1. Introduction
Mealybugs (Hemiptera: Pseudococcidae) are prevalent pests present in the grape growing regions around the world [
1]. They are the phloem feeders that use their piercing and sucking mouthparts to feed on different parts of grapevine including roots. Phloem sap contains sugar in a relatively higher proportion than needed by these insects and hence a large amount of sticky, sugary fluid is excreted by these insects. This excreted fluid is called honeydew, and it contains sugars, aminoacids, proteins, minerals, and vitamins.
Pseudococcus maritimus (Ehrhorn) has been the predominant mealybug pest of grapes in Virginia [
2]. An earlier research project on grapevine leafroll-associated viruses in wine grape varieties and native grape species has identified
P. maritimus,
P. viburni (Signoret), and
Ferrisia gilli (Gullan) (Hemiptera: Pseudococcidae) in Virginia vineyards (USA) [
3]. The densities, feeding locations, and movement patterns of various mealybug species fluctuate with the seasons, influenced by regional temperatures and vineyard management strategies [
4,
5]. One of the primary management of grape pests including mealybugs is chemical control [
6]; often the repetitive use of synthetic chemicals ([
7]). The presence of waxy covering on the body and their habit of hiding in the concealed locations within the vine often complicates management of mealybugs with conventional insecticides ([
7,
8]). Systemic insecticides demonstrate significant efficacy in mealybug management [
9]; however, further investigation into alternative control strategies remains essential to ensure sustainable and comprehensive pest mitigation.
Ants (Hymenoptera: Formicidae) belonging to subfamilies Myrmicinae, Dolichoderinae, and Formicinae are often found in close association with honeydew-producing hemipterans. Ants have a trophobiotic relation with the honeydew-producing hemipterans [
10]. In trophobiosis, ants receive honeydew from mealybugs (and other honeydew-producing hemipterans), and in return clean mealybug colony, provide protection against the natural enemies and transport them to new feeding sites [
10,
11,
12]. Initially considered as only reason for sooty mold growth in grape vines, honeydew also serves as a food source for natural enemies [
13].
Research have been conducted utilizing association between ants and mealybugs to control ants and its subsequent effect on mealybug populations in the vineyard [
14,
15,
16,
17]. Granular insecticides, liquid baits and insecticide-laced sugar provisioning have been tested in the field to control ant activity [
14,
15,
16,
17,
18] and have provided effective control of ants and mealybugs. Artificial sugar dispensers have been deployed in the field with or without insecticides [
14,
15,
16,
17,
19]. Notably, the use of dispensers target both the forager ants and the entire nest, as food is shared among the colony members through trophallaxis [
20,
21,
22]. Trophallaxis is a nutritive fluid-exchange observed in social insects and some nonsocial insects. When forager ants consume insecticide-laced sugar, the toxicant is not immediately lethal but are gradually disseminated throughout the colony, ensuring widespread exposure [
20,
22]. It helps baits with a low concentration of toxicants to reach from vines to ant nests and recruit more ants to the bait [
20,
21]. This indirect mechanism provides low-concentration toxicants to travel from vines to ant nests, recruiting additional ants to the baits and hence facilitating large-scale control.
Therefore, our study aimed at identifying the species and monitoring the distribution of populations of ants and mealybugs before and after deploying dispensers in the vineyard. During the harvest season, we also assess the level of mealybug infestations in the sampled area with and without the dispensers. The analysis reported here has shed some light on important ant genera in vicinity of mealybugs and the potential of sugar dispensers in controlling ants and mealybugs infestations in the vineyard.
2. Materials and Methods
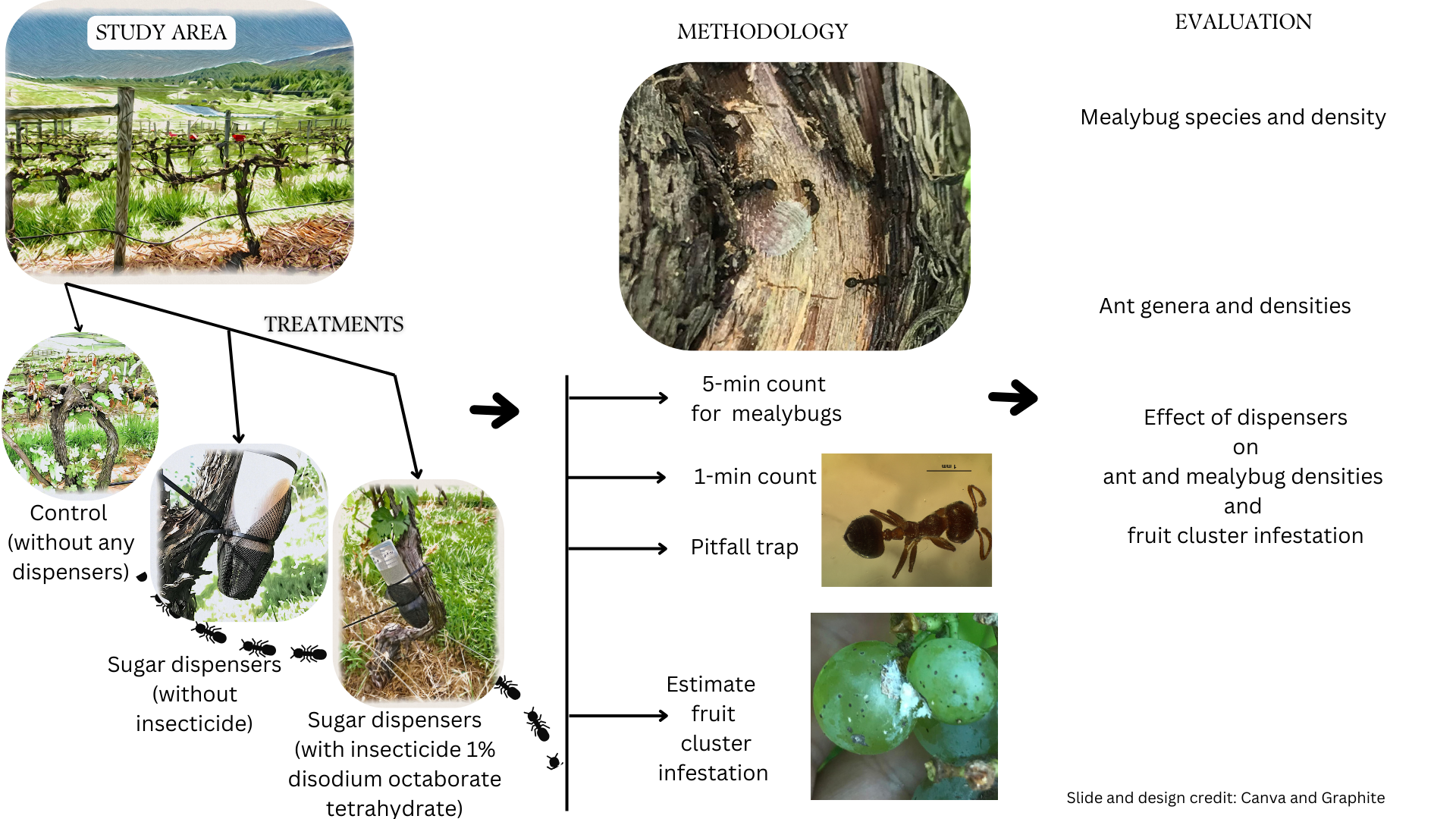
2.1. Field Sites and Experimental Design
The ant-mealybug experiment was carried out in two vineyard sites: Orange County (vineyard HV) (38° 13’ N, 78° 6’ W), and Fauquier County (vineyard PC) (38° 47’ N, 77° 44’ W), Virginia, USA. Vineyards were selected based on the availability and pest pressure recorded by the researcher in the previous years. These are conventional vineyards, relying on synthetic insecticides for pest management. Each vineyard block was more than 10 years old and had a previous history of mealybug infestations.
The research trial was carried out in an area of 0.283 to 0.081 hectare inside each vineyard. Each experimental area was divided into three plots: control plots, sugar dispenser plots (SD), and sugar dispenser with insecticide (SDI) plots (
Figure 1). The control plots were separated from other treatment plots by 10 to 20 meters. Two to three vine rows distance were maintained between the SD and SDI plots.
2.2. Sugar Dispensers
The sugar dispensers used in the field is based on earlier research by Daane, et al. [
15] and repeated by Parrilli, et al. [
17]. 250 ml HDPE narrow mouth bottles, assembled with white polypropylene closure were modified into dispenser (The Lab Depot, Dawsonville, GA). A 1 cm circular hole was drilled in the cap of the tube and a permeable mesh was placed between the tube and the cap. A 5.08 cm garden slotted mesh net cup was placed outside the cap with a plastic mesh outside to allow the entry of ants, but not bees (
Figure 1). Sugar dispensers, if improperly set up, could have a detrimental effect on bees in the vineyard.
We deployed 12-16 dispensers (
Figure 1) in four rows of vines, evenly placing them after every 5-10 plants through the experimental plots. They were deployed at the beginning of June and removed in the second week of September. A gap of 2-5 rows of grapevines was maintained in between each of the two treatments. Each of the dispensers was refilled and cleaned every one to two weeks. The insecticide used for ant control was Greenway liquid ant-killing bait with the active ingredient 1% disodium octaborate tetrahydrate. As per the manufacturer’s instruction, the Gourmet liquid ant bait could either be used in full strength or diluted with equal amount of distilled water. During the first one and half months of the research, we used liquid bait in full concentrations and after that, they were diluted to 50% strength.
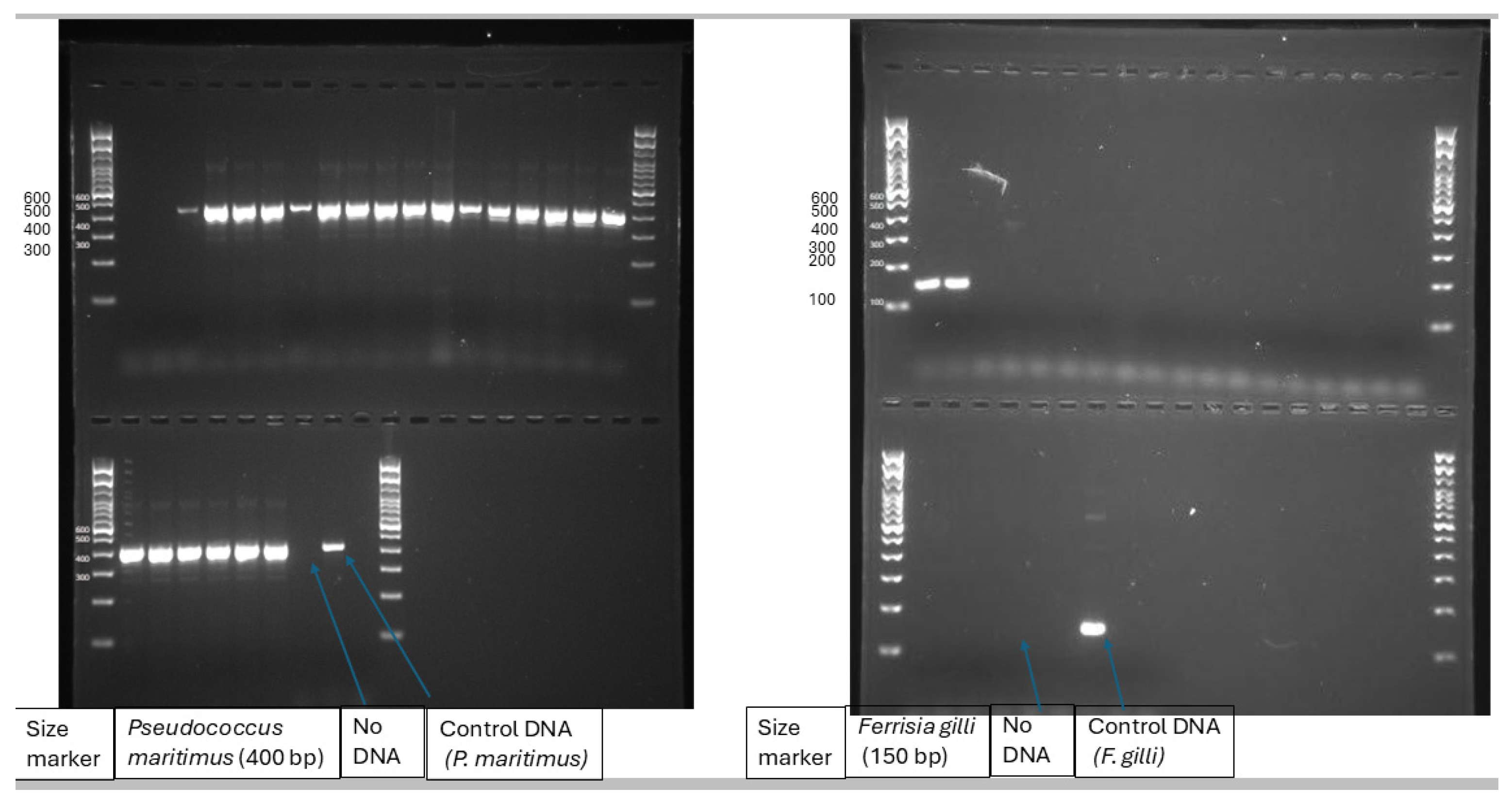
2.3. Mealybug Species
Most of the mealybug species were identified and photographed in the field based on morphology. Some representatives were taken back to the laboratory for identification. Some of the samples were pooled out and identified by multiplex PCR using mitochondrial cytochrome oxidase subunit one gene based on Daane
, et al. [
23]. The mealybug numbers were also counted in the vineyard by using a 5-minute visual count.
2.4. Ant Activity
Ant populations in the vineyard were monitored and sampled weekly in all three treatment plots by both 1. Pitfall trapping 2. 1-minute visual count. 50 ml falcon centrifuge tubes were used for pitfall trapping, with 75% alcohol and a few drops of ethylene glycol as the preservative [
24,
25,
26,
27,
28]. The advantage of using ethanol is that it does not attract ant species differentially. A total of 5-8 pitfall traps were placed randomly per experimental plot per site. The pitfall traps were set up at the field sites in the morning between 8:00 and 9:00 a.m. and retrieved after approximately one hour.
Ant activity was also monitored by counting the number of ants crossing an imaginary line of 20 cm in length and in between the vine canopy and above the dispensers on the trunk for 1-minute. The vines for the visual count and pitfall trap placement were selected randomly to represent the whole plot. The ants were collected in 70% ethanol and taken back to the lab for identification using the identification keys [
29].
2.5. Fruit Cluster Infestation
During the harvest season, 15 grape clusters per treatment were examined for the presence of mealybugs in the field. 10 grape clusters per treatment were taken back to the laboratory to examine the presence of mealybugs.
2.6. Data Analysis
The foraging activity of the different ant species was calculated by the mean number of each ant species collected throughout the season. The data were first transformed using log transformation to check for homogeneity. Since the data wasn't normally distributed, Steel's method, a nonparametric approach, was used for comparisons with the control. This method employs the Wilcoxon test to compare the control sample with each treatment group. [
30]. Data were analyzed based on count per sampling date and total count throughout the season.
Before the commercial harvest, 25 fruit clusters per treatment in each replicate were evaluated using the following scoring system: 0= no mealybug or honeydew, 1=honeydew and five or fewer mealybugs, 2=honeydew and six to nine mealybugs, 3=honeydew and more than ten mealybugs, and 3=honeydew and egg mass). Fruit clusters with a score of 2 and 3 were considered unmarketable or extremely infested. Fruit cluster infestation was analyzed using Wilcoxon paired test [
31].
Multivariate analysis was used to analyze the relation between the number of ants per minute, the number of mealybugs per vine, and the percentage of cluster damage for each of the sites. The multivariate analysis on JMP® 17 Pro was performed under default setting, that uses Pearson Correlation to calculate relationship between variables. For the statistical analysis, we used the JMP® 17 Pro and JMP® 18 software package [
32,
33].
3. Results
3.1. Mealybug Species in the Vineyard
Two species of mealybugs were identified in the field and confirmed by multiplex PCR as
Pseudococcus maritimus and
Ferrisia gilli (Figure 2).
In general, Pseudococcus maritimus remains the predominant species throughout our survey. The exact proportion of each species will vary seasonally with changes in proportion of the less common of the two species i.e., Ferrisia gilli. However, the population of Ferrisia gilli reaches up to 47% in the peak season (July-August) in our study.
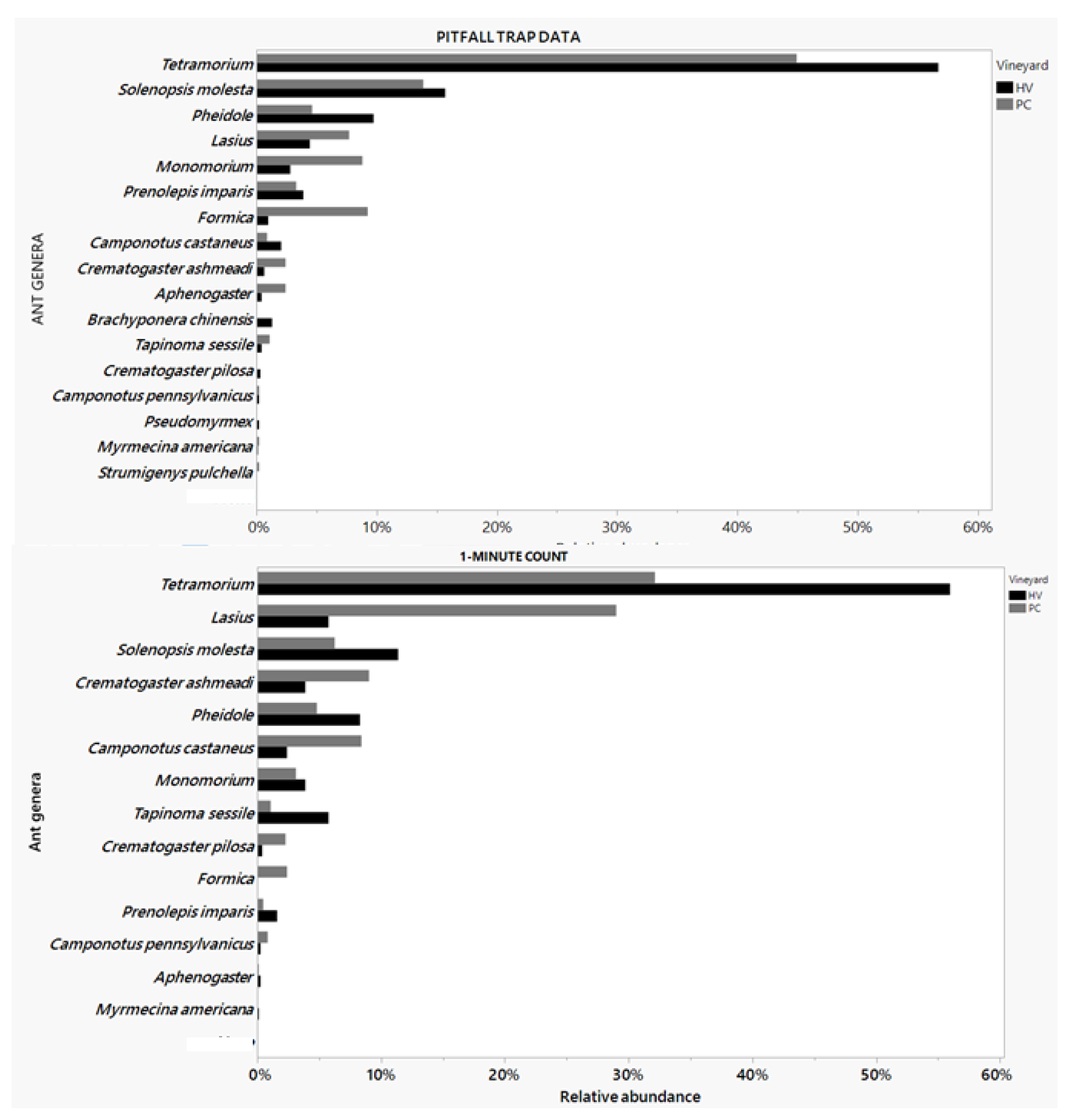
3.2. Ant Species in the Vineyard
A total number of 1131 specimens of ants were collected in total (674 samples from HV vineyard and 457 from PC vineyard) over the whole field season, representing 12 genera of ants. Ants were identified up to genus level due to time constraint. The top five leading foragers in the vineyards include
Tetramorium Mayr,
Lasius Fabricius,
Solenopsis molesta (Say),
Pheidole Westwood, and
Crematogaster Lund based on 1-minute count and pitfall trap data (
Figure 3).
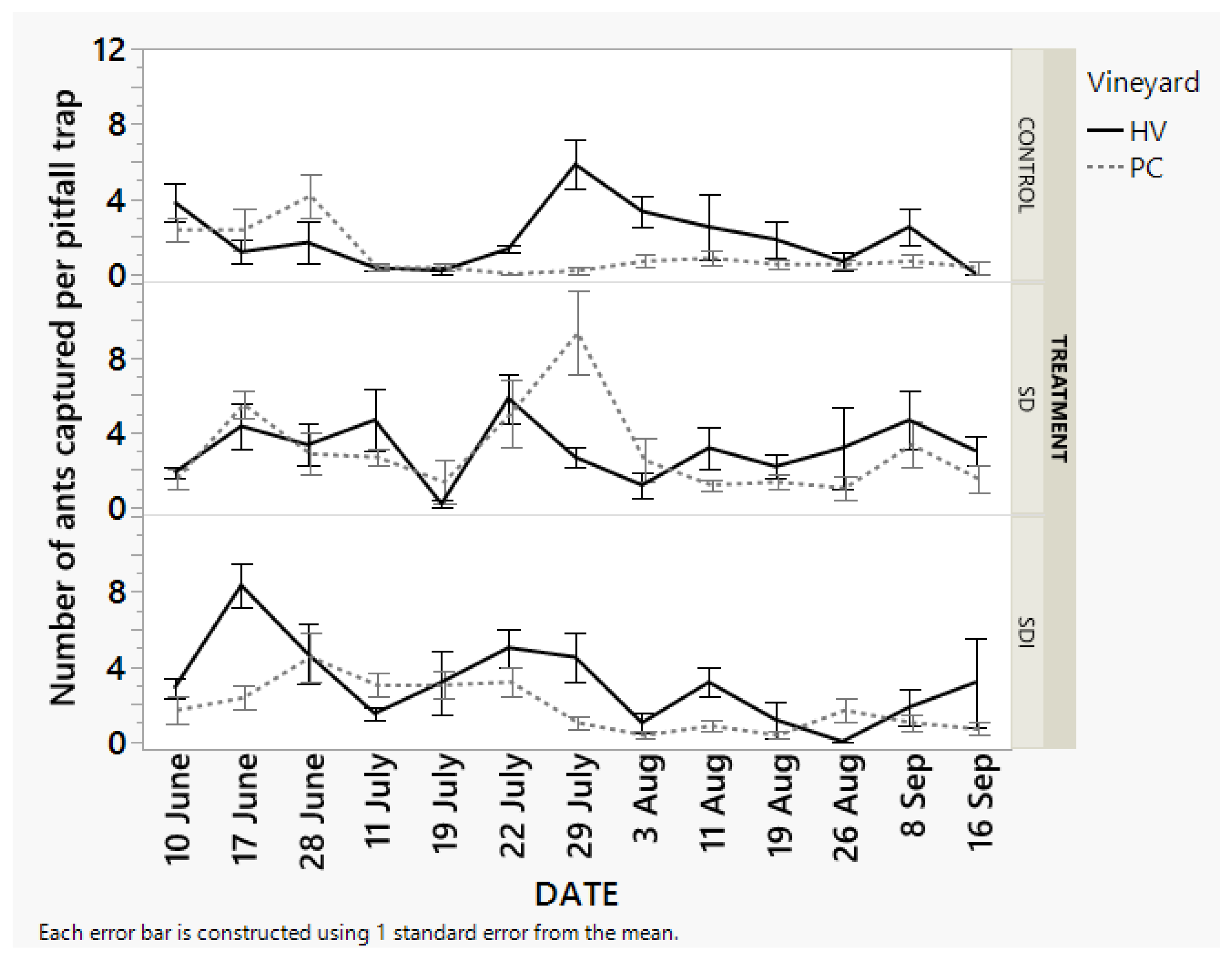
3.2.1. Ant Densities in the Field with or without Sugar Dispensers
Fewer number of ants were observed and captured in the control treatment throughout the season. The sugar dispensers with insecticide bait initially attracted a higher number of ants during the initial few weeks of deployment and started decreasing throughout the season. In comparison to the other treatments, sugar baits attracted a higher number of ants throughout the season (
Figure 4).
3.2.2. 1-Minute Count on the Trunk
Field analysis of the mean number of ants was calculated as average value for the entire season as well as per sampling dates. During the 1-minute count data analyses, the mean densities of ants in the SD and the SDI treatments were not significantly different from the density in the control before the placement of dispensers (
Table 1: vineyard PC:
Z: -0.274,
p=0.946 for SD: control;
Z: -0.508,
p=0.829 for SDI: control; and vineyard HV:
Z= -0.168,
p=0.397 for SD: control;
Z= -1.509,
p=0.226 for SDI: control) for both vineyards.
When data were compared per sampling dates, the densities were not statistically significant for most of the sampling dates (
Table 1) in both vineyards HV and site PC during the deployment of sugar dispensers. The mean densities of ants for the entire [i]sampling period have different data analysis results. In vineyard PC, there was no significant difference in the mean densities of ants present in SDI treatment compared to the control (
Table 2:
Z=-0.17,
p=0.97). However, there was a significant difference between the mean densities of ants present in the SD treatment in comparison to the control (
Table 2:
Z=4.02, p=0.0001). The result was just the opposite for vineyard HV. There is a significant difference in the mean densities of ants present in SDI treatment compared to the control (
Table 2:
Z=2.41, p=0.04). However, there was no significant difference between the mean densities of ants present in the SD treatment in comparison to the control (
Table 2:
Z=1.42,
p=0.33).
3.2.3. Pitfall Trap Data
The mean densities of ants in the sugar bait and the ant bait treatments were not significantly different from the density in the control before the placement of ant dispensers in vineyard PC (
Table 3:
Z=-0.86, p=0.59 for SD: control;
Z=-0.68,
p=0.65 for SDI: control); and vineyard HV (
Table 3:
Z=-0.57, p=0.78 for SD: control;
Z=-1.56,
p=0.205 for SDI: control).
Like the one-minute data count, the pitfall trap data were analyzed for each of the sampling dates as well as the total sampling duration. When data were compared per sampling dates, the densities were not statistically significant for most of the dates (
Table 3 and 4) in both vineyards during the deployment of sugar dispensers. The mean densities of ants for the entire sampling period have different results compared to the one-minute count. There was a significant difference in the mean densities of ants present in both the SDI and SD treatment compared to the control in vineyard PC (
Table 4:
Z=5.04,
p=<0.001 for SD: control, and
Z=3.604,
p=0.0006 for SDI: control). There was also a significant difference in the mean densities of ants present in both the SDI and SD treatments compared to the control in vineyard HV (
Table 4:
Z=3.03,
p=0.0047 for SD: control, and Z- score: 2.52, p=0.022 for SDI: control).
3.2.4. Fruit Cluster Injury Due to the Presence of Mealybugs
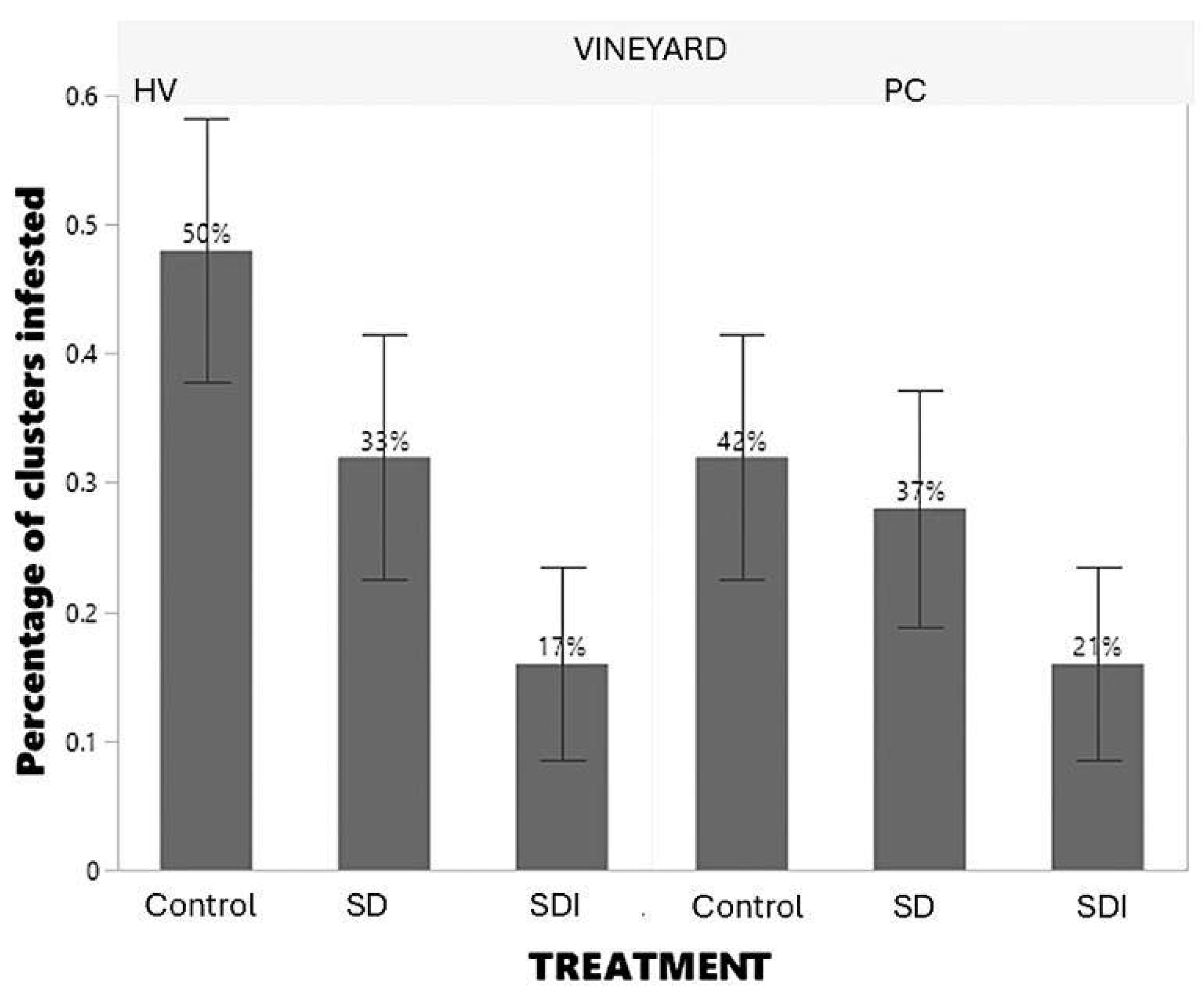
In vineyard HV, 50% of the infested clusters evaluated were from the control treatment, while 33% were from the SD treatment and 17% from the SDI treatment. In vineyard PC, 42% of the infested clusters evaluated were from the control treatment, while 37% were from the SD treatment and 21% from the SDI treatment (
Figure 5). The nonparametric test for the fruit cluster infestation had similar results in both vineyards. The cluster infestation level was significantly different for sugar-toxicant bait in comparison to the control for both sites (vineyard PC:
Z=2.034,
p=0.0419; and vineyard HV:
Z=3.005,
p=0.0027 for SDI: control). On the other hand, the cluster infestation level between SD treatment in comparison to the control in both vineyards were not significantly different (vineyard PC:
Z= -1.634,
p=0.1023; vineyard H:
Z=-1.863,
p=0.0624 for SD: control).
3.2.5. Relation Between Ants, Mealybugs and Cluster Infestation
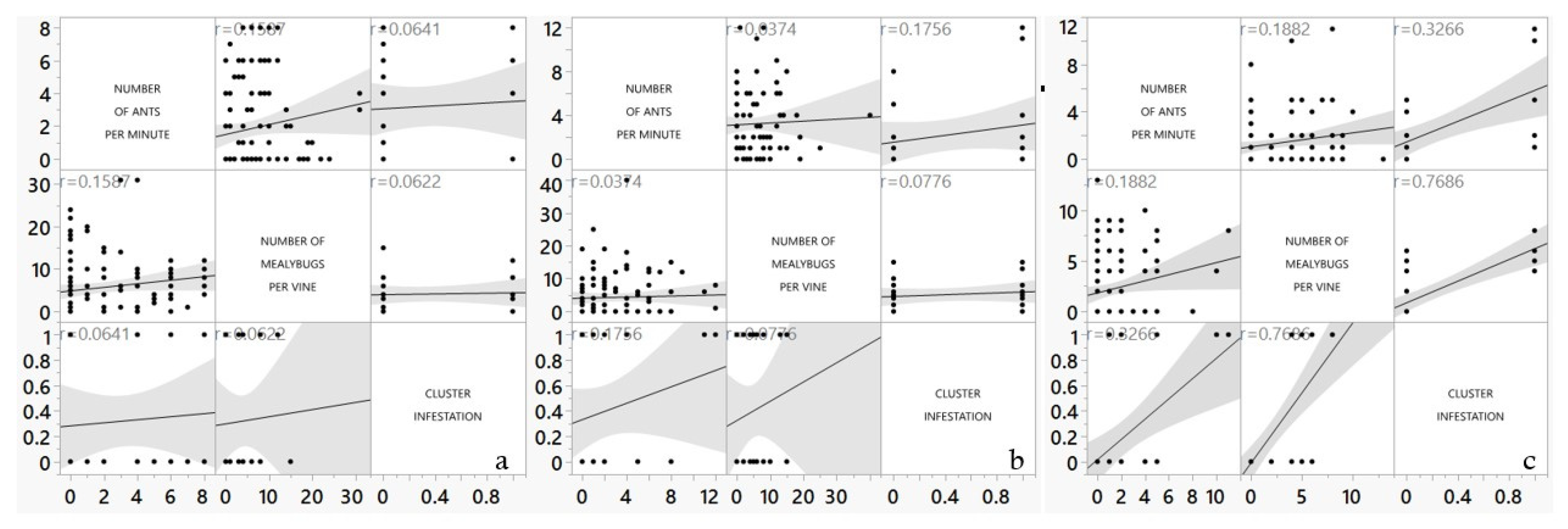
For the multivariate data analysis, we compared the data of ant densities, mealybug densities, and cluster injury data for the whole sampling season. For vineyard PC, the correlation coefficient between the number of ants per minute and the number of mealybugs per vine is low, positive, and not significant (
r=0.158;
p=0.097) in the control treatment (
Figure 6). In the same treatment, the correlation coefficient between both the number of ants per minute as well as the number of mealybugs per vine and cluster damage is low, positive, and not significant (
r=0.0641;
p=0.76 for ant number;
r=0.06; p=0.77 for mealybugs number). These weak-to-low correlation coefficient values imply that changes in one domain are not correlated strongly with changes in the related domain.
For the SDI treatment in vineyard PC, the correlation coefficient between the number of ants per minute and the number of mealybugs per vine is low, positive, and significant (r=0.1882; p=0.0489). In the same treatment, the correlation coefficient between both the number of ants per minute and the cluster damage is weak, positive, and not significant (r=0.326; p=0.11). The number of mealybugs per vine and cluster damage has a moderate correlation coefficient with a highly significant effect (r=0.768, p<0.0001).
For the SD treatment in vineyard PC, the correlation coefficient between the number of ants per minute and the number of mealybugs per vine is low, positive, and not significant (r=0.037; p=0.69). In the same treatment, the correlation coefficient between both the number of ants per minute as well as the number of mealybugs per vine and cluster damage is low, positive, and not significant (r=0.175; p=0.401 for ant number; r=0.077; p=0.71 for mealybugs number).
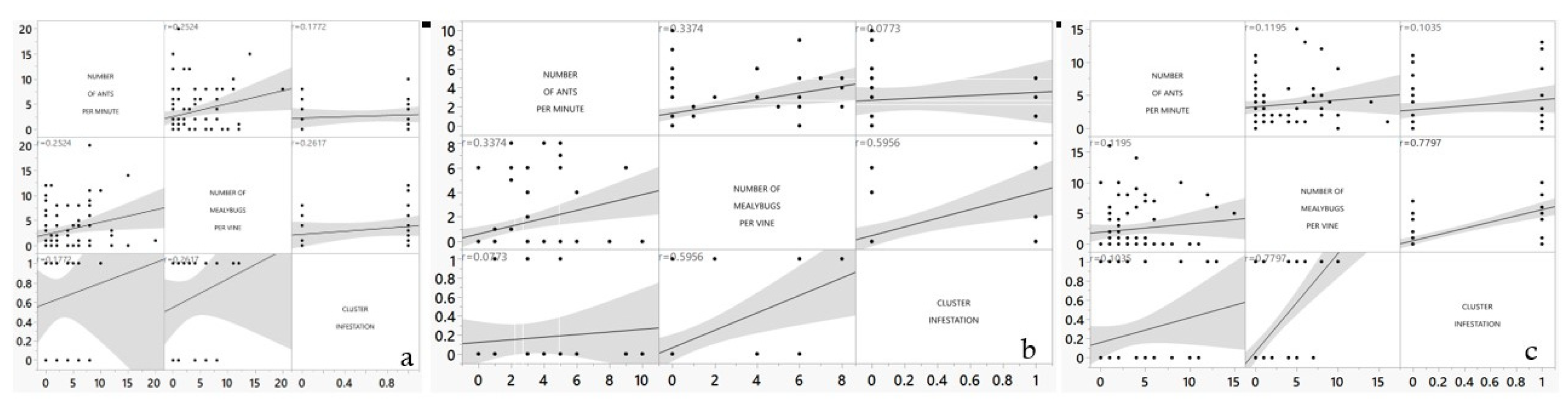
For vineyard HV, the correlation coefficient between the number of ants per minute and the number of mealybugs per vine is weak, positive, and significant (
r=0.2524;
p=0.0239) in the control treatment (
Figure 7). In the same treatment, the correlation coefficient between both the number of ants per minute as well as the number of mealybugs per vine and cluster damage is weak, positive, and not significant (
r=0.177;
p=0.397 for ant number;
r=0.261; p=0.206 for mealybugs number).
For the SDI treatment in vineyard HV, the correlation coefficient between the number of ants per minute and the number of mealybugs per vine is weak, positive, and significant (r=0.337; p=0.0022). In the same treatment, the correlation coefficient between the number of ants per minute and the cluster damage is weak, positive, and not significant (r=0.0773; p=0.713). The number of mealybugs per vine and cluster damage has a moderate correlation coefficient with a highly significant effect (r=0.595, p=0.0017).
For the SD treatment in vineyard HV, the correlation coefficient between the number of ants per minute and the number of mealybugs per vine is weak, positive, and not significant (r=0.119; p=0.291). In the same treatment, the correlation coefficient between the number of ants per minute and the cluster damage is weak, positive, and not significant (r=0.1035; p=0.47). The number of mealybugs per vine and cluster damage has a moderate correlation coefficient with a highly significant effect (r=0.779, p<0.0001).
4. Discussion
This study has explored the diversity of ants and mealybug populations in the vineyard setting and identified some of the important ant genera in close association with mealybugs. When most of the available control methods for mealybugs available on the market heavily rely on chemical spray, our findings have highlighted the potential of sugar dispensers as an alternative.
Dispensers containing sugar solution as well as ant insecticide i.e., Greenway Liquid Ant Killing Bait (1% disodium octaborate tetrahydrate) were deployed from the first week of June until the second week of September. This is a commercially available low-toxicity liquid bait, approved by United States Environmental Protection Agency (EPA) for both agricultural and non-agricultural use [
34]. This is a broad spectrum, which can be harmful to pollinators. To mitigate unintended harm, we incorporated permeable mesh inside the dispensers and a garden-slotted mesh net over the cap, allowing ant access while preventing unnecessary liquid drips.
The dispensers were deployed coinciding with the peak ant activity and mealybugs-
Pseudococcus maritimus and
Ferrisia gilli infestations in the vineyard [
17]. Grapevine infesting mealybugs usually overwinter under the bark as egg mass or first instars and move from the trunk and start infesting new buds next spring [
35]. Our timed-visual count results showed significant reduction in ant activities due to the provisioning of areas with dispensers containing insecticide.
One of the first important aspects of the study is the consistently lower number of ants in the control plots throughout the sampling period in comparison to the plots containing dispensers. Another interesting observation from this study has been significantly different ant numbers in different treatment plots in comparison to the control based on the pitfall trap data and timed-visual count. The ant numbers are not significantly different when compared on each of the sampling dates. The ant numbers were consistently lowest in the control plots in comparison to the one with the dispensers. The sampling plots with sugar dispensers had consistently a higher number of ants.
During the initial days of dispenser deployment, ant densities were numerically higher in plots with sugar dispensers containing insecticide, likely due to attraction before toxicity effects took hold. However, by mid-July, most ant nests in treated areas had disappeared, with only a few wandering ants remaining or appearance of few new nests. The effect of dispensers on the field population of ants might be more than the data observed via pitfall. The data evaluation for the entire sampling period for timed-visual count data revealed a significant reduction in ant densities inplots having dispensers compared to the control. This result is consistent with the previous study by Beltrà, et al. [
14], Daane, et al. [
15], Perez-Rodriguez, et al. [
19], and Parrilli, et al. [
17] where control of ants in association with mealybugs were achieved by the use of liquid baits.
Contrary to sugar dispensers having insecticide treatment, sugar dispensers lacking insecticide treatment had lower densities of ants during the initial days of dispenser deployment and slowly the ant number starts increasing in a few weeks. By the third week of August, even in the trunk containing dispensers, more ants were seen around mealybugs than on the dispensers. The number of ant foragers at ant baits increasing over time can be explained by pheromone recruitment and the establishment of foraging trails [
36]
One of the drawbacks of using pitfall traps is the underrepresentation of subterranean ants and the high representation of epigeic ants. Some of the arboreal ants (e.e.
Crematogaster and
Lasius) included in the sample are also active on the surface. Some of the epigeic and subterranean ants are underrepresented in the one-minute visual count for ants in the trunk [
28,
37]. The pitfall traps were installed as soon as they reached the field early in the morning and taken back when leaving the field, the same day. In addition, the vineyards are mostly surrounded by wooded areas and hence may have contained ant species present in the forests as well.
The use of sugar dispensers with or without insecticide decreased Parrilli, et al. [
17] demonstrated that the use of liquid sugar dispensers decreased the percentage of infested grapes bunches in comparison to control. Beltrà, et al. [
14],Daane, et al. [
15],Perez-Rodriguez, et al. [
19] also demonstrated that sugar provisioning decreases mealybug infestation.
Fifteen genera of ants were recorded foraging around commercial vineyards in Virginia in 2022. Among those ants, Tetramorium ant remains the dominant ant in both vineyards followed by the thief ant, the Lasius genus (garden ant), the odorous house ant (Tapinoma sessile), and the Pheidole genus (big-headed ants). During the field research, some of the ant species seen in close association to and tending after the mealybugs include genus like Crematogaster- the acrobat ant, especially species C. ashmeadi Emery and C. pilosa Emery, Tetramorium- the pavement ant, Lasius- the garden ant, and Solenopsis molesta- the thief ant.
Crematogaster ashmeadi and
C. pilosa are arboreal ants native to the southeastern United States [
38,
39,
40]. They are commonly known as acrobat ants because of the way the workers hold up their abdomen over the rest of the body when disturbed. They mostly nest in trees, logs, fallen branches, and in hollow stems of plants. These ants were seen in the grapevine under the bark, actively tending mealybugs and raising their abdomen up when alarmed or disturbed. As seen on the field, they also pick mealybugs up and transfer them to safe places when disturbed.
Tetramorium and
Lasius have been two of the dominant genera seen in association with mealybugs, the former is subterranean, and the latter is arboreal [
41]. Like
Crematogaster, they were seen actively defending and moving mealybugs around to safer sections of the grapevines. Among the common genera of ants in the vineyard,
Pheidole, and
S. molesta are widespread generalists [
42,
43].
The use of sugar/ant dispensers has often been combined with other methods of biological control like the use of predators or parasitoids [
14,
17]. The parasitization rate and predation rates on different treatments were not included in our study due to time constraints. Previous studies have recorded a significant increase in the predation pressure and parasitization rates in mealybugs when sugar dispensers were deployed [
14,
17,
19].
While studying the relationship between ant and mealybug population densities and fruit cluster infestation rate, a strong correlation between the number of ants per minute, and the number of mealybugs per vine with the cluster infestation was lacking in our data. Although there is a weak correlation, the field observation after the use of dispensers led to numerically lesser ant activities and lesser number of ant nests, mealybugs numbers and cluster injury in the treatment area having sugar dispensers with insecticide.
A further study might be needed to study the effect of multiple years of placement of dispensers on ant activity, mealybug levels and cluster infestation rate on the vineyard. The dispensers should be continuously deployed for more than two consecutive years for increasing their efficacy against vineyard-dwelling ant populations [
44].
One major drawback encountered during the research was the significant amount of time required to assemble and deploy the dispensers. This limitation made the process labor-intensive and potentially less practical for larger-scale applications. The existing dispensers, however, demonstrated functionality and effectiveness when utilized in vineyards of small to medium sizes, suggesting their potential suitability for more localized or moderately scaled settings. Optimization of the assembly and deployment process could enhance their usability for larger operations in the future. For small- to medium-sized vineyards, management of ants and mealybugs can be achieved via dispenser-based control method.
5. Conclusions
Our research emphasizes the use of sugar dispensers in managing ants and mealybug population in the vineyard. It also highlights important mealybug species and ant genera in close association with mealybugs. The ants and mealybug population in the field can be managed effectively using sugar dispensers, especially the one having ant insecticide as reported from previous similar studies [
14,
15,
16,
17]. One of the time-consuming aspects of the dispensers used in the field started from the assemblage of all the tiny pieces of the sugar dispenser and its delivery. The current dispenser is more suitable for small- to medium-sized vineyards, which require a limited number of dispensers. More research should be carried out to optimize and improve the installation and maintenance to make it more friendly for vineyards of varying sizes. Further study should focus on use of sugar dispensers for multiple years to study its long-term effect on managing mealybug population in various conditions in vineyards.
Author Contributions
The following statements should be used “Conceptualization, P.C., D.G.F., T.K., M.N., C.B. and T.J.; methodology, P.C., D.G.F., T.K., M.N., C.B. and T.J.; software, P.C.; validation, P.C., D.G.F., T.K., M.N., C.B. and T.J.; formal analysis, P.C.; investigation, P.C., D.G.F., T.K., M.N., C.B. and T.J.; resources, P.C., R.M.; data curation, P.C.; writing—original draft preparation, P.C.; writing—review and editing, P.C., D.G.F., T.K., M.N., C.B. and T.J.; visualization, P.C.; supervision, P.C., D.G.F., T.K., M.N., C.B. and T.J.; project administration, R. M.; funding acquisition, D.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Virginia Wine Board (Virginia, United States).
Data Availability Statement
Our data are available online at MDPI page.
Acknowledgments
We would like to thank Dr. Donald Mullins, and Sandra Gabbert for allowing us to use their laboratory. We would also like to thank Dr. Chin-Cheng Scotty Yang and Virginia Tech Insect ID lab for help in ant identification.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mani, M.; Amala, U. Fruit Crops: Grapevine. In Mealybugs and their Management in Agricultural and Horticultural crops, Mani, M., Shivaraju, C., Eds.; Springer India: New Delhi, 2016; pp. 329-351.
- Pfeiffer, D.G. Major insect and mite pests of grape in eastern North America. In Wine Grape Production Guide for Eastern North America, Wolf, T.K., Ed.; Natural Resource, Agriculture, and Engineering Service (NRAES): Ithaca, New York, 2008; pp. 241-261.
- Jones, T.J. Grapevine viruses and associated vectors in Virginia: survey, vector management, and development of efficient grapevine virus testing methods. Virginia Polytechnic Institute and State University, Blacksburg, Virginia, 2016.
- Grasswitz, T.R.; James, D.G. Movement of grape mealybug, Pseudococcus maritimus, on and between host plants. Entomologia Experimentalis et Applicata 2008, 129, 268-275. [CrossRef]
- Geiger, C.A.; Daane, K.M. Seasonal Movement and Distribution of the Grape Mealybug (Homoptera: Pseudococcidae): Developing a Sampling Program for San Joaquin Valley Vineyards. Journal of Economic Entomology 2001, 94, 291-301. [CrossRef]
- Franco, J.C.; Zada, A.; Mendel, Z. Novel approaches for the management of mealybug pests. In Biorational Control of Arthropod Pests: Application and Resistance Management, Ishaaya, I., Horowitz, A.R., Eds.; Springer Netherlands: Dordrecht, 2009; pp. 233-278.
- Daane, K.; Almeida, b.; Bell, V.; Walker, b.; Botton, M.; Fallahzadeh, M.; Mani, M.; Daane, K.; Almeida, R.; Bell, V.; et al. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions, Bostanian, N.J., Vincent, C., Issacs, R., Eds.; Springer Dordrecht, Heidelberg, New York, London, 2012; pp. 271-307.
- Mani, M.; Krishnamoorthy, A.; Shivaraju, C. Biological suppression of major mealybug species on horticultural crops in India. Journal of Horticultural Sciences 2011, 6, 85-100. [CrossRef]
- Mansour, R.; Belzunces, L.P.; Suma, P.; Zappalà, L.; Mazzeo, G.; Grissa-Lebdi, K.; Russo, A.; Biondi, A. Vine and citrus mealybug pest control based on synthetic chemicals. A review. Agronomy for Sustainable Development 2018, 38, 1-20. [CrossRef]
- Styrsky, J.D.; Eubanks, M.D. Ecological consequences of interactions between ants and honeydew-producing insects. Proceedings of Biological sciences / The Royal Society 2006, 274, 151-164. [CrossRef]
- Wilder, S.M.; Holway, D.A.; Suarez, A.V.; LeBrun, E.G.; Eubanks, M.D. Intercontinental differences in resource use reveal the importance of mutualisms in fire ant invasions. Proc Natl Acad Sci U S A 2011, 108, 20639-20644. [CrossRef]
- Xu, C.; Su, J.; Qu, X.; Zhou, A. Ant-mealybug mutualism modulates the performance of co-occurring herbivores. Scientific Reports 2019, 9, 13004. [CrossRef]
- Fernández de Bobadilla, M.; Ramírez, N.M.; Calvo-Agudo, M.; Dicke, M.; Tena, A. Honeydew management to promote biological control. Current Opinion in Insect Science 2024, 61, 101151. [CrossRef]
- Beltrà, A.; Navarro-Campos, C.; Calabuig, A.; Estopà, L.; Wäckers, F.L.; Pekas, A.; Soto, A. Association between ants (Hymenoptera: Formicidae) and the vine mealybug (Hemiptera: Pseudococcidae) in table-grape vineyards in Eastern Spain. Pest Manag Sci 2017, 73, 2473-2480. [CrossRef]
- Daane, K.M.; Cooper, M.L.; Sime, K.R.; Nelson, E.H.; Battany, M.C.; Rust, M.K. Testing baits to control Argentine ants (Hymenoptera: Formicidae) in vineyards. Journal of Economic Entomology 2008, 101, 699-709. [CrossRef]
- Daane, K.M.; Sime, K.R.; Hogg, B.N.; Bianchi, M.L.; Cooper, M.L.; Rust, M.K.; Klotz, J.H. Effects of liquid insecticide baits on Argentine ants in California's coastal vineyards. Crop Protection 2006, 25, 592-603. [CrossRef]
- Parrilli, M.; Profeta, M.; Casoli, L.; Gambirasio, F.; Masetti, A.; Burgio, G. Use of sugar dispensers to disrupt ant attendance and improve biological control of mealybugs in vineyard. Insects 2021, 12, 330. [CrossRef] [PubMed]
- Nondillo, A.; Andzeiewski, S.; Bello Fialho, F.; Bueno, O.C.; Botton, M. Control of Linepithema micans (Hymenoptera: Formicidae) and Eurhizococcus brasiliensis (Hemiptera: Margarodidae) in vineyards using toxic baits. Journal of Economic Entomology 2016, 109, 1660-1666. [CrossRef] [PubMed]
- Perez-Rodriguez, J.; Pekas, A.; Tena, A.; Wäckers, F. Sugar provisioning for ants enhances biological control of mealybugs in citrus. Biological Control 2021, 157, 104573. [CrossRef]
- Rust, M.K.; Reierson, D.A.; Klotz, J.H. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling Argentine ants (Hymenoptera: Formicidae). J Econ Entomol 2004, 97, 1017-1024. [CrossRef]
- Wcislo, W.T. Trophallaxis in weakly social bees (Apoidea). Ecological Entomology 2015, 41, 37-39. [CrossRef]
- Klimes, P.; Borovanska, M.; Plowman, N.S.; Leponce, M. How common is trophobiosis with hoppers (Hemiptera: Auchenorrhyncha) inside ant nests (Hymenoptera: Formicidae)? Novel interactions from New Guinea and a worldwide overview. Myrmecological News 2018, 26, 31-45.
- Daane, K.M.; Middleton, M.C.; Sforza, R.; Cooper, M.L.; Walton, V.M.; Walsh, D.B.; Zaviezo, T.; Almeida, R.P. Development of a multiplex PCR for identification of vineyard mealybugs. Environ Entomol 2011, 40, 1595-1603. [CrossRef] [PubMed]
- Bestelmeyer, B.T.; Wiens, J.A. The effects of land use on the structure of ground-foraging ant communities in the Argentine Chaco. Ecological Applications 1996, 6, 1225-1240. [CrossRef]
- Calixto, A.; Marvin, K.; Dean, A. Sampling ants with pitfall traps using either propylene glycol or water as a preservative. Southwestern Entomologist 2007, 32, 87-91. [CrossRef]
- Wike, L.D.; Martin, F.D.; Paller, M.H.; Nelson, E.A. Impact of forest seral stage on use of ant communities for rapid assessment of terrestrial ecosystem health. Journal of Insect Science 2010, 10, 1-16. [CrossRef] [PubMed]
- Johnson, J.; Adkins, J.; Rieske, L.K. Canopy vegetation influences ant (Hymenoptera: Formicidae) communities in headwater stream riparian zones of Central Appalachia. Journal of Insect Science 2014, 14, 1-5. [CrossRef]
- Sheikh, A.; Ganaie, G.; Thomas, M.; Bhandari, R.; Rather, Y.A. Ant pitfall trap sampling: An overview. Journal of Entomological Research 2018, 42, 421-436. [CrossRef]
- Fisher, B.L.; Cover, S.P.; Kirsch, G.; Kane, J.; Nobile, A. Ants of North America: A guide to the genera, 1 ed.; University of California Press: 2007.
- Douglas, C.E.; Michael, F.A. On distribution-free multiple comparisons in the one-way analysis of variance. Communications in Statistics - Theory and Methods 1991, 20, 127-139. [CrossRef]
- Beatty, W. Decision Support Using Nonparametric Statistics; 2018.
- JMP® 17 Pro JMP Statistical Discovery LLC, Cary, NC, USA, 2024.
- JMP® 18 JMP Statistical Discovery LLC, Cary, NC, USA, 2024.
- AntPro, K. AntPro Insect Control System ™. Available online: (accessed on.
- Varela, L.G. Grape mealybug (Pseudococcus maritimus) life cycle in the North Coast. University of California Cooperative Extension 2005.
- Greenberg, L.; Klotz, J.H. Argentine Ant (Hymenoptera: Formicidae) Trail Pheromone Enhances Consumption of Liquid Sucrose Solution. Journal of Economic Entomology 2000, 93, 119-122. [CrossRef]
- Ward, D.F.; New, T.R.; Yen, A.L. Effects of Pitfall Trap Spacing on the Abundance, Richness and Composition of Invertebrate Catches. Journal of Insect Conservation 2001, 5, 47-53. [CrossRef]
- Tschinkel, W. The natural history of the arboreal ant, Crematogaster ashmeadi. Journal of Insect Science 2002, 2, 1-12. [CrossRef]
- Saarinen, E.V. Acrobat Ant Crematogaster ashmeadi Emery (Insecta: Hymenoptera: Formicidae: Myrmicinae); IFAS/University of Florida: Gainsville, Florida, 2021.
- MacGown, J.A. Ants (Formicidae) of the southeastern United States. Available online: (accessed on.
- Stock, T.; Gouge, D. Integrated pest management for ants in schools; Pacific Northwest Extension: 2022; p. 10.
- Wilson, E.O. Pheidole in the New World: A dominant, hyperdiverse ant genus; Harvard University Press: 2003.
- Delabie, J.H.C.; Fowler, H.G. Soil and litter cryptic ant assemblages of Bahian cocoa plantations. Pedobiologia 1995, 39, 423-433. [CrossRef]
- Daane, K.M.; Sime, K.R.; Fallon, J.; Cooper, M.L. Impacts of Argentine ants on mealybugs and their natural enemies in California’s coastal vineyards. Ecological Entomology 2007, 32, 583-596. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).