Submitted:
11 March 2025
Posted:
13 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Evolving Hypotheses of Milk Fever: A Historical and Contemporary Overview
2.1. Historical Context and Early Theories
2.1.1. Hibbs Review and Subsequent Hypotheses
2.1.2. Development of the Hypocalcemia Theory
2.1.3. The DCAD and Potassium Hypothesis: A Shift in Thinking
2.1.4. The Endotoxin Hypothesis and Systemic Inflammation: A New Perspective
2.1.5. Endotoxins and Systemic Inflammation
3. Inflammatory Response in Milk Fever Cows
4. Re-Evaluating Traditional Theories in Light of the Endotoxin Hypothesis: The Calci-Inflammatory Axis
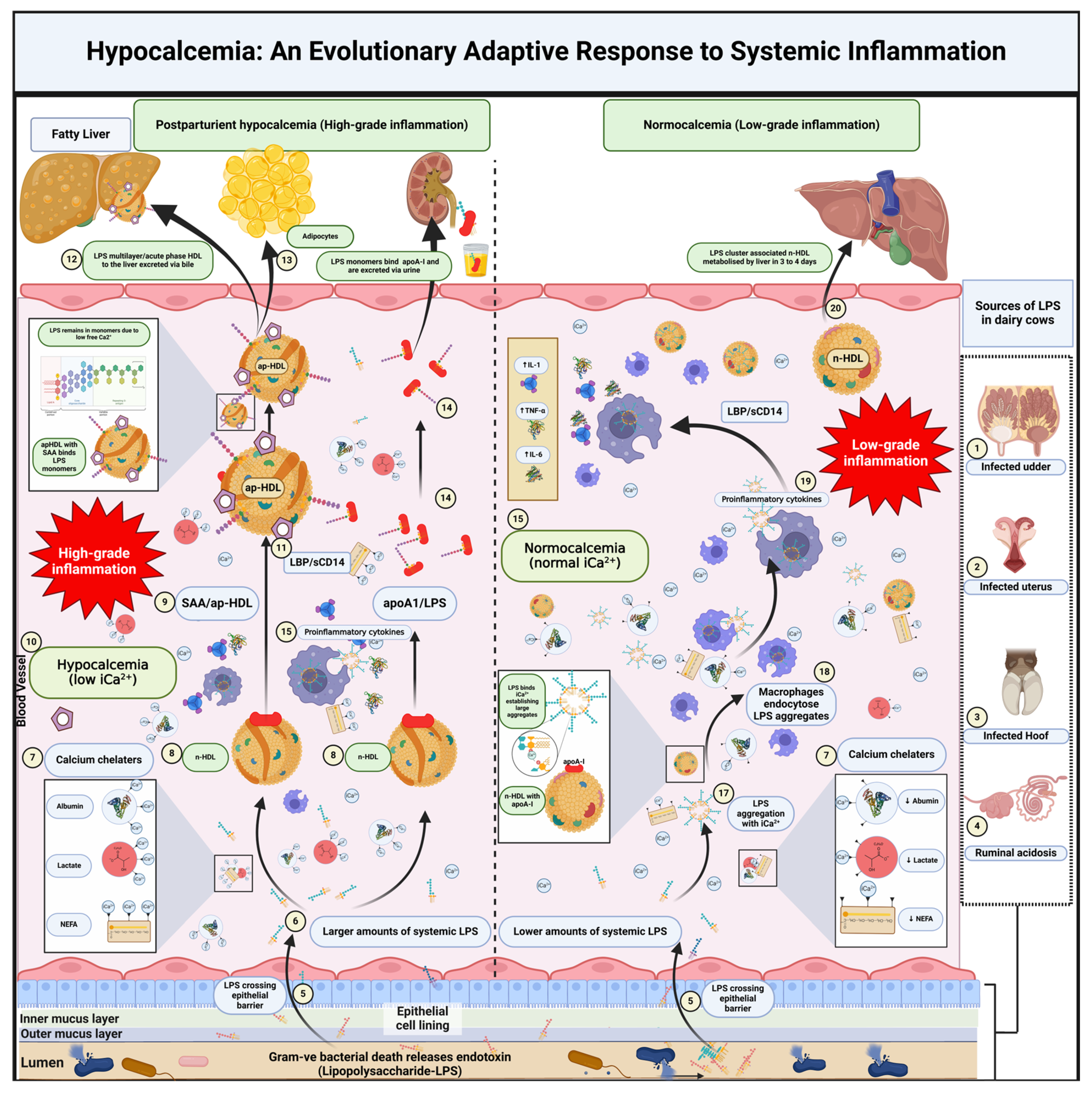
4.1. Multiple Sources of Endotoxin and Early Immune Activation
4.2. Mechanisms of the Calci-Inflammatory Axis
4.3. Clinical Consequences
4.4. Hypocalcemia in Multiple Periparturient Diseases: The Common Role of Inflammation
5. A New Hypothesis on Hypocalcemia and Its Role in Inflammation
5.1. Fate of Endotoxin in the Circulation
5.2. Calcium’s Role in LPS Aggregation and Immune Activation
5.3. Hypocalcemia as a Protective Mechanism
5.4. Lipoproteins and LPS Clearance
5.5. Modulation of the Inflammatory Response
5.6. Interactions of Calcium with Lactate, Fatty Acids, and Albumin
5.7. Lactate Binding to Ionized Calcium
5.8. Non-Esterified Fatty Acids Binding to Ionized Calcium
5.9. Albumin Binding to Ionized Calcium
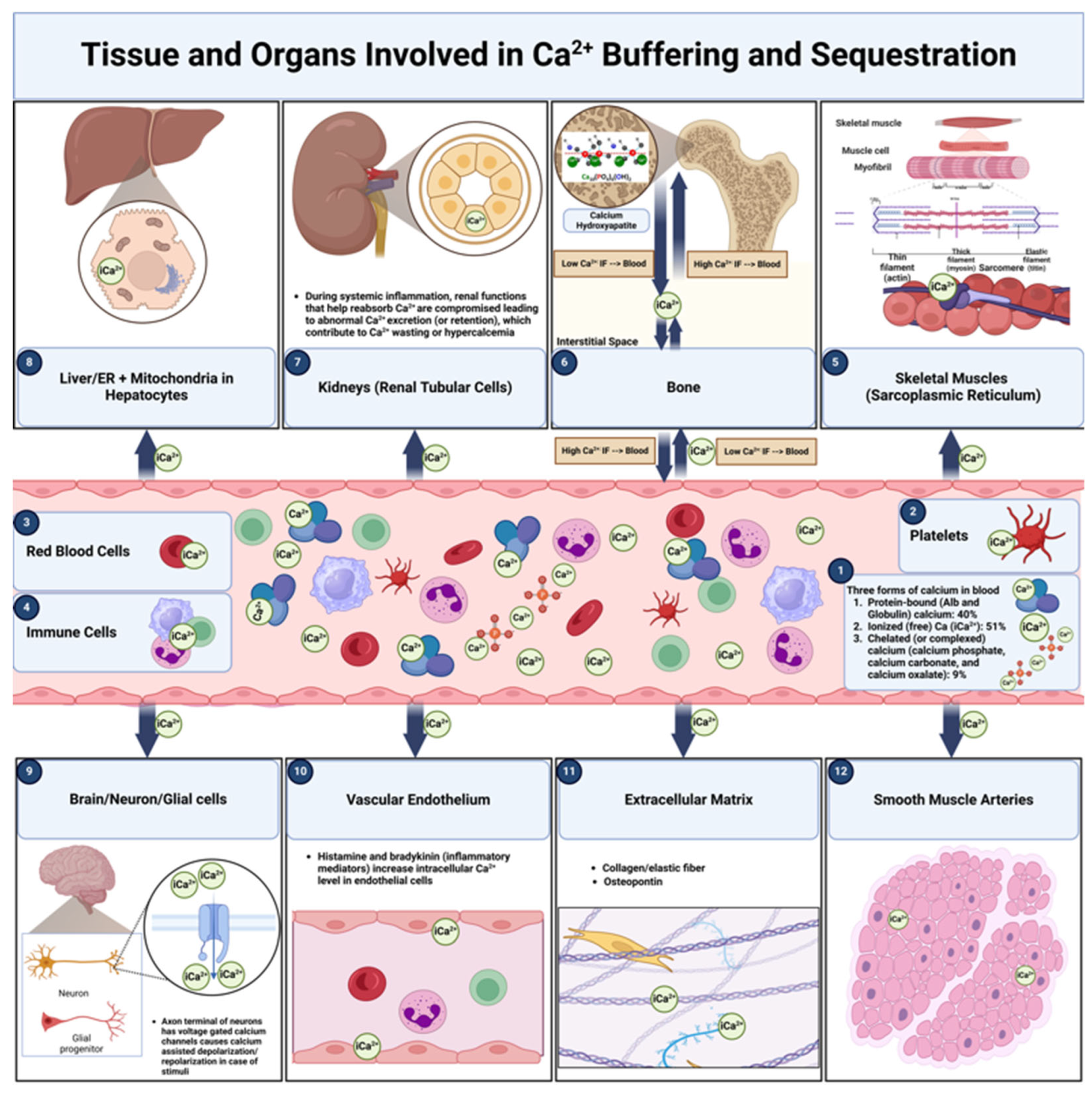
6. Calcium Buffering and Sequestration: Distinct Mechanisms and Key Locations in Calcium Homeostasis During Inflammation
6.1. Calcium Buffering
6.1.1. Primary Sites of Calcium Buffering
6.1.1.2. Blood Plasma
6.1.1.3. Extracellular Matrix (ECM)
6.1.1.4. Intracellular Compartments
6.2. Calcium Sequestration
6.2.1. Primary Sites of Calcium Sequestration
6.2.1.2. Bones
6.2.1.3. Skeletal Muscles (Sarcoplasmic Reticulum)
6.2.1.4. Liver (Hepatocytes)
6.2.1.5. Kidneys (Renal Tubular Cells)
6.2.1.5. Brain (Neurons and Glial Cells)
6.2.1.6. Mitochondria
7. Impact of Systemic Inflammation on Calcium Buffering and Sequestration
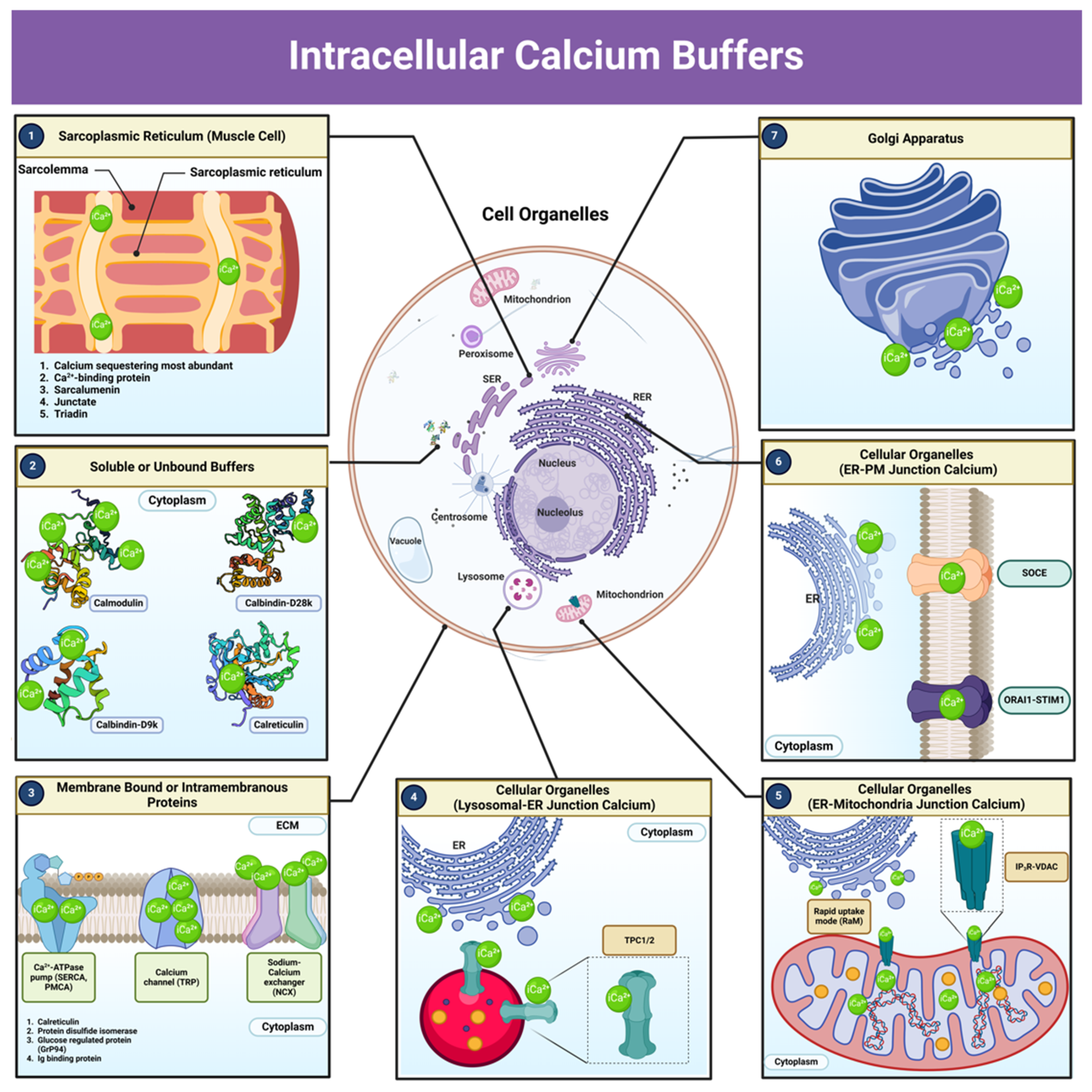
8. Intracellular Calcium Buffers: Pathways and Components Involved in Calcium Homeostasis
9. Mechanistic Insights into the Calci-Inflammatory Axis
9.1. Adaptive Hypocalcemia as a Protective Mechanism
9.2. Calcium Signaling Pathways in Inflammation
9.3. The Calcium-Sensing Receptor (CaSR): Function and Mechanisms
9.4. CaSR in Immune Modulation
10. Evidence Linking Hypocalcemia to Inflammatory Responses in Dairy Cows
10.1. Recent Research in Dairy Cows Supporting the Hypothesis
10.2. Exacerbation of Inflammation by High Calcium Levels
10.3. Experimental Designs Inducing Endotoxemia
10.4. Hypocalcemia as a Response to Endotoxin-Induced Systemic Inflammation
10.5. Pre-Existing Systemic Inflammation in Cows Prone to Milk Fever
10.6. Calcium Oral Supplementation and Its Role in Postpartum Inflammation in Dairy Cows
11. How Hypocalcemia Eases Inflammation: A Mechanistic Overview
12. The Role of Calcium in LPS Aggregation and Clearance Without Inducing Inflammation
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of subclinical hypocalcemia in dairy herds. J. Dairy Sci. 2011, 94, 2928–2934. [Google Scholar] [CrossRef]
- Block, E. Manipulating dietary anions and cations for prepartum dairy cows to reduce incidence of milk fever. J. Dairy Sci. 1984, 67, 2939–2948. [Google Scholar] [CrossRef]
- Lean, I.J.; DeGaris, P.J.; McNeil, D.M.; Block, E. Hypocalcemia in dairy cows: Pathophysiology and preventive strategies. J. Dairy Sci. 2006, 89, 1094–1108. [Google Scholar]
- Block, E. Manipulation of dietary cation-anion difference on nutritionally related production diseases, productivity, and metabolic responses of dairy cows. J. Dairy Sci. 1994, 77, 1437–1450. [Google Scholar] [CrossRef]
- Venjakob, P.L.; Borchardt, S.; Heuwieser, W. Hypocalcemia—cow-level prevalence and preventive strategies in German dairy herds. J. Dairy Sci. 2017, 100, 9258–9266. [Google Scholar] [CrossRef]
- DeGaris, P.J.; Lean, I.J. Milk fever in dairy cows: A review of pathophysiology and control principles. Vet. J. 2008, 176, 58–69. [Google Scholar] [CrossRef]
- Couto Serrenho, R.; DeVries, T.J.; Duffield, T.F.; LeBlanc, S.J. Graduate student literature review: What do we know about the effects of clinical and subclinical hypocalcemia on health and performance of dairy cows? J. Dairy Sci. 2021, 104, 6304–6326. [Google Scholar] [CrossRef]
- Zebeli, Q.; Beitz, D.C.; Bradford, B.J.; Dunn, S.M.; Ametaj, B.N. Peripartal alterations of calcitonin gene-related peptide and minerals in dairy cows affected by milk fever. Vet. Clin. Pathol. 2013, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ametaj, B.N.; Zebeli, Q.; Iqbal, S. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Rev. Bras. Zootec. 2010, 39, 433–444. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef] [PubMed]
- Hibbs, J.W. Milk fever (parturient paresis) in dairy cows: A review. J. Dairy Sci. 1950, 33, 758–789. [Google Scholar]
- Hutyra, F.; Marek, J.; Manniger, R. Special pathology and therapeutics of the diseases of domestic animals, 4th ed.; Greig, J.R., Ed.; 1938.
- Dryerre, H.; Greig, J.R. Milk fever: Its possible association with derangements in the internal secretions. Vet. Rec. 1925, 5, 225–231. [Google Scholar]
- Auger, M.L. Réalisation expérimentale de la fièvre vitulaire. Compt. Rend. 1926, 182, 348–350. [Google Scholar]
- Little, W. L., and N. C. Wright. The aetiology of milk fever in cattle. Br. J. Exp. Pathol. 1925, 6, 129.
- Dryrre, H.; Greig, J.R. The specific chemotherapy of milk fever by the parenteral administration of Ca-boro-gluconate. Vet. Med. 1935, 30, 234–238. [Google Scholar]
- Ender, F.; Dishington, I.W.; Helgebostad, A. Calcium balance studies in dairy cows under experimental induction and prevention of hypocalcaemic paresis puerperalis. The solution of the aetiology and the prevention of milk fever by dietary means. Z. Tierphysiol. Tierernaehr. Futtermittelkd. 1971, 28, 233. [Google Scholar]
- Dishington, I.W. Prevention of milk fever (hypocalcaemia paresis puerperalis) by dietary salt supplements. Acta Vet. Scand. 1975, 16, 503–512. [Google Scholar]
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A.; Buxton, D.R. Strategies for preventing milk fever in dairy cattle. J. Dairy Sci. 1997, 80, 1269–1280. [Google Scholar]
- Aiumlamai, S.; Kindahl, H.; Fredriksson, G.; Edqvist, L.E.; Kulander, L.; Eriksson, O. The role of endotoxins in induced ruminal acidosis in calves. Acta Vet. Scand. 1992, 33, 117–127. [Google Scholar] [CrossRef]
- Andersen, P.H. Bovine endotoxicosis: Some aspects of relevance to production diseases. A review. Acta Vet. Scand. Suppl. 2003, 98, 141–155. [Google Scholar] [PubMed]
- Ametaj, B.N. A new understanding of the causes of fatty liver in dairy cows. Adv. Dairy Technol. 2005, 17, 97–112. [Google Scholar]
- Ametaj, B.N. Strong relationships between mediators of the acute phase response and fatty liver in dairy cows. Can. J. Dairy Sci. 2005, 85, 165–175. [Google Scholar]
- Zhang, G.; Dervishi, E.; Ametaj, B.N. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res. Vet. Sci. 2018, 117, 167–177. [Google Scholar] [CrossRef]
- Shandilya, U.; Sharma, A.; Mallikarjunappa, S.; Guo, J.; Mao, Y.; Meade, K.; Karrow, N. CRISPR-Cas9-mediated knockout of TLR4 modulates Mycobacterium avium ssp. paratuberculosis cell lysate-induced inflammation in bovine mammary epithelial cells. J. Dairy Sci. 2021. [Google Scholar] [CrossRef]
- Carroll, J.; Reuter, R.; Chase, C.; Coleman, S.; Riley, D.; Spiers, D.; Arthington, J.; Galyean, M. Profile of the bovine acute-phase response following an intravenous bolus-dose lipopolysaccharide challenge. Innate Immun. 2009, 15, 81–89. [Google Scholar] [CrossRef]
- Jermann, P.; Wagner, L.; Fritsche, D.; Gross, J.; Wellnitz, O.; Bruckmaier, R. Acute phase reaction to LPS-induced mastitis in early lactation dairy cows fed nitrogenic, glucogenic or lipogenic diets. J. Dairy Sci. 2023. [CrossRef]
- Augustine, M.; Leonard, M.; Thayu, M.; Baldassano, R.; De Boer, I.; Shults, J.; Denson, L.; DeBoer, M.; Herskovitz, R.; Denburg, M. Changes in vitamin D-related mineral metabolism after induction with anti-tumor necrosis factor-α therapy in Crohn’s disease. J. Clin. Endocrinol. Metab. 2014, 99, E991–E998. [Google Scholar] [CrossRef]
- Meurer, M.; Höcherl, K. Endotoxaemia differentially regulates the expression of renal Ca2+ transport proteins in mice. Acta Physiol. 2018, 225. [Google Scholar] [CrossRef]
- Klein, G. The role of calcium in inflammation-associated bone resorption. Biomolecules 2018, 8. [Google Scholar] [CrossRef]
- Dervishi, E.; Ametaj, B.N. Milk fever: Reductionist versus systems veterinary approach. In: Ametaj, B.N., ed. Periparturient Diseases of Dairy Cows; Springer: Berlin, Germany, 2017; pp. 247–266.
- Ametaj, B.N.; Goff, J.P.; Horst, R.L.; Bradford, B.J.; Beitz, D.C. Presence of acute phase response in normal and milk fever dairy cows around parturition. Acta Vet. Scand. Suppl. 2003, 98, 241. [Google Scholar]
- Thaveeratitham, P.; Khovidhunkit, W.; Patumraj, S. High-density lipoproteins (HDL) inhibit endotoxin-induced leukocyte adhesion on endothelial cells in rats: Effect of the acute-phase HDL. Clin. Hemorheol. Microcirc. 2007, 36, 1–12. [Google Scholar]
- Collage, R.D.; Howell, G.M.; Zhang, X.; Stripay, J.L.; Lee, J.S.; Angus, D.C.; Rosengart, M.R. Calcium supplementation during sepsis exacerbates organ failure and mortality via calcium/calmodulin-dependent protein kinase kinase signaling. Crit. Care Med. 2013, 41, e352–e360. [Google Scholar] [CrossRef]
- Emmanuel, D.G.; Madsen, K.L.; Churchill, T.A.; Dunn, S.M.; Ametaj, B.N. Acidosis and lipopolysaccharide from Escherichia coli B:055 cause hyperpermeability of rumen and colon tissues. J. Dairy Sci. 2007, 90, 5552–5557. [Google Scholar] [CrossRef]
- Su, G.; Klein, R.; Aminlari, A.; Zhang, H.; Steinstraesser, L.; Alarcon, W.; Remick, D.; Wang, S. Kupffer cell activation by lipopolysaccharide in rats: Role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 2000, 31, —. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Mu, R.; Guo, J.; Zhao, C.; Cao, Y.; Zhang, N.; Fu, Y. The rumen microbiota contributes to the development of mastitis in dairy cows. Microbiol. Spectr. 2022, 10(1): e0251221. [CrossRef]
- Papaioannou, N.; Voutsas, I.; Samara, P.; Tsitsilonis, O. A flow cytometric approach for studying alterations in the cytoplasmic concentration of calcium ions in immune cells following stimulation with thymic peptides. Cell. Immunol. 2016, 302, 32–40. [Google Scholar] [CrossRef]
- Yang, R.; Mark, M.; Gray, A.; Huang, A.; Xie, M.; Zhang, M.; Goddard, A.; Wood, W.; Gurney, A.; Godowski, P. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 1998, 395, 284–288. [Google Scholar] [CrossRef]
- Iamartino, L.; Brandi, M. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef]
- Hendy, G.; Canaff, L. Calcium-sensing receptor, proinflammatory cytokines and calcium homeostasis. Semin. Cell Dev. Biol. 2016, 49, 37–43. [Google Scholar] [CrossRef]
- Brown, E.M. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Nat. Rev. Endocrinol. 2013, 9, 391–403. [Google Scholar] [CrossRef]
- Kehrli, M.E. Jr.; Goff, J.P. Periparturient hypocalcemia in cows: Effects on peripheral blood neutrophil and lymphocyte function. J. Dairy Sci. 1989, 72, 1188–1196. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Canaff, L.; Hendy, G. Human calcium-sensing receptor gene. J. Biol. Chem. 2002, 277, 30337–30350. [Google Scholar] [CrossRef]
- Venjakob, P.; Staufenbiel, R.; Heuwieser, W.; Borchardt, S. Association between serum calcium dynamics around parturition and common postpartum diseases in dairy cows. J. Dairy Sci. 2020. [CrossRef]
- Venjakob, P.; Staufenbiel, R.; Heuwieser, W.; Borchardt, S. Serum calcium dynamics within the first 3 days in milk and the associated risk of acute puerperal metritis. J. Dairy Sci. 2019. [CrossRef]
- Rodríguez, E.M.; Arís, A.; Bach, A. Associations between subclinical hypocalcemia and postparturient diseases in dairy cows. J. Dairy Sci. 2017, 100, 7427–7434. [Google Scholar] [CrossRef]
- Atalay, H. The effect of serum β-hydroxybutyric acid and calcium levels on left displaced abomasum in Holstein cows on transition period. J. Istanbul Vet. Sci. 2019, 3, 43–48. [Google Scholar] [CrossRef]
- Curtis, C.; Erb, H.; Sniffen, C.; Smith, R.; Powers, P.; Smith, M.; Me, W.; Rb, H.; Pearson, E. Association of parturient hypocalcemia with eight periparturient disorders in Holstein cows. J. Am. Vet. Med. Assoc. 1983, 183, 559–561. [Google Scholar]
- Kantham, L.; Quinn, S.; Egbuna, O.; Baxi, K.; Butters, R.; Pang, J.; Pollak, M.; Goltzman, D.; Brown, E. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E915–E923. [Google Scholar] [CrossRef]
- Marmalyuk, D.; Runova, G.; Fadeyev, V. The role of the calcium-sensing receptor in the regulation of parathyroid hormone secretion in physiology and in calcitropic diseases. Osteoporos. Bone Dis. 2024, 13. [Google Scholar] [CrossRef]
- Zhang, B.X.; Huang, B.; Jiang, Q.; Loor, J.; Lv, X.; Zhang, W.; Li, M.; Wen, J.; Yin, Y.; Wang, J.; Yang, W.; Xu, C. Transcriptomics of circulating neutrophils in dairy cows with subclinical hypocalcemia. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Martinez, N.; Sinedino, L.; Bisinotto, R.; Ribeiro, E.; Gomes, G.; Lima, F.; Greco, L.; Risco, C.; Galvão, K.; Taylor-Rodriguez, D.; Driver, J.; Thatcher, W.; Santos, J. Effect of induced subclinical hypocalcemia on physiological responses and neutrophil function in dairy cows. J. Dairy Sci. 2014, 97, 874–887. [Google Scholar] [CrossRef]
- Abuajamieh, M.; Kvidera, S.K.; Fernandez, M.V.; Nayeri, A.; Upah, N.C.; Nolan, E.A.; Lei, S.M.; DeFrain, J.M.; Green, H.B.; Schoenberg, K.M.; Trout, W.E.; Baumgard, L.H. Inflammatory biomarkers are associated with ketosis in periparturient Holstein cows. Res. Vet. Sci. 2016, 109, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Reinhardt, T.; Goff, J. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Trebak, M.; Kinet, J. Calcium signalling in T cells. Nat. Rev. Immunol. 2019, 19, 154–169. [Google Scholar] [CrossRef]
- Ghoshal, S.; Witta, J.; Zhong, J.; de Villiers, W.; Eckhardt, E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009, 50, 90–97. [Google Scholar] [CrossRef]
- Trebicka, J.; Krag, A.; Gansweid, S.; Appenrodt, B.; Schiedermaier, P.; Sauerbruch, T.; Spengler, U. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur. J. Gastroenterol. Hepatol. 2011, 23, 1218–1225. [Google Scholar] [CrossRef]
- Munford, R.S.; Hall, C.L.; Dietschy, J.M. Binding of Salmonella typhimurium lipopolysaccharides to rat high-density lipoproteins. Infect. Immun. 1981, 34, 835–843. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Wright, S.D. Lipopolysaccharide (LPS) binding protein catalyzes binding of LPS to lipoproteins. Prog. Clin. Biol. Res. 1995, 392, 287–295. [Google Scholar]
- Kitchens, R.L.; Wolfbauer, G.; Albers, J.J.; Munford, R.S. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J. Biol. Chem. 1999, 274, 34116–34122. [Google Scholar] [CrossRef]
- Tobias, P.S.; Soldau, K.; Ulevitch, R.J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J. Biol. Chem. 1989, 264, 10867–10871. [Google Scholar]
- Wright, S.D.; Ramos, R.A.; Tobias, P.S.; Ulevitch, R.J.; Mathison, J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990, 249, 1431–1433. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Vogel, S.N. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002, 4, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Zaloga, G.P. Ionized hypocalcemia during sepsis. Crit. Care Med. 2000, 28, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Skarnes, R.C.; Chedid, L. Biological degradation and inactivation of endotoxin (chromate-labeled). In: Landy, M.; Braun, W., Eds. Bacterial Endotoxins; Rutgers Univ. Press: New Brunswick, NJ, USA, 1964; pp. 575–587. Braun, W. Bacterial Endotoxins, Ed.;
- Hotchkiss, R.S.; Karl, I.E. Calcium: A regulator of the inflammatory response in endotoxemia and sepsis. New Horiz. 1996, 4, 58–71. [Google Scholar] [PubMed]
- Feingold, K.R.; Grunfeld, C. Lipids: A key player in the battle between the host and microorganisms. J. Lipid Res. 2012, 53, 2487–2489. [Google Scholar] [CrossRef]
- Gibot, S. On the origins of lactate during sepsis. Crit. Care 2012, 16, 151. [Google Scholar] [CrossRef]
- Toffaletti, J.; Abrams, B. Effects of in vivo and in vitro production of lactic acid on ionized, protein-bound, and complex-bound calcium in blood. Clin. Chem. 1989, 35, 935–938. [Google Scholar]
- Khatua, B.; Yaron, J.R.; El-Kurdi, B.; Kostenko, S.; Papachristou, G.I.; Singh, V.P. Ringer’s lactate prevents early organ failure by providing extracellular calcium. J. Clin. Med. 2020, 9, 263. [Google Scholar] [CrossRef]
- Zaloga, G.P.; Willey, S.; Tomasic, P.; Chernow, B. Free fatty acids alter calcium binding: A cause for misinterpretation of serum calcium values and hypocalcemia in critical illness. J. Clin. Endocrinol. Metab. 1987, 64, 1010–1014. [Google Scholar] [CrossRef]
- Whitsett, J.; Tsang, R.C. In vitro effects of fatty acids on serum-ionized calcium. J. Pediatr. 1977, 91, 233–236. [Google Scholar] [CrossRef]
- Chapinal, N.; Leblanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.O. Protein-bound calcium in human serum: Quantitative examination of binding and its variables by a molecular binding model and clinical chemical implications for measurement of ionized calcium. Scand. J. Clin. Lab. Investig. 1972, 30, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Dimeski, G.; Treacy, O. The influence of albumin and pH on total and ionized calcium and magnesium. Point Care 2018, 17, 123–126. [Google Scholar] [CrossRef]
- Dalal, P.; Muller, W.; Sullivan, D. Endothelial cell calcium signaling during barrier function and inflammation. Am. J. Pathol. 2019, 189, 1529–1542. [Google Scholar] [CrossRef]
- Ridefelt, P.; Helmersson-Karlqvist, J. Albumin adjustment of total calcium does not improve the estimation of calcium status. Scand. J. Clin. Lab. Investig. 2017, 77, 442–447. [Google Scholar] [CrossRef]
- Buege, M.; Do, B.; Lee, H.; Weber, D.; Horowitz, S.; Feng, L.; Qing, Y.; Shank, B. Corrected calcium versus ionized calcium measurements for identifying hypercalcemia in patients with multiple myeloma. Cancer Treat. Res. Commun. 2019, 21, 100159. [Google Scholar] [CrossRef]
- Gilabert, J. Cytoplasmic calcium buffering: An integrative crosstalk. Adv. Exp. Med. Biol. 2020, 1131, 163–182. [Google Scholar] [CrossRef]
- Kraus-Friedmann, N. Calcium sequestration in the liver. Cell Calcium 1990, 11, 625–640. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Baird, G.S. Ionized calcium. Clin. Chim. Acta 2011, 412, 696–701. [Google Scholar] [CrossRef]
- Contri, M.; Boraldi, F.; Taparelli, F.; Paepe, A.; Ronchetti, I. Matrix proteins with high affinity for calcium ions are associated with mineralization within the elastic fibers of pseudoxanthoma elasticum dermis. Am. J. Pathol. 1996, 148, 569–577. [Google Scholar] [PubMed]
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Bazil, J.N.; Blomeyer, C.A.; Pradhan, R.K.; Camara, A.K.; Dash, R.K. Modeling the calcium sequestration system in isolated guinea pig cardiac mitochondria. J. Bioenerg. Biomembr. 2013, 45, 177–188. [Google Scholar] [CrossRef]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; Brandi, M. Calcium intake in bone health: A focus on calcium-rich mineral waters. Nutrients 2018, 10, 102645. [Google Scholar] [CrossRef]
- Zikan, V.; Stepan, J.J. Marker of bone resorption in acute response to exogenous or endogenous parathyroid hormone. Biomark. Insights 2008, 3, 19–24. [Google Scholar] [CrossRef]
- Cianferotti, L.; Gomes, A.R.; Fabbri, S.; Tanini, A.; Brandi, M.L. The calcium-sensing receptor in bone metabolism: From bench to bedside and back. Osteoporos. Int. 2015, 26, 2055–2071. [Google Scholar] [CrossRef]
- Hatate, K.; Kawashima, C.; Hanada, M.; Kayano, M.; Yamagishi, N. Short communication: Serum osteoprotegerin concentrations in periparturient dairy cows. J. Dairy Sci. 2018, 101, 6622–6626. [Google Scholar] [CrossRef]
- Roux, E.; Marhl, M. Role of sarcoplasmic reticulum and mitochondria in Ca2+ removal in airway myocytes. Biophys. J. 2004, 86, 2583–2595. [Google Scholar] [CrossRef]
- Stammers, A.; Susser, S.; Hamm, N.; Hlynsky, M.; Kimber, D.; Kehler, D.; Duhamel, T. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA). Can. J. Physiol. Pharmacol. 2015, 93, 843–854. [Google Scholar] [CrossRef]
- Treves, S.; Jungbluth, H.; Muntoni, F.; Zorzato, F. Congenital muscle disorders with cores: The ryanodine receptor calcium channel paradigm. Curr. Opin. Pharmacol. 2008, 8, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; García-Castañeda, M.; Boncompagni, S.; Dirksen, R.T. Role of STIM1/ORAI1-mediated store-operated Ca2+ entry in skeletal muscle physiology and disease. Cell Calcium 2018, 76, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Barritt, G. Calcium signalling in liver cells; in Calcium Signalling in Liver Cells; 2000; pp. 73–94. [CrossRef]
- Rieusset, J. Endoplasmic reticulum–mitochondria calcium signaling in hepatic metabolic diseases. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Vilarnau, N.; Hankeova, S.; Vorrink, S.U.; Mkrtchian, S.; Andersson, E.R.; Lauschke, V.M. Calcium signaling in liver injury and regeneration. Front. Med. (Lausanne) 2018, 5, 192. [Google Scholar] [CrossRef]
- Jagtap, Y.; Adlakha, N. Simulation of buffered advection diffusion of calcium in a hepatocyte cell. Math. Biol. Bioinform. 2018. [CrossRef]
- Kang, Y.; McKenna, T.; Watson, L.; Williams, R.; Holt, M. Cytochemical changes in hepatocytes of rats with endotoxemia or sepsis: Localization of fibronectin, calcium, and enzymes. J. Histochem. Cytochem. 1988, 36, 665–678. [Google Scholar] [CrossRef]
- Bernardo, J.; Friedman, P. Renal calcium metabolism. In: Renal Physiology; pp. 2225–2247. [CrossRef]
- Lim, D.; Semyanov, A.; Genazzani, A.; Verkhratsky, A. Calcium signaling in neuroglia. Int. Rev. Cell Mol. Biol. 2021, 362, 1–53. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, T.; Pereira, A.; Verkhratsky, A.; Huang, J. Glial cells and synaptic plasticity. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef]
- Clementi, E.; Racchetti, G.; Melino, G.; Meldolesi, J. Cytosolic Ca2+ buffering, a cell property that in some neurons markedly decreases during aging, has a protective effect against NMDA/nitric oxide-induced excitotoxicity. Life Sci. 1996, 59, 389–397. [Google Scholar] [CrossRef]
- Yamada, A.; Watanabe, A.; Yamamoto, T. Regulatory mechanisms of mitochondrial calcium uptake by the calcium uniporter complex. Biophys. Physicobiol. 2023, 20. [Google Scholar] [CrossRef]
- Denton, R. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 2009, 1787, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Evelson, P.; Vanasco, V.; Magnani, N.; Cimolai, M.; Marchini, T. The role of mitochondria in inflammatory syndromes; in Mitochondria in Inflammation; pp. 233–247. [CrossRef]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta 2015, 1852, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Rieusset, J.; Fauconnier, J.; Paillard, M.; Belaidi, E.; Tubbs, E.; Chauvin, M.; Durand, A.; Bravard, A.; Teixeira, G.; Bartosch, B.; Michelet, M.; Theurey, P.; Vial, G.; Demion, M.; Blond, E.; Zoulim, F.; Gomez, L.; Vidal, H.; Lacampagne, A.; Ovize, M. Disruption of calcium transfer from ER to mitochondria links alterations of mitochondria-associated ER membrane integrity to hepatic insulin resistance. Diabetologia 2016, 59, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guan, Z.; Gao, Y.; Bai, Y.; Zhan, X.; Ji, X.; Xu, J.; Zhou, H.; Rao, Z. ER stress promotes mitochondrial calcium overload and activates the ROS/NLRP3 axis to mediate fatty liver ischemic injury. Hepatol. Commun. 2024, 8. [Google Scholar] [CrossRef]
- Briones-Suarez, L.; Cifuentes, M.; Bravo-Sagua, R. Secretory factors from calcium-sensing receptor-activated SW872 pre-adipocytes induce cellular senescence and a mitochondrial fragmentation-mediated inflammatory response in HepG2 cells. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Williams, G.S.; Boyman, L.; Chikando, A.C.; Khairallah, R.J.; Lederer, W.J. Mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA 2013, 110, 10479–10486. [Google Scholar] [CrossRef]
- Zhou, Y.; Greka, A. Calcium-permeable ion channels in the kidney. Am. J. Physiol. Renal Physiol. 2016, 310, F1157–F1167. [Google Scholar] [CrossRef]
- Woudenberg-Vrenken, T.; Bindels, R.; Hoenderop, J. The role of transient receptor potential channels in kidney disease. Nat. Rev. Nephrol. 2009, 5, 441–449. [Google Scholar] [CrossRef]
- Klein, G.; Castro, S.; Garofalo, R. The calcium-sensing receptor as a mediator of inflammation. Semin. Cell Dev. Biol. 2016, 49, 52–56. [Google Scholar] [CrossRef]
- Zhao, B. Does TNF promote or restrain osteoclastogenesis and inflammatory bone resorption? Crit. Rev. Immunol. 2018, 38, 253–261. [Google Scholar] [CrossRef]
- Tupling, A. The sarcoplasmic reticulum in muscle fatigue and disease: Role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can. J. Appl. Physiol. 2004, 29, 308–329. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Premkumar, P.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H. Restoration of SERCA ATPase as an intervention to muscle impairment associated with oxidative stress. FASEB J. 2018, 32, —. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; Schmid, J.A. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Esmon, C. The impact of the inflammatory response on coagulation. Thromb. Res. 2004, 114, 321–327. [Google Scholar] [CrossRef]
- Mohanty, J.; Barodka, V.; Berkowitz, D.; Rifkind, J. Intracellular calcium mediated stiffness of red blood cells is reversed by hypoxic pre-incubation with nitrite ions. Biophys. J. 2010, 98. [Google Scholar] [CrossRef]
- Lacy, P.; Stow, J. Cytokine release from innate immune cells: Association with diverse membrane trafficking pathways. Blood 2011, 118, 9–18. [Google Scholar] [CrossRef]
- Kirdajova, D.; Kriska, J.; Tureckova, J.; Anděrová, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Muller, W. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am. J. Pathol. 2014, 184, 886–896. [Google Scholar] [CrossRef]
- Dalal, P.; Muller, W.; Sullivan, D. Endothelial cell calcium signaling during barrier function and inflammation. Am. J. Pathol. 2019. [CrossRef]
- Shannon, T.; Ginsburg, K.; Bers, D. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys. J. 2000, 78, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Giacomo, V. Extracellular matrix: Immunity and inflammation; in Extracellular Matrix; pp. 83–109. [CrossRef]
- Touyz, R.; Alves-Lopes, R.; Rios, F.; Camargo, L.; Anagnostopoulou, A.; Arner, A.; Montezano, A. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Qu, J.; He, L.; Zhang, F.; Zhou, Z.; Yang, S.; Zhou, Y. Calcium in vascular smooth muscle cell elasticity and adhesion: Novel insights into the mechanism of action. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Carlstedt, F.; Lind, L. Hypocalcemic syndromes. Crit. Care Clin. 2001, 17, 139–153, vii–viii. [CrossRef]
- Klein, G. Phosphate as an adjunct to calcium in promoting coronary vascular calcification in chronic inflammatory states. eLife 2024, 13. [Google Scholar] [CrossRef]
- Edwards, F.; Taheri, A.; Dann, S.; Dye, J. Characterization of cytolytic neutrophil activation in vitro by amorphous hydrated calcium phosphate as a model of biomaterial inflammation. J. Biomed. Mater. Res. Part A 2011, 96, 552–565. [Google Scholar] [CrossRef]
- Chen, J.; Sitsel, A.; Benoy, V.; Sepúlveda, M.; Vangheluwe, P. Primary active Ca2+ transport systems in health and disease. Cold Spring Harb. Perspect. Biol. 2019. [CrossRef]
- Bers, D.M. Cardiac Excitation-Contraction Coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Wong, P.T. Isolation of a High Capacity Ca²⁺-Binding Protein from Sarcoplasmic Reticulum. Nature New Biology 1971, 231, 198–200. [Google Scholar]
- Beard, N.A.; Laver, D.R.; Dulhunty, A.F. Calsequestrin and the Calcium Release Channel of Skeletal and Cardiac Muscle. Prog. Biophys. Mol. Biol. 2004, 85, 33–69. [Google Scholar] [CrossRef]
- Berridge, M.J. Neuronal Calcium Signaling. Neuron 1998, 21, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Chin, D.; Means, A.R. Calmodulin: A Prototypical Calcium Sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, M.; Kalyanasundaram, A. SERCA Pump Isoforms: Their Role in Calcium Transport and Disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef]
- Nilius, B.; Voets, T. The Puzzle of TRPV4 Channelopathies. EMBO Rep. 2013, 14, 152–163. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Lederer, W.J. Sodium/Calcium Exchange: Its Physiological Implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. The Plasma Membrane Ca²⁺ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium. Cold Spring Harb. Perspect. Biol. 2011, 3, a004168. [Google Scholar] [CrossRef]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca²⁺ Signalling in the Golgi Apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pozzan, T. Microdomains of Intracellular Ca²⁺: Molecular Determinants and Functional Consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Pizzo, P.; Pozzan, T. Golgi Ca²⁺, Messengers and Function. Cell Calcium 2007, 42, 405–412. [Google Scholar] [CrossRef]
- Feske, S.; Wulff, H.; Skolnik, E. Ion channels in innate and adaptive immunity. Annu. Rev. Immunol. 2015, 33, 291–353. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Soto, G.; Rocher, A.; García-Rodríguez, C.; Núñez, L.; Villalobos, C. The calcium-sensing receptor in health and disease. Int. Rev. Cell Mol. Biol. 2016, 327, 321–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Suqin, H.; Zhu, W. Calcium sensing receptor: Signaling pathways and physiological functions. Chin. J. Anim. Nutr. 2015, 27, 703–714. [Google Scholar]
- Chavez-Abiega, S.; Mos, I.; Centeno, P.; Elajnaf, T.; Schlattl, W.; Ward, D.; Goedhart, J.; Kallay, E. Sensing extracellular calcium—An insight into the structure and function of the calcium-sensing receptor (CaSR). Adv. Exp. Med. Biol. 2020, 1131, 1031–1063. [Google Scholar] [CrossRef]
- Chakravarti, B.; Chattopadhyay, N.; Brown, E. Signaling through the extracellular calcium-sensing receptor (CaSR). Adv. Exp. Med. Biol. 2012, 740, 103–142. [Google Scholar] [CrossRef]
- Bird, G.; Putney, J. Differential effects of PLC-coupled receptors on intracellular calcium oscillations in HEK293 cells. Biophys. J. 2015, 108. [Google Scholar] [CrossRef]
- Chen, R.; Goodman, W. Role of the calcium-sensing receptor in parathyroid gland physiology. Am. J. Physiol. Renal Physiol. 2004, 286, F1005–F1011. [Google Scholar] [CrossRef]
- Kinoshita, Y. The functions of calcium-sensing receptor in regulating mineral metabolism. Clin. Calcium 2017, 27, 491–497. [Google Scholar]
- Liu, W.; Guo, Y.; Liu, Y.; Sun, J.; Yin, X. Calcium-sensing receptor of immune cells and diseases. 2021, —. [CrossRef]
- Chandler, T.L.; Westhoff, T.A.; Behling-Kelly, E.L.; Sipka, A.S.; Mann, S. Eucalcemia during lipopolysaccharide challenge in postpartum dairy cows: I. Clinical, inflammatory, and metabolic response. J. Dairy Sci. 2023, 106, 3586–3600. [Google Scholar] [CrossRef]
- Horst, E.A.; Mayorga, E.J.; Al-Qaisi, M.; Abeyta, M.A.; Portner, S.L.; McCarthy, C.S.; Goetz, B.M.; Kvidera, S.K.; Baumgard, L.H. Effects of maintaining eucalcemia following immunoactivation in lactating Holstein dairy cows. J. Dairy Sci. 2020, 103, 7472–7486. [Google Scholar] [CrossRef]
- Waldron, M.R.; Nonnecke, B.J.; Nishida, T.; Horst, R.L.; Overton, T.R. Effect of lipopolysaccharide infusion on serum macromineral and vitamin D concentrations in dairy cows. J. Dairy Sci. 2003, 86, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Seminara, J.A.; Seely, C.R.; McArt, J.A.A. Acute phase responses in clinically healthy multiparous Holsteins with and without calcium dysregulation during the early postpartum period. J. Dairy Sci. 2024. [CrossRef]
- Couto Serrenho, R.; Morrison, E.; Bruinjé, T.C.; LeBlanc, S.J. Assessment of systemic inflammation following oral calcium supplementation in healthy postpartum multiparous dairy cows—a randomized controlled trial. JDS Commun. 2023, 5, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Garidel, P.; Rappolt, M.; Schromm, A.; Howe, J.; Lohner, K.; Andrä, J.; Koch, M.; Brandenburg, K. Divalent cations affect chain mobility and aggregate structure of lipopolysaccharide from Salmonella minnesota reflected in a decrease of its biological activity. Biochim. Biophys. Acta 2005, 1715, 122–131. [Google Scholar] [CrossRef]
- Redeker, C.; Briscoe, W. Interactions between mutant bacterial lipopolysaccharide (LPS-Ra) surface layers: Surface vesicles, membrane fusion, and effect of Ca2+ and temperature. Langmuir 2019. [CrossRef]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; Kim, H.M. Reconstruction of LPS transfer cascade reveals structural determinants within LBP, CD14, and TLR4-MD2 for efficient LPS recognition and transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodríguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Wright, S.D. Lipopolysaccharide-binding protein and soluble CD14 transfer lipopolysaccharide to phospholipid bilayers: Preferential interaction with particular classes of lipid. J. Immunol. 1997, 158, 3925–3934. [Google Scholar]
- Tsukamoto, H.; Takeuchi, S.; Kubota, K.; Kobayashi, Y.; Kozakai, S.; Ukai, I.; Shichiku, A.; Okubo, M.; Numasaki, M.; Kanemitsu, Y.; Matsumoto, Y.; Nochi, T.; Watanabe, K.; Aso, H.; Tomioka, Y. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent toll-like receptor 4 internalization and LPS-induced TBK1-IKKϵ-IRF3 axis activation. J. Biol. Chem. 2018, 293, 10186–10201. [Google Scholar] [CrossRef]
- Uhrig, A.; Banafsche, R.; Kremer, M.; Hegenbarth, S.; Hamann, A.; Neurath, M.; Gerken, G.; Limmer, A.; Knolle, P.A. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 2005, 77, 626–633. [Google Scholar] [CrossRef]
- Baranova, I.N.; Vishnyakova, T.G.; Bocharov, A.V.; Leelahavanichkul, A.; Kurlander, R.; Chen, Z.; Souza, A.C.; Yuen, P.S.; Star, R.A.; Csako, G.; Patterson, A.P.; Eggerman, T.L. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J. Immunol. 2012, 188, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Van Bossuyt, H.; Wisse, E. Structural changes produced in Kupffer cells in the rat liver by injection of lipopolysaccharide. Cell Tissue Res. 1988, 251, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Osuga, J.; Adachi, H.; Tozawa, R.; Takanezawa, Y.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Koizumi, H.; Inaba, T.; Yagyu, H.; Kamada, N.; Suzuki, H.; Shimano, H.; Kadowaki, T.; Tsujimoto, M.; Arai, H.; Yamada, N.; Ishibashi, S. Scavenger receptor expressed by endothelial cells I (SREC-I) mediates the uptake of acetylated low density lipoproteins by macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 2004, 279, 30938–30944. [Google Scholar] [CrossRef] [PubMed]
- König, V.; Hopf, U.; Möller, B.; Lobeck, H.; Assmann, G.; Freudenberg, M.; Galanos, C. The significance of high-density lipoproteins (HDL) in the clearance of intravenously administered bacterial lipopolysaccharides (LPS) in mice. Hepatogastroenterology 1988, 35, 111–115. [Google Scholar]
- Hersoug, L.G.; Møller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Kumar, P.; Schroder, E.A.; Rajaram, M.V.S.; Harris, E.N.; Ganesan, L.P. The battle of LPS clearance in host defense vs. inflammatory signaling. Cells 2024, 13, 1590. [Google Scholar] [CrossRef]
- Safavi, K.; Nichols, F. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J. Endod. 1994, 20, 127–129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




