1. Introduction
Agmatine, a naturally occurring biogenic amine, is synthesized through the amino acid L-arginine by the enzyme arginine decarboxylase. Since its discovery in mammalian brains in the 20
th century, agmatine has garnered considerable scientific attention due to its diverse biological activities and therapeutic potential [
1,
2]. One of the primary biological functions of agmatine is inhibition of the nitric oxide (NO) synthase (NOS) [
3,
4], particularly, of the neuronal form (nNOS) of this enzyme [
5]. Since NO is an important neuromodulator, and nNOS is its primary source within the central nervous system (CNS), this enzyme modulates multiple brain neurotransmitter systems [
6]. Among the CNS neurotransmitter systems modulated by nNOS, there is a serotonergic (5-HT) system, playing a key role in pathophysiology and treatment of depression and related stress-related anxiety and mood disorders [
7].
It was reported that nNOS molecularly binds to and creates a heterodimer with the 5-HT transporter (SERT) [
8]. The latter plays a key role in the regulation of the extracellular 5-HT concentrations in the dorsal raphe nucleus (DRN), a brain area containing the cell bodies of 5-HT-secreing neurons. Extracellular 5-HT negatively regulates the firing activity of 5-HT neurons,
via a mechanism primarily involving somatodendritic serotonin-1A (5-HT
1A) autoreceptors. The 5-HT
1A autoreceptors-mediated inhibition of 5-HT neurons of the DRN is responsible, at least in part, for the delayed behavioral response and/or for the lack of adequate therapeutic response to antidepressant drugs, such as the selected serotonin (5-HT) reuptake inhibitors [
7]. Consistently, molecules dissociating the nNOS-SERT dimerization were reported to have robust and rapid antidepressant-like effect [
9] This molecular interaction between nNOS and SERT represents a potential therapeutic target, as disruption of this complex has been shown to produce rapid antidepressant effects.
Agmatine, as a natural inhibitor of the NOS/nNOS, exhibits significant psychoactive effects through modulation of 5-HT neurotransmission. Chronic agmatine treatment produces robust antidepressant-like behavioral effect in mice, accompanied by increased extracellular brain concentrations of 5-HT and glutamate along with elevated expression of neuroplasticity markers such as brain derived neurotrophic factor (BDNF) and synaptotagmin I [
10]. Additional studies have confirmed both anxiolytic and antidepressant-like effects following chronic agmatine treatment [
11,
12]. Notably, even acute agmatine treatment demonstrates antidepressant-like effect and increases BDNF levels [
13] with similar anxiolytic effects reported in mice [
14]. The mechanism underlying these behavioral effects may be linked to agmatine’s influence on 5-HT neuronal activity, as previous studies have shown that DRN 5-HT neurons expressing nNOS exhibit lower spontaneous firing rates compared to non-NOS expressing neurons. This is further supported by the observation that the nitric oxide donor diethylamineNONOate directly inhibits 5-HT neuronal firing
in vitro electrophysiology [
15]. These findings suggest that agmatine's psychoactive effects might be mediated through disinhibition of serotonergic neurotransmission via nNOS inhibition.
Bulding on previous findings suggesting NO’s role in serotonergic signaling, weaimed to investigate, using in vivo electrophysiology, both acute and chronic effects of agmatine on 5-HT neuronal firing activity. We further investigated potential molecular adaptations following chronic agmatine treatment by analyzing the expression of nNOS, serotonin transporter (SERT), and key 5-HT receptor subtypes (serotonin-1A/AB receptors (5-HT1A/1B) and serotonin-2A/2B/2C (5-HT2A/2B/2C)) in the rat dorsal raphe nucleus (DRN).
3. Discussion
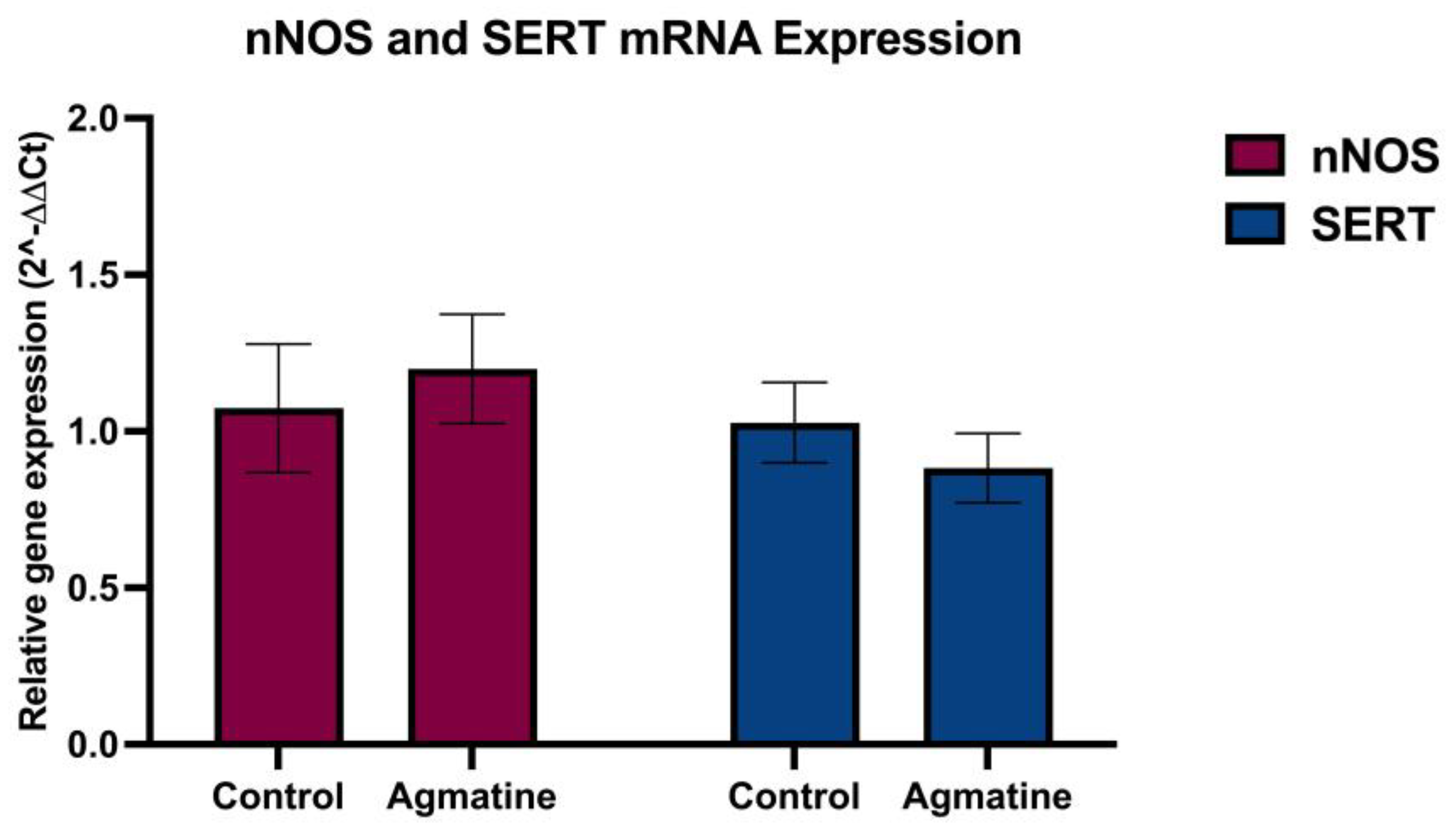
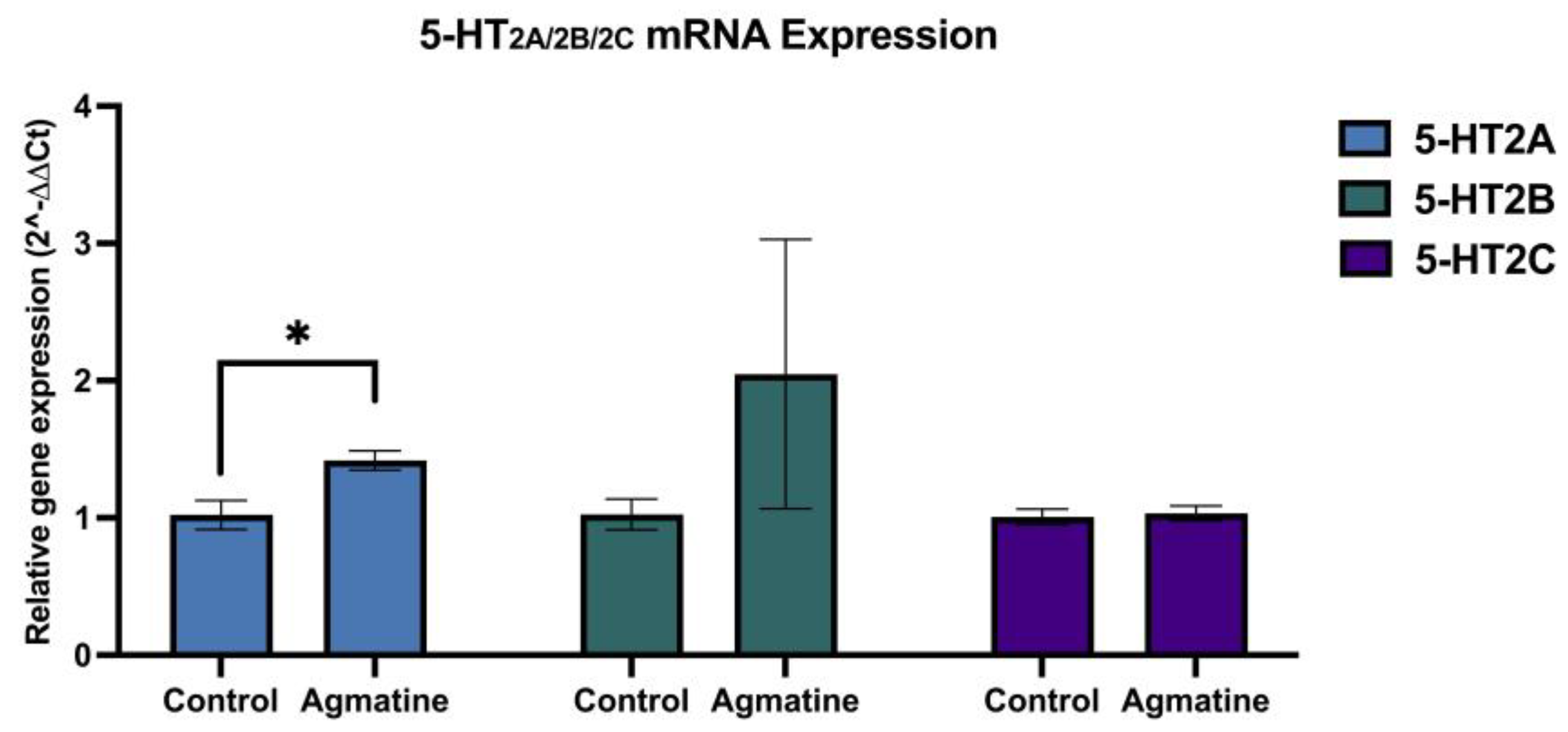
We found that both acute and chronic agmatine stimulated 5-HT neurons of the DRN. Chronic agmatine also enhances the expression of 5-HT2A receptors in the DRN. The DRN expressions of other subtypes of 5-HT receptors, belonging to the 5-HT1 and 5-HT2 subfamilies, as well as the expression of nNOS and SERT, were not affected by chronic agmatine treatment.
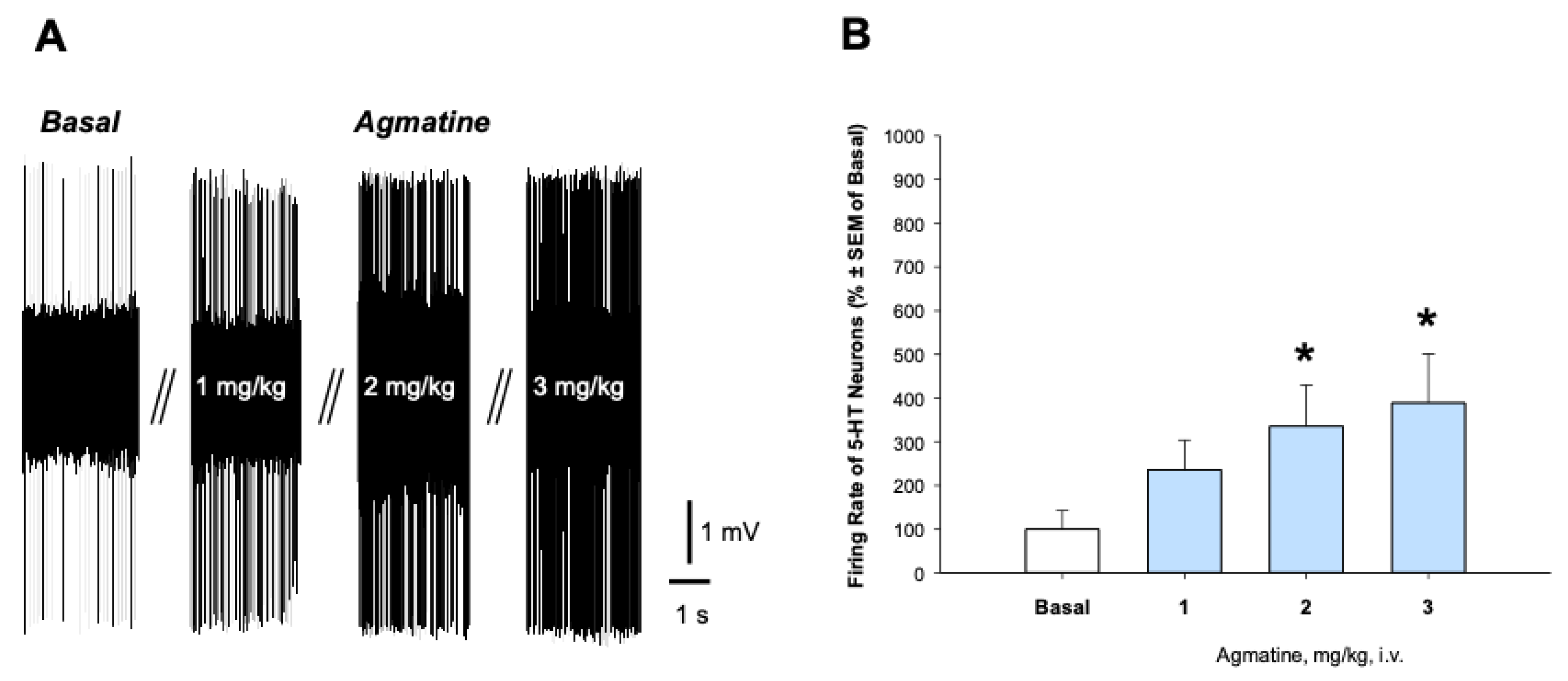
It was found that acute administration of agmatine led to the activation of 5-HT neurons of the DRN (Fig 1). It is consistent with the inhibitory effect of the NO donor on 5-HT neurons, reported in the previous study [
15]. Since 5-HT neurotransmission is fundamental in antidepressant drug response [
7], our findings may explain the antidepressant-like behavioral effect of acute agmatine, reported in a previous study [
13]. Since 5-HT stimulates BDNF expression [
16], excitation of 5-HT neurons by acute agmatine may explain its ability to enhance BDNF levels, reported in the same study. Our findings on agmatine’s bi-directional effects on 5-HT neuronal firing is consistent with the previous work showing that 5-HT
1B autoreceptor expression in the DRN can either decrease or increase anxiety-like behaviors depending on stress context, suggesting that modulation of serotonergic signaling has complex state-dependent effects on emotional behavior [
17].
The acute effect of agmatine on the excitability of the DRN 5-HT neurons was observed within seconds after the drug injection. It is therefore unlikely that any alteration in a protein and/or mRNA expression underlines the acute agmatine effect of the excitability of 5-HT neurons. It is however possible that the rapid dissociation of the nNOS/SERT dimmer and subsequent 5-HT
1A-autoreceptor-mediated disinhibition of 5-HT neurons is involved [
9]. Further experiments should be performed to test this hypothesis.
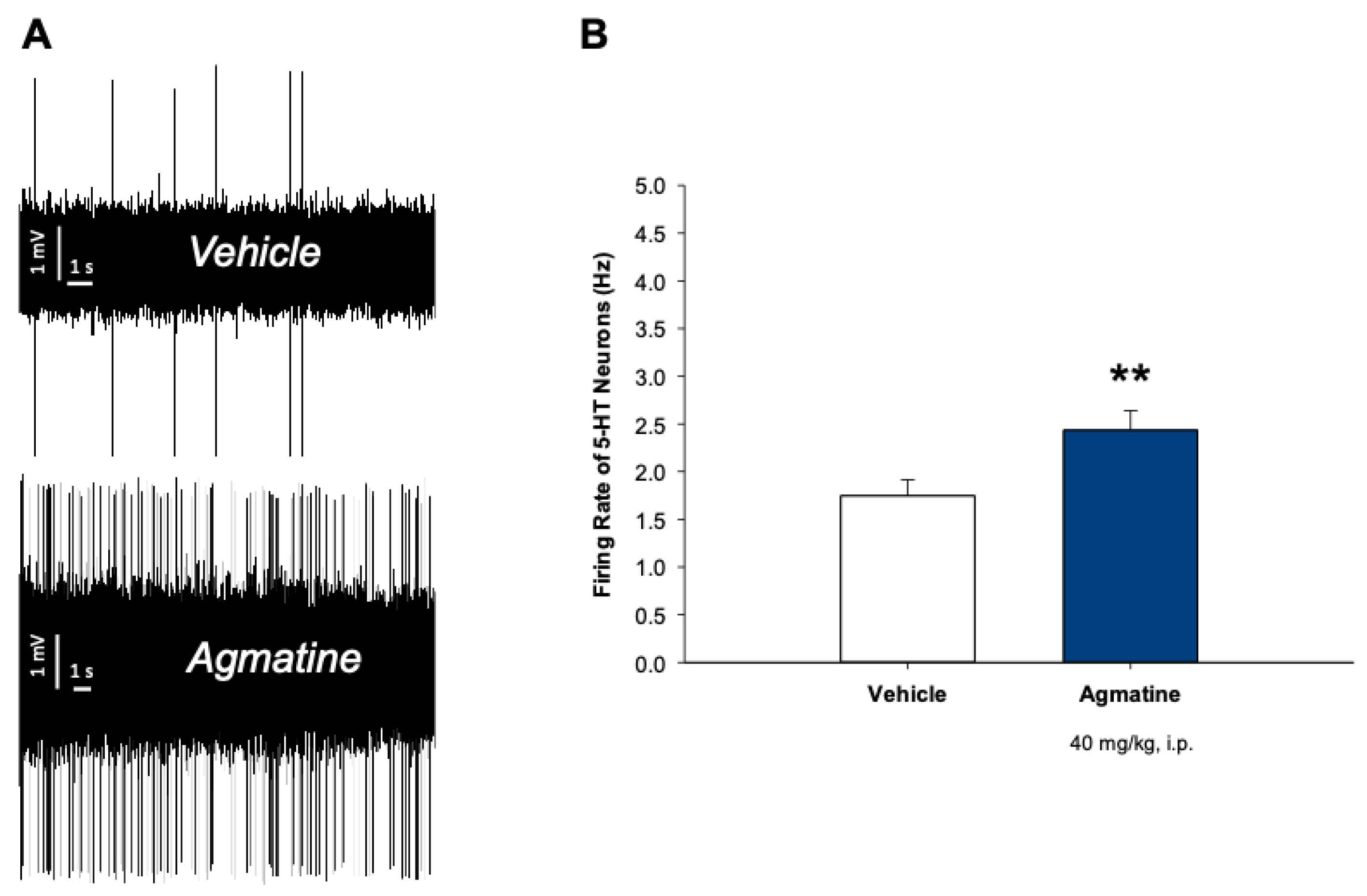
The mean spontaneous firing activity of 5-HT neurons in the agmatine-treated rats was significantly higher than in the vehicle-treated control (Fig 2). This finding is consistent with the previously observed antidepressant, anxiolytic, neuroprotective, and BDNF expression-enhancing effects of chronic agmatine [
10,
11,
14]. It is also consistent with the previous finding that 5-HT neurons expressing the nNOS have lower spontaneous firing activity than the non-nNOS expressing ones [
15].
It is known that the certain psychoactive drugs have distinct acute and chronic effects on the excitability of 5-HT neurons. Thus, acute selective serotonin reuptake inhibitor (SSRI) escitalopram [
18] or trace amine associated receptor 1 (TAAR1) agonist RO5256390 [
19] inhibit 5-HT neurons, when the chronic administration of these drugs have no effect on the firing activity of 5-HT neurons. It is also well established that the adaptive changes in the expression and/or activity of the SERT and 5-HT
1A receptors are responsible for the different response of 5-HT neurons to the acute and chronic administration of the same psychoactive drugs [
7]. In the present study, the persistence of the ability of agmatine to stimulate 5-HT neurons after its chronic administration (Fig 2) is consistent with the lack of adaptive changes in the expression of the SERT and 5-HT
1A receptors after chronic treatment with this drug (Figs 3 and 4).
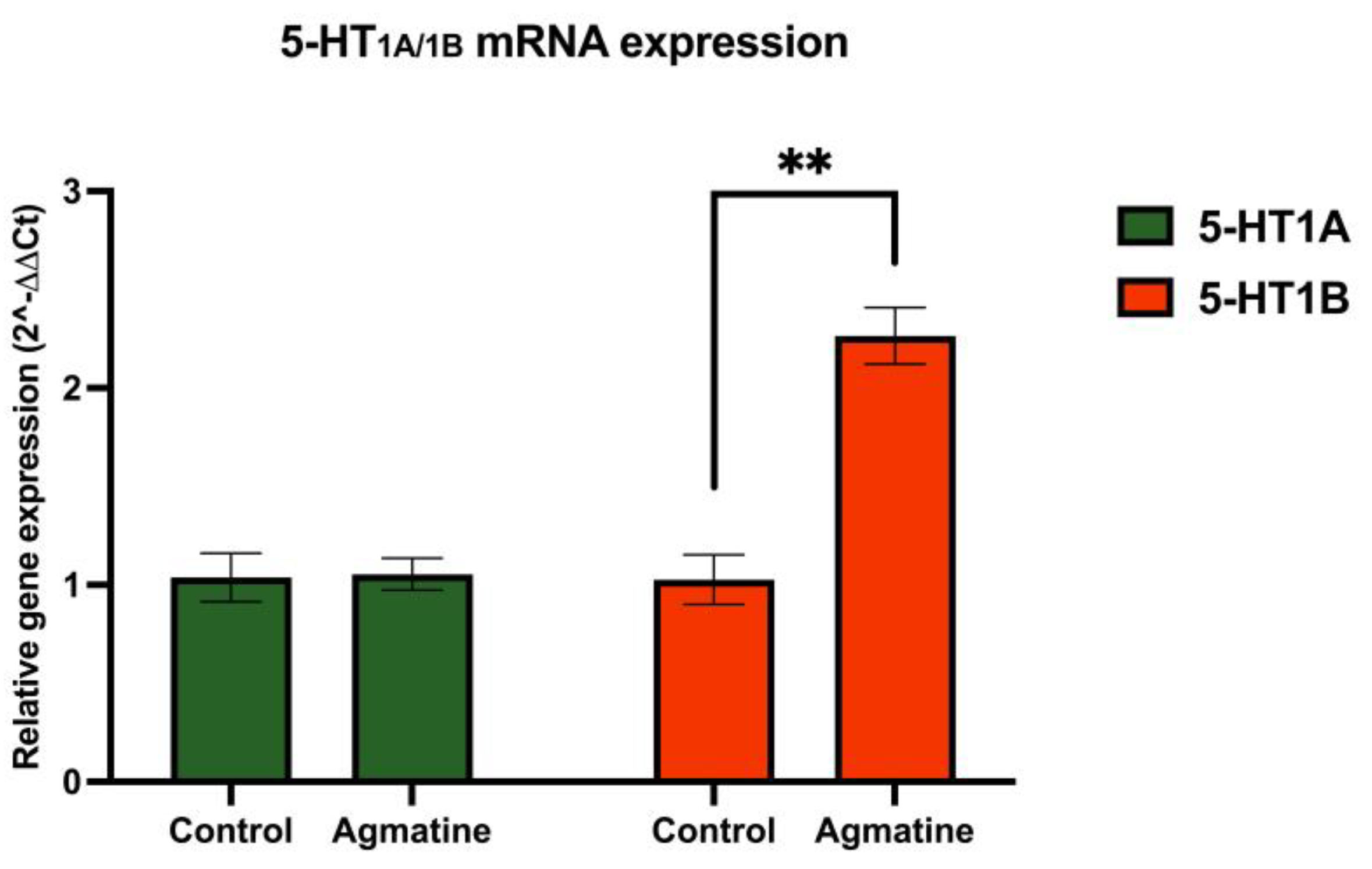
It was found that chronic agmatine treatment led to the increased expression of 5-HT
1B receptor-coding mRNA in the DRN (Fig 4). The 5-HT
1B receptor-coding mRNA, detected in the DRN, is likely to be expressed within the cell bodies of 5-HT neurons. This mRNA is latterly translated into the HT
1B receptor protein, expressed in the nerve terminals of 5-HT neurons in various brain areas, such as hippocampus. These nerve terminal 5-HT
1B autoreceptors negatively regulate 5-HT neurotransmission [
20]. It was however reported that the 5-HT
1B autoreceptor-mediated suppression of 5-HT release from the nerve terminal did not necessarily involve suppression of firing of 5-HT neurons. Furthermore, a 5-HT
1B agonist even increased, and a 5-HT
1B antagonist decreased the firing rate of 5-HT neurons [
21,
22]. The increased expression of HT
1B autoreceptors, observed after chronic agmatine, does not therefore contradict the increase in the tonic firing activity of 5-HT neurons.
The observed upregulation of 5-HT
1B receptor expression following chronic agmatine treatment warrants careful interpretation. While this effect could be directly related to agmatine’s action, several alternative mechanisms should be considered. First, the chronic intravenous administration protocol itself may have introduced a stress component that could influence 5-HT
1B receptor expression, as previous studies have shown stress-dependent modulation of serotonergic systems [
17]. Additionally, the increased 5-HT
1B receptor expression may represent a compensatory homeostatic response to enhanced serotonergic transmission induced by agmatine. Since 5-HT
1B receptor function as inhibitory autoreceptors on serotonergic terminals, their upregulation could serve as a negative feedback mechanism to maintain appropriate serotonin release. Furthermore, given that DRN serotonergic neurons receive substantial dopaminergic and glutamatergic inputs, agmatine’s effects on 5-HT
1B expression might be mediated indirectly through these neurotransmitter systems. To complex interplay between these various mechanisms deserves further investigation, particularly using selective antagonists and region-specific manipulations to dissect the relative contributions of direct and indirect pathways.
We found that the sustained treatment with agmatine led to increased expression of mRNA coding for 5-HT
2A receptors in the DRN (Fig 5). Since 5-HT
2A receptors are not known to act as autoreceptors, the 5-HT
2A receptor-coding mRNA, detected in the DRN, is likely to be expressed in the non-5-HT neurons, such as GABAergic and/or opioidergic interneurons [
23,
24]. It was reported that the local administration of 5-HT
2A/2C receptor agonist (+)-DOI hydrochloride (DOI) into the DRN activated local 5-HT neurons. Another study suggested that the 5-HT
2 receptor-modulated stimulation of the DRN 5-HT neurons involves the excitation of opioidergic interneurons [
23]. It is therefore possible that the chronic agmatine-induced stimulation of 5-HT neurons involves a 5-HT
2A receptor-based mechanism. The involvement of 5-HT
2A receptors in the beneficial CNS effect of agmatine has been previously reported as well. Santos and colleagues [
25] found that the agmatine-induced antinociception in mice was significantly attenuated by 5-HT
2A receptor antagonist ketanserin. Freitas and co-authors [
26] reported that ketanserin abolished the neuroprotective effect of agmatine in the hippocampal neuronal cell culture. The ability of chronic agmatine to upregulate both 5-HT
1B and 5-HT
2A receptor expression while stimulating 5-HT neuronal firing aligns with previous evidence that baseline serotonergic signaling may play a protective role against anxiety under normal conditions, though these relationships become more complex following stress exposure.
The primary limitation of this study is that the expression of 5-HT receptors was assessed in the DRN only. It is however known that 5-HT
1A/1B receptors expressed in other brain areas, such as prefrontal cortex [
27], are also involved in the regulation of excitability of the DRN 5-HT neurons. The effect of chronic agmatine on the nNOS, SERT, and 5-HT receptors in brain areas other than the DRN should be assessed in future studies.
4. Materials and Methods
4.1. Animals
Male Wistar rats (initial weight 250–350 g; 2–3 months old) were obtained from the Department of Toxicology and Laboratory Animals Breeding, Centre of Experimental Medicine of the Slovak Academy of Sciences, Dobra Voda, Slovak Republic. All the animals were housed (38 × 59 × 25 cm large cages) under standard laboratory conditions (temperature: 22 ± 2°C, humidity: 55 ± 10%) with a 12 h light/12 h dark cycle (lights on at 7.00 a.m.). Pelleted food and tap water were available ad libitum. The State Veterinary and Food Administration of the Slovak Republic approved all the experimental procedures. The rats were handled according to the Guide for the Care and Use of Laboratory Animals (N.R.C., 1996) and the European Communities Council Directive of September 22, 2010 (2010/63/EU, 74).
4.2. Chemicals
Agmatine sulfate salt (A7127-5G, ≥97% purity) was purchased from Sigma-Aldrich. DNA/RNA Shield (R1100-50, 50 ml) and Quick-RNA Microprep Kit (R1050), obtained from Zymo Research. SOLIScript® 1-step Multiplex Probe Kit purchased from Solis Biodyne. Primers and probes were synthesized by MultiplexDX s.r.o. (Bratislava, Slovakia). All chemicals were of analytical grade and stored per manufacturer guidelines.
4.3. Electrophysiology
In vivo electrophysiological experiments were performed as previously described [
19,
28,
29]. The rats were anesthetized with chloral hydrate (0.4 g/kg, i. p) and mounted in the stereotaxic frame (David Kopf Instruments, Tujunga, CA). Their scalp was opened, and a 3 mm hole was drilled in the skull for insertion of electrodes. Glass electrodes pulled with a DMZ-Universal Puller (Zeitz-Instruments GmbH, Martinsried, Germany) to a fine tip of ∼1 µM and filled with 2M sodium chloride (NaCl). The impedance of the electrodes was 4–6 MΩ. The electrodes were lowered through the DRN using the hydraulic micro-positioner (David Kopf Instruments, Tujunga, CA). The action potentials generated by the neurons were recorded using the AD Instruments Extracellular Recording System (Dunedin, New Zealand). The 5-HT neurons of the DRN were identified according to the waveform of their action potentials and the pattern of their generation, as explained in our previous works [
19,
28,
29]. During the experiment, the rats’ body temperature was maintained at 37°C with a heating pad (Gaymor Instruments, Orchard Park, NY, United States).
4.4. Assessment of the Acute Agmatine Effect on 5-HT Neuronal Firing Activity
In experiments aiming to assess the effect of acute agmatine on the firing activity of 5-HT neurons, after a first DRN 5-HT neuron with a stable firing activity was found, its basal firing was recorded for two minutes. Thereafter, 1 mg/kg of agmatine was administered via a catheter placed in the femoral vein. The neuronal firing activity was recorded for another two minutes. Subsequently, additional dose of 1 mg/kg of agmatine was administered (the cumulative dose 2 mg/kg) and the neuronal firing activity was recorded for another two minutes. Finally, the last administration of 1 mg/kg of agmatine was performed (the cumulative dose 2 mg/kg), and the neuron was recorded for the last two minutes. Thereafter, the animal was euthanized by overdose of chloralhydrate.
4.6. Assessment of the Chronic Agmatine Effect on 5-HT Neuronal Firing Activity
In experiments aiming to assess the effect of chronic agmatine on the firing activity of 5-HT neurons, rats were randomly divided into two groups. One group received daily injections of agmatine (40 mg/kg/day, i.p.). The control animals received daily i.p. injections of the vehicle (saline: 0.9% sodium chloride: NaCl in water). On the 15th day, electrophysiological experiments were performed. The electrode was lowered through the DRN 3-4 times and the spontaneously active 5-HT neurons detected during each electrode descend were recorded. After the completion of the electrophysiological recording, the rats were euthanized by overdose of chloralhydrate.
4.7. Assessment of Target Gene Expression in DRN Using Multiplex RT-qPCR
Following electrophysiological recordings, rats were euthanized by decapitation, and their brains were rapidly removed. Fresh brains were placed on a cold plate and sectioned coronally with razor blade. The DRN region was precisely isolated from these coronal sections using a punch tool (1.5 mm diameter) and transferred to 1.5 ml tubes. The samples were immediately flash-frozen in liquid nitrogen and stored at -80°C until further analysis. On the day of PCR experiments, DNA/RNA Shield solution (300 µL; Zymo Research) was added to the samples immediately upon removal from -80°C storage to prevent RNA degradation. Total RNA was extracted using the Quick-RNA Miniprep Kit with DNase I treatment (Zymo Research) according to the manufacturer's protocol.
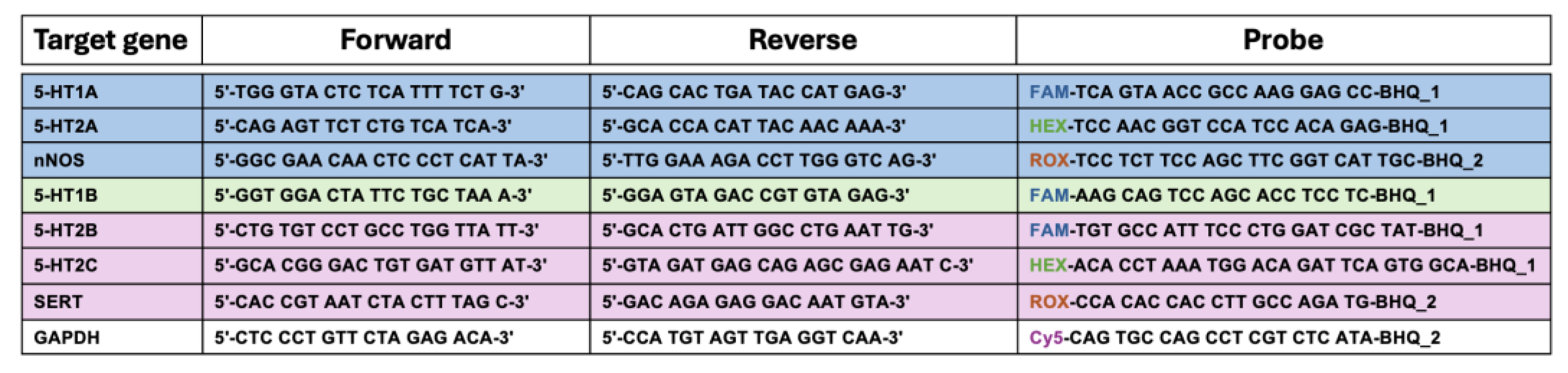
RT-qPCR primer and probe sequences were designed for multiplex analysis. Primers and dual-labeled hydrolysis probes were obtained from MultiplexDX s.r.o. (Bratislava, Slovakia). The sequences are detailed in
Table 1. Each probe was labeled at the 5' end with a fluorescent reporter dye (FAM, HEX, ROX and Cy5) and at the 3' end with a quencher dye (BHQ1, BHQ2). BLAST analysis confirmed the specificity of the primer/probe sets to their target sequences.
First multiplex reaction contains 5-HT1A, 5-HT2A, nNOS, and GAPDH. Second multiplex reaction contain 5-HT1B and GAPDH. Third multiplex reaction contains 5-HT2B, 5-HT2C, SERT, and GAPDH. For first and third multiplex reaction, the thermal cycling conditions were: cDNA synthesis at 50°C for 30 minutes, initial denaturation at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds and combined annealing/extension at 60°C for 1 minute with plate read. For second multiplex reaction, the protocol was modified with an annealing temperature of 56°C for 1 minute during the cycling steps, while maintaining the same initial cDNA synthesis and denaturation conditions. Gene expression was analyzed using CFX Maestro Software (Bio-Rad) using the 2^-ΔΔCt method, with GAPDH serving as the reference gene. Relative expression values were calculated and normalized to control samples.
4.8. Statistical Analysis
To examine the effect of different doses of acute agmatine on 5-HT neuronal firing activity, one-way RM ANOVA with a time as a factor of comparison (basal and after the administration of 1, 2, and 3 mg/kg of agmatine) , followed by the Bonferroni post hoc test, was used. To examine the effect of chronic agmatine on the firing rate of 5-HT neurons and DRN expression of the NOS, SERT, 5-HT1A/1B, and 5-HT2A/2B/2C mRNA, two-tailed Student’s t-test was applied to assess the difference between the neuronal firing rate or mRNA expression in agmatine- and vehicle-treated animals. The results are expressed as the mean ± standard error of the mean (SEM), and a value of p ≤ 0.05 was considered statistically significant.