Submitted:
20 February 2025
Posted:
21 February 2025
You are already at the latest version
Abstract
MRNA vaccines have been shown to provide strong immune responses against SARS-CoV-2, but the extent of their antibody cross-reactivity against human seasonal coronaviruses, such as NL63, remains unclear. Furthermore, it is unknown whether pre-existing antibody responses against NL63 might affect the outcome of SARS-CoV-2 mRNA vaccination. This study used a flow cytometry-based serological assay and an in vitro neutralization assay to analyze NL63 antibody responses in sera from SARS-CoV-2 mRNA-vaccinated mice and plasma samples from a vaccinated human cohort. We found that the Moderna mRNA-1273 vaccine can generate cross-reactive antibodies against NL63. Importantly, SARS-CoV-2 mRNA vaccination did not boost pre-existing anti-NL63 responses in humans, and pre-existing NL63 antibody levels did not affect the antibody response induced by SARS-CoV-2 mRNA vaccination. These findings suggest that while SARS-CoV-2 mRNA vaccination can induce cross-reactive antibodies against NL63, pre-existing immunity to this seasonal coronavirus does not appear to significantly impact the effectiveness of the vaccine. This contributes to our understanding of the complex interplay between pre-existing immunity to seasonal coronaviruses and the immune response generated by SARS-CoV-2 mRNA vaccines.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Ethics Statement and Study Population
2.3. Virus
2.4. Spike Protein Flow Cytometry-Based (SFB) Assay
2.5. Neutralization Assay
2.5. Statistical Analysis
3. Results
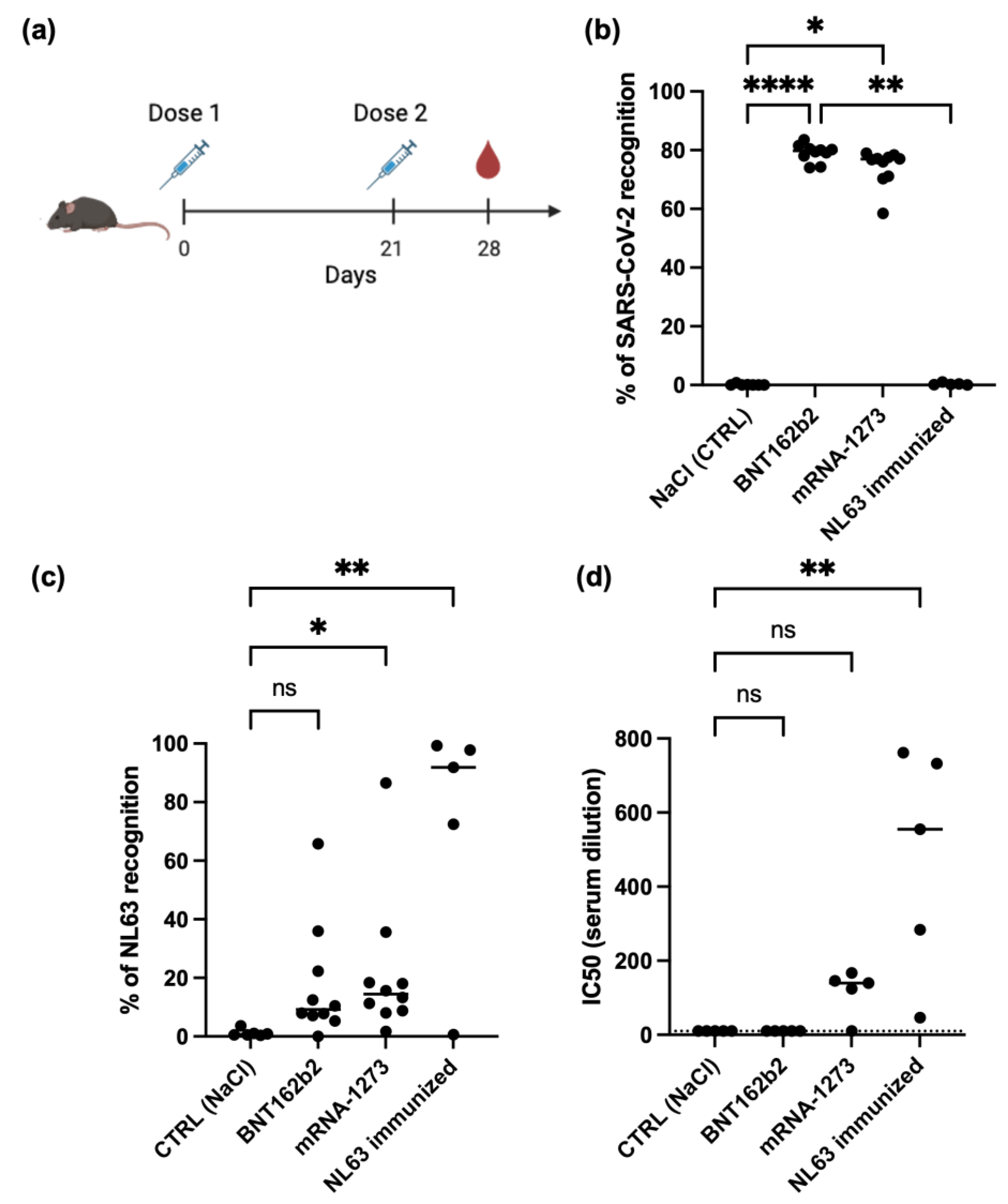
3.1. SARS-CoV-2 mRNA Vaccine Can Generate Cross-Reactive Antibody Against Human Seasonal Coronavirus NL63
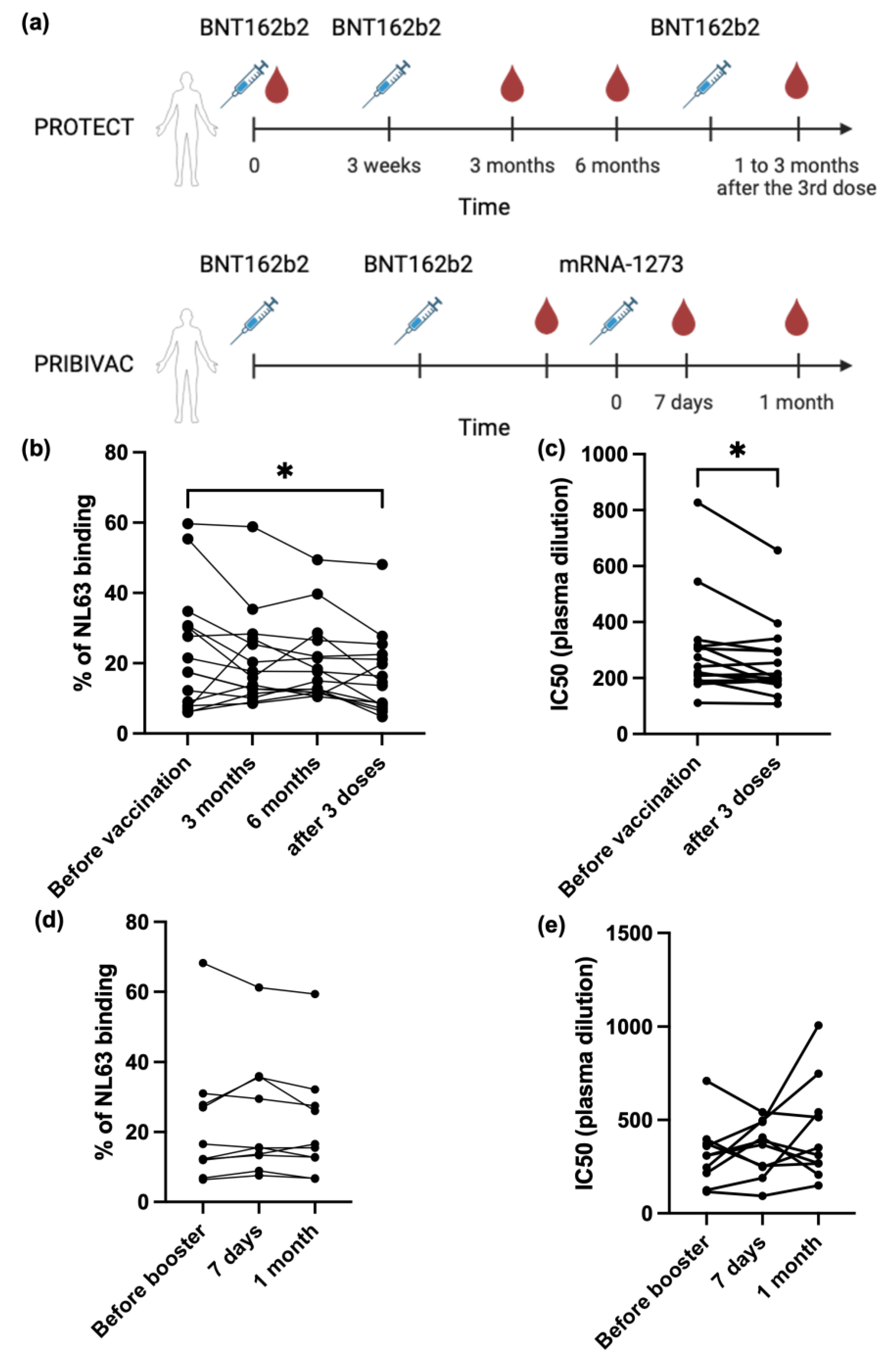
3.2. SARS-CoV-2 mRNA Vaccine Cannot Boost Pre-Existing NL63 Antibody Responses
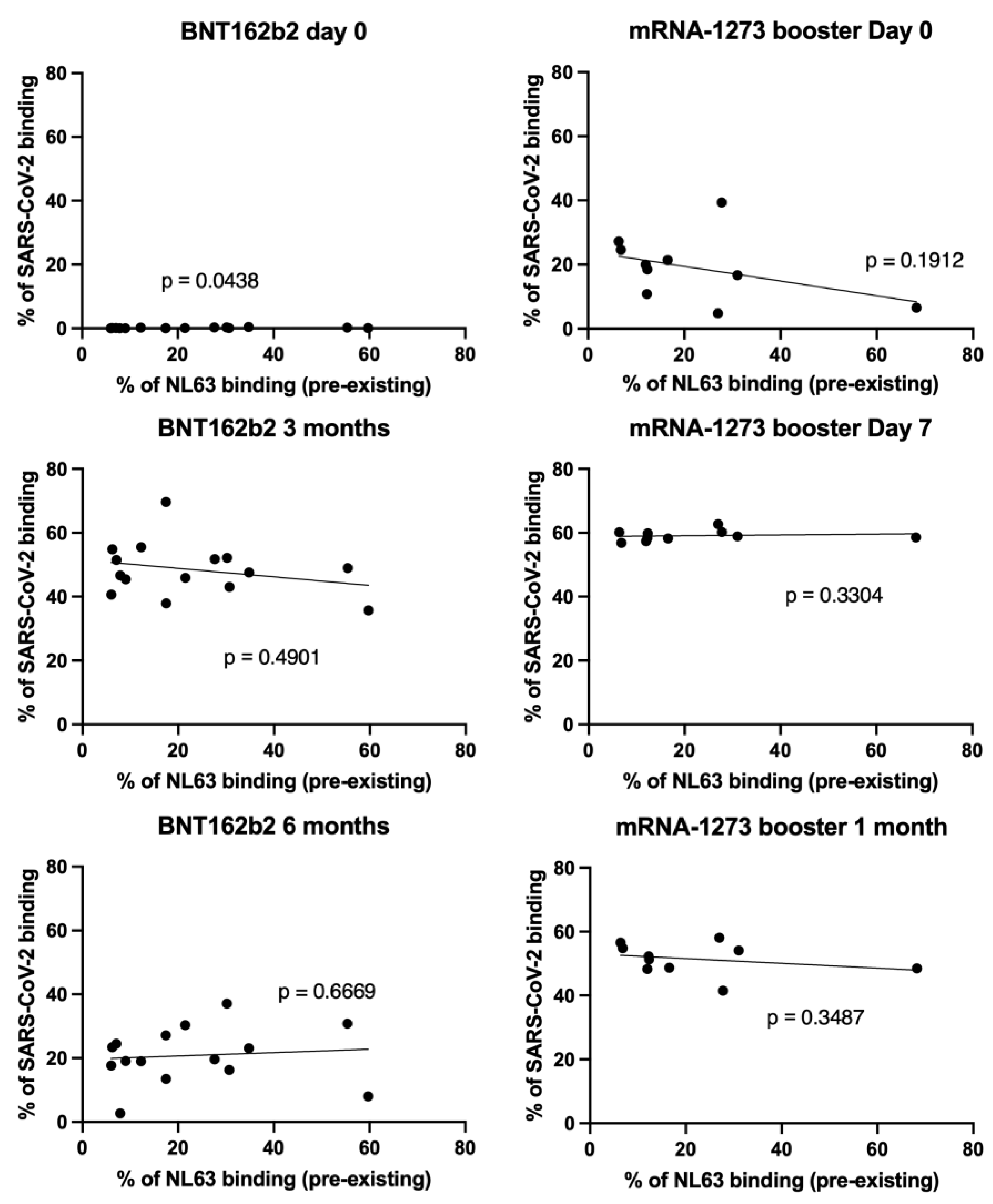
3.3. NL63 Pre-Existing Immune Response Did Not Affect Anti SARS-CoV-2 Antibody Response Imduced by Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dijkman, R.; Jebbink, M.F.; El Idrissi, N.B.; Pyrc, K.; Muller, M.A.; Kuijpers, T.W.; Zaaijer, H.L.; van der Hoek, L. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol 2008, 46, 2368–2373. [CrossRef]
- Pyrc, K.; Berkhout, B.; Van Der Hoek, L. Antiviral strategies against human coronaviruses. IDDT 2007, 7, 59–66. [CrossRef]
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.; Zeglen, S.; Baster, Z.; et al. Entry of human coronavirus NL63 into the cell. J Virol 2018, 92, e01933-17. [CrossRef]
- Amanat, F.; Clark, J.; Carreño, J.M.; Strohmeier, S.; Yellin, T.; Meade, P.S.; Bhavsar, D.; Muramatsu, H.; Sun, W.; Coughlan, L.; et al. Immunity to seasonal coronavirus spike proteins does not protect from SARS-CoV-2 challenge in a mouse model but has no detrimental effect on protection mediated by COVID-19 mRNA Vaccination. J Virol 2023, 97, e0166422. [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T Cell Responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest 2021, 131, e149335. [CrossRef]
- Hu, C.; Wang, Z.; Ren, L.; Hao, Y.; Zhu, M.; Jiang, H.; Wang, S.; Li, D.; Shao, Y. Pre-Existing Anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination. Front Cell Infect Microbiol 2022, 12, 978440. [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The Impact of pre-Existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat Rev Immunol 2023, 23, 304–316. [CrossRef]
- Adami, F.L.; de Castro, M.V.; Almeida, B. da S.; Daher, I.P.; Yamamoto, M.M.; Souza Santos, K.; Zatz, M.; Naslavsky, M.S.; Rosa, D.S.; Cunha-Neto, E.; et al. Anti-RBD IgG antibodies from endemic coronaviruses do not protect against the acquisition of SARS-CoV-2 infection among exposed uninfected individuals. Front Immunol 2024, 15. [CrossRef]
- Shrwani, K.; Sharma, R.; Krishnan, M.; Jones, T.; Mayora-Neto, M.; Cantoni, D.; Temperton, N.J.; Dobson, S.L.; Subramaniam, K.; McNamara, P.S.; et al. Detection of serum cross-reactive antibodies and memory response to SARS-CoV-2 in prepandemic and post–COVID-19 convalescent samples. J Infect Dis 2021, 224, 1305–1315. [CrossRef]
- Renia, L.; Goh, Y.S.; Rouers, A.; Le Bert, N.; Chia, W.N.; Chavatte, J.-M.; Fong, S.-W.; Chang, Z.W.; Zhuo, N.Z.; Tay, M.Z.; et al. Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose. Nat Commun 2022, 13, 4615. [CrossRef]
- Poh, X.Y.; Tan, C.W.; Lee, I.R.; Chavatte, J.-M.; Fong, S.-W.; Prince, T.; Hartley, C.; Yeoh, A.Y.Y.; Rao, S.; Chia, P.Y.; et al. Antibody response of heterologous vs homologous messenger RNA vaccine boosters against the severe acute respiratory syndrome coronavirus 2 omicron variant: interim results from the PRIBIVAC study, a randomized clinical trial. Clin Inf Dis 2022, 75, 2088–2096. [CrossRef]
- Poh, X.Y.; Lee, I.R.; Lim, C.; Teo, J., Rao, S.; Chia, P.Y.; Ong, S.W.X.; Lee, T.H.; Lin, R.J.H.; Ng, L.F.P.; et al. Evaluation of the safety and immunogenicity of different COVID-19 vaccine combinations in healthy individuals: study protocol for a randomized, subject-blinded, controlled phase 3 trial [PRIBIVAC]. Trials 2022, 23, 498. [CrossRef]
- Goh, Y.S.; Ng, L.F.P.; Renia, L. A Flow cytometry-based assay for serological detection of anti-spike antibodies in COVID-19 patients. STAR Protoc 2021, 2, 100671. [CrossRef]
- Goh, Y.S.; Chavatte, J.M.; Lim, J.A.; Lee, B.; Hor, P.X.; Amrun, S.N.; Lee, C.Y., Chee, R.S.; Wang, B., Lee, C.Y.; et al. Sensitive detection of total anti-spike antibodies and isotype switching in asymptomatic and symptomatic individuals with COVID-19. Cell Rep Med 2021, 16, 100193. [CrossRef]
- Grobben, M.; van der Straten, K.; Brouwer, P.J.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Appelman, B.; Lavell, A.A.; van Vught, L.A.; Burger, J.A.; et al. Cross-reactive antibodies after SARS-CoV-2 infection and vaccination. eLife 2021, 10, e70330. [CrossRef]
- Imai, K.; Matsuoka, M.; Tabata, S.; Kitagawa, Y.; Nagura-Ikeda, M.; Kubota, K.; Fukada, A.; Takada, T.; Sato, M.; Noguchi, S.; et al. Cross-reactive humoral immune responses against seasonal human coronaviruses in COVID-19 patients with different disease severities. Int J Infect Dis 2021, 111, 68–75. [CrossRef]
- Denninger, V.; Xu, C.K.; Meisl, G.; Morgunov, A.S.; Fiedler, S.; Ilsley, A.; Emmenegger, M.; Malik, A.Y.; Piziorska, M.A.; Schneider, M.M.; et al. Microfluidic antibody affinity profiling reveals the role of memory reactivation and cross-reactivity in the defense against SARS-CoV-2. ACS Infect Dis 2022, 8, 790–799. [CrossRef]
- Pattinson, D.; Jester, P.; Guan, L.; Yamayoshi, S.; Chiba, S.; Presler, R.; Rao, H.; Iwatsuki-Horimoto, K.; Ikeda, N.; Hagihara, M.; et al. A novel method to reduce ELISA serial dilution assay workload applied to SARS-CoV-2 and seasonal HCoVs. Viruses 2022, 14, 562. [CrossRef]
- Adams, O.; Andrée, M.; Rabl, D.; Ostermann, P.N.; Schaal, H.; Lehnert, E.; Ackerstaff, S.; Müller, L.; Fischer, J.C. Humoral response to SARS-CoV-2 and seasonal coronaviruses in COVID-19 patients. J Med Virol 2022, 94, 1096–1103. [CrossRef]
- Guo, L.; Wang, Y.; Kang, L.; Hu, Y.; Wang, L.; Zhong, J.; Chen, H.; Ren, L.; Gu, X.; Wang, G.; et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microb Infect 2021, 10, 664–676. [CrossRef]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol 2020, 5, eabe0367. [CrossRef]
- Wells, D.A.; Cantoni, D.; Mayora-Neto, M.; Genova, C.D.; Sampson, A.; Ferrari, M.; Carnell, G.; Nadesalingam, A.; Smith, P.; Chan, A.; et al. Human seasonal coronavirus neutralization and COVID-19 severity. J Med Virol 2022, 94, 4820–4829. [CrossRef]
- Camerini, D.; Randall, A.Z.; Trappl-Kimmons, K.; Oberai, A.; Hung, C.; Edgar, J.; Shandling, A.; Huynh, V.; Teng, A.A.; Hermanson, G.; et al. Mapping SARS-CoV-2 antibody epitopes in COVID-19 patients with a multi-coronavirus protein microarray. Microbiol Spectr 2021, 9, e01416-21. [CrossRef]
- Struck, F.; Schreiner, P.; Staschik, E.; Wochinz-Richter, K.; Schulz, S.; Soutschek, E.; Motz, M.; Bauer, G. Incomplete IgG avidity maturation after seasonal coronavirus infections. J Med Virol 2022, 94, 186–196. [CrossRef]
- Peng, Y.; Liu, Y.; Hu, Y.; Chang, F.; Wu, Q.; Yang, J.; Chen, J.; Teng, S.; Zhang, J.; He, R.; et al. Monoclonal Antibodies constructed from COVID-19 convalescent memory B cells exhibit potent binding activity to MERS-CoV spike S2 subunit and other human coronaviruses. Front Immunol 2022, 13. [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-Existing Humoral Immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 2022, 30, 83-96.e4. [CrossRef]
- Wang, J.; Li, D.; Zhou, Q.; Wiltse, A.; Zand, M.S. Antibody mediated immunity to SARS-CoV-2 and human coronaviruses: multiplex beads assay and volumetric absorptive microsampling to generate immune repertoire cartography. Front Immunol 2021, 12. [CrossRef]
- Stanley, A.M.; Aksyuk, A.A.; Wilkins, D.; Green, J.A.; Lan, D.; Shoemaker, K.; Tieu, H.-V.; Sobieszczyk, M.E.; Falsey, A.R.; Kelly, E.J. Seasonal human coronavirus humoral responses in AZD1222 (ChaAdOx1 nCoV-19) COVID-19 vaccinated adults reveal limited cross-immunity. Front Immunol 2024, 15. [CrossRef]
- Kolehmainen, P.; Huttunen, M.; Iakubovskaia, A.; Maljanen, S.; Tauriainen, S.; Yatkin, E.; Pasternack, A.; Naves, R.; Toivonen, L.; Tähtinen, P.A.; et al. Coronavirus spike protein-specific antibodies indicate frequent infections and reinfections in infancy and among BNT162b2-vaccinated healthcare workers. Sci Rep 2023, 13, 8416. [CrossRef]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936-3948.e10. [CrossRef]
- Cantoni, D.; Siracusano, G.; Mayora-Neto, M.; Pastori, C.; Fantoni, T.; Lytras, S.; Di Genova, C.; Hughes, J.; Lopalco, L.; Temperton, N. Analysis of antibody neutralisation activity against SARS-CoV-2 variants and seasonal human coronaviruses NL63, HKU1, and 229E induced by three different COVID-19 vaccine platforms. Vaccines 2022, 11, 58. [CrossRef]
- Asamoah-Boaheng, M.; Grunau, B.; Karim, M.E.; Jassem, A.N.; Bolster, J.; Marquez, A.C.; Scheuermeyer, F.X.; Goldfarb, D.M. Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination? Front Immunol 2022, 13. [CrossRef]
- Singh, G.; Abbad, A.; Kleiner, G.; Srivastava, K.; Gleason, C.; Group, P.S.; Carreño, J.M.; Simon, V.; Krammer, F. The Post-COVID-19 population has a high prevalence of cross-reactive antibodies to spikes from all orthocoronavirinae genera. mBio 2024. [CrossRef]
- Jacob-Dolan, C.; Feldman, J.; McMahan, K.; Yu, J.; Zahn, R.; Wegmann, F.; Schuitemaker, H.; Schmidt, A.G.; Barouch, D.H. coronavirus-specific antibody cross reactivity in rhesus macaques following SARS-CoV-2 vaccination and infection. J Virol 2021, 95, 10.1128/jvi.00117-21. [CrossRef]
- Wirsching, S.; Harder, L.; Heymanns, M.; Gröndahl, B.; Hilbert, K.; Kowalzik, F.; Meyer, C.; Gehring, S. Long-Term, CD4+ memory t cell response to SARS-CoV-2. Front Immunol 2022, 13. [CrossRef]
- Soni, M.K.; Migliori, E.; Fu, J.; Assal, A.; Chan, H.T.; Pan, J.; Khatiwada, P.; Ciubotariu, R.; May, M.S.; Pereira, M.R.; et al. The prospect of universal coronavirus immunity: characterization of reciprocal and non-reciprocal T cell responses against SARS-CoV2 and common human coronaviruses. Front Immunol 2023, 14. [CrossRef]
- Lineburg, K.E.; Grant, E.J.; Swaminathan, S.; Chatzileontiadou, D.S.M.; Szeto, C.; Sloane, H.; Panikkar, A.; Raju, J.; Crooks, P.; Rehan, S.; et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity 2021, 54, 1055-1065.e5. [CrossRef]
- Naghibosadat, M.; Babuadze, G.G.; Pei, Y.; Hurst, J.; Salvant, E.; Gaete, K.; Biondi, M.; Moloo, B.; Goldstein, A.; Avery, S.; et al. Vaccination against SARS-CoV-2 provides low-level cross-protection against common cold coronaviruses in mouse and non-human primate animal models. J Virol 2025, 0, e01390-24. [CrossRef]
- Klompus, S.; Leviatan, S.; Vogl, T.; Mazor, R.D.; Kalka, I.N.; Stoler-Barak, L.; Nathan, N.; Peres, A.; Moss, L.; Godneva, A.; et al. Cross-reactive antibodies against human coronaviruses and the animal coronavirome suggest diagnostics for future zoonotic spillovers. Sci Immunol 2021, 6, eabe9950. [CrossRef]
- Devaux, C.A.; Fantini, J. Unravelling antigenic cross-reactions toward the world of coronaviruses: extent of the stability of shared epitopes and SARS-CoV-2 anti-spike cross-neutralizing antibodies. Pathogens 2023, 12, 713. [CrossRef]
- Yin, D.; Han, Z.; Lang, B.; Li, Y.; Mai, G.; Chen, H.; Feng, L.; Chen, Y.; Luo, H.; Xiong, Y.; et al. Effect of seasonal coronavirus immune imprinting on the immunogenicity of inactivated COVID-19 vaccination. Front Immunol 2023, 14. [CrossRef]
- da Silva Antunes, R.; Pallikkuth, S.; Williams, E.; Dawen Yu, E.; Mateus, J.; Quiambao, L.; Wang, E.; Rawlings, S.A.; Stadlbauer, D.; Jiang, K.; et al. Differential T-cell reactivity to endemic coronaviruses and SARS-CoV-2 in community and health care workers. J Infect Dis 2021, 224, 70–80. [CrossRef]
- Loyal, L.; Braun, J.; Henze, L.; Kruse, B.; Dingeldey, M.; Reimer, U.; Kern, F.; Schwarz, T.; Mangold, M.; Unger, C.; et al. Cross-Reactive CD4+ T Cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science 2021, 374, eabh1823. [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific t cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [CrossRef]
- Shimizu, K.; Iyoda, T.; Sanpei, A.; Nakazato, H.; Okada, M.; Ueda, S.; Kato-Murayama, M.; Murayama, K.; Shirouzu, M.; Harada, N.; et al. Identification of TCR repertoires in functionally competent cytotoxic t cells cross-reactive to SARS-CoV-2. Commun Biol 2021, 4, 1365. [CrossRef]
- Coulon, P.-G.; Prakash, S.; Dhanushkodi, N.R.; Srivastava, R.; Zayou, L.; Tifrea, D.F.; Edwards, R.A.; Figueroa, C.J.; Schubl, S.D.; Hsieh, L.; et al. High frequencies of alpha common cold coronavirus/SARS-CoV-2 cross-reactive functional CD4+ and CD8+ memory T cells are associated with protection from symptomatic and fatal SARS-CoV-2 infections in unvaccinated COVID-19 patients. Front Immunol 2024, 15, 1343716. [CrossRef]
| Experiment group | Control group | Antibody or T cells? | Tested antigen on NL63 | Result | Ref |
| PCR-positive | PCR-negative close contacts | IgG antibody | RBD | No difference | [8] |
| PCR-positive | Pre-COVID samples | IgG antibody | RBD | No difference | [9] |
| COVID-19 severe | COVID-19 mild | IgG antibody | S and N | No difference | [16] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S1 | No difference | [17] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S and N | No difference | [18] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | N | No difference | [19] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S | No difference | [20] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | RBD | No difference | [21] |
| COVID-19 patients | Healthy individuals | IgG antibody | Pseudovirus | Higher neutralization | [22] |
| COVID-19 convalescents | Pre-COVID samples | IgG antibody | S2 and N | Higher response | [23] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | N | Higher response | [24] |
| COVID-19 convalescents | COVID-19 admission | IgG antibody | S and N | Higher against N in severe patients | [25] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S | Higher response | [15] |
| COVID-19 convalescents | Healthy individuals | IgG mAbs | S | 3 strongly cross-reactive mAbs | [25] |
| Health care workers with direct/indirect contact to COVID-19 patients | Health care workers with no contact to COVID-19 patients | IgG, IgM and IgA antibody | S | Higher IgM between direct and no contact group | [26] |
| COVID-19 convalescents or BNT162b2 vaccination (2 doses) | Pre-COVID samples | IgG antibody | S and N | No difference | [27] |
| AZD1222 primary vaccination and/or booster | Placebo | IgG antibody | S | No difference | [28] |
| BNT162b2 vaccination (2 and 3 doses) | Before vaccination (same individuals) | IgG antibody | S1 | No difference | [29] |
| BNT162b2 vaccination (2 doses) | Before vaccination (same individuals) | IgG antibody | S | No difference | [30] |
| AZD1222, BNT162b2 or mRNA-1273 vaccinated (2 doses) | AZD1222, BNT162b2 or mRNA-1273 vaccinated (1 dose) | IgG antibody | Pseudovirus | Higher neutralization for mRNA-1273 | [31] |
| BNT162b2 or mRNA-1273 | Healthy individuals | IgG antibody | S | No difference | [32] |
| BNT162b2 or mRNA-1273 (2 doses) | Before vaccination (same individuals) | IgG antibody | S | No difference | [33] |
| After 1-dose vaccination (did not mention what vaccine) | Before vaccination (same individuals) | IgG antibody | Pseudovirus | Higher neutralization | [22] |
| BBIBP-CorV vaccinated (2 doses) | Before vaccination (same individuals) | IgG antibody | S | Higher response | [6] |
| Infected, Ad26-vaccinated or DNA-vaccinated + re-infected macaques | Before treatment (same animals) | IgG antibody | S | Higher response | [33] |
| COVID-19 convalescents | Healthy individuals | CD4+ T cells | Pool peptide library S1 and S2 | No difference | [34] |
| COVID exposed individuals | Healthy individuals | CD4+ T cells | Pool peptide library S1, S2, M and NP | Higher against S1, M and NP | [35] |
| BNT162b2 or mRNA-1273 vaccinated | Healthy individuals | CD4+ T cells | Pool peptide library (whole virus) | Higher response | [36] |
| COVID-19 convalescents | Healthy individuals | CD8+ T cells | Homologous N105–113 (PPKVHFYYL) | No difference | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





