Submitted:
15 December 2024
Posted:
16 December 2024
You are already at the latest version
Abstract
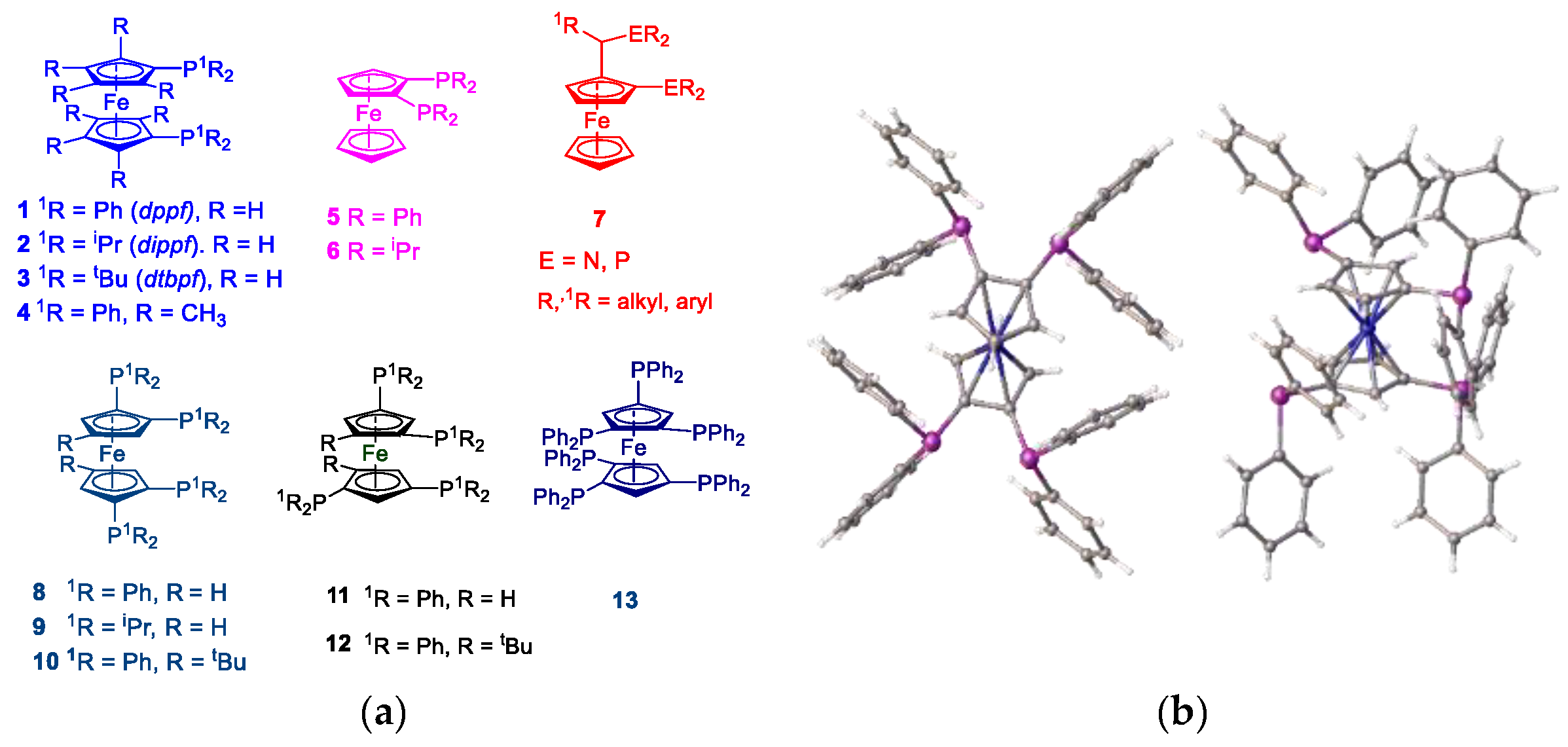
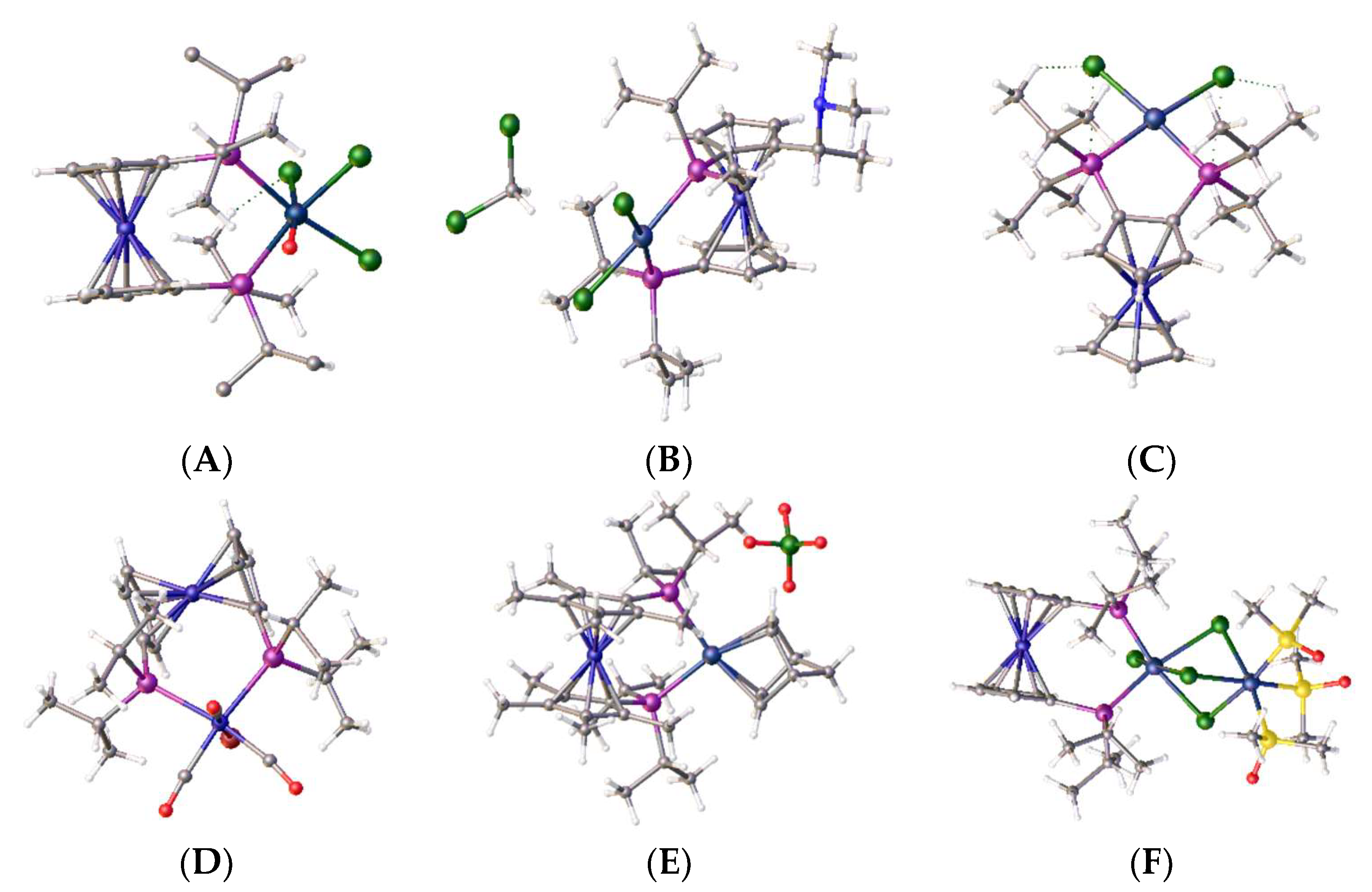
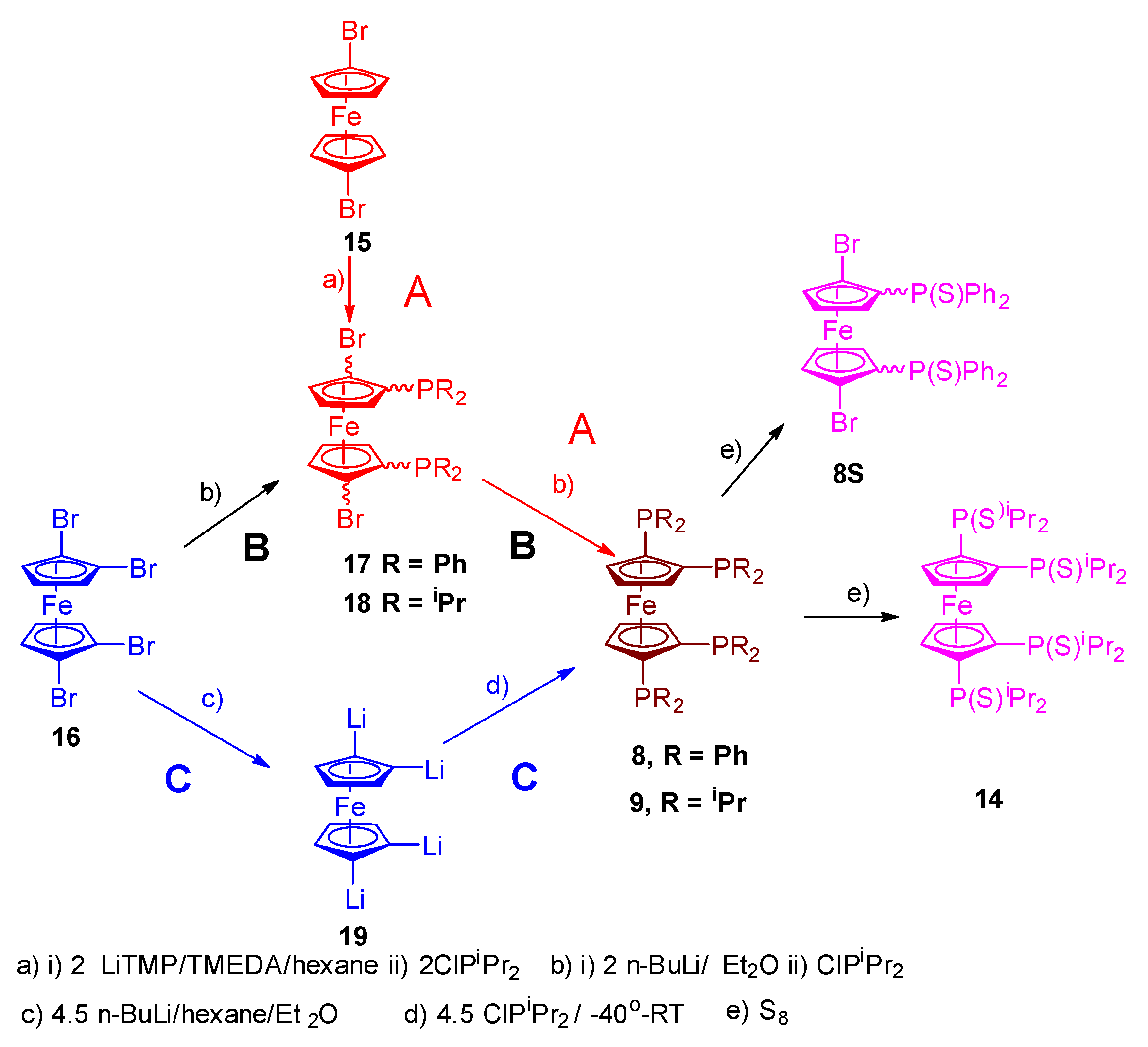
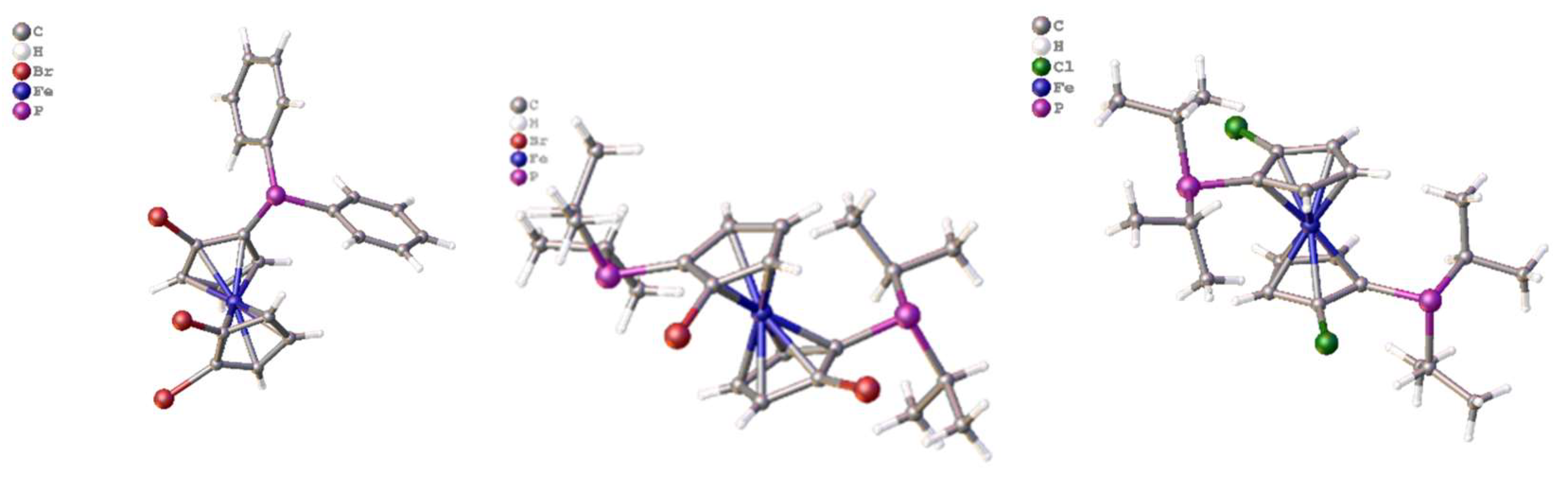
The clean high yielding, synthesis and structure of the tetraphosphine ligand, 1,1´,2,2´-tetrakis-(di-isopropyl-phosphino)ferrocene, (tdipf), is described. In addition, an improved synthesis of the 1,1´,2,2´-tetrakis(diphenylphosphino)ferrocene, (tppf) and 2,2´-bis-(diphenylphosphino)-1,1´-dibromoferrocene are also reported and the synthetic method is generalised to include the synthesis of 3,3’-bis-(diphenylphosphino)-1,1´,2,2´-tetrabromoferrocene. The related ligands 2,2´-bis-(iso-propylphosphino)-1,1´-bis-diphenylphosphinoferrocene (diprdppf) and 2,2´-bis-(di-isopropylphosphino)-dibromoferrocene have also been prepared and characterised. The crystal structure of the square planar bimetallic nickel (II) dichloride of tdipf is also described, together with a brief NMR study investigating the synthesis of this and related metal complexes. The crystal structures of the palladium and platinum dichloride complexes of 2,2’-bis-(di-isopropylphosphino)-1,1’-dibromoferrocene, bpdbf, are also discussed in the context of comparison with previously known crystal structures in the same general family. A general discussion on the synthetic methodology is given and pointers to future research other researchers might explore.
Keywords:
1. Introduction
1.1. Ligand Diversity
1.2. Tetra-Dialkylphosphinoferrocenes or Tetra-Diarylphosphinoferrocenes.
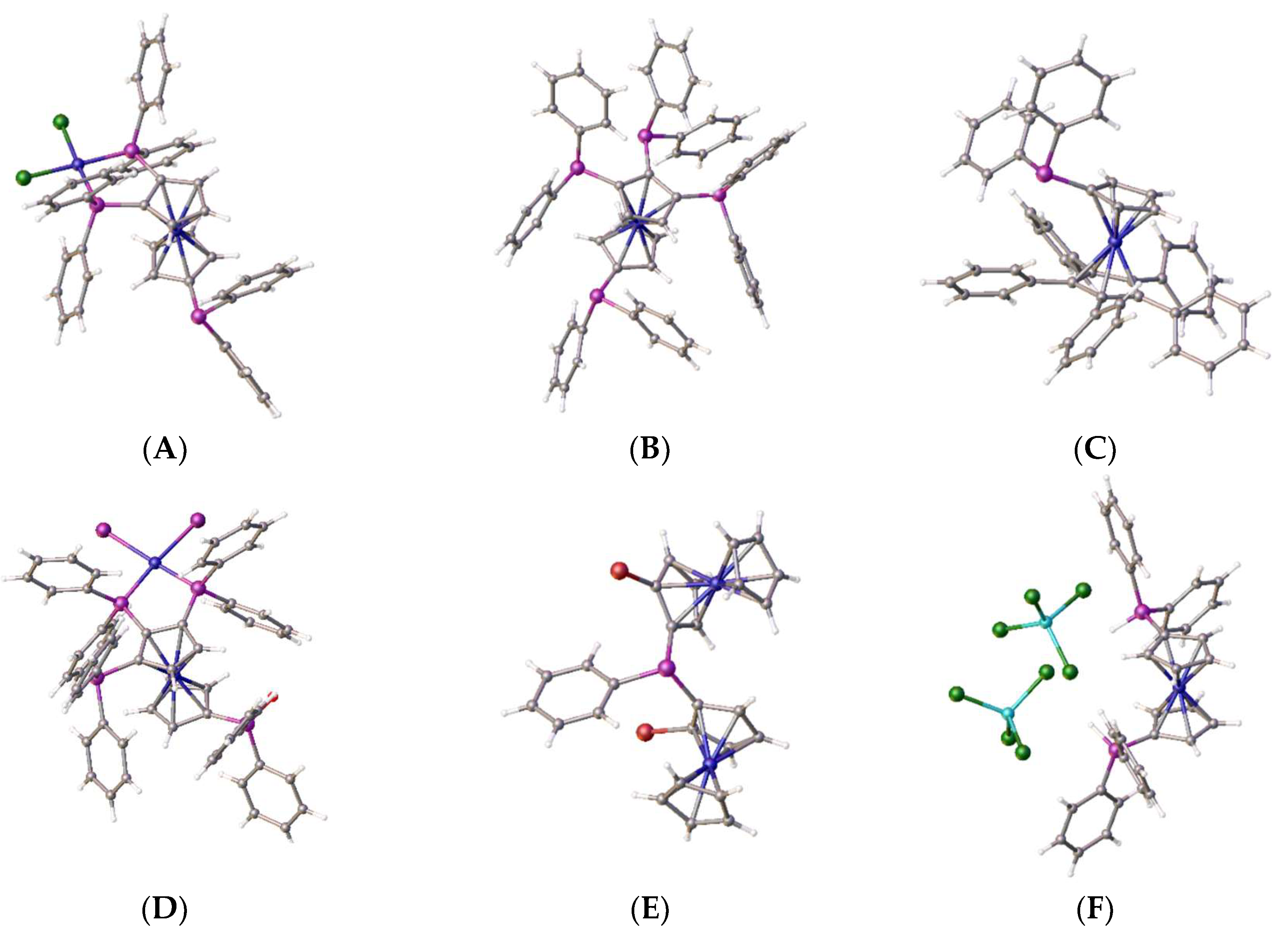
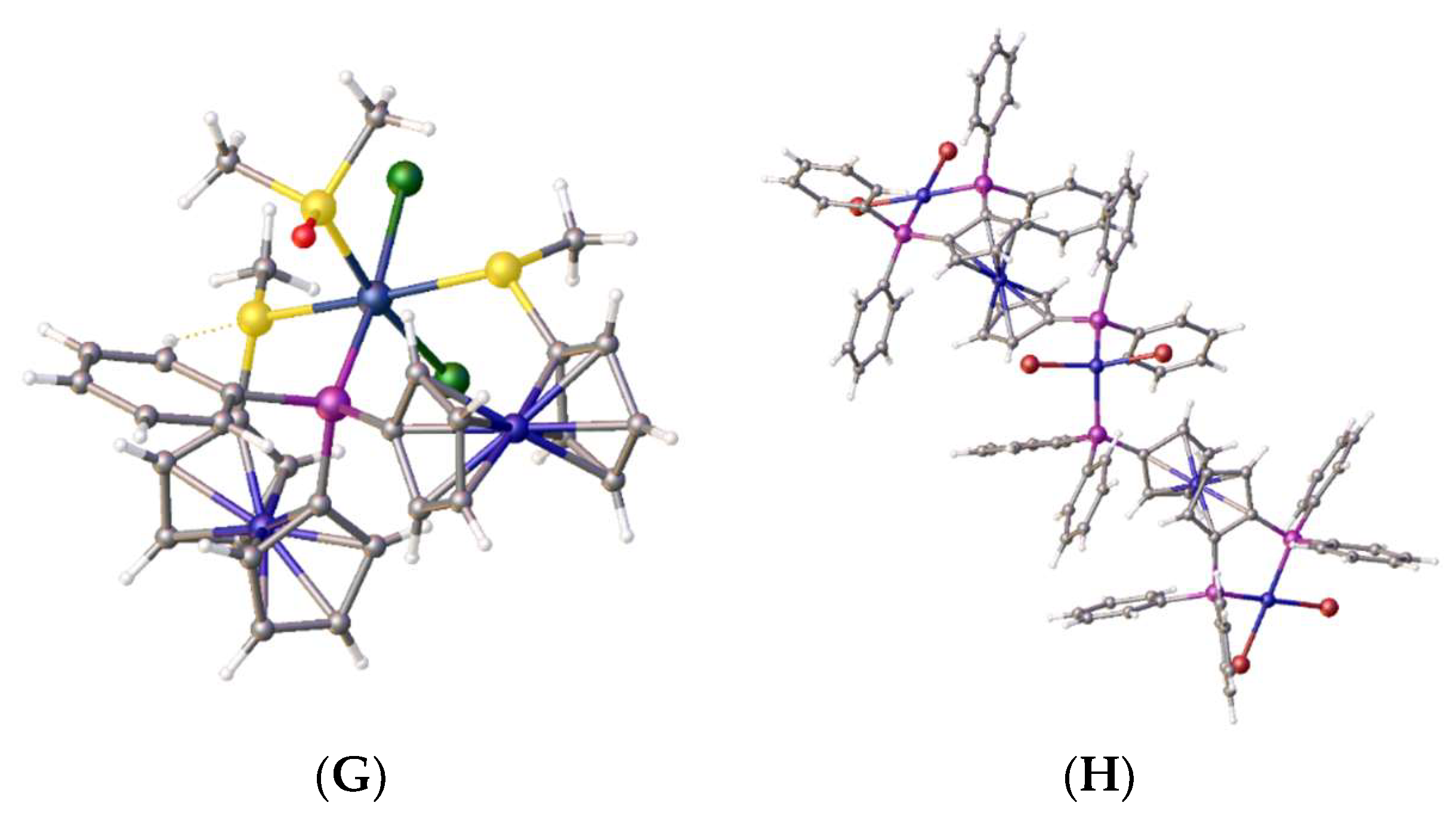
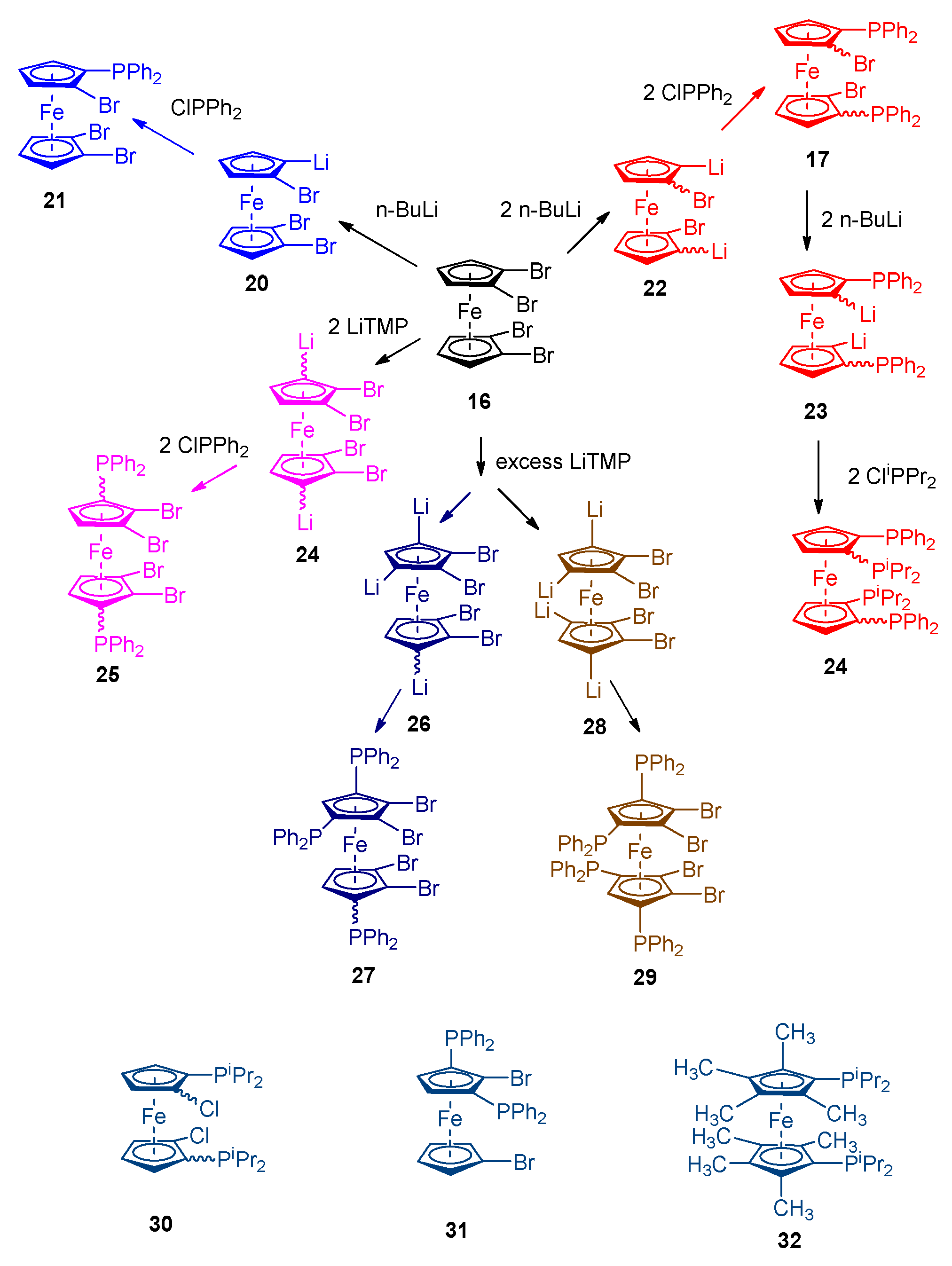
2. Results and Discussion
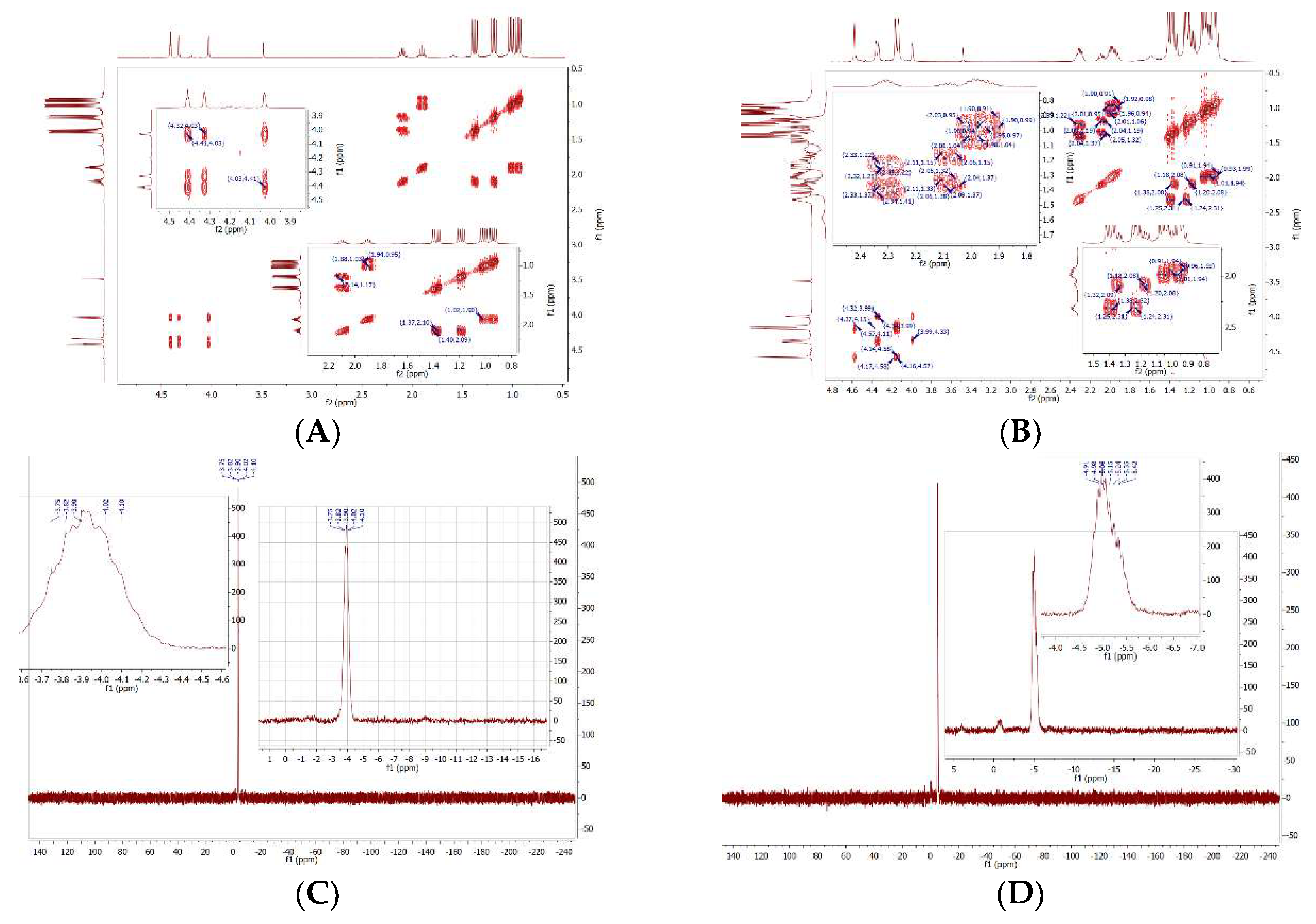
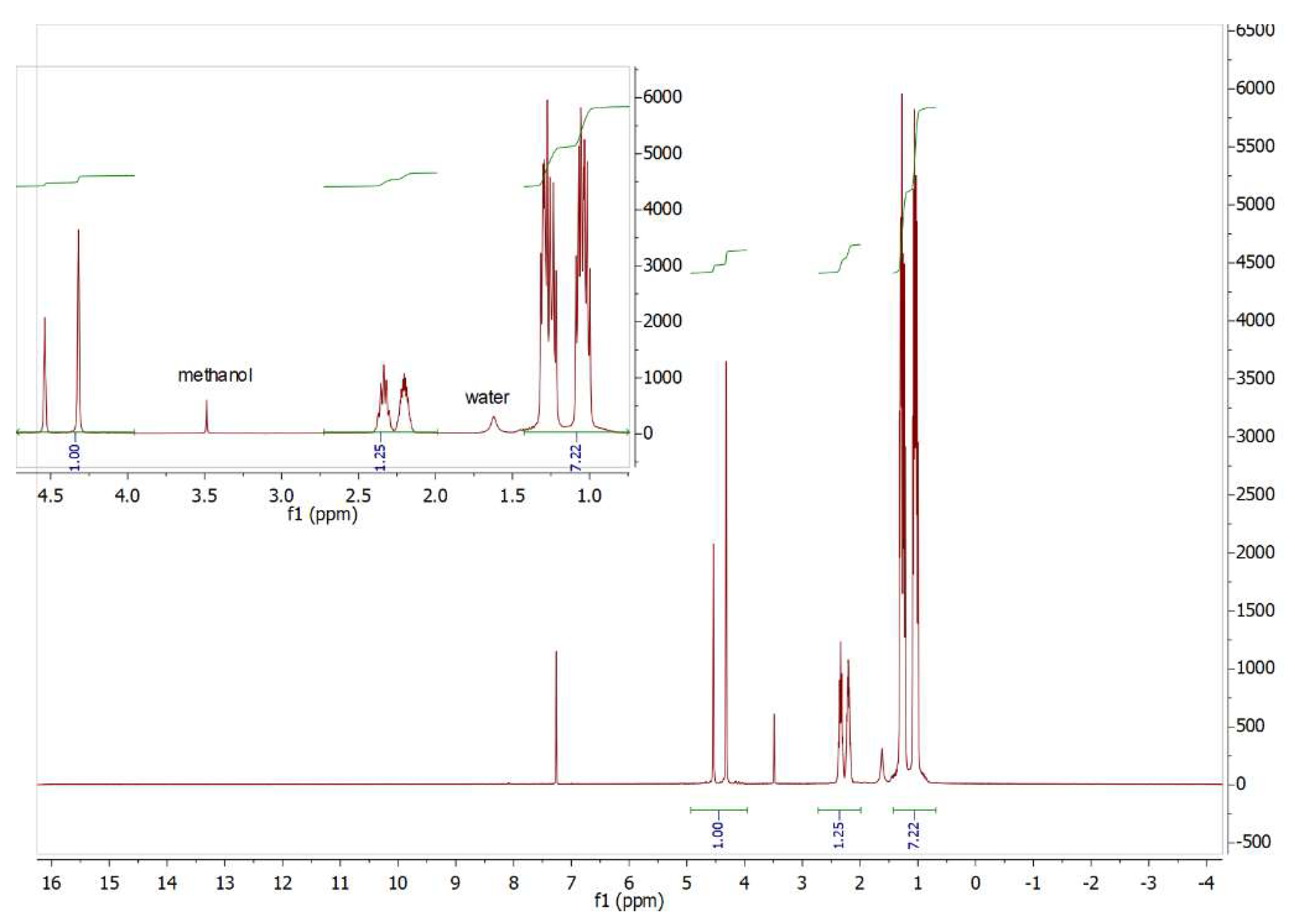
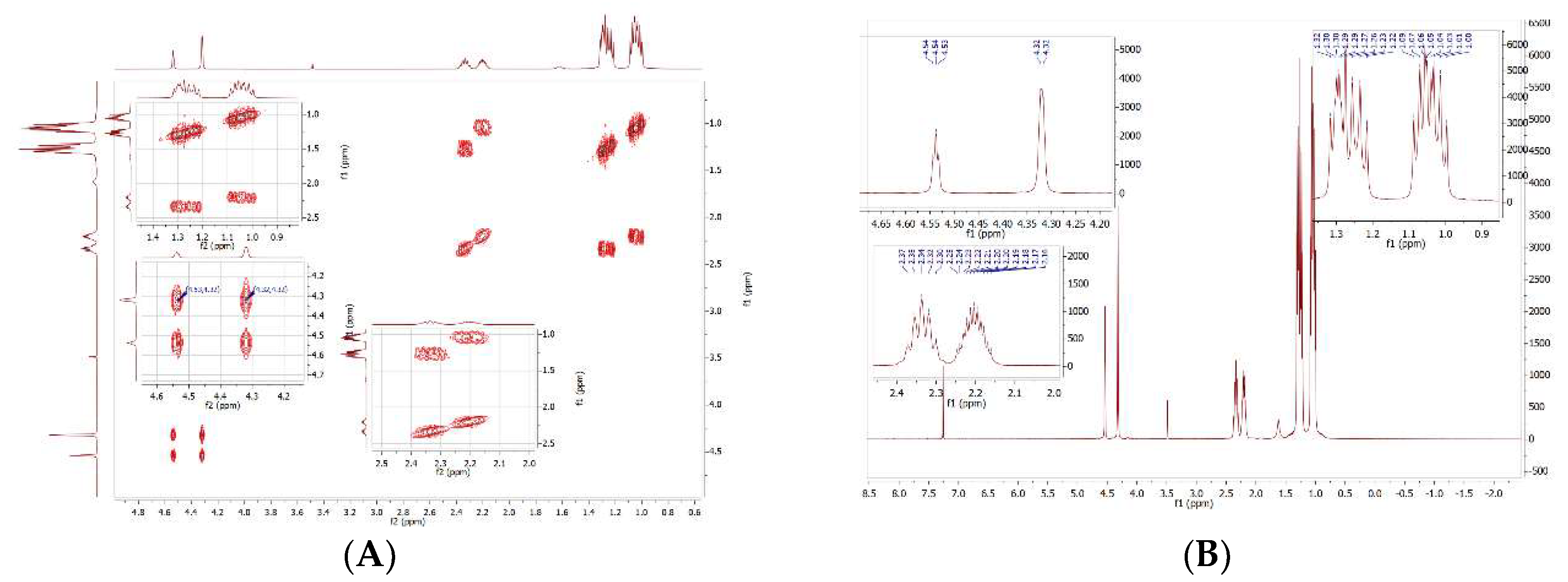
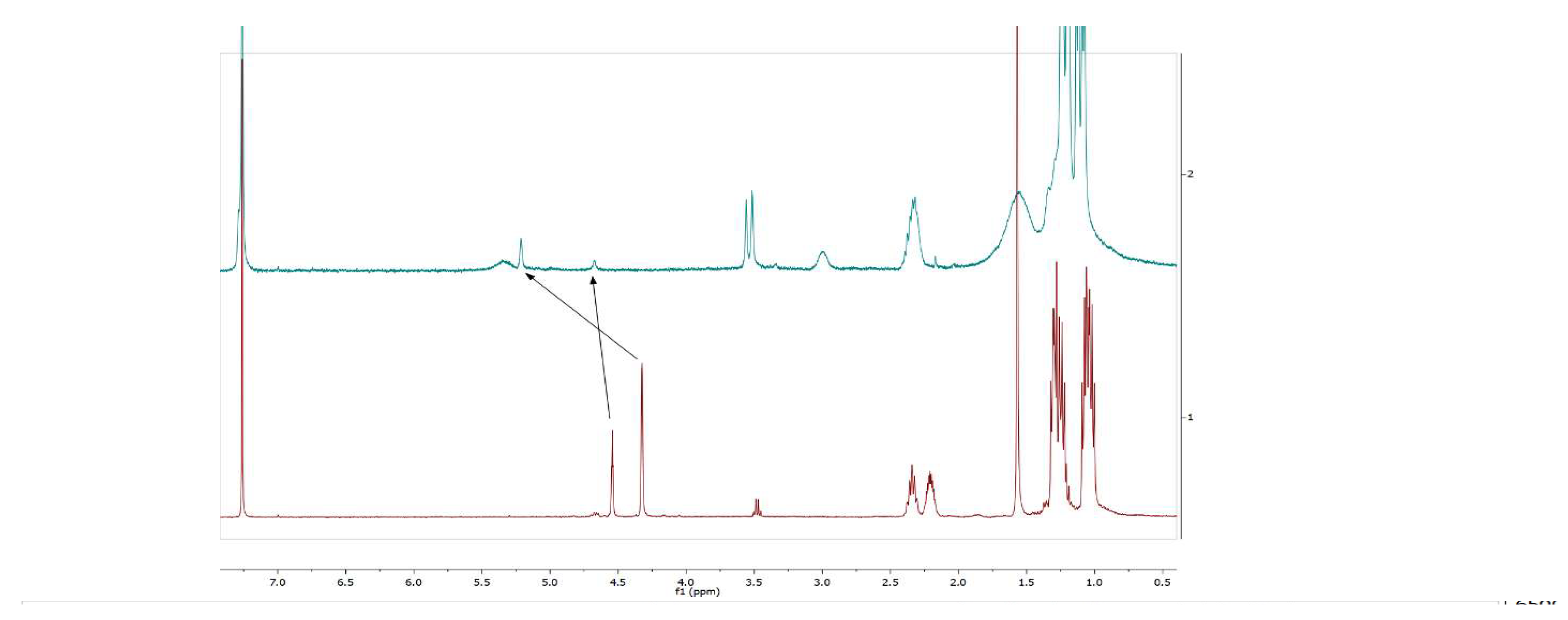
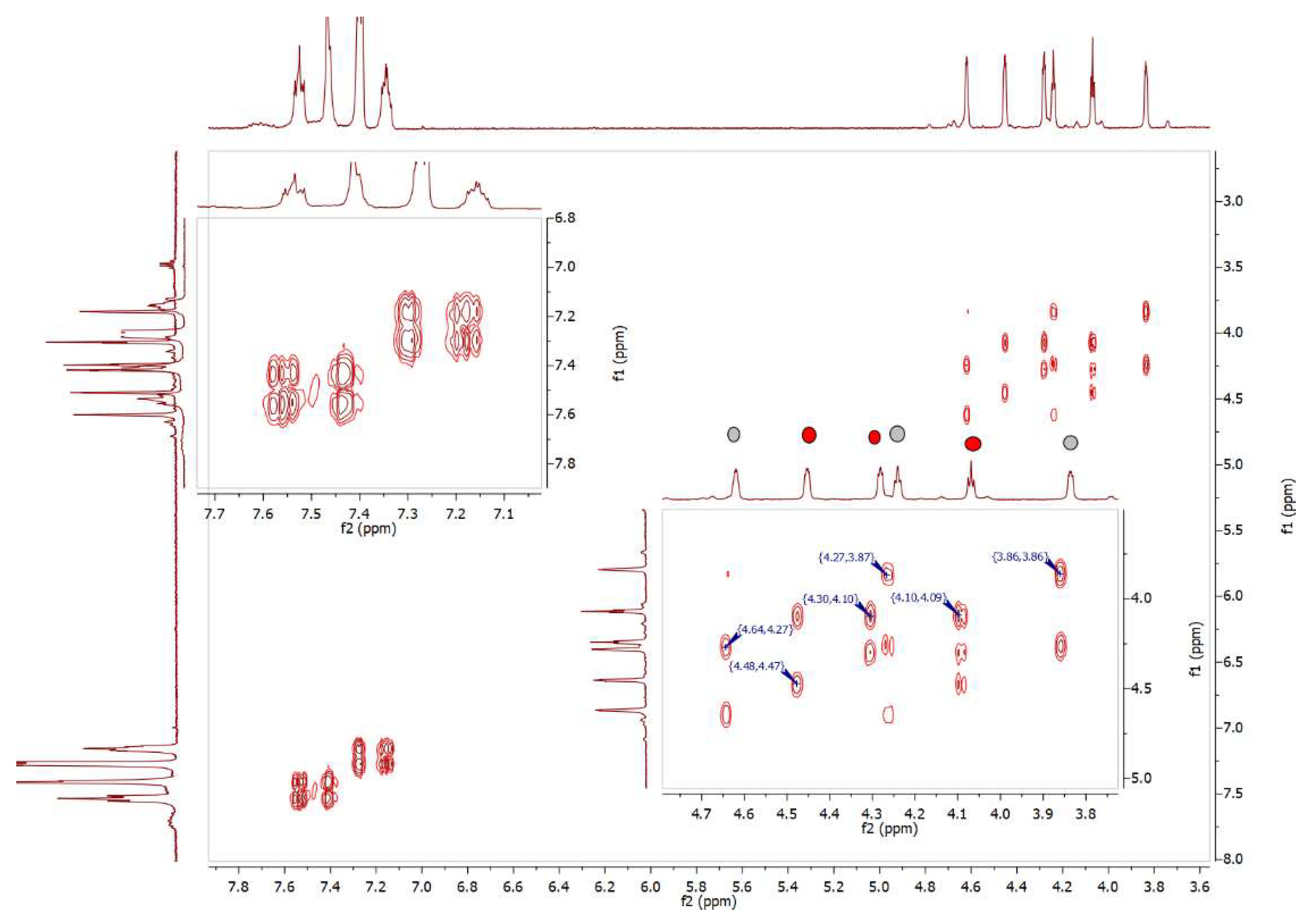
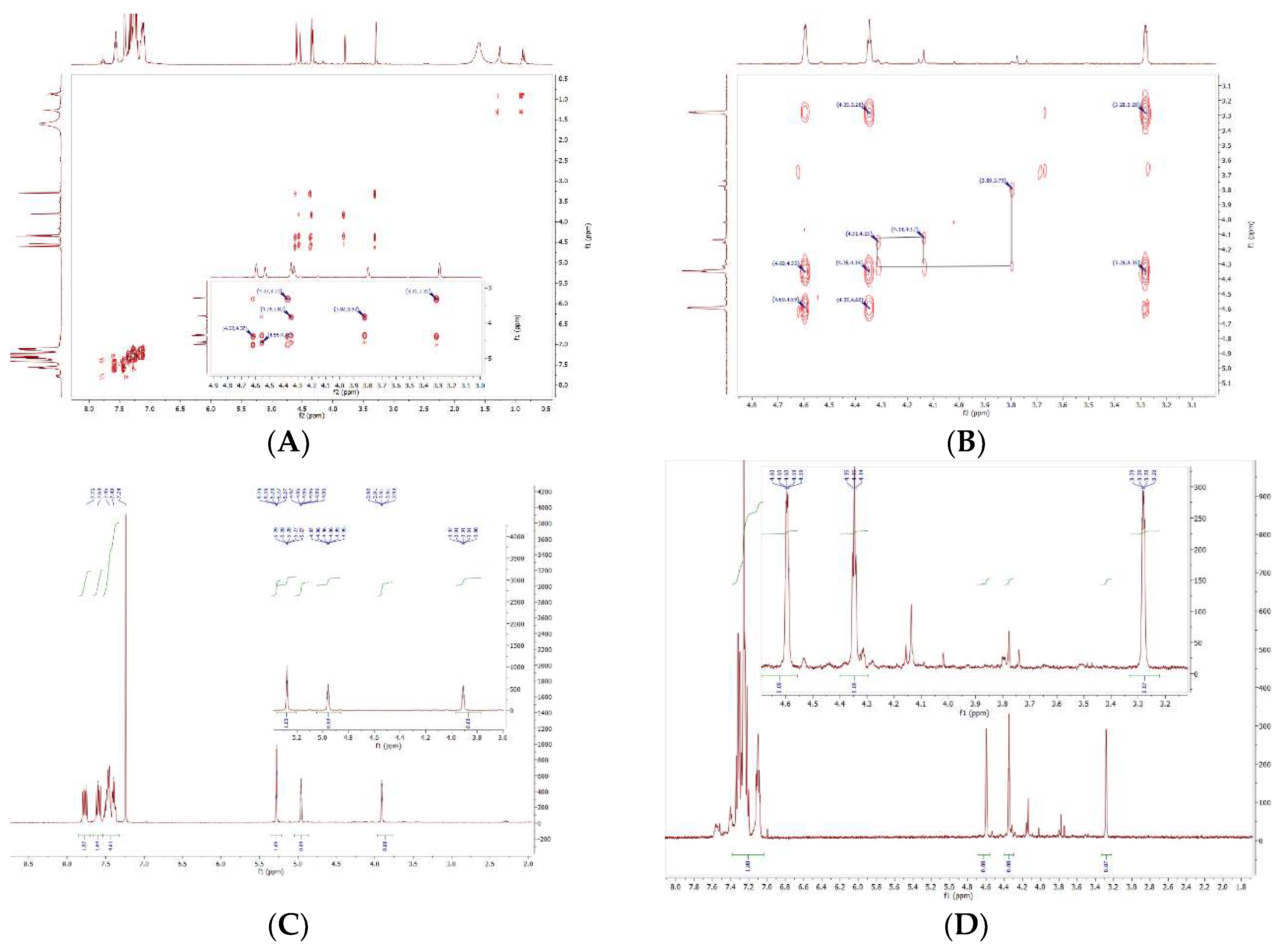
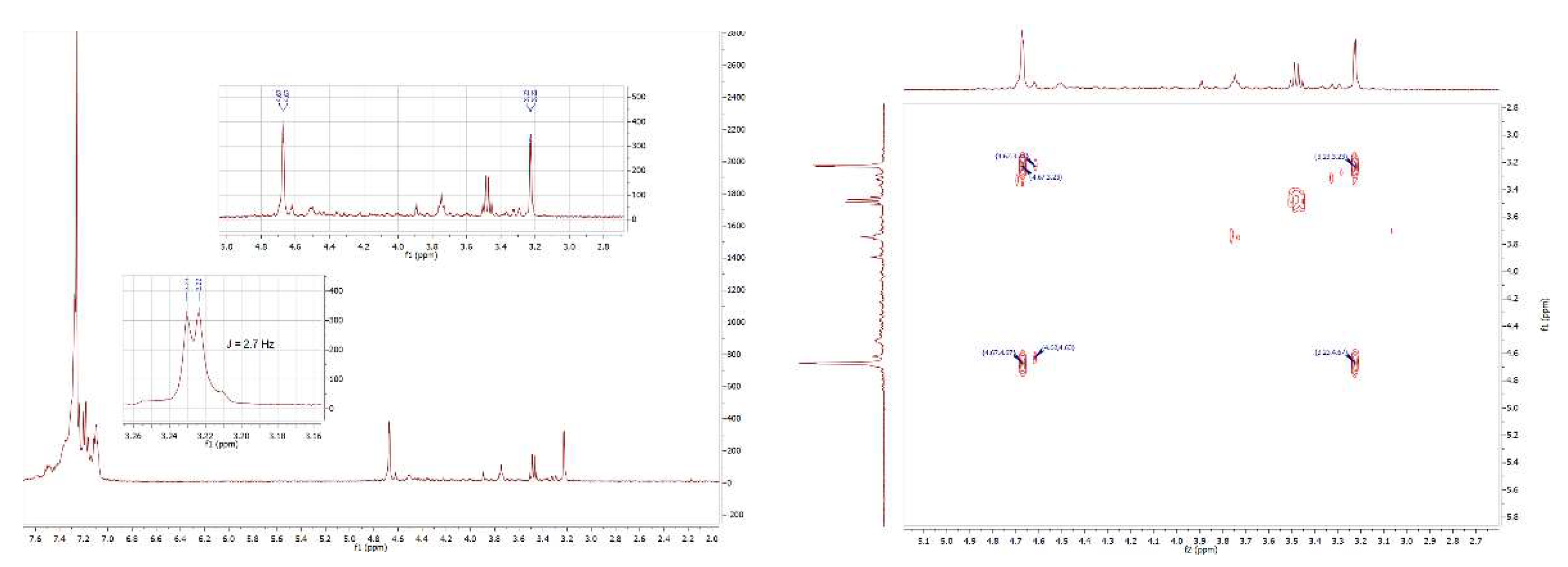
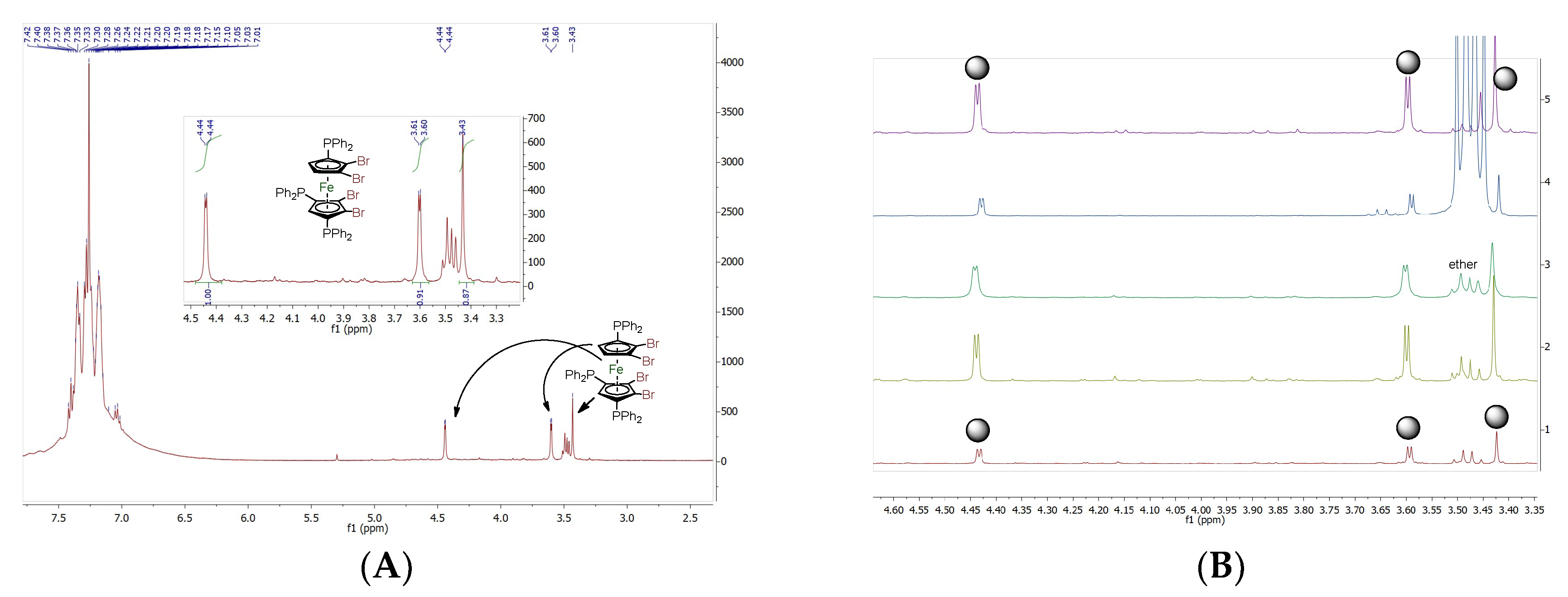
2.1:. In Situ NMR-Based Coordination Studies of 2,2´-Bis-(diphenylphosphino)-1,1’-dibromoferrocene, 17 with Palladium and Platinum.
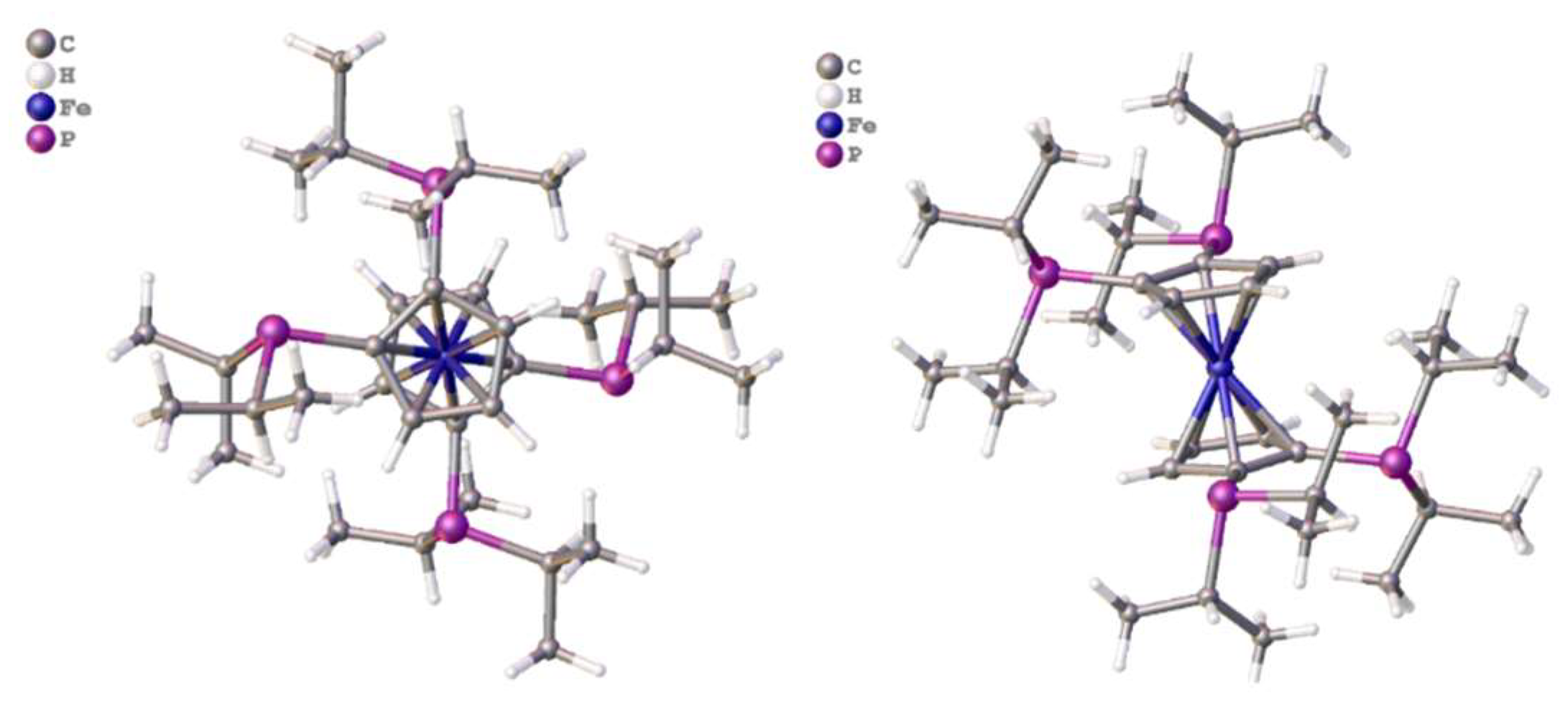
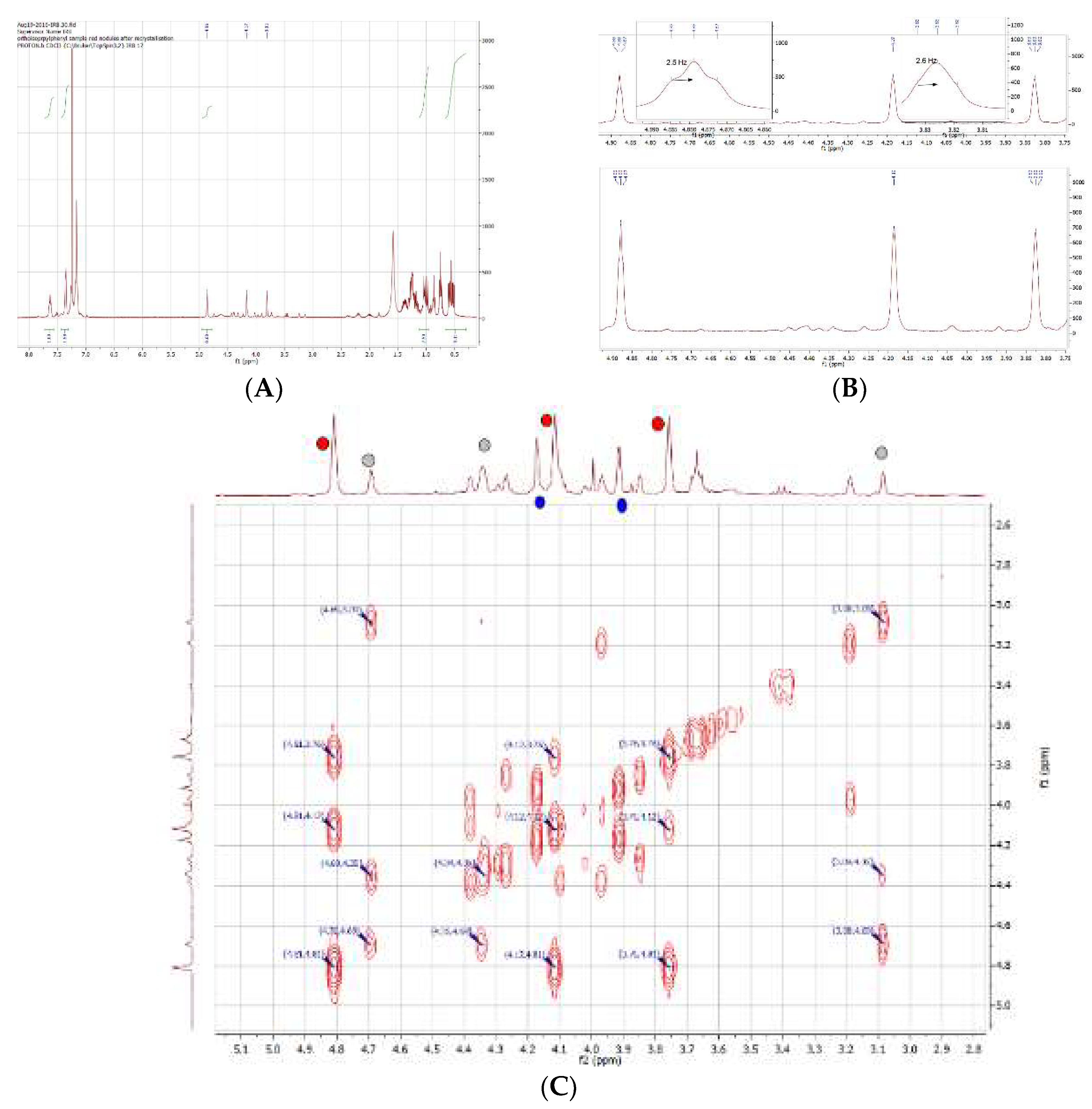
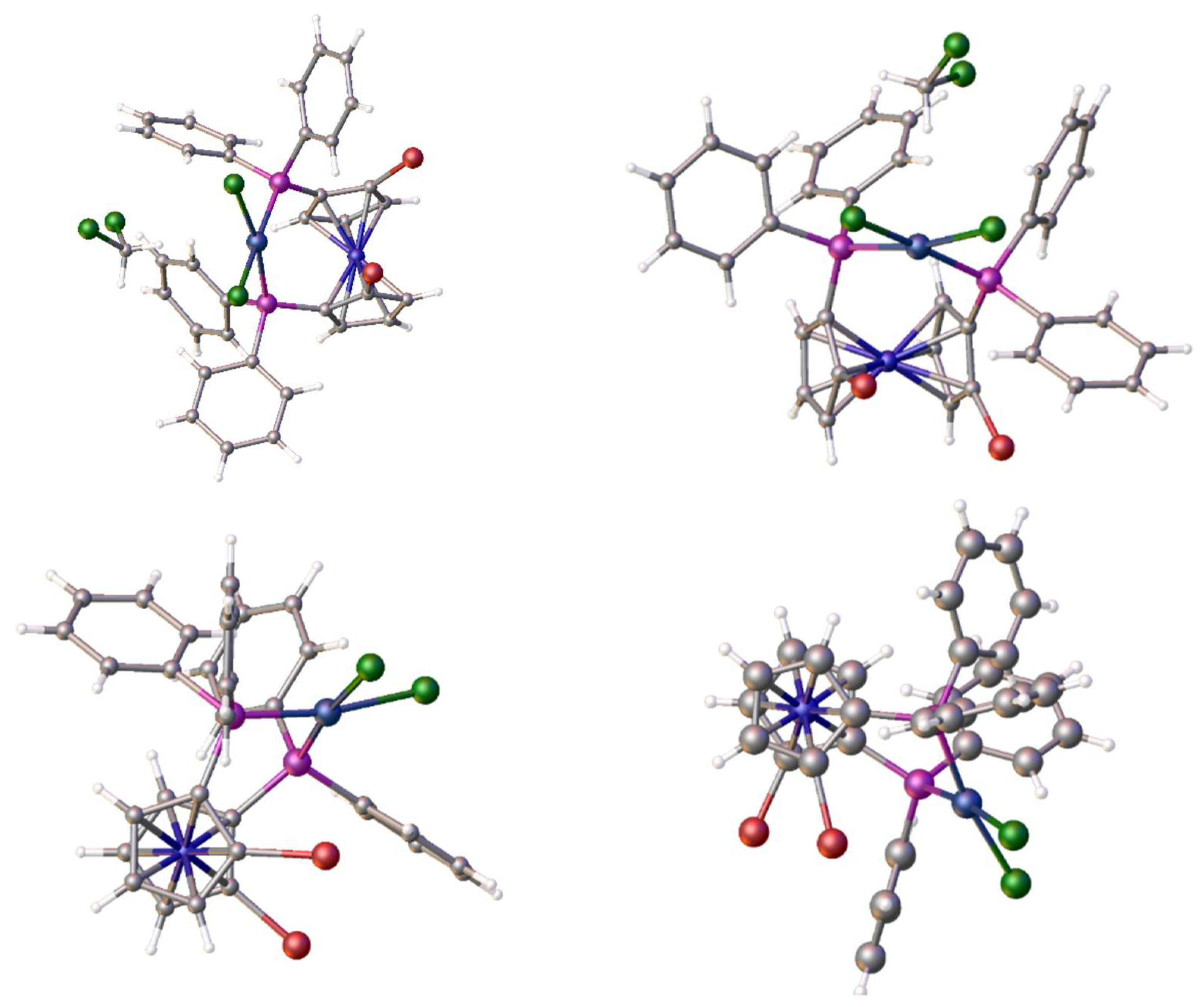
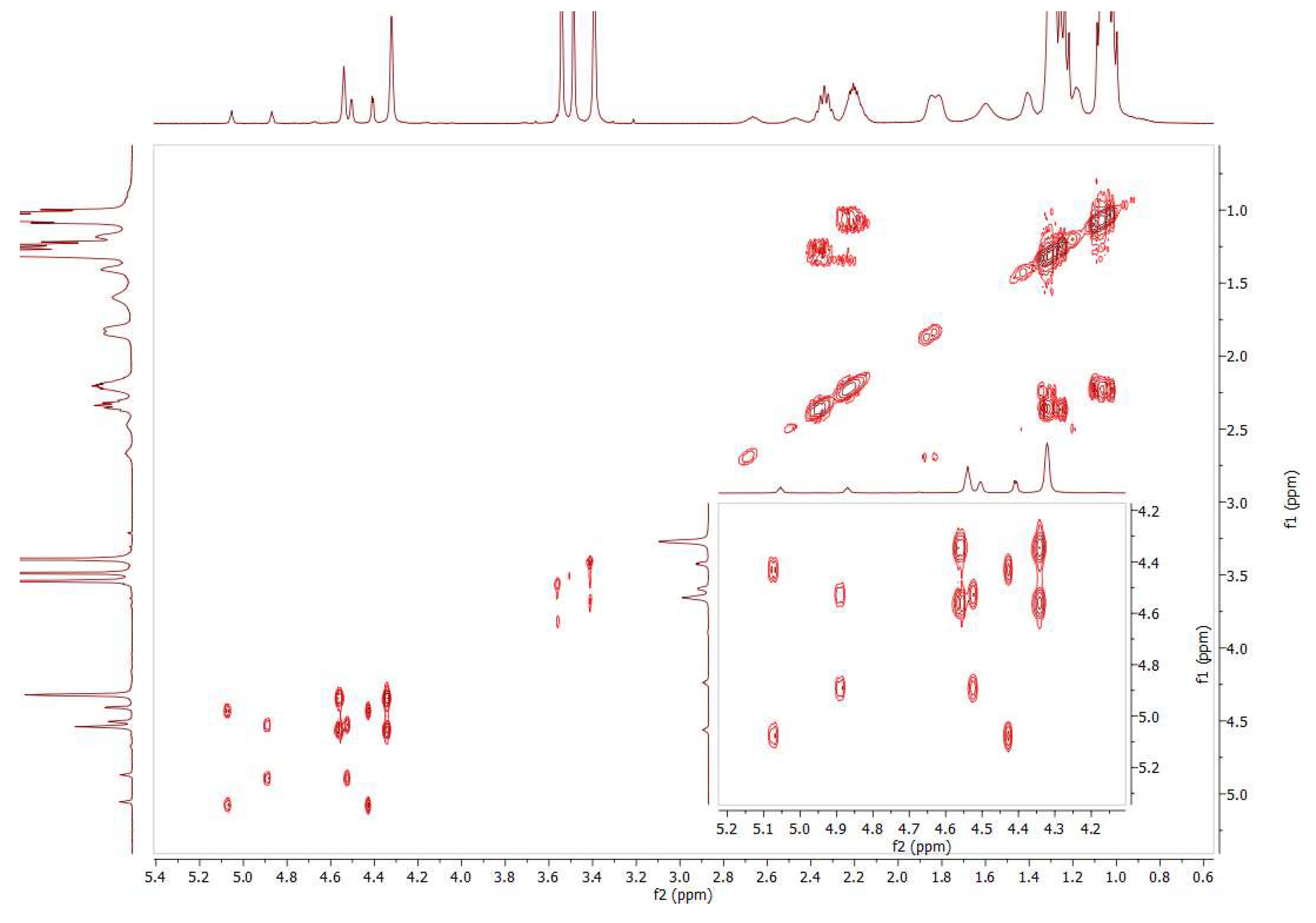
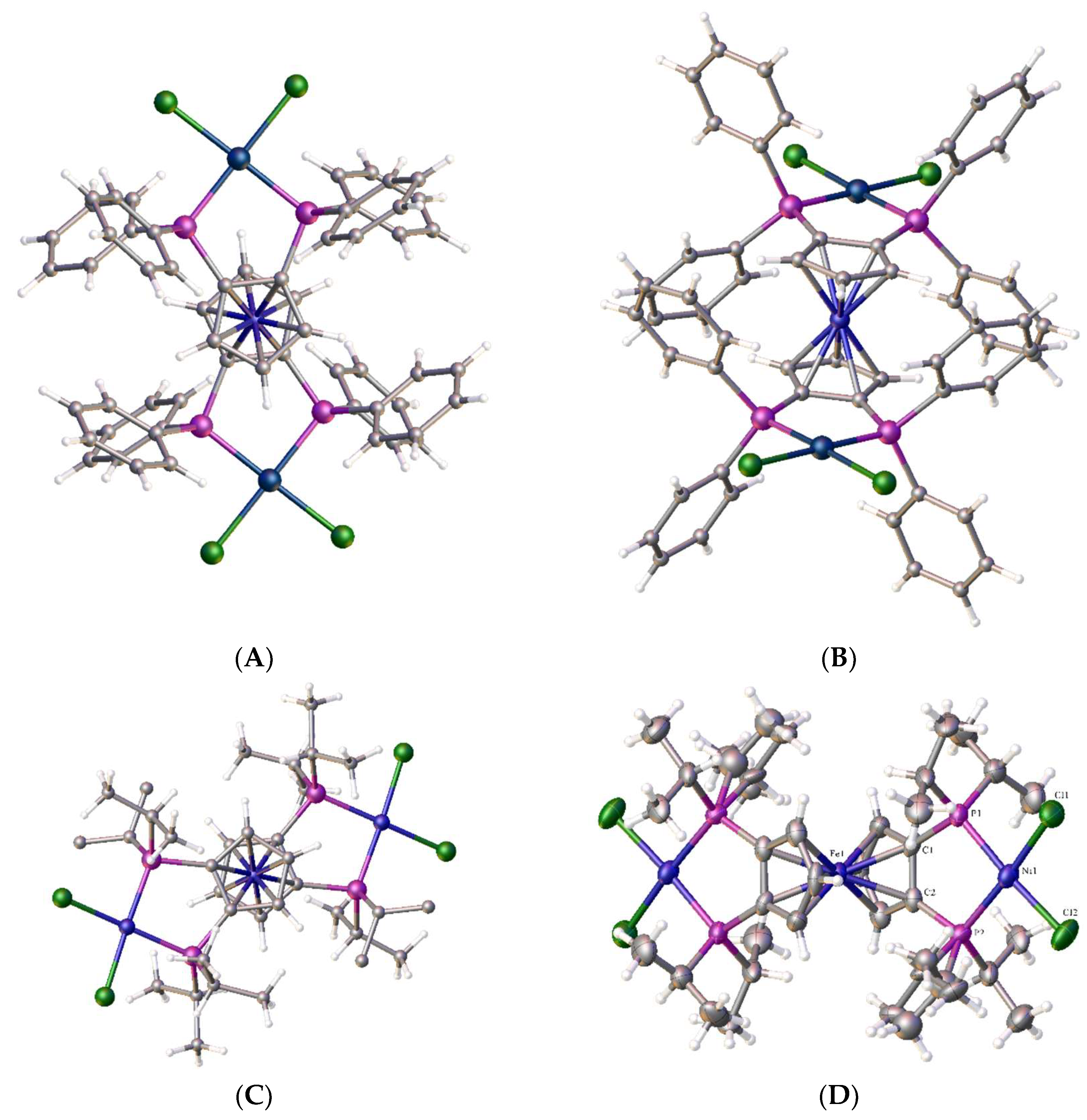
2.11. Coordination of 1,1´,2,2´-Tetrakis-(di-isopropyl-phosphino)ferrocene, (Tdipf), 9.
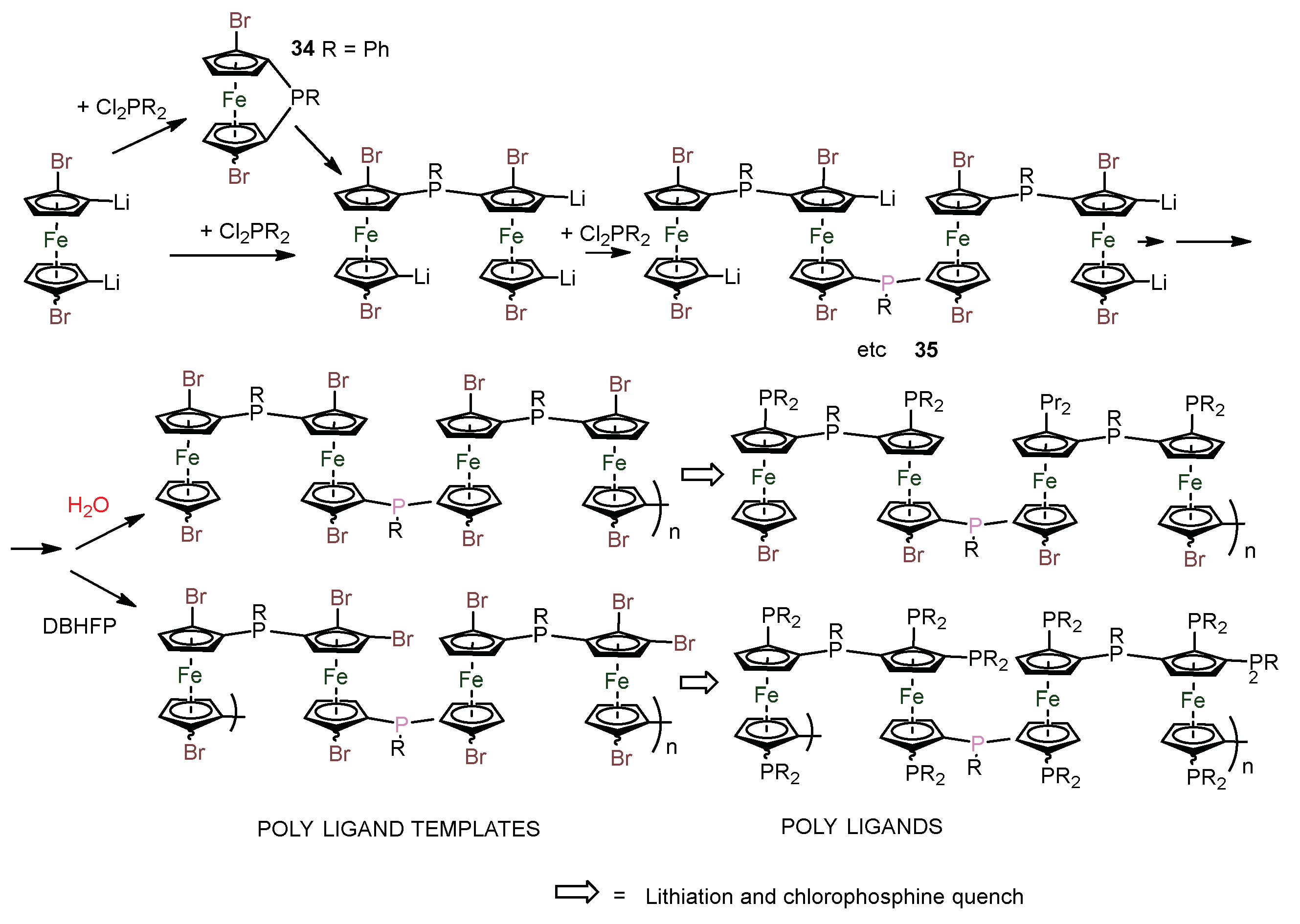
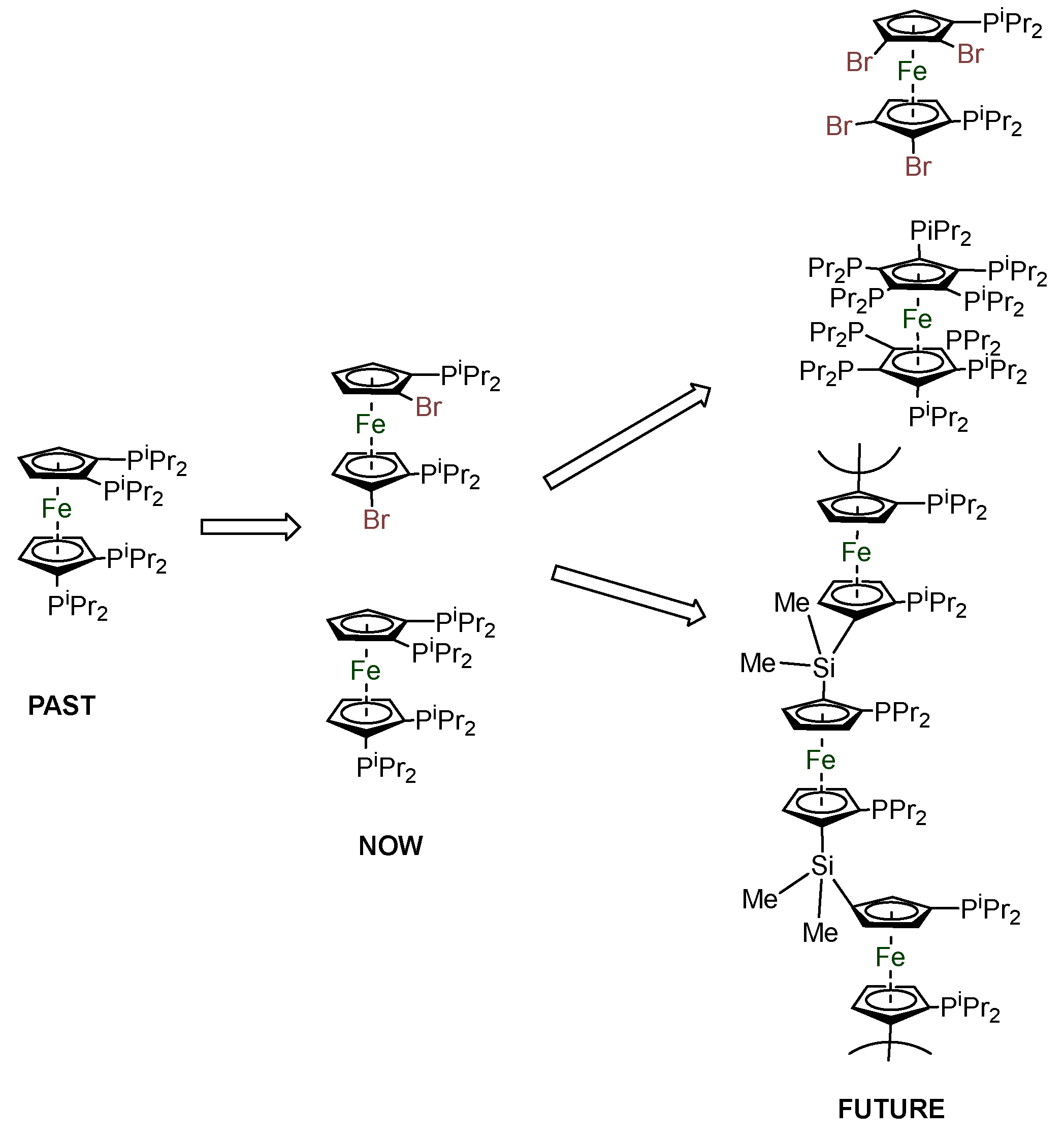
2.3. Extension of Methodology.
3. Summary
4. Materials and Methods
4.1. General Experimental Details.
4.2. Lithiation of 1,1’-Dibromoferrocene. [104]
4.2.1. Preparation of 2,2’-Dilithio-1,1’-dibromoferrocene, General Method.
4.3. Lithiation of 1,1’,2,2’-Tetrabromoferrocene: Preparation of 3,3’-Dilithio-1,1’,2,2’-tetrabromoferrocene. General Method. [104]
4.4. Tetra-Lithiation of 1,1’,2,2’-Tetrabromoferrocene. [108]
4.4.1. A Solution of 1,1’,2,2’-Tetrabromoferrocene (2.5g, 5 mmol) in Diethyl Ether Maintained at -40-50oC Was Treated with a Slight Excess of t-Butyllithium and the Deep Red Solution Was Used After 5-10 Minutes.4.4.2. A Slurry of 1,1’,2,2’-Tetralithioferrocene Was Prepared at Room Temperature by Stirring a Solution of 1,1’,2,2’-Tetrabromoferrocene in Hexane with n-BuLi or t-BuLi. Addition of a Few mL of Diethyl Ether Facilitates the Production of 1,1’,2,2’-Tetralithioferrocene Which Gradually Precipitates. The Slurry Was Used After 1h.
4.5. Quench Methodology
4.5.1. Work-Up: Isopropylphosphine Quench Reagents.
4.5.2. Work-Up: Phenylphosphine Quench Reagents.
4.6. Compound Preparations
4.6.1. Preparation of the 1,1’,2,2’-Tetrakis-(diphenylphosphino)ferrocene, Tdppf, 8. [5g Scale]
4.6.2. Preparation of 1,1’,2,2’-Tetrakis-Diisopropylphosphinoferrocene, 9. Method 1.
4.6.3. Preparation of 1,1’,2,2’-Tetrakis-Diisopropylphosphinoferrocene, 9. Method 2.
4.6.4. Preparation of Compounds 2.2’-Bis-diphenylphosphino-1,1’-dibromoferrocene, 17 and 2,2’-Bis-diisopropylphosphino-1,1’-dibromoferrocene. 18.
4.6.5. Compound 24, 2,2’-Bis-(Di-isopropylphosphino)-bis-1,1’-(diphenylphosphino)ferrocene.
4.9. Preparation of 2’-Diphenylphosphino-1,1’,2-tribromoferrocene, compound 21.
4.10. Preparation of 3,3’-Bis-(diphenylphosphino)-1,1’,2,2’-tetrabromoferrocene, 25, (maj)
5. Overall Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kealy, T. J.; Pauson, P. L. A New Type of Organo-Iron Compound. Nature, 1951, 168, 1039−1040. [CrossRef]
- Miller, S. A.; Tebboth, J. A.; Tremaine, J. F. Dicyclopentadienyliron. J. Chem. Soc. 1952, 114, 632−635. [CrossRef]
- Hayashi, T., Togni, A., Eds. Ferrocenes; VCH: Weinheim, Germany, 1995. [CrossRef]
- Togni, A., Haltermann, R. L., Eds. Metallocenes; VCH: Weinheim, Germany, 1998. [CrossRef]
- Stepnic ̌ ka, P., Ed. ̌ Ferrocenes; Wiley: Chichester, U.K., 2008. [CrossRef]
- Fu, G. C. Enantioselective Nucleophilic Catalysis with “Planar-Chiral” Heterocycles. Acc. Chem. Res., 2000, 33, 412−420. [CrossRef]
- Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Asymmetric Catalysis with Chiral Ferrocene Ligands. Acc. Chem. Res., 2003, 36, 659−667. [CrossRef]
- Blaser, H.-U.; Brieden, W.; Pugin, B.; Spindler, F.; Studer, M.; Togni, A. Solvias Josiphos Ligands: From Discovery to Technical Applications. Top. Catal., 2002, 19, 3−16. [CrossRef]
- Blaser, H.-U.; Pugin, B.; Spindler, F. Progress in Enantioselective Catalysis Assessed from An Industrial Point of View. J. Mol. Catal. A: Chem., 2005, 231, 1−20. [CrossRef]
- Dai, L.-X.; Hou, X.-L., Eds. Chiral Ferrocenes in Asymmetric Catalysis; Wiley: Weinheim, Germany, 2010. [CrossRef]
- Schaarschmidt, D.; Lang, H. Selective Syntheses of Planar-Chiral Ferrocenes., Organometallics, 2013, 32, 5668−5704. Publication Date:September 18, 2013. [CrossRef]
- Dey, S., Buzsáki, D., Bruhn, C., Kelemen, Z., Pietschnig, R. Bulky 1,1′-bisphosphanoferrocenes and their coordination behaviour towards Cu(I), Dalton Trans., 2020, 49, 6668-6681. [CrossRef]
- Dey, S., Pietschnig, R. Chemistry of sterically demanding dppf-analogs. Coordination Chemistry Reviews, 2021, 437, 213850. [CrossRef]
- Vosáhlo, P., Císařová, I., Štěpnička, P. Comparing the asymmetric dppf-type ligands with their semi-homologous counterparts. J. Organometal. Chem., 2018, 860, 14-29. [CrossRef]
- Hartlaub, S.F., Lauricella, N.K., Ryczek, C.N., Furneaux, A.G., Melton, J.D., Piro, N.A., Kassel, W.S., Nataro, C. Late Transition Metal Compounds with 1,1′-Bis(phosphino)ferrocene Ligands, Eur. J. Inorg. Chem., 2017, 424-432. [CrossRef]
- Karakaş, D.E., Durap, F., Aydemir, M., Baysal, A. Synthesis, characterization and first application of chiral C2-symmetric bis(phosphinite)-Pd(II) complexes as catalysts in asymmetric intermolecular Heck reactions, Appl. Organometal. Chem., 2017, 30, 193-198. [CrossRef]
- Siddiqui, M.M., Radhakrishna, L., Mague, J.T., Balakrishna, M.S. 1,1′-Bis(dipyrrolylphosphino)ferrocene: Synthesis, coordination chemistry and structural studies, J. Organometal. Chem., 2018, 824, 15-24. [CrossRef]
- Trivedi, M., Singh, G., Kumar, A., Rath, N.P. 1,1′-Bis(di-tert-butylphosphino)ferrocene copper(i) complex catalyzed C-H activation and carboxylation of terminal alkynes, Dalton Trans.., 2015, 44, 20874-20882. [CrossRef]
- Trivedi, M., Ujjain, S.K., Singh, G., Kumar, G., Dubey, S.K., Rath, N.P. Syntheses, characterization, and electrochemistry of compounds containing 1-diphenylphosphino-1′-(di-tert-butylphosphino)ferrocene (dppdtbpf), J. Organometal. Chem., 2014, 772-773, 202-209. [CrossRef]
- Sabounchei, S.J., Ahmadi, M., Azizi, T., Panahimehr, M. A robust, moisture- and air-stable phosphine mono-ylide palladacycle precatalyst: A simple and highly efficient system for mizoroki-heck reactions, Synlett, 2014, 25, 336-342. [CrossRef]
- Meriҫ, N., Aydemir, M., Işik, U., Ocak, Y.S., Rafikova, K., Paşa, S. Kayan, C., Durap, F., Zazybin, A., Temel, H. Cross-coupling reactions in water using ionic liquid-based palladium(II)-phosphinite complexes as outstanding catalysts, Appl. Organometal. Chem., 2014, 28, 818-825. [CrossRef]
- Rao, S., Mague, J.T., Balakrishna, M.S. Synthesis, transition metal chemistry and catalytic reactions of ferrocenylbis(phosphonite), [FeC5H4P(OC6H 3(OMe-o)(C3H5-p))22], Dalton Trans., 2013, 42, 11695-11708. [CrossRef]
- Butler, I.R. The simple synthesis of ferrocene ligands from a practitioner's perspective, Eur. J. Inorg. Chem., 2012, 4387-4406. [CrossRef]
- Lohan, M., Milde, B., Heider, S., (...), Rüffer, T., Lang, H. Synthesis, electrochemistry, spectroelectrochemistry, and solid-state structures of palladium biferrocenylphosphines and their use in C,C cross-coupling reactions, Organometallics, 2012, 31, 2310-2326. [CrossRef]
- Irandoust, M., Joshaghani, M., Rafiee, E., Pourshahbaz, M. 31P NMR study of the stoichiometry, stability and thermodynamics of complex formation between palladium(II) acetate and bis(diphenylphosphino)ferrocene, Spectrochim. Acta - Part A: Mol. Biomol. Spectroscopy, 2009, 74, 855-859. [CrossRef]
- Pourshahbaz, M., Irandoust, M., Rafiee, E., Joshaghani, M. Kinetics of complex formation between palladium(II) acetate and bis(diphenylphosphino)ferrocene, Polyhedron, 2009, 28, 609-613. [CrossRef]
- Jakob, A., Milde, B., Ecorchard, P., Schreiner, C., Lang, H. Palladium dichloride (ferrocenylethynyl)phosphanes and their use in Pd-catalyzed Heck-Mizoroki- and Suzuki-Miyaura carbon-carbon cross-coupling reactions, J. Organometal. Chem., 2008, 693, 3821-3830. [CrossRef]
- Colacot, T.J., Parisel, S. Synthesis, Coordination Chemistry and Catalytic Use of dppf Analogs (Book Chapter), Ferrocenes: Ligands, Materials and Biomolecules, 2008, 117-140. [CrossRef]
- Butler, I.R. The conversion of 1,1′-dibromoferrocene to 1,2-dibromoferrocene: The ferrocene-chemist's dream reaction, Inorg. Chem. Commun., 2008, 11, 15-19. [CrossRef]
- Performances of symmetrical achiral ferrocenylphosphine ligands in palladium-catalyzed cross-coupling reactions: A review of syntheses, catalytic applications and structural properties. Fihri, A., Meunier, P., Hierso, J.-C., Coord. Chem. Revs, 2007, 251, 2017-2055. [CrossRef]
- Bianchini, C., Oberhauser, W., Orlandini, A., Giannelli, C., Frediani, P. Operando high-pressure NMR and IR study of the hydroformylation of 1-hexene by 1,1′-bis(diarylphosphino)metallocene-modified rhodium(I) catalysts, Organometallics, 2005, 24, 3692-3702. [CrossRef]
- Fihri, A., Hierso, J.-C., Vion, A., Nguyen, D.H., Urrutigoity, M., Kalck, P., Amardeil, R., Meunier, P. Diphosphines of dppf-type incorporating electron-withdrawing furyl moieties substantially improve the palladium-catalysed amination of allyl acetates, Adv. Synth.Cat., 2005, 347, 1198-1202. [CrossRef]
- Sliger, M.D., Broker, G.A., Griffin, S.T., Rogers, R.D., Shaughnessy, K.H. Di-t-butyl(ferrocenylmethyl)phosphine: Air-stability, structural characterization, coordination chemistry, and application to palladium-catalyzed cross-coupling reactions, J. Organometal. Chem., 2005, 690, 1478-1486. [CrossRef]
- Schaarschmidt, D., Lang, H. Selective Syntheses of Planar-Chiral Ferrocenes. Organometallics, 2013, 32, 5668−5704. [CrossRef]
- Gao, D.-W., Gu, Q., Zheng, C., You, S.-L., Enantioselective Synthesis of Planar Chiral Ferrocenes via Au/Pt-Catalyzed Cycloisomerization, Acc. Chem. Res., 2017, 50, 351-365. [CrossRef]
- Fihri, A., Hierso, J.-C., Ivanov, V.V., Rebiere, B., Amardeil, R., Broussier, R., Meunier, P. Enlarging the family of ferrocenylphosphine dinuclear rhodium complexes: Synthesis and X-ray structure of a novel "A-frame"-type trimetallic Rh/Fe/Rh complex, Inorg. Chim. Acta, 2004, 357, 3089-3093. [CrossRef]
- Atkinson, R.C.J., Gibson, V.C., Long, N.J. , The syntheses and catalytic applications of unsymmetrical ferrocene ligands, Chem. Soc. Revs, 2004, 33, 313-328. [CrossRef]
- Ong, J.H.L., Nataro, C., Golen, J.A., Rheingold, A.L. Electrochemistry of Late Transition Metal Complexes Containing the Ligand 1,1′-Bis(diisopropylphosphino)ferrocene (dippf), Organometallics, 2003, 22, 5027-5032. [CrossRef]
- Bianchini, C., Meli, A., Oberhauser, W., Zuideveld, M.A., Freixa, Z., Kamer, P.C.J., Spek, A.L., Gusev, O.V., Kal'sin, A.M. Methoxycarbonylation of ethene by palladium(II) complexes with 1,1′-bis(diphenylphosphino)ferrocene (dppf) and 1,1′-bis(diphenylphosphino)octamethylferrocene (dppomf), Organometallics, 2003, 22, 2409-2421. [CrossRef]
- Butler, I.R., Coles, S.J., Hursthouse, M.B., Roberts, D.J., Fujimoto, N. Ferrocene-based ligands in ruthenium alkylidene chemistry, Inorg. Chem. Commun., 2003, 6, 760-762. [CrossRef]
- Sollot, G.P.; Snead, J.P.; Portnoy, S.; Peterson, W.R.; Mertoy, H.E.; Chem. Abstr., 1965, 63, 18147b.
- Bishop, J.J.; Davison, A.; Katcher, M.L.; Lichtenberg, D.W.; Merrill, R.E.; Smart, J.C. J. Organomet. Chem., 1971, 27, 241.
- Rudie, A.W.; Lichtenberg, D.W.; Katcher, M.L.; Davidson, A. Inorg. Chem., 1978, 17, 28592863. [CrossRef]
- Davison, A.; Bishop, J., Symmetrically disubstituted ferrocenes. II. Complexes of ferrocene-1,1'-bis(dimethylarsine) and ferrocene-1,1'-bis(diphenylarsine) with Group VI carbonyls, Inorg. Chem ., 1971, 10, 826-831. [CrossRef]
- Davison, A.; Bishop, J.J. Inorg. Chem ., Symmetrically disubstituted ferrocenes. III. Complexes of ferrocene-1,1'-bis(dimethylarsine) and ferrocene-1,1'-bis(diphenylarsine) with the Group VIII metals, Inorg. Chem. 1971, 10, 832-837. [CrossRef]
- Butler, I.R., Cullen, W.R., Kim, T.-J. Synthesis of Some Isopropylphosphinoferrocenes, Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 1985, 15:1, 109-116. [CrossRef]
- Cullen, W.R.; Kim, T.J.; Einstein, Jones T. Structures of three hydrogenation catalysts [(P-P)Rh(NBD)]ClO4 and some comparative rate studies where (P-P) = (.eta.5-R1R2PC5H4)(.eta.5-R3R4PC5H4)Fe (R1 = R2 = R3 = R4 = Ph; R1 = R2 = Ph, R3 = R4 = CMe3; R1 = R3 = Ph, R2 = R4 = CMe3), Organometallics, 1985, 4, 346-351. [CrossRef]
- Butler, I.R.; Cullen, W.R.; Kim, T.-J.; Retting, S.J.; Trotter, J. 1,1'-Bis(alkylarylphosphino)ferrocenes: Synthesis, metal complex formation, and crystal structure of three metal complexes of Fe(ƞ5-C5H4PPh2)2, Organometallics, 1985, 4, 972-980. [CrossRef]
- Gokel, G.W. and Ugi, I.K. Preparation and resolution of N,N-dimethyl-o-ferrocenylethylamine. An advanced organic experiment., J. Chem. Ed. 1972, 49, 294. [CrossRef]
- Štěpnička, P. Forever young: The first seventy years of ferrocene, Dalton Trans., 2022, 51, 8085-8102. [CrossRef]
- Marquarding, D., Klusacek, H., Gokel, G., Hoffmann, P,. Ugi, I. Stereoselective syntheses. VI. Correlation of central and planar chirality in ferrocene derivatives, J. Am. Chem. Soc., 1970, 92, 5389-5393. [CrossRef]
- Battelle, L.F., Bau, R., Gokel, G.W., Oyakawa, R.T., Ugi, I. Absolute Configuration of a 1,2-Disubstituted Ferrocene Derivative with Two Different Chiral Substituents, Angew, Chem., 1972, 11, 138-140. [CrossRef]
- Hayashi, T., Konishi, M., Fukushima, M., Mise, T., Kagotani, T., M. Kumada, M. Asymmetric synthesis catalyzed by chiral ferrocenylphosphine-transition metal complexes. 2. Nickel- and palladium-catalyzed asymmetric Grignard cross-coupling, J. Am. Chem. Soc ., 1982, 104, 4962. [CrossRef]
- Hayashi, T. Chiral monodentate phosphine ligand MOP for transition-metal-catalyzed asymmetric reactions, Acc. Chem. Res., 2000, 33, 354-362. [CrossRef]
- Hayashi, T., Konishi, M., Kobori,Y., Kumada, M., Higuchi, T., Hirotsu, K. Dichloro[1,1'-bis(diphenylphosphino)ferrocene]palladium(II): An effective catalyst for cross-coupling of secondary and primary alkyl Grignard and alkylzinc reagents with organic halides. J. Am. Chem. Soc., 1984 106, 158-163. [CrossRef]
- Ogasawara, M., Yoshida, K., Hayashi, T., 2,2‘-Bis(diphenylphosphino)-1,1‘-biphenyl: New Entry of Bidentate Triarylphosphine Ligand to Transition Metal Catalysts, Organometallics, 2000, 19, 1567-71. [CrossRef]
- Hayashi, T., Konishi, M., Ito, H., Kumada, M., Optically active allylsilanes. 1. Preparation by palladium-catalyzed asymmetric Grignard cross-coupling and anti stereochemistry in electrophilic substitution reactions. J. Am. Chem. Soc., 1982, 104, 4962-3. [CrossRef]
- Tamio Hayashi, Mitsuo Konishi, Motoo Fukushima, Takaya Mise, Masahiro Kagotani, Masatoyo Tajika, and Makoto Kumada, Asymmetric synthesis catalyzed by chiral ferrocenylphosphine-transition metal complexes. 2. Nickel- and palladium-catalyzed asymmetric Grignard cross-coupling. J. Am. Chem. Soc. 1982, 104, 180-186. [CrossRef]
- Yoshihisa, K., Keiji Yamamoto, Kohei, T., Kumada, M., Asymmetric homogeneous hydrosilylation with chiral phosphine-palladium complexes, J. Amer. Chem. Soc. 1972, 94, 4373-4374. [CrossRef]
- Cullen, W.R., Einstein, F.W.B., Huang, C-H., Willis, A. C., Yeh, E.S., Asymmetric hydrogenation catalyzed by cationic ferrocenylphosphine rhodium(I) complexes and the crystal structure of a catalyst precursor, J. Am. Chem. Soc., 1980,102 , 988-993. [CrossRef]
- Hampton, C., Cullen, W.R., James, B.R., Charland, J.P. The preparation and structure of a dinuclear .eta.2-H2 complex (P-N)(.eta.2-H2)Ru(.mu.-Cl)2(.mu.-H)Ru(H)(PPh3)2, P-N = Fe[.eta.-C5H3(CHMeNMe2){P(iso-Pr)2}-1,2](.eta.-C5H5), J. Am. Chem. Soc., 1988, 110, 6918-6919. [CrossRef]
- Togni, A., Breutel, C., Schnyder, A., Spindler, F., Landert, H., Tijani. A., A Novel Easily Accessible Chiral Ferrocenyldiphosphine for Highly Enantioselective Hydrogenation, Allylic Alkylation, and Hydroboration Reactions, J. Am. Chem. Soc. 1994, 116, 4062. [CrossRef]
- A. Togni. Angew. Chem. Int. Ed. Eng. 1996, 35, 1475; Planar-Chiral Ferrocenes: Synthetic Methods and Applications. [CrossRef]
- H. C. L. Abbenhuis, A. Togni, B. Müller, A. Albinati, U. Burckhardt, V. Gramlich, A New Stereoselective Approach to Chiral Ferrocenyl Ligands for Asymmetric Catalysis, Organometallics, 1994, 13, 4481-4493. [CrossRef]
- A. Togni, F. Spindler, G. Rihs, N. Zanetti, M. C. Soares, T. Gerfin, V. Gramlich, Synthesis and structure of new chiral ferrocenylphosphines for asymmetric catalysis, Inorg. Chim. Acta, 1994, 222, 213-224. [CrossRef]
- Togni, Planar-Chiral Ferrocenes: Synthetic Methods and Applications, Angew. Chem. Int. Ed. 1996, 35, 1475-1477. [CrossRef]
- Togni, Developing New Chiral Ferrocenyl Ligands for Asymmetric Catalysis: A Personal Account, Chimia, 1996, 50, 86-93. [CrossRef]
- Blaser, H.U., Brieden, W., Pugin, B., Spindler, F., Studer, M, Togni A., Solvias Josiphos Ligands: From Discovery to Technical Applications. Topics in Catalysis 19, 3–16 (2002). [CrossRef]
- Bianchini, C., Meli, A., Oberhauser, Parisel, S., Gusev, O.V., Kal'sin, A.M., W., Vologdin, N.V., Dolgushin, F.M. Methoxycarbonylation of styrene to methyl arylpropanoates catalyzed by palladium(II) precursors with 1,1′-bis(diphenylphosphino)metallocenes, J. Mol. Catal. A: Chem., 2004, 224, 35-49. [CrossRef]
- Gusev, O.V.; Kalsin, A.M.; Petrovskii, P.V.; Lyssenko, K.A.; Oprunenko, Y.F.; Bianchini, C.; Meli, A.; Oberhauser, W. Synthesis, Characterization, and Reactivity of 1,1‘-Bis(diphenylphosphino)osmocene: Palladium(II) Complexes and Their Use as Catalysts in the Methoxycarbonylation of Olefins. Organometallics, 2003, 22, 5, 913–915. [CrossRef]
- Gusev, O.V.; Kal’sin, A.M.; Peterleitner, M.G.; Petrovskii, P.V.; K.A. Lyssenko, K.A.; Akhmedv, N.G.; Bianchini, C.; Meli, A. Palladium(II) Complexes with 1,1‘−Bis(diphenylphosphino)ferrocenes [Fe(η5-C5R4PPh2)2]n+ (dppf, R = H, n = 0; dppomf, R = Me, n = 0; dppomf+, R = Me, n = 1). Synthesis, Characterization, and Catalytic Activity in Ethene Methoxycarbonylation. Organometallics, 2002, 21, 3637-3649. [CrossRef]
- Bianchini, C.; Meli, A.; Oberhauser, W.; Parisel, S.; Passaglia, E.; Ciardelli, F.; Gusev, O.V.; Kal’sin, A.M.; Vologdin, N.V. Ethylene Carbonylation in Methanol and in Aqueous Media by Palladium(II) Catalysts Modified with 1,1'-Bis(dialkylphosphino)ferrocenes. Organometallics 2005, 24, 1018–1030. [Google Scholar] [CrossRef]
- Kalsin, A.M.; Vologdin, N.V.; Peganova, T.A.; Petrovskii, P.V.; Lyssenko, K.A.; Dolgushin, F.M.; Gusev, O.V. Palladium(II) complexes with o-aryl substituted 1,1'-bis(phosphino)ferrocenes [Fe(η5-C5H4PR2)2Pd(NCMe)n](OTf)2 (R = o-MeOC6H4, o-MeC6H4, o-PriC6H4, C6F5): Synthesis, structure and catalytic properties in methoxycarbonylation of ethylene. J. Organomet. Chem. 2006, 691, 921–927. [Google Scholar] [CrossRef]
- Broussier, R., Ninoreille, S., Bourdon, C., Blacque, O., Ninoreille, C., Kubicki, M.M., Gautheron, B., Ferrocenic polyphosphines and polythioethers: Synthesis, reactivity and structure, J. Organometal. Chem., 1998, 561, 85-96. [CrossRef]
- Bentabet, E.A., Broussier, R., Amardeil, R., Jean-Cyrille Hierso, J.-C., Richard, P., Fasseur, D., Gautheron, B., Meunier, P., Different coordination modes of a 1,1′,2,2′-ferrocenyltetraphosphine: Bi- and tri-dentate behaviour with group 6 and 7 transition metals, J. Chem. Soc., Dalton Trans., 2002, 2322-2327. [CrossRef]
- Broussier, R., Bentabet, E., Amardeil, R., Richard, P., Meunier, P., Kalck, P., Gautheron, B., 1,1′,2,2′-Tetrakis(diphenylphosphino)-4,4′-di-tert-butylferrocene, a new cisoid arrangement of phosphino groups, J. Organometal. Chem., 2001, 637–639, 126-133. [CrossRef]
- Broussier, R., Bentabet, E., Mellet, P. Blacque, O., Boyer, P., Kubicki, M.M., Gautheron, B. New 1,1′- or 1,2- or 1,3-bis(diphenylphosphino)ferrocenes, J. Organometal. Chem., 2000, 598, 365-373. [CrossRef]
- Hierso, J.-C.; Fihri, A.; Amardeil, R.; Meunier, P.; Doucet, H.; Santelli, M.; Ivanov V. V. Catalytic Efficiency of a New Tridentate Ferrocenyl Phosphine Auxiliary: Sonogashira Cross-Coupling Reactions of Alkynes with Aryl Bromides and Chlorides at Low Catalyst Loadings of 10-1 to 10-4 Mol %., Org. Lett. 2004, 6, 3473-3476. [CrossRef]
- Ivanov, V. V.; Hierso, J.-C.; Amardeil, R.; Meunier, P. A New Tetratertiary Phosphine Ligand and Its Use in Pd-Catalyzed Allylic Substitution, Organometallics, 2006, 25, 989. 2. [CrossRef]
- Hierso, J.-C.; Fihri, A.; Ivanov, V. V.; Hanquet, B.; Pirio, N.; Donnadieu, B.; Rebière, B.; Amardeil, R.; Meunier, P. A Palladium−Ferrocenyl Tetraphosphine System as Catalyst for Suzuki Cross-Coupling and Heck Vinylation of Aryl Halides: Dynamic Behavior of the Palladium/Phosphine Species. J. Am. Chem. Soc. 2004, 126, 11077. [CrossRef]
- Mom, S.; Beaupérin, M.; Roy, D.; Royer, S.; Amardeil, R.; Cattey, H.; Doucet, H.; Hierso, J.-C. 31P NMR study of the stoichiometry, stability and thermodynamics of complex formation between palladium(II) acetate and bis(diphenylphosphino)ferrocene. Inorg. Chem., 2011, 50, 1159. [CrossRef]
- Hierso J.C, Smalliy R, Amardeil R, Meunier P. New concepts in multidentate ligand chemistry: Effects of multidentarity on catalytic and spectroscopic properties of ferrocenyl polyphosphines. Chemical Society Reviews. 2007, 36,1754-1769. [CrossRef] [PubMed]
- Roy, D.; Mom, S.; Beaupérin, M.; Doucet, H.; Hierso, J.-C. Palladium-Based Catalytic Systems for the Synthesis of Conjugated Enynes by Sonogashira Reactions and Related Alkynylations. Angew. Chem. Int. Ed. 2010, 49, 6650–6671. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Mom, S.; Lucas, D.; Cattey, H.; Hierso, J.-C.; Doucet, H. Direct Arylation of Heteroaromatic Compounds with Congested, Functionalised Aryl Bromides at Low Palladium/Triphosphane Catalyst Loading. Chem.−Eur. J. 2011, 17, 6453-61. [CrossRef]
- Platon, M., Cui, L., Mom, S., Richard, P., Saeys, M., & Hierso, J.-C. (2011). Etherification of functionalized phenols with chloroheteroarenes at low palladium loading: Theoretical assessment of the role of triphosphane ligands in C?O reductive elimination. ADVANCED SYNTHESIS & CATALYSIS, 353(18), 3403–3414. [CrossRef]
- Evrard, D.; Lucas, D.; Mugnier, Y.; Meunier, P.; Hierso, J.-C. Organometallics On the Mechanistic Behavior of Highly Efficient Palladium−Tetraphosphine Catalytic Systems for Cross-Coupling Reactions: First Spectroscopic and Electrochemical Studies of Oxidative Addition on Pd(0)/Multidentate Ferrocenylpolyphosphine Complexes. Organometallics, 2008, 27, 2643-2653. [CrossRef]
- Thomas, D.A., Ivanov, V.V., Butler, I.R., Horton, P.N., Meunier, P., Hierso, J.-C., Coordination chemistry of tetra- and tridentate ferrocenyl polyphosphines: An unprecedented [1,1'-heteroannular and 2,3-homoannular]-phosphorus-bonding framework in a metallocene dinuclear coordination complex. Inorganic Chemistry. 2008, 47, 1607-1615. [CrossRef]
- Butler, I.R.; Beaumont, M.; Bruce, M.I.; Zaittseva, N.N.; Iggo, J.A.; Robertson, C.; Horton, P.N.; Coles, S.J. Synthesis and Structures of 1,1′,2-Tribromoferrocene, 1,1′,2,2′-Tetrabromoferrocene, 1,1′,2,2′-Tetrabromoruthenocene: Expanding the Range of Precursors for the Metallocene Chemist’s Toolkit. Aust. J. Chem. 2021, 74, 204–210. [Google Scholar] [CrossRef]
- Butler, I.R. 1,2,3,4,5-Pentabromoferrocene and related compounds: A simple synthesis of useful precursors, Inorg, Chem. Commun, 2008,11, 484-486. [CrossRef]
- Butler, I.R.; Drew, M.G.B.; Greenwell, C.H.; Lewis, E.; Plath, M.; Mussig, S.; Szewczyk, J. 1,3-Bisdiphenylphosphinoferrocenes: The unexpected 2,5-dilithiation of dibromoferrocene towards a new area of ferrocene-ligand chemistry. Inorg. Chem. Commun. 1999, 2, 576–580. [Google Scholar] [CrossRef]
- Rupf, S.M., Schröder, G., Sievers, R., Malischewski, M. Tenfold Metalation of Ferrocene: Synthesis, Structures, and Metallophilic Interactions in FeC10(HgX)10, Chem. Eur. J., 2021, 27, 5125-5129. [CrossRef]
- Sünkel, K.; Bernhartzeder, S. Coordination chemistry of perhalogenated cyclopentadienes and alkynes. Part 30. New high-yield syntheses of monochloroferrocene and 1,2,3,4,5-pentachloroferrocene. Molecular structures of 1,2-dichloroferrocene and 1,2,3-trichloroferrocene. J. Organometal Chem., 2012, 716, 146-149. [CrossRef]
- Sünkel, K.; Bernhartzeder, S. Coordination chemistry of perhalogenated cyclopentadienes and alkynes. XXVIII [1] new high-yield synthesis of monobromoferrocene and simplified procedure for the synthesis of pentabromoferrocene. Molecular structures of 1,2,3-tribromoferrocene and 1,2,3,4,5-pentabromoferrocene. J. Organometal Chem., 2011, 696, 1536-15440. [CrossRef]
- Tazi, M.; Erb, W.; Roisnel, R.; Dorcet, V.; Mongin, F.; Low, P. From ferrocene to fluorine-containing penta-substituted derivatives and all points in-between; or, how to increase the available chemical space. Org. Bio-mol. Chem., 2019, 17, 9352-9359. [CrossRef]
- Sünkel, K.; Weigand, S.; Hoffmann, A.; Blomeyer, S.; Reuter, C.G.; Vishnevskiy, Y.V.; Mitzel, N.W. Synthesis and Characterization of 1,2,3,4,5-Pentafluoroferrocene. J. Am. Chem. Soc., 2015, 137, 126-129. [CrossRef]
- Tazi, M., Hedidi, M., Erb, W., Halauko, Y.S., Ivashkevich, O.A., Matulis, V.E., Roisnel, T., Dorcet, V., Bentabed-Ababsa, G., Mongin. F. Fluoro- and Chloroferrocene: From 2- to 3-Substituted Derivatives. Organometallics, 2018, 37, 2207-2211. [CrossRef]
- Blockhaus, T., Bernhartzeder, S., Kempinger, W., Klein-Heßling, C. , Weigand, S., Sünkel. K. Evidence for “Halogen-Dance” and Ring-Exchange Reactions in Chloro-methylthio-ferrocenes. Eur. J. Org. Chem., 2020, 42, 6576-6587. [CrossRef]
- Butenschön, H. Haloferrocenes: Syntheses and Selected Reactions. Synthesis, 2018, 50, 3787. [CrossRef]
- Wen, M., Erb, W., Mongin, F., Halauko, Y.S., Ivashkevich, O.A., Matulis, V.E., Roisnel, T., Synthesis of Polysubstituted Ferrocenesulfoxides. Molecules, 2022, 27, 1798. [CrossRef]
- A. Zirakzadeh, A. Herlein, M. A. Gross, K. Mereiter, Y. Wang, W. Weissensteiner. Halide-Mediated Ortho-Deprotonation Reactions Applied to the Synthesis of 1,2- and 1,3-Disubstituted Ferrocene Derivatives. Organometallics, 2015, 34, 3820-3832. [CrossRef]
- Erb, W.; Mongin, F. Twofold Ferrocene C–H Lithiations For One-Step Difunctionalizations. Synthesis 2019, 51, 146–160. [Google Scholar] [CrossRef]
- Tazi, M.; Erb, W.; Halauko, Y.S.; Ivashkevich, O.A.; Matulis, V.E.; Roisnel, T.; Dorcet, V.; Mongin, F. From 2- to 3-Substituted Ferrocene Carboxamides or How to Apply Halogen “Dance” to the Ferrocene Series. Organometallics 2017, 36, 4770–4778. [Google Scholar] [CrossRef]
- Rupf, S.M., Dimitrova, I.S., Schröder, G., Malischewski, M.,Preparation and One-Electron Oxidation of Decabromoferrocene Organometallics, 2022, 41, 1261-1267. [CrossRef]
- Rupf, S.M., Sievers, R., Riemann, P.S., Reimann, M., Kaupp, M., Fasting, C., Malischewski, M., Persilylation of ferrocene: The ultimate discipline in sterically overcrowded metal complexes. Dalton Transactions 2023, 52 (20, 6870-687. [CrossRef]
- Boyes, A.L., Butler, I.R., Quayle, S.C., Palladium (II) complexes of (diisopropylphosphino)-ferrocenes: Improved catalysts for the Heck reaction, Tet. Lett.,1998, 39, 7763-7766. [CrossRef]
- Butler, I.R., Evans, D.M., N. Horton, P.N., Coles, S.J., Murphy, P.J. 1,1′,2,2′-Tetralithioferrocene and 1,1′,2,2′,3,3′-Hexalithioferrocene: Useful Additions to Ferrocene Precursor Compounds, Organometallics, 2021, 40, 600-605. [CrossRef]
- Butler, I.R. Sitting Out the Halogen Dance. Room-Temperature Formation of 2,2′-Dilithio-1,1′-dibromoferrocene. TMEDA and Related Lithium Complexes: A Synthetic Route to Multiply Substituted Ferrocenes, Organometallics, 2021, 40, 3240-3244. [CrossRef]
- Fortune, K.M.; Castel, C.; Robertson, C.M.; Horton, P.N.; Light, M.E.; Coles, S.J.; Waugh, M.; Clegg, W.; Harrington, R.W.; Butler, I.R. Ferrocenylmethylphosphanes and the Alpha Process for Methoxycarbonylation: The Original Story. Inorganics 2021, 9, 57. [CrossRef]
- Langer, J., Fischer, R., Görls, H., Theyssen, N., Walther, D. Nickel(I)-Komplexe mit 1,1-Bis(phosphino)ferrocenen als Liganden.
- Nickel(I) Complexes with 1,1-Bis(phosphino)ferrocenes as Ligands. Zeitschrift für anorganische und allgemeine Chemie, 2007, 633, 557-562. [CrossRef]
- Evans, D. M.,Hughes, D.D., Murphy, P.J., Horton, P.N., Coles, S.J., de Biani, F.F., Corsini, M., Butler, I.R. Synthetic Route to 1,1′,2,2′-Tetraiodoferrocene That Avoids Isomerization and the Electrochemistry of Some Tetrahaloferrocenes, Organometallics, 2021, 40, 2496-2503. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




