Submitted:
18 February 2025
Posted:
18 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
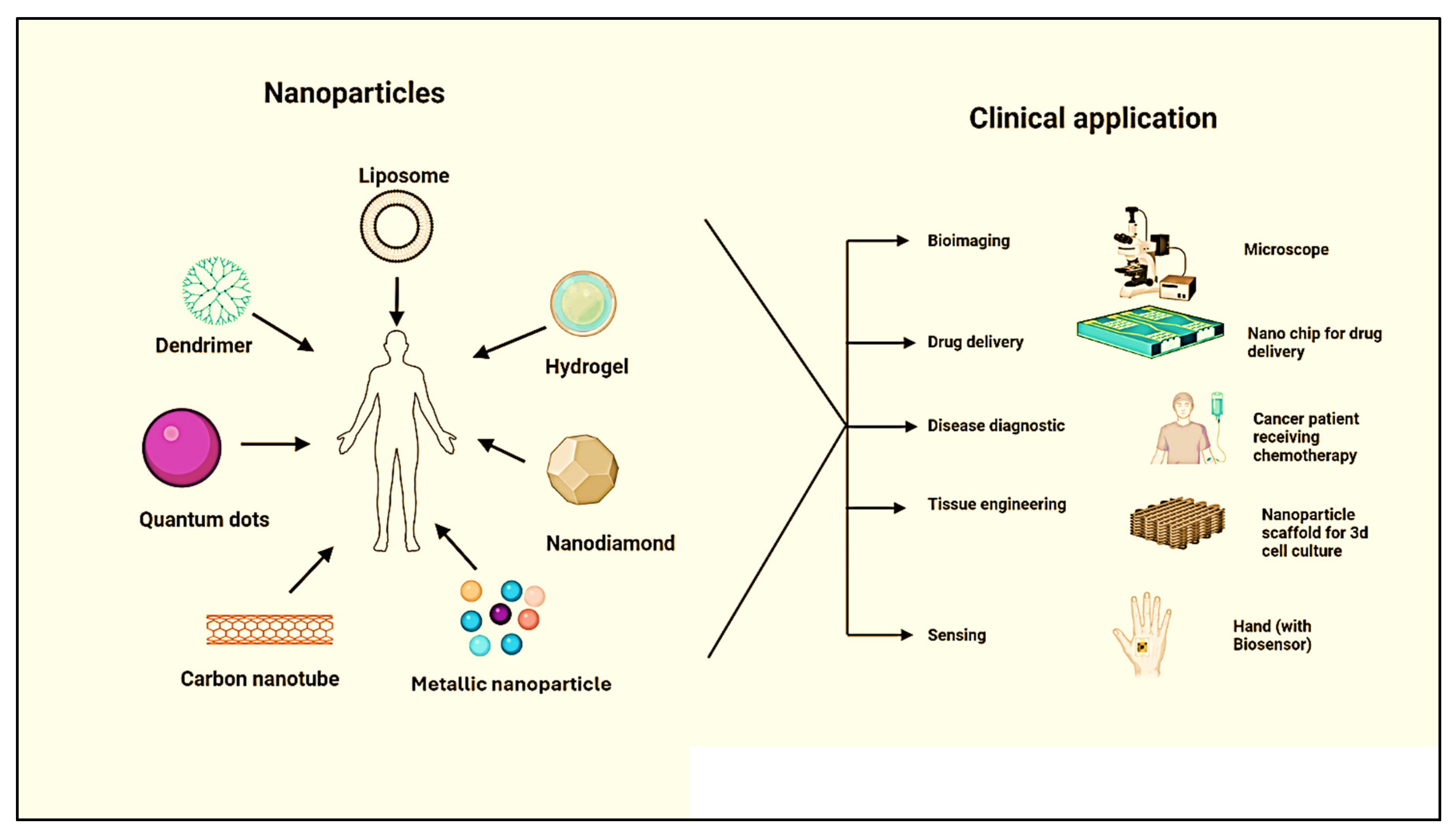
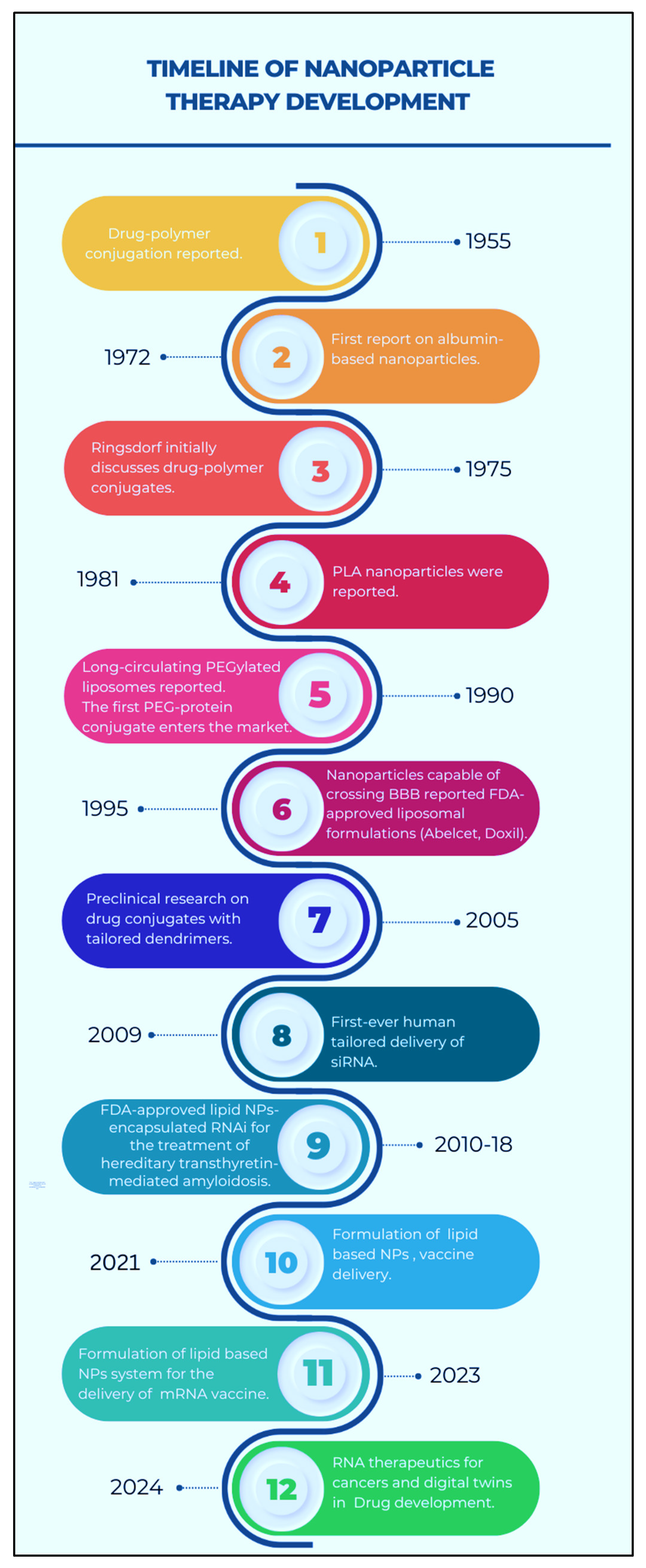
2. Historical Background of Programmable Nanomaterials
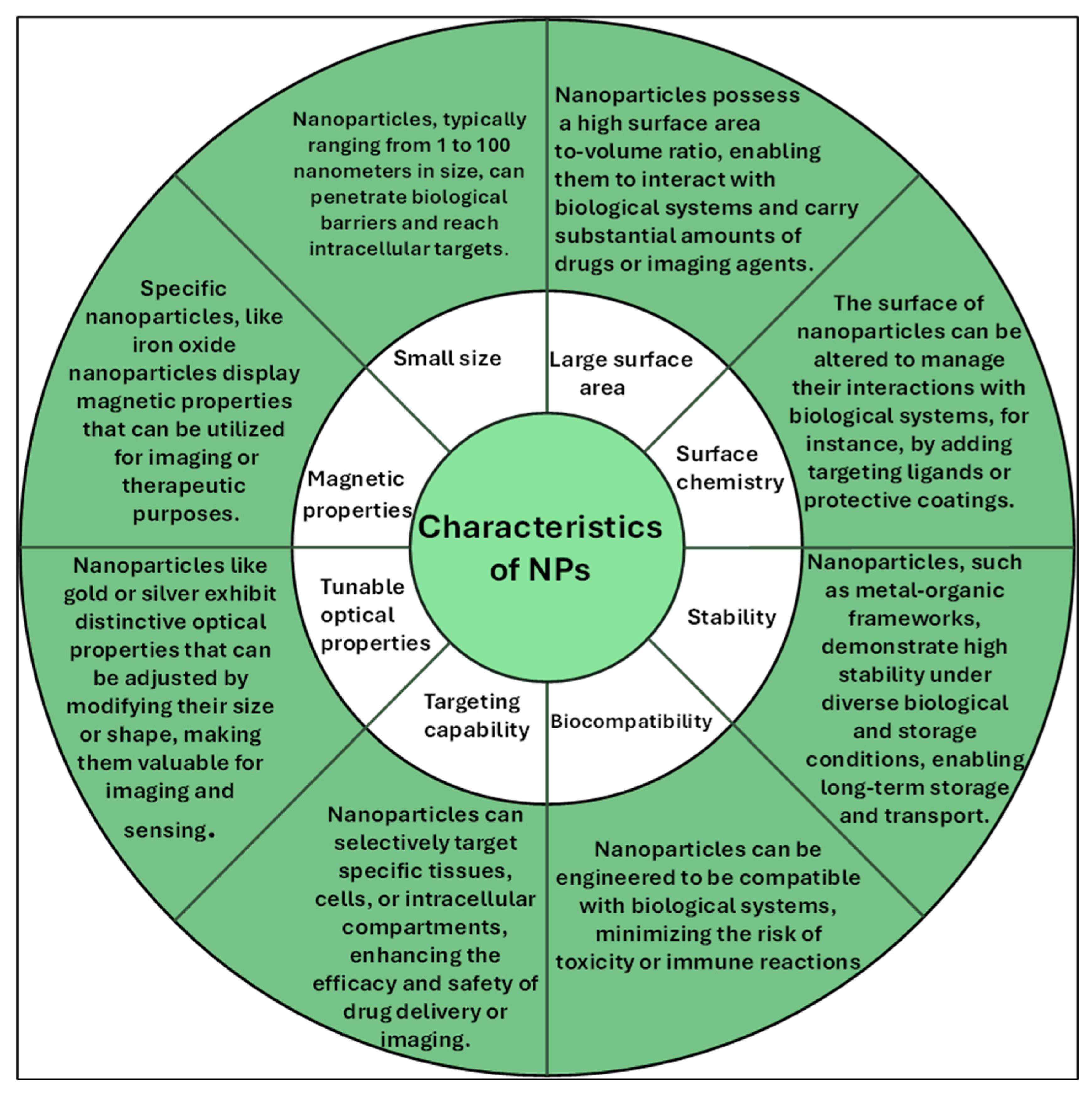
3. Characteristics of Nanomaterials
4. What is Needed to Program Nanoparticles
5. Different Types of Programmable Nanomaterials
5.1. Polymeric Nanomaterials
5.1.1. Liposomes
Doxil®
Vyxeos™ and Onivyde™
Marqibo®
5.1.2. Dendrimers
5.1.3. Nano Hydrogel
5.2. Carbon-Based Nanomaterials
5.2.1. Nanodiamonds (NDs)
5.2.2. Quantum Dots (QDs)
5.2.3. Carbon Nanotube (CNTs)
5.3. Metallic Nanoparticles
| Name | Drug | Carrier property | Indications | Manufacture Company |
| Myocet® | Doxorubicin | Liposome | Breast Cancer | Teva |
| Marqibo® | Vincristine sulphate | Liposome | Acute lymphoblastic leukemia (ALL) | Talon Therapeutics |
| Ambisome | Amphotericin B | Liposome | Fungal infection | Gilead Sciences |
| Onivyde® | Irinotecan | Liposome | Pancreatic cancer | Merrimack Pharmaceuticals |
| Doxil® | Doxorubicin | Liposome | Various cancers, ALL, AML, Breast cancer, Ovarian cancer | Johnson &Johnson |
| Vyxeos® | Cytarabine and daunorubicin |
Liposome | AML | Jazz Pharmaceuticals |
| Abraxane® | Paclitaxel | Albumin | Various cancers, Breast cancer | Abraxis BioScience |
| Name | Drug | Carrier property | Indications | Manufacture Company |
| Genexol-PM® | Paclitaxel | Polymeric micelles | Breast,lung, ovarian cancer | Samyang |
| NK-105® | Paclitaxel | Micelle: PEG- poly aspartate |
Metastatic Breast Cancer | Nippon Kayaku Co. |
| NanoTherm | Aminosilane- coated SPIONs |
Metallic nanoparticle | GBM & prostate cancer | MagForce Nanotechnologies |
| ThermoDox | Doxorubicin | Thermosensitive liposome | Hepatocellular carcinoma | Celsion |
| Lipoplatin® | Cisplatin | Liposome | Breast, pancreatic, urinary bladder, and gastrointestinal cancer |
Regulon Inc. |
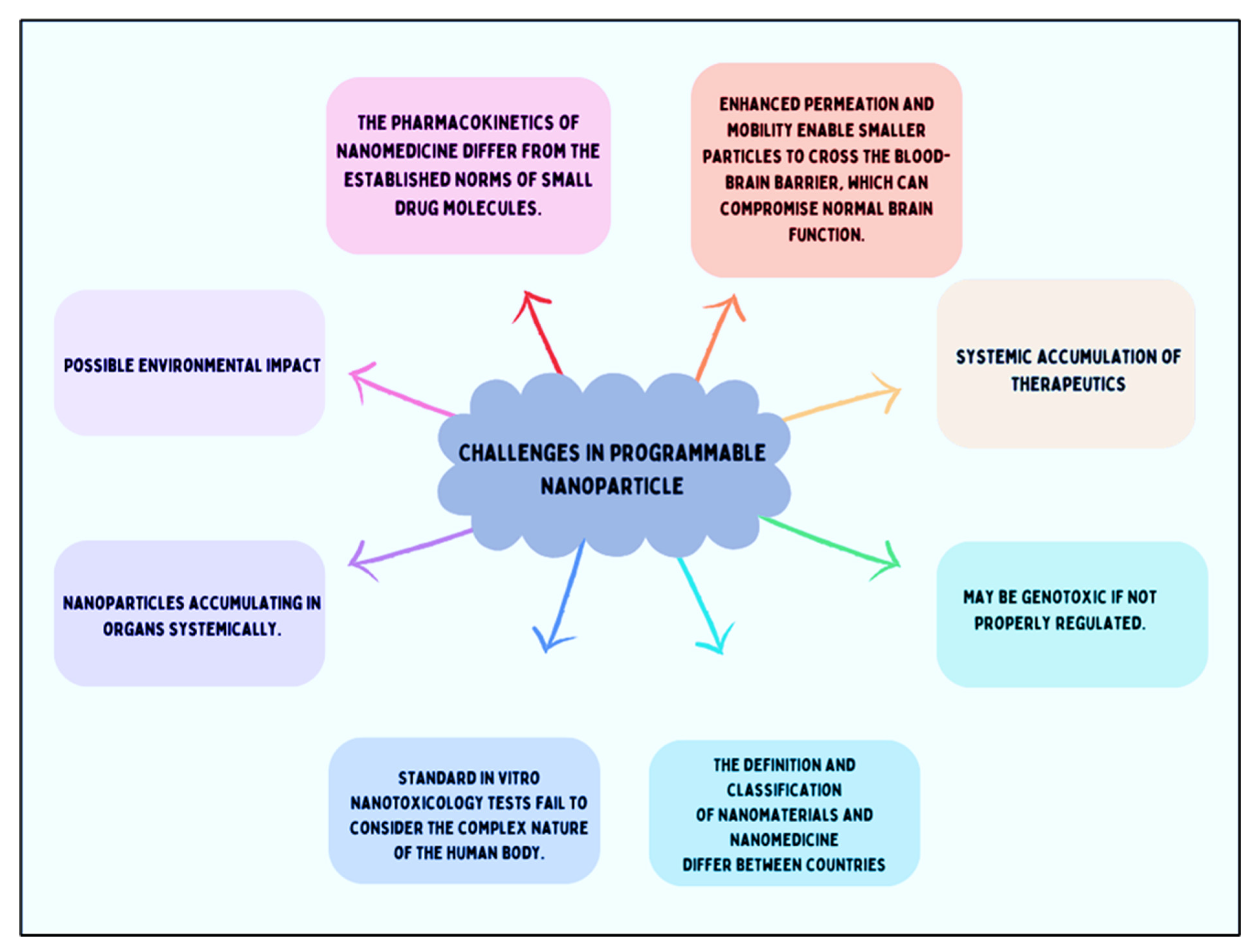
6. The Challenge in Programming the Nanoparticles
7. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacological Reports 2012, 64, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanotechnology, Biology, and Medicine 2015, 11, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Chiari-Andréo, B.G.; et al. Nanoparticles for cosmetic use and its application. In Nanoparticles in Pharmacotherapy; Elsevier, 2019; pp. 113–146. [Google Scholar] [CrossRef]
- Wang, J. Nanoparticle-based electrochemical DNA detection. Anal Chim Acta 2003, 500, 247–257. [Google Scholar] [CrossRef]
- Peng, W.; et al. Improvement of magnetorheological finishing surface quality by nanoparticle jet polishing. 2013, 52, 043401. [Google Scholar] [CrossRef]
- Ghosh, S.; et al. Nanoparticle-enhanced multifunctional nanocarbons—recent advances on electrochemical energy storage applications. J Phys D Appl Phys 2022, 55, 413001. [Google Scholar] [CrossRef]
- Hanigan, D.; et al. Trade-offs in ecosystem impacts from nanomaterial versus organic chemical ultraviolet filters in sunscreens. Water Res 2018, 139, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Baron, R.; Willner, B. Integrated nanoparticle–biomolecule systems for biosensing and bioelectronics. Biosens Bioelectron 2007, 22, 1841–1852. [Google Scholar] [CrossRef]
- Szelenyi, I. Nanomedicine: evolutionary and revolutionary developments in the treatment of certain inflammatory diseases. [CrossRef]
- Seigneuric, R.; et al. From Nanotechnology to Nanomedicine: Applications to Cancer Research. Curr Mol Med 2010, 10, 640–652. [Google Scholar] [CrossRef]
- Peer, D.; et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Park, T.; Jeong, J.; reviews, S.K.-A. drug delivery; 2006, undefined. Current status of polymeric gene delivery systems. ElsevierTG Park, JH Jeong, SW KimAdvanced drug delivery reviews, 2006•Elsevier.
- Park, T.; Jeong, J.; reviews, S.K.-A. drug delivery & 2006, undefined. Current status of polymeric gene delivery systems. ElsevierTG Park, JH Jeong, SW KimAdvanced drug delivery reviews, 2006•Elsevier.
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J Drug Target 2008, 16, 108–123. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- TORCHILIN, V. Multifunctional nanocarriers☆. Adv Drug Deliv Rev 2006, 58, 1532–1555. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. Ageing Int 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- FURUMOTO, K.; et al. Effect of coupling of albumin onto surface of PEG liposome on its in vivo disposition. Int J Pharm 2007, 329, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Mok, H.; Oh, Y.; chemistry, T.P.-B. & 2009, undefined. siRNA conjugate delivery systems. ACS PublicationsJH Jeong, H Mok, YK Oh, TG ParkBioconjugate chemistry, 2009•ACS Publications 2009, 20, 5–14. [Google Scholar]
- Elegbede, A.I.; et al. Mechanistic studies of the triggered release of liposomal contents by matrix metalloproteinase-9. ACS PublicationsAI Elegbede, J Banerjee, AJ Hanson, S Tobwala, B Ganguli, R Wang, X Lu, DK SrivastavaJournal of the American Chemical Society, 2008•ACS Publications 2008, 130, 10633–10642. [Google Scholar] [CrossRef]
- Patil, M.; Zhang, M.; nano, T.M.-A. & 2011, undefined. Multifunctional triblock nanocarrier (PAMAM-PEG-PLL) for the efficient intracellular siRNA delivery and gene silencing. ACS PublicationsML Patil, M Zhang, T MinkoACS nano, 2011•ACS Publications 2011, 5, 1877–1887. [Google Scholar]
- Malpure, P.S.; Patil, S.S.; More, Y.M.; Nikam, P.P. A Review On-Hydrogel. A Review On-Hydrogel. American Journal of PharmTech Research 2018, 8. [Google Scholar] [CrossRef]
- Coonrod, A.; Li, F.Q.; Horwitz, M. On the mechanism of DNA transfection: efficient gene transfer without viruses. Gene Ther 1997, 4, 1313–1321. [Google Scholar] [CrossRef]
- Mattoussi, H.; Palui, G.; reviews, H.N.-A. drug delivery & 2012, undefined. Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes. ElsevierH Mattoussi, G Palui, HB NaAdvanced drug delivery reviews, 2012•Elsevier.
- Smith, A.; Duan, H.; Mohs, A.; reviews, S.N.-A. drug delivery & 2008, undefined. Bioconjugated quantum dots for in vivo molecular and cellular imaging. ElsevierAM Smith, H Duan, AM Mohs, S NieAdvanced drug delivery reviews, 2008•Elsevier.
- Khursheed, R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomedicine & Pharmacotherapy 2022, 150, 112951. [Google Scholar]
- Elahi, N.; Kamali, M.; Talanta, M.B.-. & 2018, undefined. Recent biomedical applications of gold nanoparticles: A review. ElsevierN Elahi, M Kamali, MH BaghersadTalanta, 2018•Elsevier 2018, 184, 537–556. [Google Scholar]
- Clinical trials. Available online: https://www.who.int/health-topics/clinical-trials#tab=tab_1.
- Davis, M.E. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm 2009, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- JATZKEWITZ, H. [Incorporation of physiologically-active substances into a colloidal blood plasma substitute. I. Incorporation of mescaline peptide into polyvinylpyrrolidone]. Hoppe Seylers Z Physiol Chem 1954, 297, 149–56. [Google Scholar] [CrossRef]
- Scheffel, U.; Rhodes, B.A.; Natarajan, T.K.; Wagner, H.N. Albumin microspheres for study of the reticuloendothelial system. J Nucl Med 1972, 13, 498–503. [Google Scholar]
- Gradishar, W.J.; et al. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared With Polyethylated Castor Oil–Based Paclitaxel in Women With Breast Cancer. Journal of Clinical Oncology 2005, 23, 7794–7803. [Google Scholar] [CrossRef] [PubMed]
- KREUTER, J. Nanoparticles—a historical perspective. Int J Pharm 2007, 331, 1–10. [Google Scholar] [CrossRef]
- Maeda, H.; Greish, K.; Fang, J. The EPR effect and polymeric drugs: A paradigm shift for cancer chemotherapy in the 21st century. Advances in Polymer Science 2006, 193, 103–121. [Google Scholar]
- Kim, T.-Y.; et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res 2004, 10, 3708–16. [Google Scholar] [CrossRef]
- Brem, H.; et al. Biocompatibility of a biodegradable, controlled-release polymer in the rabbit brain. Sel Cancer Ther 1989, 5, 55–65. [Google Scholar] [CrossRef]
- Brem, H.; et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 1995, 345, 1008–12. [Google Scholar] [CrossRef]
- Malik, N.; Evagorou, E.G.; Duncan, R. Dendrimer-platinate: a novel approach to cancer chemotherapy. Anticancer Drugs 1999, 10, 767–76. [Google Scholar] [CrossRef] [PubMed]
- Danson, S.; et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer 2004, 90, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Kukowska-Latallo, J.F.; et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Davis, M.E. The First Targeted Delivery of siRNA in Humans via a Self-Assembling, Cyclodextrin Polymer-Based Nanoparticle: From Concept to Clinic. [CrossRef]
- Laina, A.; Vlachogiannis, N.; Stamatelopoulos, K.; Stellos, K. RNA therapies for cardiovascular disease. The Vasculome: From Many, One 2022, 413–425. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Expediting Drug Development of Novel Therapeutics: Regulatory and Commercialization Implications of Digital Twin Technology in Clinical Trials | Vanderbilt JETLaw | Vanderbilt University. Available online: https://www.vanderbilt.edu/jetlaw/2024/01/24/expediting-drug-development-of-novel-therapeutics-regulatory-and-commercialization-implications-of-digital-twin-technology-in-clinical-trials/.
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on In Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv Drug Deliv Rev 2002, 54, 135–147. [Google Scholar] [CrossRef]
- Ilium, L.; et al. Blood clearance and organ deposition of intravenously administered colloidal particles. The effects of particle size, nature and shape. Int J Pharm 1982, 12, 135–146. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hedeman, H.; Muir, I.S.; Illum, L.; Davis, S.S. An investigation of the filtration capacity and the fate of large filtered sterically-stabilized microspheres in rat spleen. Biochimica et Biophysica Acta (BBA) - General Subjects 1993, 1157, 233–240. [Google Scholar] [CrossRef]
- Porter, C.J.H.; Moghimi, S.M.; Illum, L.; Davis, S.S. The polyoxyethylene/polyoxypropylene block co-polymer Poloxamer-407 selectively redirects intravenously injected microspheres to sinusoidal endothelial cells of rabbit bone marrow. FEBS Lett 1992, 305, 62–66. [Google Scholar] [CrossRef]
- Mitragotri, S.; Lahann, J. Physical approaches to biomaterial design. Nat Mater 2009, 8, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Rege, K. Cancer Cell Phenotype Dependent Differential Intracellular Trafficking of Unconjugated Quantum Dots. [CrossRef]
- Frank, M.M.; Fries, L.F. The role of complement in inflammation and phagocytosis. Immunol Today 1991, 12, 322–326. [Google Scholar] [CrossRef]
- Leu, D.; Manthey, B.; Kreuter, J.; Speiser, P.; Delucax, P.P. Distribution and Elimination of Coated Polymethyl [2-14C]Methacrylate Nanoparticles After Intravenous Injection in Rats. J Pharm Sci 1984, 73, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Göppert, T.M.; Müller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J Drug Target 2005, 13, 179–187. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Patel, H.M. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system-The concept of tissue specificity. Adv Drug Deliv Rev 1998, 32, 45–60. [Google Scholar] [CrossRef] [PubMed]
- DOBROVOLSKAIA, M.A.; McNEIL, S.E. Immunological properties of engineered nanomaterials. Nanoscience and Technology 2009, 278–287. [Google Scholar] [CrossRef]
- Serda, R.E. 1:2;1-J Volume 1 | Number 2 | 2009 Nanoscale Pages 173-288 www. rsc. 2009, 1, 173–288. [Google Scholar]
- Xia, Y.; Halas, N.J. Shape-Controlled Synthesis and Surface Plasmonic Properties of Metallic Nanostructures. MRS Bull 2005, 30, 338–348. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Trubetskoy, V.S. Which polymers can make nanoparticulate drug carriers long-circulating? Adv Drug Deliv Rev 1995, 16, 141–155. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J Pharm Sci 2003, 92, 1343–1355. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv Drug Deliv Rev 2003, 55, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Allouni, Z.E.; Cimpan, M.R.; Høl, P.J.; Skodvin, T.; Gjerdet, N.R. Agglomeration and sedimentation of TiO2 nanoparticles in cell culture medium. Colloids Surf B Biointerfaces 2009, 68, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y.; Liang, X.-J. Theranostic Nanoparticles Engineered for Clinic and Pharmaceutics. Acc Chem Res 44, 1114. [CrossRef] [PubMed]
- Zhang, X.Q.; et al. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv Drug Deliv Rev 2012, 64, 1363–1384. [Google Scholar] [CrossRef]
- Singh, N.; et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Smolkova, B.; Dusinska, M.; Gabelova, A. Nanomedicine and epigenome. Possible health risks. Food Chem Toxicol 2017, 109, 780–796. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int 2013, 2013. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Balharry, D.; Wallin, H.; Loft, S.; Møller, P. Nanomaterial translocation--the biokinetics, tissue accumulation, toxicity and fate of materials in secondary organs--a review. Crit Rev Toxicol 2015, 45, 837–872. [Google Scholar] [CrossRef]
- Nagati, V.; Tenugu, S.; Pasupulati, A.K. Stability of therapeutic nano-drugs during storage and transportation as well as after ingestion in the human body. In Advances in Nanotechnology-Based Drug Delivery Systems; Elsevier, 2022; pp. 83–102. [Google Scholar] [CrossRef]
- Tang, M.; Lei, L.; Guo, S.; Cancer, W.H.-C. J. & 2010, undefined. Recent progress in nanotechnology for cancer therapy. scholar.archive.orgMF Tang, L Lei, SR Guo, WL HuangChin J Cancer, 2010•scholar.archive.org.
- Hu, X.; et al. Biodegradable block copolymer-doxorubicin conjugates via different linkages: preparation, characterization, and in vitro evaluation. ACS PublicationsX Hu, S Liu, Y Huang, X Chen, X JingBiomacromolecules, 2010•ACS Publications 2010, 11, 2094–2102. [Google Scholar] [CrossRef]
- Hans, M.; Materials, A.L.-C. O. in S. S. and & 2002, undefined. Biodegradable nanoparticles for drug delivery and targeting. Elsevier.
- Wang, L.; Zeng, R.; Li, C.; biointerfaces, R.Q.-C. and S. B. & 2009, undefined. Self-assembled polypeptide-block-poly (vinylpyrrolidone) as prospective drug-delivery systems. ElsevierL Wang, R Zeng, C Li, R QiaoColloids and Surfaces B: biointerfaces, 2009•Elsevier.
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J Drug Target 2008, 16, 108–123. [Google Scholar] [CrossRef]
- Gilding, D.K.; Reed, A.M. Biodegradable polymers for use in surgery—polyglycolic/poly(actic acid) homo- and copolymers: 1. Polymer (Guildf) 1979, 20, 1459–1464. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med 2009, 234, 123–131. [Google Scholar] [CrossRef]
- Mao, C.; et al. A biodegradable amphiphilic and cationic triblock copolymer for the delivery of siRNA targeting the acid ceramidase gene for cancer therapy. ElsevierCQ Mao, JZ Du, TM Sun, YD Yao, PZ Zhang, EW Song, J WangBiomaterials, 2011•Elsevier.
- Park, M.R.; et al. Degradable polyethylenimine-alt-poly(ethylene glycol) copolymers as novel gene carriers. Journal of Controlled Release 2005, 105, 367–380. [Google Scholar] [CrossRef]
- Tam, Y.T.; Gao, J.; Kwon, G.S. Oligo(lactic acid) n -Paclitaxel Prodrugs for Poly(ethylene glycol)- block -poly(lactic acid) Micelles: Loading, Release, and Backbiting Conversion for Anticancer Activity. J Am Chem Soc 2016, 138, 8674–8677. [Google Scholar] [CrossRef]
- Cho, H.; Gao, J.; Kwon, G.S. PEG- b -PLA micelles and PLGA- b -PEG- b -PLGA sol–gels for drug delivery. Journal of Controlled Release 2016, 240, 191–201. [Google Scholar] [CrossRef]
- Werner, M.E.; et al. Preclinical Evaluation of Genexol-PM, a Nanoparticle Formulation of Paclitaxel, as a Novel Radiosensitizer for the Treatment of Non-Small Cell Lung Cancer. International Journal of Radiation Oncology*Biology*Physics 2013, 86, 463–468. [Google Scholar] [CrossRef]
- Bernabeu, E.; Cagel, M.; Lagomarsino, E.; Moretton, M.; Chiappetta, D.A. Paclitaxel: What has been done and the challenges remain ahead. Int J Pharm 2017, 526, 474–495. [Google Scholar] [CrossRef]
- Kim, T.-Y.; et al. Phase I and Pharmacokinetic Study of Genexol-PM, a Cremophor-Free, Polymeric Micelle-Formulated Paclitaxel, in Patients with Advanced Malignancies. Clinical Cancer Research 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Negishi, T.; et al. NK105, a paclitaxel-incorporating micellar nanoparticle, is a more potent radiosensitising agent compared to free paclitaxel. Br J Cancer 2006, 95, 601–606. [Google Scholar] [CrossRef]
- Hamaguchi, T.; et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br J Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef]
- Yardley, D.A. nab-Paclitaxel mechanisms of action and delivery. Journal of Controlled Release 2013, 170, 365–372. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. NEJM.org. N Engl J Med 2013, 18, 1691–703. [Google Scholar] [CrossRef]
- Basu, B.; Prajapati, B.; Dutta, A.; Paliwal, H. Medical Application of Liposomes. J Explor Res Pharmacol 2024, 9, 30–39. [Google Scholar] [CrossRef]
- Wagner, A.; et al. GMP production of liposomes--a new industrial approach. J Liposome Res 2006, 16, 311–319. [Google Scholar] [CrossRef]
- Weiner, N.; Martin, F.; Riaz, M. Liposomes as a drug delivery system. Drug Dev Ind Pharm 1989, 15, 1523–1554. [Google Scholar] [CrossRef]
- Anderson, M.; Omri, A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Jr, E.G.; Mar, N. Del, Owens, J.; letters, E.H.-N. & 1995, undefined. Transfecting neurons and glia in the rat using pH-sensitive immunoliposomes. ElsevierEE Geisert Jr, NA Del Mar, JL Owens, EG HolmbergNeuroscience letters, 1995•Elsevier.
- Christie, J.; Today, U.K.-D. D. & 2008, undefined. Ophthalmic light sensitive nanocarrier systems. ElsevierJG Christie, UB KompellaDrug Discovery Today, 2008•Elsevier.
- Suzuki, R.; Oda, Y.; … N. U.-Y. Z., J. & 2010, undefined. Development of ultrasonic cancer therapy using ultrasound sensitive liposome. europepmc.orgR Suzuki, Y Oda, N Utoguchi, K MaruyamaYakugaku Zasshi: Journal Of The Pharmaceutical Society Of Japan, 2010•europepmc.org.
- Lehner, R.; Wang, X.; Wolf, M.; release, P.H.-J. of controlled & 2012, undefined. Designing switchable nanosystems for medical application. ElsevierR Lehner, X Wang, M Wolf, P HunzikerJournal of controlled release, 2012•Elsevier.
- Goins, B.; Bao, A.; Phillips, W.T. Techniques for Loading Technetium-99m and Rhenium-186/188 Radionuclides into Preformed Liposomes for Diagnostic Imaging and Radionuclide Therapy. in 155–178 (2017). [CrossRef]
- Barenholz, Y. (Chezy). Doxil® — The first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of Pegylated Liposomal Doxorubicin. Clin Pharmacokinet 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Lancet, J.E.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. Journal of Clinical Oncology 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Passero, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev Anticancer Ther 2016, 16, 697–703. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J Intern Med 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Pavan, G.M.; et al. PAMAM dendrimers for siRNA delivery: Computational and experimental Insights. Chemistry - A European Journal 2010, 16, 7781–7795. [Google Scholar] [CrossRef]
- Navarro, G.; Nanotechnology, C. de Il.-N.; and, B. & 2009, undefined. Activated and non-activated PAMAM dendrimers for gene delivery in vitro and in vivo. ElsevierG Navarro, CT de ILarduyaNanomedicine: Nanotechnology, Biology and Medicine, 2009•Elsevier.
- Jia, L.; Xu, J.; Wang, H.; Biointerfaces, J.J.-C. and S. B. & 2011, undefined. Polyamidoamine dendrimers surface-engineered with biomimetic phosphorylcholine as potential drug delivery carriers. ElsevierL Jia, JP Xu, H Wang, J JiColloids and Surfaces B: Biointerfaces, 2011•Elsevier.
- Zhou, W.; et al. Zwitterionic phosphorylcholine as a better ligand for gold nanorods cell uptake and selective photothermal ablation of cancer cells. pubs.rsc.orgW Zhou, J Shao, Q Jin, Q Wei, J Tang, J JiChemical communications, 2010•pubs.rsc.org 2010, 46, 1479–1481. [Google Scholar] [CrossRef]
- Mccarthy, T.D.; et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. ACS PublicationsTD McCarthy, P Karellas, SA Henderson, M Giannis, DF O’Keefe, G Heery, JRA PaullMolecular pharmaceutics, 2005•ACS Publications 2005, 2, 312–318. [Google Scholar] [CrossRef]
- O’Loughlin, J.; Millwood, I.; … H., M.-S. transmitted & 2010, undefined. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel®): a dose ranging, phase I study. journals.lww.comJ O’Loughlin, IY Millwood, HM McDonald, CF Price, JM Kaldor, JRA PaullSexually transmitted diseases, 2010•journals.lww.com.
- Maulvi, F.; Soni, T.; delivery, D.S.-D. & 2016, undefined. A review on therapeutic contact lenses for ocular drug delivery. Taylor & FrancisFA Maulvi, TG Soni, DO ShahDrug delivery, 2016•Taylor & Francis 2016, 23, 3017–3026. [Google Scholar]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. Journal of Cosmetic and Laser Therapy 2008, 10, 35–42. [Google Scholar] [CrossRef]
- Culver, H.R.; Wechsler, M.E.; Peppas, N.A. Label-Free Detection of Tear Biomarkers Using Hydrogel Coated Gold Nanoshells in a Localized Surface Plasmon Resonance-Based Biosensor HHS Public Access. ACS Nano 2018, 12, 9342–9354. [Google Scholar] [CrossRef]
- Liechty, W.B.; Scheuerle, R.L.; Vela Ramirez, J.E.; Peppas, N.A. Uptake and function of membrane-destabilizing cationic nanogels for intracellular drug delivery. Bioeng Transl Med 2019, 4, 17–29. [Google Scholar] [CrossRef]
- Liechty, W.B.; Scheuerle, R.L.; Vela Ramirez, J.E.; Peppas, N.A. Cytoplasmic delivery of functional siRNA using pH-Responsive nanoscale hydrogels. Int J Pharm 2019, 562, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; et al. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Advanced Materials 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nature Nanotechnology 2011 7:1 2011, 7, 11–23. [Google Scholar]
- Hsiao, W.W.-W.; Lin, H.-H.; Chang, H.-C. Diamond Nanoparticles for Drug Delivery and Monitoring. 2017; 119–140. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Vlasov, I.I.; Burikov, S.A.; Dolenko, T.A.; Shenderova, O.A. Nanodiamond-Based Composite Structures for Biomedical Imaging and Drug Delivery. J Nanosci Nanotechnol 2014, 15, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-J.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. ( 2017. [CrossRef]
- Boon Toh, T.; Jieh Lim, J.; Kai-Hua Chow, E. Epigenetics in cancer stem cells. [CrossRef]
- Giammarco, J.; Mochalin, V.N.; Haeckel, J.; Gogotsi, Y. The adsorption of tetracycline and vancomycin onto nanodiamond with controlled release. J Colloid Interface Sci 2016, 468, 253–261. [Google Scholar] [CrossRef]
- Giammarco, J.; Mochalin, V.N.; Haeckel, J.; Gogotsi, Y. The adsorption of tetracycline and vancomycin onto nanodiamond with controlled release. J Colloid Interface Sci 2016, 468, 253–261. [Google Scholar] [CrossRef]
- Chen, M.; et al. Nanodiamond-mediated delivery of water-insoluble therapeutics. ACS Nano 2009, 3, 2016–2022. [Google Scholar] [CrossRef]
- Lim, D.G.; Jung, J.H.; Ko, H.W.; Kang, E.; Jeong, S.H. Paclitaxel-Nanodiamond Nanocomplexes Enhance Aqueous Dispersibility and Drug Retention in Cells. ACS Appl Mater Interfaces 2016, 8, 23558–23567. [Google Scholar] [CrossRef]
- Chow, E.K.; et al. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci Transl Med 2011, 3. [Google Scholar] [CrossRef]
- Dong, X.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: current progress. Nanomedicine (Lond) 2010, 5, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; et al. Mechanism-independent optimization of combinatorial nanodiamond and unmodified drug delivery using a phenotypically driven platform technology. ACS Nano 2015, 9, 3332–3344. [Google Scholar] [CrossRef]
- Chen, M.; et al. Nanodiamond vectors functionalized with polyethylenimine for siRNA delivery. Journal of Physical Chemistry Letters 2010, 1, 3167–3171. [Google Scholar] [CrossRef]
- Alhaddad, A.; et al. Nanodiamond as a vector for siRNA delivery to Ewing sarcoma cells. Small 2011, 7, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Alwani, S.; et al. Lysine-functionalized nanodiamonds as gene carriers: development of stable colloidal dispersion for in vitro cellular uptake studies and siRNA delivery application. Int J Nanomedicine 2016, 11, 687. [Google Scholar] [PubMed]
- Zhang, X.Q.; et al. Polymer-functionalized nanodiamond platforms as vehicles for gene delivery. ACS Nano 2009, 3, 2609–2616. [Google Scholar] [CrossRef]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Girija Aswathy, R.; Yoshida, Y.; Maekawa, T.; Sakthi Kumar, D. Near-infrared quantum dots for deep tissue imaging. SpringerRG Aswathy, Y Yoshida, T Maekawa, DS KumarAnalytical and bioanalytical chemistry, 2010•Springer 2003, 397, 1417–1435. [Google Scholar]
- Aswathy: Near-infrared quantum dots for deep tissue imaging - Google Scholar. Available online: https://scholar.google.com/scholar_lookup?journal=Anal.+Bioanal.+Chem&title=Near-infrared+quantum+dots+for+deep+tissue+imaging&author=RG+Aswathy&author=Y+Yoshida&author=T+Maekawa&author=DS+Kumar&volume=397&issue=4&publication_year=2010&pages=1417-1435&pmid=20349348&.
- Li, C.; et al. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. ElsevierC Li, Y Zhang, M Wang, Y Zhang, G Chen, L Li, D Wu, Q WangBiomaterials, 2014•Elsevier 2014, 35, 393–400. [Google Scholar] [CrossRef]
- Wang, Q.; et al. Quantum dot bioconjugation during core-shell synthesis. ics.purdue.eduQ Wang, Y Liu, Y Ke, H YanANGEWANDTE CHEMIE-INTERNATIONAL EDITION IN ENGLISH-, 2008•ics.purdue.edu. [CrossRef]
- Medintz, I.L.; Mattoussi, H.; Clapp, A.R.; Clapp, A.; Medintz, I. Potential clinical applications of quantum dots. Taylor & FrancisIL Medintz, H Mattoussi, AR ClappInternational journal of nanomedicine, 2008•Taylor & Francis 2008, 3, 151–167. [Google Scholar]
- Derfus, A.; Chan, W.; letters, S.B.-N. & 2004, undefined. Probing the cytotoxicity of semiconductor quantum dots. ACS PublicationsAM Derfus, WCW Chan, SN BhatiaNano letters, 2004•ACS Publications 2004, 4, 11–18. [Google Scholar]
- Gao, S.; Xu, B.; Sun, J.; Zhang, Z. Nanotechnological advances in cancer: therapy a comprehensive review of carbon nanotube applications. Front Bioeng Biotechnol 2024, 12. [Google Scholar] [CrossRef]
- Naik, S.; Lee, S.; Theerthagiri, J.; … Y., Y.-J. of H. & 2021, undefined. Rapid and highly selective electrochemical sensor based on ZnS/Au-decorated f-multi-walled carbon nanotube nanocomposites produced via pulsed laser technique. ElsevierSS Naik, SJ Lee, J Theerthagiri, Y Yu, MY ChoiJournal of Hazardous Materials, 2021•Elsevier.
- Mohanta, D.; Patnaik, S.; Sood, S.; analysis, N.D.-J. of pharmaceutical & 2019, undefined. Carbon nanotubes: Evaluation of toxicity at biointerfaces. ElsevierD Mohanta, S Patnaik, S Sood, N DasJournal of pharmaceutical analysis, 2019•Elsevier.
- Liu, J.; Li, X.; psychiatry, X.L.-B. & 2021, undefined. Proteome-wide association study provides insights into the genetic component of protein abundance in psychiatric disorders. Elsevier.
- Kucukayan-Dogu, G.; Gozen, D.; Bitirim, V.; … K. A.-A., S. & 2015, undefined. A new tool for differentiating hepatocellular cancer cells: Patterned carbon nanotube arrays. ElsevierG Kucukayan-Dogu, D Gozen, V Bitirim, KC Akcali, E BenguApplied Surface Science, 2015•Elsevier 2015, 351, 27–32. [Google Scholar]
- Kim, M.; et al. Detection of ovarian cancer via the spectral fingerprinting of quantum-defect-modified carbon nanotubes in serum by machine learning. nature.comM Kim, C Chen, P Wang, JJ Mulvey, Y Yang, C Wun, M Antman-Passig, HB Luo, S ChoNature biomedical engineering, 2022•nature.com.
- Zhang, Q.; Yu, H.; Barbiero, M.; … B. W.-L., S. & & 2019, undefined. Artificial neural networks enabled by nanophotonics. nature.comQ Zhang, H Yu, M Barbiero, B Wang, M GuLight: Science & Applications, 2019•nature.com. [CrossRef]
- Kumar, R.; Kumar, A.; Structures, D.K.-C. & 2023, undefined. Buckling response of CNT based hybrid FG plates using finite element method and machine learning method. ElsevierR Kumar, A Kumar, DR KumarComposite Structures, 2023•Elsevier.
- science, J.H.-I. encyclopedia of statistical & 2011, undefined. Logistic regression. encyclopediaofmath.org 2008, 117, 2395–2399. [Google Scholar]
- Kumar, R.; Kumar, A.; Structures, D.K.-C. & 2023, undefined. Buckling response of CNT based hybrid FG plates using finite element method and machine learning method. ElsevierR Kumar, A Kumar, DR KumarComposite Structures, 2023•Elsevier.
- Quinlan, J.R. Learning Decision Tree Classifiers. ( 1996. [CrossRef]
- Greenhill, S.; Rana, S.; Gupta, S.; … P., V.-I. & 2020, undefined. Bayesian optimization for adaptive experimental design: A review. ieeexplore.ieee.orgS Greenhill, S Rana, S Gupta, P Vellanki, S VenkateshIEEE access, 2020•ieeexplore.ieee.org.
- Yan, H.; et al. Toxicity of Carbon Nanotubes as Anti-Tumor Drug Carriers. Int J Nanomedicine 2019, 14, 10179–10194. [Google Scholar] [CrossRef]
- Wang, J.; Sun, P.; Bao, Y.; Liu, J.; An, L. Cytotoxicity of single-walled carbon nanotubes on PC12 cells. Toxicol In Vitro 2011, 25, 242–250. [Google Scholar] [CrossRef]
- Tian, L.; et al. Multi-talented applications for cell imaging, tumor cells recognition, patterning, staining and temperature sensing by using egg white-encapsulated gold nanoclusters. ElsevierL Tian, W Zhao, L Li, Y Tong, G Peng, Y LiSensors and Actuators B: Chemical, 2017•Elsevier.
- Saeed, A.; Sánchez, J.; O’Sullivan, C.; Bioelectrochemistry, M.A.-. & 2017, undefined. DNA biosensors based on gold nanoparticles-modified graphene oxide for the detection of breast cancer biomarkers for early diagnosis. Elsevier.
- Liu, G.; et al. Aptamer-nanoparticle strip biosensor for sensitive detection of cancer cells. Anal Chem 2009, 81, 10013–10018. [Google Scholar] [CrossRef]
- Chen, M.; et al. Three-dimensional electrochemical DNA biosensor based on 3D graphene-Ag nanoparticles for sensitive detection of CYFRA21-1 in non-small cell lung cancer. ElsevierM Chen, Y Wang, H Su, L Mao, X Jiang, T Zhang, X DaiSensors and Actuators B: Chemical, 2018•Elsevier.
- Benvidi, A.; Chemistry, S.J.-J. of E. & 2016, undefined. Self-assembled monolayer of SH-DNA strand on a magnetic bar carbon paste electrode modified with Fe3O4@ Ag nanoparticles for detection of breast cancer. Elsevier.
- Mirabello, V.; Calatayud, D.G.; Arrowsmith, R.L.; Ge, H.; Pascu, S.I. Metallic nanoparticles as synthetic building blocks for cancer diagnostics: from materials design to molecular imaging applications. pubs.rsc.orgV Mirabello, DG Calatayud, RL Arrowsmith, H Ge, SI PascuJournal of Materials Chemistry B, 2015•pubs.rsc.org. [CrossRef]
- Sánchezsánchez, A.; et al. Hybrid decorated core@ shell janus nanoparticles as a flexible platform for targeted multimodal molecular bioimaging of cancer. ACS PublicationsA Sánchez, K Ovejero Paredes, J Ruiz-Cabello, P Martínez-Ruíz, JM Pingarrón, R VillalongaACS applied materials & interfaces, 2018•ACS Publications 2018, 10, 31032–31043. [Google Scholar]
- Materials, S.S.-A. & 2006, undefined. Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Wiley Online LibraryS SunAdvanced Materials, 2006•Wiley Online Library 2006, 18, 393–403. [Google Scholar]
- Mahalunkar, S.; et al. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Taylor & FrancisS Mahalunkar, AS Yadav, M Gorain, V Pawar, R Braathen, S Weiss, B Bogen, SW GosaviInternational journal of nanomedicine, 2019•Taylor & Francis 2019, 14, 8285–8302. [Google Scholar]
- Luan, X.; et al. Anisamide-targeted PEGylated gold nanoparticles designed to target prostate cancer mediate: Enhanced systemic exposure of siRNA, tumour growth suppression and a synergistic therapeutic response in combination with paclitaxel in mice. European Journal of Pharmaceutics and Biopharmaceutics 2019, 137, 56–67. [Google Scholar] [CrossRef] [PubMed]
- El-Boubbou, K. Magnetic Iron Oxide Nanoparticles As Drug Carriers: Clinical Relevance. Nanomedicine 2018, 13, 953–971. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J Neurooncol 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Cardoso, V.F.; et al. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv Healthc Mater 2018, 7. [Google Scholar] [CrossRef]
- Vol. Handbook of Clinical Nanomedicine : Law, Business, Regulation, Safety, and Risk. In Handbook of Clinical Nanomedicine; 2017. [CrossRef]
- Bawa, R. Regulating nanomedicine - can the FDA handle it? Curr Drug Deliv 2011, 8, 227–234. [Google Scholar] [CrossRef]
- Rannard, S.; Owen, A. Nanomedicine: Not a case of “One size fits all”. Nano Today 2009, 4, 382–384. [Google Scholar] [CrossRef]
- Ali, F. Regulatory perspectives of nanomaterials for theranostic application. Nanotheranostics for Treatment and Diagnosis of Infectious Diseases 2022, 373–384. [Google Scholar] [CrossRef]
- Paradise, J. Regulating Nanomedicine at the Food and Drug Administration. AMA J Ethics 2019, 21, 347–355. [Google Scholar]
- Kroll, A.; et al. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part Fibre Toxicol 2011, 8, 9. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Dickinson, A.M.; Godden, J.M.; Lanovyk, K.; Ahmed, S.S. Assessing the safety of nanomedicines: A mini review. https://eprints.ncl.ac.uk 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Flühmann, B.; Ntai, I.; Borchard, G.; Simoens, S.; Mühlebach, S. Nanomedicines: The magic bullets reaching their target? Eur J Pharm Sci 2019, 128, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, S.; et al. Preclinical hazard evaluation strategy for nanomedicines. Nanotoxicology 2019, 13, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, S.; et al. Preclinical hazard evaluation strategy for nanomedicines. Nanotoxicology 2019, 13, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Hussain, S.; Schlager, J.; Ali, S.F.; Sharma, A. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl 2010, 106, 359–364. [Google Scholar]
- Agrahari, V.; Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine (Lond) 2017, 12, 819–823. [Google Scholar] [CrossRef]
- Limaye, V.; Fortwengel, G.; Limaye, D. REGULATORY ROADMAP FOR NANOTECHNOLOGY BASED MEDICINES. International Journal of Drug Regulatory Affairs 2014, 2, 33–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





