1. Introduction
The geological team of the Hungarian State Geological Institute discovered the country's first alginite deposit in November 1973 [
1]. The formation is similar to conventional oil shale. Alginite colonies are defined more specifically as forming in volcanic craters and are primarily made of algae [
1]. We currently know of four volcanic craters in Hungary that contain alginite based on research by the Hungarian State Geological Institute. All locations are in Transdanubia: Pula in Bakony, Gérce in Kemeneshát, in Várkesz villages, and the southwest edge of Egyházaskesz village [
1].
Alginite mineral rock formed through the fossilisation of accumulated organic (algae) and inorganic materials such as quartz, carbonates, clay and modified amorphous silicic acid in the watery environment. Humic acids, as a component of alginite's are organic molecules which naturally formed during long-term decomposition and transformation of biomass residues [
2,
3].
Humic substances are complex organic materials that arise from the decomposition of plant and animal matter, and they exhibit a variety of properties that are crucial for their roles in soil health, plant growth, and microbial activity. These substances can be broadly categorized into three main fractions: humic acid, fulvic acid, and humin, each with distinct characteristics and functions [
4,
5]. The numerous positive research results are not surprising since humic substances have many beneficial properties such as redox activity, surface activity and nutrient source, thanks to which they can participate in many microbiological processes [
6]. Humic substances also exhibit strong antioxidant properties, which can mitigate oxidative stress in biological systems. They contain polyphenolic components that contribute to their ability to scavenge free radicals, thus protecting cells from oxidative damage [
7]. In addition to their effects on soil and plants, humic substances play a crucial role in microbial ecology. They serve as electron donors and acceptors in various redox reactions, facilitating anaerobic respiration in microorganisms [
8,
9]. This electron transfer capability is vital for the metabolic processes of many soil bacteria and can enhance the degradation of organic matter, thus contributing to nutrient cycling within ecosystems [
8,
9]. Furthermore, humic substances can stimulate microbial growth and activity (in the cow’s rumen), increasing fermentation efficiency and producing beneficial metabolites such as short-chain fatty acids [
10]. Moreover, the humic substance showed an increase in the number of LABs found in chicks that were ten days old and had an inclusion level of 0.45% HS. According to this finding, a humic compound derived from worm compost can be utilised as a growth enhancer component in broiler feeds.
Thanks to its unique composition, alginite has many benefits and uses in agriculture because of its water adsorption capability and pH stabilising effect. Numerous studies on this mineral have reported that, depending on the dose, alginite can significantly boost agricultural productivity [
1,
2,
3]. Recent studies have explored innovative approaches to using alginite. For instance, turned out that alginite is efficient in demulsification. The mineral contributed significantly to the demulsification of W/O emulsion, which was stable over two months - the emulsion was prepared from 50 wt.% crude oil (Brent type) and 50 wt.% of brine. Furthermore, chemical analysis of the separated oil showed compliance with the industrial standards [
11,
12].
Other researchers have found alginite beneficial with Lactobacilli (LABs) gastroenterally in animal models [
13,
14,
15]. In 2017, Hlubeňová K. and colleagues demonstrated the immunomodulatory potential of lactobacilli and humic substances present in alginite. The administration of
L. reuteri and alginite in the group infected with
S. Typhimurium significantly stimulated the cellular immune response, particularly in the mesenteric lymph nodes of mice. This was evidenced by the activation of CD4+CD8+ lymphocytes, natural killer and natural killer T cells, as well as the activation of phagocytosis within the innate immune system component [
13]. In the same year in a separate study, alginite was introduced into canine diet at a dose of 1% for 14 days, along with probiotic supplementation. The combination of alginite and LAB decreased coliform and Clostridium-like bacteria while increased the lactic acid bacteria of the gastrointestinal system, as well as haemoglobin concentration in blood. At the same time, the treatment stimulated cellular immunity parameters and an improvement in serum mineral levels [
14]. Another research study reported comprehensive and comparable positive results as before regarding the alginite combination with probiotic strain [
15]. Their research concentrated on the impact of a probiotic strain combined with alginite on the intestinal milieu of SPF mice infected with
Salmonella typhimurium. The group supplemented with
Lactobacillus reuteri CCM 8617 and alginite exhibited a significant decrease in the growth of
Salmonella typhimurium in mouse faeces at 24 and 72 hours (P < 0.001) post-infection. The addition of additives significantly influenced nitrogen, enzymatic, hepatic, and energy metabolism in mice.
Typhimurium infection on the morphology examined in the jejunum and ileum of the LAB group of mice. Mice livers subjected to treatment with both alginite and
Lactobacillus reuteri CCM 8617 exhibited a significant decrease in overall inflammation, hepatocyte necrosis, and the size of typhoid nodules. These promising findings make us one step closer to making better non-dairy probiotic products. A review by Chaudhary et al. (2024) emphasizes the individual roles of site-specific microbiomes in maintaining health and preventing diseases; furthermore, the complex interactions between different microbiomes across the body are also highlighted [
16]. These interactions originate with the mouth and lungs, extending to the vagina, skin, and the central hub of the digestive system. Comprehensive research has demonstrated that the gut microbiota significantly influences the health of other microbiome sites and vice versa. Previously, the health benefits of probiotics were provided by milk/ other dairy products. However, the growth of dairy probiotics is limited by lactose intolerance, and health concerns related to cholesterol, allergic milk proteins and fat content in dairy products [
17,
18,
19]. Silanikove N. et al. (2015) state that 75% of the world's population suffers from lactose intolerance [
20]. The investigation of non-dairy probiotic products, which utilise food matrices derived from fruit, vegetables and cereals, has received significant attention and widely studied due to a growing number of persons with lactose intolerance [
21]. The following LABs, such as
L. acidophilus,
L. casei,
L. plantarum,
L. rhamnosus, and
B. lactis are the most employed in the development of novel probiotic products [
22,
23,
24,
25]. Hence, we have chosen
Lactococcus lactis,
Lactobacillus rhamnosus, and
Lactobacillus acidophilus for our research.
Given the positive influence of alginite and lactic acid bacteria (LABs) on gastrointestinal function in animal models, as well as the growing need for lactose-free probiotic food products, we aimed to conduct an experimental study to examine the impact of alginite on the fermentation by probiotic strains. The assessment involved quantifying the fermentation process using multiple methodologies, like applying two cultivation systems (Bactrac® 4100 and fermentor), monitoring impedance levels, determining the specific colony-forming units (CFU/ml) and employing an online living cell sensor to monitor the fermentation progress.
2. Materials and Methods
2.1. Used Strains and Media
The bacteria were ordered from the National Collection of Agricultural and Industrial Microorganisms (NCAIM). The following microorganisms and media were used in this study: L. rhamnosus (NCAIM B.02274; ATCC 7469; DSMZ 20021) in MRS medium, L. acidophilus (NCAIM B.02085; ATCC:4356; DSM:20079) in MRS medium, L. lactis (NCAIM B.0212; DSM 20661) in M17 medium.
MRS medium: Glucose 20 g/L, Peptone 10g/L, Meat extract 10 g/L, yeast extract 5,0g/L, Sodium acetate 2,0 g/L; K2HPO4 2,0 g/L, Ammonium citrate 2,0 g/L, MgSO4 ∙ 7∙ H2O 0,2 g/L; MnSO4 ∙ H2O 0,05 g/L, Tween 80 1,08 g/L.
M17 medium: Lactose 5g/L, Disodium glycerol-β-phosphate 10 g/L, Tryptone 5,0 g/L, Soy peptone 5,0 g/L, Beef extract 5,0 g/L, Yeast extract 2,5 g/L, L-Ascorbic acid 0,5 g/L, MgSO4 0,25 g/L.
In case of alginite added fermentations media were supplemented with 10g/L of alginite mineral before sterilisation. Alginite’s particle size was 20-40 microns, determined via sieving after mining near Gérce, Transdanubian region, Hungary. The used alginite mineral had an average 20 micron particle size. The used alginite contained according to Kádár et al. [
26] 4% moisture, 15% CaCO3 and 4.6% organic matter. Total-N was 0.15%, K 63 mg/kg, Al-K
2O 386 mg/kg, Al-P
2O
5 216 mg/kg. The alginite used contained approximately 5% elemental Ca; 3.6% Al; 2.9% Fe; 1.9% Mg; 0.82% K; 0.15% P; 0.12% S. Aqua regia soluble content: Ca 49942 mg/kg, Al 36026 mg/kg, Fe 28501 mg/kg, Mg 19188 mg/kg, K 8166 mg/kg, P 1501 mg/kg, S 1237 mg/kg, Mn 587 mg/kg, Na 454 mg/kg, Sr 419 mg/kg, Ba 281 mg/kg, Ni 75,0 mg/kg, Zn 65,8 mg/kg, Cr 63,9 mg/kg, B 26,8 mg/kg, Cu 19,2 mg/kg, Co 15,9 mg/kg, Pb 9,75 mg/kg, As 8,84 mg/kg, Sn 2,84 mg/kg, Mo 1,86 mg/kg, Se 1,02 mg/kg, Cd 0,12 mg/kg.
2.2. Fermentations
The small-scale fermentations were conducted in 10 mL reusable aerobic glass vials of BacTrac® 4100 (Sy-Lab, Austria) instrument at a temperature of 37°C, with a total liquid volume of 10 mL. In each vial, after sterilisation with distilled water in an autoclave (121°C, 20min), the distilled water was replaced by 10 mL of the corresponding media (including or free from alginite), followed by inoculation with 20 μL cell suspension (1 sterile loop from fresh agar plate culture in 1 mL media). The fermentations were monitored using relative impedance changes of the medium (M%) as we had previously reported [
27]. Uninoculated vials served as a reference since the relative impedance of the media may also vary. BacMonitor Y 1.39Er program presented the results. Sigma Plot 7.0 was used for curve fitting of the Weibull equation
. Weibull was the best fitting model among Log-logistic, Gamma, Log-normal, and exponential in survival analysis using the Akaike information and Bayesian information criterion [
28].
The bioreactor experiments were conducted in a 1 L benchtop bioreactor with a working volume of 0.8 L (Biostat Q fermenter, B. Braun Biotech International, Melsungen, Germany) with 5% v/v inoculum. The temperature for production was set to 37 °C with an agitation speed of 300 rpm. The pH was regulated using 25% H
3PO
4 and 25% NaOH, the initial pH was set and held at 6.5 [
29]. The cell count was followed during fermentation online and observed time courses were compared by paired t-test with Statistica 13.5.
2.3. Analytic
A Waters Breeze HPLC system determined glucose and lactic acid content from samples. The instrument consisted of a Waters 717 Plus Autosampler, Waters 1515 Isocratic Pump, Biorad Aminex HPX 87H (300 × 7.8 mm, 9 µm) column (65 °C) and Waters 2414 RI detector (40 °C). After appropriate dilution steps, the samples were filtered with a 0.2-micron mixed ester syringe filter (ViaLab Ltd). The mobile phase was 5 mM H2SO4 in ion-exchanged water (Simplicity, Millipore, USA), and the elution rate was 0.5 mL/min.
The colony-forming unit (CFU) determination was carried out as follows: Samples (1 ml/sample) were diluted tenfold in a sterile Falcon tube with 9 ml of sterile saline (9,0g/L NaCl). The ten-fold dilution was repeated six and seven times based on the expected cell count, and then 100microL of the diluted samples were spread on MRS agar Petri dishes. The petri dishes were then incubated at 37°C for 24 hours.
The dry matter was quantified by transferring 10 ml of fermentation broth from the BacTrac tubes into Falcon Conical Centrifuge Tubes, which were then centrifuged at 6000 rpm for 10 minutes using a Hermle Z200A centrifuge. The supernatant was decanted, after which the cells were resuspended in distilled water and subjected to centrifugation again. The supernatant was decanted once again, and the biomass was placed into the crystallisation cup with 2-3 ml of distilled water and dried expeditiously using the Sartorius MA35 moisture analyser until mass constancy. [
29].
For
in-line living cell numbers, we used a Hamilton Incyte (Hamilton, Switzerland) viable cell density sensor. It is a capacitance-based detection of living cells, since it uses alternating current for polarising biomass (dead cells cannot be polarised). Applying different frequencies, dielectric spectroscopy can be applied to determine the volume, size, and size distribution of living cells [
30]. The registered permittivity is proportional to the living cell concentration. Moreover, capacitance sensors are insensitive to micro-carriers, gas bubbles, cell debris and other particles in suspension [
31].
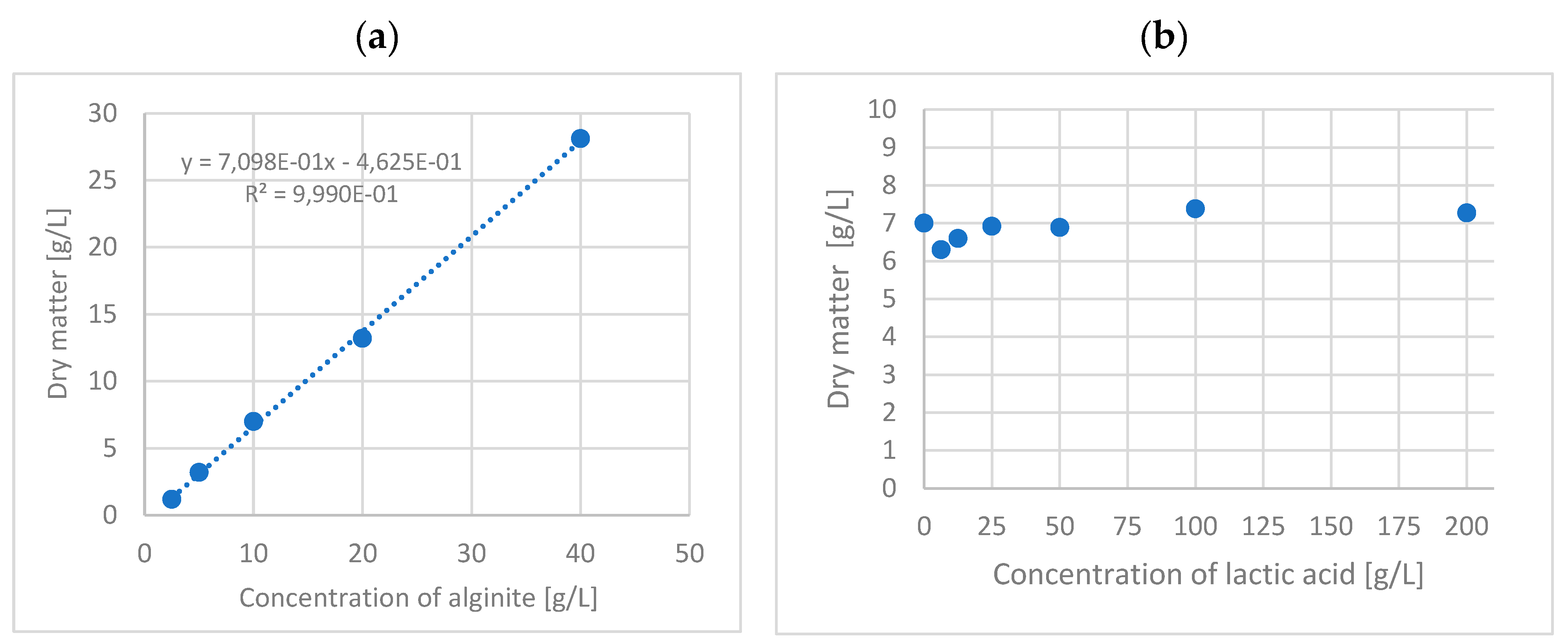
To determine what percentage of alginite mineral total mass was lost via solubilisation during fermentation and via dry matter measurement, where the water content and volatile compounds could vaporise, a calibration curve was made using 100g/L lactic acid solution with 2.5, 5, 10, 20 and 40g/L alginite. Solutions were placed into BacTrac, where they could also be checked to see whether the alginite samples contained contaminants. After three days, the solutions were poured into Falcon tubes, then centrifuged, and the sediments were washed with distilled water as described above. Finally, the dry matter content of the sediment was measured and plotted versus alginite concentrations (
Figure 1a). Furthermore, another solubilisation test was prepared with lactic acid solutions of different concentrations, to which 10g/L of alginite was added uniformly, modelling alginite solubilisation during the lactobacillus fermentation. The concentration of lactic acid solutions was 12.5, 25, 50, 100, and 200 g/L; the sample processing method was the same as the previous one. Finally, the dry matter content of the samples was measured and plotted versus lactic acid concentration (
Figure 1b)
3. Results
Alginite, characterised by its notable humin and humic acid content, exhibits the potential to enhance the availability of trace elements to plants [
32]. Furthermore, its buffering capacity contributes to the soil’s pH shift from acidic to neutral. These factors promote optimal plant growth and maximise crop yield [
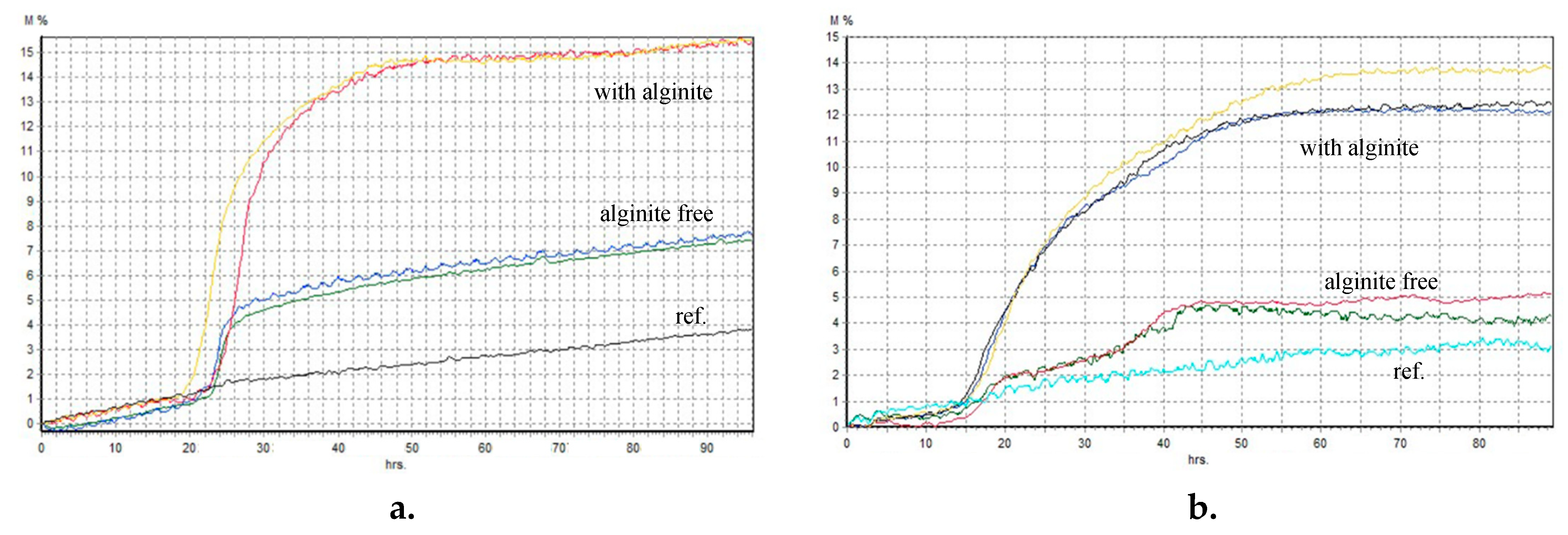
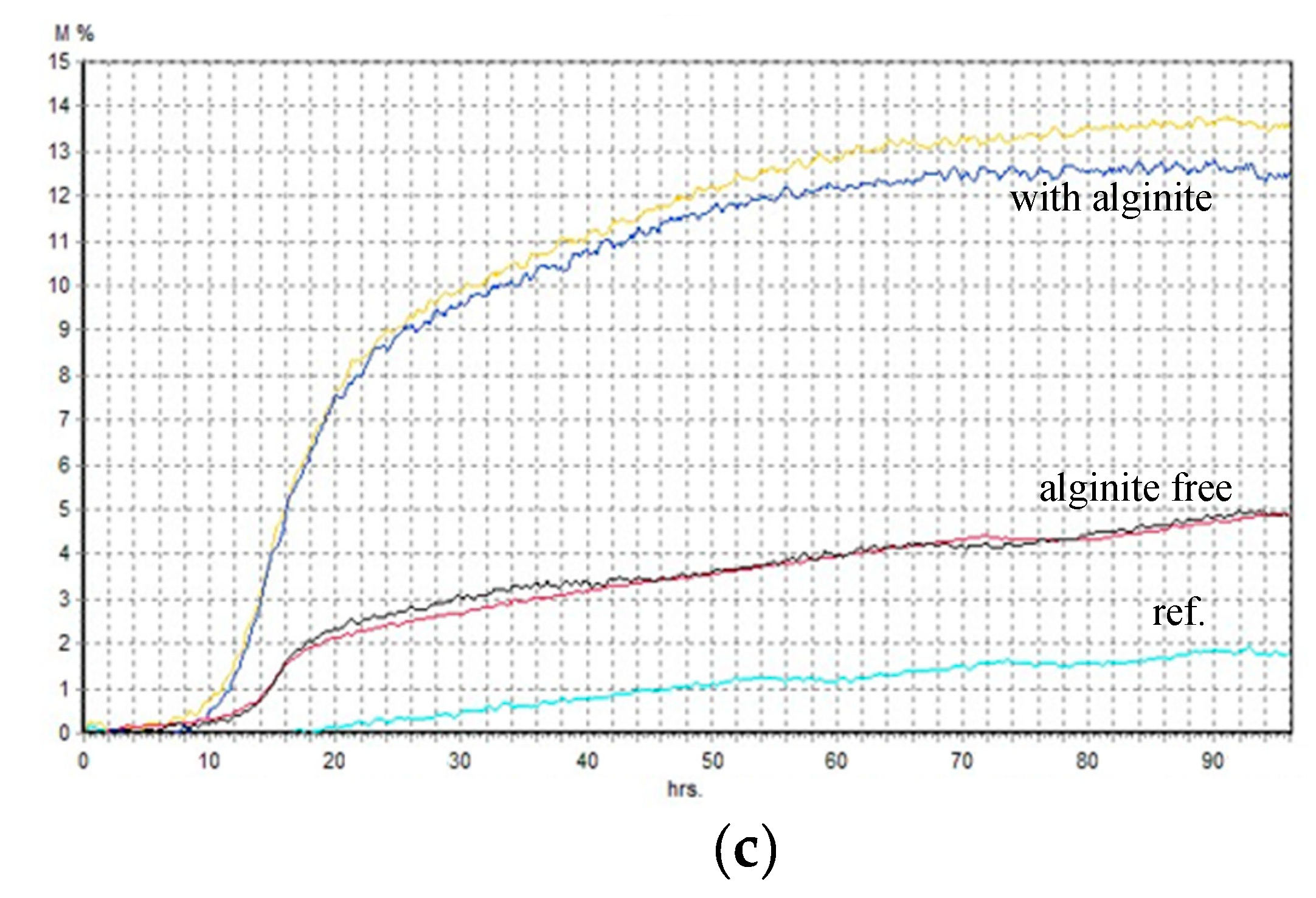
3]. Our expectations were similar in the fermentation experiments. Firstly, we examined the processes in static cultures using BacTrac. We observed a considerable difference between the fermentations supplemented with alginite (with A.) and without it (
Figure 2). It suggests that the alginite positively affected these lactic acid-producing bacteria’s (LABs) cell growth because they have 2-3 times higher impedance levels than the alginite-free ones.
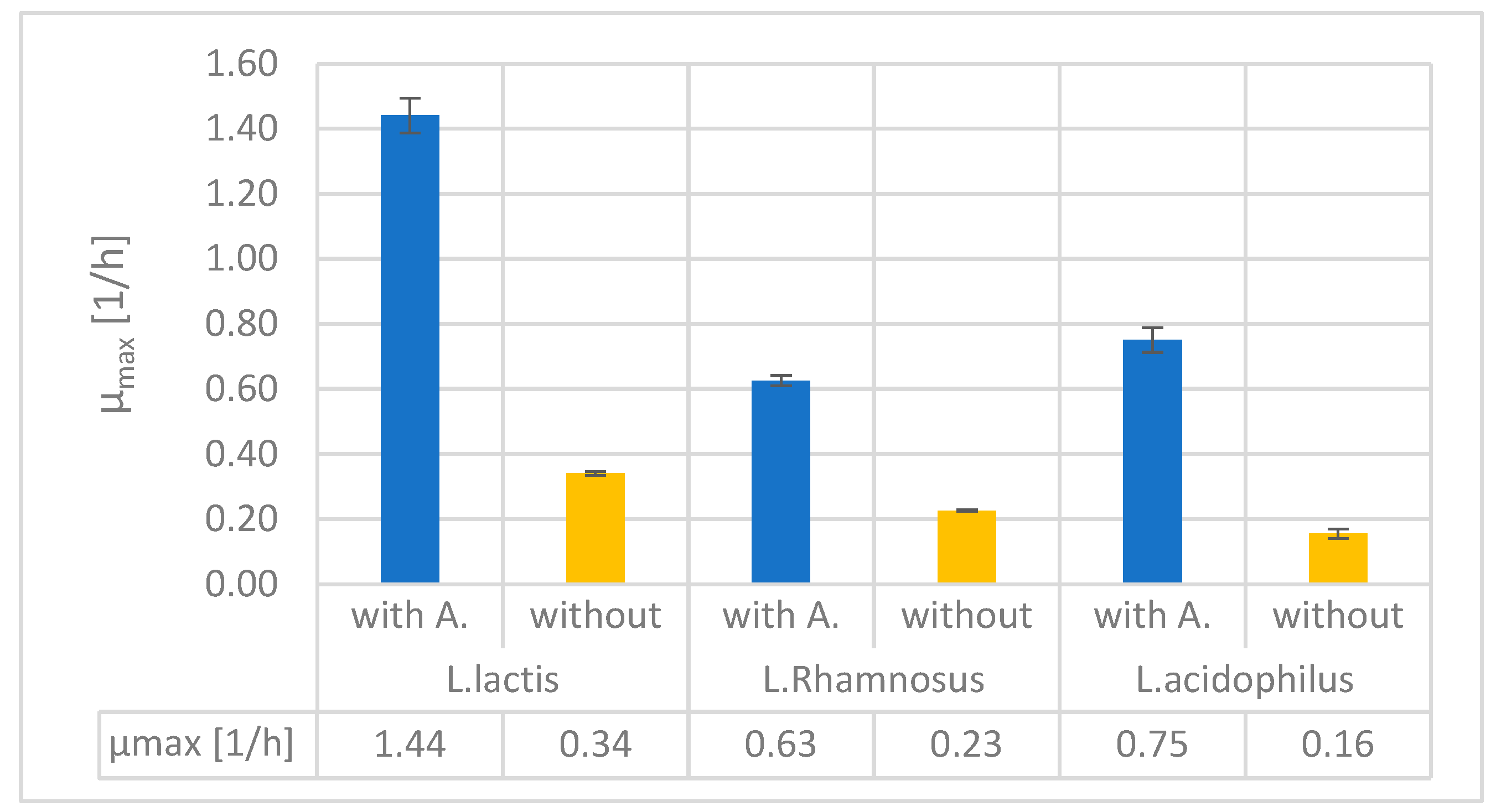
Maximum specific growth rates were determined through curve fitting and are shown in
Figure 3. The results were similar in the case of particular growth rates to maximal impedance values: the µmax is significantly higher in all cases where alginite was used in the fermentation medium compared to the alginite-free cases.
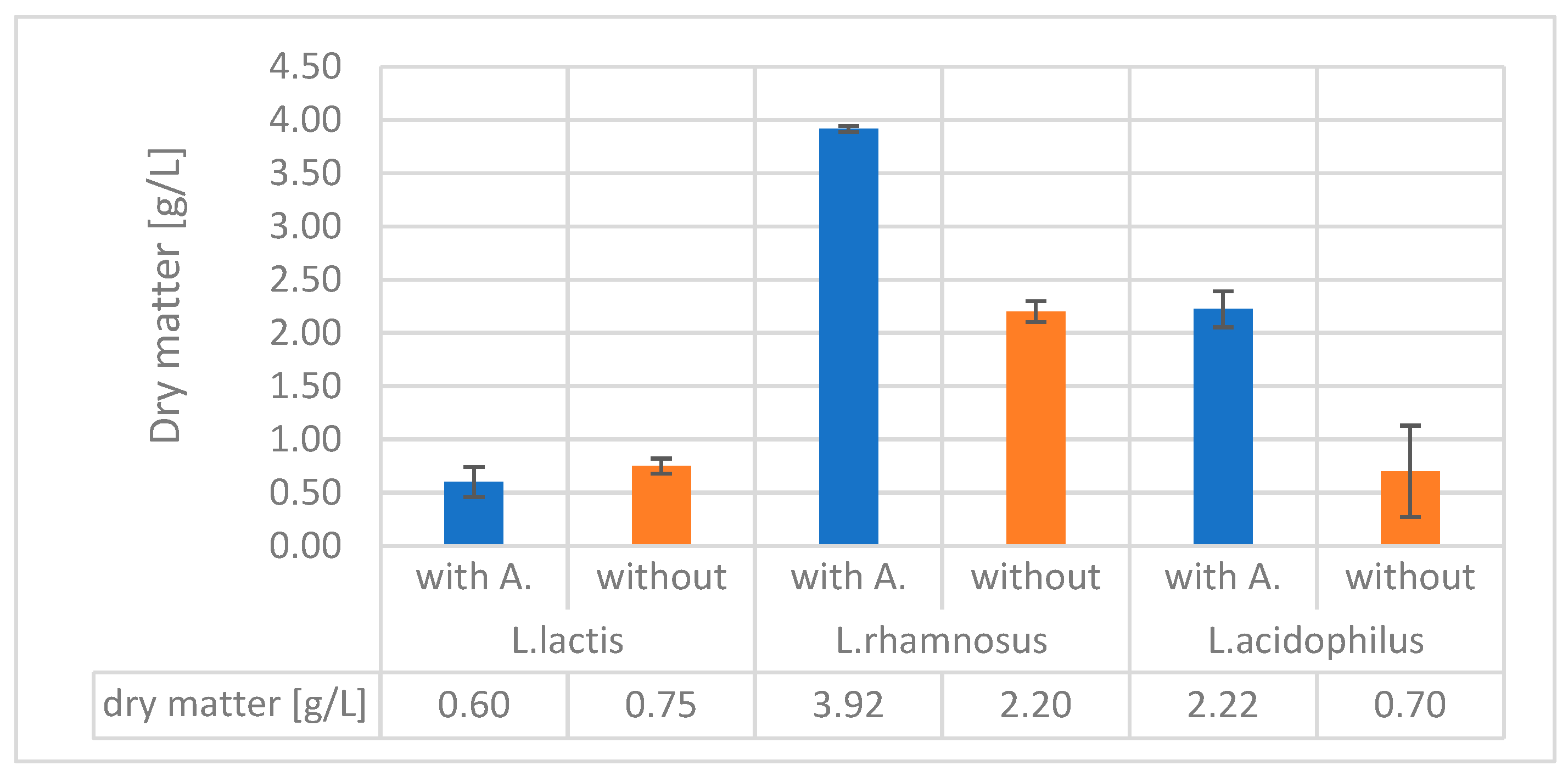
To validate the observations above, we conducted measurements of biomass dry matter. In order to accurately determine the dry weight of biomass without interference originating from solid alginite, we used solubilisation curves (see
Figure 1). These curves revealed that 70% of the alginate remained as carbonates after reacting with lactic acid. Additionally, soluble and volatile compounds were released regardless of the lactic acid concentration at each alginite concentration tested. So, according to the solubilization curves, the remainder of the alginite was subtracted, and the final data is represented in
Figure 4.
The dry mass results were significantly not different in the case of alginite-containing and alginite-free runs of L. lactis. In all other cases, alginite-containing fermentations resulted in significantly higher biomass dry weight, especially in the case of L. acidophilus, reaching three times higher biomass dry weight than in the normal alginite-free fermentations, which was considered a significant enhancement since p-values were lower than 0.05.
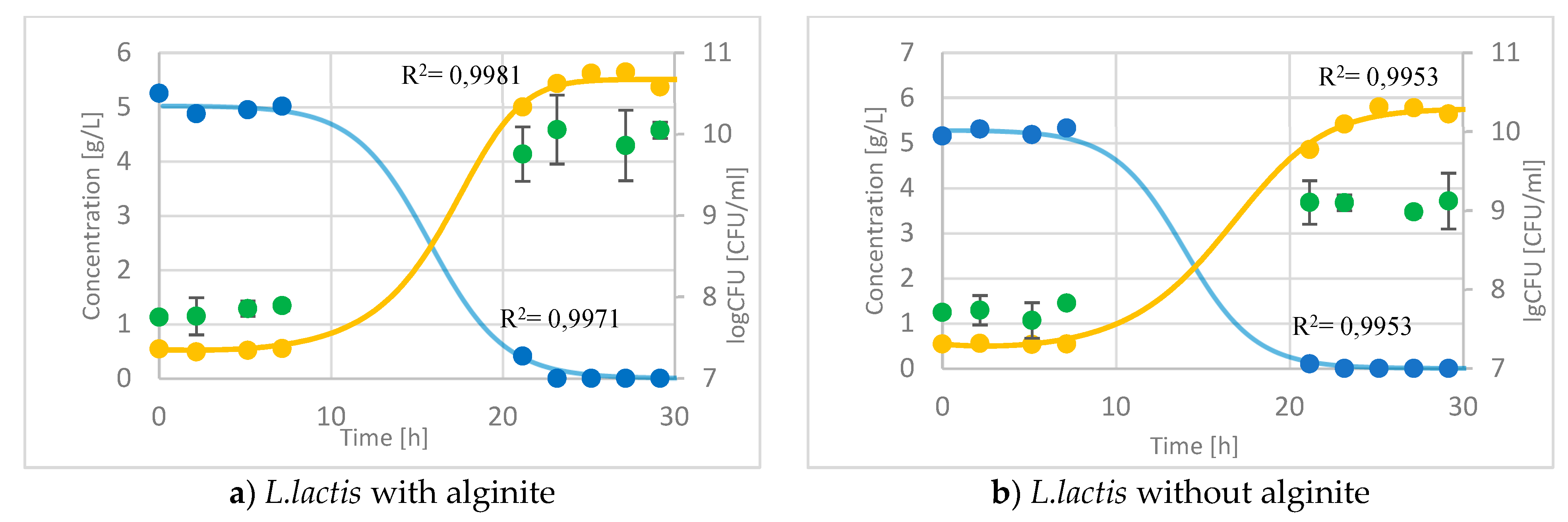
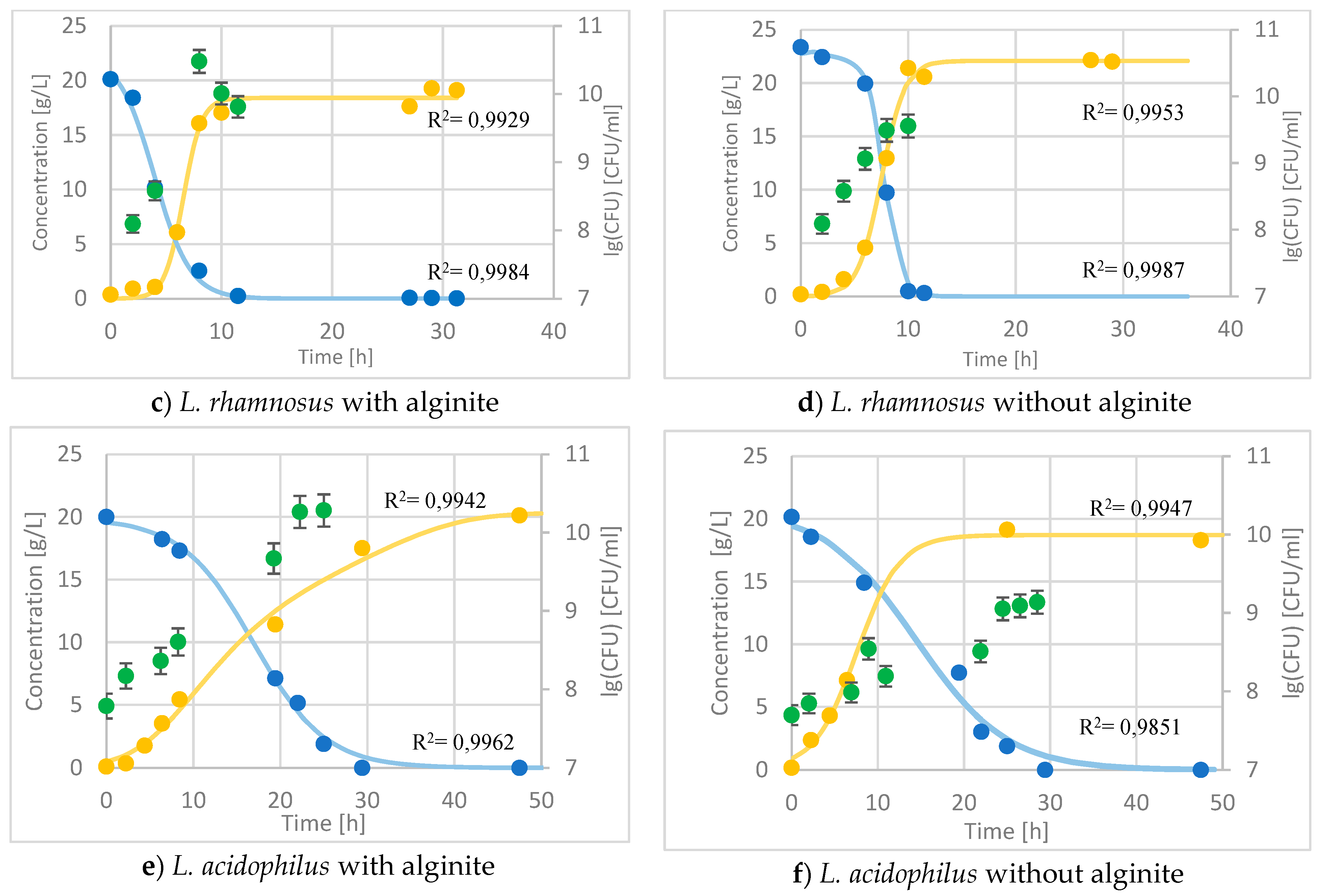
The effect of alginite addition to LAB fermentations was also compared in 1L laboratory fermenters since probiotics are rarely produced in static cultures like BacTrac. While offline HPLC measurements monitored substrate and product concentrations, the biomass was followed by CFU determinations since solid alginite disturbed the commonly used optical density and cell dry weight measurements.
In the case of
Lactococcus lactis fermentation, the sugar (lactose) consumption and the lactic acid production are the same (
Figure 5a,b). Nevertheless, the CFU in alginite fermentation is ca.1 order of magnitude higher (on a logarithmic scale) than in alginite-free fermentation.
During the fermentation of
Lactobacillus rhamnosus with alginite, the CFU reached a higher value by one order of magnitude, even though, for some reason, the starting sugar amount was less than in the alginite-free fermentation (
Figure 5c,d). However, the product reached a higher concentration of 20% in the alginite-free fermentation, following the higher substrate amount consumed, and there was no more production after the 10th hour of fermentation.
We may infer that the production of lactic acid was slower from the flat curve, showing product formation in the Lactobacillus acidophilus alginite supplemented fermentation (
Figure 5e,f). Interestingly, while the lactic acid production was slower, the CFUs were actually higher in the alginite-supplemented fermentation compared to the alginite-free fermentation, indicating a greater bacterial population despite the slower acid production.
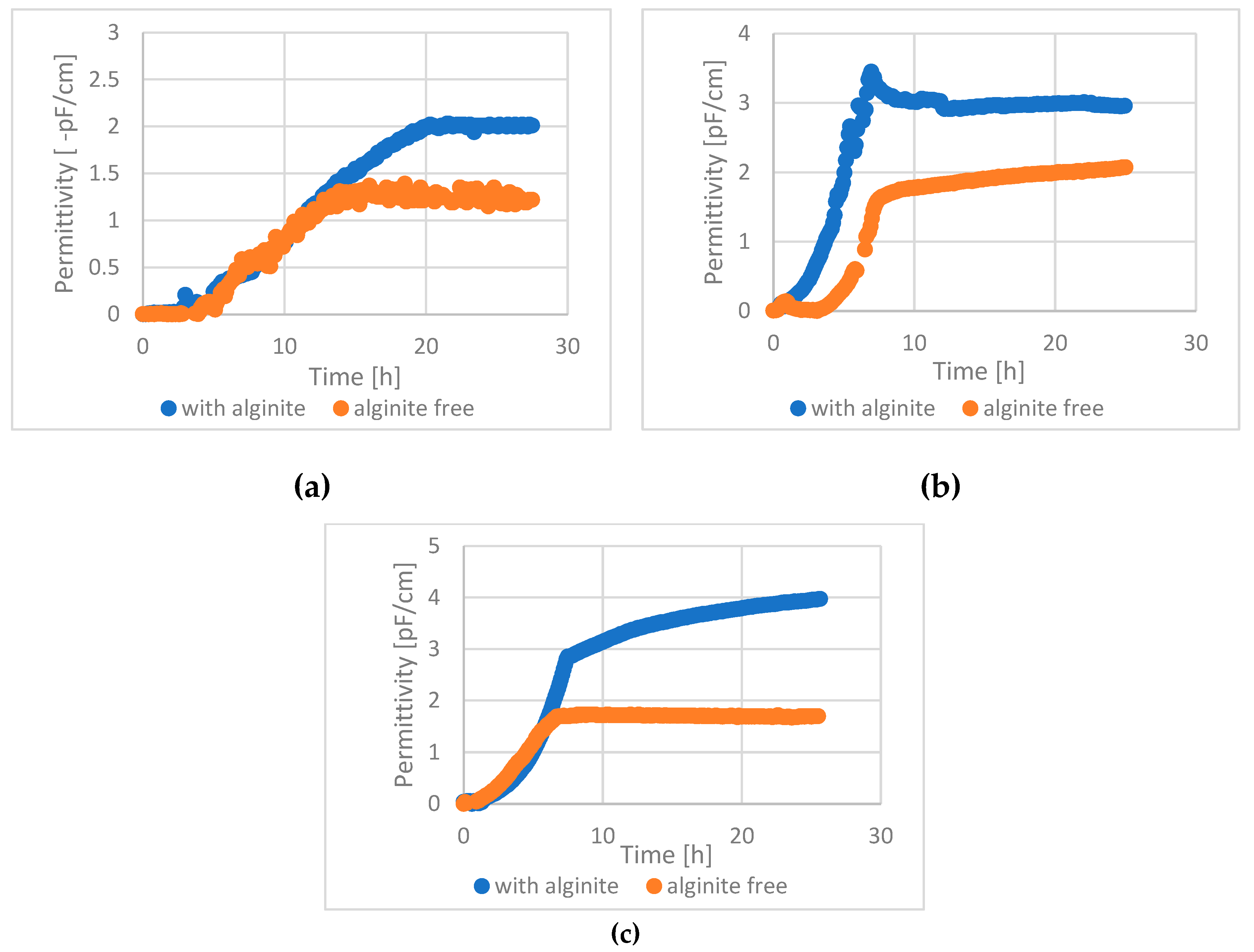
The BacTrac static cultures' cell dry weight (CDW) and bioreactor-based dynamic cultures' CFU results are in full accordance, despite this a third method was used to confirm the results: an online capacitance-based living cell sensor was also used and evaluated in bioreactor experiments (permittivity was shown, which correlates with cell density),
Figure 6. This method shows only the living cells; other particles and the dead cells do not give a signal during the measurement.
In case of all tested lactic acid bacteria, 1,5-2 fold higher cell density could be reached with alginite supplementation compared to alginite free cultures.
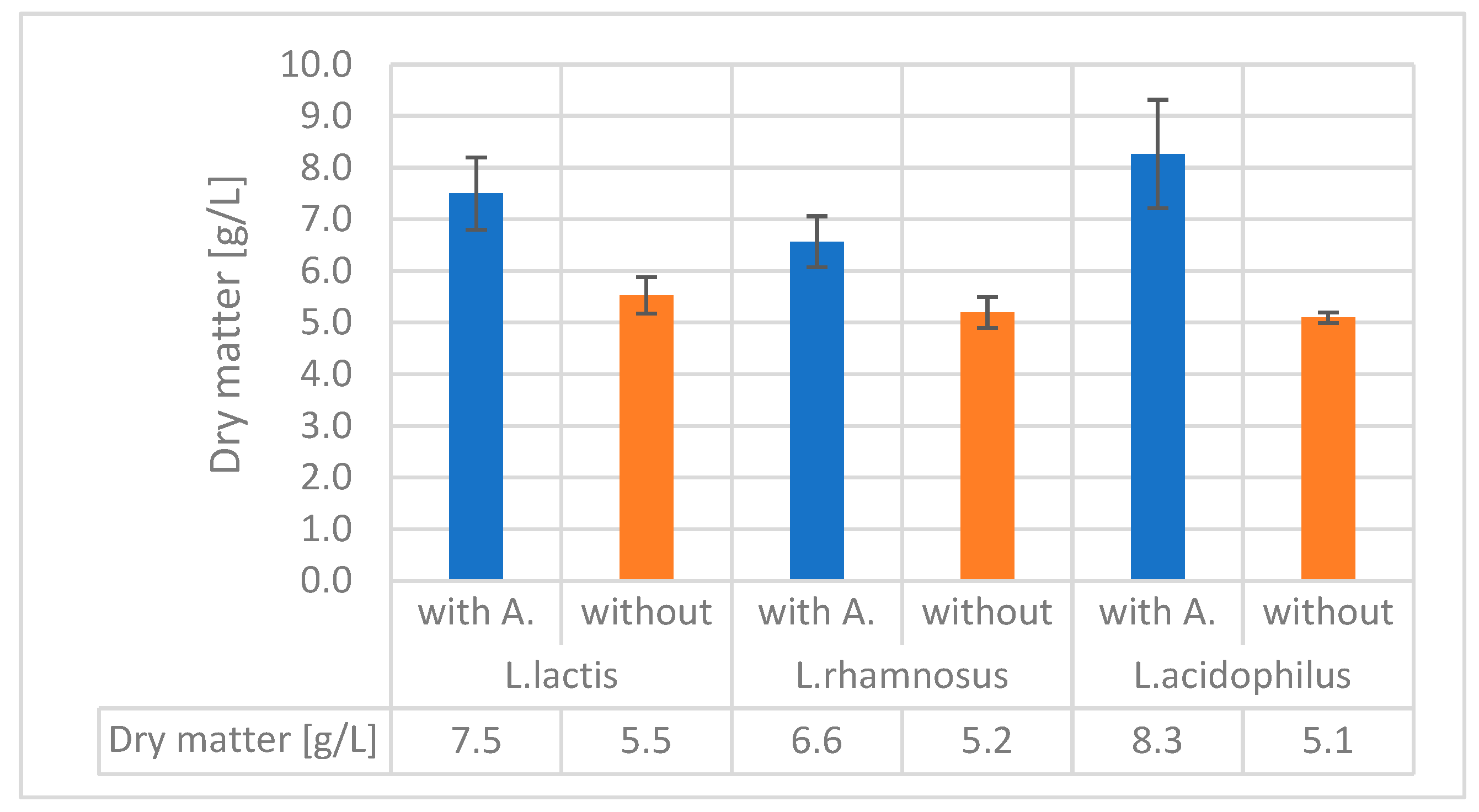
We also measured dry matter during the fermentation processes to prevent the potential measurement-disturbing effect of alginite-released ions. These measurements consistently confirmed that fermentations involving alginite resulted in higher cell biomass at the end of the process (see
Figure 7).
The results were corrected with the previously determined alginite residual amounts (
Figure 1). So, in all cases of tested strains' fermentations, the alginite-supplemented ones significantly reached higher biomass dry matter. These observations are based on
Figure 7 and contribute to declaring that alginite is beneficial for probiotic biomass production, but are based on
Figure 5. alginite does not affect the fermentation products.
4. Discussion
Facilitating microbial growth, alginite has been demonstrated to improve the overall fermentation environment by buffering capacity of alginite helps stabilise pH levels during fermentation, hence facilitating ideal conditions for microbial activity [
15]. This stabilisation can enhance fermentation rates and product yields, as pH changes can negatively impact microbial metabolism and fermentation efficiency [
15]. Following the completion of our preliminary BacTrac investigations, we got to the same result as well, given that the experiments lacked pH control. For this exact reason, the trials were carried out in a fermenter with a capacity of one litre, with constant mixing (300 rpm) and pH (6.5) that was controlled. Because of this, the buffer capacity that alginite offers cannot be taken into consideration in these experiments. The results were very remarkable in terms of the number of living cells as well as the amount of biomass that was produced.
We assume that the presence of humic substances (HS) in alginite plays a crucial role in enhancing the amount of LAB biomass. Among the several types of soils, alginite has the lowest amount of aromatic carbon and the highest quantity of aliphatic carbon. The compared soils include lignite, lignohumate, acadian, compost, and alginite [
33]. The source of alginite humic acids (HA) are marine organic materials with some contribution of algae and not higher plants, as in soil or lignite. Lubica P. et al. (2015) found that alginite-derived humic acid, as well as humic acids obtained from compost, lignohumates, and acadian sources, are newly formed humic acids with a lower level of aromaticity, estimated to be around 40% [
33]. Conversely, the humic acids obtained from soils and lignite demonstrate a notable degree of aromaticity, causing enhanced stability and reduced vulnerability to oxidation. The information on alginite is significant in comprehending our findings, as previous studies have demonstrated that humic compounds, regardless of their source, can be utilised by 70-80% of bacterial strains [
34]. Initially, when Visser [
35] introduced this area of study, it was proposed that bacteria might not have easy access to humic compounds. However, further studies conducted by Tranvik and Sieburth [
36], Moran and Hodson [
37], and Donderski and Wodkowska [
38] highlighted the significant role of humic chemicals as nourishment for bacteria. The findings have resulted in diverse studies on the interactions between microorganisms and humic chemicals. The investigation of a strain of
Lactococcus lactis subsp.
lactis,
Propionibacterium freudenreichii, and
Enterococcus cecorum with humic compounds were investigated and they found that these microorganisms were able to reduce humic substances and produce more oxidised products [
39]. Notwithstanding, we haven’t experienced over-oxidised products (for example, pyruvic acid from lactic acid, etc.). Nevertheless, laboratory experiments resembling the ones detailed in our study have not yet been investigated, and according to our information, no one has investigated the effect of alginite mineral on the living cell number and biomass.
In summary, based on our living cell-sensor supported results and other reports, it is assumed that the presence of humic and fulvic acid compounds and minerals (i.e. inorganic metal ions) in alginite leads to a higher total amount of LAB biomass. In addition to humic substances, alginite also contains other useful components for microbes, such as minerals, amino acids, lipids, polyphenols, and proteins, which the microbes are able to release from the macromolecules of humic- and fulvic acid in alginite [
39].
To gain information, on what type of substances were consumed by the tested LAB strains, we compared initial and final samples of LAB fermentations with and without alginite by Near Infrared Spectroscopy (see Supplementary S1.). While these are only preliminary tests, they suggested, that the spectra had lower transmittance at wavelength of 1020, 1410, 1570 1/cm indicating more soluble in initial alginite supplemented fermentations compared to their final samples and alginite-free pairs.
To conclude, it seems, that the strains we are studying may be able to utilize certain sugar components found in alginite. In addition, alginite has organic content between 4.6-13%, which may result maximum 1,3g/L organic material increasment. Since LAB biomass increased in several case more that 1.3g/L, alginite effect is rather considered, as source of minerals, which resolved media bottleneck compounds resulting in higher utilization of media protein. LAB converts carbohydrate almost fully into lactic acids, and build their own biomass from organic N-source (like amino acids and proteins). Without alginite from MRS (having overall protein content above 25g/L) only ca 5g/L of L.acidophilus, L.rhamnosus and L. lactis was obtained, but beside alginite the 25g/L protein was converted into 8, 6.5 and 7.5g/L LAB biomass respectively.
Since studies have shown furthermore, that alginite and lactic acid bacteria (LABs) together have positive and synergic effects on gastrointestinal function in animal models [
13,
14,
15], our findings on alginite enhancing effect may result more effective probiotic goods with higher living cell number. However, in order to utilise this new useful result, it first needs to be clarified that alginite maintains its positive synergic effects with LABs even if alginite took part in the LAB fermentation, not only added post fermentative as in the above cited earlier reports (
Figure 8).
The potential for fermented alginite to maintain its beneficial effects on the gastrointestinal system has not been investigated. If this is later confirmed, it could lead to the development of a groundbreaking probiotic product. Prior to that time, we can only emphasise that the use of alginite in LAB fermentation has the potential to enhance biomass production yields without drastically altering lactic acid yield. This, in turn, may contribute to meeting the growing demand for lactose-free probiotics [
40].
These findings support the future development and production of probiotic non-dairy-based superfoods or dietary supplements. They also add to our research on LAB-based cosmetics combined with alginite minerals.
Author Contributions
P.T. methodology, investigation, formal analysis, visualisation, writing original draft; Á.N conceptualisation, writing – review &editing, supervision, validation, project administration
Funding
This research received no external funding. APC was funded by MÉL Biotech K+F Kft (Budapest, Hungary).
Institutional Review Board Statement
No animals and human experiments are involved; therefore, N/A.
Data Availability Statement
Authors confirm that all data are involved in the manuscript; further data are available upon request from the corresponding author.
Acknowledgments
Authors are grateful to MEL Biotech K+F Kft (Afo Biotech R&D Ltd., Hungary) for supporting our research with Hamilton Arc View unit for online cell number monitoring. We are also expressing our greatest gratitude to prof. Csaba Fehér at Biorefinery Research group of Applied Biotechnology and Food Science for providing us a 2nd living cell sensor. The used Alginite sample was a kind gift of Alginit Kft, Hungary. Authors have deepest respect and are very grateful to Szilveszter Gergely professor making possible the near infra-red measurements and giving full support in their evaluation.
Conflicts of Interest
The authors declare no competing interests.
References
- G. Solti, Az alginit. Budapest, A Magyar Áll Föld Int (occasional publication). 1987, ISBN 963 671 073 2.
- Jan C., Lukáš L., Zdeněk V., Martin B., Rostislav L. The effects of alginite fertilisation on selected tree species seedlings performance on afforested agricultural lands, Cent. Eur. For. J. ,2017,63, 48–56. [CrossRef]
- M. Tužinský, I. Kupka, V. Podrázský, H. Prknová, Influence of the mineral rock alginite on survival rate and re-growth of selected tree species on agricultural land, J For Sci, 2015, 61, (9): pp 399–405. [CrossRef]
- Shah A. , Sah R. , Sharir S. , Zulkipli N. , Mohamad A. , Farinordin F. et al.. Untitled. Pertanika Journal of Tropical Agricultural Science 2022;45(3). [CrossRef]
- Gerke J.. Concepts and misconceptions of humic substances as the stable part of soil organic matter: a review. Agronomy 2018;8(5):76. [CrossRef]
- Kulikova N.A., Perminova, I.V., Interactions between Humic Substances and Microorganisms and Their Implications for Nature-like Bioremediation Technologies. Molecules, 2021, 26, 2706. [CrossRef]
- Hudák M. , Semjon B. , Marcinčáková D. , Bujňák L. , Naď P. , Koréneková B. et al.. Effect of broilers chicken diet supplementation with natural and acidified humic substances on quality of produced breast meat. Animals 2021;11(4):1087. [CrossRef]
- Coates J. , Cole K. , Chakraborty R. , O’Connor S. , & Achenbach L.. Diversity and ubiquity of bacteria capable of utilizing humic substances as electron donors for anaerobic respiration. Applied and Environmental Microbiology 2002;68(5):2445-2452. [CrossRef]
- Terry S. , Ramos A. , Holman D. , McAllister T. , Breves G. , & Chaves A.. Humic substances alter ammonia production and the microbial populations within a rusitec fed a mixed hay – concentrate diet. Frontiers in Microbiology 2018;9. [CrossRef]
- Sheng P. , Ribeiro G. , Wang Y. , & McAllister T.. Humic substances reduce ruminal methane production and increase the efficiency of microbial protein synthesisin vitro. Journal of the Science of Food and Agriculture 2018;99(5):2152-2157. [CrossRef]
- Sebastian H., Sangar S. A., Peter F., Martin B., Demulsification of water/crude oil emulsion using natural rock alginite, Colloids and Surfaces A: Physicochem Eng Asp., 2018, 553, pp 71-79, ISSN 0927-7757. [CrossRef]
- Sangar S. A., Sebastian H., Quirina I. R.-G., Peter F., Martin B., Alginite rock as effective demulsifier to separate water from various crude oil emulsions, Colloids and Surfaces A: Physicochem Eng Asp, 2021, 611, 125830. [CrossRef]
- Hlubeňová, K., Mudroňová, D., Nemcová, R., Gancarčíková, S., Maďar, M., Sciranková, The Effect of Probiotic Lactobacilli and alginite on the Cellular Immune Response in Salmonella Infected MiceĽ., Folia Vet, 2017, 61, pp 2: 61—66. [CrossRef]
- Viola S., Ivana K., Jana F., Aladár M., Soňa G., Dagmar M., Andrea L. Evaluation of Probiotic Lactobacillus fermentum CCM 7421 administration with alginite in Dogs, Probiotics Antimicro, 2017, 10, 3, pp 577-588, . [CrossRef]
- Gancarčíková S., Nemcová R., Popper M., et al. The Influence of Feed-Supplementation with Probiotic Strain Lactobacillus reuteri CCM 8617 and alginite on Intestinal Microenvironment of SPF Mice Infected with Salmonella Typhimurium CCM 7205. Probiotics & Antimicro. Prot., 2018, 11, pp 493–508,. [CrossRef]
- Chaudhary, P.P., Kaur, M. & Myles, I.A., Does "all disease begin in the gut"? The gut-organ cross talk in the microbiome. Appl Microbiol Biotechnol, 2024, 108, 339. [CrossRef]
- Kumar D. , Lal M. , Dutt S. , Raigond P. , Changan S. , Tiwari R. et al.. Functional fermented probiotics, prebiotics, and synbiotics from non-dairy products: a perspective from nutraceutical. Molecular Nutrition &Amp; Food Research 2022;66(14). [CrossRef]
- Natt S. and Katyal P.. Current trends in non-dairy probiotics and their acceptance among consumers: a review. Agricultural Reviews 2021(Of). [CrossRef]
- Aysegul K., Ilkin Y. S., Traditional Non-Dairy Fermented Products: A Candidate for Probiotics, Food Rev Int., 2023. [CrossRef]
- Silanikove N. Leitner G., Merin U., The Interrelationships between Lactose Intolerance and the Modern Dairy Industry: Global Perspectives in Evolutional and Historical Backgrounds. Nutrients, 2015, 7, pp 7312-7331. [CrossRef]
- Panghal A., Janghu S., Virkar K., Gat Y., Kumar V., Chhikara N., Potential non-dairy probiotic products – A healthy approach. Food Biosci, 2018, 21, pp 80–89. [CrossRef]
- M.J. Martin, F. Lara-Villoslada, M.A. Ruiz, M.E. Morales, Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716, LWT - J Food Sci Tech, 2013, 53, 2, pp 480-486. [CrossRef]
- Petrova P., Petrov K., Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients, 2020, 12, 1118. [CrossRef]
- Lasta E.L., da Silva Pereira Ronning E., Dekker R.F.H., da Cunha M.A.A., Encapsulation and dispersion of Lactobacillus acidophilus in a chocolate coating as a strategy for maintaining cell viability in cereal bars. Sci Rep., 2021, 11, 20550. [CrossRef]
- Zhao, F., Bai, X., Zhang, J., Kwok L., Shen L., Jin H., Sun T., Sun Z., Zhang H. Gut Bifidobacterium responses to probiotic Lactobacillus casei Zhang administration vary between subjects from different geographic regions. Appl Microbiol Biotechnol 2022, 106, 2665–2675. [CrossRef]
- Kádár, I., Ragályi, P., Murányi, A., Radinszky, L., & Gajdó, A., Effect of Gérce alginit on the fertility of an acid sandy soil., Agrokémia és talajtan Agrokem, 2015, 64(2), 437-452. [CrossRef]
- Tóth P.; Németh Á., Investigations Into the usage of the mineral alginite fermented with Lactobacillus paracasei for cosmetic purposes, Hung J Ind Chem, 2022, Vol. 50(2), pp. 16-20. [CrossRef]
- George, B., Seals, S., & Aban, I. Survival analysis and regression models. J Nucl Cardiol, 2014, 21(4), 686–694. [CrossRef]
- Tóth P.; Németh Á., Investigation and Characterisation of New Eco-Friendly Cosmetic Ingredients Based on Probiotic Bacteria Ferment Filtrates in Combination with Alginite Mineral. Processes, 2022, 10, 2672. [CrossRef]
- Kiss, B., Németh, Áron “Application of a High Cell Density Capacitance Sensor to Different Microorganisms”, Periodica Polytechnica Chemical Engineering, 60(4), pp. 290–297, 2016. [CrossRef]
- Incyte sensor manufacturer’s webpage: https://www.hamiltoncompany.com/process-analytics/sensors/cell-density-sensors/viable-cell-density-sensors/incyte-arc.
- Barančíková G., Litavec T., Comparison of Chemical Structure of Alginite Humic Acids Isolated with Two Different Procedures with Soil Humic Acids. Agr (Polnohospodárstvo), 2016,62(4), pp 138–148. [CrossRef]
- Lubica P., Markéta K., Ondřej Z., René K., Gabriela B., Tadeáš L., Tomáš L., Jaroslav H., Anna M., Tibor L., Fate of humic acids isolated from natural humic substances, Acta Agr Scand, Section B — Soil & Plant Science, 2015, 65:6, pp 517-528. [CrossRef]
- W. Donderski, A. Burkowska, Metabolic Activity of Heterotrophic Bacteria in the Presence of Humic Substances and Their Fractions. Pol J Environ Stud, 2000,9(4), pp.267-271. ISSN: 1230-1485.
- Visser S. A., Effect of humic acids of number and activities of microorganisms within physiological groups. Org. Geochem., 1984, 8, pp 81. [CrossRef]
- Travnik L.J., Sieburth J., Effect of flocculated humic matter on free and attached pelagic microorganisms. Lim nol. Oceanogr, 1989, 34 (4), pp 688. [CrossRef]
- Moran M. A., Hodson R. E., Bacterial production on humic and nonhumic components of dissolved organic carbon. Limnol. Oceanogr., 1990, 35, pp 1744.
- Donderski W., Wodkowska A., Humic substances as a source of carbon and nitrogen for heterotrophic bacteria isolated from lakes of different trophy. Pol. J. Environ. Stud., 1997, 6 (4), pp 9.
- Benz M, Schink B, Brune A. Humic Acid Reduction by Propionibacterium freudenreichii and Other Fermenting Bacteria. Appl Environ Microbiol 1998, 64. [CrossRef]
- Dahiya, D.; Nigam, P.S. Therapeutic and Dietary Support for Gastrointestinal Tract Using Kefir as a Nutraceutical Beverage: Dairy-Milk-Based or Plant-Sourced Kefir Probiotic Products for Vegan and Lactose-Intolerant Populations. Fermentation 2023, 9, 388. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
 glucose (at L.lactis lactose), ● lactic acid, ● CFU.
glucose (at L.lactis lactose), ● lactic acid, ● CFU.
 glucose (at L.lactis lactose), ● lactic acid, ● CFU.
glucose (at L.lactis lactose), ● lactic acid, ● CFU.