1. Introduction
Bananas (Musa spp.) are a crucial crop in global agriculture, playing a significant role in food security and economic stability, especially in tropical and subtropical regions [
1]. Over 100,000 farmers in Malawi are involved in banana production and more than 40% are women. The volume of production ranges from 569,510 to 701,173 metric tons [
2]. Despite its importance, the production of banana in Malawi is declining due to diseases, poor agronomic practices and lack of disease-free planting materials. Diseases such as banana leaf streak (Black sigatoka) and banana bunchy top disease (BBTD), as well as pests like the corm weevil (Cosmopolites sordidus), nematodes, and banana aphid (Pentalonia nigronervosa), are easily transmitted from one farmer to another . This is largely because the crop is propagated vegetatively, with farmers sharing suckers [
3]. Currently almost 70% of bananas in Malawi have been wiped out due to diseases and pests, mainly transmitted through sharing or purchase of infected planting materials. The situation has become so dire that over 70% of the desert bananas consumed in the country are imported from Tanzania [
3]. Agricultural extension advice for farmers to uproot and burn infected plants has mostly been ignored due to labour implications involved in destroying banana marts and limited access to clean planting materials to replace the ones uprooted. The lack of clean planting materials has also negatively affected adherence to phytosanitary measures by Department of Agricultural Research Services (DARS) of Malawi.
Tissue culture remains a popular method for rapidly producing disease-free banana planting materials. Plants produced from tissue culture are uniform, true-to-type, disease-free, and can be produced regardless of season[
4]. However, tissue culture plants are produced in laboratory conditions under sterile media, requiring expertise and expensive equipment. Additionally, plants from tissue culture are prone to somaclonal variation and require a prolonged period of hardening before they can be accessed by farmers in the field[
5]. Therefore, for the case of Malawi where banana production is largely done by resource-constrained smallholder farmers, there is need to explore affordable and sustainable methods of producing disease-free banana planting materials [
1].
Macropropagation has emerged as a method that can effectively compliment tissue culture efforts in Malawi. In macropropagation, healthy corms are dug out, prepared to the appropriate size, sterilized, and covered with plastic sheets after being placed in locally available media [
6]. These corms produce plantlets within six weeks. Although the number of plants produced is not as high as that produced in tissue culture, the plants produced in macropropagation are simple and take a shorter period to harden. In addition, the plants from macropropagation, just like those from tissue culture, are free from pests and diseases [
1]. Importantly, macropropagation is particularly applicable to smallholder farmers in Malawi, as it is a simple technique that requires affordable and locally available materials[
6]. Therefore, macropropagation has potential to improve sustainable access of planting materials within local communities.
Although macropropagation is known to be more affordable than tissue culture, the standard recommended macropropagation units (using wooden planks, thick plastic sheets and sawdust as a substrate) are still unaffordable for many resource-poor farming communities [
7]. Macropropagation units made from local materials have failed to gain the anticipated level of adoption due to the low availability of sawdust as a standard substrate [
7]. Simpler methods of producing plantlets have been demonstrated with enset in Ethiopia. Large numbers of plantlets were produced using a mixture of soil and farmyard manure covered with mulch. This approach may also be effective for bananas. Recently, the exploration of low-cost propagation methods has focused on using available local materials [
8]. To increase adoption within resource-poor communities, like those in Malawi, an alternative low-cost propagation method, requiring low skills to implement and use local materials, is urgently needed. The objective of the study was, therefore to investigate different proagation structutres and locally available substrates for banana macropropagaiton in Malawi.
2. Materials and Methods
2.1. Source of Plant Materials
The Cv Williams corms of sword suckers used in this study were sourced from the LUANAR Bunda College Horticulture Farm, which was established two years earlier with virus-free tissue-culture sourced suckers. At the time of sourcing the corms, there were no visible signs of banana bunchy top virus (BBTV) or any other diseases in the banana field.
2.2. Experimental Site
Macro-propagation experiments were conducted at Bunda College of Lilongwe University of Agriculture and Natural Resources (LUANAR) (14° 11’S, 33° 46’E) from 6th June 2022 to 19th October in 2022. Therefore total period from placement of corms in the chamber to harvest of final suckers was 135 days. The site is situated at 14˚11'S latitude and 33˚46'E longitude, with an altitude of 1100 meters above sea level. It falls within the mid-elevation, upland plateau agro-ecological zone of Malawi. The mean annual temperature is 19.1˚C, with a maximum of 27.8˚C and a minimum of 17.2˚C.
2.3. Experimental Design
2.3.1. Experimental Design
The substrates investigated in the study are rice husks (S1), sawdust (S2), and loam soil (S3). The study included three structural setups: a standard chamber (SC), a standard chamber with a black net (SBN), and mulched open bed (MOB). The decision to include black netting was based on our initial studies, which revealed that plastic without netting was causing a hot microclimate that resulted in scorching of the suckers. The experimental design followed a Split Plot Design with three replicates and eight corms per replicate. Conditions in the SC and SBN, including temperature, humidity, and light, were recorded. The primary aim of the study is to identify optimal conditions for macropropagation using accessible and cost-effective materials. The statistical model employed for the analysis is expressed as follows:
Where: Yijk = the response variable for the kth replicate of the ith main plot and jth sub-plot, μ = overall mean, Ai = effect of the ith level of the main plot factor (macro-propagation structure), Bj = effect of the jth level of the sub-plot factor (substrate), (AB)ij = interaction effect between the main plot and sub-plot factors, ϵijk = random error term associated with the kth replicate of the ith main plot and jth sub-plot, assumed to be normally distributed.
2.3.2. Macropropagation Structures and Management
The structures were constructed by building three identical beds measuring 8m in length, 1m in width, and 30cm in height using bricks, sand, and cement. Quarry stones were placed in all beds to separate the soil and substrate and enhance drainage. Two standard structures (SC and SBN) were then constructed using bamboo sticks covered with a clear plastic sheet (
Figure 1a,b). These standard structures were 1.8m high, 2.5m wide, and 8m long. One of the standard structures was covered with a black blacknet to create a humidity chamber with the blacknet (
Figure 1b). A third bed featured a smaller structure measuring 0.5m in height made from poles, nails, and strings, and thatched with grass (
Figure 1c). Each 8m bed inside and outside the humidity chambers was divided into nine 1m
2 beds. The standard chamber (SC) served as the control in the experiment. A temperature and humidity meter were placed in each Macropropagation structure to monitor temperature and humidity levels (
Table 1 and
Table S1).
2.3.3. Substrate Preparation and Characterization
Three types of substrate were used in this study: sawdust, rice husks, and loam soil (
Figure S1). Fresh sawdust was obtained from local timber processors, while fresh rice husks were collected from rice mills in the same area. Loam soil was sourced from Bunda College forest. The samples of the substrates were sent to the soil lab for water holding capacity characterization. The air-dried substrates were packed gently in core rings (5.3 diameter and 6 length) in 3 replicates. The rings were placed on a pressure plate and water was applied gently around the rings. The rings were left for 48 hours until saturation (water appeared at the surface). Immediately the rings with the plate were placed in the pressure plate apparatus and subjected to 33kPa (i.e. field capacity) and 1500kPa (i.e permanent wilting point). Water content at each suction was determined gravimetrically (
Table 2). Each substrate was sterilised by placing it in a sack and haning in a drum of 200-litres capacity. The sack did not touch the bottm of the the drum. The drum was then filled with about 30 litres of water and closed. These were heated for about six hours so that the heat from the boling water could kill bacyeria, fungus, nematodes, weevils and other insects within the substarte. The sterilised substrates were then cooled. Each cooled submstrate was used to fill a 8m
2 beds, as illustrated in
Figure 1d.
2.3.4. Banana Corms Preparation and Planting
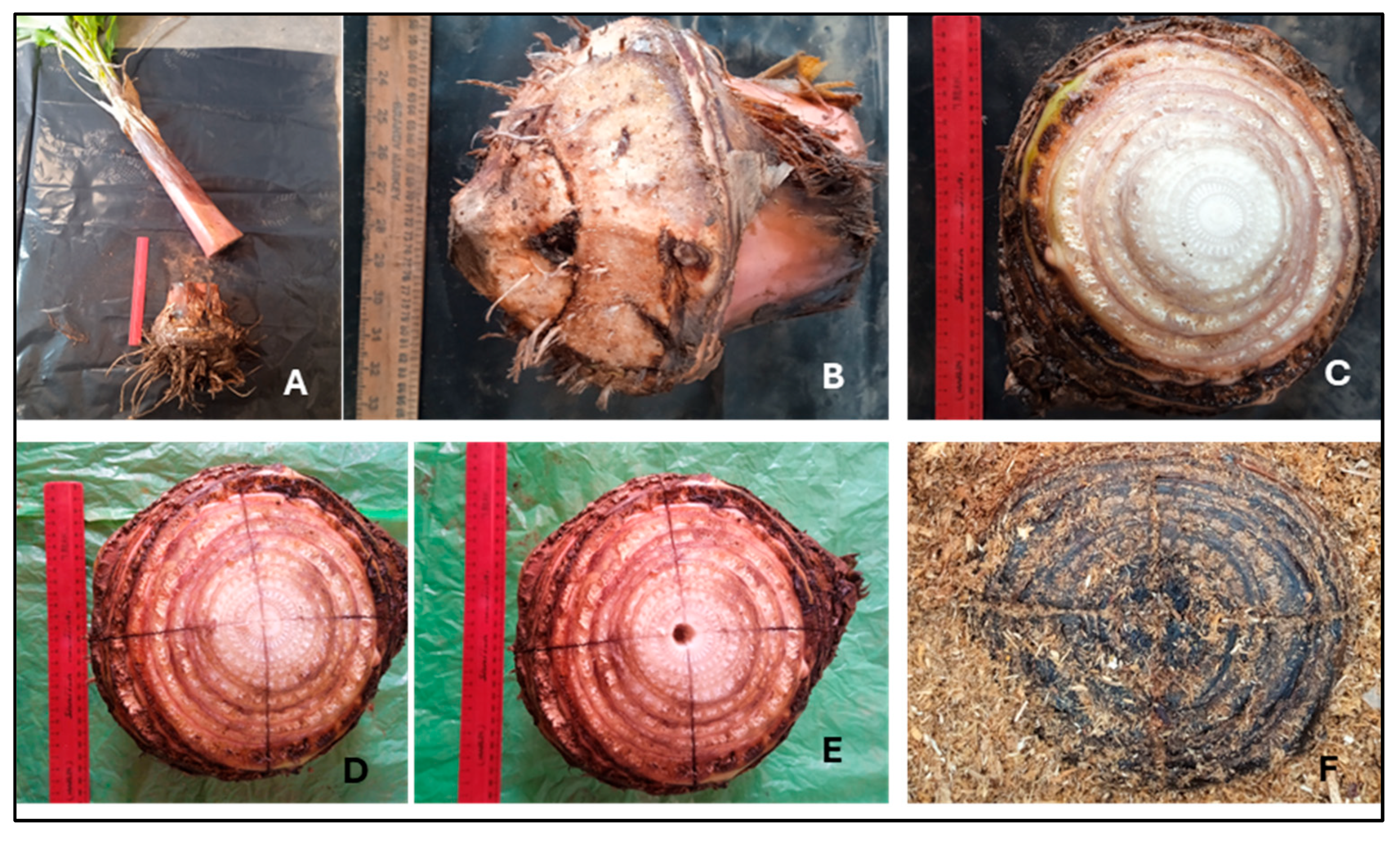
Banana Cv. Williams Corms were obtained from sword suckers. Corms were obtained from farms that did not have visibly detectable pests and diseases. The farms had been established from tissue-culture sourced suckers. The banana corms were processed using the whole corm method, where roots and excess pseudo stem were removed, and apical dominance was eliminated [
1] (
Figure 2B,C). A sharp knife was was used to make incisions on top of the corm. These incisions were 5 cm deep into the corm. The incisions were made in such a way that the two lines crossed at the center of the corm, forming the pattern of a cross.. The central meristem was killed by inserting a knife vertically and screeing into the flesh of the corm until the knife had transitioned from the pseoudo-leaf section and reached the underground stem section (
Figure 2D,E). The circumference, height, and diameter of all corms were similar. Subsequently, the corms were sterilized by washing them in a 50% Copper Oxychloride solution for 15 minutes, prepared by diluting 50g of Copper Oxychloride powder in 10L of water. After sterilization, the corms were cured under a shed for 48 hours before being transferred to three propagation structures filled with sawdust, rice husks, and loam soil. Corms were spaced 5cm apart, and covered with a 2-5cm layer of the respective medium (
Figure 2F). Eight corms prepared as above were planted in each replicate of 8m
2 bed. The substrates were watered to field capacity throughout the macropropagation period.
2.3.5. Decapitation Process
Due the killing of the central meristem of the corm, some lateral buds rapidly produced primary suckers within four weeks. Primary suckers that had a large base at the connection to the corm were decapitated when they had two or three leaves. Decapitation involved cutting the pseudostem of the sucker at 2cm distance from the connection to the corm. This was followed by scarifying the central meristem of the primary sucker by making two deep cross-wise incisions to destroy apical dominance (
Figure 2E). Primary suckers that had a narrow connection to the corm were harvested even when only one root was present. This was done to induce other dormant lateral buds from producing shoots before the corm lost vigour in propagation.
2.4. Data Collection and Analysis
The collected data underwent statistical analysis using ANOVA in Genstat 18th Edition [
2] to evaluate the effects of different treatments. The variable factors analyzed were
substrate, macropropagation structure, and decapitation. ANOVA was selected for its capability to compare means across multiple groups and determine significant differences. Significant differences among treatment groups were further identified using Tukey's test at a significance level of 0.05.
Principal component analysis (PCA) was conducted using R software version 4.2.2 [
3] along with factoextra [
4] and ggforce [
5] packages to explore relationships among variables and identify patterns within the dataset. Pearson correlation analysis was performed using corrplot [
6] and metan [
7] packages to assess linear relationships between variables.
Regression analysis was conducted using the stats package in R [
3] to model the relationship between variables and further elucidate their impacts. This comprehensive statistical approach ensures the reliability and validity of the findings, providing clear insights into the effects of various substrates and structural setups on banana growth parameters.
3. Results
3.1. Effect of Macropropagation Structure
The study compared the effect of different macropropagation structures on primary suckers, specifically analyzing the standard chamber (SC), standard chamber with a black net (SBN), and mulched open bed (MOB) (
Table 3). The number of leaves per plant did not vary significantly among the structures (Fpr. = 0.159), with the MOB having the highest mean (4.76±0.15) and the Standard Chamber the lowest (4.03±0.14). Plant height exhibited significant variation (Fpr. = 0.011), with the standard chamber and standard chamber with a black net structures producing taller plants (20.50±0.83 cm and 20.26±1.02 cm, respectively) compared to the mulched open bed (16.59±0.79 cm). The number of roots also showed significant differences (Fpr. = 0.010), with the standard chamber having the highest (6.78±0.52) and the standard chamber with a black net the lowest (4.18±0.52). Root length did not vary significantly (Fpr. = 0.545), ranging from 7.78±1.04 cm in standard chamber with a black net to 9.25±1.09 cm in the mulched open bed. The number of harvested suckers exhibited a highly significant difference (Fpr. < 0.001), with the standard chamber yielding the most (102.22±14.09) compared to standard chamber with a black net (59.11±7.32) and the mulched open bed (38.33±3.78). Overall, the grand mean values were 4.42±0.26 leaves, 19.12±0.92 cm height, 5.35±0.56 roots, 8.73±1.04 cm root length, and 66.60±9.84 harvested suckers, with the highest coefficient of variation observed for the number of harvested suckers (44.3%) and the lowest for the number of leaves (17.6%). The least significant difference values highlighted the statistical significance thresholds for each measured trait (
Table 3).
3.1. Effect of Substrate
The effect of substrate on various growth parameters of primary suckers were assessed using loam soil, rice husks, and sawdust (
Table 4). Significant differences were observed in plant height (Fpr = 0.013) and root length (Fpr < 0.001) among the substrates. Suckers grown in loam soil and sawdust exhibited similar plant heights (20.44±0.64 cm and 20.27±1.04 cm, respectively), which were significantly greater than those grown in rice husks (16.27±0.96 cm). Root length was longest in suckers grown in rice husks (11.49±0.69 cm), significantly surpassing those in loam soil (6.28±0.38 cm) and sawdust (8.40±0.91 cm). The number of leaves and roots per sucker did not differ significantly among substrates (Fpr = 0.690 and Fpr = 0.284, respectively). However, the number of harvested suckers varied significantly (Fpr = 0.009), with sawdust (85.33±13.81) and loam soil (79.00±11.95) producing more suckers compared to rice husks (35.33±3.62). These findings underscore the differential effects of substrate on growth parameters of primary suckers, highlighting implications for optimizing cultivation practices in similar agricultural contexts.
3.1. Combined Effects of Macropropagation Structure and Substrate
The study showed significant variations in key growth parameters of primary suckers based on the macropropagation structure and substrate used (
Table 5). Plant height varied significantly across treatments (Fpr. = 0.001), with the tallest plants in the standard chamber with sawdust substrate (22.33 cm) and the shortest in the mulched open bed with rice husks (13.40 cm). The number of roots was highest in the standard chamber with rice husks (8.43 roots) and lowest in the standard chamber with black net and rice husks (3.38 roots) (Fpr. < 0.001). Root length followed a similar trend (Fpr. < 0.001), with the longest roots in the standard chamber with rice husks (11.83 cm) and the shortest in the standard chamber with black net and sawdust (5.70 cm). The number of harvested suckers was also significantly affected by the treatments (Fpr. < 0.001), with the standard chamber with sawdust yielding the highest number of suckers (135.33) and the standard chamber with black net and rice husks producing the lowest (32). These results highlight the importance of selecting suitable macropropagation structures and substrates to enhance the growth and yield of primary suckers.
3.1. Regression Analysis of the Relationship Between Decapitated Suckers and Secondary Suckers
A regression analysis was conducted to investigate the correlation between the number of decapitated suckers and the production of secondary suckers. The independent variable was the number of decapitated suckers, and the dependent variable was the number of secondary suckers. The results, as shown in
Table 6, indicated a significant positive relationship (p < 0.001) with a regression coefficient of 2.867. This suggests that for each additional decapitated sucker, the number of secondary suckers increased by approximately 2.87. The intercept was -0.844, which was not statistically significant (p = 0.843). The R-squared value of 0.741 indicates that 74.1% of the variability in secondary sucker production can be explained by the number of decapitated suckers. The F-statistic of 80.94 (p < 0.001) further supports the significance of the relationship, highlighting the effectiveness of decapitation in promoting sucker proliferation (
Figure 3).
3.1. Correlation Coefficients
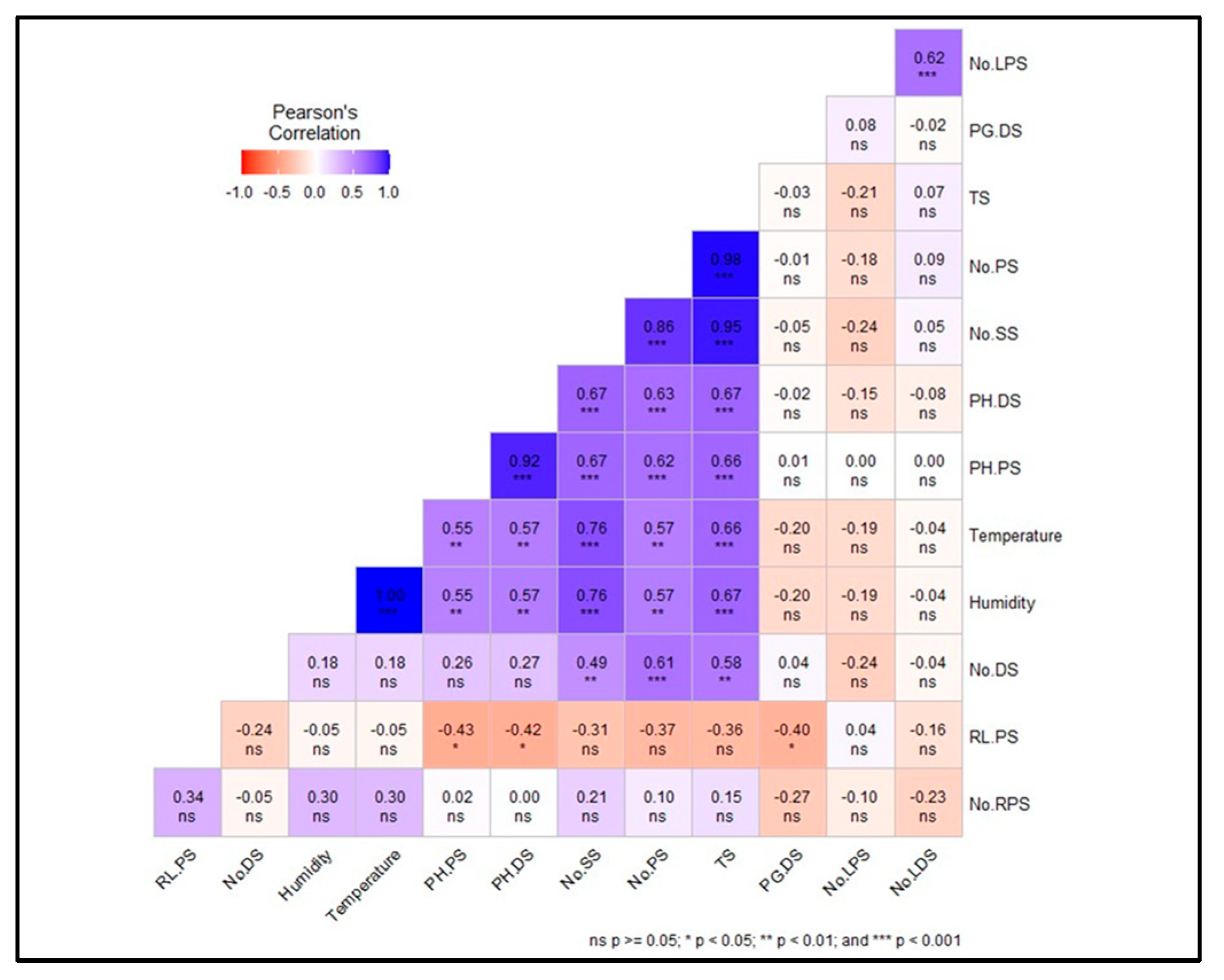
The Pearson correlation matrix (
Figure 4) provides insights into the relationships between various environmental factors and performance metrics of macro-propagation units. Strong positive correlations are observed among several performance metrics, such as PH.PS (Plant Height for Primary suckers) and PH.DS (Plant Height for Decapitated Suckers) (r = 0.92, p < 0.001), PH.DS and No.SS (Number of Secondary Suckers) (r = 0.86, p < 0.001), and No.SS and PH.PS (r = 0.95, p < 0.001), indicating a high degree of interdependence among these variables. Temperature shows a significant positive correlation with No.LPS (Number of Leaves for Primary Suckers) (r = 0.62, p < 0.001) and PG.DS (Plant Girth of Decapitated Suckers) (r = 0.55, p < 0.01), suggesting that higher temperatures enhance the performance of these units. Similarly, Humidity is positively correlated with PG.DS (r = 0.57, p < 0.01) and shows a strong correlation with Temperature (r = 0.76, p < 0.001), highlighting a compounded effect on macro-propagation performance.
Conversely, negative correlations are observed between Humidity and PH.PS (r = -0.43, p < 0.05) as well as PH.DS (r = -0.42, p < 0.05), indicating that higher humidity may adversely impact these specific performance metrics. Additionally, RL.PS (Root Length for Primary Suckers) shows a moderate positive correlation with No.RPS (r = 0.34, p < 0.05), while No.DS (Number of Decapitated Suckers) is negatively correlated with No.LDS (Number of Leaves for Decapitated Suckers) (r = -0.24, p < 0.05). These correlations suggest the need for a nuanced approach in managing environmental factors to optimize the performance of different macro-propagation units, considering the diverse and sometimes opposing influences of temperature and humidity on various performance outcomes.
3.1. Principal Component Analysis
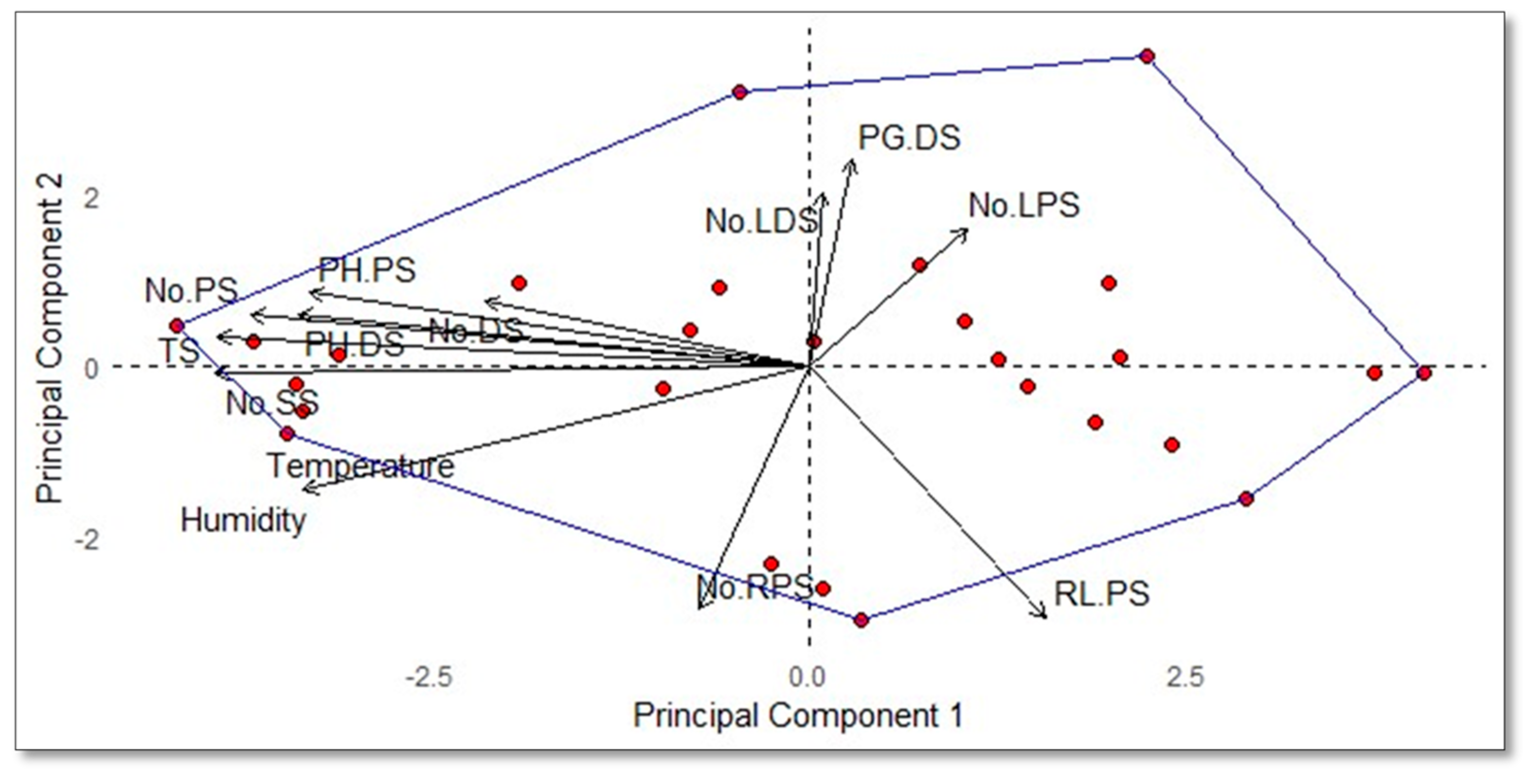
The principal component analysis (PCA) biplot (
Figure 4) illustrates the intricate relationships between environmental factors (Temperature and Humidity) and the performance metrics of various macro-propagation units (e.g., No.RPS, RL.PS, and No.LDS). The vectors representing Temperature and Humidity, oriented towards the lower left quadrant, indicate a significant contribution to the second principal component (PC2) and a potential negative correlation with the principal components. The substantial lengths of these vectors suggest that both factors are critical in explaining the variability in the dataset. Notably, the positioning of macro-propagation unit-related variables along both principal components suggests a compounded effect of Temperature and Humidity on their performance. Variables such as No.RPS and RL.PS, aligned significantly with both PC1 and PC2, indicate that the efficacy of the macro-propagation units is not solely dependent on one environmental factor but rather the combined influence of both. This compounded effect underscores the necessity of optimizing both Temperature and Humidity conditions to enhance the performance of macro-propagation units. The observed clustering patterns and distinct variable contributions highlight the complex interplay between these environmental factors, suggesting that their combined impacts are crucial in driving the performance outcomes of the macro-propagation units.
4. Discussion
Micropropagation through tissue culture techniques is an efficient method of producing large quantities and good quality Musa suckers, but is constrained by high capital and skills requirements. As such macropropagation is being advocated as an effective alternative method which requires less capital and skills to produce large numbers of better-quality banana suckers [
1].However, macropropagation is a relatively new technology in Malawi and needs to be adapted so that it can produce many suckers at an affordable cost. The objective of the study was, therefore to investigate different proagation structutres and locally available substrates for banana macropropagaiton in Malawi. It has been established, under our conditions, that using the Standard Chamber with sawdust as the substarate leads to high product of suckers.
This study represents a pioneering evaluation of structured macropropagation systems and substrates aimed at optimizing the growth of Cv. Williams banana suckers. The results have potential application to other banana cultivars, plantain, and ensete. By systematically comparing various macropropagation structures and substrates, our findings reveal significant differences in growth metrics, underscoring the critical role of macropropagation structures in banana sucker production. Notably, the standard chamber and standard chamber with black net consistently outperformed the mulched open bed structure, highlighting the efficacy of macropropagation structures in promoting superior growth outcomes. These results emphasize the importance of macropropagation structures in maximizing propagation efficiency, aligning with previous studies that underscore the role of temperature and humidity in banana development [
9], [
10].
A novel aspect of our study is the investigation into decapitation as a method to enhance sucker proliferation, providing new insights into banana sucker production practices. We observed a significant positive correlation between decapitated suckers and the subsequent production of secondary suckers, indicating the potential of targeted decapitation to increase sucker yields per banana corm. This innovative approach offers practical strategies to enhance productivity and sustainability in commercial banana farming, advancing our understanding beyond traditional propagation methods. The robustness of this finding is supported by a high R-squared value (0.741) and a significant regression coefficient (2.867), affirming decapitation as an effective technique for enhancing sucker proliferation.
Moreover, our study emphasizes the critical role of substrate selection in banana cultivation, with loam soil identified as highly effective for enhancing sucker yield. The robust support for increased plant height and sucker production in loam soil stems from its favorable physical properties, including optimal porosity and excellent water retention capacity. Furthermore, loam soil exhibits a near-neutral pH of 6.7, a balanced nitrogen content of 0.095%, and a notably high phosphorus content of 113.93 mg/kg (
Table S5). These factors collectively optimize nutrient availability, microbial activity, and root development, crucial for promoting vigorous vegetative growth and sustaining plant health. The balanced texture of loam soil, consisting of sand, silt, and clay, facilitates efficient drainage and adequate water retention, thereby preventing waterlogging and supporting robust growth [
11]. This balanced water regime reduces irrigation frequency and minimizes the risk of overwatering, offering practical benefits for sustainable farming practices that enhance productivity while reducing costs [
12]. Moreover, the enhanced root environment provided by loam soil contributes to stronger plants capable of withstanding environmental stresses, thereby improving overall plant resilience and health. These findings underscore the importance of considering substrate physical characteristics alongside nutrient content, providing valuable insights for farmers seeking to optimize yield and economic returns through informed substrate selection [
1].
In terms of macropropagation structures, our results demonstrate significant variations in plant height, root development, and sucker production. The Standard Chamber with sawdust consistently produced the tallest plants and highest sucker yields, underscoring its effectiveness due to optimal moisture retention, aeration, and temperature regulation critical for plant vigor. Conversely, the Open Bed with rice husks yielded the shortest plants, suggesting a need for improvements to support overall plant vigor effectively. These findings underscore the necessity of optimizing both environmental conditions and substrate choice to maximize banana cultivation outcomes, providing clear guidance for farmers aiming to enhance yield and economic returns.
The findings from this study are consistent with and extend upon previous research on the influence of environmental factors on plant growth and macropropagation performance [
1]. The strong positive correlations among key growth parameters such as plant height, number of secondary suckers, and number of leaves observed in our study align with the results of Pinar et al., [
13] , who reported that favorable environmental conditions, particularly optimal temperature and humidity levels, significantly enhance vegetative growth and sucker proliferation in banana plants Tumuhimbise & Talengera,[
14]. Similarly, our observation that higher temperatures positively correlate with vegetative growth metrics echoes the findings of Turner et al.,[
15], Joshi et al.,[
16], and Hatfield et al.,[
17], who found that elevated temperatures within a specific range promote cell expansion and leaf area development in Banana and Maize, respectively.
The dual nature of humidity, with its positive correlation to some metrics and negative impact on plant height, corroborates the nuanced findings of previous studies[
18].For instance, while high humidity can enhance leaf turgor and overall plant biomass, it can also lead to reduced stem elongation and increased susceptibility to fungal diseases [
19,
20]. This dual impact underscores the necessity of balanced humidity management to optimize growth without incurring adverse effects. Furthermore, the PCA biplot in our study, illustrating the compounded effects of temperature and humidity on various performance metrics. This supports the idea of simultaneously optimizing multiple environmental factors to achieve the best outcomes in plant macropropagation and general horticulture practices.
Overall, the alignment of our findings with prior research underscores the importance of integrated environmental management in macropropagation units. The study confirms that both temperature and humidity play crucial roles, and their combined effects need careful balancing to maximize the growth and productivity of macropropagation efforts. These insights not only validate previous studies but also provide a more comprehensive understanding of the environmental dynamics influencing macropropagation, with practical implications for improving agricultural practices and optimizing plant production.
5. Conclusions
In conclusion, the study demonstrates that both the macropropagation structure and substrate significantly influence the growth and number of primary banana suckers. The standard chamber proved to be the most effective structure in promoting sucker production, resulting in the highest number of harvested suckers. The addition of a black net to the standard chamber did not show significant improvements in plant performance. Conversely, the mulched open bed, while encouraging leaf production, led to lower number of banana suckers and reduced plant height. The choice of substrate also played a crucial role in plant growth, with loam soil and sawdust proving beneficial for plant height and sucker production. In contrast, rice husks promoted better root growth but resulted in lower overall sucker production. The combination of the standard chamber with sawdust yielded the best results in terms of plant growth and yield, producing the highest number of harvested suckers. Additionally, the regression analysis revealed a positive correlation between decapitated suckers and secondary sucker production, highlighting the importance of decapitation in enhancing sucker proliferation. These findings provide valuable insights for enhancing banana macropropagation techniques to improve sucker production and overall banana cultivation in similar agricultural settings. Future research should focus on investigating the long-term effects of these treatments and the underlying mechanisms influencing growth patterns.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Substrates; Table S1: Temperature and Humidity of macropropagation structures.
Author Contributions
AS acquired the funding. AS and MN designed experiments, analysed the data, supervised all the experiments, and drafted the manuscript. KP, RMK, EN, LM, EC, NN, MH, YG, JZ, and JC were involved in performing the experiments. All authors have read and agreed to publish the manuscript.
Funding
Please add: The study was supported by the Malawi Government’s Ministry of Agriculture and the European Union under Agreement No. MW/FED/038-578. It was implemented under the Kutukula Ulimi M’malawi (KULIMA) Programme (Promoting Farming in Malawi).
Data Availability Statement
Data supporting the results of this study are available from the corresponding authors (Abel Sefasi) upon request.
Acknowledgments
The authors are grateful for the support received from various offices in the Ministry of Agriculture. These include the Programmes Coordinating Unit (PCU), the Department of Crops Production, and the Department of Agricultural Research Services
Conflicts of Interest
We the authors of this paper hereby declare that there are no competing interests in this publication.
References
- Ntamwira, J., Sivirihauma, C., Ocimati, W., Bumba, M., Vutseme, L., Kamira, M., & Blomme, G. (2017). Macropropagation of banana/plantain using selected local materials: A cost-effective way of mass propagation of planting materials for resource-poor households. European Journal of Horticultural Science, 82(1), 38–53. [CrossRef]
- Changadeya, W., & Kambewa, D. (2012). Farmers’ adoption potential of improved banana production techniques in Malawi. International Journal of Physical and Social Sciences, 2(4), 32–48.
- E. Jooste and M. Theyse, FEASABILITY STUDY Report of the workshop and field visits of the feasibility study on developing virus indexing capacity for banana planting materials in Malawi (STDF/PPG/404).
- S. Subramaniam, R. Xavier, R. Poobathy, and U. Sinniah, “In Vitro Production of Multiple Bud Clumps (Mbcs) from Cavendish Banana Cultivar, Brasilian (AAA),” American-Eurasian Journal of Sustainable Agriculture, vol. 3, no. 2, pp. 300–307, 2008, [Online]. Available: https://www.researchgate.net/publication/228501601.
- S. R. Bhalsing, N. P. Teli, P. K. Pawar, P. V Saindane, M. P. Baviskar, and V. L. Maheshwari, “Tissue Culture Grown Banana : A Cost Effective _ Strategy for Hardening,” no. May, 2017.
- R. Njeri Njau, M. Mwangi, R. Gathu, J. Mbaka and Muasya, “Potential challenges facing macropropagation technique in banana,” in 4th International e-Conference on Agricultural Biosciences 2011, 2011, pp. 32–34. [CrossRef]
- P. Lepoint, F. Iradukunda, and G. Blomme, “Macropropagation of Musa spp. in Burundi: a preliminary study,” CABI, pp. 58–65, 2013. [CrossRef]
- Kasyoka, M. Mwangi, N. Kori, N. Gitonga, and R. Muasya, “Evaluating the macropropagation efficiency of banana varieties preferred by farmers in Eastern and Central Kenya Résumé,” Second RUFORUM Biennial Meeting, Entebbe, Uganda, no. May, pp. 499–503, 2010.
- J. van Wesemael, E. Kissel, D. Eyland, T. Lawson, R. Swennen, and S. Carpentier, “Using growth and transpiration phenotyping under controlled conditions to select water efficient banana genotypes,” Front Plant Sci, vol. 10, no. March, pp. 1–14, 2019. [CrossRef]
- O. R. Salau, M. Momoh, O. A. Olaleye, and R. S. Owoeye, “Effects of Changes in Temperature, Rainfall and Relative Humidity on Banana Production in Ondo State, Nigeria,” World Sci News, vol. 44, no. March 2016, pp. 143–154, 2016, [Online]. Available: www.worldscientificnews.com.
- S. Melero, J. C. R. Porras, J. F. Herencia, and E. Madejon, “Chemical and biochemical properties in a silty loam soil under conventional and organic management,” Soil Tillage Res, vol. 90, no. 1, pp. 162–170, 2006. [CrossRef]
- B. O. Olivares, J. Calero, J. C. Rey, D. Lobo, B. B. Landa, and J. A. Gómez, “Correlation of banana productivity levels and soil morphological properties using regularized optimal scaling regression,” Catena (Amst), vol. 208, p. 105718, 2022. [CrossRef]
- H. Pinar, N. Kılınc, and A. Uzun, “Effect of Different Temperature and Moisture on Development of in Vitro Derived Banana Plantlets,” Current Trends in Natural Sciences, vol. 9, no. 17, pp. 216–221, 2020. [CrossRef]
- R. Tumuhimbise and D. Talengera, “Improved propagation techniques to enhance the productivity of Banana (Musa spp.),” Open Agric, vol. 3, no. 1, pp. 138–145, 2018. [CrossRef]
- D. W. Turner, J. A. Fortescue, and D. S. Thomas, “Environmental physiology of the bananas (Musa spp.),” Brazilian Journal of Plant Physiology, vol. 19, no. 4, pp. 463–484, 2007. [CrossRef]
- R. U. Joshi, A. K. Singh, V. P. Singh, R. Rai, and P. Joshi, “A review on adaptation of banana (Musa spp.) to cold in subtropics,” Plant Breeding, vol. 142, no. 3, pp. 269–283, 2023. [CrossRef]
- J. Hatfield, J. L. Hat, and J. H. Prueger, “Temperature extremes : Effect on plant growth and development Temperature extremes : Effect on plant growth and development,” Weather Clim Extrem, vol. II, no. August, pp. 1–7, 2015. [CrossRef]
- M. Chowdhury et al., “Effects of temperature, relative humidity, and carbon dioxide concentration on growth and glucosinolate content of kale grown in a plant factory,” Foods, vol. 10, no. 7, pp. 2–22, 2021. [CrossRef]
- S. Y. Chia and M. W. Lim, “A critical review on the influence of humidity for plant growth forecasting,” IOP Conf Ser Mater Sci Eng, vol. 1257, no. 1, p. 012001, 2022. [CrossRef]
- F. Romero, S. Cazzato, F. Walder, S. Vogelgsang, S. F. Bender, and M. G. A. van der Heijden, “Humidity and high temperature are important for predicting fungal disease outbreaks worldwide,” New Phytologist, vol. 234, no. 5, pp. 1553–1556, 2022. [CrossRef]
Figure 1.
Macropropagation Structures. (a) Standard Chamber (SC), (b) Standard chamber with a black net (SBN), (c) Mulched open bed (MOB), and (d) Substrate allocation.
Figure 1.
Macropropagation Structures. (a) Standard Chamber (SC), (b) Standard chamber with a black net (SBN), (c) Mulched open bed (MOB), and (d) Substrate allocation.
Figure 2.
Preparation of corm for macropropagation: A: Corm with pseudostem removed; B: Corm after scraping the roots with a sterile panga knife to remove weevils, weevil eggs, and nematodes; C: Corm with peeled leaf sheaths guided by the V-junction of leaves to expose lateral buds; D: Corm with central meristem scarified by making two deep cross-wise incisions to destroy apical dominance; E: Central meristem destroyed by mechanical removal through screwing with sharp knife on the centre of the pseudo stem to remove any apical dominance; F: Corm placed 3cm deep in sawdust in humid chamber.
Figure 2.
Preparation of corm for macropropagation: A: Corm with pseudostem removed; B: Corm after scraping the roots with a sterile panga knife to remove weevils, weevil eggs, and nematodes; C: Corm with peeled leaf sheaths guided by the V-junction of leaves to expose lateral buds; D: Corm with central meristem scarified by making two deep cross-wise incisions to destroy apical dominance; E: Central meristem destroyed by mechanical removal through screwing with sharp knife on the centre of the pseudo stem to remove any apical dominance; F: Corm placed 3cm deep in sawdust in humid chamber.
Figure 3.
Banana development process in sawdust after decapitation of primary suckers; A: Corms develop roots two weeks after placement in sawdust substrate; B: Primary shoots emerge above substrate from two to five weeks; C: Decapitated primary shoots develop multiple secondary shoots; D: Shoot developing after decapitation are harvested and potted for aclimatisation after developing roots; E: Well-acclimatised suckers with three to five leaves after three weeks in pots; F: Well-developed roots of banana sucker after successful acclimatisation.
Figure 3.
Banana development process in sawdust after decapitation of primary suckers; A: Corms develop roots two weeks after placement in sawdust substrate; B: Primary shoots emerge above substrate from two to five weeks; C: Decapitated primary shoots develop multiple secondary shoots; D: Shoot developing after decapitation are harvested and potted for aclimatisation after developing roots; E: Well-acclimatised suckers with three to five leaves after three weeks in pots; F: Well-developed roots of banana sucker after successful acclimatisation.
Figure 4.
Shows Pearson's correlation coefficients for the variables studied. No.RPS (Number of roots for primary suckers), No.LPS (Number of leaves for primary suckers), TS (Total suckers), No.PS (Number of Primary suckers), No.SS (Number of Secondary suckers), RL.PS (Root length for primary suckers), No.DS (Number of decapitated suckers), PH.DS (Plant height for decapitated suckers), PG.DS (Plant girth of decapitated suckers), No.LDS (number of leaves for decapitated suckers).
Figure 4.
Shows Pearson's correlation coefficients for the variables studied. No.RPS (Number of roots for primary suckers), No.LPS (Number of leaves for primary suckers), TS (Total suckers), No.PS (Number of Primary suckers), No.SS (Number of Secondary suckers), RL.PS (Root length for primary suckers), No.DS (Number of decapitated suckers), PH.DS (Plant height for decapitated suckers), PG.DS (Plant girth of decapitated suckers), No.LDS (number of leaves for decapitated suckers).
Figure 5.
A biplot illustrating the distribution of various variables across different types of macro-propagation units.
Figure 5.
A biplot illustrating the distribution of various variables across different types of macro-propagation units.
Table 1.
Average temperature and humidity conditions inside different macro-propagation structures over the study period.
Table 1.
Average temperature and humidity conditions inside different macro-propagation structures over the study period.
| Structure |
Temperature (°C) |
Humidity (%) |
| Standard Chamber |
40.77 |
72.22 |
| Standard Chamber with BlackBlack Net |
36.29 |
59.61 |
| Open Bed |
28.56 |
38.51 |
Table 2.
Substrate water holding capacity at field capacity ( and permanent wilting point ().
Table 2.
Substrate water holding capacity at field capacity ( and permanent wilting point ().
| Substrate |
|
|
| Rice husk |
71.5±3.2 |
58.9±2.7 |
| Saw dust |
109.9±2.1 |
92.1±0.3 |
| Loam soil |
25.6±0.3 |
9.9±0.2 |
Table 3.
Effect of Macropropagation Structures on primary suckers.
Table 3.
Effect of Macropropagation Structures on primary suckers.
| Macropropagation Structure |
No. of leaves |
Plant height (cm) |
No. of roots |
Root Length (cm) |
No. of Harvested suckers |
| Standard Chamber |
4.03±0.14a
|
20.50±0.83b
|
6.78±0.52b
|
9.15±0.85a
|
102.22±14.09b
|
| SBN |
4.48±0.44a
|
20.26±1.02b
|
4.18±0.52a
|
7.78±1.04a
|
59.11±7.32a
|
| Open Bed |
4.76±0.15a
|
16.59±0.79a
|
5.08±0.60b
|
9.25±1.09a
|
38.33±3.78a
|
| Grand Mean |
4.42±0.26 |
19.12±0.92 |
5.35±0.56 |
8.73±1.04 |
66.60±9.84 |
| LSD |
0.762 |
2.708 |
1.631 |
3.041 |
28.85 |
| CV% |
17.6 |
14.5 |
31.2 |
35.6 |
44.3 |
| Fpr. |
0.159 |
0.011 |
0.010 |
0.545 |
<.001 |
Table 4.
Effect of substrate on total banana suckers.
Table 4.
Effect of substrate on total banana suckers.
| Substrate |
No. of leaves |
Plant height (cm) |
No. of roots |
Root Length (cm) |
No. of Harvested suckers |
| Loam Soil |
4.23±0.21a
|
20.44±0.64b
|
5.44±0.54a
|
6.28±0.38a
|
79.00±11.95b
|
| Rice Husks |
4.55±0.24a
|
16.27±0.96a
|
6.04±0.85a
|
11.49±0.69b
|
35.33±3.62a
|
| Saw Dust |
4.49±0.34a
|
420.27±1.04b
|
4.56±0.43a
|
8.40±0.91a
|
85.33±13.81b
|
| Grand Mean |
4.42±0.28 |
19.12±0.932 |
5.35±0.65 |
8.73±0.72 |
66.60±11.22 |
| LSD |
0.815 |
2.732 |
1.895 |
2.099 |
32.92 |
| CV% |
18.8 |
14.6 |
36.3 |
24.6 |
50.6 |
| Fpr. |
0.690 |
0.013 |
0.284 |
<.001 |
0.009 |
Table 5.
Effects of Macropropagation structure and substrate.
Table 5.
Effects of Macropropagation structure and substrate.
| Macropropagation structure |
Substrate |
No. of leaves |
Plant Height (cm) |
No. of roots |
Root length (cm) |
No. of harvested Suckers |
| Standard chamber |
Loam Soil |
3.72a
|
21.03b
|
6.35ab
|
6.84abc
|
124.33d
|
| Rice husks |
4.00a
|
18.13ab
|
8.43b
|
11.83c
|
47ab
|
| Saw Dust |
4.38a
|
22.33b
|
5.56ab
|
8.78abc
|
135.33d
|
| Standard Chamber with Black Net |
Loam Soil |
4.24a
|
20.71b
|
5.08ab
|
6.17ab
|
66.33bc
|
| Rice husks |
5.07a
|
18.36ab
|
3.38a
|
11.49bc
|
32a
|
| Saw Dust |
4.97a
|
21.70b
|
4.07a
|
5.70a
|
79c
|
| Open Bed |
Loam Soil |
4.73a
|
19.58b
|
4.88ab
|
5.85ab
|
46.33ab
|
| Rice husks |
4.59a
|
13.40a
|
6.31ab
|
11.16abc
|
27a
|
| Saw Dust |
4.12a
|
16.78ab
|
4.04a
|
10.73abc
|
41.67a
|
| |
Mean |
4.42±0.48 |
19.12±1.17 |
5.36±0.86 |
8.73±1.12 |
66.6±4.79 |
| |
LSD |
1.429 |
3.512 |
2.583 |
3.367 |
14.25 |
| CV |
18.7 |
10.6 |
27.9 |
22.3 |
12.5 |
| Fpr. |
0.594 |
0.001 |
<.001 |
<.001 |
<.001 |
Table 6.
Regression Analysis of the Relationship between Decapitated Suckers and Secondary Suckers.
Table 6.
Regression Analysis of the Relationship between Decapitated Suckers and Secondary Suckers.
| Parameter |
Estimate |
Std. Error |
t-Value |
P-value |
95% confidence interval |
| Intercept |
-0.844 |
4.221 |
-0.200 |
0.843 |
-9.523,7.836 |
| Decapitated suckers |
2.867 |
0.318 |
8.933 |
<.001 |
2.213, 3.520 |
| R-squared |
0.741 |
|
|
|
|
| Adj. R-squared |
0.732 |
|
|
|
|
| F-statistic |
80.94 |
|
|
<.001 |
|
| Prob(F-Statistic) |
|
|
|
<.001 |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).