1. Introduction
Several studies have reported an association between insufficient iodine intake and abnormal serum lipid profile. Pregnancy predisposes to insufficient iodine nutrition status through increased physiological demand and increased renal filtration [
1,
2]. Iodine deficiency is also thought to predispose to dyslipidaemia with the proposed mechanism being the elevation of serum TSH as it occurs in overt and subclinical hypothyroidism [
3]. Pregnancy simulates a metabolic syndrome-like states and is associated with increased levels of serum lipids especially in the third trimester and among the obese women [
4]. Hence pregnant women with obesity and iodine deficiency are likely to have abnormal lipid profiles. Obesity, dyslipidaemia and hypothyroidism are known risk factors of preeclampsia [
5,
6]. It is not quite certain whether iodine deficiency is an independent predictor of dyslipidaemia. The pathological mechanisms through which iodine deficiency may independently predispose to dyslipidaemia has not yet been described. This study set out to establish whether insufficient iodine intake is an independent risk factor for dyslipidaemia among preeclamptic and normotensive pregnant women with or without iodine deficiency and/or subclinical hypothyroidism, and the likely pathophysiological mechanisms involved.
2. Materials and Methods
2.1. Study Setting
Lomo Medical Centre (LMC) is a tertiary hospital located in Kinshasa province of the Democratic Republic of Congo. The Democratic Republic of Congo has the third highest age-standardized rate of iodine deficiency in the world estimated at 16,385/ 100,000 people [
7].
2.2. Study Design
This analytical cross-sectional study was carried out as a secondary analysis of archived data from participants enrolled in the Communicable Disease, Nutritional, Environmental Epidemiological Risk study carried out in Kinshasa Province, Democratic Republic of Congo, conducted at Lomo Medical Centre between 2007 and 2008. The study was approved by the Lomo Medical Centre Institutional Review Board (Reference no. LMDE031LMB02). The data of 240 preeclamptic women and 120 randomly selected women who remained normotensive till delivery and had complete results of thyroid function status, lipid profile, and demographic characteristics was used for the current study.
Preeclampsia was defined according to the International Society for the Study of Hypertension in Pregnancy [
8] as new onset of hypertension (SBP>140 mmHg systolic or DBP >90 mmHg diastolic) after 20 weeks gestation, measured at least 4 hours apart, with proteinuria and or end-organ dysfunction Severe preeclampsia is gestational hypertension (SBP>160 mmHg or DBP>110 mmHg with or without systemic organ involvement. Eclampsia is hypertension (SBP>140 mmHg systolic or DBP >90 mmHg) after 20 weeks gestation with convulsions.
The height, weight, systolic (SBP) and diastolic (DBP) blood pressure, of the participants were measured according to standardized procedures. Overnight fasting venous blood was drawn from the cubital fossa between 7:00 and 9:00 a.m. The blood samples were assayed immediately to measure the concentrations of high-density cholesterol (HDL), total cholesterol, triglycerides, low density lipoprotein (LDL), oxidized low density lipoprotein (oxLDL). Laboratory data were obtained using calibrated and standard routine procedures and specific protocols of manufacturers’ such as kits of Biomérieux (Marcy l’Etoile, France) and Mercodia AB (Silveniusgatan 8 A, SE754, Uppsala, Sweden, and a caloric Sensor Hach DR/2010 spectrophotomer (HACH, USA). T3, T4 and TSH were measured by enzyme linked immunosorbent assay method purchased from DIALAB GmbH IZ-NOE Sued Company, Hondastrasse, Objekt M55, A- 2351 wr, Neudorf, Austria. Urinary iodine concentration was measured using the Sandell-Kolthof method.
2.3. Statistical Analysis
Data analysis was performed using the IBM SPSS STATISTICS software package version 29 for windows (IBM Inc., Chicago IL, USA). The data was summarized into proportions (%) for categorical variables, means ± standard deviation (SD) for normally distributed, and as median (25th-75th percentiles) for non-normally distributed continuous variables, respectively. The Chi-square test was used for the comparison of categorical variables between groups. The student’s t-test was used to compare means between two groups, and one-way analysis of variance (ANOVA), to compare means involving more than two groups. Univariate Odds ratios (OR) were computed for the association between potential contributing factors and preeclampsia. A p <0.05 was considered significant. Exploratory component analysis was conducted to delineate the patterns of interaction between UIC, TSH, HDL, LDL, oxidized LDL and nitric oxide in participants with preeclampsia. Eigenvalues ≥1 were used to identify the main latent interactions of these variables among participants with preeclampsia in the study population.
3. Results
3.1. General Characteristics of the Participants
The mean chronological ages were 32.4 ± 6.0 and 34.0 ± 4.5 years and the mean gestational ages were 30.8 ± 7.9 and 38.6 ± 2.3 weeks’ gestation respectively for preeclamptic and normotensive women. Eighty-seven percent (209/240) of the preeclamptic and 27% (32/120) of the normotensive pregnant women had insufficient iodine nutrition (UIC <150µg/l) (p <0.0001). Seventy-five percent (180/240) of the preeclamptic women and 54.2 % (65/120) of the normotensive women had TSH above the upper range of the normal third trimester pregnancy levels of 3.0 UI/L (p <0.0001).
3.2. Serum Lipid and Nitric Oxide Levels of Normotensive and Preeclamptic Participants
Normotensive pregnant women had higher HDL, LDL, and nitric oxide while preeclamptic participants had higher BMI, oxidized LDL, and triglycerides (
Table 1).
3.3. The Relationship Between Iodine Nutrition Status, TSH, Nitric Oxide, and Serum Lipids
Both preeclamptic and normotensive pregnant women with elevated TSH had higher oxidized LDL and lower nitric oxide levels than respondents with TSH levels within normal pregnancy levels. HDL was significantly lower while LDL and triglycerides were significantly higher among preeclamptic women with insufficient iodine nutrition status (UIC<150 µg/L) than those with sufficient iodine intake (
Table 2 and
Table 3).
UIC positively and strongly correlated HDL and also showed a moderate positive correlation with LDL, Triglycerides, and NO; and a mild to moderate negative correlation with TSH, and oxidised LDL. TSH was positively correlated with oxidized LDL but negatively correlated with NO, HDL, LDL, and UIC. There was a strong and negative correlation between NO (an antioxidant biomarker) with TSH and oxidized LDL (
Table 4).
Univariable and multivariable logistic regression were carried out to establish the independent predictors of low HDL (below the lower range of normal of 40.3 mg/dL). Preeclampsia status and TSH > 3 IU/L were independent predictors of low serum HDL. UIC >150 µg/l. was protective against low HDL (
Table 5).
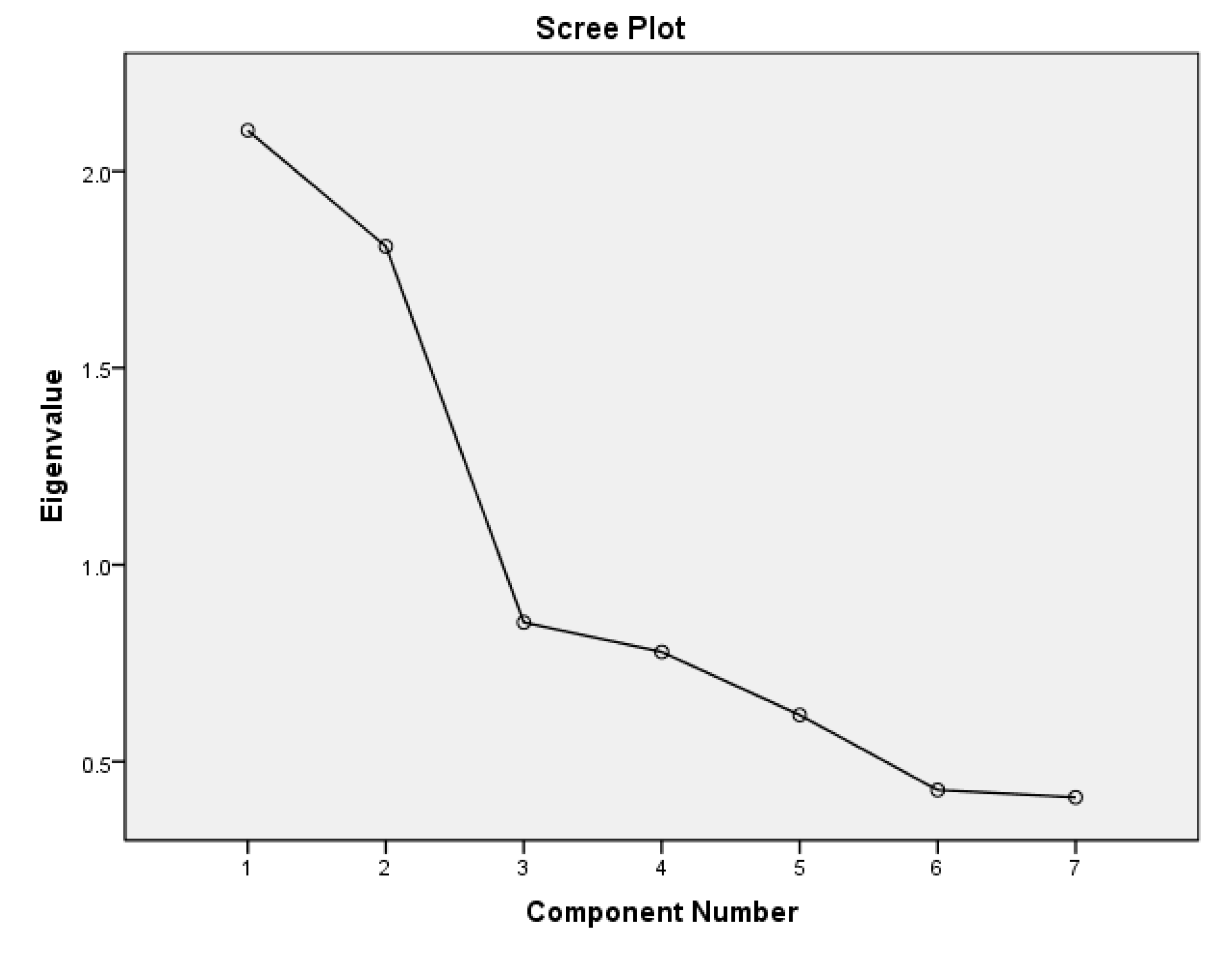
Exploratory factor analysis revealed two major patterns through which UIC, thyroid function parameters, and lipid profile variables interacted in participants with preeclampsia. The first includes the urine iodine concentration that correlates positively with HDL cholesterol but negatively with triglycerides and LDL, and the second involves a positive correlation between serum TSH and oxidized LDL but low serum NO (components 1 and 2 in the scree plot-
Figure 1 as well as
Table 6 below).
4. Discussion
This study finds that preeclampsia was associated with low UIC, elevated TSH, and higher but normal levels of T3 and T4 which are features consistent with chronic iodine deficiency, thyroid hyperstimulation, and subclinical hypothyroidism. The serum levels of HDL and the UIC of preeclamptic women were much lower than those of normotensive women. Furthermore, insufficient iodine intake in pregnancy was associated with low HDL and elevated triglycerides only among preeclamptic women. Exploratory factor analysis for patterns of association revealed sufficient iodine intake is associated with higher serum levels of HDL and lower levels of LDL and triglycerides (latent factor 1 in
Figure 1). Therefore, we hypothesize that iodine may be a necessary factor in the synthesis and/or metabolism of HDL which is associated with a less atherogenic lipid profile.
The elevated serum LDL and triglycerides may result from low serum HDL which in the current study seems to be secondary to a low iodine nutrition status. HDL cholesterol is known for transporting LDL and VLDL cholesterol from the circulation towards the liver. High serum concentrations of LDL and VLDL cholesterol result in lipid peri-oxidation and endothelial dysfunction [
9], increasing the risk of preeclampsia. According to the World Health Organization criteria, the median UIC of 98 µg/L of the preeclamptic participants in the current study reveals an insufficient iodine nutrition status [
10]. Furthermore, Iodine is one of the exogenous antioxidants hence the level of iodine deficiency among preeclamptic participants in the current study may partially explain the concurrent elevation in serum oxidised LDL observed in the current study [
11].
The findings of the current study are consistent with previous studies. In two clinical trials and two observation studies [
12,
13,
14,
15]. Iodine supplementation was only able to reduce the level of LDL without significant impact on HDL or triglycerides [
12,
13]. However, in both clinical trials all the participants’ UIC remained well below 100 µg/L, the cut-off value for adequate iodine nutrition [
16] after the iodine supplementation period. The findings in the current study may imply that only individuals with sufficient iodine intake in pregnancy will have HDL within the normal range.
Previously it has been suggested that the mechanism by which iodine deficiency predisposes to low HDL is through elevated TSH [
17,
18]. However, the current study seems to suggest that iodine deficiency may increase the risk of low serum HDL independent of TSH but this seems true only among preeclamptic women.
During normal pregnancy, there is a progressive increase of serum levels of VLDL, LDL, and triglycerides from the first to the third trimester while HDL cholesterol increases from the first to the second but tends to slowly decline in the third trimester but not reach the pre-pregnancy levels [
19,
20]. In addition, there is a progressive rise in oxidised lipids but this is variably matched with antioxidant enzymatic activity [
19,
20].
Both normotensive and preeclamptic women had median TSH above the upper range expected in the third trimester despite T3 and T4 in the normal range which is consistent with subclinical hypothyroidism [
21]. Although only the preeclamptic women had median UIC consistent with insufficient iodine nutrition status, the median UIC of the normotensive pregnant women was near the lower limit of the normal range. This implies a high prevalence of subclinical hypothyroidism in the study population secondary to insufficient iodine nutrition in pregnancy especially among the preeclamptic women.
In the current study, high levels of serum TSH were associated with high serum oxidised LDL cholesterol and low Nitric oxide among both preeclamptic and normotensive women. Chronic iodine deficiency seems to have led to persistent TSH stimulation of the thyroid gland which predisposes to increased production of hydrogen peroxide and superoxide radicals. Hydrogen peroxide and superoxide radicals react with and rapidly diminish the levels of Nitric Oxide (NO) forming peroxynitrite (ONOO-) a more potent oxidant that can cause lipid oxidation as well as accentuate endothelial dysfunction [
22]. Furthermore, elevated serum TSH is known to stimulate extra-thyroidal endothelial-TSH receptors leading to increased tumour necrotic factor alpha (TNF-α) and endothelin production, reduced prostacyclin and nitric oxide production. Low serum NO predisposes to endothelial dysfunction, reduction in flow-mediated dilatation, endothelial activation and increased carotid media-intima thickness (cIMT) which are pathological features of preeclampsia [
23,
24,
25,
26,
27,
28,
29] and atherosclerosis which is a herald of cardiovascular disease [
30,
31]. These mechanisms may account for the high levels of oxidised LDL and low levels of Nitric Oxide observed among participants with preeclampsia in the current study.
5. Conclusions
Insufficient iodine nutrition in pregnancy is associated with subclinical hypothyroidism and preeclampsia. Among both normotensive and preeclamptic women subclinical hypothyroidism predisposes to elevated serum oxidised LDL and decreased NO while insufficient iodine nutrition among preeclamptic women predisposes to reduced HDL and increased serum Triglycerides which are risk factors of atherosclerosis and cardiovascular disease.
Author Contributions
Conceptualization, BCB and BLM.; methodology, BCB and BLM.; software, BCB.; validation, BLM.; formal analysis, BCB.; investigation, BLM resources, BLM.; data curation, BLM.; writing—original draft preparation, BCB.; writing—review and editing, BLM.; supervision, and project administration, BLM. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Helsinki declaration and was approved by the Lomo Medical Centre Institutional Review Board (Reference no. LMDE031LMB02).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article is available on the website of the journal
Conflicts of Interest
The authors declare no conflicts of interest
Abbreviations
The following abbreviations are used in this manuscript:
| BMI |
Body Mass Index |
| HDL |
High density lipoprotein |
| LDL |
Low density Lipoprotein |
| OxLDL |
Oxidized low density lipoprotein |
| T3 |
Triiodothyronine |
| T4 |
Thyroxine |
| Trigli |
Triglycerides |
| TSH |
Thyroid stimulating Hormone |
References
- Leung, A.M. ,E.N. Pearce, and L.E. Braverman, Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am, 2011. 40(4): p. 765-77.
- Delshad, H. , Iodine nutrition in pregnancy. Annals of Thyroid, 2018. 3.
- Pearce, E.N. , M. Andersson, and M.B. Zimmermann, Global iodine nutrition: Where do we stand in 2013? Thyroid, 2013. 23(5): p. 523-8.
- Formisano, E. , et al., Characteristics, Physiopathology and Management of Dyslipidemias in Pregnancy: A Narrative Review. Nutrients, 2024. 16(17).
- Sharami, S.H. , et al., Role of dyslipidemia in preeclamptic overweight pregnant women. Iran J Reprod Med, 2012. 10(2): p. 105-12.
- Hosier, H. , et al., Dyslipidemia and Risk of Preeclampsia: A Multiancestry Mendelian Randomization Study. Hypertension, 2023. 80(5): p. 1067-1076.
- Han, X. , et al., Global, regional, and national burdens of common micronutrient deficiencies from 1990 to 2019: A secondary trend analysis based on the Global Burden of Disease 2019 study. EClinicalMedicine, 2022. 44: p. 101299.
- Magee, L.A. , et al., The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens, 2022. 27: p. 148-169.
- Higashi, Y. , Endothelial Function in Dyslipidemia: Roles of LDL-Cholesterol, HDL-Cholesterol and Triglycerides. Cells, 2023. 12(9): p. 1293.
- Andersson, M. , et al., Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr, 2007. 10(12a): p. 1606-11.
- Aceves, C. , et al., Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int J Mol Sci, 2021. 22(3).
- Zimmermann, M.B. , et al., Iodine treatment in children with subclinical hypothyroidism due to chronic iodine deficiency decreases thyrotropin and C-peptide concentrations and improves the lipid profile. Thyroid, 2009. 19(10): p. 1099-104.
- Herter-Aeberli, I. , et al., Iodine Supplementation Decreases Hypercholesterolemia in Iodine-Deficient, Overweight Women: A Randomized Controlled Trial. J Nutr, 2015. 145(9): p. 2067-75.
- Asvold, B.O., T. Bjøro, and L.J. Vatten, Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11-year follow-up of the HUNT study. Eur J Endocrinol, 2013. 169(1): p. 73-82.
- Al-Odat, I., S. Al-Fawaeir, and M.H. Al-Mahmoud, Study of the association between thyroid dysfunction and serum lipid abnormalities. Biomed Rep, 2024. 21(4): p. 138.
- WHO, Assessment of iodine deficiency disorders and monitoring their elimination : a guide for programme managers. 2007, World Health Organization: Geneva.
- Pearce, E.N. , Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab, 2012. 97(2): p. 326-33.
- Liu, J. , et al., Alteration of Lipid Profile Between Subclinical Hypothyroidism and Well-Matched Controls: A Meta-Analysis. Horm Metab Res, 2023. 55(7): p. 479-486.
- Wankasi, M.M. , et al., Lipid Profile and Some Parameters of Lipid Peroxidation in Pregnancy Trimesters. Asian Pacific Journal of Health Sciences, 2024. 11(1): p. 12-16.
- Bassi, R., M. Kaur, and S. Sharma. Study of Changes in Lipid Profile, Lipid Peroxidation and Superoxide Dismutase during Normal Pregnancy. 2011.
- Alexander, E.K. , et al., 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid®, 2017. 27(3): p. 315-389.
- Johansen, J.S. , et al., Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol, 2005. 4: p. 5.
- Dardano, A. , et al., Recombinant human thyrotropin reduces endothelium-dependent vasodilation in patients monitored for differentiated thyroid carcinoma. J Clin Endocrinol Metab, 2006. 91(10): p. 4175-8.
- Jiang, F. , et al., Thyrotropin Regulates eNOS Expression in the Endothelium by PGRN Through Akt Pathway. Frontiers in Endocrinology, 2018. 9.
- Lu, M. , et al., Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review). Exp Ther Med, 2015. 9(1): p. 3-10.
- Taddei, S. , et al., Impaired endothelium-dependent vasodilatation in subclinical hypothyroidism: beneficial effect of levothyroxine therapy. J Clin Endocrinol Metab, 2003. 88(8): p. 3731-7.
- Cabral, M.D. , et al., Effects of thyroxine replacement on endothelial function and carotid artery intima-media thickness in female patients with mild subclinical hypothyroidism. Clinics, 2011. 66(8): p. 1321-1327.
- Razvi, S. , et al., Thyroid Hormones and Cardiovascular Function and Diseases. Journal of the American College of Cardiology, 2018. 71(16): p. 1781-1796.
- Tian, L. , et al., Effects of TSH on the function of human umbilical vein endothelial cells. J Mol Endocrinol, 2014. 52(2): p. 215-22.
- Fernández-Alvarez, V. , et al., Evaluation of Intima-Media Thickness and Arterial Stiffness as Early Ultrasound Biomarkers of Carotid Artery Atherosclerosis. Cardiology and Therapy, 2022. 11(2): p. 231-247.
- Darabian, S. , et al., The role of carotid intimal thickness testing and risk prediction in the development of coronary atherosclerosis. Curr Atheroscler Rep, 2013. 15(3): p. 306.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).