Introduction
Left ventricular dysfunction is a key determinant of prognosis in patients with non-ST elevation myocardial infarction (NSTEMI), a common and clinically significant form of acute coronary syndrome (ACS) [
1]. Despite advances in treatment strategies, NSTEMI patients remain at high risk for long-term adverse cardiovascular events, including mortality and major adverse cardiovascular events (MACE) [
2,
3]. Effective risk stratification is essential for identifying high-risk individuals and tailoring management strategies to reduce morbidity and mortality. However, traditional risk markers, such as left ventricular ejection fraction (LVEF), while helpful, have limitations in the acute setting, as they may not fully capture the complexity of left ventricular (LV) performance, particularly in the early phases of myocardial injury [
4]. Moreover, LVEF primarily reflects systolic function and may fail to detect subtle abnormalities in diastolic function or the progressive changes in ventricular remodeling that occur after NSTEMI [
5].
The Left Ventricular Global Function Index (LVGFI) is a novel composite marker that integrates multiple dimensions of LV function, including both volumetric and functional parameters. By combining stroke volume, LV total volume, and myocardial mass, LVGFI offers a more holistic assessment of cardiac performance, potentially providing more accurate prognostic information than traditional indices [
6]. Previous studies have demonstrated the value of LVGFI in healthy population, chronic cardiovascular diseases, including heart failure, hypertrophic cardiomyopathy, and chronic kidney disease, where it has shown superior predictive power for adverse outcomes compared to traditional measures like LVEF [
6,
7,
8,
9]. Despite these findings, the role of LVGFI in predicting outcomes in acute settings, particularly in NSTEMI, remains underexplored. Given the dynamic nature of myocardial stress and recovery in NSTEMI, a more comprehensive index like LVGFI may have unique advantages for identifying patients at higher risk for adverse events in the short and long term.
This study aims to evaluate the predictive value of LVGFI for three-year mortality and MACE in NSTEMI patients, hypothesizing that LVGFI offers superior prognostic information compared to traditional markers.
Materials and Methods
Ethical approval for this retrospective cohort study was obtained from ethics committee and the study was conducted in accordance with the principles outlined in the Declaration of Helsinki. The study included a total of 432 patients diagnosed with NSTEMI. Patients were categorized into three groups based on their LVGFI values: T1 (low), T2 (intermediate), and T3 (high), with each group consisting of 144 patients. Baseline demographic and clinical characteristics, including age, sex, body mass index (BMI), and comorbidities such as hypertension, diabetes mellitus, and smoking status, were recorded for all patients. Laboratory parameters including glucose levels, lipid profiles, hemoglobin, platelet count, and creatinine levels were also collected.
Patients eligible for inclusion in this study were those aged 18 years or older with a diagnosis of NSTEMI, confirmed by clinical presentation, elevated cardiac biomarkers (such as troponins or creatine kinase MB), electrocardiographic findings indicative of ischemia without ST-segment elevation, received standard care for NSTEMI, including medical management (e.g., antiplatelet therapy, anticoagulants, and statins) and invasive procedures (e.g., coronary angiography and percutaneous coronary intervention) were included, provided they had complete follow-up data over a three-year period to assess clinical outcomes such as mortality and MACE. MACE was defined as cardiovascular death, myocardial infarction, target vessel revascularization (TVR), and stroke during follow-up.
Exclusion criteria included patients with ST-elevation myocardial infarction (STEMI), as it involves different pathophysiology and prognosis. Additionally, those with significant structural heart conditions including severe valvular diseases, hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy, and dilated cardiomyopathy (DCM), or advanced heart failure (defined as NYHA Class III/IV symptoms with LVEF< 35% and persistant symptoms despite optimal medical therapy) were excluded, as these could confound the assessment of LV function. Patients with inadequate echocardiographic data due to poor image quality, as well as those who could not provide informed consent due to cognitive impairment or other factors, were also excluded. Other exclusions included patients with active inflammatory or infectious diseases, end-stage renal disease (ESRD) requiring dialysis, and those who were lost to follow-up or had insufficient follow-up data.
The LVGFI was estimated using a comprehensive echocardiographic protocol based on established guidelines, specifically the American Society of Echocardiography (ASE) recommendations for chamber quantification [
10]. Transthoracic echocardiography was performed using advanced imaging techniques with a Philips EPIQ 7G ultrasound system and a high-frequency 1-5 MHz transducer. Measurements were obtained from parasternal long-axis, short-axis, and apical four-chamber views to ensure accuracy and reproducibility. To calculate LVGFI, left ventricular volumes were measured using the biplane method of discs (modified Simpson’s rule) from the apical four-chamber and two-chamber views. Left ventricular end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were derived by tracing the endocardial border in both diastole and systole, with the papillary muscles excluded. Stroke volume (SV) was calculated as the difference between LVEDV and LVESV. LVEF was derived by dividing the stroke volume by LVEDV, providing a measure of systolic function. Left ventricular mass (LVM) was calculated using the ASE formula: LVM = 1.04 × [(LVEDD + PWT + IVS)^3 - LVEDD^3] - 13.6 g, where LVEDD is the left ventricular end-diastolic diameter, PWT is the posterior wall thickness, and IVS is the interventricular septal thickness. The left ventricular mass index (LVMI) was calculated by dividing LVM by body surface area (BSA) to normalize for individual size. The LVGFI was calculated by multiplying the LVEF, the ratio of SV to LVEDV, and the LVMI, and then dividing the result by 100.
2.1. Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics, with continuous variables presented as mean ± standard deviation and categorical variables as percentages. Between-group comparisons were made using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. The primary outcome measures were three-year mortality and MACE, with differences between groups assessed using Kaplan-Meier survival curves and log-rank tests.
To evaluate the independent associations between LVGFI tertiles and clinical outcomes, Cox proportional hazards models were used. Model 1 was unadjusted, while Models 2, 3, and 4 adjusted for various covariates, including demographic characteristics (age, sex, BMI), clinical factors (systolic blood pressure, heart rate, previous coronary artery disease), and laboratory values (creatinine, hemoglobin, CRP, LDL). Hazard ratios (HR) with 95% confidence intervals (CI) were reported for mortality and MACE. A p-value of less than 0.05 was considered statistically significant.
Results
This study included a total of 432 NSTEMI patients with a mean age of 68 years, and 65% were male. There were no significant differences in key cardiovascular risk factors, including the prevalence of hypertension, diabetes mellitus, and smoking status. BSA was higher in T3 compared to T1 and T2 (p = 0.003). Platelet counts were highest in compared to T2 and T3 (p = 0.049). Triglyceride levels were lowest in T3 relative to T1 and T2 (p = 0.011). Other laboratory parameters, such as glucose, hemoglobin, and lipid profiles, were not significantly different between the groups (
Table 1).
Table 2 highlights the hemodynamic and echocardiographic characteristics of patients across LVGFI tertiles. Systolic and diastolic blood pressures, as well as heart rate, were similar among the groups (p > 0.05). LVEF showed no significant differences between groups (p = 0.901). However, SV was significantly lower in T1 (p < 0.001). LVM and LVMI showed a decreasing trend from T1 to T3, with LVM highest in T1 and lowest in T3, and LVMI declining from T1 to T3 (both p < 0.001). Left ventricular end-diastolic diameter (LVEDD) and LVEDV were consistent across tertiles (p = 0.167 and p = 0.407, respectively), while LVESV was slightly higher in T1, nearing significance (p = 0.050).
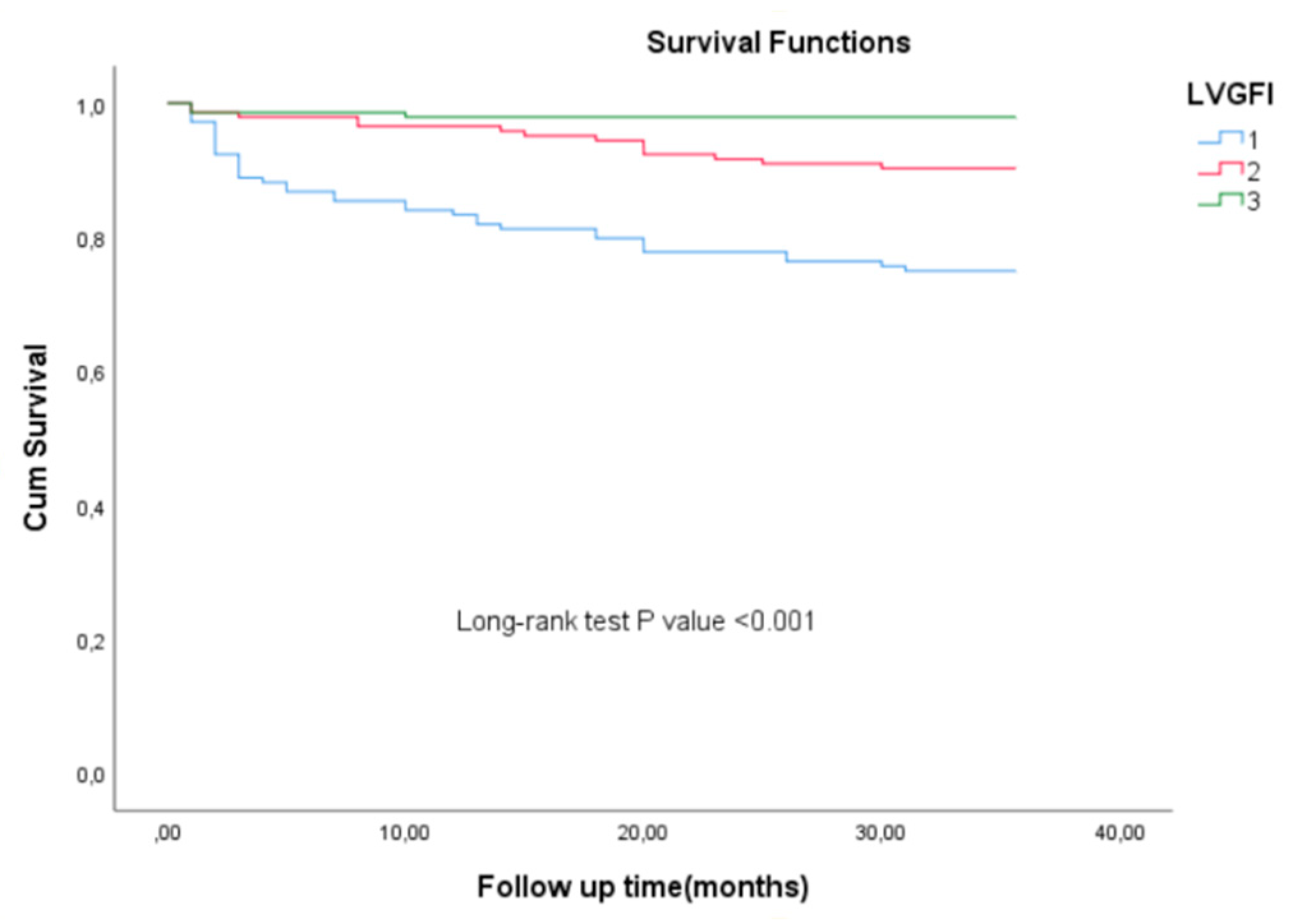
Three-year clinical outcomes across the LVGFI tertiles (T1, T2, T3) were presented in
Table 3. The mean follow-up duration was 35 months. The rates of TVR and recurrent myocardial infarction (RMI) were similar among the three tertiles, with TVR occurring in 3.5% of patients in both T1 and T2, and 4.2% in T3 (p = 0.937), while RMI rates were 6.3%, 3.5%, and 2.8%, respectively (p = 0.296). In contrast, significant differences were observed in the rates of MACE and mortality. MACE occurred in 27.1% of patients in T1, 14.6% in T2, and 7.6% in T3 (p < 0.001), while mortality were significantly more frequent in T1 (25.0%) compared to T2 (9.7%) and T3 (2.1%) (p < 0.001) (
Figure 1).
Cox proportional hazards analysis for three-year mortality and MACE based on LVGFI tertiles was presented in
Table 4. For three-year mortality, the unadjusted HR was highest in T1, with a HR of 14.45 (95% CI: 4.44–46.98) compared to T3. After adjusting for age, sex, BMI, systolic blood pressure, heart rate, and previous coronary artery disease (CAD), the HR remained significantly elevated in T1 (HR 11.86; 95% CI: 3.60–39.10), indicating a substantially higher risk of mortality. The risk of mortality in T2 was also significantly increased compared to T3, with HRs ranging from 4.86 (95% CI: 1.39–16.94) to 4.36 (95% CI: 1.23–15.47) across different models. For MACE, T1 also had the highest risk, with an unadjusted HR of 4.33 (95% CI: 2.21–8.48), and after adjustment for covariates, the HR remained elevated at 3.44 (95% CI: 1.71–6.90). The HR for MACE was significantly lower in T2, ranging from 2.02 (95% CI: 0.97–4.21) in the unadjusted model to 1.86 (95% CI: 0.87–4.01) after full adjustment, while T3 remained the reference group with the lowest event risk.
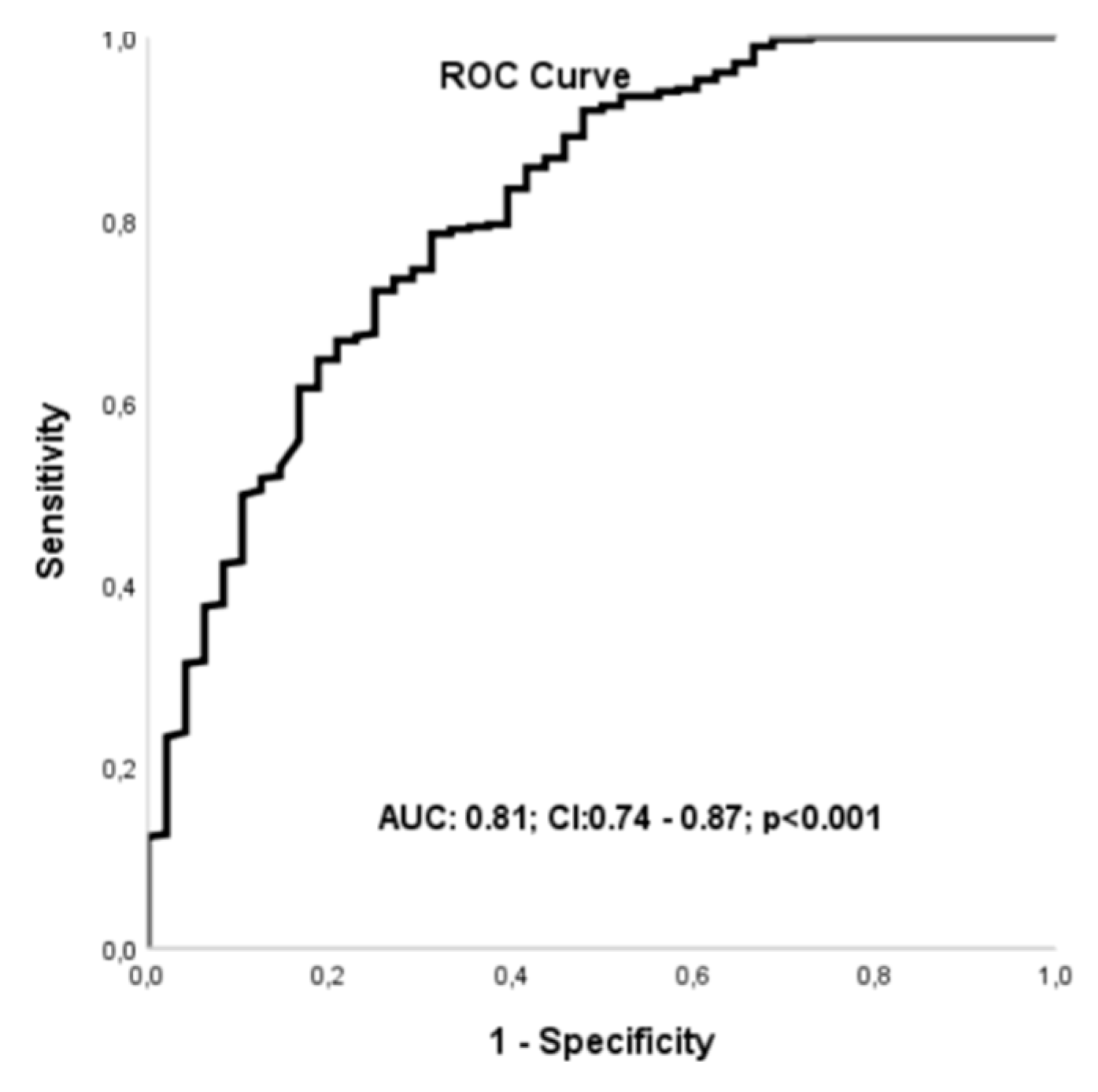
The ROC analysis also demonstrated that a LVGFI cut-off value of 23.22 predicted three-year mortality with a sensitivity of 72% and specificity of 75% (AUC: 0.81; 95% CI: 0.74-0.87; p < 0.001) (
Figure 2).
Discussion
In this study, we investigated the prognostic value of the LVGFI in predicting 3-year mortality and MACE among patients diagnosed with NSTEMI. Our findings demonstrate that a lower LVGFI is significantly associated with higher mortality and MACE rates, underscoring its potential as a valuable prognostic tool in clinical practice.
In our study, the LVGFI was selected over traditional indices, such as LVEF and global longutidunal strain (GLS), as it provides a more comprehensive assessment of cardiac function. Unlike LVEF, which primarily evaluates systolic function, LVGFI integrates multiple dimensions of left ventricular performance, encompassing volumetric and functional parameters [
11]. The advantages of LVGFI also extend to its sensitivity in detecting early or subclinical left ventricular dysfunction, which is often missed by traditional metrics [
6]. In a study, Diaz-Navarro et al. [
5] characterized three patients groups including acute myocarditis, takotsubo cardiomyopathy, and acute myocardial infarction and found that the LVGFI offered incremental value over traditional metrics LVEF and GLS. Our study corroborates this advantage, as the LVEF did not differ significantly across tertiles, whereas LVGFI demonstrated significant predictive value for mortality and MACE.
Our results align with and extend the findings of several studies that have explored the prognostic value of LVGFI in ACS patients. Reinstadler et al. [
12] demonstrated that LVGFI was a strong predictor of adverse events in patients with STEMI. In their study of 226 STEMI patients, they found that LVGFI independently predicted and had better prognostic value than traditional parameters such as LVEF. Eitel et al. [
13] conducted a larger study with 795 STEMI patients and found that the LVGFI was strongly associated with markers of significant myocardial and microvascular injury in STEMI, providing superior prognostic value compared to traditional cardiac risk factors, including LVEF. Similar to our study, Doganay et al. [
14] evaluated the prognostic role of the LVGFI in predicting MACE in patients with acute coronary syndrome after 3-year follow-up. Decreased LVGFI levels were identified as independent predictors of MACE in both STEMI and NSTEMI groups.
LVGFI has demonstrated its utility in various clinical settings, extending beyond its application in acute coronary syndrome. Studies have explored its prognostic value in diverse patient populations, including those with chronic kidney disease (CKD)
, heart failure
, hypertrophic cardiomyopathy (HCM), and amyloidosis [
7,
8,
9,
15,
16]. For instance, Liu et al.[
7] investigated the association between the LVGFI and clinical outcomes in patients with DCM. They found that lower LVGFI was linked to higher rates of death and heart failure events. Similarly, Schober et al. [
16] revealed that in patients with implanted cardioverter-defibrillators (ICD) for secondary prevention, a reduced LVGFI was identified as an independent predictor of both mortality and rehospitalization. A prospective study including 158 patients with ESRD undergoing maintenance dialysis showed that a 10% decrease in LVGFI increased the risk of MACE by 114%, and the predictive model including LVGFI had significantly better performance in forecasting MACE compared to other cardiac parameters like native T1 mapping and GLS, with these findings remaining consistent even in patients with LVEF above the median [
9]. Huang et al [
15] also demonstrated that LVGFI had excellent diagnostic performance in differentiating cardiac amyloidosis from HCM. Interestingly, a multicenter prospective cohort study evaluated the predictive value of the LVGFI for cardiovascular events in 5004 healthy participants with a median follow-up of 7.2 years. The results showed that LVGFI was significantly associated with heart failure, hard cardiovascular events, and all cardiovascular events, with lower LVGFI values independently predicting higher risk for these outcomes, highlighting its potential as a powerful prognostic tool in a multiethnic population without prior cardiovascular disease [
6].
The primary distinction of our study lies in its focus on the prognostic value of LVGFI specifically within an acute NSTEMI patient population, whereas much of the existing literature has primarily explored LVGFI in more chronic or stable cardiovascular conditions. While LVGFI's ability to capture subtle left ventricular dysfunction in these chronic settings is well-established, the pathophysiological dynamics in acute NSTEMI patients, characterized by rapid and significant myocardial stress, require distinct prognostic approaches. Our study contributes to the current literature by demonstrating LVGFI's predictive value in an acute myocardial infarction context, thus broadening its clinical applicability. Notably, our findings highlight LVGFI's strong association with three-year mortality and MACE, complementing and extending previous research, and reinforcing its potential as a versatile prognostic tool across diverse cardiovascular conditions.
Despite these promising results, our study has limitations. First of all, the retrospective design and single-center setting may limit the generalizability of our findings. Second, we utilized echocardiographic measurements for LVGFI, which may have a lower degree of accuracy compared to MRI. However, this choice was deliberate, as LVGFI has the potential to be assessed using echocardiography, a more readily accessible imaging modality in the acute phase of NSTEMI. Third, while we adjusted for several confounders, the possibility of residual confounding cannot be entirely excluded. Multi-center, prospective studies would be valuable in confirming and extending our results.
Conclusion
Our findings suggest that LVGFI is an independent predictor of three-year mortality and MACE in patients with NSTEMI, supporting its potential integration into clinical risk stratification models. Unlike traditional markers, LVGFI provides a holistic assessment of ventricular function, making it a valuable tool for identifying high-risk patients and guiding post-NSTEMI management. Future prospective studies are warranted to validate LVGFI’s clinical utility across diverse populations and to optimize its application in acute care settings.
Author Contributions
Conceptualization, I.I, M.K.; Methodology, I.I, M.K, K.E.S.; Software, M.K.; Validation, , I.I, M.K, C.S.; Formal Analysis, K.E.S, M.K, K.E.S.; Investigation, C.S, I.I, M.K.; Resources, M.K, I.I, C.S.; Data Curation, K.E.S, M.K.; Writing – Original Draft Preparation, I.I, M.K, C.S.; Writing – Review & Editing, I.I, K.E.S, M.K, C.S.; Visualization, M.K, C.S.; Supervision, I.I.; Project Administration, M.K.; Funding Acquisition, I.I, M.K, C.S, K.E.S.
Ethics approval
Approved by ethics committee (Date: 22.01.2025, Decision number: MSTH/20859).
Informed Consent
Informed written consent was obtained from all participants.
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- Yoshioka, G.; Tanaka, A.; Watanabe, N.; Nishihira, K.; Natsuaki, M.; Kawaguchi, A.; Shibata, Y.; Node, K. Prognostic impact of incident left ventricular systolic dysfunction after myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 1009691. [Google Scholar] [CrossRef]
- Nadarajah, R.; Ludman, P.; Appelman, Y.; Brugaletta, S.; Budaj, A.; Bueno, H.; Huber, K.; Kunadian, V.; Leonardi, S.; Lettino, M.; et al. Cohort profile: the ESC EURObservational Research Programme Non-ST-segment elevation myocardial infraction (NSTEMI) Registry. Eur. Hear. J. - Qual. Care Clin. Outcomes 2022, 9, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, R.; Ludman, P.; Laroche, C.; Appelman, Y.; Brugaletta, S.; Budaj, A.; Bueno, H.; Huber, K.; Kunadian, V.; Leonardi, S.; et al. Presentation, care, and outcomes of patients with NSTEMI according to World Bank country income classification: the ACVC-EAPCI EORP NSTEMI Registry of the European Society of Cardiology. Eur. Hear. J. - Qual. Care Clin. Outcomes 2023, 9, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, F.; Longo, G.; Henein, M.Y. Left ventricular ejection fraction: clinical, pathophysiological, and technical limitations. Front. Cardiovasc. Med. 2024, 11, 1340708. [Google Scholar] [CrossRef]
- Diaz-Navarro, R.A.; Kerkhof, P.L.M. Left Ventricular Global Function Index and the Impact of its Companion Metric. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Harsha, D.W.; Bray, G.A. Weight loss and blood pressure control (Pro). Hypertension 2008, 51, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, Z.; Bo, K.; Gao, Y.; Wang, H.; Wang, R.; Liu, W.; Chang, S.; Liu, Y.; Sun, Y.; et al. Association Between Left Ventricular Global Function Index and Outcomes in Patients With Dilated Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Mentias, A.; Alashi, A.; Flamm, S.D.; Thamilarasan, M.; Lever, H.M.; Popovic, Z.B. LV Global Function Index Provides Incremental Prognostic Value Over LGE and LV GLS in HCM. JACC: Cardiovasc. Imaging 2020, 13, 2052–2054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; An, D.; Fang, Y.; Zhou, H.; Yan, H.; Chen, B.; Lu, R.; Fang, W.; Wang, Q.; Che, X.; et al. Assessment of the Prognostic Value of MRI Left Ventricular Global Function Index (LVGFI) in Patients With End-Stage Renal Disease Under Maintenance Dialysis. J. Magn. Reson. Imaging 2023, 59, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Pezel, T.; Horts, T.B.D.; Schaaf, M.; Croisille, P.; Bière, L.; Garcia-Dorado, D.; Jossan, C.; Roubille, F.; Cung, T.-T.; Prunier, F.; et al. Predictive value of early cardiac magnetic resonance imaging functional and geometric indexes for adverse left ventricular remodelling in patients with anterior ST-segment elevation myocardial infarction: A report from the CIRCUS study. Arch. Cardiovasc. Dis. 2020, 113, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Reinstadler, S.J.; Klug, G.; Feistritzer, H.-J.; Kofler, M.; Pernter, B.; Göbel, G.; Henninger, B.; Müller, S.; Franz, W.-M.; Metzler, B. Prognostic value of left ventricular global function index in patients after ST-segment elevation myocardial infarction. Eur. Hear. J. - Cardiovasc. Imaging 2015, 17, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Pöss, J.; Jobs, A.; Eitel, C.; de Waha, S.; Barkhausen, J.; Desch, S.; Thiele, H. Left ventricular global function index assessed by cardiovascular magnetic resonance for the prediction of cardiovascular events in ST-elevation myocardial infarction. J. Cardiovasc. Magn. Reson. 2015, 17, 62–9. [Google Scholar] [CrossRef] [PubMed]
- Doganay, B.; Celebi, O.O. Prognostic Role of the Left Ventricular Global Function Index in Predicting Major Adverse Cardiovascular Events in Acute Coronary Syndrome Patients. Biomarkers Med. 2023, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, H.-Y.; Diao, K.-Y.; Shi, K.; He, Y.; He, S.; Zhang, Y.; Gao, Y.; Shen, M.-T.; Guo, Y.-K.; et al. Left ventricular global function index by magnetic resonance imaging — a novel marker for differentiating cardiac amyloidosis from hypertrophic cardiomyopathy. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schober, A.L.; Jungbauer, C.; Poschenrieder, F.; Schober, A.D.; Hubauer, U.; Keyser, A.; Fredersdorf-Hahn, S.; Debl, K.; Maier, L.S.; Sossalla, S.; et al. Cardiac MRI Based Left Ventricular Global Function Index: Association with Disease Severity in Patients with ICD for Secondary Prevention. J. Clin. Med. 2021, 10, 4980. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).