133.2 (2*C), 133.2 (2*C), 131.6 (2*CH=), 128.9 (2*CH=), 128.7 (4*CH=), 128.0 (4*CH=), 32.8 (2*SCH2), 24.6 (2*SCH2), 17.9 (2*CH3). IRνmax = 3103.22; 3036.47; 2929.45; 1591.22; 1567.16; 1497.84; 1491.49; 1454.25; 1416.03; 1397.99; 1332.63; 1276.56; 1232.78; 1164.59; 1091.60; 1069.42; 1038.22; 1013.86; 921.36; 888.32; 843.82; 801.25; 785.33; 758.73; 742.69; 709.13; 692.17; 672.19; 613.42; 553.44; 503.60; 456.57 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C28H29N6S4+ 577.1337; Found 577.1335.
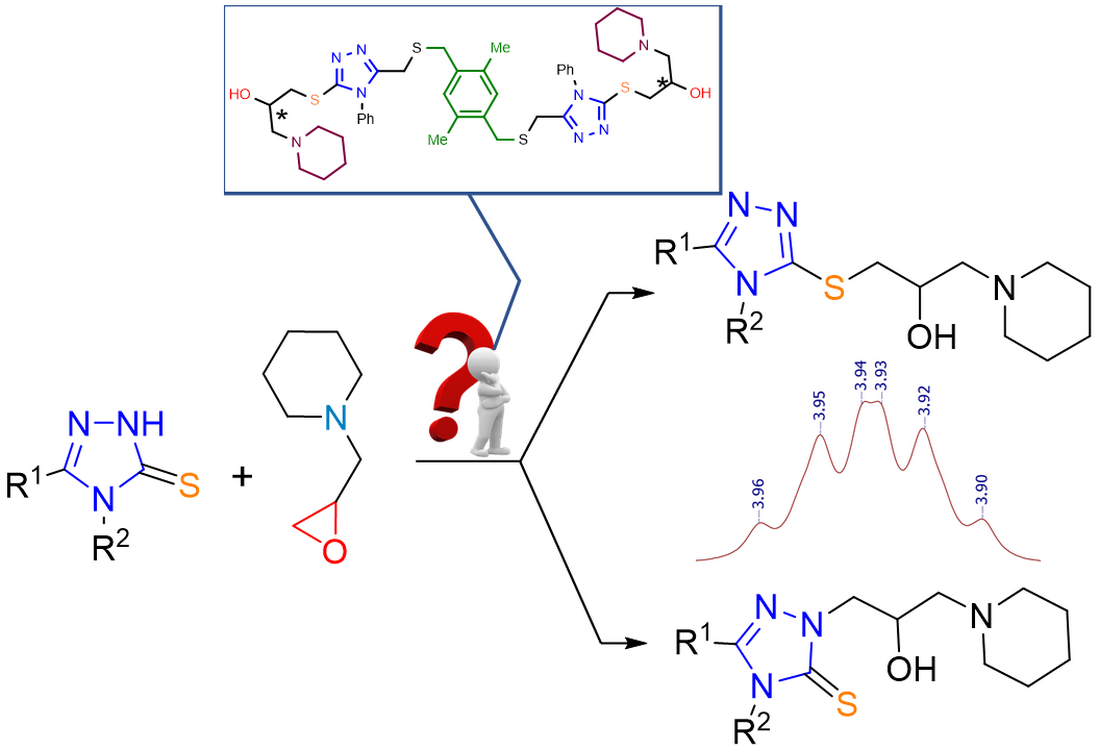
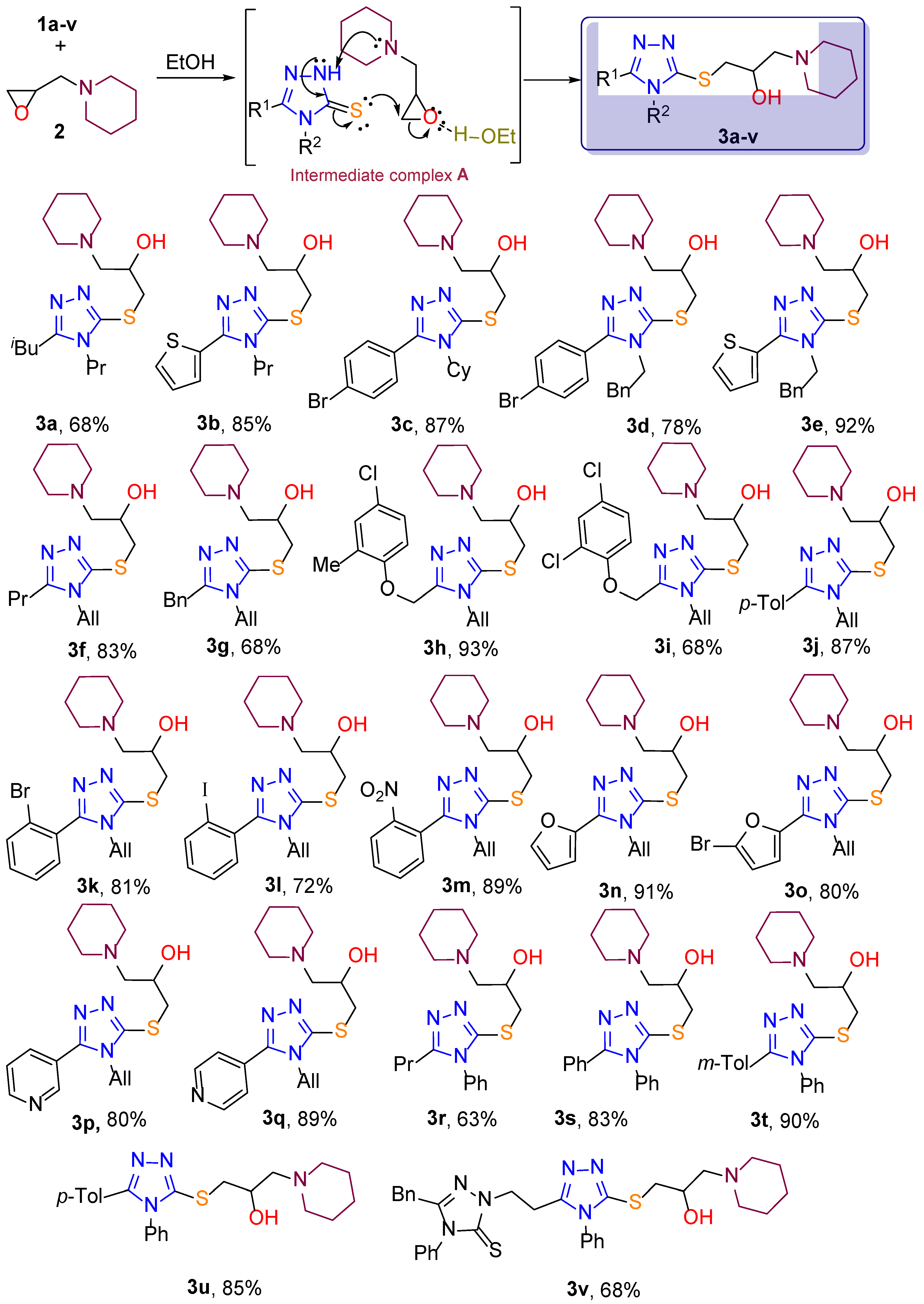
General procedure of synthesys 1-((4,5-disubstituted-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ols. Mixture of the corresponding triazole (2 mmol) and 1-(oxiran-2-ylmethyl)piperidine (2.2 mmol, 310 mg) was stirred in 50 °C 4 hours. Then the mixture was cooled down to r.t. and purified with flash chromatography (eluent: C6H6:MeOH:Et3N 50:1:1).
1-((5-isobutyl-4-propyl-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3a). Yield 68%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 4.74 (br.s., 1H, OH), 3.90 – 3.80 (m, 1H, CHOH), 3.80 – 3.72 (m, 2H, NCH2CH2CH3), 3.30 (dd, J = 13.2, 4.2 Hz, 1Ha, SCH2), 3.08 (dd, J = 13.2, 6.7 Hz, 1Hb, SCH2), 2.49 (d, J = 7.1 Hz, 2H, CH2CH(CH3)2), 2.46 – 2.33 (m, 4H, N(CH2CH2)2CH2), 2.31 (dd, J = 6.4, 1.8 Hz, 2H, NCH2CH), 2.16 – 2.05 (m, 1H, CH(CH3)2), 1.72 – 1.61 (m, 2H), 1.56 – 1.44 (m, 4H, N(CH2CH2)2CH2), 1.42 – 1.30 (m, 2H, N(CH2CH2)2CH2), 0.97 (d, J = 6.7 Hz, 6H, CH(CH3)2), 0.91 (t, J = 7.4 Hz, 3H, CH2CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.6 (SC=N), 149.3 (C=N), 66.8 (OCH), 63.3 (NCH2), 54.6 (2*NCH2), 44.4 (NCH2), 38.2 (SCH2), 33.2 (CH2), 26.5 (CH), 25.5 (2*CH2), 23.8 (CH2), 22.8 (CH2), 22.1 (2*CH3), 10.6 (CH3). IRνmax = 3258.63; 2931.75; 2871.01; 2853.17; 2796.28; 2757.23; 1514.33; 1465.15; 1442.49; 1428.03; 1398.14; 1383.68; 1366.80; 1351.86; 1342.21; 1300.75; 1280.02; 1244.83; 1225.06; 1202.40; 1168.17; 1156.60; 1116.58; 1088.62; 1039.93; 995.09; 962.79; 924.70; 898.18; 862.02; 804.17; 787.78; 745.83; 688.46; 656.16; 614.22; 592.52; 555.88; 514.90; 476.81; 469.58; 444.51; 430.05; 423.78; 416.07; 408.35 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C17H33N4OS+ 341.2375; Found 341.2388.
1-(piperidin-1-yl)-3-((4-propyl-5-(thiophen-2-yl)-4H-1,2,4-triazol-3-yl)thio)propan-2-ol (3b). Yield 85%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.58 (dd, J = 5.1, 1.2 Hz, 1Hthph), 7.45 (dd, J = 3.7, 1.2 Hz, 1Hthph), 7.16 (ddd, J = 5.0, 3.6, 1.2 Hz, 1Hthph), 4.70 (br.s., 1H, OH), 4.06 (dd, J = 8.6, 6.8 Hz, 2H, NCH2CH2CH3), 4.00 – 3.87 (m, 1H, CHOH), 3.44 (dd, J = 13.2, 4.2 Hz, 1Ha, SCH2), 3.27 – 3.14 (m, 1Hb, SCH2), 2.42 (br.t., J = 5.1 Hz, 4H, N(CH2CH2)2CH2), 2.37 (dd, J = 6.4, 1.2 Hz, 2H, NCH2CHOH), 1.82 – 1.69 (m, 2H, NCH2CH2CH3), 1.59 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.46 – 1.36 (m, 2H, N(CH2CH2)2CH2), 0.95 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 151.4 (SC=N), 149.0 (C=N), 128.0, 127.6 (CH=), 127.3 (CH=), 126.6 (CH=), 66.6 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 45.6 (NCH2), 38.4 (SCH2), 25.6 (2*CH2), 23.9 (CH2), 22.6 (CH2), 10.5 (CH3). IRνmax = 3297.68; 3100.01; 3078.32; 2931.27; 2876.31; 2851.72; 2797.24; 2757.23; 1652.70; 1565.43; 1467.56; 1458.89; 1432.37; 1416.94; 1386.57; 1366.32; 1351.86; 1334.98; 1300.75; 1278.09; 1250.13; 1232.77; 1209.63; 1155.63; 1141.17; 1115.62; 1086.21; 1039.44; 994.61; 962.30; 943.02; 896.74; 850.45; 787.30; 710.64; 648.93; 582.88; 543.83; 505.74; 490.79; 478.26; 461.39; 444.03; 420.89 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C17H27N4OS2+ 367.1626; Found 367.1648.
1-((5-(4-bromophenyl)-4-cyclohexyl-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3c). Yield 87%, white solid, m.p. 109 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.70 – 7.55 (m, 2Harom), 7.46 – 7.33 (m, 2Harom), 4.73 (br.s., 1H, OH), 4.02 – 3.85 (m, 2H, 1H CHOH, 1H NCH), 3.47 (dd, J = 13.2, 4.3 Hz, 1Ha, SCH2), 3.25 (dd, J = 13.2, 6.7 Hz, 1Hb, SCH2), 2.42 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.36 (dd, J = 6.4, 1.5 Hz, 2H, NCH2CHOH), 2.19 – 2.02 (m, 2H, Cy), 1.89 – 1.74 (m, 4H, Cy), 1.68 – 1.58 (m, 1H, Cy), 1.57 – 1.47 (m, 4H, N(CH2CH2)2CH2), 1.43 – 1.34 (m, 2H, N(CH2CH2)2CH2), 1.33 – 1.09 (m, 3H, Cy). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.8 (SC=N), 150.1 (C=N), 131.4 (2*CH), 130.7 (2*CH), 126.8, 123.5, 66.6 (OCH), 63.5 (NCH2), 56.2 (NCH), 54.6 (2*NCH2), 38.3 (SCH2), 30.6 (2*CH2), 25.6 (2*CH2), 25.2 (2*CH2), 24.3 (CH2), 23.9 (CH2). IRνmax = 3185.83; 3062.41; 3036.37; 2931.27; 2856.54; 2796.76; 2781.81; 2757.71; 2745.17; 2694.07; 2668.52; 1595.81; 1567.36; 1465.15; 1440.08; 1410.67; 1389.94; 1362.46; 1345.11; 1288.70; 1272.79; 1258.32; 1213.49; 1200.95; 1183.60; 1157.56; 1146.47; 1129.12; 1105.01; 1061.62; 1041.37; 1025.94; 1010.52; 999.43; 971.47; 947.84; 928.07; 900.59; 892.88; 870.22; 855.28; 837.92; 823.46; 788.74; 764.64; 751.62; 731.37; 712.57; 690.39; 626.27; 592.52; 561.18; 530.81; 515.38; 483.08; 456.56; 443.07; 433.90; 421.37; 405.94 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C22H3279BrN4OS+ 479.1480; Found 479.1489.
1-((5-(4-bromophenyl)-4-phenethyl-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3d). Yield 78%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.62 – 7.54 (m, 2Harom), 7.35 – 7.31 (m, 2Harom), 7.21 – 7.14 (m, 3Harom), 6.97 – 6.91 (m, 2Harom), 4.73 (br.s., 1H, OH), 4.21 (t, J = 7.4 Hz, 2H, NCH2CH2Ph), 4.00 – 3.90 (m, 1H, CHOH), 3.45 (dd, J = 13.2, 4.2 Hz, 1Ha, SCH2), 3.21 (dd, J = 13.1, 6.9 Hz, 1Hb, SCH2), 2.93 (t, J = 7.4 Hz, 2H, CH2Ph), 2.44 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.39 (dd, J = 6.5, 1.5 Hz, 2H, NCH2CHOH), 1.61 – 1.50 (m, 4H, N(CH2CH2)2CH2), 1.48 – 1.38 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.5 (SC=N), 151.3 (C=N), 136.2, 131.3 (2*CH=), 129.8 (2*CH=), 128.3 (2*CH=), 128.1 (2*CH=), 126.3 (CH=), 126.2, 123.3, 66.6 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 45.3 (NCH2), 38.4 (SCH2), 35.0 (CH2Ph), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3282.73; 3086.51; 3062.89; 3027.21; 3001.18; 2931.75; 2851.72; 2797.72; 2757.23; 1599.18; 1567.36; 1496.49; 1454.55; 1432.37; 1385.60; 1353.78; 1334.02; 1300.75; 1276.16; 1240.97; 1206.74; 1177.33; 1155.63; 1115.14; 1091.99; 1067.89; 1039.44; 1007.14; 995.57; 970.98; 931.45; 891.43; 860.58; 827.79; 786.81; 764.64; 750.17; 728.48; 720.28; 698.10; 678.82; 625.79; 577.58; 549.13; 522.61; 496.10; 477.30; 452.71; 438.24; 430.53; 425.71; 419.44; 410.28 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C24H3079BrN4OS+ 501.1324; Found 501.1328.
1-((4-phenethyl-5-(thiophen-2-yl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3e). Yield 92%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.58 (dd, J = 5.1, 1.1 Hz, 1Harom), 7.36 (dd, J = 3.7, 1.1 Hz, 1Harom), 7.28 – 7.14 (m, 4Harom), 7.12 – 7.06 (m, 2Harom), 4.68 (br.s., 1H, OH), 4.38 – 4.21 (m, 2H, CH2CH2Ph), 3.98 – 3.85 (m, 1H, CHOH), 3.40 (dd, J = 13.2, 4.2 Hz, 1Ha, SCH2), 3.16 (dd, J = 13.2, 6.8 Hz, 1Hb, SCH2), 3.01 (t, J = 7.8 Hz, 2H, CH2Ph), 2.43 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.37 (dd, J = 6.3, 0.9 Hz, 2H, NCH2CHOH), 1.60 – 1.50 (m, 4H, N(CH2CH2)2CH2), 1.46 – 1.37 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 151.4, 149.0, 136.2, 128.3 (2*CH=), 128.2 (2*CH=), 127.7 (CH=), 127.3 (CH=), 126.9 (CH=), 126.4 (CH=), 66.6 (OCH), 63.4 (NCH2), 54.6 (NCH2), 45.4 (NCH2), 38.6 (SCH2), 35.1 (CH2Ph), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3302.98; 3102.90; 3085.55; 3064.82; 3026.73; 3001.66; 2931.27; 2851.24; 2797.24; 2757.71; 1603.52; 1583.75; 1565.43; 1496.49; 1474.31; 1454.06; 1431.40; 1416.94; 1386.57; 1354.27; 1333.05; 1301.23; 1276.65; 1255.43; 1240.49; 1224.58; 1210.60; 1175.88; 1155.63; 1115.14; 1086.21; 1039.93; 994.61; 961.82; 942.54; 906.86; 891.92; 850.94; 808.51; 786.81; 764.15; 748.73; 697.14; 648.93; 643.14; 620.48; 571.31; 539.49; 495.13; 478.74; 429.57; 420.41; 407.39 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C22H29N4OS2+ 429.1783; Found 429.1796.
1-((4-phenyl-5-(m-tolyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3f). Yield 83%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 5.88 (ddt, J = 17.2, 10.1, 4.9 Hz, 1H, CH=), 5.20 (dq, J = 10.4, 1.5 Hz, 1Ha, =CH2), 4.92 (dq, J = 17.1, 1.6 Hz, 1Hb, =CH2), 4.71 (br.s., 1H, OH), 4.53 (dt, J = 5.0, 1.9 Hz, 2H, NCH2 in Allyl), 3.92 – 3.80 (m, 1H, CHOH), 3.29 (dd, J = 13.2, 4.2 Hz, 1Ha, SCH2), 3.07 (dd, J = 13.2, 6.8 Hz, 1Hb, SCH2), 2.60 (t, J = 7.5 Hz, 2H, CH2CH2CH3), 2.40 (br.t, J = 5.2 Hz, 4H, N(CH2CH2)2CH2), 2.37 – 2.27 (m, 2H, NCH2CHOH), 1.82 – 1.69 (m, 2H, CH2CH2CH3), 1.58 – 1.47 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.35 (m, 2H, N(CH2CH2)2CH2), 1.01 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 154.4 (SC=N), 149.5 (C=N), 131.7 (CH=CH2), 116.6 (=CH2), 66.7 (OCH), 63.3 (NCH2), 54.5 (2*NCH2), 45.0 (NCH2), 38.5 (SCH2), 26.2 (CH2), 25.5 (2*CH2), 23.8 (CH2), 19.7 (CH2), 13.4 (CH3). IRνmax = 3245.61; 3086.03; 2931.75; 2873.42; 2852.69; 2796.28; 2757.23; 1645.46; 1518.67; 1460.81; 1441.05; 1431.40; 1411.16; 1399.58; 1375.48; 1352.34; 1332.09; 1300.27; 1279.54; 1255.91; 1242.41; 1215.90; 1196.13; 1156.12; 1116.10; 1088.62; 1039.93; 994.12; 962.79; 915.06; 891.92; 862.02; 812.85; 786.81; 739.08; 685.09; 664.36; 615.18; 553.47; 478.74; 462.83; 442.58; 420.89; 411.25 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C16H29N4OS+ 325.2062; Found 325.2083.
1-((4-allyl-5-benzyl-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3g). Yield 68%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.31 – 7.15 (m, 5Harom), 5.62 (ddt, J = 17.2, 10.3, 5.2 Hz, 1H, CH=), 5.09 (dt, J = 10.4, 1.3 Hz, 1Ha, =CH2), 4.87 (m, 2H, 1H, OH, 1Hb, =CH2), 4.41 (dt, J = 5.3, 1.7 Hz, 2H, NCH2 in Allyl), 4.09 (s, 2H, PhCH2), 3.95 – 3.82 (m, 1H, CHOH), 3.31 (dd, J = 13.2, 4.3 Hz, 1Ha, SCH2), 3.10 (dd, J = 13.2, 6.7 Hz, 1Hb, SCH2), 2.57 – 2.31 (m, 6H, NCH2), 1.62 – 1.47 (m, 4H, N(CH2CH2)2CH2), 1.47 – 1.37 (m, 2H N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.3 (SC=N), 150.4 (C=N), 135.4, 131.0 (CH=CH2), 128.1 (2*CH), 128.1 (2*CH), 126.3 (CH=), 117.2 (=CH2), 66.5 (OCH), 63.1 (NCH2), 54.4 (2*NCH2), 45.3 (NCH2), 38.4 (SCH2), 30.6 (CH2Ph), 25.3 (2*CH2), 23.6 (CH2). IRνmax = 3257.66; 3086.03; 3063.37; 3029.14; 2931.75; 2851.72; 2796.28; 2758.67; 1644.98; 1603.52; 1517.70; 1495.53; 1463.71; 1452.62; 1440.56; 1420.32; 1392.84; 1374.03; 1352.82; 1329.20; 1300.75; 1278.57; 1242.90; 1203.36; 1155.63; 1115.62; 1087.66; 1075.60; 1039.44; 993.64; 962.30; 928.07; 915.54; 862.02; 787.30; 751.62; 725.59; 695.69; 680.75; 616.15; 572.75; 553.95; 493.69; 478.74; 445.96; 425.23; 402.09 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C20H29N4OS+ 373.2062; Found 373.2090.
1-((4-allyl-5-((4-chloro-2-methylphenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3h). Yield 93%, colorless oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.17 – 7.05 (m, 3Harom), 5.89 (ddt, J = 17.0, 10.4, 5.2 Hz, 1H, CH=), 5.24 – 5.15 (m, 3H, 1Ha, =CH2, 2H OCH2), 5.00 (dq, J = 17.1, 1.4 Hz, 1Hb, =CH2), 4.80 – 4.55 (m, 3H, 2H NCH2 in Allyl, 1H OH), 3.96 – 3.85 (m, 1H, CHOH), 3.39 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.16 (dd, J = 13.1, 6.9 Hz, 1Hb, SCH2), 2.42 (br.t., J = 5.1 Hz, 4H, N(CH2CH2)2CH2), 2.38 – 2.34 (m, 2H, NCH2CHOH) 2.16 (s, 3H, CH3), 1.58 – 1.50 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.38 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 154.1 (SC=N), 152.0, 150.6, 131.2 (CH=CH2), 129.8 (CH=), 127.8, 126.0 (CH=), 125.0, 117.5 (=CH2), 112.6 (CH=), 66.5 (OCH), 63.3 (NCH2), 60.1 (OCH2), 54.5 (2*NCH2), 45.7 (NCH2), 38.3 (SCH2), 25.5 (2*CH2), 23.8 (CH2), 15.7 (CH3). IRνmax = 3299.13; 3085.55; 2932.72; 2852.69; 2797.72; 2758.19; 1645.46; 1597.73; 1489.74; 1467.08; 1440.08; 1418.87; 1396.21; 1330.16; 1295.93; 1276.65; 1239.52; 1225.06; 1186.49; 1154.67; 1133.46; 1088.62; 1071.26; 1039.44; 1013.89; 994.12; 962.79; 930.49; 919.88; 877.93; 838.40; 803.69; 786.81; 746.80; 687.98; 654.23; 641.22; 590.11; 554.43; 513.94; 481.63; 468.14; 460.90; 441.14; 404.50 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C21H30ClN4O2S+ 437.1778; Found 437.1794.
1-((4-allyl-5-((2,4-dichlorophenoxy)methyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3i). Yield 68%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.38 – 7.32 (m, 2Harom), 7.26 (dd, J = 8.9, 2.6 Hz, 1Harom), 5.93 (ddt, J = 17.2, 10.6, 5.5 Hz, 1H, CH=), 5.31 (s, 2H, OCH2), 5.21 (dq, J = 10.3, 1.3 Hz, 1Ha, =CH2), 5.07 (dq, J = 17.0, 1.5 Hz, 1Hb, =CH2), 4.79 – 4.57 (m, 3H, 1H OH, 2H NCH2 in Allyl), 3.96 – 3.85 (m, 1H, CHOH), 3.39 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.16 (dd, J = 13.1, 6.8 Hz, 1Hb, SCH2), 2.41 (br.t., 4H, N(CH2CH2)2CH2), 2.35 (d, J = 6.4 Hz, 2H, NCH2CHOH), 1.57 – 1.50 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.37 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 152.2, 151.6, 150.0, 131.0 (CH=CH2), 129.1 (CH=), 127.4 (CH=), 125.6, 122.7, 117.9 (=CH2), 115.1 (CH=), 66.5 (OCH), 63.3 (NCH2), 60.9 (OCH2), 54.6 (2*NCH2), 45.9 (NCH2), 38.3 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3248.02; 3081.69; 3029.14; 2934.16; 2886.43; 2853.65; 2796.28; 2757.71; 1642.57; 1587.13; 1575.08; 1528.31; 1481.06; 1469.49; 1455.51; 1441.53; 1418.39; 1390.42; 1355.23; 1329.20; 1284.84; 1266.04; 1244.83; 1232.77; 1203.85; 1154.19; 1115.14; 1105.01; 1092.96; 1059.69; 1040.89; 1006.18; 995.09; 962.79; 932.90; 914.58; 890.95; 863.47; 833.58; 815.74; 802.72; 785.85; 722.69; 698.10; 687.02; 651.34; 641.70; 593.00; 555.40; 507.67; 490.79; 486.46; 467.65; 440.65; 421.85; 409.80 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C20H27Cl2N4O2S+ 457.1232; Found 457.1252.
1-((4-phenethyl-5-(thiophen-2-yl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3j). Yield 87%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.52 – 7.46 (m, 2Harom), 7.28 (d, J = 7.9 Hz, 2Harom), 5.95 (ddt, J = 17.2, 10.5, 4.6 Hz, 1H, CH=), 5.31 – 5.18 (m, 1Ha, =CH2), 5.00 – 4.88 (m, 1Hb, =CH2), 4.71 (br.s., 1H, OH), 4.60 (dt, J = 4.3, 1.9 Hz, 2H, NCH2 in Allyl), 3.98 – 3.87 (m, 1H, CHOH), 3.41 (dd, J = 13.2, 4.3 Hz, 1Ha, SCH2), 3.18 (dd, J = 13.2, 6.8 Hz, 1Hb, SCH2), 2.47 – 2.38 (m, 7H, 4H, N(CH2CH2)2CH2, 3H CH3), 2.37 (dd, J = 6.4, 2.1 Hz, 2H, NCH2CHOH), 1.60 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.37 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 154.5(SC=N), 151.3 (C=N), 139.0, 131.8 (CH=CH2), 128.9 (2*CH=), 127.7 (2*CH=), 124.1, 117.0 (=CH2), 66.6 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 46.1 (NCH2), 38.4 (SCH2), 25.5 (2*CH2), 23.8 (CH2), 20.8 (CH3). IRνmax = 3275.50; 3085.07; 3025.76; 2931.27; 2852.20; 2796.28; 2757.23; 1645.46; 1617.02; 1574.11; 1478.65; 1452.62; 1431.40; 1386.09; 1352.82; 1330.64; 1300.75; 1279.54; 1256.40; 1201.43; 1186.01; 1155.63; 1114.65; 1087.66; 1039.44; 1021.61; 993.64; 983.52; 962.79; 916.50; 891.92; 861.54; 821.53; 786.81; 762.23; 727.03; 685.09; 639.77; 626.75; 579.50; 552.99; 530.81; 491.28; 438.73; 430.53; 414.14; 405.94 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C20H29N4OS+ 373.2062; Found 373.2068.
1-((4-allyl-5-(2-bromophenyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3k). Yield 81%, colorless oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.72 – 7.67 (m, 1Harom), 7.49 – 7.37 (m, 3Harom), 5.69 (ddt, J = 17.2, 10.4, 5.3 Hz, 1H, CH=), 5.07 (dt, J = 10.2, 1.3 Hz, 1Ha, =CH2), 4.83 (dq, J = 17.0, 1.5 Hz, 1Hb, =CH2), 4.69 (br.s., 1H, OH), 4.43 – 4.30 (m, 2H, NCH2 in Allyl), 3.96 – 3.86 (m, 1H, CHOH), 3.38 (dd, J = 13.1, 4.4 Hz, 1Ha, SCH2), 3.17 (dd, J = 13.1, 6.8 Hz, 1Hb, SCH2), 2.51 – 2.28 (m, 6H, NCH2), 1.58 – 1.48 (m, 4H, N(CH2CH2)2CH2), 1.44 – 1.34 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.2 (SC=N), 150.8 (C=N), 132.4 (CH=), 132.2 (CH=), 131.6 (CH=CH2), 130.9 (CH=), 128.7, 127.2 (CH=), 123.3, 117.6 (=CH2), 66.5 (OCH), 63.3 (NCH2), 54.5 (2*NCH2), 45.9 (NCH2), 38.5 (SCH2), 25.4 (2*CH2), 23.8 (CH2). IRνmax = 3295.75; 3085.07; 3064.33; 3019.02; 2931.27; 2851.24; 2798.21; 2758.19; 2701.30; 1644.98; 1597.25; 1563.99; 1528.31; 1446.83; 1428.99; 1387.05; 1352.34; 1328.23; 1301.23; 1276.65; 1245.79; 1203.85; 1156.12; 1115.14; 1087.66; 1040.41; 1026.91; 993.64; 981.59; 962.30; 916.02; 891.92; 862.02; 768.98; 725.59; 712.57; 684.12; 647.96; 587.70; 567.45; 552.51; 511.04; 471.03; 451.26; 422.82; 403.53 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C19H2679BrN4OS+ 437.1011; Found 437.1019.
1-((4-allyl-5-(2-iodophenyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3l). Yield 72%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.98 (dd, J = 8.0, 1.1 Hz, 1Harom), 7.51 (td, J = 7.5, 1.2 Hz, 1Harom), 7.39 (dd, J = 7.6, 1.7 Hz, 1Harom), 7.28 (td, J = 7.7, 1.8 Hz, 2Harom), 5.73 (ddt, J = 17.1, 10.5, 5.3 Hz, 1H, CH=), 5.12 (dq, J = 10.4, 1.3 Hz, 1Ha, =CH2), 4.88 (dq, J = 17.0, 1.5 Hz, 1Hb, =CH2), 4.69 (br.s., 1H, OH), 4.46 – 4.29 (m, 2H, NCH2 in Allyl), 3.99 – 3.86 (m, 1H, CHOH), 3.42 (dd, J = 13.1, 4.4 Hz, 1Ha, SCH2), 3.20 (dd, J = 13.1, 6.8 Hz, 1Hb, SCH2), 2.43 (br.t., J = 4.8 Hz, 4H, N(CH2CH2)2CH2), 2.38 (dd, J = 6.4, 2.1 Hz, 2H, NCH2CHOH), 1.60 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.47 – 1.37 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 155.3 (SC=N), 150.8 (C=N), 138.7 (CH=), 132.7, 131.4 (CH=CH2), 131.3 (CH=), 130.9 (CH=), 127.5 (CH=), 117.7 (=CH2), 99.1 (=CI), 66.6 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 46.0 (NCH2), 38.6 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3293.34; 3085.55; 3058.55; 3033.48; 2983.34; 2930.79; 2850.76; 2796.28; 2757.71; 1644.98; 1590.50; 1559.65; 1525.42; 1441.53; 1428.03; 1386.57; 1351.86; 1328.23; 1300.75; 1272.79; 1245.79; 1203.36; 1155.63; 1115.14; 1087.66; 1039.44; 1016.78; 993.64; 980.63; 962.30; 916.02; 891.43; 861.54; 768.98; 720.76; 705.82; 679.78; 640.73; 587.22; 553.47; 509.12; 468.62; 459.94; 444.03; 421.85; 418.00 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C19H26IN4OS+ 485.0872; Found 485.0896.
1-((4-allyl-5-(2-nitrophenyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3m). Yield 89%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 8.25 – 8.14 (m, 1Harom), 7.91 – 7.76 (m, 2Harom), 7.70 – 7.59 (m, 1Harom), 5.77 (ddt, J = 17.1, 10.5, 5.4 Hz, 1H, CH=), 5.14 (dt, J = 10.3, 1.3 Hz, 1Ha, =CH2), 4.97 (dq, J = 17.1, 1.5 Hz, 1Hb, =CH2), 4.68 (br.s., 1H, OH), 4.52 – 4.37 (m, 2H, NCH2 in Allyl), 3.98 – 3.86 (m, 1H, CHOH), 3.44 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.20 (dd, J = 13.1, 6.9 Hz, 1Hb, SCH2), 2.43 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.41 – 2.33 (m, 2H, NCH2CHOH), 1.62 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.47 – 1.38 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 151.3, 150.8, 148.4, 133.1 (CH=), 132.2 (CH=), 131.3 (CH=CH2), 131.0 (CH=), 124.4 (CH=), 121.9, 117.8 (=CH2), 66.5 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 46.1 (NCH2), 38.6 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3293.34; 3084.58; 2932.72; 2852.20; 2799.17; 2758.67; 1644.98; 1617.50; 1575.08; 1527.83; 1452.14; 1441.05; 1432.37; 1419.83; 1388.50; 1344.14; 1301.23; 1278.57; 1253.50; 1203.85; 1155.63; 1115.14; 1087.66; 1070.78; 1039.44; 993.16; 983.04; 962.79; 930.00; 891.43; 852.86; 786.33; 751.14; 728.00; 716.91; 697.14; 652.30; 589.63; 559.26; 534.19; 511.04; 479.22; 459.94; 442.10; 423.30; 576.13; 525.99; 484.53; 465.24; 444.03; 420.89; 404.50 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C19H26N5O3S+ 404.1756; Found 404.1772.
1-((4-allyl-5-(furan-2-yl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3n). Yield 91%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.70 (d, J = 1.8 Hz, 1Hfur), 6.95 (d, J = 3.5 Hz, 1Hfur), 6.59 (dd, J = 3.5, 1.9 Hz, 1Hfur), 5.94 (ddt, J = 17.2, 10.3, 5.1 Hz, 1H, CH=), 5.26 – 5.13 (m, 1Ha, =CH2), 5.00 (dd, J = 17.2, 2.2 Hz, 1Hb, =CH2), 4.81 (dt, J = 5.2, 1.8 Hz, 2H, NCH2 in Allyl), 4.67 (br.s., 1H, OH), 3.97 – 3.85 (m, 1H, CHOH), 3.40 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.17 (dd, J = 13.1, 6.9 Hz, 1Hb, SCH2), 2.42 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.36 (dd, J = 6.3, 1.5 Hz, 2H, NCH2CHOH), 1.58 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.44 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 151.2 (SC=N), 146.5 (C=N), 143.5 (CH=), 141.7, 131.5 (CH=CH2), 117.2 (=CH2), 111.2 (CH=), 110.8 (CH=), 66.5 (OCH), 63.3 (NCH2), 54.6 (2*NCH2), 46.4 (NCH2), 38.6 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3293.34; 3148.22; 3121.22; 3088.92; 2983.34; 2932.23; 2852.20; 2797.72; 2758.67; 2697.44; 1645.46; 1612.20; 1515.29; 1466.60; 1440.56; 1428.51; 1420.80; 1378.85; 1352.82; 1331.61; 1301.23; 1278.57; 1257.84; 1224.09; 1205.77; 1158.04; 1115.14; 1087.66; 1070.30; 1039.44; 1015.82; 993.16; 962.30; 915.54; 902.04; 885.17; 862.02; 821.53; 785.37; 742.94; 712.09; 684.12; 648.45; 592.52; 546.24; 496.58; 475.85; 444.03; 429.08; 413.66; 403.53 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C17H25N4O2S+ 349.1698; Found 349.1708.
1-((4-allyl-5-(5-bromofuran-2-yl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3o). Yield 80%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.01 – 6.92 (m, 1Hfur), 6.64 – 6.55 (m, 1Hfur), 5.94 (ddt, J = 17.3, 10.3, 5.1 Hz, 1H, CH=), 5.28 – 5.18 (m, 1Ha, =CH2), 5.09 – 4.98 (m, 1Hb, =CH2), 4.83 – 4.72 (m, 2H, NCH2 in Allyl), 4.61 (br.s., 1H, OH), 3.96 – 3.83 (m, 1H, CHOH), 3.41 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.17 (dd, J = 13.1, 6.8 Hz, 1Hb, SCH2), 2.41 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.35 (d, J = 6.4 Hz, 2H, NCH2CHOH), 1.59 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.46 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 151.6 (SC=N), 145.6 (C=N), 143.6, 131.2 (CH=CH2), 122.9, 117.5 (=CH2), 113.2 (2*CH=), 66.5 (OCH), 63.3 (NCH2), 54.6 (2*NCH2), 46.4 (NCH2), 38.6 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3308.77; 3140.03; 3120.74; 3087.96; 2984.30; 2932.23; 2851.72; 2796.28; 2757.23; 1645.46; 1613.64; 1514.81; 1465.63; 1441.05; 1426.58; 1420.32; 1378.85; 1353.30; 1331.12; 1301.23; 1278.09; 1256.40; 1205.29; 1155.63; 1114.65; 1091.99; 1039.44; 1012.45; 993.64; 962.30; 928.56; 892.40; 861.54; 786.33; 762.71; 709.19; 684.61; 649.41; 587.70; 548.65; 534.67; 510.08; 477.30; 459.94; 433.90; 414.14; 408.83; 403.0594 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C17H2479BrN4O2S+ 427.0803; Found 427.0818.
1-((4-allyl-5-(pyridin-3-yl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3p). Yield 80%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 8.80 (d, J = 2.2 Hz, 1Hpyr), 8.66 (dd, J = 4.9, 1.7 Hz, 1Hpyr), 8.01 (dt, J = 8.0, 2.0 Hz, 1Hpyr), 7.48 (dd, J = 7.9, 4.8 Hz, 1Hpyr), 6.04 – 5.89 (m, 1H, CH=), 5.27 (dd, J = 10.5, 2.0 Hz, 1Ha, =CH2), 4.94 (dd, J = 17.2, 2.1 Hz, 1Hb, =CH2), 4.81 – 4.47 (m, 3H, 1H OH, 2H NCH2 in Allyl), 3.99 – 3.86 (m, 1H, CHOH), 3.44 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.20 (dd, J = 13.1, 6.9 Hz, 1Hb, SCH2), 2.43 (br.t., J = 5.0 Hz, 4H, N(CH2CH2)2CH2), 2.37 (dd, J = 6.4, 1.5 Hz, 2H, NCH2CHOH), 1.59 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.46 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 152.3, 152.1, 150.1 (CH=N), 148.1 (CH=N), 135.0 (CH=), 131.7 (CH=CH2), 123.2, 123.1 (CH=), 117.1 (=CH2), 66.6 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 46.3 (NCH2), 38.5 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3291.41; 3086.03; 3072.05; 3035.41; 2982.37; 2931.75; 2851.72; 2796.76; 2759.64; 1644.98; 1598.70; 1571.22; 1513.85; 1453.10; 1432.37; 1412.60; 1386.09; 1352.34; 1328.71; 1301.23; 1278.57; 1257.84; 1241.45; 1201.43; 1191.79; 1155.63; 1115.14; 1088.14; 1039.44; 1024.98; 994.12; 981.59; 962.79; 918.43; 891.43; 861.54; 812.37; 786.33; 764.64; 709.19; 687.02; 651.34; 620.00; 590.11; 551.54; 505.26; 479.22; 437.76; 424.26 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C18H26N5OS+ 360.1858; Found 360.1876.
1-((4-allyl-5-(pyridin-4-yl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3q). Yield 89%, m.p. 86 °C . 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 8.77 – 8.60 (m, 2Hpyr), 7.71 – 7.52 (m, 2Hpyr), 5.99 (ddt, J = 17.2, 10.5, 4.5 Hz, 1H, CH=), 5.34 – 5.23 (m, 1Ha, =CH2), 5.01 – 4.90 (m, 1Hb, =CH2), 4.79 – 4.58 (m, 3H, 2H NCH2 in Allyl, 1H OH), 3.99 – 3.86 (m, 1H, CHOH), 3.45 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.20 (dd, J = 13.1, 6.9 Hz, 1Hb, SCH2), 2.49 – 2.25 (m, 6H, NCH2), 1.60 – 1.47 (m, 4H, N(CH2CH2)2CH2), 1.46 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.0, 152.3, 149.8 (2*CH=N), 134.1, 131.6 (CH=CH2), 121.4 (2*CH=), 117.2 (=CH2), 66.5 (OCH), 63.4 (NCH2), 54.6 (2*NCH2), 46.4 (NCH2), 38.4 (SCH2), 25.5 (2*CH2), 23.8 (CH2). IRνmax = 3190.17; 3084.58; 3045.53; 2985.75; 2969.84; 2929.34; 2917.29; 2849.79; 2806.40; 2774.58; 2755.30; 2730.71; 2669.48; 2552.81; 2519.54; 1644.50; 1604.97; 1557.24; 1515.29; 1451.65; 1428.03; 1411.16; 1379.34; 1367.28; 1326.30; 1312.32; 1301.72; 1284.36; 1255.91; 1235.67; 1221.68; 1201.43; 1186.01; 1154.19; 1116.10; 1087.17; 1066.44; 1049.57; 1039.44; 1016.30; 998.46; 988.82; 962.30; 925.66; 870.70; 857.69; 825.87; 790.19; 762.23; 728.48; 705.82; 699.07; 658.57; 582.88; 574.68; 557.81; 536.11; 511.04; 478.74; 457.53; 447.89; 422.82; 408.35; 403.53 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C18H26N5OS+ 360.1858; Found 360.1862.
1-((4-phenyl-5-propyl-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3r). Yield 63%, colorless oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.58 – 7.45 (m, 3Harom), 7.36 – 7.28 (m, 2Harom), 4.63 (br.s., 1H, OH), 3.91 – 3.77 (m, 1H, CH), 3.28 (dd, J = 13.2, 4.4 Hz, 1Ha, SCH2), 3.04 (dd, J = 13.2, 6.7 Hz, 1Hb, SCH2), 2.49 – 2.44 (m, 2H, CH2CH2CH3), 2.38 (br.t., J = 5.2 Hz, 4H, N(CH2CH2)2CH2), 2.29 (dd, J = 6.7, 2.4 Hz, 2H, NCH2CHOH), 1.62 – 1.53 (m, 2H, CH2CH2CH3), 1.53 – 1.45 (m, 4H, N(CH2CH2)2CH2), 1.41 – 1.32 (m, 2H, N(CH2CH2)2CH2), 0.87 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 154.5 (SC=N), 150.3 (C=N), 133.2 (=CH), 129.3 (2*CH=), 129.2, 126.9 (2*CH=), 66.5 (OCH), 63.3 (NCH2), 54.5 (2*NCH2), 37.4 (SCH2), 26.4 (CH2), 25.4 (2*CH2), 23.7 (CH2), 19.7 (CH2), 13.3 (CH3). IRνmax = 3281.77; 3062.89; 2931.27; 2872.45; 2851.72; 2796.76; 2757.71; 1597.25; 1589.06; 1521.56; 1498.42; 1441.53; 1431.40; 1396.69; 1351.86; 1327.75; 1300.27; 1276.16; 1156.12; 1116.10; 1087.66; 1074.64; 1039.44; 1009.07; 994.61; 962.79; 891.43; 862.02; 808.99; 785.85; 770.90; 742.46; 696.18; 614.22; 602.65; 562.63; 499.95; 480.19; 470.55; 445.48; 419.92; 411.73; 405.94 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C19H29N4OS+ 361.2062; Found 361.2073.
1-((4,5-diphenyl-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3s). Yield 83%, white solid, m.p. 97 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.59 – 7.45 (m, 3Harom), 7.37 – 7.24 (m, 7Harom), 4.68 (br.s., 1H, OH), 4.01 – 3.87 (m, 1H, CHOH), 3.43 (dd, J = 13.1, 4.4 Hz, 1Ha, SCH2), 3.18 (dd, J = 13.2, 6.8 Hz, 1Hb, SCH2), 2.44 (br.t., J = 5.4 Hz, 4H, N(CH2CH2)2CH2), 2.39 – 2.31 (m, 2H, NCH2CHOH), 1.61 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.46 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.6, 152.4, 134.0, 129.3 (2*CH=), 128.9 (CH=), 127.9 (2*CH=), 127.4 (2*CH=), 127.1 (2*CH=), 126.6, 66.4 (OCH), 63.3 (NCH2), 54.5 (2*NCH2), 37.4 (SCH2), 25.4 (2*CH2), 23.7 (CH2). IRνmax = 3450.51; 3104.35; 3085.55; 3049.87; 3012.27; 2934.16; 2918.25; 2855.10; 2787.12; 2757.71; 2736.01; 2696.96; 2647.79; 1596.29; 1497.94; 1473.35; 1457.44; 1445.87; 1425.62; 1377.89; 1356.68; 1330.16; 1304.61; 1282.91; 1271.82; 1257.36; 1246.27; 1214.45; 1176.85; 1153.71; 1118.99; 1095.85; 1073.19; 1043.30; 1032.21; 1006.66; 995.09; 970.98; 922.29; 906.38; 889.02; 861.54; 849.49; 834.06; 808.99; 791.64; 771.87; 729.92; 710.16; 694.25; 620.00; 614.22; 602.65; 584.33; 558.29; 514.42; 504.29; 482.12; 465.72; 425.71; 406.42 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C22H27N4OS+ 395.1906; Found 395.1912.
1-((4-phenyl-5-(m-tolyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3t). Yield 90%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.57 – 7.46 (m, 3Harom), 7.33 – 7.29 (m, 3Harom), 7.14 – 7.08 (m, 2Harom), 7.04 – 6.99 (m, 1Harom), 4.63 (br.s., 1H, OH), 4.01 – 3.87 (m, 1H, CHOH), 3.43 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.18 (dd, J = 13.1, 6.8 Hz, 1Hb, SCH2), 2.42 (br.t, 4H, N(CH2CH2)2CH2), 2.37 – 2.31 (m, 2H, NCH2CHOH), 2.28 (s, 3H, CH3), 1.59 – 1.49 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.6, 152.2, 137.2, 134.0, 129.6 (CH=), 129.3 (2*CH=), 129.1 (CH=), 128.2 (CH=), 127.7 (2*CH=), 126.4, 124.4 (CH=), 66.5 (OCH), 63.4 (NCH2), 54.5 (2*NCH2), 37.5 (SCH2), 25.5 (2*CH2), 23.8 (CH2), 20.8 (CH3). IRνmax = 3303.95; 3060.48; 3036.37; 2931.27; 2851.24; 2797.24; 2757.23; 1607.86; 1595.81; 1497.45; 1485.40; 1465.15; 1453.10; 1432.37; 1375.96; 1324.37; 1303.16; 1268.45; 1242.90; 1210.11; 1156.12; 1116.10; 1088.14; 1073.19; 1038.96; 1009.55; 994.61; 962.79; 911.20; 887.09; 861.06; 849.97; 788.26; 768.49; 717.87; 692.32; 680.75; 667.73; 608.43; 545.27; 525.03; 502.85; 481.15; 442.10; 429.57; 418.96; 410.28 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C23H29N4OS+ 409.2062; Found 409.2082.
1-((4-phenyl-5-(p-tolyl)-4H-1,2,4-triazol-3-yl)thio)-3-(piperidin-1-yl)propan-2-ol (3u). Yield 85%, white solid, m.p. 115 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.56 – 7.46 (m, 3Harom), 7.34 – 7.26 (m, 2Harom), 7.26 – 7.18 (m, 2Harom), 7.11 – 7.03 (m, 2Harom), 4.60 (br.s., 1H, OH), 3.98 – 3.85 (m, 1H, CHOH), 3.41 (dd, J = 13.1, 4.3 Hz, 1Ha, SCH2), 3.16 (dd, J = 13.1, 6.8 Hz, 1Hb, SCH2), 2.41 (br.t., J = 5.3 Hz, 4H, N(CH2CH2)2CH2), 2.36 – 2.25 (m, 5H, 2H NCH2CH, 3H CH3), 1.59 – 1.47 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 153.6, 152.1, 138.6, 134.1, 129.3 (2*CH=), 129.1 (CH=), 128.5 (2*CH=), 127.4 (2*CH=), 127.1 (2*CH=) 123.7, 66.5 (OCH), 63.4 (NCH2), 54.5 (2*NCH2), 37.4 (SCH2), 25.5 (2*CH2), 23.8 (CH2), 20.7 (CH3). IRνmax = 3410.01; 3099.53; 3069.64; 3048.91; 2932.23; 2920.18; 2858.47; 2803.51; 2759.64; 2665.14; 1613.64; 1593.40; 1494.56; 1479.62; 1430.44; 1403.92; 1374.51; 1356.68; 1335.95; 1322.93; 1305.09; 1284.36; 1264.11; 1243.38; 1227.95; 1205.77; 1185.53; 1158.04; 1152.26; 1120.92; 1106.94; 1081.39; 1062.10; 1039.93; 1020.64; 1012.45; 1005.70; 992.20; 970.02; 947.84; 917.47; 890.95; 867.81; 847.08; 822.97; 798.87; 782.48; 769.46; 748.73; 725.59; 708.23; 694.25; 645.55; 633.50; 612.77; 583.84; 552.51; 525.51; 514.90; 498.51; 484.05; 478.74; 444.03; 424.26; 406.91 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C23H29N4OS+ 409.2062; Found 409.2062.
5-benzyl-2-(2-(5-((2-hydroxy-3-(piperidin-1-yl)propyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)ethyl)-4-phenyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (3v). Yield 68%, yellow oil. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.61 – 7.52 (m, 3Harom), 7.48 – 7.40 (m, 5Harom), 7.21 – 7.09 (m, 5Harom), 6.93 – 6.85 (m, 2Harom), 4.62 (br.s., 1H, OH), 4.44 (t, J = 7.3 Hz, 2H, NNCH2), 3.92 – 3.86 (m, 1H, CHOH), 3.83 (s, 2H, CH2Ph), 3.34 (dd, J = 13.2, 4.3 Hz, 1Ha, SCH2), 3.16 – 3.06 (m, 3H, 1Hb, SCH2, 2H NNCH2CH2), 2.39 (br.t., J = 5.1 Hz, 4H, N(CH2CH2)2CH2), 2.31 (d, J = 6.4 Hz, 2H, NCH2CHOH), 1.57 – 1.47 (m, 4H, N(CH2CH2)2CH2), 1.45 – 1.36 (m, 2H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 167.4, 151.5, 151.0, 149.3, 133.7, 133.6, 132.9, 129.4, 129.3, 128.9, 128.7, 128.2, 127.9, 127.7, 127.0, 126.4, 66.5 (OCH), 63.4 (NCH2), 54.5 (2*NCH2), 45.5 (NCH2), 37.6 (SCH2), 31.2 (CH2), 25.5 (2*CH2), 23.8 (CH2), 23.3 (CH). IRνmax = 3363.73; 3088.44; 3062.89; 3033.00; 2932.72; 2851.72; 2799.17; 2757.71; 1596.77; 1567.84; 1522.52; 1497.45; 1478.17; 1454.55; 1442.01; 1415.01; 1344.62; 1333.53; 1295.45; 1256.88; 1213.97; 1155.63; 1115.62; 1072.71; 1038.00; 1016.30; 995.09; 962.79; 918.91; 890.95; 862.02; 767.53; 722.69; 694.25; 679.30; 638.80; 614.22; 566.00; 533.22; 503.81; 477.30; 458.01; 436.80; 418.96 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C33H38N7OS2+ 612.2579; Found 612.2579.
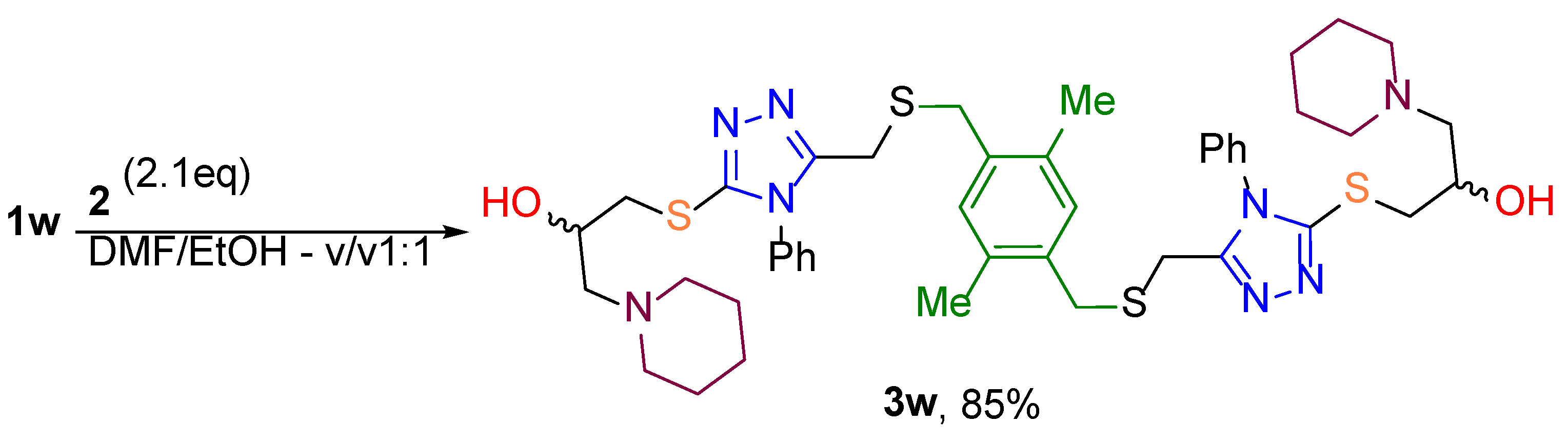
3,3′-((((((2,5-dimethyl-1,4-phenylene)bis(methylene))bis(sulfanediyl))bis(methylene))bis(4-phenyl-4H-1,2,4-triazole-5,3-diyl))bis(sulfanediyl))bis(1-(piperidin-1-yl)propan-2-ol) (3w). Yield 73%, white solid, m.p. 186 °C. 1H NMR (400 MHz, DMSO/CCl4 -1/3) δ 7.59 – 7.46 (m, 6Harom), 7.39 – 7.28 (m, 4Harom), 7.00 (s, 2Harom), 4.58 (br.s., 2H, OH), 3.94 – 3.83 (m, 2H, CHOH), 3.65 (s, 4H, CH2CNN), 3.53 (s, 4H, SCH2Ar), 3.38 (dd, J = 13.1, 4.3 Hz, 2Ha, SCH2CHOH), 3.12 (dd, J = 13.1, 6.8 Hz, 2Hb, SCH2CHOH), 2.40 (br.t., J = 5.3 Hz, 8H, N(CH2CH2)2CH2), 2.32 (d, J = 6.4 Hz, 4H, NCH2CHOH), 2.22 (s, 6H, CH3), 1.61 – 1.46 (m, 8H, N(CH2CH2)2CH2), 1.46 – 1.32 (m, 4H, N(CH2CH2)2CH2). 13C NMR (101 MHz, DMSO/CCl4 -1/3) δ 152.3 (2*N=CCH2), 151.5 (2*N=CS), 133.7 (2*C=CCH3), 133.2 (2*C=CCH2), 132.7 (2*NC=CH), 131.7 (2*CH=CCH3), 129.5 (2*CH=), 129.3 (4*CH=), 126.9 (4*CH=CN), 66.4 (2*OCH), 63.5 (2*NCH2CH), 54.5 (4*NCH2), 37.6 (2*SCH2CH), 32.6 (2*SCH2(C6H2)), 25.4 (4*NCH2), 24.0 (2*SCH2(C2N3)), 23.8 (2*CH2), 17.9 (2*CH3). IRνmax = 3447.62; 3070.12; 3055.17; 2960.68; 2948.63; 2920.66; 2851.72; 2803.03; 2759.15; 2735.53; 1496.49; 1445.39; 1435.26; 1409.23; 1403.44; 1388.01; 1374.03; 1354.75; 1325.82; 1321.00; 1303.16; 1279.06; 1253.02; 1238.56; 1222.65; 1171.54; 1151.78; 1116.10; 1090.07; 1069.82; 1044.75; 1028.84; 1007.62; 991.23; 964.23; 923.25; 916.02; 891.43; 859.61; 843.22; 833.58; 797.42; 781.03; 748.25; 702.93; 689.43; 663.87; 615.66; 582.40; 554.92; 486.94; 462.35; 426.19 cm-1. HRMS (ESI) m/z: [M + H]+ Calcd for C44H59N8O2S4+ 859.3644; Found 859.3639.