1. Introduction
The coordination chemistry of nickel(II) dichlorides with bidentate phosphines is one of the most studied areas of coordination chemistry with the variation in coordination geometries [
1,
2,
3] between square planar and tetrahedral [
4,
5] being used as a vehicle to teach the differences between strong and weak ligand fields [
6]. In most cases the colour of a metal complex is enough to assign whether the complex is low- or high-field: a larger energy gap (low-field) means the colour of the complex is the complementary one i.e. blue or green wavelengths missing, therefore a yellow, orange or red [
7,
8,
9,
10]. Some complexes exist in both coordination modes and some are switchable. In rare cases a complex may be observed as a Zwitterion, which is the subject of this work. The ligand we used was 1,2-bis-(di-
tbutylphosphinomethyl)benzene [
11]
1, which is the classic so-called alpha ligand [
12]. We have extensive experience in the coordination of these ligands, particularly the ferrocene class [
13,
14,
15]. It is known that palladium is coordinated by the ferrocene alpha ligand,
butphos,
2, in a square-planar fashion (
Figure 1) [
13].
We decided to examine the complexation reaction of nickel with the simple alpha ligand. Much is known about the similar square-planar coordination chemistry with palladium and platinum, as these complexes are useful industrial catalysts [
16,
17,
18]. To visualise the alpha ligand more clearly the disulfide derivative was prepared to ensure air-stability, and its crystal structure was determined. This is shown in
Figure 2. The pendant methylene-phosphines are located above and below the plane of the benzene ring. On reaction of the alpha ligand with molybdenum hexacarbonyl the molybdenum tetracarbonyl complex is formed [
19,
20].
2. Results and Discussion
The phosphorus atoms move to the same side of the benzene ring to adopt a pseudo-octahedral coordination mode (
Figure 3). It is interesting to observe that axial carbonyl ligands are bent back away from the perpendicular plane, a consequence of the steric crowding due to the proximity of the bulky tert-butyl groups on the ligand.
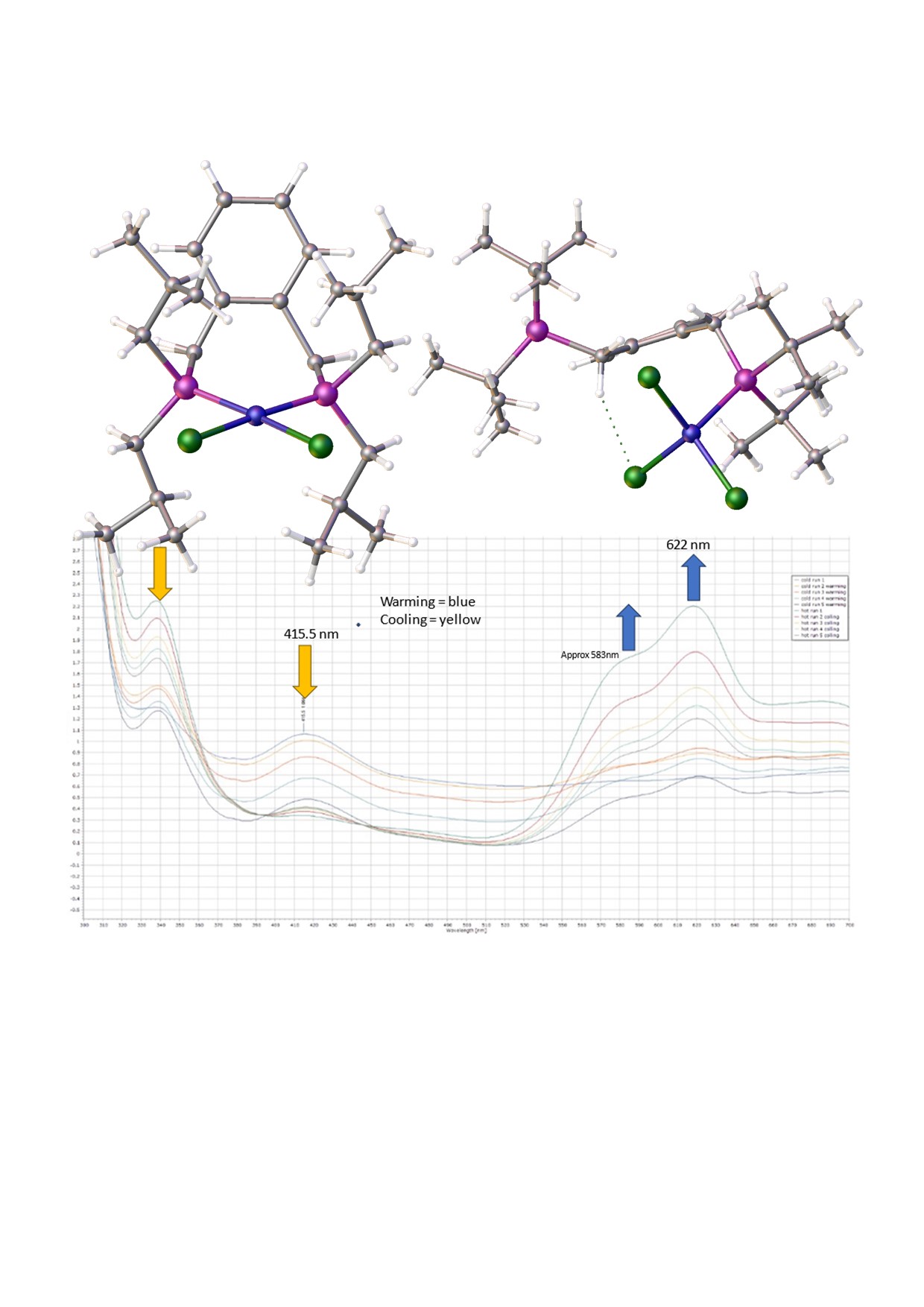
With these data in hand the reaction of this ligand with hydrated nickel(II) chloride in alcoholic solvents was attempted, but this led only to amorphous yellow powders. However, when a solution of the ligand is added to the [Ni(DME)Cl
2] complex [
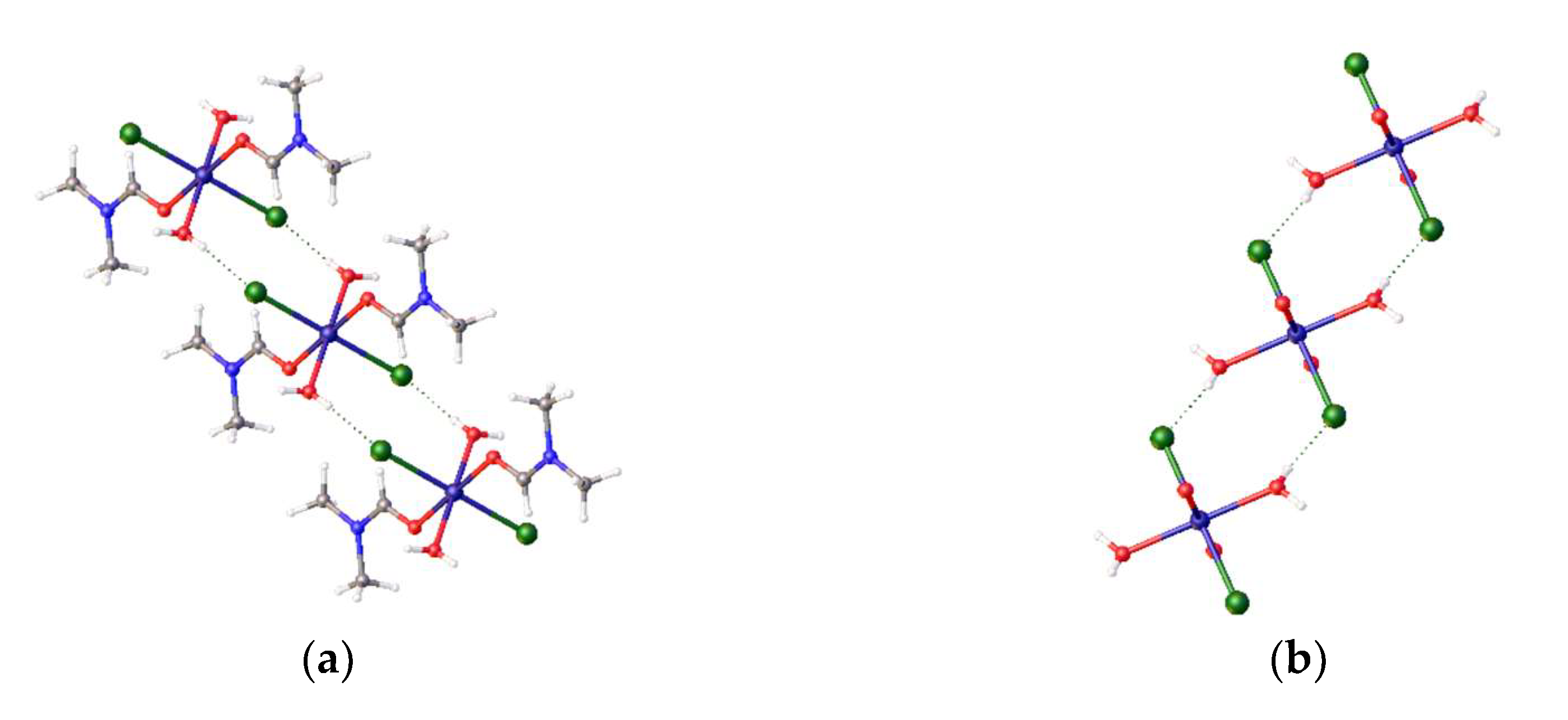
22] in dichloromethane the solution develops a blue colour and, when left for several days, deep blue crystals begin to grow. These crystals were collected after 2 weeks and examined by single-crystal X-ray diffraction; the product is a rare Zwitterionic compound, which accounts for its poor solubility in organic solvents. Only one phosphine is bound to nickel, which has 3 chloro ligands attached in a pseudo-tetrahedral coordination (
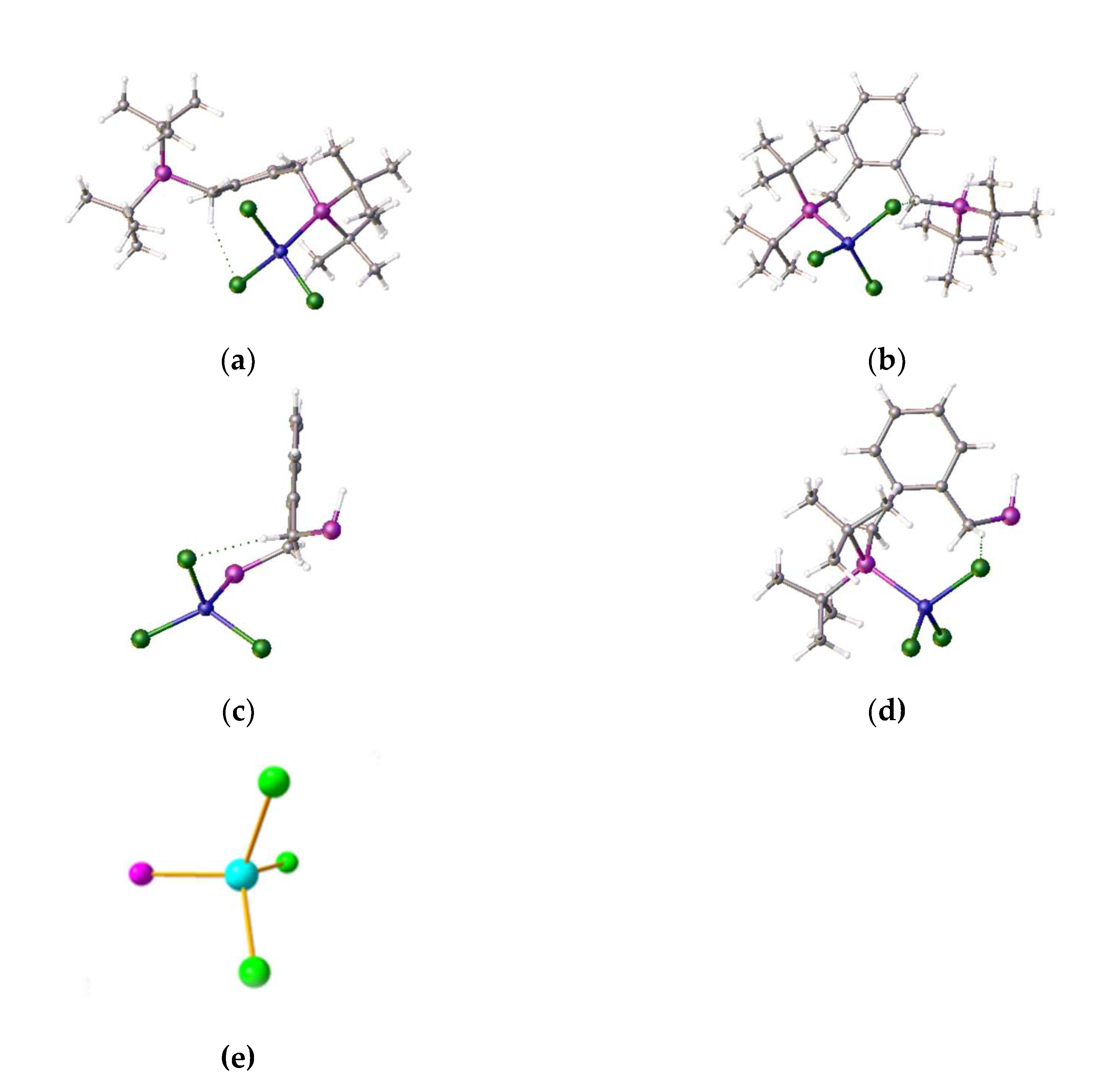
Figure 4).
The other phosphorus atom is protonated, forming a phosphonium pendant arm; this probably explains why the crystals grow so slowly. The Cl-Ni-Cl angles are 101.00(3), 120.89(3), 109.76(3)°, while there are two hydrogen bonding interactions (
Figure 4), between a chloro ligand and the proton on the phosphorus (Cl…H = 2.70(4) Å), and between another chloro ligand and phenyl ring H atom (Cl…H = 2.7943(7) Å). The origin of the proton and the additional chloride attached to nickel is unclear: the proton may come from the solvent or trace water, while the chloride may come from the solvent or excess nickel chloride starting material. It is likely that nickel plays a role in the proton transfer process. The product complex decomposes in methanol and forms a pale-yellow solution/slurry. It is sparingly soluble in DMF and forms a blue solution at room temperature. On cooling this solution to −20°C, the blue colour (ƛ
max,
~ 621nm) changes to yellow (ƛ
max,
~ 417nm) totally reversibly. We postulate that the proton on phosphorus is transferred to DMF to form its quaternary cation. Interestingly this compound is one which crystallised well in regimented fashion in local magnetic fields [
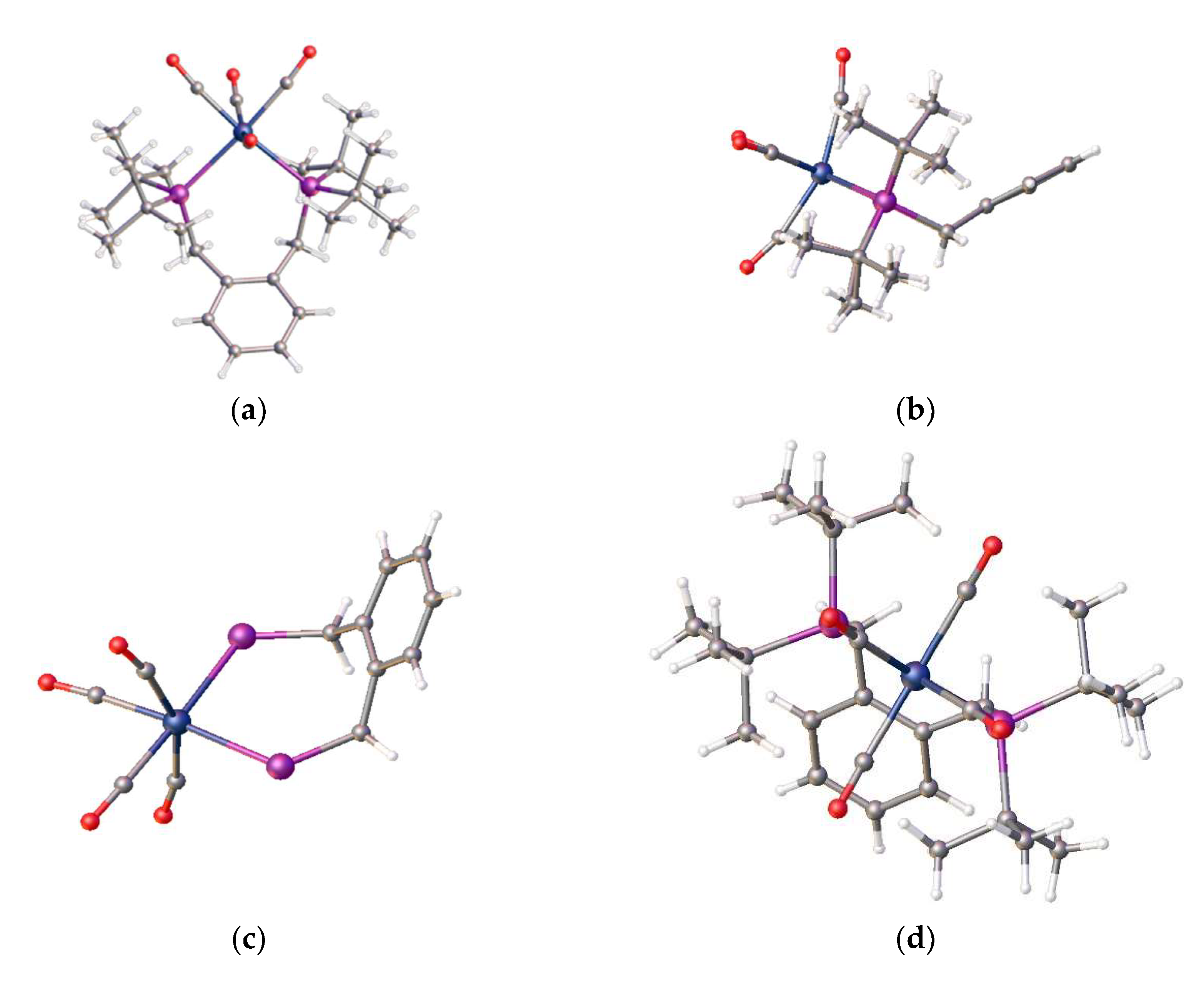
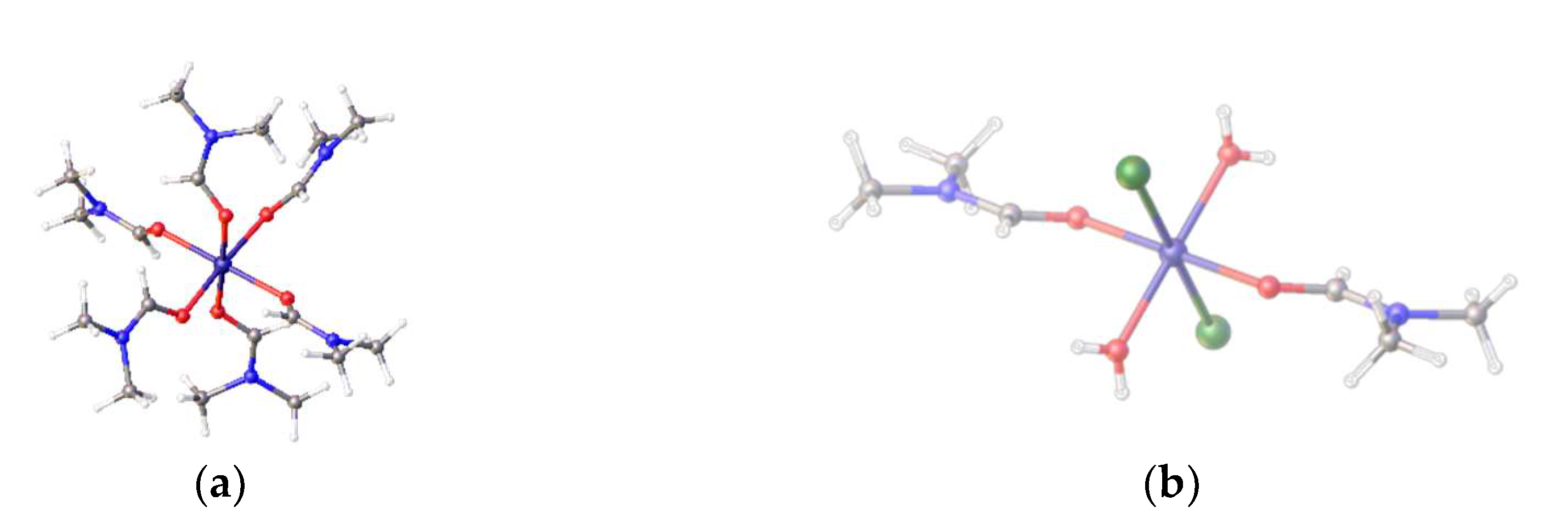
23]. It is known that complex equilibria exist in DMF solutions of nickel chlorides and thus DMF binding to nickel may occur. We have also been able to isolate two nickel complexes from the reaction of [Ni(DME)Cl
2] with DMF in magnetic fields [
23]. These complexes are the octahedral complexes, all-
trans- diaqua-bis(dimethylformaldehydo)dichloronickel(II), [Ni(Cl)
2(H
2O)
2(DMF)
2] (
Figure 5b) and the known hexadimethylformaldehydo-nickel(II) tetrachloronickelate(II), [Ni(DMF)
6)]
2+[NiCl
4]
2− (
Figure 5a).
These are both formed at room temperature, which demonstrates the lability of the chloro ligands. [NiCl
2(H
2O)
2(DMF)
2] exists in hydrogen-bonded ribbons which are also of general interest. Cutaway sections of a packing diagram are shown in
Figure 6.
These results confirm that DMF can bind to the nickel centre at room temperature. The other alternative is that DMF functions as a base to deprotonate the phosphonium phosphorus atom, which may than coordinate to the nickel to displace a chloro ligand. During these coordination studies we have non-definitive evidence for the formation of the anticipated square-planar Ni-alpha complex as small red crystals, which were isolated during one of our room-temperature coordination reactions, but the initial crystallographic results indicated a disordered structure. However, we note that we have recently been able to determine the structure of the red nickel dichloride complex of the related ligand [1,2-C
6H
4-(CH
2P
iPr
2)
2]. The structure is disordered but it clearly shows that nickel adopts a square-planar coordination mode (
Figure 7).
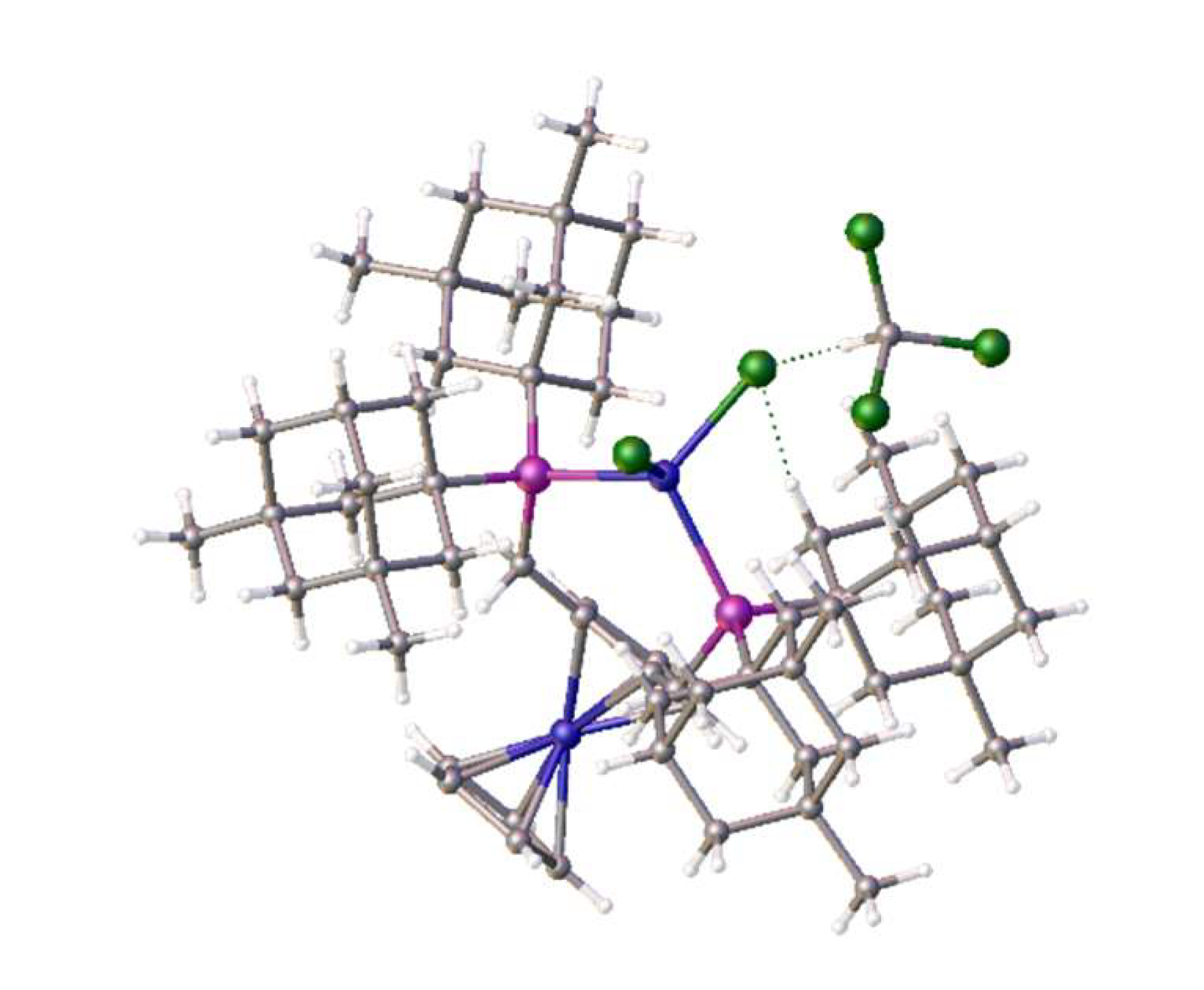
Clearly this ligand is less hindered, which may account for the difference in coordination properties and its relative ease of formation. We had previously observed similar disorder in the nickel dichloride complex of the bis-1,2-{(1,3-dimethyladamantyl)phosphino}ferrocene [
7], which interestingly exhibited tetrahedral coordination (
Figure 8). The research reported here, and these observations make this area of research worthy of a more though investigation, as this limited work was done without external funding.
A reaction was attempted in which d5-pyridine was used in an NMR experiment: in this case it was clear to see partial de-ligation of the alpha ligand with the appearance of the resonances of the free ligand in solution. There were two phosphorus resonances observed at approximately 27.5 and 62.3 ppm (several resonances). The former is due to free ligand, which indicates partial de-ligation possibly due to trace water in the d5-pyridine.
3. Materials and Methods
The protocols used in this work were essentially standard coordination and crystallization at room temperature, conditions which were developed with a view to keeping the methodology as simple as possible. Of course, we could add that crystallization is one of the main methods of self-assembly. All metal complex crystallizations were carried out with the compound dissolved in a solution of reagent-grade dichloromethane with a top layer of diethyl ether added carefully. Crystals formed in all cases after the solvents diffused together over several days. Additional experimental detail is given in the Supplementary Information.
4. Conclusions
Although the coordination of the alpha ligand with palladium and platinum is relatively straightforward, the coordination with nickel is less so: the natural propensity towards tetrahedral coordination may be difficult because of the steric bulk of the ligand in a relatively small coordination sphere. However, it is interesting to observe the formation of a new Zwitterionic complex. Given that there is a readily available supply of related ligands, it will be of interest to observe the general trends in coordination chemistry when the synthetic method described here is applied. Clearly, given that these nickel complexes are so much cheaper to prepare than the corresponding palladium and platinum derivatives and the former of these is used in such large quantities in industrial acrylic formation, it is clearly imperative to test them in carboxy-alkylation reactions. We would encourage interested research groups to contact us to develop such work.
Supplementary Materials
Crystallographic data for [1,2-(C
6H
4-CH
2PtBu
2)
2Mo(CO)
4], [1,2-(C
6H
4-H
2P(S)
tBu
2)
2], [2-(C
6H
4-CH
2P(H)tBu
2-1-(CH
2PtBu
2NiCl
3)Cl, [1,2-(C
6H
4-CH
2PiPr2)
2NiCl
2], [Ni(DMF)
6)]
2+[NiCl
4]
2-, [Ni(Cl)
2(H
2O)
2(DMF)
2] are available as supplementary materials. CCDC 2421627, 2421631-5 respectively also contain the supplementary crystallographic data for this paper. These CCDC data can be obtained free of charge via
http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from CCDC, 12 Union Road, Cambridge, CB2 1EZ. Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Author Contributions
I.R.B.: investigations, experimental work, writing, reviewing and editing, supervision, methodology, conception. P.N.H.: crystallography, data deposition, writing; W.C.: proofing, crystallography work, director of synchrotron crystallographic facilities; S.J.C.: Director of National Crystallographic Services and project overseer; K.M.F. synthesis, thesis work; M.G.B.D. early crystallography (2004); K. S. synthetic work. All authors have read and agreed to the published version of the manuscript.
Funding
We thank EPSRC for funding the National Crystallography Service (both Southampton.
Data Availability Statement
All requisite data are available in the Supplementary Information.
Acknowledgments
The authors thank Dr Mohammed Al-mashhadani, who helped us with the many thermochromic data runs. We all thank the EPSRC Crystallographic Services for grants and facilities and the EPSRC mass spectroscopic service Crystallographic work was carried out at the EPSRC National Crystallography Centre based at the University of Southampton and at the Daresbury Laboratory Synchrotron Radiation Source. Mass spectroscopic measurements were carried out in house and at the EPSRC National Mass Spectrometry Centre based at Swansea University. We thank the staff of both these institutions for their excellent and painstaking work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicholls, D. The Chemistry of Iron, Cobalt and Nickel: Comprehensive Inorganic Chemistry, Pergamon texts in Inorg. Chem. 2013, 24, Elsevier–2013. (199 pages). eBook ISBN: 9781483146430. [Google Scholar]
- Blanchard, S.; Neese, F.; Bothe, E.; Weyhermüllerb E., B.T. Wieghardt, Square planar vs tetrahedral coordination in diamagnetic complexes of nickel(II) containing two bidentate pi-radical monoanions. K. Inorg. Chem. 2005, 44, 3636–3656. [Google Scholar] [CrossRef] [PubMed]
- Collinson, S.R.; Schröder, M. Nickel: Inorganic & Coordination Chemistry, Encyclopaedia of Inorganic Chemistry. 2006. Wiley. [CrossRef]
- Lomjanský, D.; Rajnák, C.; Titiš, J.; Moncoľ, J.; Smolko, L.; Boča, R. Impact of tetrahedral and square planar geometry of Ni(II) complexes with (pseudo)halide ligands to magnetic properties, Inorg. Chim. Acta 2018, 483, 352–358. [Google Scholar]
- Cope, J.D.; Denny, J.A.; Lamb, R.W.; McNamara, L.E.; Hammer, N.I.; Webster, C.E.; Hollis, T.K. Electrocatalytic reduction of CO2 with CCC-NHC pincer nickel complexes, J. Organometal. Chem. 2017, 845, 258–265. [Google Scholar] [CrossRef]
- Venanzi, L.M. Tetrahedral complexes of nickel (II) and the factors determining their formation, J. Inorg. Nucl. Chem. 1958, 8, 137–142. [Google Scholar] [CrossRef]
- Balakrishnan, K.P. J. Chem. Eng. Data. 1980, 25, 186–187, Preparation and properties of nickel(II) complex
dyes,. [CrossRef]
- W. Clegg, W.; Eastham, G.R.; Elsegood, M.R.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R.; Zacchini, S. Highly active and selective catalysts for the production of methyl propanoate via the methoxycarbonylation of ethene, Organometallics 2002, 21, 1832–1840. [CrossRef]
- Bellabarba, R.M.; Tooze, R.P.; Slawin, A.M.Z. Synthesis, X-ray characterisation and reactions of a trigonal planar palladium(0) carbonyl complex, (tbpx)PdCO, Chem Commun. 2003, 15, 1916–1917. [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Heaton, B.T.; Iggo, J.A.; Tooze, R.P.; Whyman, R. ; Zacchini,S. Synthesis and reactivity of palladium hydrido-solvento complexes, including a key intermediate in the catalytic methoxycarbonylation of ethene to methyl propanoate, J. Chem. Soc. Dalton Trans. 2002, 17, 3300–3308. [Google Scholar] [CrossRef]
- Fanjul, T.; G. Eastham, G.R.; Fey, N.; Hamilton.; Orpen, A.G.; Pringle, P.G.; Waugh, M. Organometallics, Characterization and Dynamics of [Pd(L−L)H(solv)]+, [Pd(L−L)(CH2CH3)]+, and [Pd(L−L)(C(O)Et)(THF)]+ (L−L = 1,2-(CH2PBut2)2C6H4): Key Intermediates in the Catalytic Methoxycarbonylation of Ethene to Methylpropanoate. 2010, 29, 2292–2305. [Google Scholar] [CrossRef]
- Krishna; M. ; Pringle, P.G.; Sparkes, H.A.; Wass, D.F. Organometallics, 2020, 39, 468–477, Transition metal cooperative Lewis pairs using platinum(0) piphosphine Mmonocarbonyl complexes as Lewis bases. [Google Scholar] [CrossRef]
- Fortune, K.M; Castel, C.; Robertson, C.M.; Horton, P.N.; Light, M.; Coles, S.J.; Waugh, M.; Clegg, W.; Harrington, R.W.; Butler, I.R. Ferrocenylmethylphosphanes and the Alpha Process for Methoxycarbonylation: The Original Story, Inorganics 2021, 9, 57. [CrossRef]
- Butler, I.R.; Baker, P.K.; Eastham, G.R.; Fortune, K.M.; Horton, P.N.; Hursthouse, M.B. Ferrocenylmethylphosphines ligands in the palladium-catalysed synthesis of methyl propionate. Inorg. Chem. Commun. 2004, 7, 1049–1052. [Google Scholar] [CrossRef]
- Butler, I.R.; Horton, P.N.; Fortune, K.M.; Morris, K.; Greenwell, C.H.; Eastham, G.R.; Hursthouse, M.B. The first 1,2,3-tris(phosphinomethyl)ferrocene. Inorg. Chem. Commun. 2004, 7, 923–928. [Google Scholar] [CrossRef]
- Clegg, W.; Eastham, G.R.; Elsegood, M.R.J.; Tooze, R.P.; Wang, L. Highly active and selective catalysts for the production of methyl propanoate via the methoxycarbonylation of ethene. Chem. Commun. 1999, 1877–1878. [Google Scholar] [CrossRef]
- Knight, J.G.; Doherty, S.; Harriman, A.; Robins, E.G.; Berham, M.; Eastham, G.R.; Tooze, R.P.; Elsegood, M.R.J.; Champkin, P.; Clegg, W. Remarkable Differences in Catalyst Activity and Selectivity fo the Production of Methyl Propanoate versus CO−Ethylene Copolymer by a Series of Palladium Complexes of Related C4-Bridged Diphosphines. Organometallics 2000, 19, 4957–4967. [Google Scholar] [CrossRef]
- Vondran, J.; Furst, M.R.L.; Eastham, G.R.; Seidensticker, T.; Cole-Hamilton, D.J. Magic of Alpha: The Chemistry of a Remarkable Bidentate Phosphine, 1,2-Bis(di-tert-butylphosphinomethyl)benzene, Chem. Rev. 2021, 121, 6610–6653. [Google Scholar] [CrossRef] [PubMed]
- Garrou, E.; Hartwell, G.E. Molybdenum carbonyl complexes of unsaturated tertiary phosphines, Organometal. Chem. 1973, 55, 331–341. [Google Scholar] [CrossRef]
- Laine, T.V.; Klinga, M.; Leskela, M. Pyridinylimine-based nickel(II) and palladium(II) complexes: preparation, structural characterization and use as alkene polymerization catalysts. Eur. J. Inorg. Chem. 1999, 6, 959–964. [Google Scholar] [CrossRef]
- Fortune, K.; PhD thesis, Bangor University, 2004. Nitrogen donor complexes of molybdenum and tungsten and new routes to bis-1,2 & tris-1,2,3 substituted ferrocenes. https://research.bangor.ac.uk/portal/files/50696506/K_M_FORTUNE_PhD_2004_OCR.pdf.
- Ward, L.G.L. Anhydrous nickel(II) halides and their tetrakis(ethanol) and 1,2-dimethoxyethane complexes, Inorganic Syntheses 1971, 13, 154–164. https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470132449.ch30?msockid=2387c07414a862d13e0ed482153763cf.
- Butler, I.R.; Williams, R.M.; Heeroma, A.; Horton, P.N.; Coles, S.J. ; Jones. L.F. The Effect of Localized Magnetic Fields on the Spatially Controlled Crystallization of Organometallics and Transition Metal Complexes. Manuscript in preparation.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).