Submitted:
25 February 2025
Posted:
26 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Application of the Spherical Agglomeration Technique (SAT)
2.3. Experimental Design for Fluoride Removal in Aqueous Models and Groundwater
2.4. Fluoride Removal from Well Water by SAT
2.5. Statistical Analysis
3. Results and discussion
3.1. Determination of Fluoride Concentration Used in Aqueous Models
3.2. Fluoride Removal in Aqueous Models by SAT Application
3.2.1. Comparison between n-Heptane and Sunflower Oil as Humectant Agent
3.2.2. SAT Optimization for Fluoride Removal in Aqueous Models, Using Different Surfactant and Humectant Doses
3.3. Removal of Fluoride from Well Water Using the Spherical Agglomeration Technique
3.3.1. Groundwater Sampling and Analysis in the City of Durango, Mexico
3.3.2. Fluoride Removal from Well Water by SAT
3.4. Statistical Analysis
3.4.1. Statistical Analysis for Sunflower Oil and n-Heptane Treatment in Aqueous Models
3.4.2. Statistical Analysis for Fluoride Removal in Aqueous Models
3.4.3. Response Surface Graph for Fluoride Removal in Aqueous Models
3.4.4. Statistical Analysis for Fluoride Removal in Well Water
3.4.5. Response Surface Graph for Fluoride Removal in Well Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analyzes of variance |
| ED | Extract dosage (mL Ext/g TMC) |
| F- | Fluoride ion |
| HD | Humectant dosage (mL Hum/g TMC) |
| TMC | Total Mixture Component (formed by the reaction between AlCl3, Ca(OH)2) |
| R2 | Pearson determination coefficient |
| SAT | Spherical agglomeration technique |
References
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride contamination, consequences and removal techniques in water: a review.Environ. Sci. Adv. 2022, 1(5), 620–661. [CrossRef]
- Shaji, E.; Sarath, K.V.; Santosh, M.; Krishnaprasad, P.K.; Arya, B.K.; Babu, M.S. Fluoride contamination in groundwater: A global review of the status, processes, challenges, and remedial measures. Geosci. Front. 2024, 15(2), 101734. [CrossRef]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022.
- Gutiérrez, M.; -Herrera, M.T. Fluoride in groundwater in the north-central region of Mexico and its possible origin. Rev. Int. Contam. Ambient. 2022, 38. https://doi.org/10.20937/rica.54307.
- Kumar, S.; Singh, R.; Venkatesh, A.S.; Udayabhanu, G.; Sahoo, P.R. Medical Geological assessment of fluoride contaminated groundwater in parts of Indo-Gangetic Alluvial plains. Sci. Rep. 2019, 9(1), 16243. [CrossRef]
- Ashong, G.W.; Ababio, B.A.; Kwaansa-Ansah, E.E.; Koranteng, S.K.; Muktar, G.D.H. Investigation of fluoride concentrations, water quality, and non-carcinogenic health risks of borehole water in bongo district, northern Ghana. Heliyon 2024, 10(6). [CrossRef]
- Podgorski, J.; Berg, M. Global analysis and prediction of fluoride in groundwater. Nat. Commun. 2022, 13(1), 4232. [CrossRef]
- Ali, S.; Thakur, S.K.; Sarkar, A.; Shekhar, S. Worldwide contamination of water by fluoride. Environ. Chem. Lett. 2016, 14, 291–315. [CrossRef]
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Hengstler, J.G. Toxicity of fluoride: critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [CrossRef]
- Rasool, A.; Farooqi, A.; Xiao, T.; Ali, W.; Noor, S.; Abiola, O.; Nasim, W. A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ. Geochem. Health, 2018, 40, 1265–1281 . [CrossRef]
- Roy Chowdhury, N.; Majumder, S.; Ghosh, S.; Satheesh Babu, S.; Vidyadharan, V.; Samanta, J.; Roychowdhury, T. A Vivid Picture of the Distribution, Impact, and Consequences of Fluoride in Indian Perspective. In Ground Water Contamination in India: Adverse Effects on Habitats. Springer Nature: Cham, Switzerland, 2024; pp. 83–103. [CrossRef]
- El Messaoudi, N.; Franco, D.S.P.; Gubernat, S.; Georgin, J.; Şenol, Z.M.; Ciğeroğlu, Z.; El Hajam, M. Advances and future perspectives of water defluoridation by adsorption technology: A review. Environ. Res. 2024, 118857. [CrossRef]
- Soni, J.; Bansal, N.; Gupta, M. Impact and removal techniques of fluoride from the drinking water. Int. J. Pharm. Chem. Biol. Sci. 2020, 10(2).
- Yadav, A.K.; Shirin, S.; Singh, V.P. (Eds.) Advanced Treatment Technologies for Flouride Removal in Water; Springer: Singapore, 2023. [CrossRef]
- Bailón-Salas, A.M.; Ordaz-Díaz, L.A.; Cháirez-Hernández, I.; Alvarado-de la Peña, A.; Proal-Nájera, J.B. Lead and copper removal from groundwater by spherical agglomeration using a biosurfactant extracted from Yucca decipiens Trel. Chemosphere 2018, 207, 278–284. [CrossRef]
- Chauhan, V.; Dalvadi, H. A Systematic Review of Spherical Agglomeration by Particle Design of Drug Formulation. Pharmacophore 2022, 13(1–2022), 83–90. [CrossRef]
- Proal-Nájera, J.B.; Tabche, L.M.; Mueller, M. Estudio sobre el tratamiento de aguas residuales industriales altamente concentradas en metales pesados bajo aglomeración esférica. J. Mex. Chem. Soc. 1997, 41(2), 51–56.
- Gonzalez-Valdez, L.S.; Almaraz-Abarca, N.; Proal-Nájera, J.B.; Robles-Martinez, F.; Valencia-Del-Toro, G.; Quintos-Escalante, M. Surfactant properties of the saponins of Agave durangensis, application on arsenic removal. Int. J. Eng. 2013, 4(2), 8269.
- Alcázar-Medina, F.A.; Valle-Cervantes, S.; Alcazar-Medina, T.L.; Rodríguez-Rosales, M.D.J. Optimization of copper removal through spherical agglomeration: Effect of pH-precipitation and the use of Agave spp. leaf extracts as biosurfactants. Rev. Mex. Ing. Quim. 2024, 23(1). [CrossRef]
- Pitt, K.; Peña, R.; Tew, J.D.; Pal, K.; Smith, R.; Nagy, Z.K.; Litster, J.D. Particle design via spherical agglomeration: A critical review of controlling parameters, rate processes and modelling. Powder Technol. 2018, 326, 327–343. [CrossRef]
- Gryglewicz, S.; Grabas, K.; Gryglewicz, G. Use of vegetable oils and fatty acid methyl esters in the production of spherical activated carbons. Bioresour. Technol. 2000, 75(3), 213–218. [CrossRef]
- Ahmed, B.; Arjmandi-Tash, O.; Litster, J.D.; Smith, R.M. Mechanistic modelling of spherical agglomeration processes. Powder Technol. 2023, 417, 118254. [CrossRef]
- Torres-Corral, O.A.; Rojas-Montes, J.C.; Valle-Cervantes, S.; Alcazar-Medina, F.A. Arsenic removal by Spherical Agglomeration Technique in groundwater using vegetable oil instead of n-heptane. Environ. Technol. Innov. 2025, 37, 103955. [CrossRef]
- Secretaría de Economía. NMX-AA-051-SCFI-2001. Water Analysis—Determination of Fluorides (Ion-Selective Electrode Method); Secretaría de Economía: Mexico City, Mexico, 2001.
- Velazquez-Jimenez, L.H.; Vences-Alvarez, E.; Flores-Arciniega, J.L.; Flores-Zuniga, H.; Rangel-Mendez, J.R. Water defluoridation with special emphasis on adsorbents-containing metal oxides and/or hydroxides: a review. Sep. Purif. Technol. 2015, 150, 292–307. [CrossRef]
- Li, Y.; Zhang, P.; Du, Q.; Peng, X.; Liu, T.; Wang, Z.; Wu, D. Adsorption of fluoride from aqueous solution by graphene. J. Colloid Interface Sci. 2011, 363, 348–354. [CrossRef]
- Wu, X.; Zhang, Y.; Dou, X.; Zhao, B.; Yang, M. Fluoride adsorption on an Fe–Al–Ce trimetal hydrous oxide: characterization of adsorption sites and adsorbed fluorine complex species. Chem. Eng. J. 2013, 223, 364–370.. [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 7; StatSoft: Tulsa, OK, USA, 2004.
- Irigoyen-Campuzano, J.R.; Barraza-Barraza, D.; Gutiérrez, M.; Torres-Castañón, L.A.; Reynoso-Cuevas, L.; Alarcón-Herrera, M.T. Hydrogeochemical Characterization of an Intermontane Aquifer Contaminated with Arsenic and Fluoride via Clustering Analysis. Hydrology 2024, 11(6), 76. [CrossRef]
- Martínez-Cruz, D.A.; Alarcón-Herrera, M.T.; Reynoso-Cuevas, L.; Torres-Castañón, L.A. Space-time variation of arsenic and fluoride in groundwater in the city of Durango, Mexico. Tecnol. Cienc. Agua 2020, 11, 309–340. [CrossRef]
- Chary, G.H.V.C.; Dastidar, M.G. Comprehensive study of process parameters affecting oil agglomeration using vegetable oils. Fuel 2013, 106, 285–292. [CrossRef]
- Aktaş, Z. Some Factors Affecting Spherical Oil Agglomeration Performance of Coal Fines. Int. J. Miner. Process. 2002, 65, 177–190. [CrossRef]
- Akafu, T.; Chimdi, A.; Gomoro, K. Removal of fluoride from drinking water by sorption using diatomite modified with aluminum hydroxide. J. Anal. Methods Chem. 2019, 2019, 4831926. [CrossRef]
- Abbasi, A.; Memon, S.A.; Qureshi, R.F.; Mehdi, M.; Khatri, M.; Ahmed, F.; Kim, I.S. Adsorptive defluoridation from aqueous solution using a novel blend of eggshell powder and chitosan nanofibers. Mater. Res. Express 2020. 7(12), 125005. [CrossRef]
- Hashemkhani, M.; Rezvani Ghalhari, M.; Bashardoust, P.; Hosseini, S.S.; Mesdaghinia, A.; Mahvi, A.H. Fluoride removal from aqueous solution via environmentally friendly adsorbent derived from seashell. Sci. Rep. 2022. 12(1), 9655. [CrossRef]
- Alhassan, S.I.; Huang, L.; He, Y.; Yan, L.; Wu, B.; Wang, H. Fluoride removal from water using alumina and aluminum-based composites: A comprehensive review of progress. Crit. Rev. Environ. Sci. Technol. 2021. 51(18), 2051–2085. [CrossRef]
- Eow, J.S.; Ghadiri, M. The behaviour of a liquid–liquid interface and drop-interface coalescence under the influence of an electric field. Colloids Surf. A Physicochem. Eng. Asp. 2003, 215(1–3), 101–123. [CrossRef]
- Ünal, İ.; Aktaş, Z. Effect of various bridging liquids on coal fines agglomeration performance. Fuel Process. Technol. 2001, 69(2), 141–155. [CrossRef]
- Garcia, A.B.; Martinez-Tarazona, M.R.; Vega, J.G. Cleaning of Spanish high-rank coals by agglomeration with vegetable oils. Fuel 1996, 75(7), 885–890. [CrossRef]
- Gebre, S.H. Synthesis and Potential Applications of Trimetallic Nanostructures. New J. Chem. 2022, 46(12), 5438–5459. [CrossRef]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Physical and chemical characterisation of conventional and nano/emulsions: Influence of vegetable oils from different origins. Foods 2022, 11(5), 681. [CrossRef]
- Tolkou, A.K.; Zouboulis, A.I. Fluoride removal from water sources by adsorption on MOFs. Separations 2023, 10(9), 467. [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Rangasamy, G.; Badawi, M.; Aminabhavi, T.M. Membrane-based removal of fluoride from groundwater. Chem. Eng. J. 2024, 150880. [CrossRef]
- Huang, H.; Zhang, H.; Xiao, F.; Liang, J.; Wu, Y. Efficient removal of fluoride ion by the composite forward osmosis membrane with modified cellulose nanocrystal interlayer. Results Eng. 2023, 20, 101449. [CrossRef]
- Zhao, M.; Wang, Q.; Krua, L.S.N.; Yi, R.; Zou, R.; Li, X.; Huang, P. Application Progress of New Adsorption Materials for Removing Fluorine from Water. Water 2023, 15, 646. [CrossRef]
- Tolkou, A.K.; Trikkaliotis, D.G.; Kyzas, G.Z.; Katsoyiannis, I.A.; Deliyanni, E.A. Simultaneous removal of As(III) and fluoride ions from water using manganese oxide supported on graphene nanostructures (GO-MnO2). Sustainability 2023, 15(2), 1179. https://www.mdpi.com/2071-1050/15/2/1179.
- Beolchini, F.; Pagnanelli, F.; De Michelis, I.; Veglio, F. Treatment of concentrated arsenic (V) solutions by micellar enhanced ultrafiltration with high molecular weight cut-off membrane. J. Hazard. Mater. 2007, 148, 116–121 . [CrossRef]
- Iqbal, J.; Kim, H.J.; Yang, J.S.; Baek, K.; Yang, J.W. Removal of arsenic from groundwater by micellar-enhanced ultrafiltration (MEUF). Chemosphere 2007, 66, 970–976. [CrossRef]
- Deng, J.; Gu, Z.; Wu, L.; Zhang, Y.; Tong, Y.; Meng, F.; Liu, H. Efficient purification of graphite industry wastewater by a combined neutralization-coagulation-flocculation process strategy: Performance of flocculant combinations and defluoidation mechanism. Sep. Purif. Technol. 2023, 326, 124771. [CrossRef]
- Pigatto, R.S.; Bizi, A.; Miyashiro, C.S.; Peruch, M.; Schadeck Netto, R.; Oliveira, V.L.; Mignoni, M.L. Thermally treated sludge obtained from a coagulation–flocculation water treatment process as a low-cost and eco-friendly adsorbent for water defluorination. Braz. J. Chem. Eng. 2021, 38, 451–460. [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manage. 2009, 91(1), 67–77. [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. 2012, 93, 154–168. [CrossRef]
- Xu, M.; Zhou, Y.; Hao, Y.; Cao, Y.; Xing, Y.; Gui, X. Enhancing Flotation Performance of Low-Rank Coal Using Environment-Friendly Vegetable Oil. Minerals 2023, 13(6), 717. [CrossRef]
- Ozairi, N.; Mousavi, S.A.; Samadi, M.T.; Seidmohammadi, A.; Nayeri, D. Removal of fluoride from water using coagulation-flocculation process: A comparative study. Desalin. Water Treat. 2020, 180, 265–270. [CrossRef]
- Vuorte, M.; Kuitunen, S.; Van Tassel, P.R.; Sammalkorpi, M. Equilibrium state model for surfactants in oils: Colloidal assembly and adsorption. J. Colloid Interface Sci. 2023, 630, 783–794. [CrossRef]
- Mureth, R.; Machunda, R.; Njau, K.N.; Dodoo-Arhin, D. Assessment of fluoride removal in a batch electrocoagulation process: A case study in the Mount Meru Enclave. Sci. Afr. 2021, 12, e00737. [CrossRef]
- Zhang, M.; Tan, X.; Ding, W.; Jiang, Z.; He, K.; Zhao, B.; Huang, Y. Aluminum-based electrocoagulation for residual fluoride removal during per- and polyfluoroalkyl substances (PFASs) wastewater treatment. Sep. Purif. Technol. 2023, 308, 122989. [CrossRef]
- Habuda-Stanić, M.; Ergović Ravančić, M.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7(9), 6317–6366. [CrossRef]
- Sivarajasekar, N.; Paramasivan, T.; Subashini, R.; Kandasamy, S.; Prakash Maran, J. Central Composite Design Optimization of Fluoride Removal by Spirogyra Biomass. Asian J. Microbiol. Biotech. Env. Sc. 2017, 19, S130–S137.
- Kumar, R.; Sharma, P.; Aman, A.K.; Singh, R.K. Equilibrium sorption of fluoride on the activated alumina in aqueous solution. Desalin. Water Treat. 2020, 197, 224–236. [CrossRef]
- Maity, J.P.; Hsu, C.M.; Lin, T.J.; Lee, W.C.; Bhattacharya, P.; Bundschuh, J.; Chen, C.Y. Removal of Fluoride from Water through Bacterial-Surfactin Mediated Novel Hydroxyapatite Nanoparticle and Its Efficiency Assessment: Adsorption Isotherm, Adsorption Kinetic and Adsorption Thermodynamics. Environ. Nanotechnol. Monit. Manag. 2018, 9, 18–28. [CrossRef]
- Solanki, Y.S.; Agarwal, M.; Maheshwari, K.; Gupta, S.; Shukla, P.; Gupta, A.B. Investigation of plausible mechanism of the synthesized inorganic polymeric coagulant and its application toward fluoride removal from drinking water. Ind. Eng. Chem. Res. 2020, 59(20), 9679–9687. [CrossRef]
- Tripathy, S.S.; Bersillon, J.L.; Gopal, K. Removal of Fluoride from Drinking Water by Adsorption onto Alum-Impregnated Activated Alumina. Sep. Purif. Technol. 2006, 50, 310–317. [CrossRef]
- Aung, S.H.; Nam, K.C. Impact of humectants on physicochemical and functional properties of jerky: A meta-analysis. Food Sci. Anim. Resour. 2024, 44(2), 464. [CrossRef]
- Liu, X.; Xu, X.; Dong, X.; Park, J. Competitive adsorption of heavy metal ions from aqueous solutions onto activated carbon and agricultural waste materials. Pol. J. Environ. Stud. 2020, 29(1), 749–761. [CrossRef]
- Jiang, L.; Cao, S.; Cheung, P.P.H.; Zheng, X.; Leung, C.W.T.; Peng, Q.; Huang, X. Real-time monitoring of hydrophobic aggregation reveals a critical role of cooperativity in hydrophobic effect. Nat. Commun. 2017, 8(1). [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Abd Aziz, A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Zoh, K.D. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023, 11(3), 109610. [CrossRef]
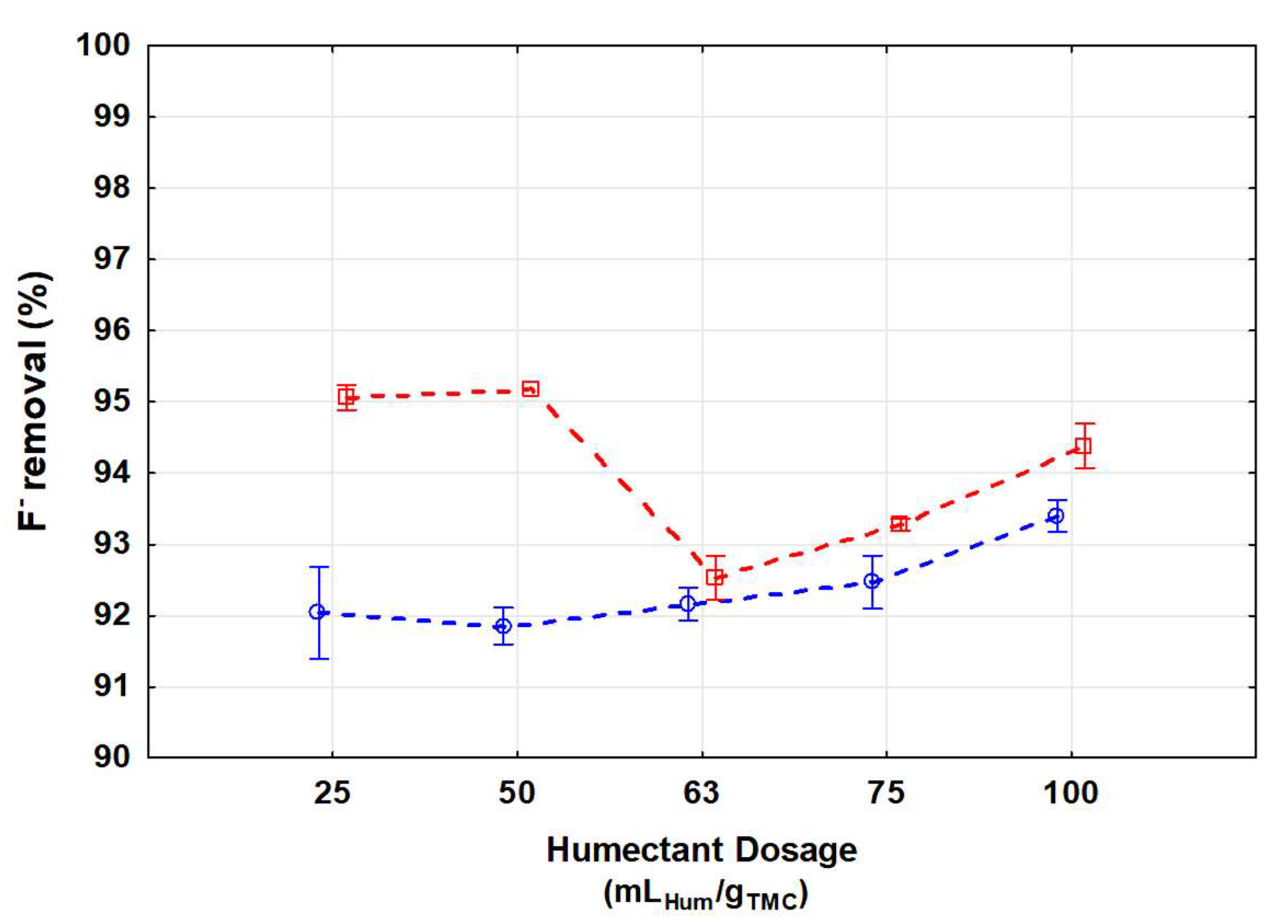
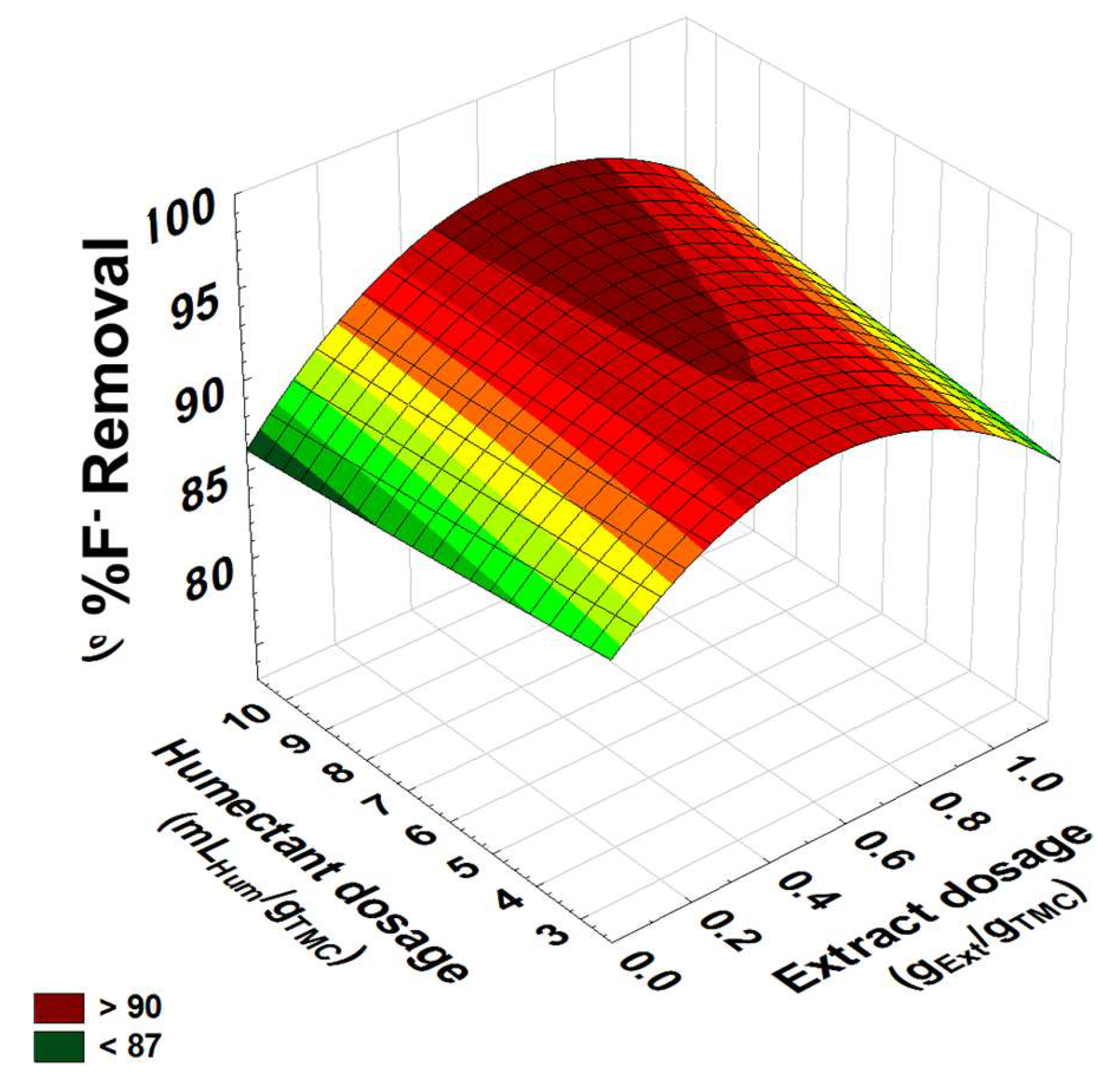
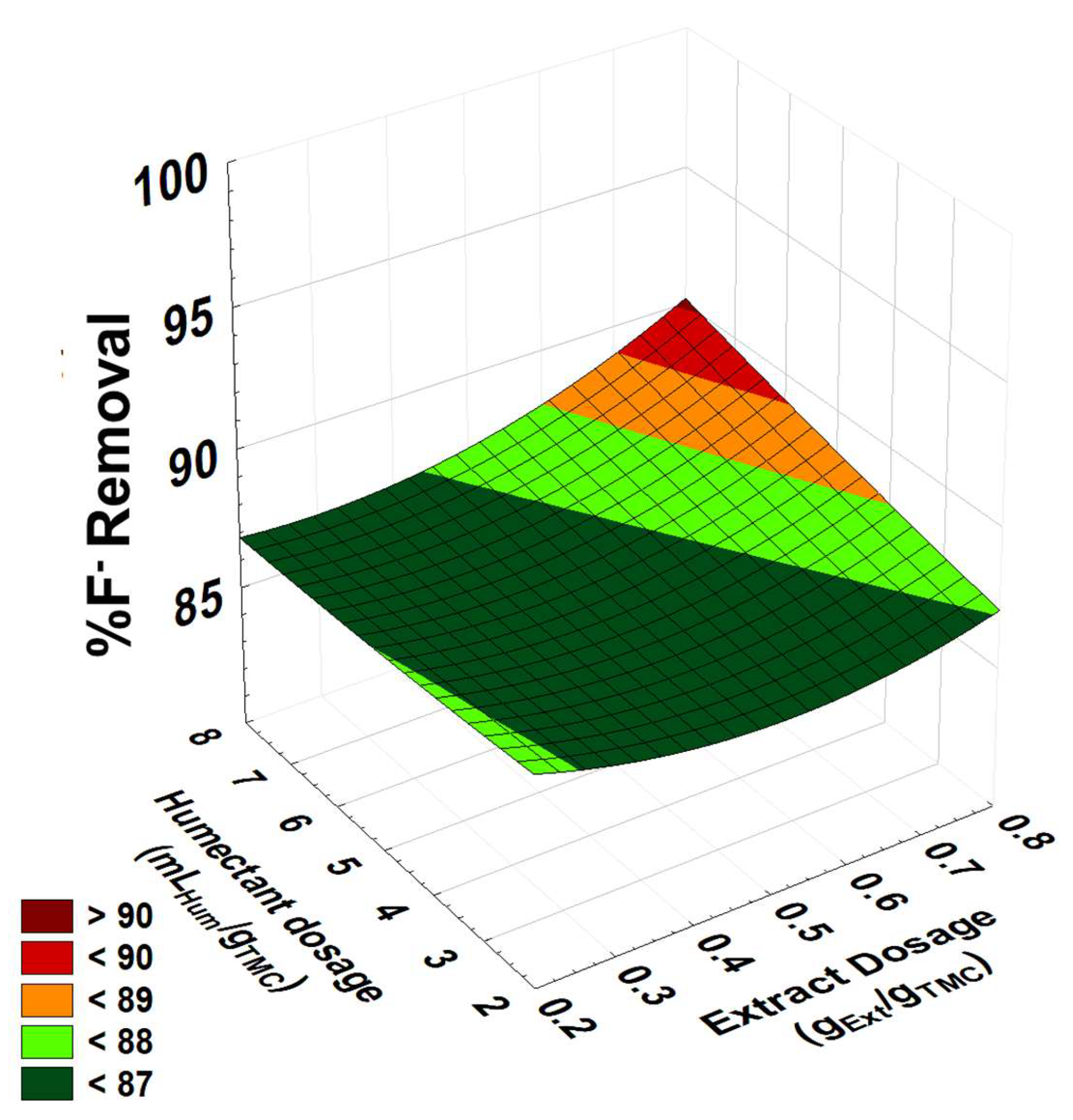
| n-Heptane | |||||
|---|---|---|---|---|---|
|
Humectant dosage (mLHum/gTMC) |
pHfinal |
[F−]final (mg/L) |
|||
| 2.5 | 7.04 | 0.43* | ± | 0.04 | |
| 5 | 7.1 | 0.44* | ± | 0.02 | |
| 6.3 | 7.11 | 0.42* | ± | 0.02 | |
| 7.5 | 7.09 | 0.41* | ± | 0.03 | |
| 10 | 7.11 | 0.36* | ± | 0.02 | |
| Sunflower oil | |||||
|
Humectant dosage (mLHum/gTMC) |
pHfinal |
[F−]final (mg/L) |
|||
| 2.5 | 7.1 | 0.27* | ± | 0.01 | |
| 5 | 7.15 | 0.26* | ± | 0.01 | |
| 6.3 | 7.04 | 0.40* | ± | 0.02 | |
| 7.5 | 7.12 | 0.36* | ± | 0.01 | |
| 10 | 7.05 | 0.30* | ± | 0.02 | |
| - Initial fluoride concentration: 5.0 mg/L; * Values below NOM-SSA-127-SSA1–2021 (1.0 µg/L); pH between 6.5 and 8. | |||||
| Surfactant dosage (g Ext/g TMC) |
Humectant dosage (mL Hum/gTMC) |
pHfinal | [F−]final (mg/L) |
||
|---|---|---|---|---|---|
| 1 | 2.5 | 7.14* | 0.61* | ± | 0.01 |
| 0.75 | 2.5 | 7.12* | 0.34* | ± | 0.03 |
| 0.5 | 2.5 | 7.10* | 0.27* | ± | 0.01 |
| 0.25 | 2.5 | 7.14* | 0.41* | ± | 0.02 |
| 0 | 2.5 | 7.0* | 0.67* | ± | 0.01 |
| 1 | 5 | 7.2* | 0.37* | ± | 0.01 |
| 0.75 | 5 | 7.25* | 0.38* | ± | 0.01 |
| 0.5 | 5 | 7.15* | 0.26* | ± | 0.04 |
| 0.25 | 5 | 7.12* | 0.45* | ± | 0.02 |
| 0 | 5 | 7.14* | 0.67* | ± | 0.01 |
| 1 | 7.5 | 7.0* | 0.51* | ± | 0.01 |
| 0.75 | 7.5 | 7.0* | 0.28* | ± | 0.02 |
| 0.5 | 7.5 | 7.12* | 0.36* | ± | 0.02 |
| 0.25 | 7.5 | 7.15* | 0.50* | ± | 0.02 |
| 0 | 7.5 | 7.11* | 0.68* | ± | 0.02 |
| 1 | 10 | 7.1* | 0.31* | ± | 0.01 |
| 0.75 | 10 | 7.09* | 0.30* | ± | 0.01 |
| 0.5 | 10 | 7.05* | 0.30* | ± | 0.01 |
| 0.25 | 10 | 7.0* | 0.57* | ± | 0.01 |
| 0 | 10 | 7.04* | 0.65* | ± | 0.02 |
| Dosis surfactante (gExt/gTMC) |
Dosis humectante (mLHum/gTMC) |
pHfinal | [F−]final (mg/L) |
||
|---|---|---|---|---|---|
| 0.25 | 2.5 | 7.0* | 0.63* | ± | 0.02 |
| 0.25 | 5 | 7.03* | 0.66* | ± | 0.01 |
| 0.25 | 7.5 | 7.05* | 0.67* | ± | 0.01 |
| 0.5 | 2.5 | 7.07* | 0.71* | ± | 0.02 |
| 0.5 | 5 | 7.03* | 0.68* | ± | 0.01 |
| 0.5 | 7.5 | 7.02* | 0.61* | ± | 0.02 |
| 0.75 | 2.5 | 7.03* | 0.64* | ± | 0.01 |
| 0.75 | 5 | 7.0* | 0.58* | ± | 0.01 |
| 0.75 | 7.5 | 7.04* | 0.55* | ± | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





