Submitted:
18 February 2025
Posted:
19 February 2025
You are already at the latest version
Abstract
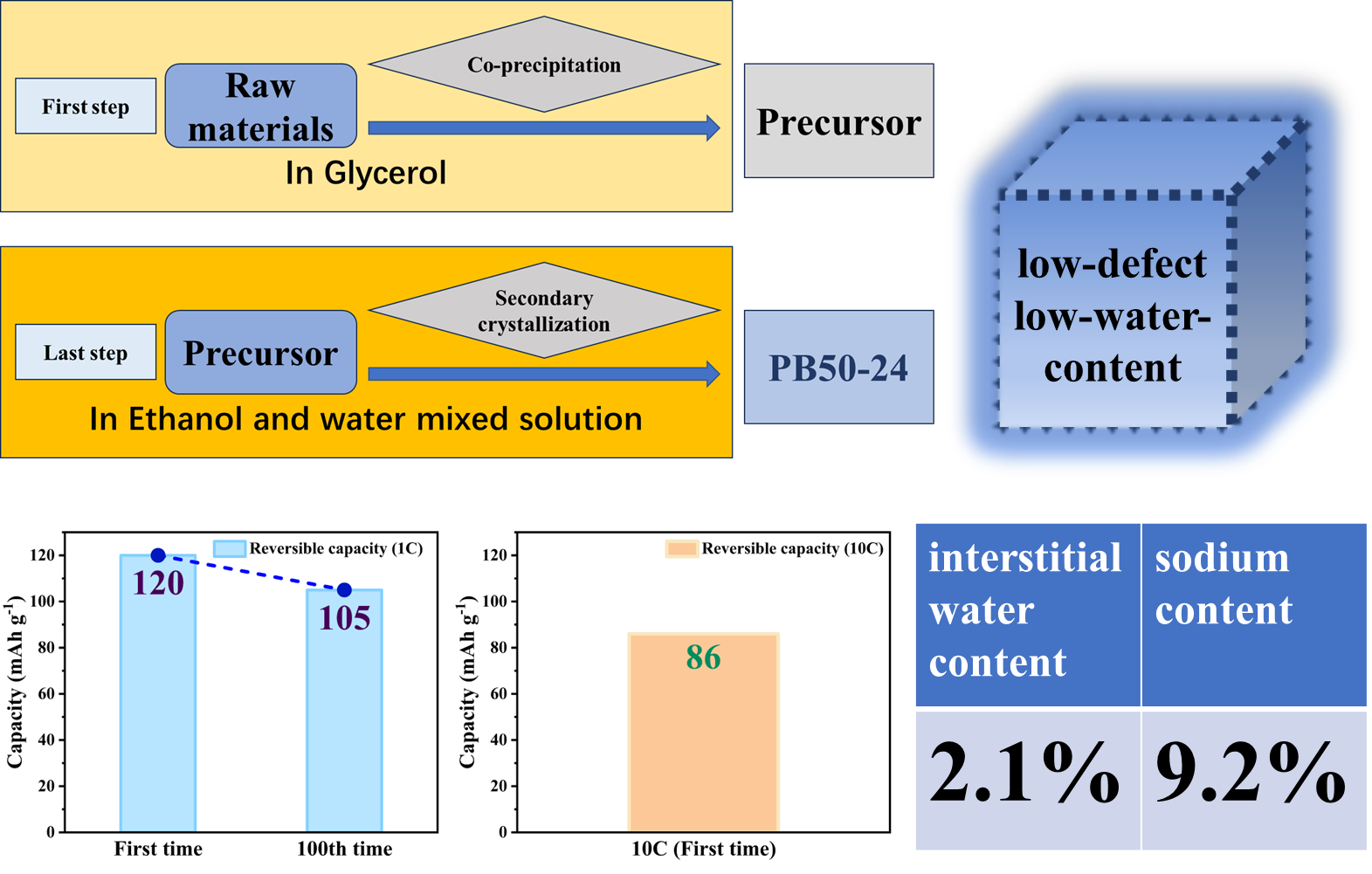
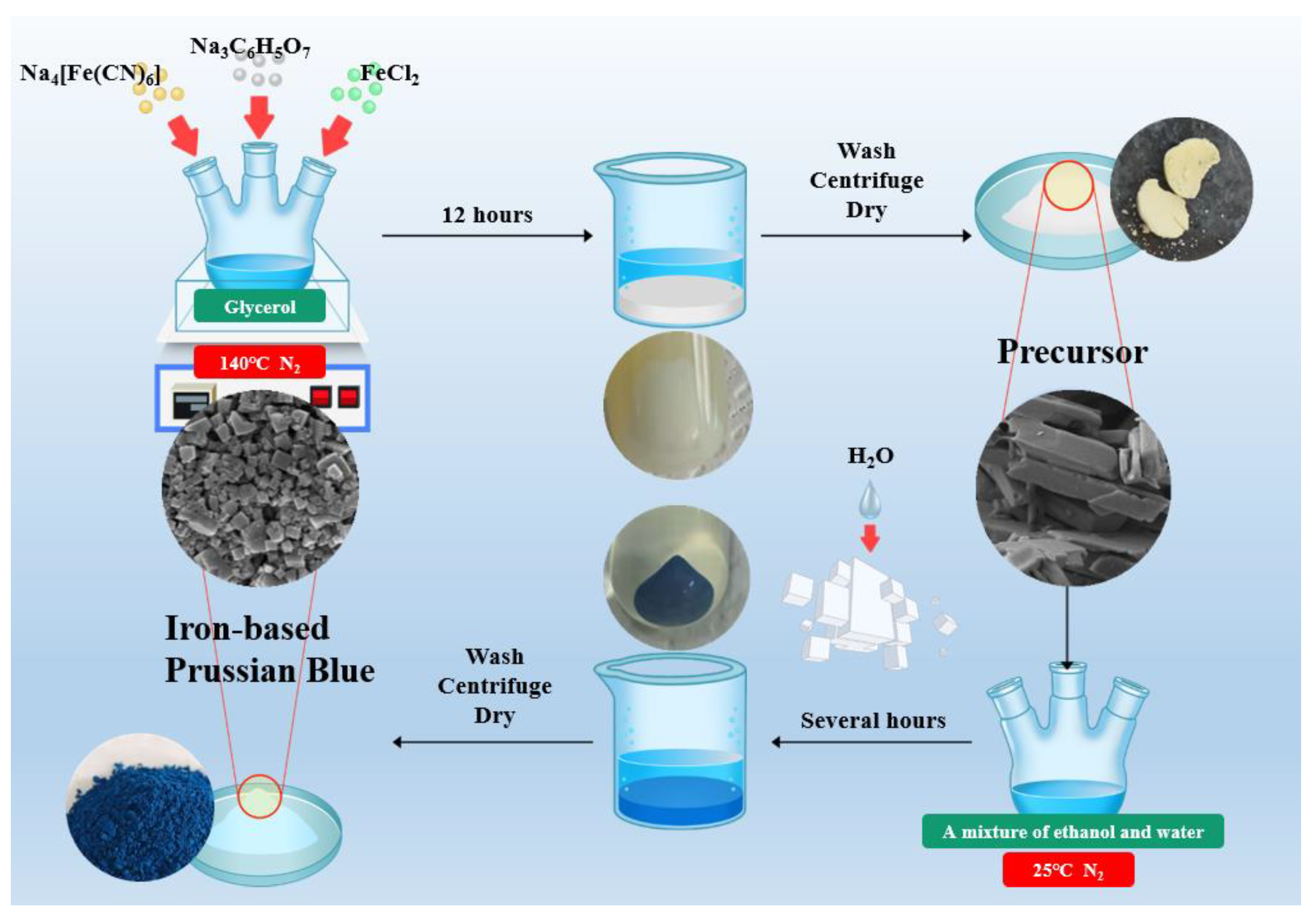
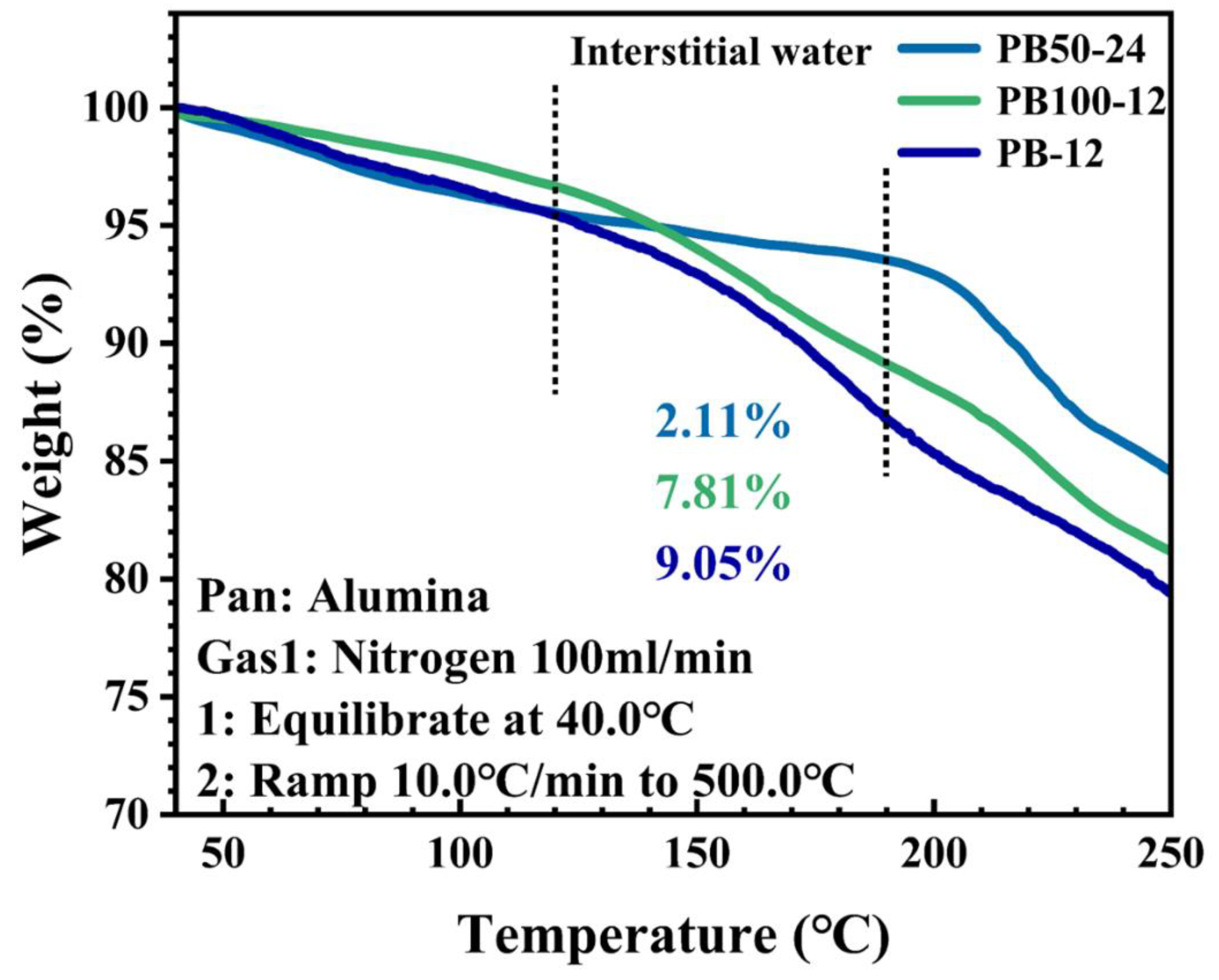
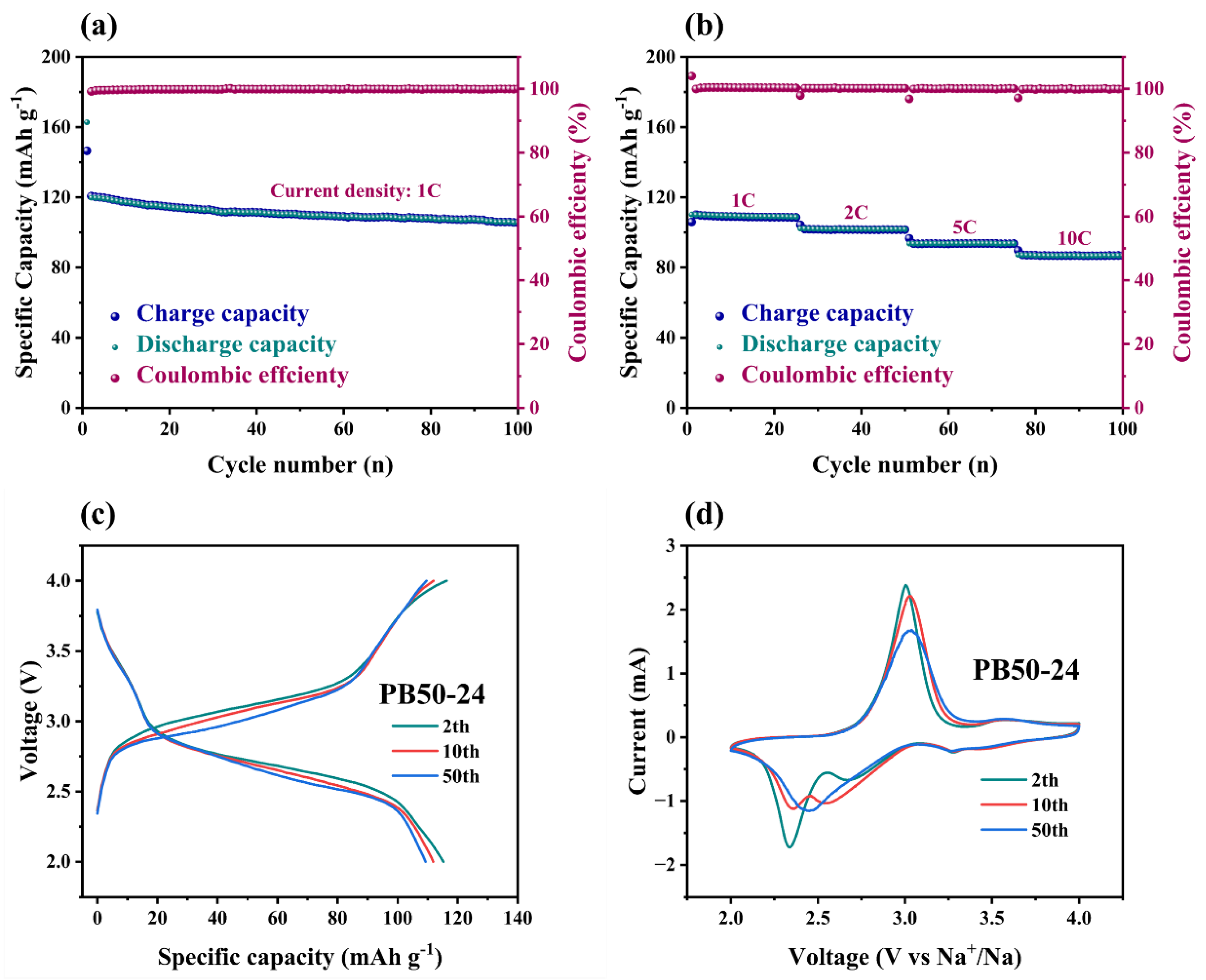
This study proposes an innovative two-step synthesis strategy to significantly enhance the performance of sodium-ion batteries by developing low-defect, low-water-content iron-based Prussian blue (PB) materials. Addressing the limitations of traditional co-precipitation methods—such as rapid reaction rates leading to excessive crystal defects and interstitial water content—the research team introduced a synergistic approach combining non-aqueous phase precursor synthesis and controlled water-concentration secondary crystallization. The process involves preparing a PB precursor in a glycerol system, followed by secondary crystallization in a water/ethanol mixed solvent with precisely regulated water content, achieving dual objectives of water-content reduction and crystal morphology optimization. Systematic characterization revealed that water concentration during secondary synthesis critically influences the materials crystal structure, morphological features, and water content. The optimized PB50-24 material exhibited a highly regular cubic morphology with a sodium content of 9.2% and a remarkably low interstitial water content of 2.1%. Electrochemical tests demonstrated outstanding performance: an initial charge-discharge capacity of 120 mAh g⁻¹ at 1C rate, retention of 105 mAh g⁻¹ after 100 cycles, and a high-rate capability of 86 mAh g⁻¹ at 10C, representing significant improvements in cycling stability and rate performance over conventional methods. This work not only establishes a cost-effective, scalable synthesis pathway for Prussian blue materials but also provides theoretical guidance for developing other metal-based Prussian blue analogs, offering substantial value for advancing the industrial application of sodium-ion batteries in next-generation energy storage systems.
Keywords:
1. Introduction
2. Experimental
2.1. Materials Synthesis
2.2. Electrode Preparation
2.3. Materials Characterization
2.4. Electrochemical Measurements
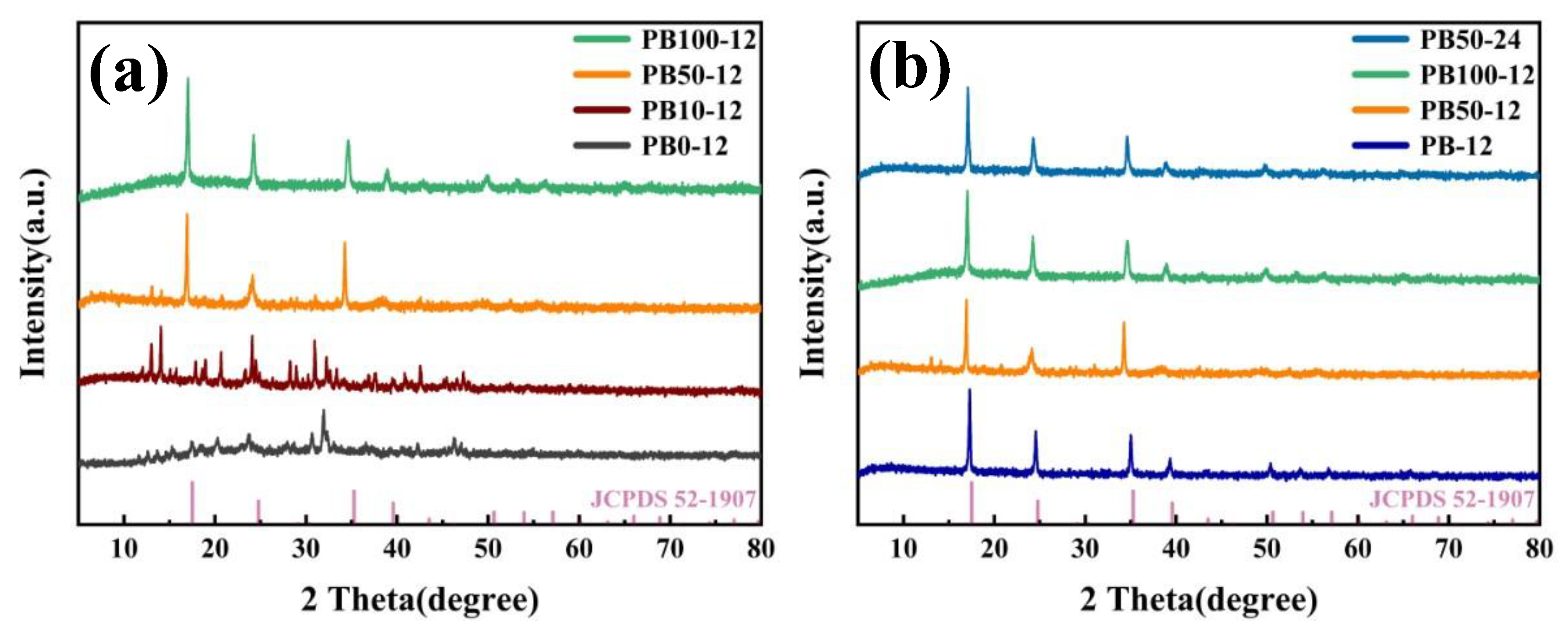
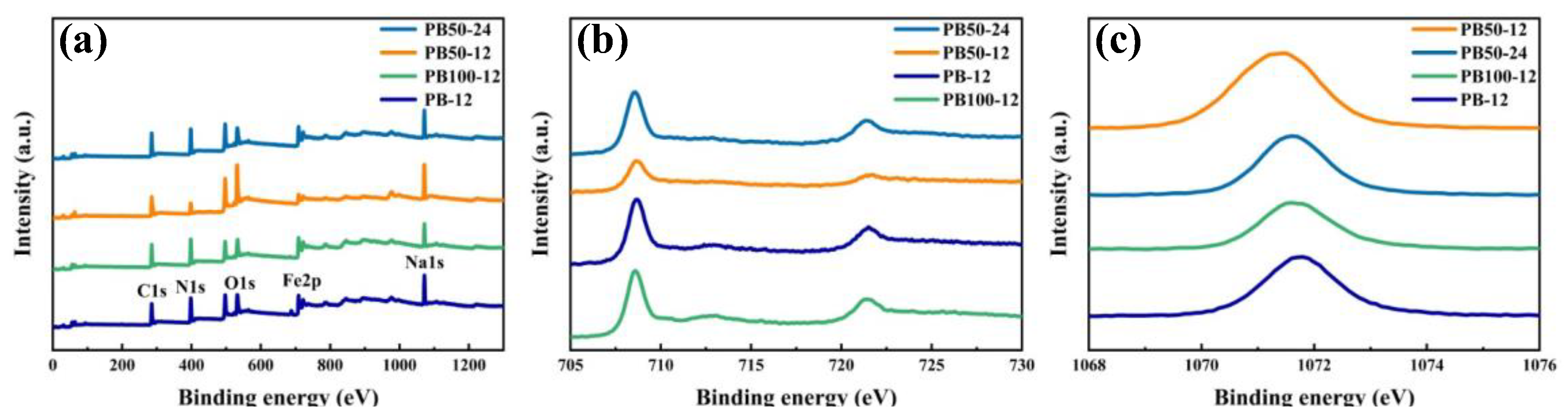
3. Results and Discussion
4. Conclusions
Author Contributions
Data Availability
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lu, Y.C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Abraham, K.M. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett. 2020, 5, 3544–3547. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and sodium-ion batteries: 50 years of research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Fang, Y.; Xiao, L.; Chen, Z.; Ai, X.; Cao, Y.; Yang, H. Recent advances in sodium-ion battery materials. Electrochem. Energy Rev. 2018, 1, 294–323. [Google Scholar] [CrossRef]
- Qian, J.; Wu, C.; Cao, Y.; Ma, Z.; Huang, Y.; Ai, X.; Yang, H. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv. Energy Mater. 2018, 8, 1702619. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian blue analogues for sodium-ion batteries: Past, present, and future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef]
- Xie, B.; Sun, B.; Gao, T.; Ma, Y.; Yin, G.; Zuo, P. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Y.; Sun, J. Defect engineering in Prussian Blue analogs for high-performance sodium-ion batteries. Adv. Energy Mater. 2022, 12, 2202532. [Google Scholar] [CrossRef]
- Wang, W.; Gang, Y.; Peng, J.; Hu, Z.; Yan, Z.; Lai, W.; Zhu, Y.; Appadoo, D.; Ye, M.; Cao, Y.; Gu, Q.F.; Liu, H.K.; Dou, S.X.; Chou, S.L. Effect of eliminating water in prussian blue cathode for sodium-ion batteries. Adv. Funct. Mater. 2022, 32, 2111727. [Google Scholar] [CrossRef]
- Li, W.J.; Han, C.; Cheng, G.; Chou, S.L.; Liu, H.K.; Dou, S.X. Chemical properties, structural properties, and energy storage applications of Prussian blue analogues. Small 2019, 15, 1900470. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian blue analogs as battery materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Jin, H.; Xin, S.; Gao, H. Prussian-blue materials: Revealing new opportunities for rechargeable batteries. InfoMat 2022, 4, e12311. [Google Scholar] [CrossRef]
- He, M.; Davis, R.; Chartouni, D.; Johnson, M.; Abplanalp, M.; Troendle, P.; Suetterlin, R.P. Assessment of the first commercial Prussian blue based sodium-ion battery. J. Power Sources 2022, 548, 232036. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Z.; Deng, Z.; Li, X.; Wang, B.; Chen, X.; Ong, S.P. Water contributes to higher energy density and cycling stability of Prussian blue analogue cathodes for aqueous sodium-ion batteries. Chem. Mater. 2019, 31, 5933–5942. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Wang, L.; Zhu, Y.; Sun, G.; Tang, Y.; Yan, M.; Jiang, Y. Structurally stable, low H2O prussian blue analogs toward high performance sodium storage. Adv. Funct. Mater. 2024, 34, 2314860. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Xie, M.; Zhang, X.; Li, L.; Wu, F. Preparation of Prussian blue submicron particles with a pore structure by two-step optimization for Na-ion battery cathodes. ACS Appl. Mater. Interfaces 2016, 8, 16078–16086. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments. Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
- Qin, M.; Ren, W.; Jiang, R.; Li, Q.; Yao, X.; Wang, S.; You, Y.; Mai, L. Highly crystallized Prussian blue with enhanced kinetics for highly efficient sodium storage. ACS Appl. Mater. Interfaces 2021, 13, 3999–4007. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Ji, L.; Wang, L.; Xu, B.B.; Shahzad, M.W.; Tang, Y.; Zhu, Y.; Yan, M.; Sun, G.; Jiang, Y. Boosting the sodium storage performance of Prussian blue analogs by single-crystal and high-entropy approach. Energy Storage Materials 2023, 58, 1–8. [Google Scholar] [CrossRef]
- Bie, X.; Kubota, K.; Hosaka, T.; Chihara, K.; Komaba, S. (2018) Synthesis and electrochemical properties of Na-rich Prussian blue analogues containing Mn, Fe, Co, and Fe for Na-ion batteries. J. Power Sources 2018, 378, 322–330. [Google Scholar] [CrossRef]
- Niu, Y.B.; Guo, Y.J.; Yin, Y.X.; Zhang, S.Y.; Wang, T.; Wang, P.; Xin, S.; Guo, Y.G. High-efficiency cathode sodium compensation for sodium-ion batteries. Adv. Mater. 2020, 32, 2001419. [Google Scholar] [CrossRef]
- Geng, W.; Zhang, Z.; Yang, Z.; Tang, H.; He, G. Non-aqueous synthesis of high-quality Prussian blue analogues for Na-ion batteries. Chem. Commun. 2020, 58, 4472–4475. [Google Scholar] [CrossRef]
- Camacho, P.S.; Wernert, R.; Duttine, M.; Wattiaux, A.; Rudola, A.; Balaya, P.; Fauth, F.; Berthelot, R.; Monconduit, L.; Carlier, D.; Croguennec, L. Impact of synthesis conditions in Na-Rich Prussian blue analogues. ACS Appl. Mater. Interfaces 2021, 13, 42682–42692. [Google Scholar] [CrossRef]
- Shen, L.; Jiang, Y.; Jiang, Y.; Ma, J.; Yang, K.; Ma, H.; Yang, K.; Ma, H.; Liu, Q.; Zhu, N. Monoclinic bimetallic prussian blue analog cathode with high capacity and long life for advanced sodium storage. ACS Appl. Mater. Interfaces 2022, 14, 24332–24340. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, M.; Zhang, G.; Shi, Y.; Su, Y.; Liu, X.; Pang, H. Structural properties, design strategies, and morphology control of micro/nanoscaled prussian blue and its analogues. Mater. Today Chem. 2024, 38, 102063. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Zhu, M.; Lin, Y.; Liu, Y.; Zhang, J.; Chen, T.; Liu, K. Yolk-shell Prussian blue nanoparticles with fast ion diffusion for sodium-ion battery. Mater. Lett. 2019, 249, 206–209. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, M.; Zhang, J.; Wang, Z.; Jiang, Y.; Xiao, G.; Li, S.; Li, L.; Wu, F.; Chen, R. A novel border-rich Prussian blue synthetized by inhibitor control as cathode for sodium ion batteries. Nano Energy 2017, 39, 273–283. [Google Scholar] [CrossRef]
- Wang, W.; Gang, Y.; Hu, Z.; Yan, Z.; Li, W.; Li, Y.; Gu, Q.F.; Wang, Z.; Chou, S.L.; Liu, H.K.; Dou, S.X. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun. 2020, 11, 980. [Google Scholar] [CrossRef]
- You, Y.; Wu, X.L.; Yin, Y.X.; Guo, Y.G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]

| Sample | Fe(ppm) | Na(ppm) |
|---|---|---|
| PB-12 | 219940 | 81776 |
| PB100-12 | 297016 | 66596 |
| PB50-24 | 203450 | 91795 |
| Sample | Synthetic medium | Specific capacity (mAh g -1) |
Current density (mA g -1) |
|---|---|---|---|
| YSPB[33] | Water | 118 | 170 |
| BR-FeHCF[34] | Water | 115 | 100 |
| PB-S3[35] | Water | 99 | 100 |
| HQ-NaFe[36] | Water | 110 | 150 |
| PB50-24 | Glycerol, 50%Ethanol | 120 | 170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




