Submitted:
01 March 2025
Posted:
03 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
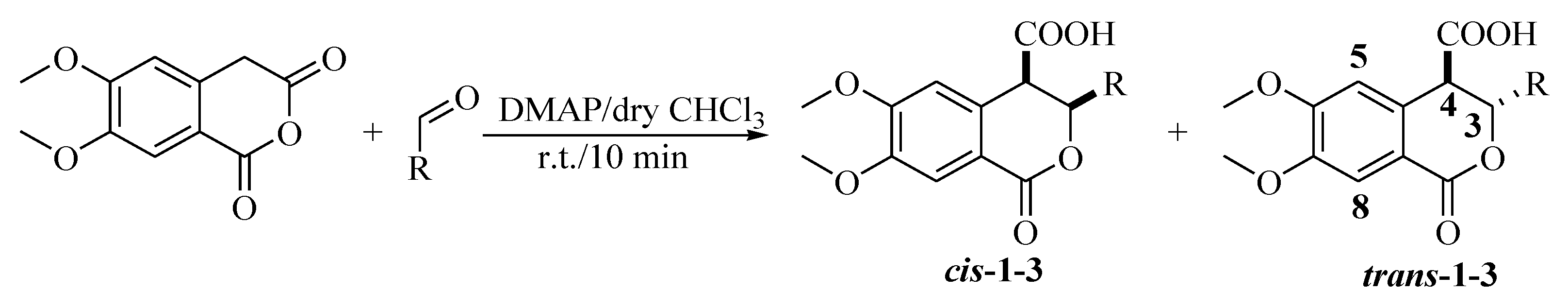
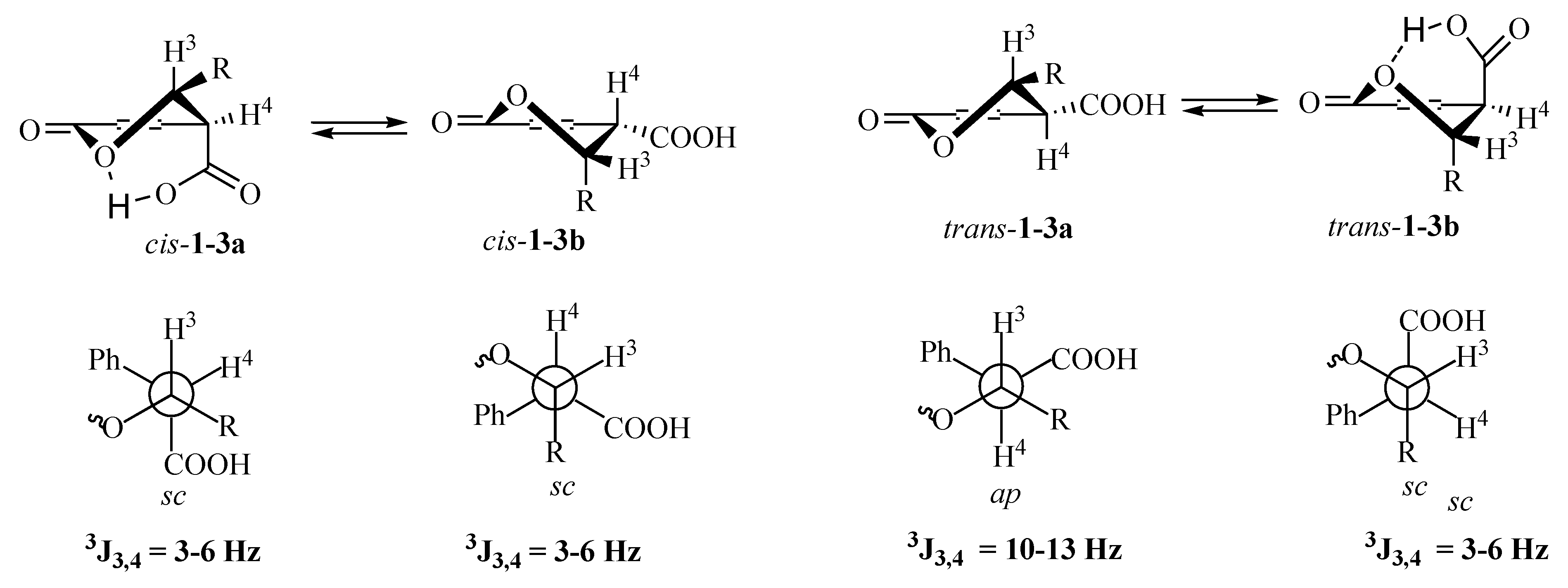
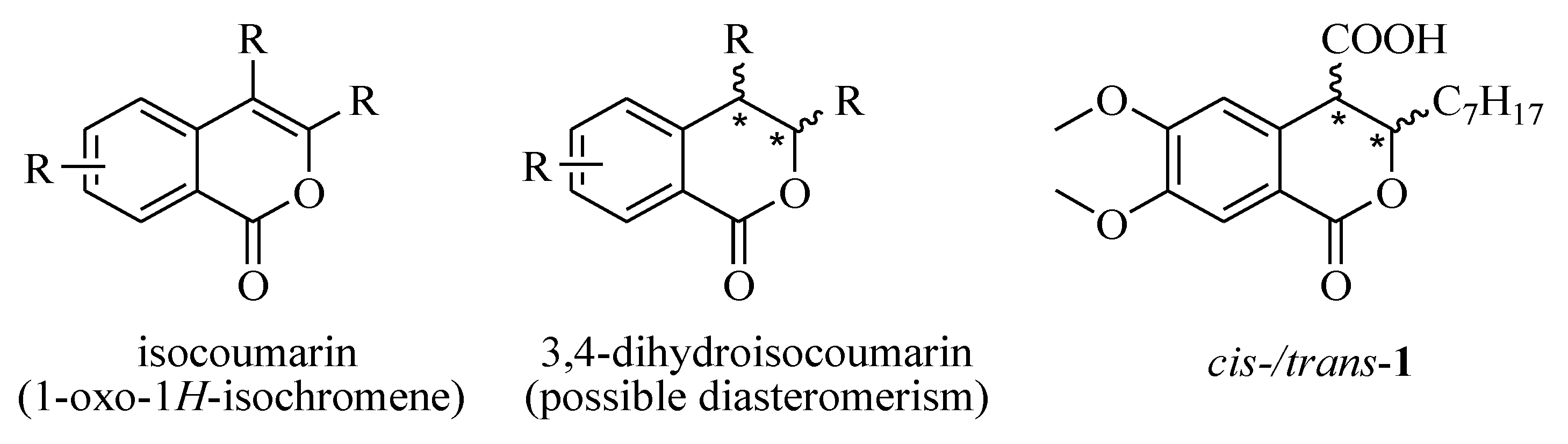
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Cis- and Trans-(±)-3-heptyl-3,4-dihydro-6,7-dimethoxy-1-oxo-1H-isochromene-4-carboxylic acids (1)
3.2.1.1. Cis-1, m.p. 132–134 ˚C (From CH2Cl2: Petroleum Ether)
3.2.1.2. Trans-1, m.p 134–136 ˚C (From CH2Cl2: Petroleum Ether)
3.2.2. Cis- and Trans-(±)-3,4-dihydro-6,7-dimethoxy-3-nonyl-1-oxo-1H-isochromene-4-carboxylic acids (2)
3.2.2.1. Cis-2, m.p. 137–139 ˚C (From CH2Cl2: Petroleum Ether)
3.2.2.2. Trans-2, m.p. 140–143 ˚C (From CH2Cl2: Petroleum Ether)
3.2.3. Cis- and Trans-(±)-3-decyl-3,4-dihydro-6,7-dimethoxy-1-oxo-1H-isochromene-4-carboxylic acids (3).
Cis-3, m.p. 143–145 ˚C (From CH2Cl2: Petroleum Ether)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, R. Naturally Occurring Isocoumarins. Fortschr. Chem. Org. Naturst. 1986, 49, 1 -78.
- Napolitano E. The synthesis of isocoumarins over the last decade. A review. Org. Prep. Proced. Int. 1997, 29, 631-664. [CrossRef]
- Orfali, R.; Perveen, S.; AlAjmI, M.F.; Ghaffar, S.; Rehman, M.T.; AlanzI, A.R.; Gamea, S.B.; Essa Khwayri, M. Antimicrobial Activity of Dihydroisocoumarin Isolated from Wadi Lajab Sediment-Derived Fungus Penicillium chrysogenum: In Vitro and In Silico Study. Molecules 2022, 27, 3630. [CrossRef]
- Hussain, M.; Hussain, M.T.; Rama, N.H.; Hameed, S.; Malik, A.; Khan, K.M. Synthesis and antimicrobial activities of some isocoumarin and dihydroisocoumarin derivatives. Nat. Prod. Res. 2003, 17, 207-214.
- Devienne, K.F.; Raddi, M.S.G.; Coelho, R.G.; Vilegas, W. Structure-antimicrobial activity of some natural isocoumarins and their analogues. Phytomedicine 2005, 12, 378-381. [CrossRef]
- Nozawa, K.; Yamada, M..; Tsuda, Y.; Kawai, K.; Nakajima, S. Antifungal activity of oosponol, oospolactone, phyllodulcin, hydrangenol, and some other related compounds. Chem. Pharm. Bull. 1981, 29, 2689-2691.
- Kovacs, T.; Sonnenbichler, I.; Sonnenbichler J. Total synthesis of the toxin oosponol and of structural analogues and investigation of their antibiotic activities. Justus. Liebigs. Ann. Chem. 1997, 4, 773-777. [CrossRef]
- Matsuda, H.; Shimoda, H.; Yamahara, J.; Yoshikawa, M. Immunomodulatory activity of thunberginol A and related compounds isolated from Hydrangeae Dulcis Folium on splenocyte proliferation activated by mitogens. Biorg. Med. Chem. Lett. 1998, 8, 215-220. [CrossRef]
- Hamada, Y.; Hara, O.; Kawai, A.; Kohno, Y.; Shioiri, T. Efficient total synthesis of AI-77-B, A gastroprotective substance from bacillus pumilus AI-77. Tetrahedron 1991, 47, 8635-8652.
- Matsuda, H.; Shimoda, H.; Yoshikawa, M. Structure-requirements of isocoumarins, phthalides and stilbenes from Hydrangeae Dulcis Folium for inhibitory activity on histamine release from rat peritoneal mast cells. Bioorg. Med. Chem. Lett. 1999, 7, 1445-1450. [CrossRef]
- Shimojima, Y.; Shirai, T.; Baba, T.; Hayashi, H. 1H-2-Benzopyran derivatives, microbial products with pharmacological activity. Conversion into orally active derivatives with antiinflammatory and antiulcer activities. J. Med. Chem. 1985, 28, 3-9.
- Patel, S. K.; Murat, K.; Py, S.; Vallee, Y. Asymmetric total synthesis and stereochemical elucidation of the antitumor agent PM-94128. Org. Lett. 2003, 5, 4081-4084.
- Rauwald, H.W.; Maucher, R.; Dannhardt, G.; Kuchta, K. Dihydroisocoumarins, Naphthalenes, and Further Polyketides from Aloe vera and A. plicatilis: Isolation, Identification and Their 5-LOX/COX-1 Inhibiting Potency. Molecules 2021, 26, 4223. [CrossRef]
- Sukandar, E.R.; Kaennakam, S.; Raab, P.; Nöst, X.; Rassamee, K.; Bauer, R.; Siripong, P.; Ersam, T.; Tip-pyang, S.; Chavasiri, W. Cytotoxic and Anti-Inflammatory Activities of Dihydroisocoumarin and Xanthone Derivatives from Garcinia picrorhiza. Molecules 2021, 26, 6626. [CrossRef]
- Koopklang, K.; Choodej, S.; Hantanong, S.; Intayot, R.; Jungsuttiwong, S.; Insumran, Y.; Ngamrojanavanich, N.; Pudhom, K. Anti-Inflammatory Properties of Oxygenated Isocoumarins and Xanthone from Thai Mangrove-Associated Endophytic Fungus Setosphaeria rostrata. Molecules 2024, 29, 603. [CrossRef]
- Saikia, P.; Gogoi, S. Isocoumarin: General aspects and recent advances in the synthesis. Adv. Synth. Catal. 2018, 360, 2063-2075.
- Bogdanov, M.; Palamareva, M. cis/trans-Isochromanones. DMAP induced cycloaddition of homophthalic anhydride and aldehydes. Tetrahedron, 2004, 60, 2525-2530.
- Bogdanov, M.; Todorov, I.; Manolova, P.; Cheshmedzhieva, D.; Palamareva, M. Configuration and conformational equilibrium of (±)-trans-1-oxo-3-thiophen-2-yl-isochroman-4-carboxylic acid methyl ester. Tetrahedron lett., 2004, 45, 8383-8386. [CrossRef]
- Miliovsky, M.; Svinyarov, I.; Mitrev, Y.; Evstatieva, Y.; Nikolova, D.; Chochkova, M.; Bogdanov, M. A novel one-pot synthesis and preliminary biological activity evaluation of cis-restricted polyhydroxy stilbenes incorporating protocatechuic acid and cinnamic acid fragments. Eur. J. Med. Chem. 2013, 66, 185-192. [CrossRef]
- Miliovsky, M.; Svinyarov, I.; Prokopova, E.; Batovska, D.; Stoyanov, S.; Bogdanov, M. Synthesis and Antioxidant Activity of Polyhydroxylated trans-Restricted 2-Arylcinnamic Acids. Molecules 2015, 20, 2555-2575. [CrossRef]
- Svinyarov, I.; Bogdanov, M. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. Eur. J. Med. Chem. 2014, 78, 198-206. [CrossRef]
- Karplus, M. Contact ElectronSpin Coupling of Nuclear Magnetic Moments. J. Chem. Phys. 1959, 30, 11-15.
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance J. Am. Chem. Soc. 1963, 85, 2870–2871.
| R | Yeald, % | cis, % | trans, % | |
|---|---|---|---|---|
| 1 | – C7H15 | 92 | 40 | 60 |
| 2 | – C9H19 | 85 | 40 | 60 |
| 3 | -– C10H21 | 77 | 41 | 59 |
| Compd. | Chemical shifts (ppm), multiplicity and coupling constants (Hz) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H-8, s | H-5, s | H-4, d | H-3, m* | 3J 3,4 | ||||||
| CDCl3 | DMSО | CDCl3 | DMSО | CDCl3 | DMSО | CDCl3 | DMSО | CDCl3 | DMSО | |
| cis-1 | 7.57 | 7.39 | 6.73 | 7.02 | 3.78 | 3.86 | 4.59 (4.62-4.57) | 4.62 (4.65-4.58) | 3.2 | 3.3 |
| cis-2 | 7.58 | 7.39 | 6.73 | 7.02 | 3.73 | 3.85 | 4.59 (4.61- 4.56) | 4.61 (4.66-4.56) | 3.0 | 3.3 |
| cis-3 | 7.58 | 7.38 | 6.73 | 7.02 | 3.73 | 3.86 | 4.58 (4.60-4.55) | 4.61 (4.66-4.55) | 3.2 | 3.3 |
| Average (cis) | 7.58 | 7.39 | 6.73 | 7.02 | 3.75 | 3.86 | 4.59 | 4.61 | 3.1 | 3.3 |
| trans-1 | 7.57 | 7.37 | 6.73 | 6.99 | 3.74 | 3.93 | 4.91 (4.95-4.87) | 4.85 (4.88-4.81) | 4.4 | 3.3 |
| trans-2 | 7.57 | 7.38 | 6.73 | 6.99 | 3.78 | 3.92 | 4.91 (4.94-4.88) | 4.85 (4.89-4.80) | 4.5 | 3.3 |
| trans-3 | 7.58 | 7.37 | 6.73 | 6.99 | 3.78 | 3.92 | 4.92 (4.95-4.88) | 4.84 (4.89-4.79) | 4.5 | 3.3 |
| Average (trans) | 7.57 | 7.37 | 6.73 | 6.99 | 3.77 | 3.92 | 4.91 | 4.85 | 4.5 | 3.3 |
| Δδ | 0.01 | 0.02 | na | 0.03 | 0.02 | 0.06 | 0.32 | 0.24 | 1.4 | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




