1. Introduction
Forests are essential to hydrological and biogeochemical cycles, providing numerous ecosystem services vital for biodiversity conservation [
1,
2]. The rise in extreme weather events like droughts and heatwaves due to climate change may lead to higher tree mortality rates, potentially causing a net release of CO
2 in the atmosphere. Among these extreme events, drought and its related disturbances have the greatest global impact on forests [
3,
4]. Drought is expected to increase in frequency and severity in many regions in the future resulting from global climate change [
5,
6]. Trees may not be able to adapt in time to increases in aridity through evolutionary mechanisms due to their long reproductive life cycles and limited capacity to move away from stressful environments. The imminent threat of extended and more severe droughts underscores the need to study drought effects on woody plants [
4,
6].
During water limitation, plants eventually undergo desiccation, leading to a decrease in cell turgor pressure and causing the stomatal pores on leaf surfaces to close. This closure significantly slows down the dehydration process [
7]. Wilting of the leaves and the loss of stem conductivity occur when drought stress becomes severe [
8,
9]. In trees, stomata generally close prior to the development of cavitation in the hydraulic conduit system, despite the negative impacts of stomatal closure, specifically lowered carbon assimilation [
10]. After stomatal closure, further water loss can still happen through the cuticula, stomatal leakiness, and through the bark [
10].The light-harvesting capacity of leaf photosynthesis is damaged only after a significant loss of hydraulic function under prolonged dehydration [
11]. During extreme drought, leaf shedding is an adaptive strategy to enhance survival chances [
12,
13]. Typically, leaf shedding occurs after stomatal closure [
10], with xylem embolism in the leaves being a primary cause of leaf mortality during drought [
14,
15,
16]. The shedding reduces the evaporative leaf surface area, helping woody perennials delay cavitation in stem conductive tissues [
17]. However, shedding leaves without fully resorbing the nutrients results in net nutrient loss, which can influence the functioning of the tree in the long run [
17]. Recovery and restoration of a damaged crown after a severe drought involves, amongst others, supplementary carbon investment, either from non-structural carbohydrate reserves or through the assimilation in residual or newly post-drought developed leaves [
18].
While much focus has been placed on understanding the physiological aspects contributing to drought-induced tree mortality, it is likewise crucial to comprehend the mechanisms that are involved in drought recovery. Resilience to dehydration can be evaluated by examining both the impact of the drought and the rate of post-drought recovery [
19]. If cavitation of the conducting tissues is minimal or non-existent, recovery after re-watering is swift, with stomata reopening and allowing new carbon to be assimilated [
18]. Though, if cavitation thresholds are surpassed, recovery of photosynthesis is slower [
18]. Many plant species can resprout vegetatively after substantial biomass loss caused by environmental stress, including drought [
18]. In woody plants, the resprouting response to disturbances has been defined in two ways: binary or continuous [
20,
21]. In a binary framework, a plant either dies or resprouts and survives. A continuous framework defines the resprouting response as a spectrum, ranging from weak to strong reactions to the disturbance. This response can vary within an individual or within a population, depending on the severity and frequency of the disturbance [
21,
22]. Quite evidently, plants require adequate storage reserves to resprout after the loss of shoots and foliage. These reserves include non-structural carbohydrates stored in roots and stems [
23,
24].
Senescence is the final stage in the life cycle of leaves, and in deciduous woody species it signals the transition from the active to the dormant stage [
13,
25]. It marks a shift from nutrient assimilation to nutrient remobilization, which is vital for plant fitness [
13]. This involves a gradual and coordinated disassembly of macromolecules, leading to nutrient accumulation, which is then mobilized away from the senescing leaves [
26]. When leaves get older, they become more permissive to the induction of senescence and at the same time remain competent for perceiving senescence-delaying or -reverting signals [
27]. Leaf senescence is governed by complex genetic programs, finely regulated at multiple levels [
26,
28] and is influenced by various environmental stresses [
13,
29]. Literature provides mixed reports on whether drought stress leads to earlier, later, or unchanged timings of autumn leaf senescence, which complicates our understanding of its effects. Drought stress can advance leaf senescence [
25,
30]. This is supported by observations of early leaf abscission due to hydraulic failure in response to drought [
13]. Other studies report that drought stress can delay autumn leaf senescence [
31,
32]. No difference in timing of leaf senescence upon drought stress has also been reported [
29].
In this study, we carried out a controlled experiment in greenhouse conditions to assess the effects of drought. The experiment was performed with three provenances of
Prunus spinosa L. (Rosaceae) in a common garden setting.
P. spinosa, commonly known as blackthorn or sloe, is a deciduous thorny shrub with small, oval, serrated leaves and dark blue-black fruits called sloes. It blooms in early spring with white flowers before leaf emergence and is known for its dense growth, providing habitat and food for wildlife [
33].
P. spinosa is a deciduous and widespread shrub species in Central and Southern Europe reaching up to Western Asia [
33]. It is found in forest margins, wooded banks and hedgerows, prefers sunny and open spaces, and is adaptable to different soil conditions [
33]. In Belgium it is often planted for species diversity, restoration of historical landscapes and to support wildlife [
34].
P. spinosa is a widespread shrub species, but it has received little attention in scientific research on woody species due to its lack of economic value. Still, some studies have emphasized key characteristics of the species regarding its morphology and genetics [
35,
36,
37] Understanding its drought tolerance can inform conservation strategies aimed at maintaining biodiversity and ecosystem services in increasingly drought prone environments. In addition, within species, responses to drought are not necessarily similar for different populations originating from diverse geographic origins, and a better knowledge on population differentiation in drought responses can help decisions on assisted migration as an anticipation to the climate change.
Potted saplings were subjected to water deprivation during the summer of 2021, followed by rewatering. We hypothesized that the responses to the water with-holding would be affected by (i) the severity of the drought and (ii) by the provenance of the saplings. Our main objective was to gain more understanding of the post-drought recovery process, with a specific focus on the ability for post-drought resprouting and on the timing of leaf senescence. The common garden allowed the assessment of the variability in responses among the different provenances. We were able to relate visual symptoms of drought stress with post-drought responses. Most strikingly, leaf senescence was advanced or delayed depending on the severity of the drought stress. We also observed population differentiation in the drought responses.
4. Materials and Methods
4.1. Plant Material
We established a common garden of potted plants that consisted of 274
P. spinosa plants derived from three provenances: 107 plants from a local Belgian provenance (Lat 50.953324, Lon 3.663467 and Alt 10m), 79 from a Spanish-Pyrenean provenance (Lat 42.630049, Lon -0.169068 and Alt 1270m) and 88 from a south Swedish provenance (Lat 55.67668, Lon 13.32481 and Alt 58m). Local climate, day length and also stone collection have been described before [
61]. In short, drupes were picked in 2016 and the stones germinated in 2017. In this year, the seedlings grew in forestry trays (54.5 x 31cm with 28 cells) using normal nursery potting soil (1.5 kg/m³ NPK 12 + 14 + 24, 20% organic matter, pH levels from 5.0 to 6.5, electrical conductivity of 450 µS/cm, and 25% dry matter content). No extra fertilizer was mixed in the soil. In 2018 a temperature experiment was conducted on the seedlings and the effects of it were extinguished in 2020 [
61]. At the end of 2018 the young plants were moved into 1 liter pots using the same soil type, and no extra nutrients was added. A common garden of the young plants was created on an outdoor container field at the Research Institute for Agriculture and Fisheries (Melle, Belgium). The plants were watered with automated sprinklers, which were watched by skilled technical personnel. The seedlings from the three provenances were intermingled in a single tree plot design. In the beginning of 2020, the plants were transferred to 4 liter pots, adding the same soil type without any extra fertilizer. The pots were again randomly intermingled and stayed on the container field throughout 2020 and into the beginning of 2021.
4.2. Drought Treatment
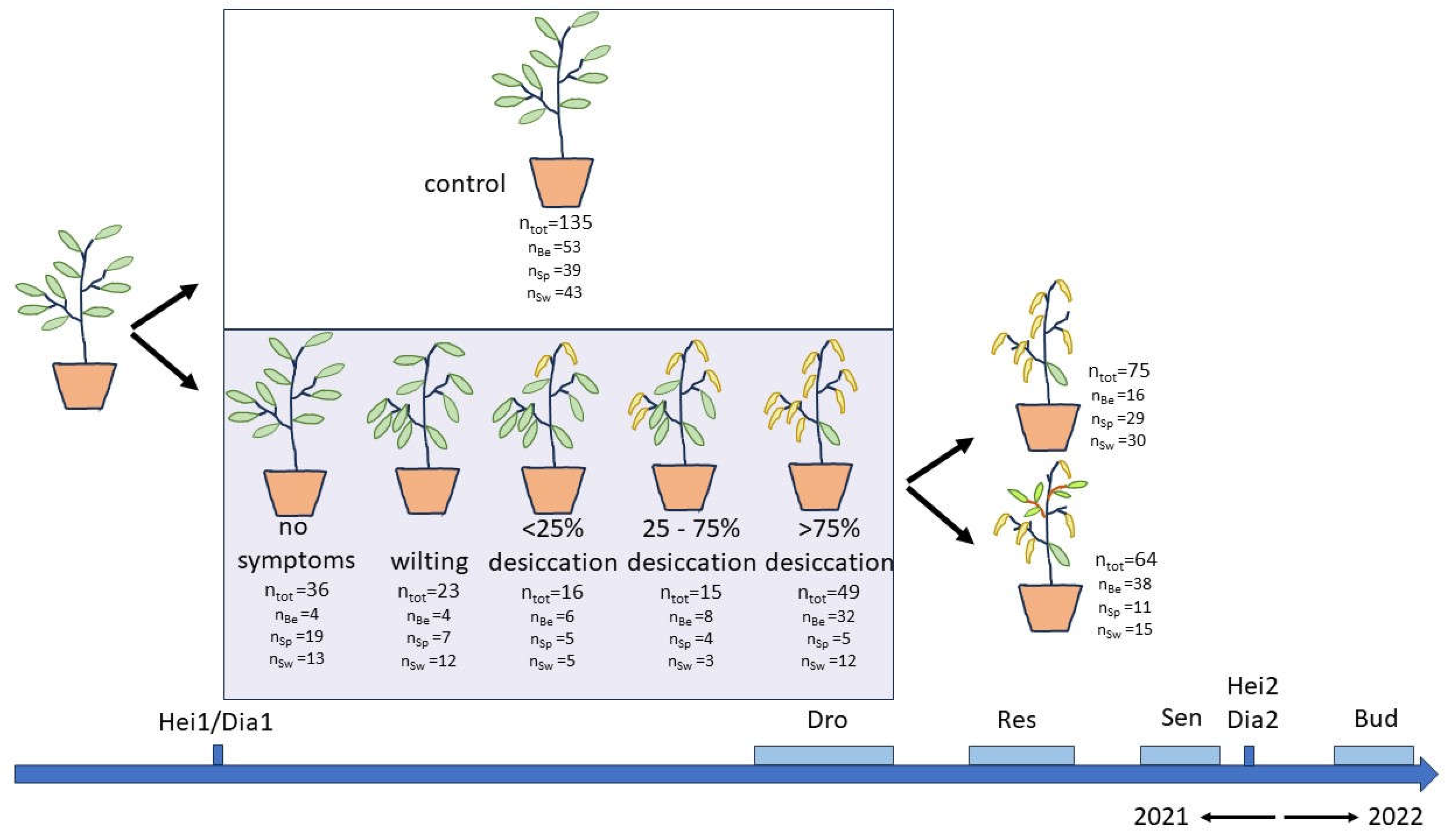
In mid-June 2021, we moved all the potted plants from the container field to a greenhouse. This greenhouse was equipped with an programmed shade net to protect the saplings from intense solar radiation. We conducted a dry-out experiment from June 29 to July 29. At the start and also at the end of the treatment, we fully hydrated all plants, including the controls, by placing the pots overnight in a water basin, while maintaining the water level at 5 cm above the base of the pots. Next morning, the excess of water was drained. By this, we could approach field capacity. From the start of the drought period, half of the plants received regular watering from experienced technicians to keep them well watered (control plants), while the other half received no water at all (droughted plants). The three provenances were evenly distributed between the control and drought group (
Figure 8) and were randomly intermingled in each group. To prevent excessive mortality, the drought treatment was stopped when several plants showed (nearly) total leaf desiccation. By this point, various visual drought stress symptoms were evident in the drought treated plants (
Figure 8).
After the treatment, the saplings were kept in a non-heated (but frost-free) greenhouse until January 2022, safeguarding they were constantly held under well-watered conditions. This was guaranteed by qualified technical employees. In January, the plants were then planted in an experimental field in Grimminge, Belgium. All treatment groups, as well as the provenances within each group, were randomly intermingled (single tree plot design).
4.3. Measurements and Observations
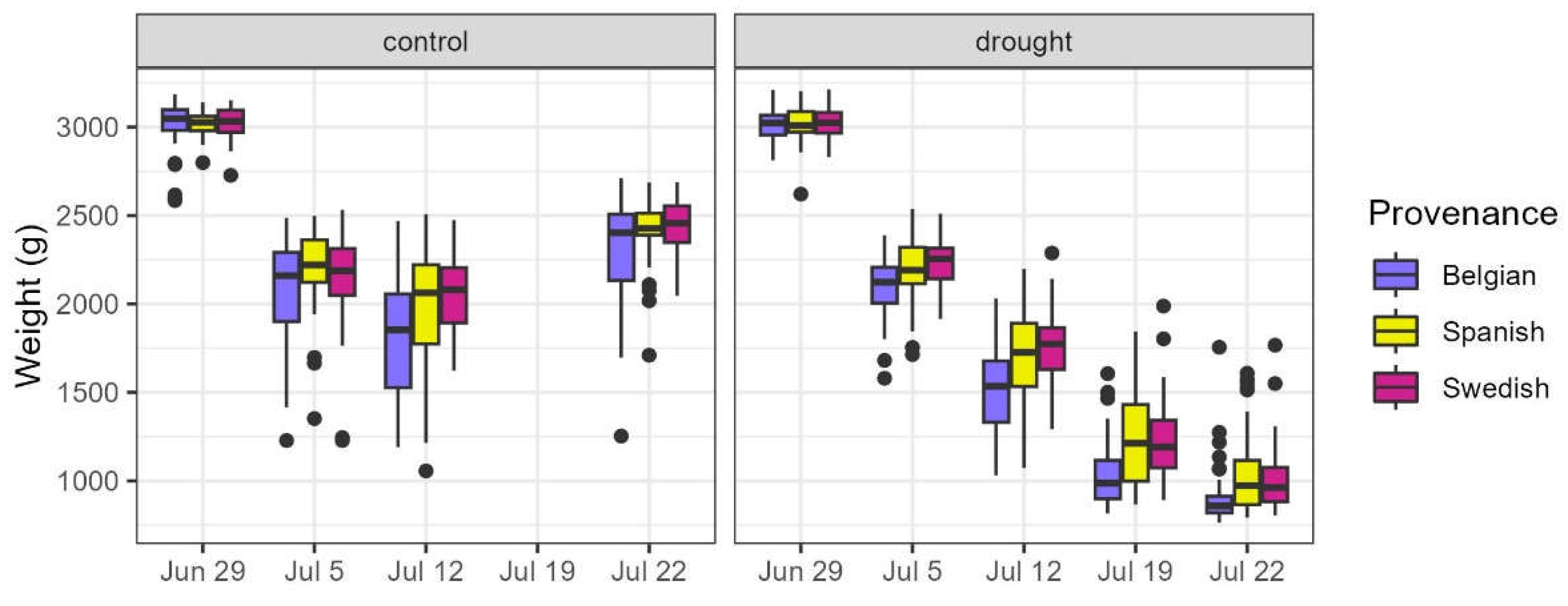
During and after the drought treatment, we performed various measurements and observations. All pots were weighted at the start of the treatment, after draining excess water (a proxy for field capacity), and about weekly thereafter during the treatment (
Figure 9). The decrease in pot weight during the drought period was an indicator of the water scarcity experienced by the drought treated plants. The relative weight loss of the pots was calculated by subtracting the last weights from the initial weights at the beginning of the treatment and then dividing this difference by the initial weights.
Height and diameter were measured on all the plants (both control and drought treated) at the start of the treatment (June 29), and when plants had entered winter rest (November 16). Height of the saplings was measured up to the place where plants were still alive. The stem diameter was measured using a rod at 2 cm above the soil level.
We assessed the visual drought symptoms in the drought treated group, i.e., the wilting and desiccation of the leaves, on July 19, 22, 26 and 29, following a scoring protocol with 1: no visual drought stress symptoms, 2: leaves wilting but not yet desiccating, 3: < 25% of the leaves desiccated, 4: 25-75% of the leaves desiccated and 5: >75% of the leaves desiccated (
Figure 8). Five plants that lost (nearly) all their leaves due to desiccation by the drought did not resprout after the rewatering and thus were not recorded in the leaf senescence scoring, but they did flush in the next spring (four Belgian and one Swedish). Three plants finally died off, two that lost more than 75% of their leaves due to the drought (both Belgian) and one with less than 25% desiccated leaves due to the drought (Swedish).
Plants in the drought treated group were scored for resprouting after the rewatering, following a scoring protocol with 1: buds not swelling, 2: buds swelling, 3: first leaves protruding but not yet unfolding, 4: first leaves unfolding, 5: first leaves unfolded but small, 6: first leaves clearly enlarging, 7: all new leaves on a plant enlarged. Resprouting was scored on August 3, 10 and 17. After august 17 no more plants started to resprout. A binary variable was deduced from the scorings on the last observation day (August 17) with 0: plants not resprouting (score 1) and 1: plants resprouting (scores > 1).
Autumn leaf senescence was scored applying a protocol with 1: green leaves, 2: light green leaves, 3: less than half of the leaves turning yellow, 4: more than half of the leaves yellowing, 5: all leaves yellow and starting to fall off [
61]. This phenophase was recorded on September 21 and October 5 and 19. Bud burst in the spring of 2022 was evaluated applying a protocol with 1: winter rest, 2: buds swell, 3: buds open and first leaves protrude but do not yet unfold, 4: leaves unfold, 5: leaves unfolded and enlarged [
61]. Bud burst was scored on April 7, 14 and 21. For both phenophases, the whole sapling (i.e., all buds or all non-desiccated leaves) was assessed and a mean score was assigned.
The relative chlorophyll content index in the leaves of a subset of plants was measured by making use of a chlorophyll content meter (CCM-200, Edaphic Scientific, Melbourne, Australia). The instrument determines the relative chlorophyll content of a leaf by calculating the ratio of optical transmission at 931 nm (near-infrared) to that at 653 nm (green). We focused on control plants at the one hand and plants that had reached severe visual drought symptoms during the drought period at the other hand. In the control group, 27 plants were chosen at random (10 Belgian, 9 Spanish-Pyrenean and 8 Swedish) and in the group of drought treated plants, 27 were chosen that displayed severe visual drought symptoms (3 with visual drought symptoms score 4 and 24 with score 5). Because more Belgian plants reached the highest score of visual drought symptoms, more Belgian plants were measured (19 Belgian, 3 Spanish-Pyrenean and 5 Swedish). In the middle of the young crown, a single mature and undamaged leaf that was representative to the plant, was carefully chosen for the measurement. Only leaves on short shoots were chosen. These leaves were indicated so that repeated measurements in time were made on the same leaves. Measurements were performed on September 21, October 5, October19 and November 2.
The leaf traits lamina length and lamina widest width were measured on the first fully developed and damage free leaf at the top of a representative long shoot at the top of the plant and on a fully developed leaf on a short shoot at the centre of the plant for all plants in the control group in the summer of 2021. For 57 randomly chosen plants (20 Belgian, 19 Spanish and 18 Swedish) in the control group, the stomatal density and stomatal length on the underside of the sampled leaves were counted and measured. A transparent nail varnish imprint was taken from the underside of the leaf at the centre of the leaf but avoiding the veins. These imprints were placed on microscope slides and examined using a Keyence VHX-7000 digital microscope (Keyence Corporation, Japan). In each nail varnish imprint two stomatal counts, each in a randomly chosen square of 0.0454 mm2, were performed. The lengths of five randomly selected stomata in each square were measured.
4.4. Statistical Analysis
We used the open-source statistical software R [
62]. Linear models were applied to analyse height and diameter and their increments, and leaf and stomatal size measurements. Logistic regression models were applied for the post-drought resprouting [
63]. For phenological observations (the timing of visual drought symptoms, timing of resprouting, timing of leaf senescence, and timing of bud burst), which were ordinal data, we utilized cumulative logistic regression with the ordinal package [
64]. Figures were created using ggplot2 [
65]. To account for repeated observations on the same plants, a unique plant identifier was included as a random effect. Mixed-effects modelling is particularly well-suited for analysing ecological data, as it can account for nested structures, handle unbalanced datasets, and incorporate random effects [
66].
Height (Hei1) and diameter (Dia1) at the start of the treatment were modelled for all the plants, to detect initial growth variations between the provenances (Pro with Belgian, Spanish-Pyrenean and Swedish provenances):
The timing of the appearance of visual drought symptoms (Dro) in the drought treated group of plants was modelled using cumulative logistic regression, with p
Dro being the chance to have reached maximally a given drought score level, Day the day of observation, Pro the provenance, Rwe the relative weight loss of the pots and Hei1 the initial height.
For the drought treated plants, the chance to resprout after the rewatering (Res1) was modelled with logistic regression. For this, scorings of resprouting on the last observation day (August 17) were transformed to binary data (resprouting or not resprouting). The first model focussed on the influence of the visual drought symptoms (Dro):
When taking into account the provenance (Pro with Be: Belgian, Sp: Spanish-Pyrenean and Sw: Swedish) in the modelling of the post-drought resprouting (Res1
Pro), some levels of visual drought symptoms were pooled to attain a higher number of plants for each provenance in each droughted group: Dro scores 1, 2 and 3 were pooled (no symptoms up to 25% of desiccated leaves, with n
Be=14, n
Sp=31 and n
Sw=30), and also score 4 and 5 (more than 25% of desiccated leaves, with n
Be=40, n
Sp=9 and n
Sw=15) were pooled. The dataset was split in two according to the two pooled levels of visual drought symptoms, to keep models as simple as possible. The two new datasets were used for modelling, applying the same model structure for both, with Hei1 the initial height:
For the droughted plants that recovered after rewatering by resprouting (n=64,
Figure 8), the timing of the resprouting (Res2) was modelled using cumulative logistic regression. Because of the low number of resprouting plants in the different score levels of visual drought symptoms, the lower drought score levels were pooled so that three levels remained: no to mild symptoms with n=9 (pooled scores 1, 2 and 3), 25 to 75% desiccated leaves (score 4, n=13) and more than 75% (score 5, n=42). p
Res2 was the chance to have maximally reached a given resprouting score (to have reached the given score or a score lower than this), Day the day of observation, Dro_adj the adjusted visual drought symptoms variable with pooled visual drought symptoms as described above, and Hei1 the initial height.
Because of the low number of plants for each provenance among the droughted plants that resprouted, the model for the timing of the resprouting (Res2
Pro) containing the provenance (Pro) in the fixed part did not retain a visual drought symptoms variable:
The timing of leaf senescence in autumn (Sen) was modelled for controls and drought treated plants together, using cumulative logistic regression. Firstly, a model was run focussing on the different categories of visual drought symptoms. p
Sen was the chance to have maximally reached a given senescence score level on a given day, with Day the day of observation, Dro the control and the visual drought symptom groups and Hei2 the height at winter rest.
Secondly, the provenances were taken into account and again we had to take care of some drought categories with limited amount of plants for each provenance. Three datasets were constructed: one with the control plants, one with the pooled categories of no to mild visual drought symptoms (scores 1, 2 and 3) and one with the pooled categories of severe visual drought symptoms (scores 4 and 5). A model was run for each dataset to keep the models as simple as possible. p
SenPro was the chance to have maximally reached a given senescence score level on the day of observation (Day), with Pro the provenance and Hei2 the plant height at winter rest. Three models were run with the following structure:
For all the leaf senescence models - the visual drought symptoms model and the provenance models - time spans were calculated between the different groups of drought categories or between the provenances based on the model statistics. The basic formula to calculate the day when 50% of the plants in a given group of plants had attained maximally a given leaf senescence score (D
50%) was based on (p
Sen/1- p
Sen) being 0 for p
Sen = 0.5. Using the mean height at winter rest (mHei2):
Time spans for the timing of leaf senescence between two groups of plants (visual drought symptom groups or provenances) were calculated by subtracting the respective D50% values.
The relative chlorophyll content measurements of the leaves (RCC) were analysed to corroborate the autumnal leaf senescence scores for the plants that displayed severe visual drought symptoms. Because of too little measurements for every provenance separately, provenance was not taken into account in the model. Because the measurements were not linear over time, the time variable (Day) was quadratic in the model. Dro_adj2 was the variable indicating whether a plant belonged to the control group or to the group of plants that were severely affected by the drought (pooled Dro scores 4 and 5: >25% of the leaves desiccated). An interaction term between the time variable and this adjusted visual drought symptoms variable allowed the relative chlorophyll content index to diminish over time at a different rate for the different levels in the Dro_adj2 variable.
In the spring of the year after the drought treatment, bud burst (Bud) was scored on all the plants, both controls and drought treated. We modelled the probability (p
Bud) that on a given day a sapling had already reached a given bud burst score, or a score higher than this. First we focussed on the different visual drought symptom categories. Day was the day of observation, Dro consisted of the controls and the different levels of visual drought symptoms and Hei2 was the height at the end of the growing season.
Similar as for the timing of leaf senescence, the timing of bud burst in the three provenances (p
BudPro) was studied in three subdatasets: controls, mild visual drought symptoms (pooled scores 1, 2 and 3) and severe visual drought symptoms (pooled scores 4 and 5), with the following model structure:
Leaf lamina length (Lle) and lamina widest width (Lww) were analysed using a linear model.
For stomatal length (Sle) a linear mixed model was applied to account for repeated measurements per plant.
Finally, for the stomatal density (Sde), a Poisson general linear mixed model was applied with a similar formula structure.
Author Contributions
Conceptualization, K.V.M., E.N.P., K.V.C. and St.M.; methodology, K.V.M., E.N.P., D.B. and K.V.C.; investigation, D.B., Sh.M. and St.M.; validation, K.V.M.; formal analysis, K.V.M., D.B. and K.V.C.; data curation, D.B., K.V.M. and Sh.M.; writing—original draft preparation, D.B. and K.V.M.; writing—review and editing, D.B., K.V.M., K.V.C. and E.N.P.; supervision, K.V.C., K.V.M. and E.N.P. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Boxplots presenting the initial height (a) and diameter (b) of the saplings at the start of the treatment. Be: Belgian, Sp: Spanish-Pyrenean, Sw: Swedish.
Figure 1.
Boxplots presenting the initial height (a) and diameter (b) of the saplings at the start of the treatment. Be: Belgian, Sp: Spanish-Pyrenean, Sw: Swedish.
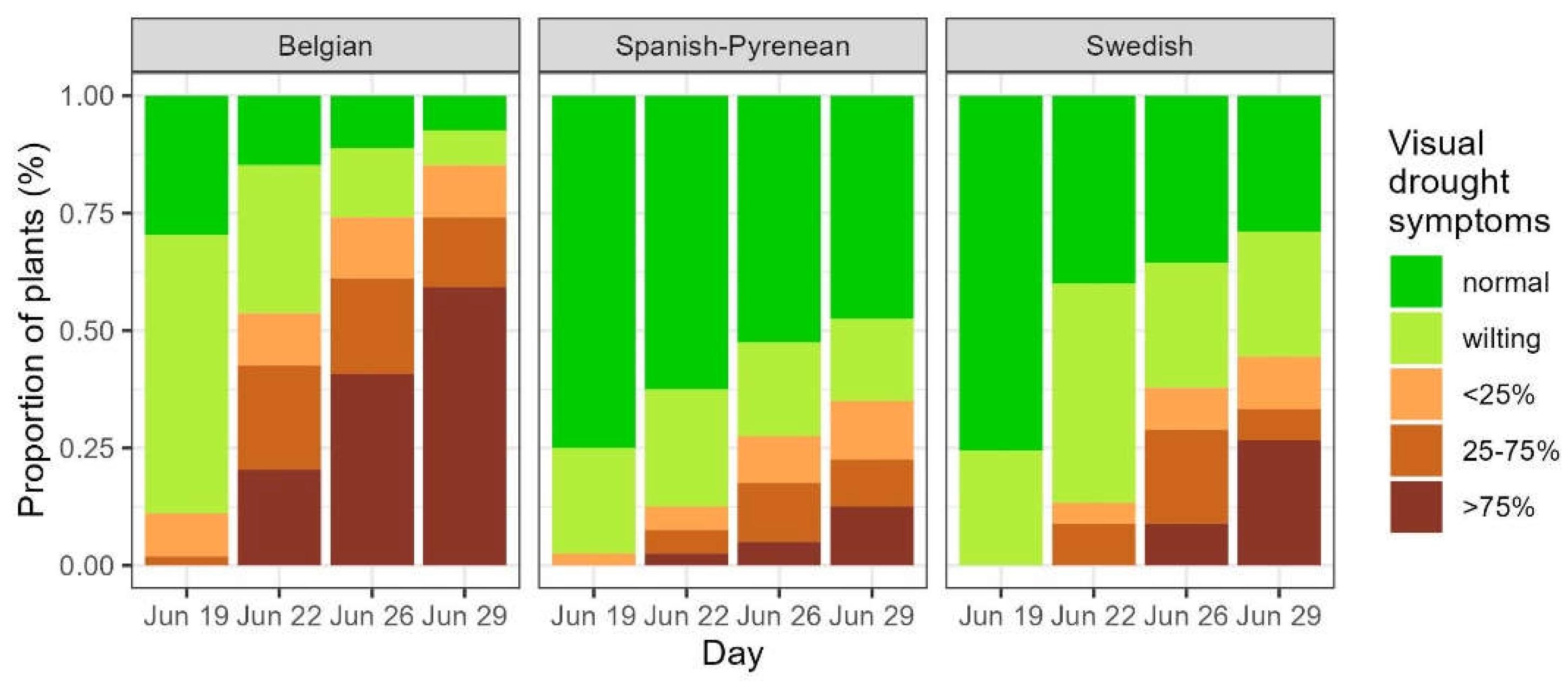
Figure 2.
Development of visual drought symptoms during the drought period in the three provenances. Normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves.
Figure 2.
Development of visual drought symptoms during the drought period in the three provenances. Normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves.
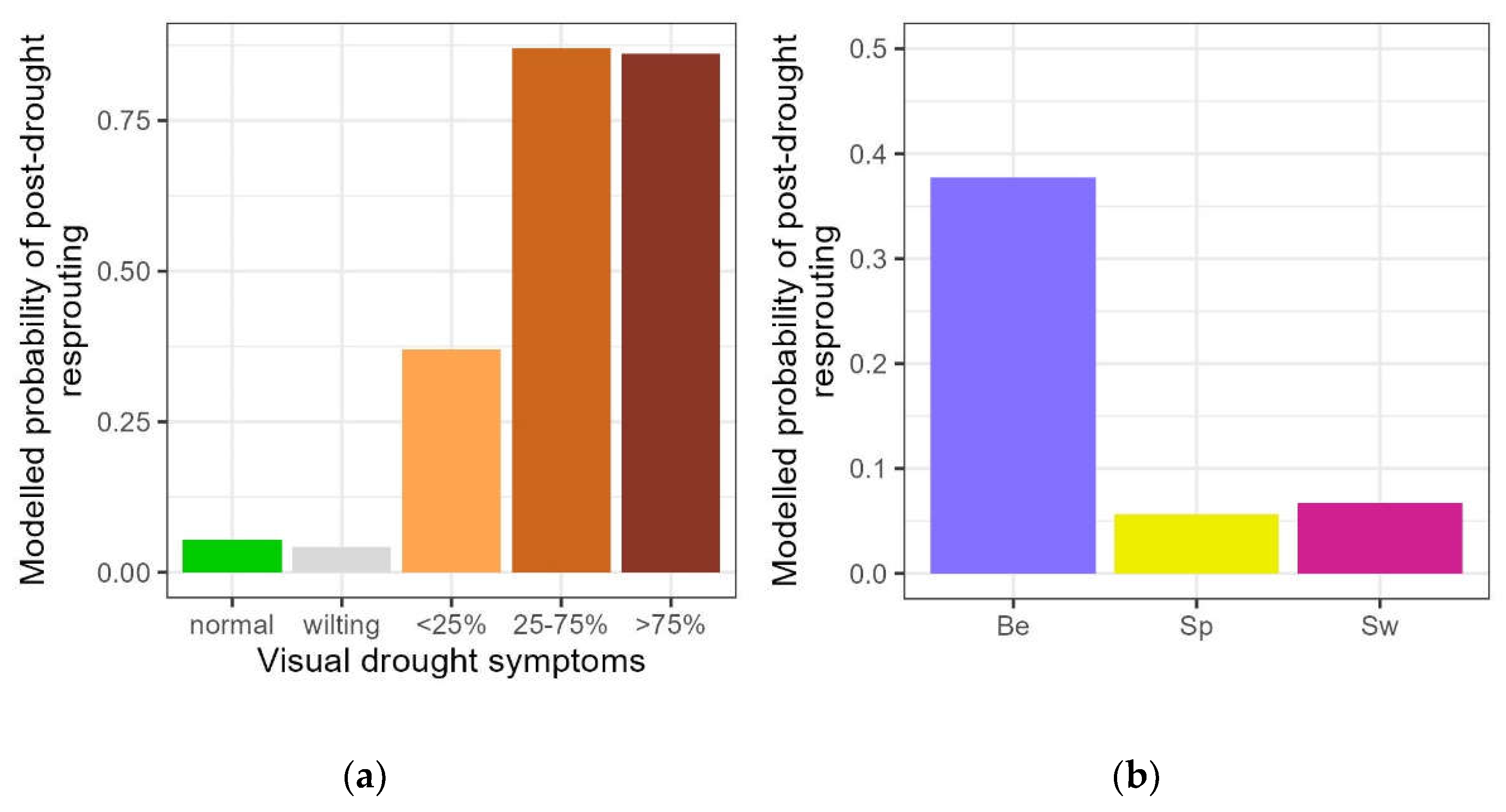
Figure 3.
Modelled probability of post-drought resprouting among the different categories of visual drought symptoms (a) and among the three provenances for the pooled visual drought categories normal, wilting leaves and <25% desiccated leaves (b). Normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves. Be: Belgian, Sp: Spanish-Pyrenean, Sw: Swedish. Categories not significantly differing from the standard (normal) are displayed in grey.
Figure 3.
Modelled probability of post-drought resprouting among the different categories of visual drought symptoms (a) and among the three provenances for the pooled visual drought categories normal, wilting leaves and <25% desiccated leaves (b). Normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves. Be: Belgian, Sp: Spanish-Pyrenean, Sw: Swedish. Categories not significantly differing from the standard (normal) are displayed in grey.
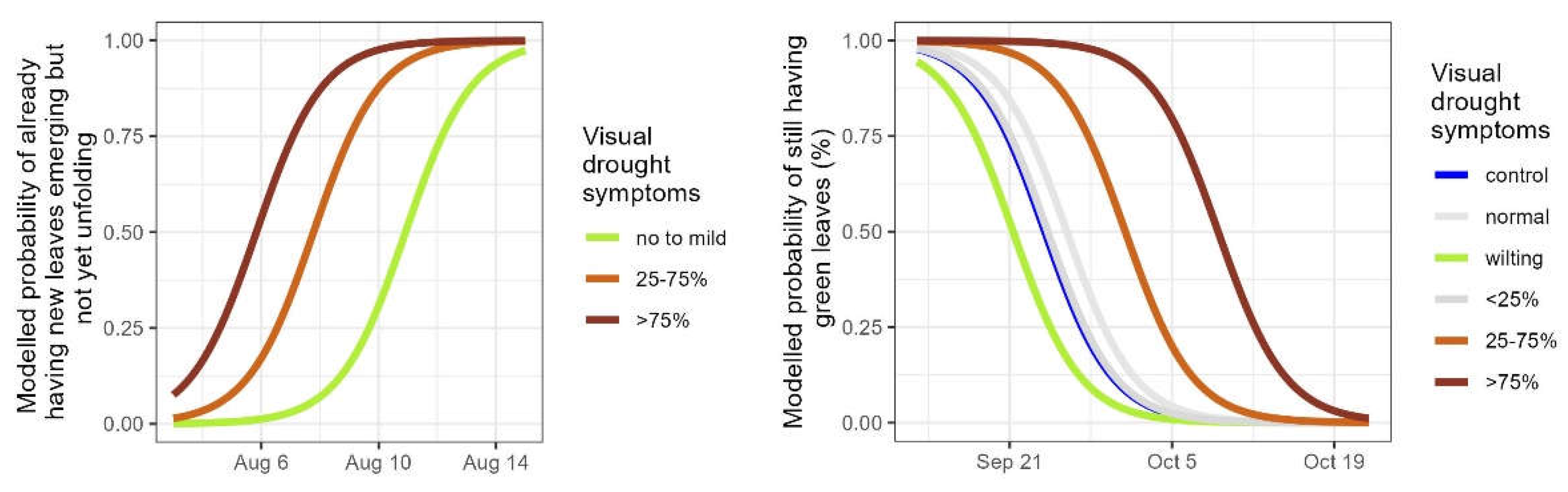
Figure 4.
Modelled probability of the timing of resprouting among the resprouting saplings (a) and modelled probability of the timing of leaf senescence for all controls and droughted plants (b), according to the visual drought symptom categories. For the resprouting (a), no to mild: pooling of no visual drought symptoms, wilting leaves and < 25% of desiccated leaves; 25-75% and >75%: percentages of desiccated leaves. For leaf senescence (b), normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves. Categories not significantly differing from the standard (control) are displayed in grey.
Figure 4.
Modelled probability of the timing of resprouting among the resprouting saplings (a) and modelled probability of the timing of leaf senescence for all controls and droughted plants (b), according to the visual drought symptom categories. For the resprouting (a), no to mild: pooling of no visual drought symptoms, wilting leaves and < 25% of desiccated leaves; 25-75% and >75%: percentages of desiccated leaves. For leaf senescence (b), normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves. Categories not significantly differing from the standard (control) are displayed in grey.
Figure 5.
Modelled probability of the timing of leaf senescence according to the provenance for controls (a), pooled categories of visual drought symptoms normal, wilting and <25% desiccated leaves (b) and pooled categories of visual drought symptoms 25-75% and >75% desiccated leaves (c). Provenances not significantly differing from the standard (Belgian) are displayed in grey.
Figure 5.
Modelled probability of the timing of leaf senescence according to the provenance for controls (a), pooled categories of visual drought symptoms normal, wilting and <25% desiccated leaves (b) and pooled categories of visual drought symptoms 25-75% and >75% desiccated leaves (c). Provenances not significantly differing from the standard (Belgian) are displayed in grey.
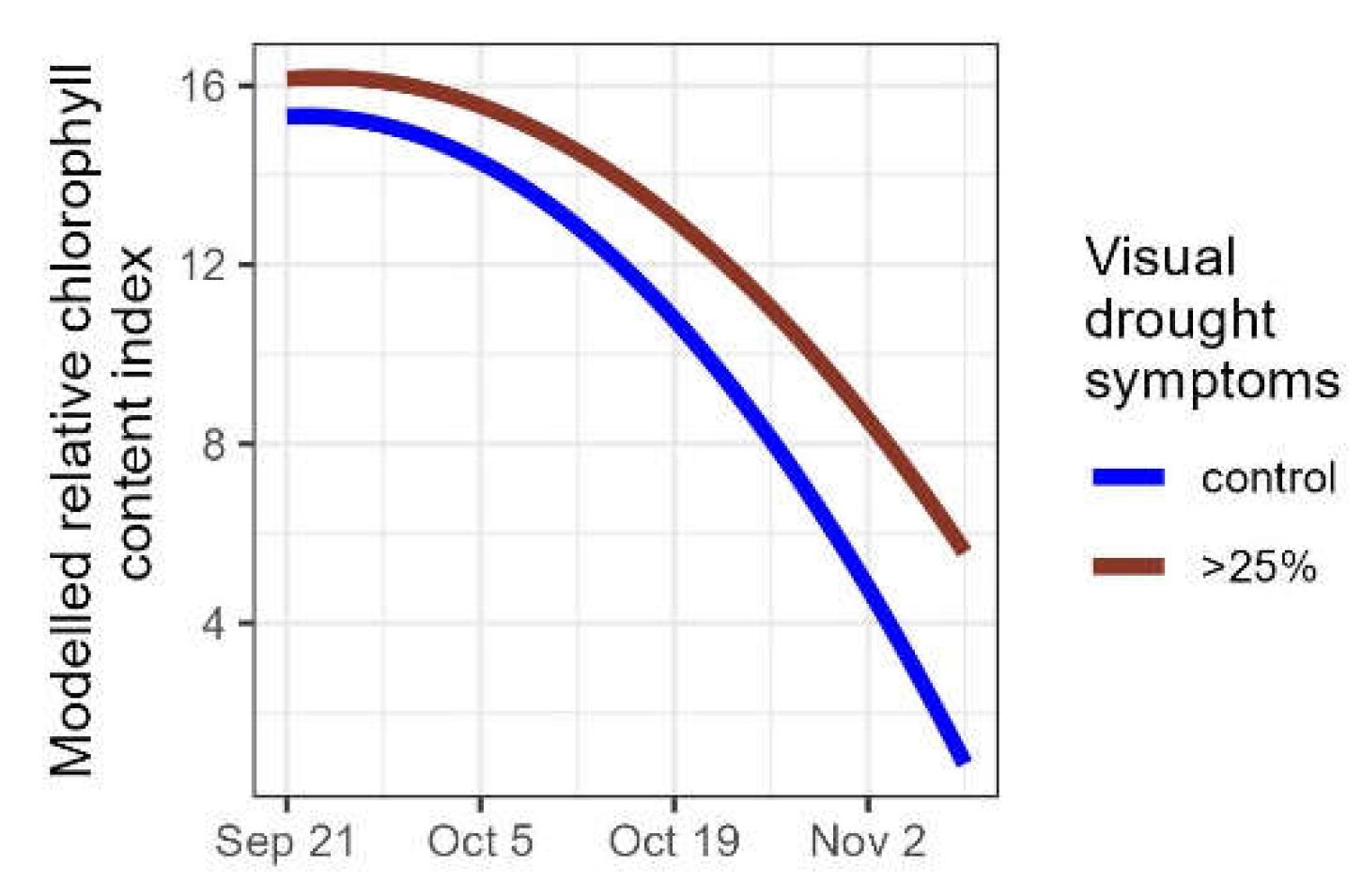
Figure 6.
Modelled relative chlorophyll content index for control plants (n=27) and for plants severely affected by the drought ,i.e., that lost more than a quarter of their foliage (n=27).
Figure 6.
Modelled relative chlorophyll content index for control plants (n=27) and for plants severely affected by the drought ,i.e., that lost more than a quarter of their foliage (n=27).
Figure 7.
Modelled probability of the timing of bud burst for controls and droughted plants, according to the visual drought symptom categories. Normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves. Categories not significantly differing from the standard (control) are displayed in grey.
Figure 7.
Modelled probability of the timing of bud burst for controls and droughted plants, according to the visual drought symptom categories. Normal: no visual drought symptoms, wilting: leaf wilting, <25%, 25-75% and >75%: percentage of desiccated leaves. Categories not significantly differing from the standard (control) are displayed in grey.
Figure 8.
Schematic representation of the drought treatment with the plants subjected to water exclusion categorized according to the level of visible drought symptoms. The number of plants in each group is indicated with a further subdivision according to the provenance (Be: Belgian, Sp: Spanish-Pyrenean, Sw: Swedish). Several variables were recorded during and after the treatment. Hei1/Hei2/Dia1/Dia2: height and diameter, Dro: visual drought stress symptoms, Res: post-drought resprouting, Sen: autumn leaf senescence, Bud: bud burst.
Figure 8.
Schematic representation of the drought treatment with the plants subjected to water exclusion categorized according to the level of visible drought symptoms. The number of plants in each group is indicated with a further subdivision according to the provenance (Be: Belgian, Sp: Spanish-Pyrenean, Sw: Swedish). Several variables were recorded during and after the treatment. Hei1/Hei2/Dia1/Dia2: height and diameter, Dro: visual drought stress symptoms, Res: post-drought resprouting, Sen: autumn leaf senescence, Bud: bud burst.
Figure 9.
Weights of the pots during the treatment for the control and drought group, according to the provenance.
Figure 9.
Weights of the pots during the treatment for the control and drought group, according to the provenance.
Table 1.
Test statistics for the height (Hei1) and diameter (Dia1) at the start of the treatment. The Spanish-Pyrenean (Sp) and the Swedish (Sw) provenances are compared to the standard Belgian provenance.
Table 1.
Test statistics for the height (Hei1) and diameter (Dia1) at the start of the treatment. The Spanish-Pyrenean (Sp) and the Swedish (Sw) provenances are compared to the standard Belgian provenance.
| |
Height |
|
|
|
Diameter |
|
|
|
| |
Estimate |
Std. error |
t value |
p value |
Estimate |
Std. error |
t value |
p value |
| (Intercept) |
75,523 |
1,055 |
71,557 |
< 0,001*** |
8,900 |
0,113 |
78,898 |
< 0,001*** |
| Sp |
-10,650 |
1,619 |
-6,576 |
< 0,001*** |
-0,617 |
0,173 |
-3,564 |
< 0,001*** |
| Sw |
-5,194 |
1,571 |
-3,306 |
0,001** |
-1,214 |
0,168 |
-7,231 |
< 0,001*** |
Table 2.
Test statistics for the development of drought symptoms during the drought treatment. The Spanish-Pyrenean (Sp) and the Swedish (Sw) provenances are compared to the standard Belgian provenance. Rew is the relative weight loss, Day the day of observation, Hei1 the plant height at the start of the treatment.
Table 2.
Test statistics for the development of drought symptoms during the drought treatment. The Spanish-Pyrenean (Sp) and the Swedish (Sw) provenances are compared to the standard Belgian provenance. Rew is the relative weight loss, Day the day of observation, Hei1 the plant height at the start of the treatment.
| |
Estimate |
Std. error |
z value |
p value |
| Day |
0,707 |
0,057 |
12,330 |
< 0,001*** |
| Sp |
-3,416 |
0,815 |
-4,194 |
< 0,001*** |
| Sw |
-1,754 |
0,713 |
-2,459 |
0,014* |
| Rew |
77,327 |
9,396 |
8,230 |
< 0,001*** |
| Hei1 |
0,053 |
0,028 |
1,930 |
0,054 |
Table 3.
Test statistics for the modelling of the chance on post-drought resprouting in the drought treated plants among the different categories of visual drought symptoms (Dro) and among the provenances. In the model of the drought categories, the category normal (no symptoms, score 1) is the standard to which the other categories are compared to. In the two provenance models, the Belgian provenance is the standard to which the other provenances are compared to. Hei1 it the plant height at the start of the drought period. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
Table 3.
Test statistics for the modelling of the chance on post-drought resprouting in the drought treated plants among the different categories of visual drought symptoms (Dro) and among the provenances. In the model of the drought categories, the category normal (no symptoms, score 1) is the standard to which the other categories are compared to. In the two provenance models, the Belgian provenance is the standard to which the other provenances are compared to. Hei1 it the plant height at the start of the drought period. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
| |
|
Estimate |
Std. error |
z value |
p value |
| Visual drought symptom categories (Dro) |
(Intercept) |
-2,477 |
1,703 |
-1,454 |
0,146 |
| Dro score 2 |
-0,238 |
1,258 |
-0,190 |
0,850 |
| Dro score 3 |
2,341 |
0,897 |
2,612 |
0,009** |
| Dro score 4 |
4,766 |
1,087 |
4,383 |
< 0,001*** |
| |
Dro score 5 |
4,692 |
0,888 |
5,284 |
< 0,001*** |
| |
Hei1 |
-0,006 |
0,024 |
-0,231 |
0,818 |
| provenances in dataset containing no to mild visual drought symptom categories(pooled Dro scores 1, 2 and 3) |
(Intercept) |
1,511 |
2,561 |
0,590 |
0,555 |
| Sp |
-2,320 |
0,972 |
-2,387 |
0,017* |
| Sw |
-2,136 |
0,933 |
-2,289 |
0,022* |
| Hei1 |
-0,031 |
0,037 |
-0,831 |
0,406 |
| provenances in dataset containing severe visual drought symptoms(pooled Dro scores 4 and 5) |
(Intercept) |
0,625 |
2,578 |
0,242 |
0,808 |
| Sp |
17,060 |
2171,000 |
0,008 |
0,994 |
| Sw |
0,310 |
0,868 |
0,358 |
0,721 |
| Hei1 |
0,012 |
0,034 |
0,362 |
0,718 |
Table 4.
Test statistics for the modelling of the timing of post-drought resprouting in the resprouting plants of the drought treated group, among the different categories of visual drought symptoms and among the provenances. In the model of the drought categories, the categories normal, wilting and <25% desiccated leaves (Dro scores 1, 2 and 3) are pooled and serve as the standard to which the other categories are compared to. In the provenance model, the Belgian provenance is the standard to which the other provenances are compared to. Day is the day of observation, Hei1 the plant height at the start of the treatment. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
Table 4.
Test statistics for the modelling of the timing of post-drought resprouting in the resprouting plants of the drought treated group, among the different categories of visual drought symptoms and among the provenances. In the model of the drought categories, the categories normal, wilting and <25% desiccated leaves (Dro scores 1, 2 and 3) are pooled and serve as the standard to which the other categories are compared to. In the provenance model, the Belgian provenance is the standard to which the other provenances are compared to. Day is the day of observation, Hei1 the plant height at the start of the treatment. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
| |
|
Estimate |
Std. error |
z value |
p value |
| visual drought symptom categories (Dro with scores 1, 2 and 3 being pooled) |
Day |
-0,885 |
0,098 |
-9,011 |
< 0,001*** |
| Dro score 4 |
-2,774 |
1,258 |
-2,206 |
0,027* |
| Dro score 5 |
-4,498 |
1,149 |
-3,916 |
< 0,001*** |
| Hei1 |
-0,029 |
0,030 |
-0,986 |
0,324 |
| provenances |
Day |
-0,883 |
0,097 |
-9,073 |
< 0,001*** |
| |
Sp |
0,879 |
1,072 |
0,820 |
0,412 |
| |
Sw |
0,344 |
0,955 |
0,360 |
0,719 |
| |
Hei1 |
-0,060 |
0,034 |
-1,775 |
0,076 |
Table 5.
Test statistics for the modelling of the timing of leaf senescence in controls and droughted plants, among the different categories of visual drought symptoms and among the provenances. In the visual drought symptoms model, the control plants are the standard to which the different categories of visual drought symptoms (Dro) are compared to. In the three provenance models, the Belgian provenance is the standard to which the other provenances are compared to. Day is the day of observation, Hei2 the plant height at the end of the growing season. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
Table 5.
Test statistics for the modelling of the timing of leaf senescence in controls and droughted plants, among the different categories of visual drought symptoms and among the provenances. In the visual drought symptoms model, the control plants are the standard to which the different categories of visual drought symptoms (Dro) are compared to. In the three provenance models, the Belgian provenance is the standard to which the other provenances are compared to. Day is the day of observation, Hei2 the plant height at the end of the growing season. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
| |
|
Estimate |
Std. error |
z value |
p value |
| visual drought symptom categories (total dataset) |
Day |
0,343 |
0,020 |
17,288 |
<0,001*** |
| Dro score1 |
-0,647 |
0,347 |
-1,864 |
0,062 |
| Dro score2 |
0,941 |
0,398 |
2,363 |
0,018* |
| Dro score3 |
-0,084 |
0,464 |
-0,180 |
0,857 |
| Dro score4 |
-2,379 |
0,524 |
-4,545 |
<0,001*** |
| Dro score5 |
-5,126 |
0,442 |
-11,609 |
<0,001*** |
| |
Hei2 |
0,013 |
0,009 |
1,516 |
0,129 |
| provenances in dataset of control plants |
Sp |
-0,872 |
0,311 |
-2,808 |
0,005** |
| Sw |
0,808 |
0,272 |
2,969 |
0,003** |
| Day |
0,334 |
0,026 |
12,699 |
<0,001*** |
| Hei2 |
0,008 |
0,008 |
0,968 |
0,333 |
| provenances in dataset of plants with no to mild visual drought symptoms (pooled Dro scores 1, 2 and 3) |
Sp |
-1,771 |
0,673 |
-2,631 |
0,009** |
| Sw |
1,239 |
0,634 |
1,954 |
0,051 |
| Day |
0,379 |
0,041 |
9,248 |
<0,001*** |
| Hei2 |
0,003 |
0,021 |
0,134 |
0,893 |
| provenances in dataset of plants with severe visual drought symptoms (pooled Dro scores 4 and 5) |
Sp |
-0,694 |
1,052 |
-0,659 |
0,510 |
| Sw |
0,569 |
0,872 |
0,652 |
0,514 |
| Day |
0,390 |
0,064 |
6,129 |
<0,001*** |
| Hei2 |
-0,006 |
0,033 |
-0,172 |
0,863 |
Table 6.
Test statistics for the modelling of the relative chlorophyll content index between control plants and plants that lost more than a quarter of their foliage due to the drought. The control plants are the standard to which the severely affected plants (Dro_adj2) are compared to. Day is the day of observation.
Table 6.
Test statistics for the modelling of the relative chlorophyll content index between control plants and plants that lost more than a quarter of their foliage due to the drought. The control plants are the standard to which the severely affected plants (Dro_adj2) are compared to. Day is the day of observation.
| |
Estimate |
Std. error |
DF |
t value |
p value |
| (Intercept) |
11,45 |
0,69 |
152 |
16,65 |
<0,001*** |
| Day |
-55,78 |
3,21 |
152 |
-17,37 |
<0,001*** |
| Day2
|
-18,04 |
3,15 |
152 |
-5,72 |
<0,001*** |
| Dro_adj2 |
1,98 |
0,97 |
52 |
2,04 |
0,047* |
| Day:Dro_adj2 |
15,49 |
4,40 |
152 |
3,52 |
0,001*** |
| Day2:Dro_adj2 |
4,00 |
4,38 |
152 |
0,91 |
0,362 |
Table 7.
Test statistics for the modelling of the timing of bud burst in controls and droughted plants, among the different categories of visual drought symptoms and among the provenances. In the visual drought symptoms model, the control plants are the standard to which the different categories of visual drought symptoms (Dro) are compared to. In the three provenance models, the Belgian provenance is the standard to which the other provenances are compared to. Day is the day of observation, Hei2 the plant height at the end of the growing season. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
Table 7.
Test statistics for the modelling of the timing of bud burst in controls and droughted plants, among the different categories of visual drought symptoms and among the provenances. In the visual drought symptoms model, the control plants are the standard to which the different categories of visual drought symptoms (Dro) are compared to. In the three provenance models, the Belgian provenance is the standard to which the other provenances are compared to. Day is the day of observation, Hei2 the plant height at the end of the growing season. Sp: Spanish-Pyrenean provenance, Sw: Swedish provenance.
| |
|
Estimate |
Std. error |
z value |
p value |
| visual drought symptom categories (total dataset) |
Dro score1 |
0,450 |
0,621 |
0,724 |
0,469 |
| Dro score2 |
2,374 |
0,756 |
3,139 |
0,002** |
| Dro score3 |
1,839 |
0,902 |
2,038 |
0,042* |
| Dro score4 |
0,366 |
0,895 |
0,409 |
0,682 |
| Dro score5 |
1,901 |
0,565 |
3,363 |
<0,001*** |
| Hei2 |
0,058 |
0,014 |
4,028 |
<0,001*** |
| |
Day |
-0,914 |
0,051 |
-17,785 |
<0,001*** |
| provenances in dataset of control plants |
Sp |
-0,713 |
0,507 |
-1,407 |
0,160 |
| Sw |
4,286 |
0,572 |
7,490 |
<0,001*** |
| Day |
-0,854 |
0,069 |
-12,427 |
<0,001*** |
| |
Hei2 |
0,061 |
0,015 |
4,033 |
<0,001*** |
| provenances in dataset of plants with no to mild visual drought symptoms (pooled Dro scores 1, 2 and 3) |
Sp |
0,350 |
0,750 |
0,466 |
0,641 |
| Sw |
4,977 |
0,920 |
5,410 |
<0,001*** |
| Day |
-0,889 |
0,099 |
-9,017 |
<0,001*** |
| |
Hei2 |
0,105 |
0,027 |
3,913 |
<0,001*** |
| provenances in dataset of plants with severe visual drought symptoms (pooled Dro scores 4 and 5) |
Sp |
-0,114 |
1,198 |
-0,095 |
0,924 |
| Sw |
4,829 |
1,170 |
4,129 |
<0,001*** |
| Day |
-1,036 |
0,131 |
-7,894 |
<0,001*** |
| Hei2 |
-0,004 |
0,037 |
-0,111 |
0,912 |
Table 8.
Schematic representation of the timing of autumn leaf senescence and spring bud burst in the droughted plants in comparison to the controls. Droughted plants are grouped according to the categories of visual drought symptoms during the summer water withholding.
Table 8.
Schematic representation of the timing of autumn leaf senescence and spring bud burst in the droughted plants in comparison to the controls. Droughted plants are grouped according to the categories of visual drought symptoms during the summer water withholding.
| Visual drought symptoms during water withholding (Dro) |
leaf senescence |
bud burst |
| Score 1: no symptoms |
= control |
= control |
| Score 2: wilting leaves |
earlier |
later |
| Score 3: <25% desiccated leaves |
= control |
later |
| Score 4: 25%-75% desiccated leaves |
later |
= control |
| Score 5: >75% desiccated leaves |
later |
later |