Submitted:
11 March 2025
Posted:
12 March 2025
You are already at the latest version
Abstract
Phytoplasma (‘Candidatus Phytoplasma’ species) diseases have been reported globally to severely limit the productivity of a wide range of economically important crops and wild plants causing different yellows-type diseases. With new molecular detection techniques, several unknown and known diseases with uncertain aetiologies or attributed to other pathogens have been identified as being caused by Phytoplasmas. In Africa, phytoplasmas have been reported in association with diseases in a broad range of host plant species. However, the few reports of phytoplasma occurrence in Africa have not been collated together to determine the status in different countries of the continent. Thus, this paper discusses the geographical distribution, detection techniques, insect vectors, alternative hosts and socio-economic impacts of phytoplasma diseases in Africa. This is to create research perspectives on the disease’s aetiology in Africa for further studies towards identifying and limiting their negative effects on the continent’s agricultural economy. In Africa, Phytoplasmas recorded in different countries affecting different crops belong to eight groups (16SrI, 16SrII, 16SrIII, 16SrIV, 16SrVI, 16SrXI, 16SrXIV and 16SrXXII) out of the 37 groups and over 150 subgroups reported worldwide on the basis of their 16S rRNA RFLP profile. Lethal yellow disease was the most destructive Phytoplasma reported in Africa and has a high socio-economic impact.
Keywords:
1. Overview and Rationale
| Acronym | Phytoplasma strain | 16Sr | Related Ca. species | Origin | DNA source |
|---|---|---|---|---|---|
| CACT BCRD KVE AYA AVUT WBDL FBP FBPSA SOYP TBB SPLL IPO PYLV GVX API JRI TLD CSPWD ULW PWB BLL CPS ASHY-1 PPWB AP-15 GSFY-1 ESFY NGS CPF STOL LYAM LYHV LYPR MPV |
Cactus aster yellows Blackcurrant reversion disease Clover phyllody-England Apricot chlorotic leaf roll Atypical aster yellows Lime witches’-broom Faba bean phyllody Crotalaria saltiana phyllody Soybean phyllody Australian tomato big bud Sweet potato little leaf Ipomoea (unspecified) Peach western X Green valley X Euscelidius variegatus Poinsettia branching factor Tanzanian lethal decline Ghanaian Cape St Paul wilt Elm witches’-broom Potato witches’-broom Brinjal little leaf Catharanthus phyllody Ash yellows Pigeon pea witches’-broom Apple proliferation German stone fruit yellows European stone fruit yellows Napier grass stunt Cordyline phytoplasma Stolbur of pepper Coconut lethal yellowing (Adonidiamerrillii) Coconut lethal yellowing (Hyophorbeverschafeltii) Coconut lethal yellowing (Phoenix rupicola) Mexican periwinkle virescence |
I-B I-C I-B I-F I-M II-B II-C II-C II-C II-D II-D II-D III-A III-A III-B III-H IV-B IV-C V-A VI-A VI-A VI-C VII-A IX X-A X-B X-B XI XII XII- A IV-A IV-A IV-A XIII |
Phytoplasma asteris Phytoplasma asteris Phytoplasma asteris Phytoplasma asteris Phytoplasma asteris Phytoplasma aurantifolia Phytoplasma aurantifolia Phytoplasma aurantifolia Phytoplasma aurantifolia Phytoplasma aurantifolia Phytoplasma aurantifolia Phytoplasma aurantifolia Phytoplasma pruni Phytoplasma pruni Phytoplasma pruni Phytoplasma pruni Phytoplasma cocostanzaniae Phytoplasma cocosnigeriae Phytoplasma ulmi Phytoplasma trifolii Phytoplasma trifolii Phytoplasma trifolii Phytoplasma fraxini Phytoplasma phoenicium Phytoplasma mali Phytoplasma prunorum Phytoplasma prunorum Phytoplasma oryzae Phytoplasma fragariae Phytoplasma solani Phytoplasma palmae Phytoplasma palmae Phytoplasma palmae – |
USA Czech. UK Spain Germany Arabia Sudan Sudan Thailand Australia Australia Fiji USA USA Italy USA Tanzania Ghana France USA India Sudan USA USA Italy Germany Germany Ethiopia Jersey, UK Serbia USA Florida, USA Florida, USA Mexico |
DNA Plant DNA DNA DNA DNA Plant Plant Plant Plant Plant DNA DNA DNA DNA DNA DNA DNA DNA DNA Plant DNA DNA DNA DNA DNA DNA Plant DNA DNA DNA DNA DNA DNA |
2. Symptoms and Spread of Phytoplasmal Diseases
2.1. Symptoms of Infection
2.2. Phytoplasma Transmission
2.2.1. Insect Vectors
2.2.2. Other Modes of Transmission
3. Detection and Classification of Phytoplasma
3.1. Detection of Phytoplasma
3.2. Classification System
4. Phytoplasma Diseases Status in Africa
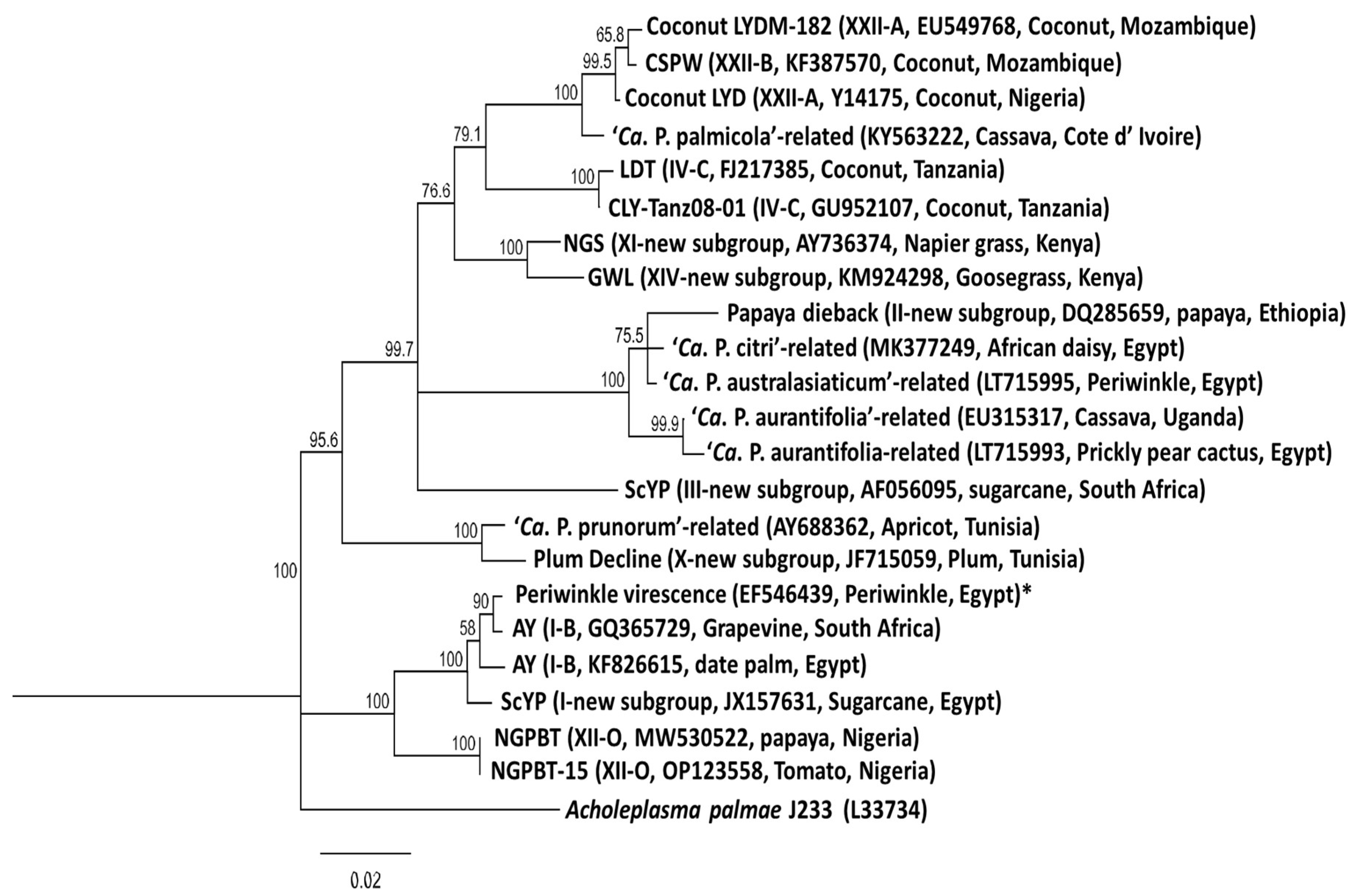
4.1. Groups and Subgroups of Phytoplasmas in Africa
4.1.1. Grapevine Yellows Disease
4.1.2. Phyllody/Witches Broom/Virescence
4.1.3. Napier Grass Stunt Phytoplasma
4.1.4. Yellow Leaf Syndrome
4.1.5. Sugarcane Grassy Shoot
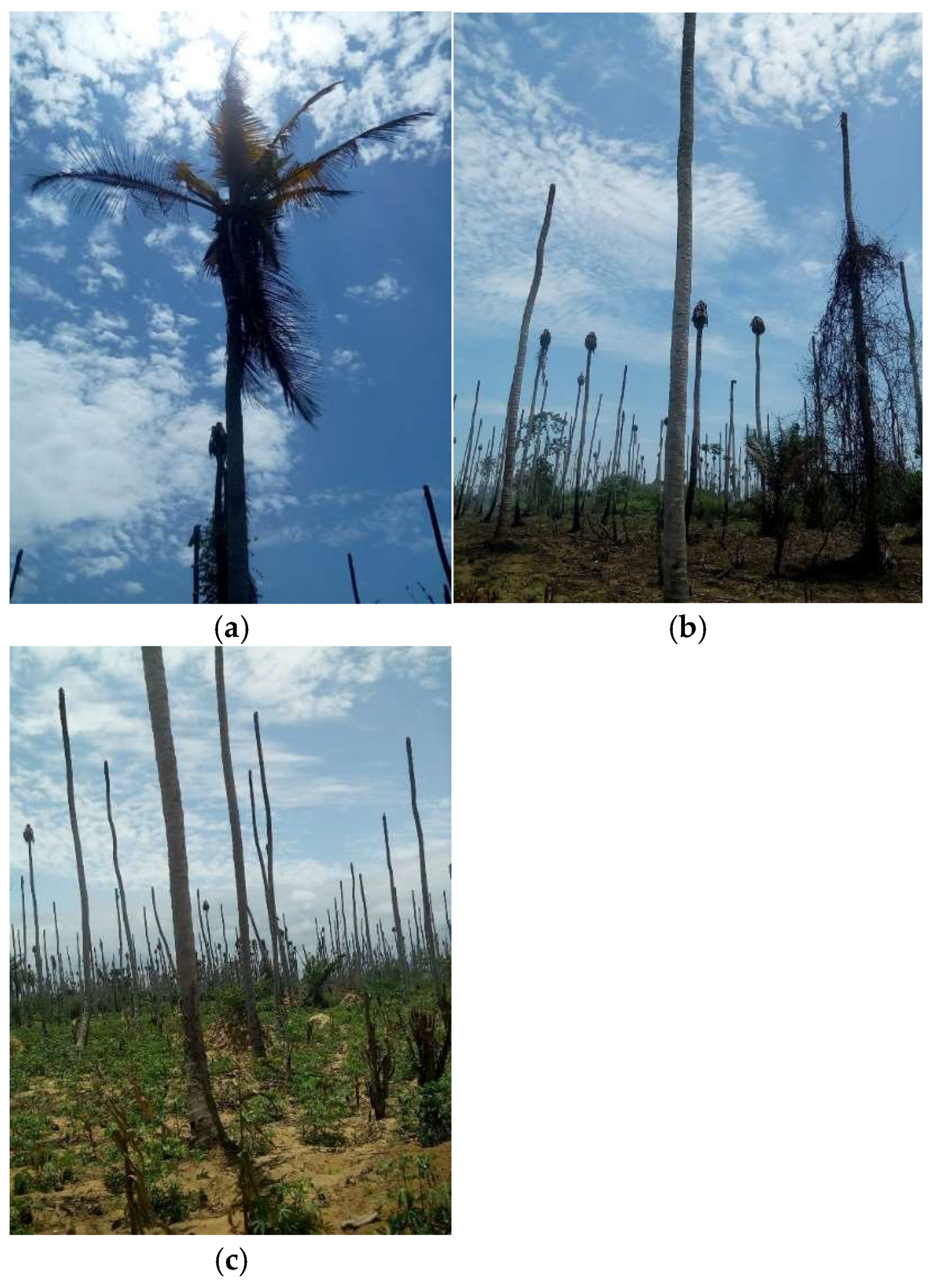
4.1.6. Lethal Yellowing Diseases of Coconut and Cassava
Lethal Yellowing Disease of Coconut
Cassava Phytoplasma
4.1.7. Bermuda and Hyparrhenia Grass White Leaf
4.1.8. Phytoplasma Disease of Date Palm
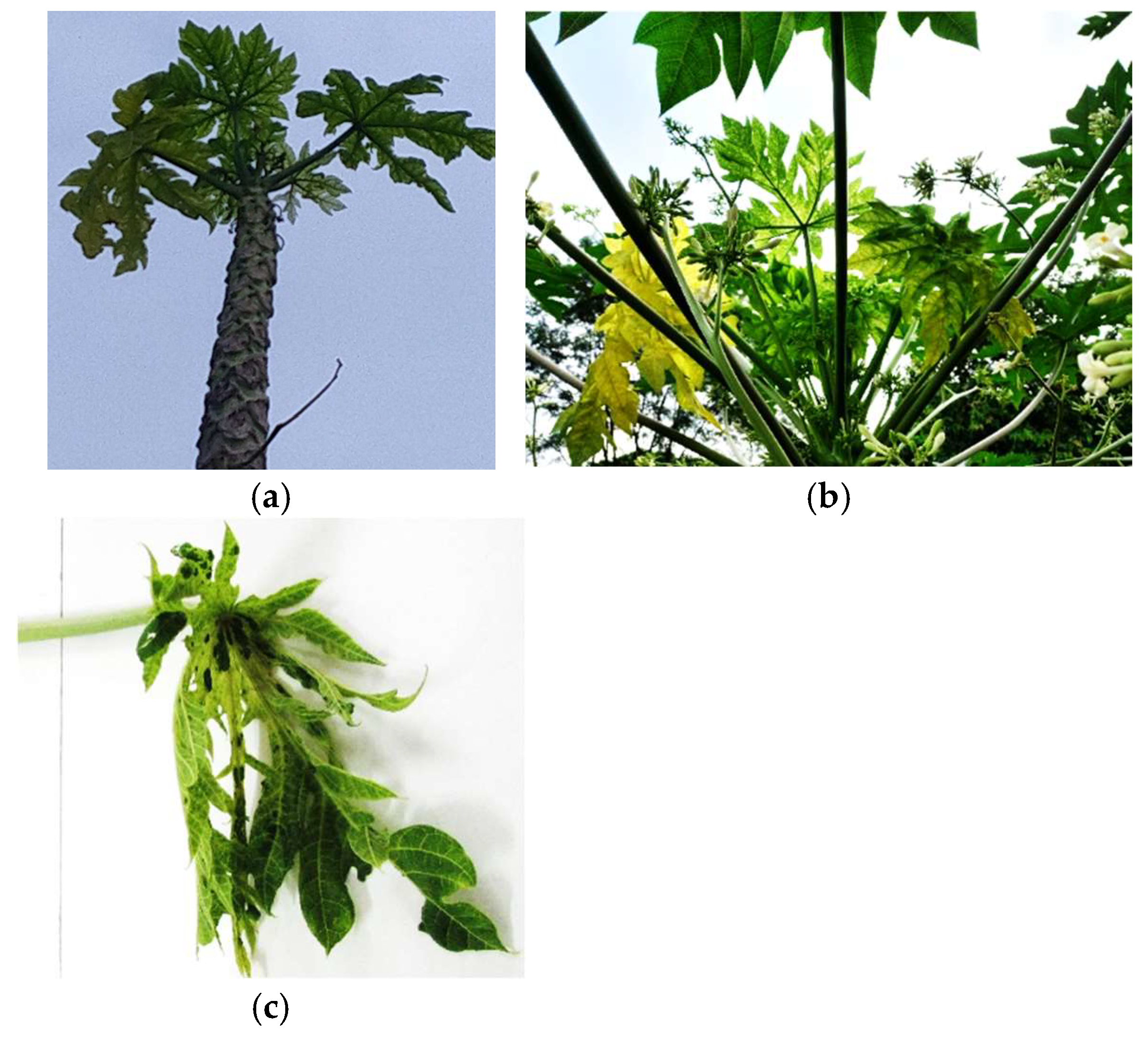
4.1.9. Phytoplasma Diseases of Papaya
4.1.10. Unclassified Phytoplasma Group
5. Impact of phytoplasmal Disease in Africa
6. Conclusion and Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, IM, Davis RE, Gundersen-Rindal, D.E. 2000. Phytoplasma: phytopathogenic mollicutes. Annual Review of Microbiology 54: 221-255.
- IRPCM, 2004. ‘Candidatus Phytoplasma’, a taxon for the wall-less, non-helical prokaryotes that colonize plant phloem and insects. International Journal of Systematic and Evolutionary Microbiology 54, 1243–1255. [CrossRef]
- Bertaccini, A. and Duduk, B. 2009. Phytoplasma and phytoplasma diseases: a review of recent research. Phytopathologia Mediterranea 48(3): 355–378. http://www.jstor.org/stable/26463360.
- Makarova, O., Contaldo, N., Paltrineri, S., Kawube, G., Bertaccini, A. and Nicolaisen, M. 2012.DNA barcoding for identification of ‘Candidatus Phytoplasma’ using a fragment of the Elongation factor Tu Gene. Plos One 7(12): e52092. [CrossRef]
- Bertaccini, A., Duduk, B., Paltrinieri, S. and Contaldo, N. 2014. Phytoplasmas and Phytoplasma Diseases: A Severe Threat to Agriculture. American Journal of Plant Sciences 5: 1763-1788. [CrossRef]
- Marcone, C. 2012. Advances in differentiation and classification of phytoplasmas. Annals of Applied Biology 160:201-203. [CrossRef]
- Marcone, C. 2014.Molecular biology and pathogenicity of phytoplasmas. Annals of Biology 165 (2): 199-221. [CrossRef]
- Marcone, C. 2019. Comparison of Different Procedures for DNA Extraction for Routine Diagnosis of Phytoplasmas P 72-81 In: Rita Musetti and Laura Pagliari (eds.), Phytoplasmas: Methods and Protocols, Methods in Molecular Biology, vol. 1875. [CrossRef]
- Liu, J, Gopurenko D, Fletcher, M.J., Johnson, A.C. and Gurr, G.M. 2017. Phytoplasmas–The “Crouching Tiger” Threat of Australian Plant Pathology. Front. Plant Sci. 8:599. [CrossRef]
- Wambua, L., Bernd, S., Allan, O., Joseph, O. W., Olive, I., Peninah, N. W., Lavender, A., Cassandra, O., Chris, S. J., Daniel, M., Charles, M., Zeyaur, K., Joerg, J. and Anne, F. 2017. Development of field-applicable tests for rapid and sensitive detection of Candidatus Phytoplasma oryzae. Molecular and Cellular Probes 35:44-56.
- Arocha, Y., Gonzalez, L., Peralta, E. L. and Jones, P. 1999. First Report of Virus and Phytoplasma Pathogens Associated with Yellow Leaf Syndrome of Sugarcane in Cuba. Plant Disease 1999 83:12, 1177-1177.
- Aljanabi, S.M., Parmessur, Y., Moutia, Y., Saumtally, S. and Dookun, A. 2001. Further evidence of the association of a phytoplasma and a virus with yellow leaf syndrome in sugarcane. Plant Pathology, 50: 628-636. [CrossRef]
- Doi, Y., Teranaka, M., Yora, K., Asuyama, H., 1967. Mycoplasma or PLT group-like microorganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’ broom, aster yellows, or paulownia witches’ broom. Ann. Phytopath.Soc. Jpn. 33, 259–266.
- ISPM 27. Annex 12. 2016. Phytoplasmas. Rome, IPPC, FAO. 12pp.
- Weisburg, W.G., Tully, J.G., Rose, D.L., Petzel, J.P., Oyaizu, H., Mandelco, L., Sechrest J., Lawrence, T.G. and Van Etten, J. 1989. A phylogenetic analysis of the mycoplasmas:basis for their classification. Journal of Bacteriology 171 (2): 6455-6467.
- Harrison N.A., Gundersen-Rinda, D., Davis, R.E., May, M. and Brown D.R. 2018. Candidatus Phytoplasma. in Bergey’s Manual of Systematics of Archaea and Bacteria, in association with Bergey’s Manual Trust. Published by John Wiley & Sons, Inc.. [CrossRef]
- Hogenhout, S.A., Kenro, O., EL-Desouky, A., Shigeyuki, K., Heather, N. K. and Shigetou, N. 2008. Phytoplasmas: bacteria that manipulate plants and insects. Molecular Plant Pathology 9 (4):403–423. [CrossRef]
- Streten, C. and Gibb, K.S. 2003. Identification of genes in the tomato big bud phytoplasma and coparison to those in sweet potato little leaf-V4 phytoplasma. Microbiology 149: 1797-1805.
- Tran-Nguyen, L.T. and Gibb, K.S. 2007. Optimizing Phytoplasma DNA purification for genome analysis. J. Biomol. Tech. 18 (2): 104-112.
- Bertaccini, A., Contaldo, N., Calari, A., Paltrinieri, S., Windsor, H.M. and Windsor, D. 2010. Preliminary Results of Axenic Growth of Phytoplasmas from Micropropagated Infected Periwinkle Shoots. 18th Congress of the International Organization for Mycoplasmology (IOM), Chianciano Terme, 11-16 July 2010. 147-153.
- Bendix C. and Lewis. J.D. 2018. The enemy within: phloem-limited pathogens. Molecular Plant Pathology 19(1): 238–254. [CrossRef]
- Bove, J. M. and Garnier, M. 2003. Phloem-and xylem-restricted plant pathogenic bacteria. Plant Sci. 164: 423–438. 10.1016/S0168-9452(03)00032-3.
- Wei, W. and Zhao, Y. 2022. Phytoplasma Taxonomy: Nomenclature, Classification, and Identification. Biology 11: 1119. [CrossRef]
- Wang, R., Bai, B., Li, D., Wang, J., Huang, W., Wu, Y. and Zhao, L. 2024. Phytoplasma: A plant pathogen that cannot be ignored in agricultural production-Research progress and outlook. Molecular Plant Pathology 25: 10.1111/mpp.13437.
- Gasparich, G. E. 2010. Spiroplasmas and phytoplasmas: Microbes associated with plant hosts. Biologicals 38 (2): 193–203. [CrossRef]
- Asudi, G. O., Van den Berg, J., Midega, C. A. O., Schneider, B., Seem¨uller, E., Pickett, J. A., and Khan, Z. R. 2016. Detection, identification, and significance of phytoplasmas in wild grasses in East Africa. Plant Dis. 100:108-115.
- Gurr, G.M., Johnson, A.C., Ash, G.J., Wilson, B.A.L, Ero, M.M., Pilotti, C.A., Dewhurst, C.F. and You, M.S. 2016. Coconut Lethal Yellowing Diseases: A Phytoplasma Threat to Palms of Global Economic and Social Significance. Front. Plant Sci. 7:1521. [CrossRef]
- Kumari, S., Nagendran, K., Rai, A.B., Singh, B., Rao, G.P. and Bertaccini, A. 2019. Global Status of Phytoplasma Diseases in Vegetable Crops. Front. Microbiol. 10:1349. [CrossRef]
- Abeysinghe, S. Kanatiwela-de Silva, C., Abeysingbe, P.D., Udagama. P., Warawichanee, K., Aljafar, N., Kawicha, P. and Dickinson, M. 2016. Refinement of the Taxonomic structure of 16SrXI and 16SrXIV phytoplasmas of gramineous plants using multilocus sequencing typing. Plant Dis. 100: 2001-2010.
- Zhao, Y., Wei, W., Lee, M., Shao, J., Suo, X., and Davis, R. E. 2009. Construction of an interactive online phytoplasma classification tool, iPhyClassifier, and its application in analysis of the peach X-disease phytoplasma group (16SrIII). International Journal of Systematic and Evolutionary Microbiology, 59, 2582–2593.
- Zhao, Y. and Davis, R. E. 2016. Criteria for phytoplasma 16Sr group/subgroup delineation and the need of a platform for proper registration of new groups and subgroups. International Journal of Systematic and Evolutionary Microbiology, 66(5), 2121–2123.
- Muirhead, K., Pérez-López, E., Bahder, B. W. Hill, J. E and Dumonceaux T. J. 2019. The CpnClassiPhyR Facilitates Phytoplasma Classification and Taxonomy Using cpn60 Universal Target Sequences. Olivier C. Y., Pérez-López, E., and Dumonceaux T. J. (eds.) in Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt, Sustainability in Plant and Crop Protection 12, . [CrossRef]
- Danet, J-L, Balakishiyeva, G., Cimerman, A., Sauvion, N., Marie-Jeanne, V., Labonne, G., Laviña, A., Batlle, A., Križanac, I., Škorić, D., Ermacora, P., Ulubaş S. Ç., Caglayan, K., Jarausch, W. and Foissac, X. 2011. Multilocus sequence analysis reveals the genetic diversity of European fruit tree phytoplasmas and supports the existence of inter-species recombination. Microbiolog 157: 438-50. 10.1099/mic.0.043547-0.
- Li, Y., Piao, C-G., Tian, G-Z., Liu, Z-X., Guo, M-W., Lin, C-L. and Wang, X.-Z. 2014. Multilocus sequences confirm the close genetic relationship of four phytoplasmas of peanut witches’-broom group 16SrII-A. J. Basic Microbiol. 54:818-827. [CrossRef]
- Pilet F, Quaicoe RN, Osagie IJ, Freire M, Foissac X. 2019. Multilocus sequence analysis reveals three distinct populations of “Candidatus Phytoplasma palmicola” with a specific geographical distribution on the African continent. Applied and Environmental Microbiology 85 (8): e02716-18. [CrossRef]
- Quaglino, F., Kube, M., Jawhari, M., Abou-Jawdah, Y., Siewart, C., Choueiri, E., Sobh, H., Casati, P., Tedeschi, R., Lova, M.M., Alma, A. and Bianco, P.A. 2015. “Candidatus Phytoplasma phoenicium’ associated with almond witches’-broom disease: from draft genome to genetic diversity among strain populations. BMC Microbiol 15:148. [CrossRef]
- Johnson WH. 1918. Annual report of the agricultural department of southern provinces Nigeria for the year 1917, Ibadan, Nigeria. Government Publication 14.
- Harrison, N., Davis, R.E., Oropeza, C., Helmick, E., Narvaez, M., Eden-Green, S., Dollet, M., Dickinson, M., Konan Konan, J.L., 2014. ‘Candidatus Phytoplasma palmicola’, a novel taxon associated with a lethal yellowing-type disease (LYD) of coconut (Cocos nucifera L.) in Mozambique. Int. J. Syst. Evol. Microbiol. 64, 1890–1899. [CrossRef]
- Contaldo, N; Bertaccini, A., Paltrinieri, S., Windsor, H.M., Windsor, D.G., 2012. Axenic culture of plant pathogenic phytoplasmas. Phytopath. Medit. 51 (3), 607–617.
- Contaldo, N., Satta, E., Zambon, Y., Paltrinieri, S., Bertaccini, A., 2016. Development and evaluation of different complex media for phytoplasma isolation and growth. J. Microbiol. Meth. 127, 105–110.
- Contaldo, N., D’Amicoa, G., Paltrinieria, S., Diallob, H.A, Bertaccinia, A., Arocha-Rosete, Y. 2019. Molecular and biological characterization of phytoplasmas from coconut palms affected by the lethal yellowing disease in Africa. Microbiological Research 223–225 (2019) 51–57. [CrossRef]
- Trivellone, V. 2019. An online global database of Hemiptera-Phytoplasma-Plant biological interactions. Biodiversity Data Journal 7: e32910. [CrossRef]
- Bertaccini, A. 2007. Phytoplasmas: diversity,taxonomy, and epidemiology. Front Biosci 12:673–689.
- Ermacora P and R. Osler, 2019. Symptoms of Phytoplasma Diseases In: Rita Musetti and Laura Pagliari (eds.), Phytoplasmas: Methods and Protocols, Methods in Molecular Biology, vol. 1875. Springer Nature 53-67. [CrossRef]
- Wei, W., Shao, J., Zhao, Y., Inaba, J., Ivanauskas, A., Bottner-Parker, K.D, Costanzo, S., Kim, B.M., Flowers, K. and Escobar, J. 2024. iPhyDSDB: Phytoplasma Disease and Symptom Database. Biology, 13: 657. [CrossRef]
- Pracros, P., Renaudin, J., Eveillard, S., Mouras, A. and Hernould, M. 2006. Tomato flower abnormalities induced by stolbur phytoplasma infection are associated with changes of expression of floral development genes. Molecular plant-microbe interactions 19(1): 62–68. [CrossRef]
- Maejima, K., Iwai, R., Himeno, M., Komatsu,K., Kitazawa, Y., Fujita, N., Ishikawa, K., Fukuoka, M., Minato,N., Yamaji, Y., Oshima, K. and Namba S. 2014. Recognition of floral homeotic MADS domain transcription factors by a phytoplasmal effector,phyllogen, induces phyllody. Plant Journal 78:541-554. 10.1111/tpj.
- Kruger, K. and Fiore, N. 2019. Sampling Methods for Leafhopper, Planthopper, and Psyllid Vectors. P37-52. In:Rita Musetti and Laura Pagliari (eds.), Phytoplasmas: Methods and Protocols, Methods in Molecular Biology, vol. 1875, Springer Nature New York. [CrossRef]
- Tedeschi, R. and Bertaccini, A. 2019. Transovarial Transmission in Insect Vectors. P 115- 130 In Bertaccini, A., Weintraub, P.G., Rao, G.P. and Mori, N. Phytoplasmas: Plant Pathogenic Bacteria – II. Springer Nature Singapore Pte Ltd. [CrossRef]
- Alma, Alberto; Federico Lessio, and Herbert Nickel 2019. Insects as Phytoplasma Vectors: Ecological and Epidemiological Aspects. P1-25 In Bertaccini, A., Weintraub, P.G., Rao, G.P. and Mori, N 2019. Phytoplasmas: Plant Pathogenic Bacteria – II. Springer Nature Singapore Pte Ltd. [CrossRef]
- Weintraub, P.G. and Beanland, L. 2006. Insect vectors of phytoplasmas. Annu Rev Entomol 51:91–111.
- Jarausch, B. Tedeschi, R. Sauvion, N. Gross J. and Jarausch W. 2019. P 54-78 In Bertaccini, A., Weintraub, P.G., Rao, G.P. and Mori, N. Phytoplasmas: Plant Pathogenic Bacteria – II. Springer Nature Singapore Pte Ltd. [CrossRef]
- Jović, J. Riedle-Bauer M. and Chuche J. 2019. Vector Role of Cixiids and Other Planthopper Species P 79-113 In Bertaccini, A., Weintraub, P.G., Rao, G.P. and Mori, N . Phytoplasmas: Plant Pathogenic Bacteria – II. Springer Nature Singapore Pte Ltd. [CrossRef]
- Weintraub, P. G., Trivellone, V. and Krüger, K. 2019. The Biology and Ecology of Leafhopper Transmission of Phytoplasmas. P27 -53 In Bertaccini, A., Weintraub, P.G., Rao, G.P. and Mori, N. Phytoplasmas: Plant Pathogenic Bacteria – II. Springer Nature Singapore Pte Ltd. [CrossRef]
- Carraro, L., Loi. N. and Ermacora, P. 2001. Transmission characteristics of the European stone fruit yellows phytoplasma and its vector Cacopsyllapruni. Eur J. Plant Pathol 107: 695–700.
- Ammar, E. and Hogenhout, S. 2006. Mollicutes associated with arthropods and plants. In: Kostas B, Miller T (Eds) Insect Symbiosis. CRC Press, Taylor & Francis Group, II. Boca Raton, FL, U.S.A., 97-118 pp.
- Kingdom, H. 2013. Insect Maintenance and Transmission. .P47-59 In: Matt Dickinson and Jennifer Hodgetts (eds.), Phytoplasma: Methods and Protocols, Methods in Molecular Biology, vol. 938. [CrossRef]
- Bosco, D. and Tedeschi, R. 2013 Insect Vector Transmission Assays. P73-85 In: Matt Dickinson and Jennifer Hodgetts (eds.), Phytoplasma: Methods and Protocols, Methods in Molecular Biology, vol. 938. [CrossRef]
- Bertin, S. and Bosco, D. 2013. Molecular Identification of Phytoplasma Vector Species P87-108 In: Matt Dickinson and Jennifer Hodgetts (eds.), Phytoplasma: Methods and Protocols, Methods in Molecular Biology, vol. 938. [CrossRef]
- Pagliari, L., Chuche, J. Bosco, D. and Thiery, D. 2019. Phytoplasma Transmission: Insect Rearing and Infection Protocols. P21-36 In: Rita Musetti and Laura Pagliari (eds.), Phytoplasmas: Methods and Protocols, Methods in Molecular Biology, vol. 1875. [CrossRef]
- Weintraub P. and Jürgen G. 2013. Capturing Insect Vectors of Phytoplasmas. P61-72 In: Matt Dickinson and Jennifer Hodgetts (eds.), Phytoplasma: Methods and Protocols, Methods in Molecular Biology, vol. 938. [CrossRef]
- Kruger, K., Stiller, M., Van Wyk, D. J. and de Klerk, A. 2018. Diversity of leafhopper and plantjopper species in South African vineyards. In: 5th European Bois Noir Workshop, Ljubljana, Slovenia, 18-19thSeptember, 2018.
- Nahdi, S., Bouhachem, S.B., Mahfoudhi, N., Paltrinieri, S. and Bertaccini, A. 2020. Identification of phytoplasmas and Auchenorryncha in Tunisian vineyards. Phytopathogenic Mollicutes 10(1):25-35.
- Jones, P., Arocha, Y., Zerfy, T., Proud, J., Abebe, G. and Hanson, J. 2007. A stunting syndrome of Napier grass in Ethiopia is associated with a 16SrIII group phytoplasma Plant Pathology 56: 345. [CrossRef]
- Arocha, Y., Pinol, B., Acosta, K., Almeida, R., Devonshire, J. Van de Meene, A., Boa., E. and Lucas, J. 2009. Detection of phytoplasma and potyvirus pathogens in papaya (Carica papaya L.) affected by Bunchy top symptom (BTS) in eastern Cuba. Crop Protection 28: 640-646.
- Obura, E., Midega, C. A. O., Masiga, D., Pickett, J. A., Hassan, M., Koji, S.,and Khan, Z. R. 2009. Recilia banda Kramer (Hemiptera: Cicadellidae), a vector of Napier stunt phytoplasma in Kenya. Naturwissenschaften 96:1169-1176.
- Philippe, R., Nkansah, J., Fabre, S., Quaicoe, R., Pilet, F. and Dollet, M. 2007. Search for the vector of Cape Saint Paul wilt (coconut lethal yellowing) in Ghana. Bulletin of Insectology. 60 (2) :179-180.
- Bila, J., Mondjana, A., Samils, B., Hogberg, H., Wilson, M.R. and Santos, L. 2017. First report of ’Candidatus Phytoplasma palmicola’ detection in the planthopper Diostrombus mkurangai in Mozambique. Bulletin of Insectology 70(1):45-48.
- Trivellone, V. and Dietrich, C. H. 2021. Evolutionary Diversification in Insect Vector–Phytoplasma–Plant Associations, Annals of the Entomological Society of America 114 (2): 137–150. [CrossRef]
- Trivellone, V., Wei, W., Filippin, L. and Dietrich, C. H. 2021. Screening potential insect vectors in a museum biorepository reveals undiscovered diversity of plant pathogens in natural areas. Ecology and evolution 11(11): 6493–6503. [CrossRef]
- Marcone, C., Hergenhahn F., Ragozzino A., Seemuller E., 1999. Dodder transmission of pear decline, European stone fruit yellows, rubus stunt, Picris echioides yellows and cotton phyllody phytoplasmas to periwinkle. Journal of Phytopathology 147: 187-92.
- Pribylova, J. and Spak, J. 2013. Dodder transmission of phytoplasmas. In: Dickinson, M and Hodgetts, J. (Eds.) Phytoplasma: Methods and Protocols. 938:41-46. [CrossRef]
- Caglayan, K., Choueiri, E. and Rao, G.P. 2023. Graft and vegetative transmission of phytoplasma-associated diseases in Asia and their management. In: Tiwari, A.K., Oshima, K., Yadav, A., Esmaeilzadeh-Hosseini, S.A., Hanboonsong, Y. and Lakhanpaul, S. (Eds) Phytoplasma Diseases in Asian Countries, Characterization, epidemiology, and management. Academic press. Vol 3:21-36. [CrossRef]
- Chang, H.C. and Chen, J.C. 2024. An efficient grafting method for phytoplasma transmission in Catharanthus roseus. Plant Methods 20: 13. [CrossRef]
- Satta, E., Paltrinieri, S. and Bertaccini, A. 2019. Phytoplasma transmission by seed. In: Phytoplasmas: Plant Pathogenic Bacteria-II. Transmission and Management of Phytoplasma Associated Diseases. Chapter 6. Eds. Bertaccini A, Weintraub PG, Rao GP, Springer, Singapore 131–147 pp.
- Khan AJ, Botti S, Paltrinieri S, Al-Subhi AM, Bertaccini AF. 2002. Phytoplasmas in alfalfa seedlings: infected or contaminated seeds? In Abstracts, 14th InternationalOrganization of Mycoplasmology Conference, p. 148. Vienna, Austria.
- Cordova I, Jones P, Harrison NA, Oropeza C. 2003. In situ PCR detection of phytoplasma DNA in embryos from coconut palms with lethal yellowing disease. Mol. Plant Pathol. 4:99–108.
- Zwolińska, A., Krawczyk, K. and Pospieszny, H. 2010. First report of “stolbur” phytoplasma infecting pea plants. In Proceedings of the 18th Congress of the International Organization for Mycoplasmology (IOM), Chianciano Terme, Italy, 11-16 July 2010; Volume 11, p16.
- Contaldo, N; and Bertaccini, A.,2019 Phytoplasma Cultivation P89-103 In: Assunta Bertaccini, Michael Kube, KenroOshima, and Govind Pratap Rao (eds) Phytoplasmas: Plant Pathogenic Bacteria - III. Springer Nature Singapore Pte Ltd. 2019. [CrossRef]
- Botti, S. and Bertaccini, A. 2006. Phytoplasma infection through seed transmission: further observations. In: Abstracts, 16th International Organization of Mycoplasmology Conference, Cambridge, UK, p. 76.
- Calari, A., Paltrinieri, S., Contaldo, N., Sakalieva, D.,Mori, N., Duduk, B. and Bertaccini, A. 2011. Molecular evidence of phytoplasmas in winter oilseed rape, tomato and corn seedlings. Bulletin of insectology 64: S157-S158.
- Hanboonsong, Y., Choosai, C., Panyim, S. and Damak, S. 2002. Transovarial transmission of sugarcane white leaf phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumara). Insect molecular biology 11:97-103. 10.1046/j.0962-1075.2001.00314.x.
- Tedeschi, R., Ferrato, V., Rossi, J. and Alma, A. 2006. Possible phytoplasma transovarial transmission in the psyllids Cacopsylla melanoneura and Cacopsylla pruni. Plant Pathology 55 (1): 18-24.
- Musetti R. and Pagliari L. (eds.) 2019. Phytoplasmas: Methods and Protocols, Methods in Molecular Biology, vol. 1875. [CrossRef]
- Pusz-Bochenska, K., Perez-lopez, e., Dumonceaux, T.J., Olivier, C., and Wist, T.J. 2020. A rapid, simple, laboratory and field adaptable DNA extraction and Diagnosis method suitable for insect transmitted plant pathogens and insect identification. Plant Health progress 21(1):63-68. [CrossRef]
- Ustun, N., Zamharir, M.G. and Al-Sadi, A.M. 2023. Updates on phytoplasma diseases management. In: Tiwari, A.K., Oshima, K., Yadav, A., Esmaeilzadeh-Hosseini, S.A., Hanboonsong, Y. and Lakhanpaul, S. (Eds) Phytoplasma Diseases in Asian Countries, Characterization, epidemiology, and management. Academic press. Vol 3:97-123. [CrossRef]
- Chiykowski, L.N. and Sinha, R.C. 1989. Differentiation of MLO disease by means of symptomatology and vector transmission. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrandheiten und Hygiene Supplement 20: 280–287.
- Errampalli, D., Fletcher, J. and Claypool, P.L. 1991. Incidence of yellows in carrot and lettuce and characterization of mycoplasmalike organism isolates in Oklahoma. Plant Disease 75 (6): 579-584.
- Cai, W., Shao, J., Zhao, Y., Davis, R.E. and Stefano, C. 2020. Draft genom Sequence of ‘Candidatus Phytoplasma pini’-related strain MDPP: A resource for comparative Genomics of Gymnosperm-infecting.
- Obura, E.; Masiga, D., Wachira, F., Gurja, B. and Khan, Z.R. 2011. Detection of phytoplasma by loop-mediated isothermal amplification of DNA (LAMP). Journal of Microbiological Methods 84: 312–316. [CrossRef]
- Tomlinson, J. A., Boonham, N. and Dickinson, M. 2010. Development and evaluation of a one-hour DNA extraction and loop-mediated isothermal amplification assay for rapid detection of phytoplasmas. Plant Pathology (2010) 59, 465–471. [CrossRef]
- Bekele, B., Hodgetts, J., Tomlinson, J., Boonham, N., Nikolić, P., Swarbrick, P.J. and Dickinson, M.J. 2011. Use of a real-time LAMP isothermal assay for detecting 16SrII and XII phytoplasmas in fruit and weeds of the Ethiopian Rift Valley. Plant Pathology, 60, 345-355.
- Hodgetts, J., Tomlinson J., Boonham, N., González-Martín, I. and Nikolić, P. 2011. Development of rapid in-field loop-mediated isothermal amplification (LAMP) assays for phytoplasmas. Bulletin of Insectology 64: S41-S42.
- Bertaccini A. N. Fiore. A. Zamorano, A. K. Tiwari and G. P. Rao 2019. Molecular and Serological Approaches in Detection of Phytoplasmas in Plants and Insects P 105-136 In: Bertaccini, A., Kube,M., Oshima, K., and Rao, G.P. (eds) Phytoplasmas: Plant Pathogenic Bacteria – III. Springer Nature Singapore Pte Ltd. 2019 . [CrossRef]
- Swarbrick P., Yankey, E.N. and Dickinson, M. 2011. Development of rapid in-field loop-mediated isothermal amplification (LAMP) assays for phytoplasmas. Bulletin of Insectology 64 (Supplement): S41-S42.
- Alič, Š., Dermastia, M., Burger, J., Dickinson, M., Pietersen, G., Pietersen, G., and Dreo, T. (2022). Genome-Informed Design of a LAMP Assay for the Specific Detection of the Strain of ’Candidatus Phytoplasma asteris’ Phytoplasma Occurring in Grapevines in South Africa. Plant disease, 106(11), 2927–2939. [CrossRef]
- Minguzzi S, Terlizzi F, Lanzoni C, Poggi Pollini C, Ratti C (2016) A Rapid Protocol of Crude RNA/DNA Extraction for RT-qPCR Detection and Quantification of ’Candidatus Phytoplasma prunorum’ PLoS ONE 11(1): e0146515. [CrossRef]
- Hodgetts, J., N. Boonham, R. Mumford, N. Harrison, and M. Dickinson. 2008. Phytoplasma phylogenetics based on analysis of the secA and 23S rRNA gene sequences for improved resolution of the ‘Candidatus Phyto plasma’ species. Int. J. Syst. Evol. Microbiol. 58:1826–1837.
- Lee, I-M., Zhao, Y. and Bottner, K.D. 2006. SecY Gene Sequence Anaylysis for finer differentiation of diverse strains in the Aster Yellows Phytoplasma group. Molecular and Cellular Probes 20: 87-91. [CrossRef]
- Dickinson, M., and Hodgetts, J. 2013. PCR Analysis of Phytoplasmas Based on the secA Gene. In: Dickinson, M., Hodgetts, J. (eds) Phytoplasma. Methods in Molecular Biology, vol 938. Humana Press, Totowa, NJ. [CrossRef]
- Wei, W., Lee, I.M., Davis, R.E., Suo, X. and Zhao, Y. 2008. Automated RFLP pattern comparison and similarity coefficient calculation for rapid delineation of new and distinct phytoplasma 16Sr subgroup lineages. International Journal of Systematic and Evolutionary Microbiology 58:2368-2377.
- Lee, I-M., Gundersen-Rindal, D.E., Davis R.E. and Bartoszyk I.M. 1998. Revised classification scheme of phytoplasma based on RFLP analysis of 16S rRNA and ribosomal protein gene sequences. International Journal system Bacteriol. 48: 1153-1169.
- Wei, W., Davis, R.E., Lee, I-M and Zhao, Y. 2007. Computer-simulated RFLP analysis of 16S rRNA genes: identification of ten new phytoplasma groups. International Journal of Systematic and Evolutionary Microbiology 57:1855-1867. [CrossRef]
- Naderali, N., Nejat, N., Vadamalai, G., Davis R. E., Wei W., Harrison N. A., Kong L., Kadir J., Tan Y-H and Zhao Y. 2017. ‘Candidatus Phytoplasma wodyetiae’, a new taxon associated with yellow decline disease of foxtail palm (Wodyetiabifurcata) in Malaysia. Int J Syst. Evol. Microbiol. 10.1099/ijsem.0.002187.
- Zhao, Y., Wei, W., Davis, R.E., Lee, I.M., and Bottner-Parker, K.D. 2021. The agent associated with blue dwarf disease in wheat represents a new phytoplasma taxon, ‘Candidatus Phytoplasma tritici’ International Journal of Systematic and Evolutionary Microbiology, p.ijsem004604.
- Bertaccini, A.; Arocha-Rosete, Y.; Contaldo, N.; Duduk, B.; Fiore, N.; Montano, H.G.; Kube, M.; Kuo, C.H.; Martini, M.; Oshima, K.; Quaglino F., Schneider, B., Wei, W. and Zamorano, A. 2022. Revision of the ‘Candidatus Phytoplasma’species description guidelines. Int. J. Syst. Evol. Microbiol. 72:005353.
- Deng, S.J. and Hiruki, C. 1991.Amplification of 16S rRNA from culturable and non-culturable mollicutes. Journal of Microbiological Method 14: 53-61. [CrossRef]
- Kirkpatrick, B. C., Smart, C. D., Gardner, S. L., Gao, J.-L., Ahrens, U., Ma ̈urer, R., Schneider, B., Lorenz, K.-H., Seemu ̈ller, E., Harrison, N. A., Namba, S. and. Daire. X. 1994. Phylogenetic relationships of plant pathogenic MLOs established by 16/23S rDNA spacer sequences. IOM Lett.3:228–229.
- Smart, C. D., Schneider, B., Blomquist, C. L., Guerra, L. J., Harrison, N. A., Ahrens, U., Lorenz, K. H., Seemüller, E., & Kirkpatrick, B. C. (1996). Phytoplasma-specific PCR primers based on sequences of the 16S-23S rRNA spacer region. Applied and environmental microbiology 62(8): 2988–2993. [CrossRef]
- Gundersen, D.E. and Lee, I.-M.1996. Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopathologia Mediterranea 35 (3): 144-151.
- Lee, I-M., Hammod, R., Davis R. and Gundersen-Rindal, D.E. 1993. Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology 83: 834-842. 10.1094/phyto-83-834.
- Tymon, A.M., Jones, P. and Harrison, N.A. 1997. Detection and differentiation of African coconut phytoplasamas: RFLP analysis of PCR-amplified 16S rDNA and DNA hybridization. Annals of Applied Biology 131:91-102.
- Rohde, W., Kullaya, A., Mpunami, A.A., and Becker, D. 1993. Rapid and sensitive diagnosis of mycoplasmalike organisms associated with lethal disease of coconut palm by a specifically primed polymerase chain reaction for the amplification of 16S rDNA. Oleagineux 48:319–322.
- Martini M., Lee I.M., Bottner K.D., Zhao Y., Botti S., Bertaccini A., Harrison N.A., Carraro L., Marcone C., Khan A.J., Osler R., 2007. Ribosomal protein gene-based phylogeny for finer differentiation and classification of phytoplasmas. International Journal of Systematic and Evolutionary Microbiology 57: 2037-51.
- Pérez-López, E., Luna-Rodríguez, M., Olivier, C.Y. and Dumonceaux, T.J., 2016. The underestimated diversity of phytoplasmas in Latin America. International Journal of Systematic and Evolutionary Microbiology, 66(1):492-513.
- Cho, S. T., Zwolińska, A., Huang, W., Wouters, R. H. M., Mugford, S. T., Hogenhout, S. A., and Kuo, C. H. 2020. Complete Genome Sequence of “Candidatus Phytoplasma asteris” RP166, a Plant Pathogen Associated with Rapeseed Phyllody Disease in Poland. Microbiology resource announcements 9:35. e00760-20. [CrossRef]
- Hugenholtz, P., Chuvochina, M., Oren, A., Parks, D.H. and Soo, R.M. 2021. Prokaryotic taxonomy and nomenclature in the age of big sequence data. ISME J., 15, 1879–1892.
- Schneider, B., Gibb, K.S. and Seemüller, E. 1997. Sequence and RFLP Analysis of the Elongation Factor Tu Gene Used in Differentiation and Classification of Phytoplasmas. Microbiology 143:3381-3389. [CrossRef]
- Hodgetts, J. and Dickson, M. 2012. T-RFLP for detection and identification of phytoplasmas in plants. Methods in Molecular Biology 938:233-244.
- Martini, M., Bottner-Parker, K.D. and Lee, I-M. 2019. PCR-Based Sequence Analysis on Multiple Genes Other than 16S rRNA Gene for Differentiation of Phytoplasmas. P97-115 In: Musetti, R. and Pagliari, L. (eds.), Phytoplasmas: Methods and Protocols, Methods in Molecular Biology, vol. 1875. [CrossRef]
- Zambon Y, Contaldo N, Richards RS, Bertaccini A, Burger J. 2015. Multigene characterization of aster yellows phytoplasmas infecting grapevine in South Africa. Phytopathogenic Mollicutes 5: S21–S22. [CrossRef]
- M’hirsi, S., Acheche, H., Fattouch, S., Boccardo, G., Marrackchi, M. and Marzouki, N. 2004. First report of phytoplasmas in the aster yellows group infecting grapevine in Tunisia. New Disease Reports 9. http:bspp.org.uk/ndr/july2004/2004-10.asp.
- IPPC, 2008. Pest reports from South Africa on Aster yellows phytoplasma on grapevine. www.ippc.int. Accessed 11th September, 2024.
- Engelbrecht, M., Joubert, J. and Burger, J.T. 2010.First report of aster yellows phytoplasma in grapevines in South Africa. Plant Disease 94:373. [CrossRef]
- Coetzee B, Douglas-Smit N, Maree HJ, Burger JT, Krüger K, Pietersen G. 2019. Draft genome sequence of a “Candidatus Phytoplasma asteris”-related strain (aster yellows, subgroup 16SrI-B) from South Africa. Microbiol Resource Announc 8:e00148-19. [CrossRef]
- Kruger, K., De Klerk, A., Douglas-Smit, N., Joubert, J., Pietersen, G. and Stiller, M. 2011. Aster yellows phytoplasma in grapevines:identification of vectors in South Africa Bulletin of insectology 64. S137-S138.
- Pietersen, G., Pietersen, G., Jnr., Pietersen, I., and Stiller, M. 2018. Identification of Mgenia fuscovaria (Stal) (Hemiptera: Cicadellidae), a vector of aster yellows disease on grapevines in South Africa, and differentiation from Mgenia augusta (Theron) by nucleotide sequences of the mitochondrial cytochrome oxidase 1 (cox1) gene. S. Afr. J. Enol. Vitic 39 (2). [CrossRef]
- Cousin M.T., Maillet P.L., Gourret J.P., 1969. La virescence du cotonnier (Gossypium hirsutum L.) nouvelle maladie à mycoplasmes. CompteRendues Académie des Sciences, Paris, Série D 268: 2382-2384.
- Laboucheix J., van Offeren A., Desmidts M., 1973. Etude de la transmission par Orosiuscellulosus (Lindberg) (Homoptera,Cicadellidae) de la virescence florale du cotonnier et de Sida sp.. Coton et Fibres Tropicaux 28: 461-471.
- Delattre R., Joly A., 1981. Résultats des enquêtes sur la virescence florale du cotonniereffectuéesen Haute-Volta de 1970 à 1978. Coton et Fibres Tropicaux 36: 167-185.
- Marzachì, C., Coulibaly, A., Coulibaly, N., Sangaré, A., Diarra, M., De Gregorio T. and Bosco, D. 2009. Cotton virescence phytoplasma and its weed reservoir in mali. Journal of Plant Pathology 91 (3): 717-721.
- El-Banna, O. H. M., Mikhail, M. S., Farag, A. G., and Mohammed, A. M. S. (2007). Detection of phytoplasma in tomato and pepper plants by electron microscopy and molecular biology-based methods. Egypt J. Virol. 4, 93–111.
- Omar, A.F. and Fossiac, X. 2012. Occurrence and incidene of phytoplasmas of the 16SrII-D subgroup on solananceous and curcurbit crops in Egypt. European Journal of Plant Pathology 133 (2):353-360.
- El-Sisi Y., Omar A.F., Sidaros, S.A. and Elsharkawy M.M. 2017. Characterization of 16SrII-D subgroup associated phytoplasmas in new host plants in Egypt. Archives of phytopathology and plant protection. [CrossRef]
- Kumar, L. P., Sharma, K., Boahen, S., Tefera, H. and Tamò, M. 2011. First Report of Soybean Witches’-Broom Disease Caused by Group 16SrII Phytoplasma in Soybean in Malawi and Mozambique. Plant Dis. 95(4):492. [CrossRef] [PubMed]
- Murithi, H., Owati, A., Madata, C. S., Joosten, M., Beed, F. and Kumar, L. P. 2015. First report of 16SrII-C subgroup phytoplasma causing phyllody and witches’-broom disease in Soybean in Tanzania: Disease notes. Plant Disease 99(6): 886. [CrossRef]
- Alfaro-Fernández, A., Ali, M. A., Abdelraheem, F. M., Saeed, E. A. E., and Font- San-Ambrosio, M. I. 2012. Molecular identification of 16SrII-D subgroup phytoplasmas associated with chickpea and faba bean in Sudan. Eur. J. Plant Pathol. 133: 791–795. [CrossRef]
- Dafalla, G.A. and Cousin, M.T. Cousin. 1988. Natural occurrence of virescence disease on Catharanthus roseus and Zinna elegans in the Gezira, Sudan. Journal of Plant Diseases and Protection 95 (4): 414-418.
- Omar, A.F., Emeran, A.A. and Abass, J.M. 2008. Detection of perinwinkle virescence in Egypt. Plant Pathology Journal 7(1): 92-97.
- Gad, S.M., Kheder, A.A. and Awad, M.A. 2019. Detection and Molecular identification of phytoplasma associated with Gazania in Egypt. J. of Virol. Sci. 6: 12-23.
- Lukuyu, B., Ngunga, D. and Bekunda, M. 2021. Improved Napier grass varieties for smallholder farmers in East Africa. Nairobi, Kenya: ILRI.
- Midega, CAO., Pittchar, J.O., Pickett, J.A., Hailu, G.W. and Khan, Z.R. 2018. A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J.E. Smith) in maize in East Africa. Crop Protection 105: 10-15.
- Scheidegger, L., Niassy, S., Midega, C., Chiriboga, X., Delabays, N., Lefort, F., Zurcher, R., Hailu, G., Khan, Z. and Subramanian, S. 2021. The role of Desmodium intortum, Brachiaria sp. and Phaseolus vulgaris in the management of fall armyworm Spodoptera frugiperda (J.E. Smith) in maize cropping system in Africa. Pest management Science 77: 2350-2357. [CrossRef]
- Tsai, Y.C., Luo, P.Q., Sung, C.L., Li, Y., Hu, F.Y, Wang, C.L., Chen, Y.N., Hsu, J.H., Liao, C.E, Chang, S.R. and Chuang, W.P.2024. Evaluating local plant species for effective fall armyworm management strategies in Taiwan. Botanical Studies 65: 18. [CrossRef]
- Asudi, G. O., Muyekho, F. N., Midega, C. A. O. and Khan Z. R. 2019. Integrated Management of Napier Grass Stunt Disease in East Africa. Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt, Sustainability in Plant and Crop Protection 12: . [CrossRef]
- Jones, P., Devonshire, B.J., Holman, T.J. and Ajanga, S. 2004. Napier grass stunt: a new disease associated with a 16SrXI group phytoplasma in Kenya. Plant Pathology 53:519.
- Nielsen, S. L., Ebong, C., Kabirizi, J., and Nicolaisen, M. 2007. First report of a 16SrXI group phytoplasma (Candidatus Phytoplasma oryzae) associated with Napier grass stunt disease in Uganda. Plant Pathology 56(6): 1039–1039.
- Asudi, G. O. 2018. The dynamics of Napier grass stunt phytoplasma in East Africa Endocytobiosis and Cell Research 29:13–17.
- Fischer A, Santana-Cruz I, Wambua L, Olds C, Midega C, Dickinson M, Kawicha P, Khan Z, Masiga D, Jores J, Schneider B. 2016. Draft genome sequence of “Candidatus Phytoplasma oryzae” strain Mbita1, the causative agent of Napier grass stunt disease in Kenya. Genome Announc 4(2):e00297-16. [CrossRef]
- Asudi, G. O., Omenge, K. M., Paulmann, M. K., Reichelt, M., Grabe, V., Mithöfer, A., Oelmüller, R., & Furch, A. C. U. 2021. The Physiological and Biochemical Effects on Napier Grass Plants Following Napier Grass Stunt Phytoplasma Infection. Phytopathology, 111(4), 703–712. [CrossRef]
- Kabirizi, J., Nielsen, S.L., Nicolaisen, M., Byenkya, S. and Alicai, T. 2007. Napier stunt disease in Uganda: farmers’ perceptions and impact on fodder production. African Crop Science Conference Proceedings. 8:895–897.
- Asudi, G. O., Van den Berg, J., Midega, C. A. O., Pickett, J. A., and Khan, Z. R. 2016. The significance of Napier grass stunt phytoplasma and its transmission to cereals and sugarcane. Journal of phytopathology 164:378-385.
- Kawube, G., Talwana, H., Nicolaisen, M., Alicai, T., Otim, M., Kabirizi, J., Mukwaya, A. and Nielsen, S.L. 2015. Napier grass stunt disease prevalence, incidence, severity and genetic variability of the associated phytoplasma in Uganda. Crop Prot. 75:63–69.
- Asudi, G.O., Van den Berg, J. Midega, C.A.O., Pittchar, J., Pickett, J. and Khan, Z. 2015. Napier grass stunt disease in East Africa: Farmers’ perspectives on disease management. Crop Protection 71:116-124.
- Ricaud, C. 1968. Yellow wilt of sugarcane in eastern Africa. Sugarcane Pathol. Newsl. 1:45-49.
- Cronje, C. P. R., Tymon, A. M., Jones, P. and Bailey, R. A. 1998. Association of a phytoplasma with a yellow leaf syndrome of sugarcane in Africa. Ann. Appl. Biol. 133:177-186. [CrossRef]
- El Sayed, A.I., Soufi, Z., Wahdan, K.M. and Komor, E. 2015. Detection and characterization of phytoplasma and sugarcane yellow leaf virus associated with leaf yellowing of sugarcane. Journal of phytopathology 164:4217-225. [CrossRef]
- Scagliusi, S.M. and Lockhart, B.E.L.2000. Transmission, characterization and serology of a luteovirus associated with yellow leaf syndrome of sugarcane. Phytopathology 90:120-124.
- Marcone, C. 2002. Phytoplasma diseases of sugarcane. Sugar Tech. 4 (3 and 4).79-85.
- Rutherford, R.S., Brune, A.E. and Nuss, K.J. 2004. Current status of research on sugarcane yellow leaf syndrome in Southern Africa. Proc S Afr Sug Technol Ass 78: 173-180.
- Rott, P., Comstock, J.C., Croft, B.J. Kusalwong, A., Saumtally, S.A. 2005. Advances and Challenges in sugarcane pathology. Proc. Inten. Soc. Sugar Cane Technol. Congr. 25:607-614.
- Rogers,P.F. 1969.Proceedings of a meeting on the yellow wilt condition of sugarcane. June 25th-26th, 1969. Nairobi Kenya: East Africa Specialist Committee on Sugarcane Research.
- Arocha, Y., López, M., Fernández, M., Piñol, B., Horta, D., Peralta, E. L., Almeida, R., Carvajal, O., Picornell, S., Wilson, M. R. and Jones, P. 2005. Plant pathology 54 (5):634-642. [CrossRef]
- Abdelmajid, N., Mohamed, A., Cronje, P., & Jones, P. (1999). First Report of Yellow Leaf Syndrome of Sugarcane in Morocco. Plant disease, 83(4), 398. [CrossRef]
- Lockhart, B.E.L. and Cronjé, P.R., 2000. Yellow leaf syndrome. In: Rott, P. Bailey, R.A. Croft, B.J., Comstock, J.C. Saumtally, A.S. (eds). Guide to Sugarcane Diseases. France: CIRAD, 291 – 5.
- Nithya, K., Kirdat, K., Parameswari, B., Tiwarekar, B., Tiwari, A.K., Rao, G.P., Nikpay, A., Hoat, T.X., Viswanathan, R. and Yadav, A. 2023. Updates on phytoplasma diseases associated with sugarcane in Asia. In: Tiwari, A.K., Oshima, K., Yadav, A., Esmaeilzadeh-Hosseini, S.A., Hanboonsong, Y. and Lakhanpaul, S. (Eds) Phytoplasma Diseases in Asian Countries, Characterization, epidemiology, and management. Academic press. Vol 2:215-232. [CrossRef]
- Nithya, K., Parameswari, B. and Viswanathan, R. 2020. Mixed infection of sugarcane yellow leaf virus and grassy shoot phytoplasma in yellow leaf affected Indian sugarcane cultivars. The Plant pathology journal 36 (4): 10.5423/PPJ.OA.06.2020.0092.
- Kirdat, K., Tiwarekar, B., Thorat, V., Narawade, N., Dhotre, D., Sathe, S., Shouche, Y. and Yadav, A. 2020. Draft genome sequences of two phytoplasma strains associated with sugarcane grassy shoot (SCGS) and Bermuda grass white leaf (BGWL) diseases. Mol. Plant-Microbe Interact. 33:715-717. 10.1094/MPMI-01-0005-A.
- Viswanathan, R. 2000. Grassy shoot. In: Rott, P., Bailey, R.A., Comstock, J.C., Croft, B.J. and Saumtally, A.S. (eds.) A guide to Sugarcane diseases. Centre de cooperation international en recherche agronomique pour le development (CIRAD) and International Society of Sugar Cane Technologists (ISSCT) Montpellier. P215-220.
- Ekpo, E.N. and Ojomo, E.E. 1990. The spread of lethal coconut diseases in West Africa: incidence of akwa disease or bronze leaf wilt in the Ishan area of Bendel State of Nigeria. Principes 34 (3): 143-146.
- Mpunami, A., Tymon, A., Jones, P. and Dickinson, M.J. 1999. Genetic diversity in the coconut lethal yellowing disease phytoplasmas of East Africa. Plant Pathol 48:109 –114. [CrossRef]
- Dollet, M., Quaicoe, R., and Pillet, F. 2009. Review of coconut “lethal yellowing” type diseases. Diversity, variability and diagnosis. Oilseed fats crops lipids 16:97-101.
- Eziashi, E. and Omamor, I. 2010. Lethal yellowing disease of the coconut palms (Cocos nucifera L.): An Overwiew of the crises. Afr. J. Biotechnol. 9: 9122-9127.
- Eden-Green, S.J. 1997. History, distribution and research on coconut lethal yellowing-like diseases of palms. In: Eden-Green, S.J. and Ofori, F.(eds.) Proceedings of the International workshop on lethal yellowing-like diseases of coconut, Elmina, Ghana. NRI: Chatham, UK. P9-25.
- Konan Konan, J.L., Allou, K., Atta Diallo, H., Yao, S. D., Koua, B., Kouassi, N., Benabid, R., Michelutti, R., Scott, J.A. and Arocha-Rosete, Y., 2013. First report on the molecular identification of the phytoplasma associated with a lethal yellowing-type disease of coconut palms in Cote d’Ivoire. New Disease Reports 28:3. [CrossRef]
- Osagie, I.J., Ojomo, E.E. and Pilet, F. 2015. Occurrence of Awka wilt disease of coconut in Nigeria for one century. Phytopathogenic Mollicutes 5: S61–S62. [CrossRef]
- Ofori F. and Nkansah-Poku J. 1997. Cape Saint Paul wilt disease of coconut in Ghana: History of its occurrence and spread. In: Eden-Green S.J. and Ofori F. (eds). Proceedings of an International Workshop on Lethal Yellowing-Like Diseases of Coconut, Elmina, Ghana, November 1995. Chatham, UK: NRI, P27-32.
- Dabek, A.J., Johnson, C.G. and Harries, H.C. 1976. Mycoplasma-like organisms associated with Kaincope and Cape St. Paul wilt diseases of coconut palms in West Africa. PANS:22 (3): 354-358. [CrossRef]
- Dollet, M., Gianotti, J., Renard, J-L and Ghosh, S.K. 1977. Study of a lethal yellowing of coconut trees in Cameroon: Kribi disease. Observations of mycoplasma-type organisms. Oleagieux 32 (7):317-322.
- Bila J, Mondjana A, Samils B, Hogberg N. 2015. High diversity, expanding populations and purifying selection in phytoplasmas causing coconut lethal yellowing in Mozambique. Plant Pathol 64:597– 604. [CrossRef]
- Cordova, I., Oropeza, C., Puch-Hau, C., Harrison, N., Colli-Rodriguez, A., Narvaez, M., Nic-Matos, G., Reyes, C. and Saenz, L. 2014. A real-time PCR assay for detection of coconut lethal yellowing phytoplasmas of group 16S IV subgroups A,D and E found in the Americas. J. Plant Pathology 96: 343-352.
- Bila, J. Hogberg, N., Mondjana, A and Samils, B. 2015. African fan palm (Borassus aethiopum) and Oil palm (Elaeis guineensis) are alternate host of coconut lethal yellowing phytoplasma in Mozambique. African Journal of Biotechnology 14 (52):3359-3367.
- Danyo, 2011. Review of scientific research into the Cape Saint Paul wilt disease of coconut in Ghana. African Journal of Agricultural Research 6(19). [CrossRef]
- Nipah, J. O., Jones, P. and Dickinson, M. J. 2007. Detection of lethal yellowing phytoplasma in embryos from coconut palms infected with cape St Paul wilt disease in Ghana. Plant Pathol. 56(5): 777–784.
- Oropeza, C., Cordova, I., Puch-Hau, C., Castillo, R., Chan, J. and Sáenz, L. 2017. Detection of lethal yellowing phytoplasma in coconut plantlets obtained through in vitro germination of zygotic embryos from the seeds of infected palms. Annals of Applied Biology, 171(1), 28–36.
- Mpunami, A., Tymon, A., Jones, P. and Dickinson, M.J. 2000. Identification of potential vectors of the coconut lethal disease phytoplasma. Plant Pathology 49 (3): 355–361. [CrossRef]
- Kwadjo, K. E., Beugré,N.’D. I., Dietrich,C. H., Kodjo, A. T. T., Diallo, H. A., Yankey,N., Dery,S., Wilson, M., Konan Konan, J. L., Contaldo, N., Paltrinieri, S. Bertaccini, A. and Arocha-Rosete, Y.2018. Identification of Nedotepa curta Dmitriev as a potential vector of the Côte d’Ivoire lethal yellowing phytoplasma in coconut palms sole or in mixed infection with a ‘Candidatus Phytoplasma asteris’-related strain. Crop Protection 110:48-56. [CrossRef]
- Bila, J. 2016. Coconut lethal yellowing phytoplasma disease in Mozambique. Doctoral Thesis, Swedish University of Agricural Sciences, Uppsal.
- Kra KD, Toualy YMN, Kouamé AC, Diallo HA, Arocha-Rosete Y, 2017. First report of a phytoplasma affecting cassava orchards in Cote d’Ivoire..New Disease Reports 35, 21. [CrossRef]
- Arocha-Rosete, Y., Diallo, H.A., Konan Konan, J.L., Yankey, N., Saleh, M., Pilet, F., Contaldo, N., Paltrinieri, S., Bertaccini, A. and Scott, J. 2017. Detection and differentiation of the coconut lethal yellowing phytoplasma in coconut growing villages of Grand-Lahou, Côte d’Ivoire. Annals of Applied Biology 170: 333–347. [CrossRef]
- Bila, J., Mondjana, A., Samils, B., Santos, L. and Hogberg, N. 2019. Integrated Management of Coconut Lethal Yellowing Phytoplasma Disease in Mozambique: Current Challenges and Future Perspectives. Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt, Sustainability in Plant and Crop Protection 12. [CrossRef]
- Álvarez E., 2019. Phytoplasma Diseases Affecting Cassava. Olivier, C. Y.. Tim J. Dumonceaux and Edel Pérez-López (eds.), Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt, Sustainability in Plant and Crop Protection 12, . [CrossRef]
- Arocha, Y and Jones, P. 2010. Phytoplasma disease of Graminae.I: Weintraub P, and Jones, P. (Eds.). Phytoplasmas: genome, plants hosts and vectors. Wallingford, UK: CABI International. P170-187.
- Obura, E., Masiga, D., Midega, C. A. O., Wachira, F., Pickett, J. A., Deng, A. L.,and Khan, Z. R. 2010.First report of a phytoplasma associated with Bermuda grass white leaf disease in Kenya New Dis. Rep. 21:23.
- Obura, E., Masiga, D., Midega, C. A. O., Otim, M., Wachira, F., Pickett, J., and Khan, Z. R. 2011. Hyparrhenia grass white leaf disease, associated with a 16SrXI phytoplasma, newly reported in Kenya. New Dis. Rep. 24:17.
- Cronje, P., Dabek, A.J., Jones, P. and Tymon, A.M. 2000. First report of a phytoplasma associated with a disease of date palms in North Africa. Plant Pathology 49(6): 801-801. [CrossRef]
- Cronje, P., Dabek, A.J., Jones, P. and Tymon, A.M. 2000. Slow decline: a new disease of matured date palms in North Africa associated with phytoplasma. Plant Pathology 49(6): 804-804. [CrossRef]
- Cronjé, P., Dabek, A. J., Jones, P. and Tymon, A. M.2008. First report of a phytoplasma associated with a disease of date palms in North Africa. Plant Pathology 49(6) 801-801. [CrossRef]
- Ammar, M.I. Amer, M.A. and Rashed, M.F. 2005. Detection of phytoplasma associated with yellow streak disease of date palms in Egypt. Egyptian J. Virol. 2:74-86.
- Alkhazinder, M., 2014.Detection and molecular identification of Aster Yellows phytoplasma in date palm in Egypt. Journal of Phytopathology. [CrossRef]
- Guthrie, J.N., White, D.T., Walsh, K.B. and Scott, P.T. 1998. Epidemiology of phytoplasma associated papaya diseases in Queensland, Australia. Plant Diseases 82: 1107-1111.
- Padovan, A. and Gibb, K. 2001. Epidemiology of phytoplasma diseases in papaya in Northern Australia. Journal of Phytopathol. 149: 649-658.
- Elder, R., Milne, J., Reid, D., Guthrie, J. and Persley, D. 2002. Temporal incidence of three phytoplasma associated diseases of Carica papaya and their potential hemipteran vectors in central and south-east Queensland. Aust. Plant. Pathol.31: 165-176.
- Gera, A., Mawassi, M., Zeidan, M., Spiegel, S. and Bar-Joseph, M. An isolate of ‘Candidatus Phytoplasma australiense’ group associated with Nivun Haamir dieback disease of papaya in Israel. Plant Pathology 54(4):560 – 560. 10.1111/j.1365-3059.2005.01236.x.
- Arocha, Y., Bekele, B., Tadesse, D. and Jones, P. 2007. First report of a 16SrII group associated with die-back diseases of papaya and citrus in Ethiopia. Plant Pathology 56:1039.
- Kazeem, S.A., Inaba, J., Zhao, Y., Zwolińska, A., Ogunfunmilayo, A.O., Arogundade, O. and Wei, W. 2021. Molecular identification and characterization of ‘Candidatus Phytoplasma convolvuli’-related strains (representing a new 16SrXII-O subgroup) associated with papaya bunchy top disease in Nigeria. Crop Protection 148: . [CrossRef]
- Lobognon N.P.A., Kra, K.D. and Toualy, M-N. 2014. First Detection of Ca. phytoplasma asteris in Papaya orchards in Ivory Coast. Pakistan Journal of phytopathology 36 (2):347-358. [CrossRef]
- Inaba, J., Kazeem, S.A., Zhao, Y., Zwolińska, A., Ogunfunmilayo, A.O., Arogundade, O. and Wei, W. 2023. Tomato and Jute Mallow are Two New Hosts of Papaya Bunchy Top Phytoplasma, a ‘Candidatus Phytoplasma convolvuli’-Related Strain in Nigeria. Plant Disease 107 (6): 1937. 10.1094/PDIS-09-22-2192-PDN.
- El-Banna, O.H.M. and El-Deeb, S.H. 2007. Phytoplasma associated with mango malformation disease in Egypt. Journal of Phytopathology: 157:639-641.
- Amr, M., Kheder, A., Ahmed, G., El-Habbaa and Mahdy, A. 2024. Identification and molecular characterization of phytoplasma associated carrot plant (Daucus carota L.) in Qalyubia Governorate, Egypt. Annals of Agricultural Science, Moshtohor 62:21-36. [CrossRef]
- SA wine Industry Statistics 2023. South Africa Wine Industry Information and systems SAWIS. www.sawis.co.za and wosa.co.za. Accessed 1st September, 2024.
- Carstens, R. 2014. The incidence and distribution of grapevine yellows diseases in South African vineyards. M.Sc Thesis Stellenbosch University. 95p.
- Kruger, K. 2020. Grapevine Yellows management in South Africa: Manangement Strategies for Aster yellows phytoplasma in grapevine in South Africa. Tropicsafe Technical Innovative Factsheet. www.tropicsafe.eu.
- Yankey, E. N., Aidoo, O.F., and Sossah, F. L. 2024. A critical review of Cape Saint Paul Wilt Disease: A devastating phytoplasma-associated infection affecting coconut trees in Ghana. Crop Protection 184:. [CrossRef]
- Khan ZR, Midega CAO, Nyang’au MI, Murage A, Pittchar J, Agutu L,Amudavi DM, Pickett JA. (2014) Farmers’ knowledge and perceptions of the stunting disease of Napier grass in western Kenya. Plant Pathol. 63(6):1426–1435.
- El Sayed, A. I. and Boulila, M., 2014. Molecular Identification and phylogenetic analysis of sugarcane yellow leaf Phytoplasma (SCYLP) in Egypt. J. Phytopathol. 162: 89-97. 10.1111/jph.12156.
- Jibrin, M. O., Olson, J. Wallace, S., Walker, N. and Marek, S.M. 2024. First Report of ‘Candidatus Phytoplasma asteris’-Related Strains (Subgroup 16SrI-A) Associated With Aster Yellows on Chrysanthemums in Oklahoma. Plant Disease 108 (11): 3406. [CrossRef]
- Singh, K., Ranebennur,H., Rawat,K., Chalam, V.C., Gupta,S., Choudhary, M., Meena, V.S., Shekhawat, N., Sharma, M., Chawla, M.P., Kumar, M., Singh, P. K. and Singh G. P. First Report of ‘Candidatus Phytoplasma asteris’ (16SrI-B Subgroup) Associated with Stunting and Little Leaves of Guar (Cyamopsis tetragonoloba) in World. Plant Disease xxx. [CrossRef]
- Dutta, D.S., Kalita, M.K. and Nath, P.D. (2024). First report of Candidatus Phytoplasma trifolii (16SrVI-D) associated with little leaf disease of Nyctanthes arbor-tristis in the world. J Plant Pathol 106: 1403–1404. [CrossRef]

| Primers | Sequence (5՜-3՜) | Reference |
|---|---|---|
| P1 | AAGAGTTTGATCCTGGCTCAGGATT | [107] |
| P4 | GAAGTCTGCAACTCGACTTC | [108] |
| P6 | CGGTAGGGATACCTTGTTACGACTTA | [107] |
| P7 | CGTCCTTCATCGGCTCTT | [109] |
| R16F2n | GAAACGACTGCTAAGACTGG | [110] |
| R16R2 | TGACGGGCGGTGTGTACAAACCCCG | [111] |
| LYDSR (Lethal Disease Tanzania) | GGTGCCATATATATTAGATTG | [112] |
| G813F (Lethal Disease Ghana) | CTAAGTGTCGGGGGTTTCC | [112] |
| AKSR (Lethal Disease Nigeria) | TTGAATAAGAGGAATGTGG | [112] |
| Rhode F (Lethal Disease Tanzania) | GAGTACTAAGTGTCGGGGCAA | [113] |
| Rhode R (Lethal Disease Tanzania) | AAAAACTCGCGTTTCAGCTAC | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





