Submitted:
13 February 2025
Posted:
14 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CNC
2.3. Synthesis of Hybrid Gels
2.4. Hydrogels Characterization
2.5. Cell Culture and Cell Viability
2.6. DLS Analysis
- in solutions (ergodic systems)
- in gels
- in glasses (completely freezed systems)
- one coming from the "freezed", averaged configurations of the scatterers,
- one due to the fluctuations around their average position.
3. Results and Discussion
Conclusions
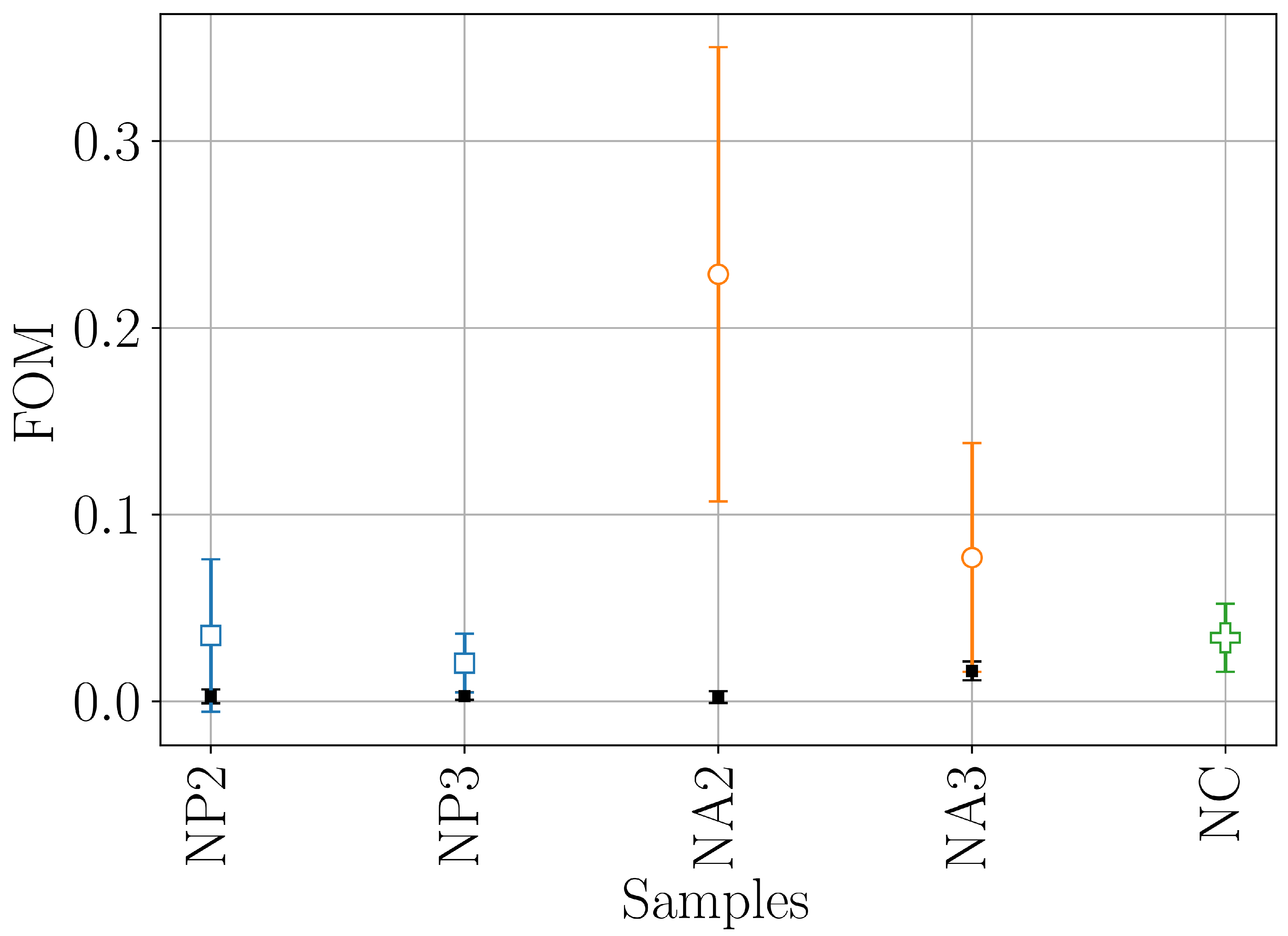
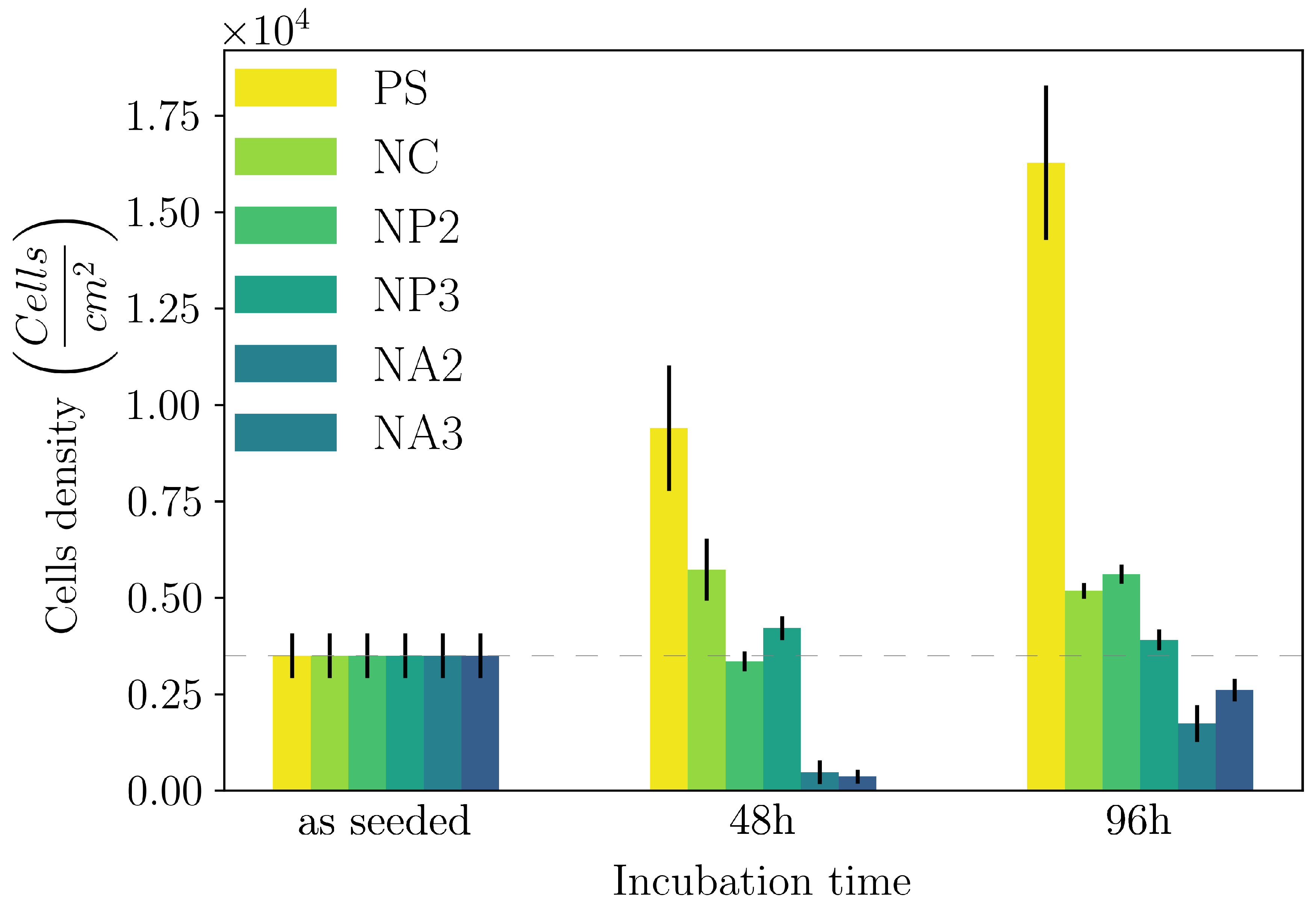
- we showed that both the composite hydrogels made of CNC/linear amino-PEG and CNC/alginate are good substrates for the growth of a model cell line. However CNC/amino-PEG induces a much faster cell proliferation, despite the similar physical macroscopic properties of the two tested materials. This result suggests that the cells respond also to dynamic environmental cues not detectable by covnentional techniques.
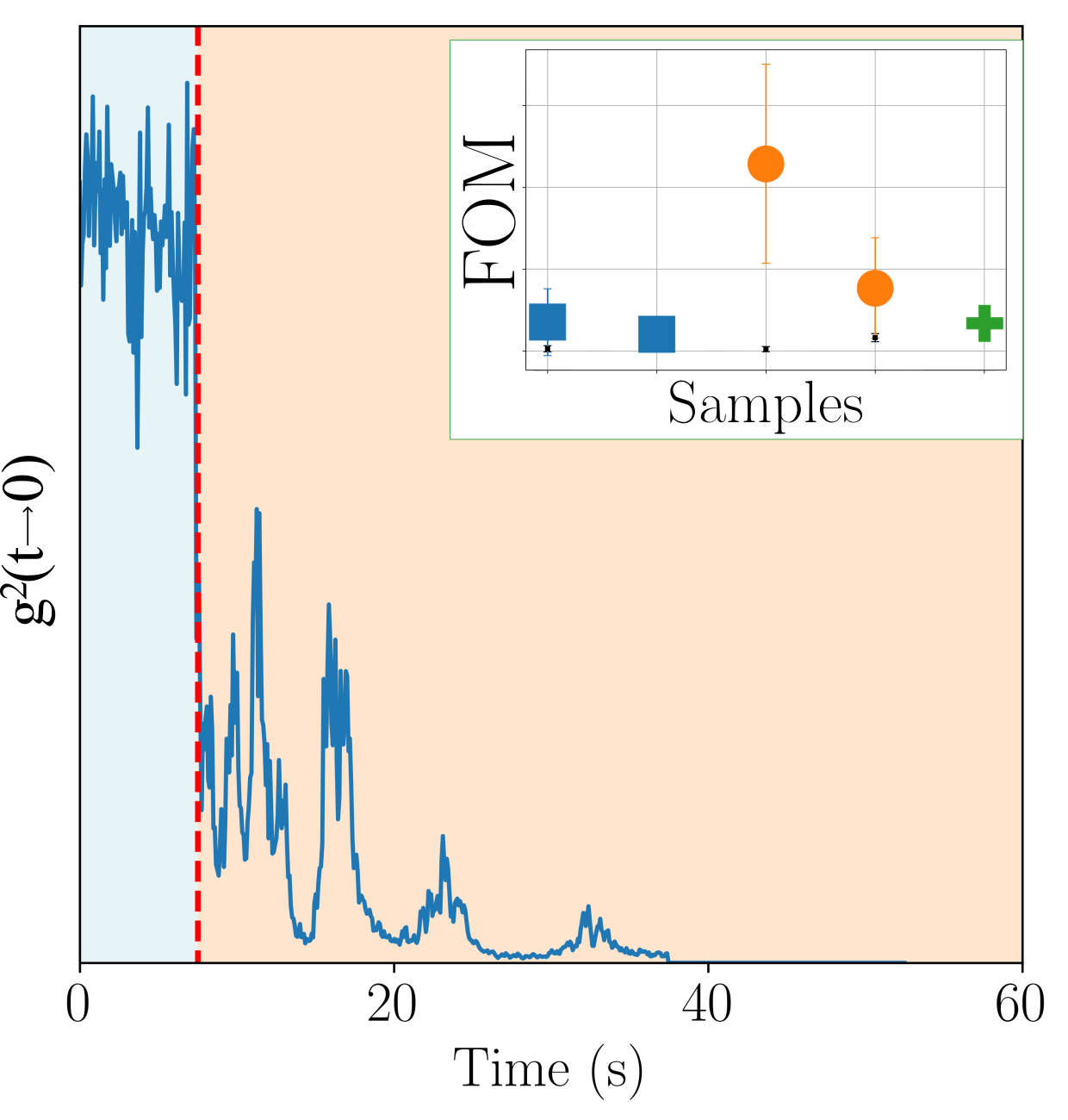
- we introduced a novel DLS analysis implemented on commercial, single angle apparatus that is able to discriminate between gels having similar macroscopic mechanical properties but differing for their chemical composition and microscopic structural dynamics. As representative tests we investigated the dynamics of CNC-PEG and CNC-alginate materials. To prove the relationship between the dynamic DLS data and the microscopic hydrogel structure we exploited the peculiar characteristic of alginates to jellify in the presence of Ca2+ but not with Mg2+ [43] since the smaller Mg2+, at difference from Ca2+, does not fit into the G boxes and establishes with alginate chains a weaker affinity-driven interaction ruled basically by Manning’s theory [69]. By defining a phenomenological Figure of Merit (FOM), based on the short-time values of the 2nd order correlation function, we showed that its statistical properties contains information about the sol-gel transition and the material composition. We observed that the nanoscale dynamical properties are in some way retained when both the hydrogels attain stable and similar macroscopic properties. We suppose that the differences in their internal dynamics causally affect the hydrogel interaction with cells in culture. In fact, while CNC-PEG composites form a stable gel structure in a relatively short time and it induces a faster cell proliferation, the slowly equilibrating CNC-alginate hydrogels shows a longer induction time. Notably, cell proliferation depends on the stability of cell adhesion to the support and our results indicate that this stability is affected by the nanoscale phenomena. Cell adhesion and proliferation is a fundamental issue for the design of innovative hydrogels for human use (i.e. dispositive for tissue regeneration wound healing, drug delivery and organoids) and at present, the nanoscale behavior is often neglected. Conversely, this behavior seems to play an important role and here we provided a simple approach for its monitoring. Our method is characterized by a significantly better temporal (10’s s) and spatial (10’s m) resolution than the current state-of-the-art techniques commonly used for such analyses, such as rheometry, SAXS/SANS, and NMR, moreover it is directly implemented on simple DLS equipment without any modification. While phenomenological in nature, our approach is the only one reported able to fully exploit the spatial and temporal resolution provided by DLS. It provides quantitative information about differences between materials that vary in specific parameters, like component ratios. Despite DLS is a well known technique, this is the first report where the statistical properties of the initial values of g(2)(t) is used to derive information about the chemical composition and the dynamical state of a material. Our proof-of-principle demonstration will pave the way for a much broader use of our approach. Dedicated models and computational tools are needed to fully understand the method’s possibilities and limitations and will be developed in the future.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. Journal of Advanced Research 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. Journal of Applied Polymer Science 2021, 138, 50376. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Materials Science and Engineering: C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Hu, L.; Chee, P.L.; Sugiarto, S.; Yu, Y.; Shi, C.; Yan, R.; Yao, Z.; Shi, X.; Zhi, J.; Kai, D.; et al. Hydrogel-Based Flexible Electronics. Advanced Materials 2023, 35, 2205326. [Google Scholar] [CrossRef] [PubMed]
- Mikhailidi, A.; Ungureanu, E.; Tofanica, B.M.; Ungureanu, O.C.; Fortună, M.E.; Belosinschi, D.; Volf, I. Agriculture 4.0: Polymer Hydrogels as Delivery Agents of Active Ingredients. Gels 2024, 10. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. European Polymer Journal 2015, 65, 252–267, 50 Years of European Polymer Journal. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Advanced Science 2018, 5, 1700527. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: a tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; E. Eriksson, J.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydrate Polymers 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Materials Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in biomedical and biosensing applications: A review. International Journal of Biological Macromolecules 2021, 166, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Maestri, C.A.; Abrami, M.; Hazan, S.; Chistè, E.; Golan, Y.; Rohrer, J.; Bernkop-Schnürch, A.; Grassi, M.; Scarpa, M.; Bettotti, P. Role of sonication pre-treatment and cation valence in the sol-gel transition of nano-cellulose suspensions. Scientific Reports 2017, 7, 11129. [Google Scholar] [CrossRef] [PubMed]
- Maestri, C.A.; Bettotti, P.; Scarpa, M. Fabrication of complex-shaped hydrogels by diffusion controlled gelation of nanocellulose crystallites. J. Mater. Chem. B 2017, 5, 8096–8104. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chemical Reviews 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Bettotti, P.; Scarpa, M. Nanocellulose and Its Interface: On the Road to the Design of Emerging Materials. Advanced Materials Interfaces 2022, 9, 2101593. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chemistry of Materials 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Advances in Colloid and Interface Science 2019, 267, 47–61. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.B.; de Azeredo, H.M.C.; Aouada, F.A.; Feitosa, J.P.A.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydrate Polymers 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhi, Y.; Shan, S.; Ni, Y. Research progress of smart response composite hydrogels based on nanocellulose. Carbohydrate Polymers 2022, 275, 118741. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xi, J.; Meng, L.; Lou, Y.; Seidi, F.; Wu, W.; Xiao, H. Stimuli-Responsive nanocellulose Hydrogels: An overview. European Polymer Journal 2022, 180, 111591. [Google Scholar] [CrossRef]
- Strnad, S.; Zemljič, L. Cellulose–Chitosan Functional Biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bai, J.; Tian, P.; Xie, R.; Duan, Z.; Lv, Q.; Tao, Y. The Application Status of Nanoscale Cellulose-Based Hydrogels in Tissue Engineering and Regenerative Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 732513. [Google Scholar] [CrossRef]
- Heidarian, P.; Kaynak, A.; Paulino, M.; Zolfagharian, A.; Varley, R.J.; Kouzani, A.Z. Dynamic nanocellulose hydrogels: Recent advancements and future outlook. Carbohydrate Polymers 2021, 270, 118357. [Google Scholar] [CrossRef]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. International Journal of Biological Macromolecules 2021, 181, 275–290. [Google Scholar] [CrossRef]
- Dong, S.; Roman, M. Fluorescently Labeled Cellulose Nanocrystals for Bioimaging Applications. Journal of the American Chemical Society 2007, 129, 13810–13811. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Palaganas, N.B.; Mangadlao, J.D.; de Leon, A.C.C.; Palaganas, J.O.; Pangilinan, K.D.; Lee, Y.J.; Advincula, R.C. 3D Printing of Photocurable Cellulose Nanocrystal Composite for Fabrication of Complex Architectures via Stereolithography. ACS Applied Materials & Interfaces 2017, 9, 34314–34324. [Google Scholar] [CrossRef]
- Kalossaka, L.M.; Mohammed, A.A.; Sena, G.; Barter, L.; Myant, C. 3D printing nanocomposite hydrogels with lattice vascular networks using stereolithography. Journal of Materials Research 2021, 36, 4249–4261. [Google Scholar] [CrossRef]
- Bai, C.; Tang, A.; Zhao, S.; Liu, W. Flexible nanocellulose/poly(ethylene glycol) diacrylate hydrogels with tunable Poisson’s ratios by masking and photocuring. BioResources 2020, 15, 3307–3319. [Google Scholar] [CrossRef]
- Tang, A.; Ji, J.; Li, J.; Liu, W.; Wang, J.; Sun, Q.; Li, Q. Nanocellulose/PEGDA Aerogels with Tunable Poisson’s Ratio Fabricated by Stereolithography for Mouse Bone Marrow Mesenchymal Stem Cell Culture. Nanomaterials 2021, 11, 603. [Google Scholar] [CrossRef]
- Monfared, M.; Mawad, D.; Rnjak-Kovacina, J.; Stenzel, M.H. 3D bioprinting of dual-crosslinked nanocellulose hydrogels for tissue engineering applications. J. Mater. Chem. B 2021, 9, 6163–6175. [Google Scholar] [CrossRef] [PubMed]
- Iman, M.; Barati, A.; Safari, S. Characterization, in vitro antibacterial activity, and toxicity for rat of tetracycline in a nanocomposite hydrogel based on PEG and cellulose. Cellulose 2020, 27, 347–356. [Google Scholar] [CrossRef]
- Yin, A.; Yang, J. Cross-Linking Dynamics of Cellulose Nanofibrils-Based Transient Network Hydrogels: A Study of pH Dependence. Macromolecular Chemistry and Physics 2017, 218, 1600584. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Ma, M.; Xu, F. Modulation of Assembly and Dynamics in Colloidal Hydrogels via Ionic Bridge from Cellulose Nanofibrils and Poly(ethylene glycol). ACS Macro Letters 2015, 4, 829–833. [Google Scholar] [CrossRef]
- Monfared, M.; Mawad, D.; Rnjak-Kovacina, J.; Stenzel, M.H. 3D bioprinting of dual-crosslinked nanocellulose hydrogels for tissue engineering applications. Journal of Materials Chemistry B 2021, 9, 6163–6175. [Google Scholar] [CrossRef]
- Tehrani, Z.; Nordli, H.R.; Pukstad, B.; Gethin, D.T.; Chinga-Carrasco, G. Translucent and ductile nanocellulose-PEG bionanocomposites—A novel substrate with potential to be functionalized by printing for wound dressing applications. Industrial Crops and Products 2016, 93, 193–202. [Google Scholar] [CrossRef]
- Silva, R.d.; Sierakowski, M.R.; Bassani, H.P.; Zawadzki, S.F.; Pirich, C.L.; Ono, L.; de Freitas, R.A. Hydrophilicity improvement of mercerized bacterial cellulose films by polyethylene glycol graft. International Journal of Biological Macromolecules 2016, 86, 599–605. [Google Scholar] [CrossRef]
- Donati, I.; Christensen, B.E. Alginate-metal cation interactions: Macromolecular approach. Carbohydrate Polymers 2023, 321, 121280. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Asaron, F.; Paoletti, S. Experimental evidence of counterion affinity in alginates: The case of nongelling ion Mg2+. Journal of Physical Chemistry B 2009, 113, 12877–12886. [Google Scholar] [CrossRef]
- Tordi, P.; Ridi, F.; Samorì, P.; Bonini, M. Cation-Alginate Complexes and Their Hydrogels: A Powerful Toolkit for the Development of Next-Generation Sustainable Functional Materials. Advanced Functional Materials, n/a, 2416390. [https://advanced.onlinelibrary.wiley.com/doi/pdf/10.1002/adfm.202416390]. [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Advances in Colloid and Interface Science 2019, 274, 102044. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Batchelor, W.; Tabor, R.F.; Garnier, G. Gelation mechanism of cellulose nanofibre gels: A colloids and interfacial perspective. Journal of Colloid and Interface Science 2018, 509, 39–46. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Beris, A.N.; Rogers, S.A.; Wagner, N.J. Dynamic shear rheology of a thixotropic suspension: Comparison of an improved structure-based model with large amplitude oscillatory shear experiments. Journal of Rheology 2016, 60, 433–450. [Google Scholar] [CrossRef]
- Jakeman, E. Photon Correlation. In Photon Correlation and Light Beating Spectroscopy; Cummins, H.Z., Pike, E.R., Eds.; Springer Science + Business Media, LLC: New York, 1974. [Google Scholar]
- Pusey, P.; Van Megen, W. Dynamic light scattering by non-ergodic media. Physica A: Statistical Mechanics and its Applications 1989, 157, 705–741. [Google Scholar] [CrossRef]
- Shibayama, M.; Norisuye, T. Gel formation analyses by dynamic light scattering. Bulletin of the Chemical Society of Japan 2002, 75, 641–659. [Google Scholar] [CrossRef]
- Abou, B.; Bonn, D.; Meunier, J. Aging dynamics in a colloidal glass. Phys. Rev. E 2001, 64, 021510. [Google Scholar] [CrossRef]
- Scheffold, F.; Skipetrov, S.; Romer, S.; Schurtenberger, P. Diffusing-wave spectroscopy of nonergodic media. Physical Review E - Statistical, Nonlinear, and Soft Matter Physics 2001, 63, 061404/1–061404/11. [Google Scholar] [CrossRef]
- Urquidi, O.; Barbosa, N.; Brazard, J.; Adachi, T.B.M. Toward time resolved dynamic light scattering microscopy: Retrieving particle size distributions at high temporal resolutions. Review of Scientific Instruments 2023, 94, 083101. [Google Scholar] [CrossRef]
- Fahimi, Z.; Aangenendt, F.J.; Voudouris, P.; Mattsson, J.; Wyss, H.M. Diffusing-wave spectroscopy in a standard dynamic light scattering setup. Physical Review E 2017, 96, 062611. [Google Scholar] [CrossRef] [PubMed]
- Badruddoza, A.Z.M.; MacWilliams, S.V.; Sebben, D.A.; Krasowska, M.; Beattie, D.; Durian, D.J.; Ferri, J.K. Diffusing wave spectroscopy (DWS) methods applied to double emulsions. Current Opinion in Colloid & Interface Science 2018, 37, 74–87, Surface analysis techniques. [Google Scholar] [CrossRef]
- Hassan, P.A.; Rana, S.; Verma, G. Making Sense of Brownian Motion: Colloid Characterization by Dynamic Light Scattering. Langmuir 2015, 31, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Höhler, R.; Cohen-Addad, S.; Durian, D.J. Multiple light scattering as a probe of foams and emulsions. Current Opinion in Colloid & Interface Science 2014, 19, 242–252. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Freshney, R., Eds. Freshney’s Culture of Animal Cells, 8 ed.; Wiley-Blackwell, 2021.
- Truong, C.; Oudre, L.; Vayatis, N. Selective review of offline change point detection methods. Signal Processing 2020, 167, 107299. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chemistry: X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Kotov, N.; Larsson, P.A.; Jain, K.; Abitbol, T.; Cernescu, A.; Wågberg, L.; Johnson, C.M. Elucidating the fine-scale structural morphology of nanocellulose by nano infrared spectroscopy. Carbohydrate Polymers 2023, 302, 120320. [Google Scholar] [CrossRef]
- Li, M.C.; Wu, Q.; Song, K.; Qing, Y.; Wu, Y. Cellulose Nanoparticles as Modifiers for Rheology and Fluid Loss in Bentonite Water-based Fluids. ACS Applied Materials & Interfaces 2015, 7, 5006–5016. [Google Scholar] [CrossRef]
- Abitbol, T.; Mijlkovic, A.; Malafronte, L.; Stevanic, J.S.; Larsson, P.T.; Lopez-Sanchez, P. Cellulose nanocrystal/low methoxyl pectin gels produced by internal ionotropic gelation. Carbohydrate Polymers 2021, 260, 117345. [Google Scholar] [CrossRef]
- Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Current Opinion in Cell Biology 2021, 72, 10–18, Cell Dynamics. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nature Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tan, Y.; Feng, J.; Huang, C.; Liu, B.; Fan, Z.; Xu, B.; Lu, T. Exploration of the Effects of Substrate Stiffness on Biological Responses of Neural Cells and Their Mechanisms. ACS Omega 2020, 5, 31115–31125. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, Q.; Li, X.; Feng, j.; Ao, Z.; Li, X.; Wang, J. Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 7118834. [Google Scholar] [CrossRef]
- Guo, A.; Wang, B.; Lyu, C.; Li, W.; Wu, Y.; Zhu, L.; Bi, R.; Huang, C.; Li, J.J.; Du, Y. Consistent apparent Young’s modulus of human embryonic stem cells and derived cell types stabilized by substrate stiffness regulation promotes lineage specificity maintenance. Cell Regeneration 2020, 9. [Google Scholar] [CrossRef]
- Borgogna, M.; Skjåk-Bræk, G.; Paoletti, S.; Donati, I. On the Initial Binding of Alginate by Calcium Ions. The Tilted Egg-Box Hypothesis. The Journal of Physical Chemistry B 2013, 117, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
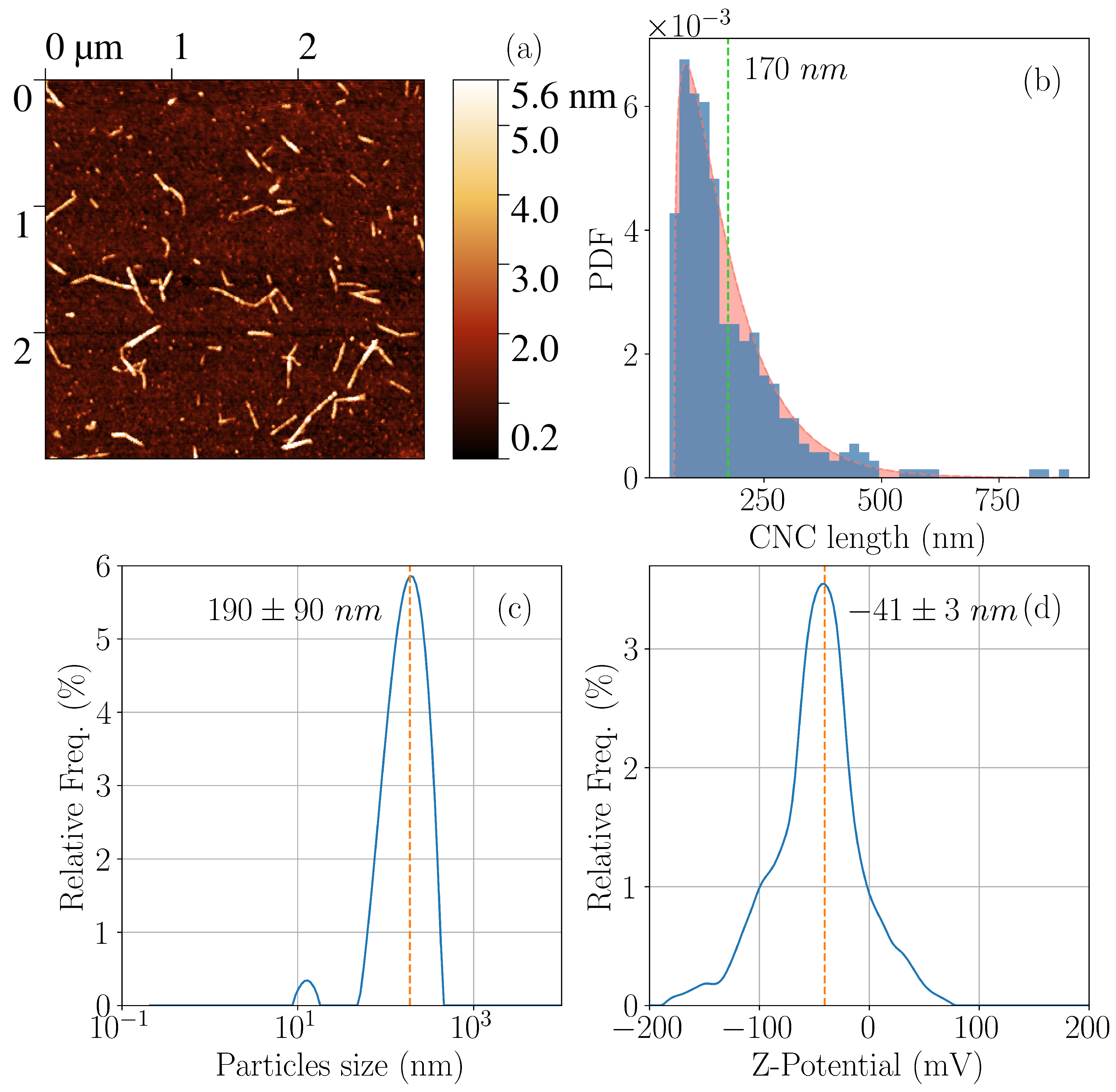
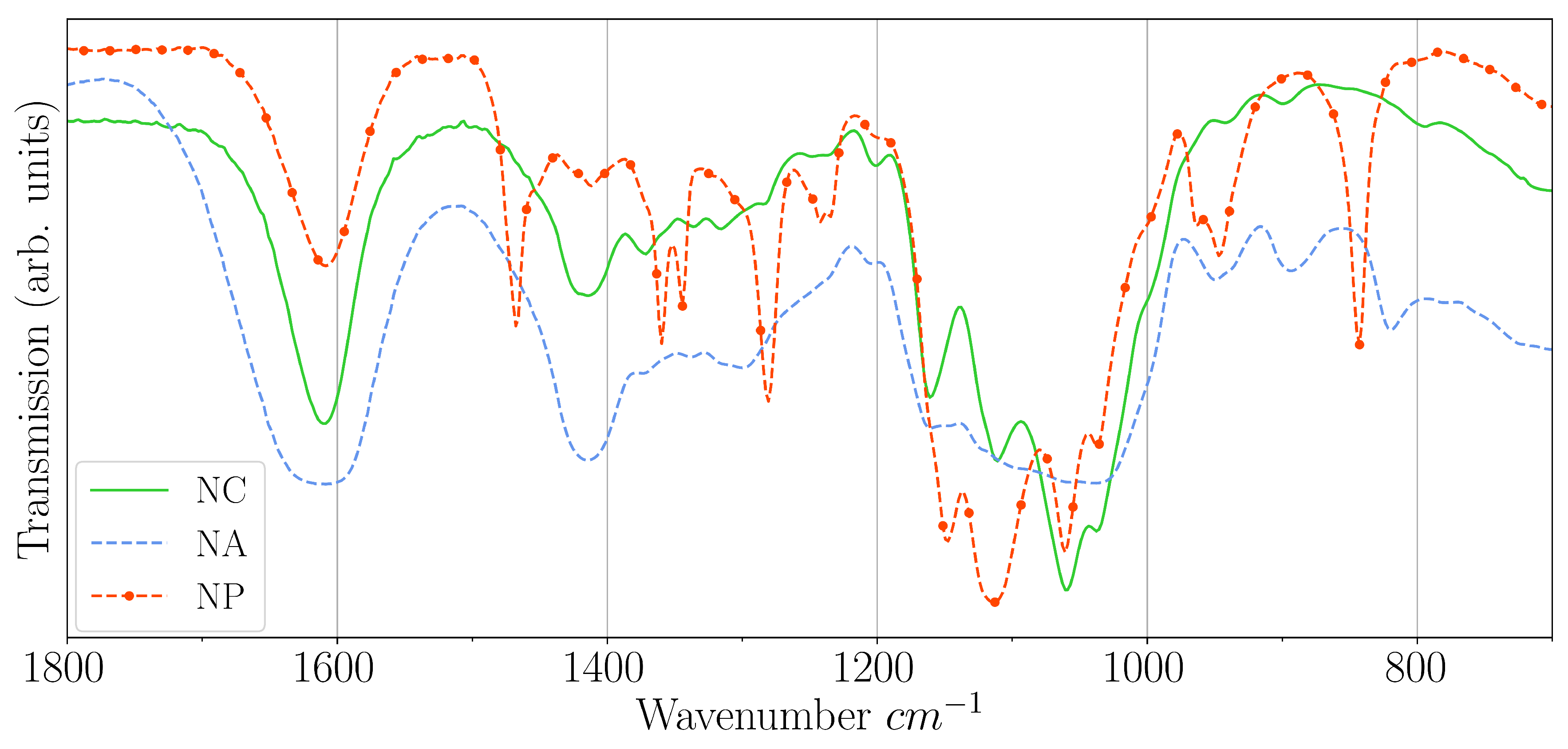
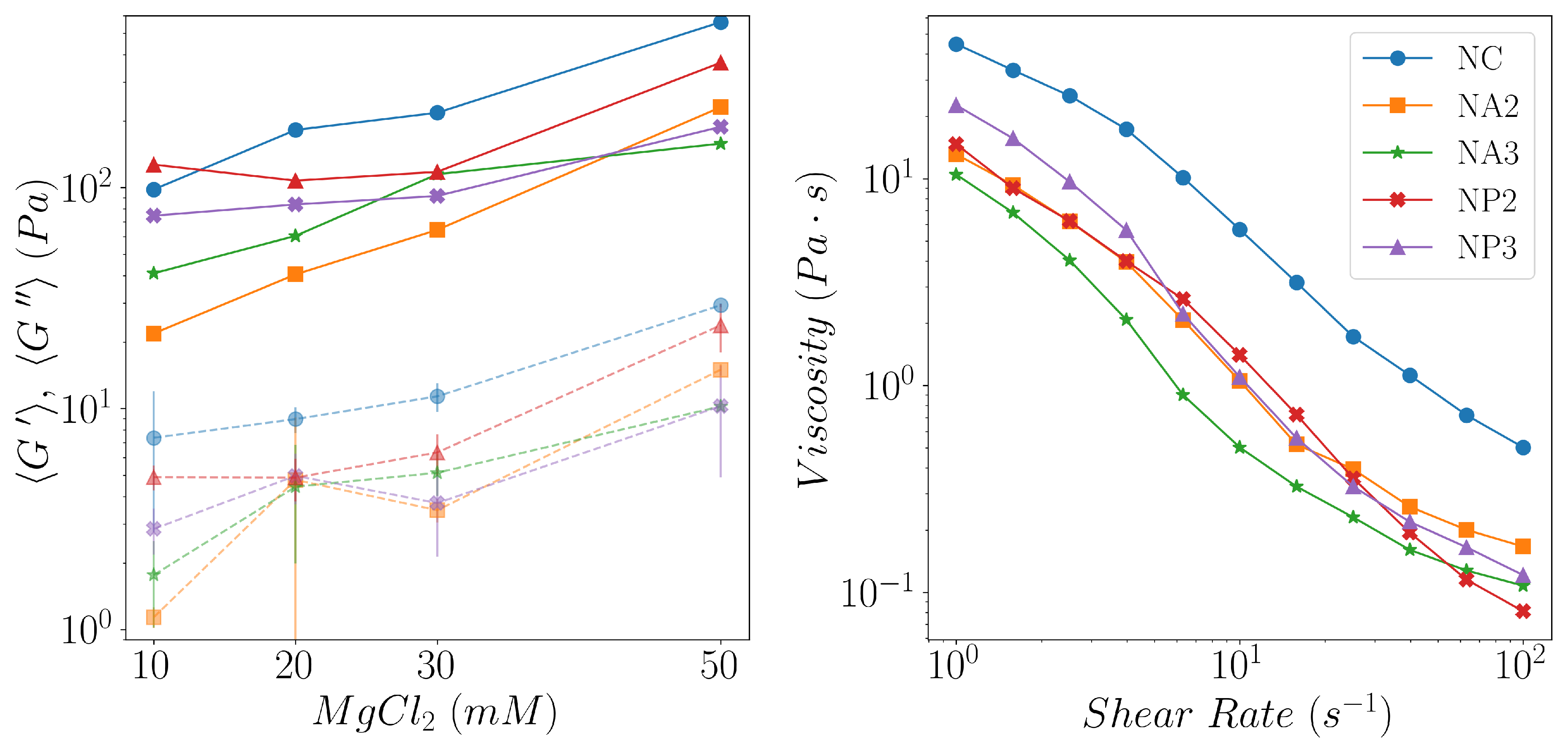

| Name | CNC | PEG | Alginate |
|---|---|---|---|
| CNC | 1 | 0 | 0 |
| NP2 | 2 | 1 | 0 |
| NP3 | 3 | 1 | 0 |
| NA2 | 2 | 0 | 1 |
| NA3 | 3 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





