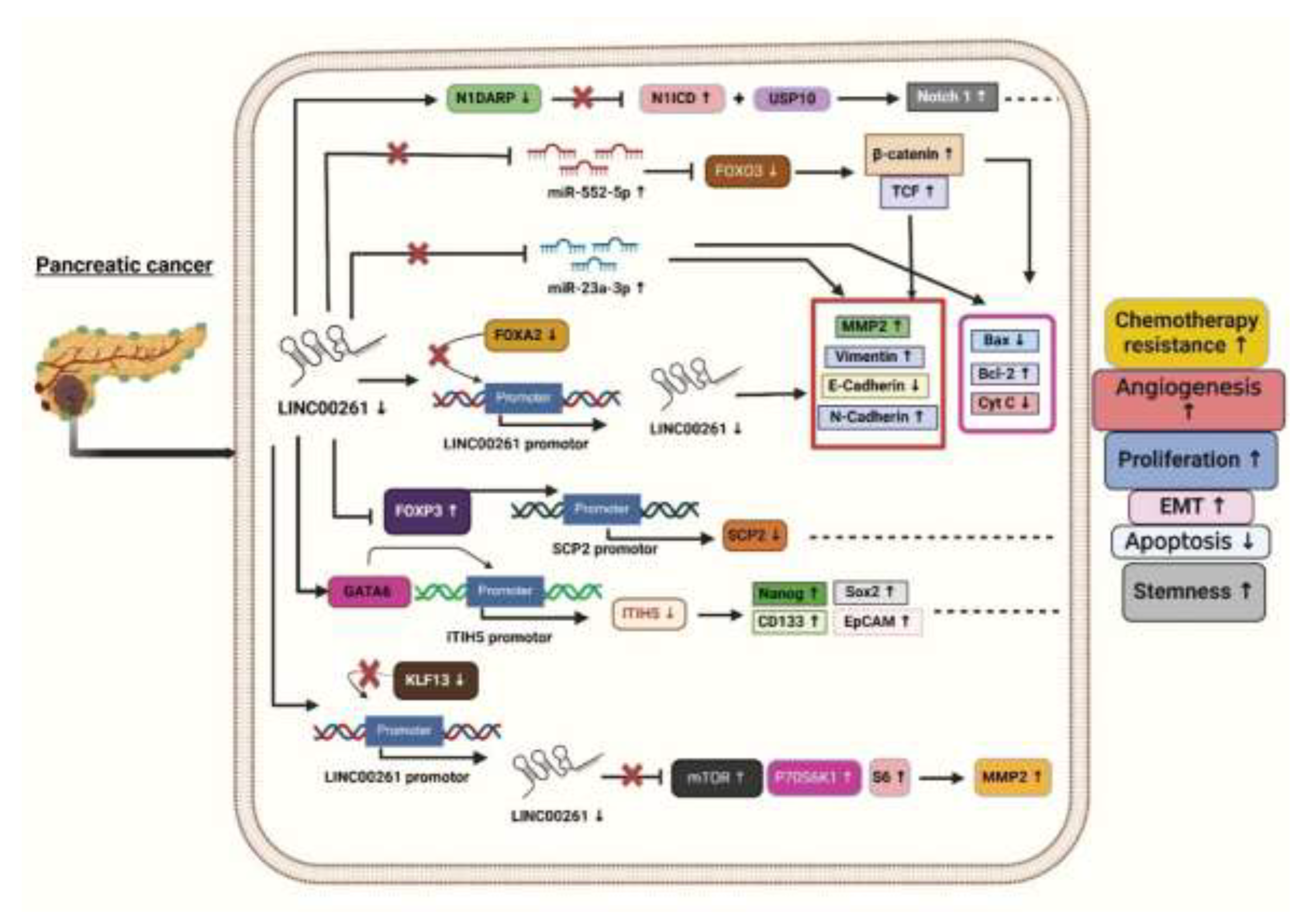
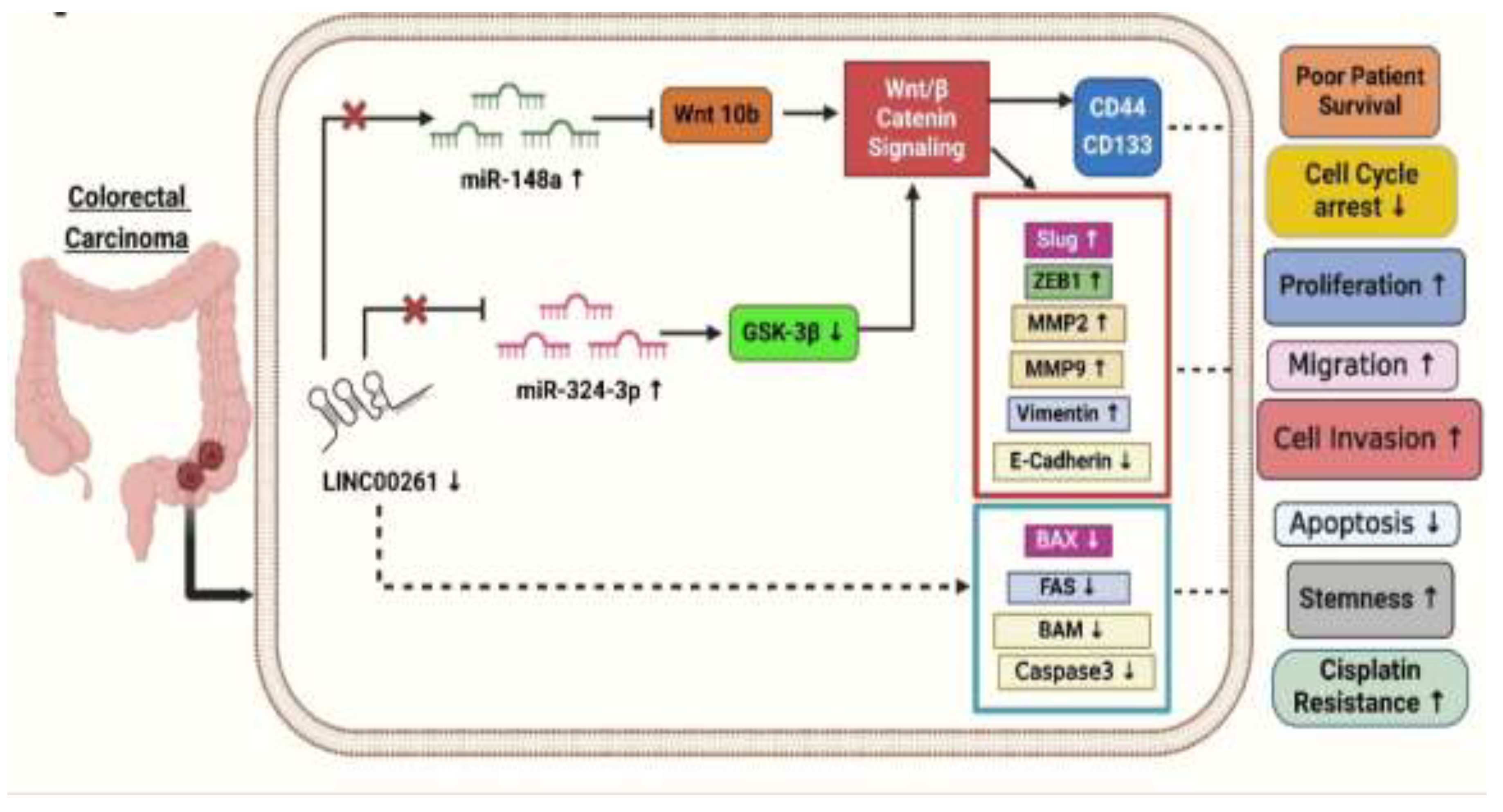
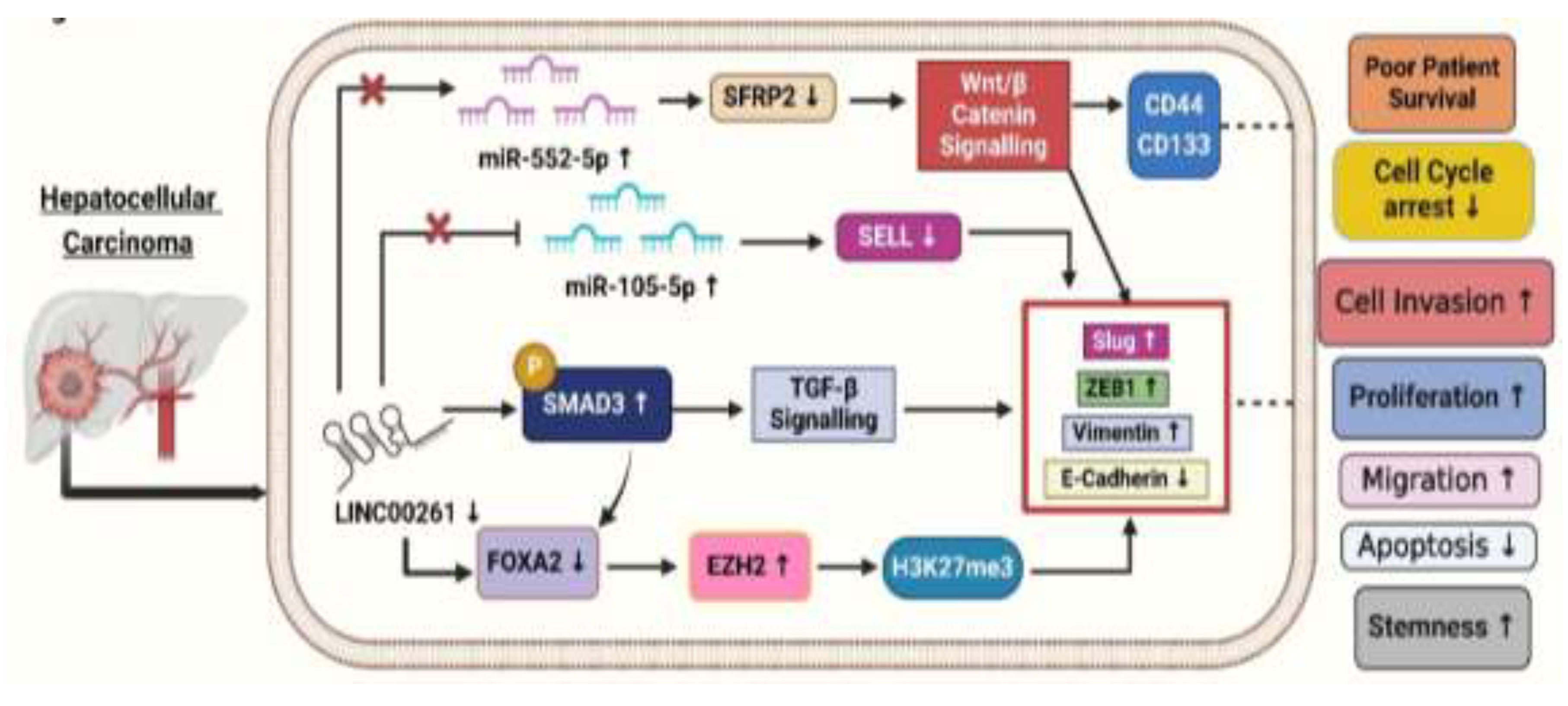
3.1. LINC00261 and Pancreatic Cancer
Multiple studies have indicated the critical involvement of diverse lncRNAs in the progression and treatment of PC. For example, HOTAIR (HOX transcript antisense intergenic RNA), MALAT1 (metastasis-associated lung adenocarcinoma transcript 1), HOTTIP (HOXA transcript at the distal tip), and PVT1 (Plasmacytoma Variant Translocation 1) have recently been found to be essential regulators linked to the progression of PC and have diagnostic and prognostic implications [
26,
27,
28].
In one of the recent studies, LINC00261 was investigated as an important regulator of PC, where authors have demonstrated that it is abnormally expressed in PC patients and cell lines compared to corresponding control samples. Most of these studies reported lower expression of LINC00261 in PC, which is associated with its tumorigenesis [
29,
30].
In one of the investigations, Wang et al. (2020) observed lower levels of LINC00261 in PC cell lines AsPC-1 (Ascites Pancreatic Cancer-1), BxPC-3 (Biopsy xenograft of Pancreatic Carcinoma line-3), PANC-1 (Pancreatic Cancer-1), and CFPAC-1 (cystic fibrosis pancreatic adenocarcinoma cell line) compared to HPDE6-C7 (Human Pancreatic Duct Epithelial cell line clone7), normal pancreatic ductal epithelial cells showing fold change of (~ 1.20) respectively. Moreover, through the TCGA (The Cancer Genome Atlas) database, the authors found low expression of LINC00261 in PC patients compared to healthy individuals, which also correlates with poor patient survival [
31].
To know how LINC00261 affects the cell's viability, invasion, and apoptotic activity in PC cells, the authors transfected the LINC00261 overexpression vector into two PC cell lines named CFPAC-1 and BxPC-3. Through the Western blot analysis, they found reduced levels of EMT marker proteins such as N-cadherin, Vimentin, and MMP2 (Matrix Metalloproteinase 2) while concurrently increasing the levels of E-cadherin (epithelial cadherin), which helped in maintaining epithelial integrity and strong cell-cell adhesion, limiting invasion and metastasis in CFPAC-1 and BxPC-3 cells. These data suggest that an increase in the expression of LINC00261 leads to less aggressive tumor phenotypes, better clinical outcomes, and improved prognosis [
31]. Moreover, through flow cytometry and transwell assays, it was found that LINC00261 also plays a crucial role in inhibiting metastasis and increasing apoptosis. To further know how this LINC00261 mechanistically suppresses (PC), LINC00261 targeted miRNA analysis was performed. For this, authors selected five miRNAs namely miR-23a-3p, miR-21, miR-222, miR-193b, and miR-221, from the LinkedOmics TCGA database. Following an in-depth analysis using starBase, an (online bioinformatics tool), they found that miR-23a-3p exhibited stronger and more effective binding sequences with LINC00261. The TCGA database from LinkedOmics displayed a slightly inverse correlation (p<0.0001) with the expression levels of LINC00261 and miR-23a-3p in PC. Later, RNA Immunoprecipitation assays and dual-luciferase reporters validated the direct interaction between LINC00261 and miR-23a-3p. Besides identifying in the plasma of patients with PC, Humeau et al. (2015) discovered that miR-23a acts as oncogenic and overexpressed in the saliva of PC patients with precursor lesions [
32]. To further check the viability, PC cells BxPC-3 and CFPAC-1 were given a transfection containing several vectors specifically, with a vector + miR-con (control), a LINC00261 overexpression vector + miR-con, or a LINC00261 overexpression vector + miR-23a-3p and was assessed using the MTT (3- (4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay) test and found that miR-23a-3p partially reversed cell viability these effects by lowering viability reduction and invasion reduction. Thus, LINC00261 may inhibit PC progression by regulating miR-23a-3p in vitro and can be considered a possible target for PC treatment. The above features of LINC00261 suggest that LINC00261 plays an important role as a tumor suppressor and can be exploited for future cancer-targeting purposes in PC. Though the authors suggested that LINC00261 plays a significant role in PC, more research is still needed to better understand its role as a tumor suppressor molecule [
31].
To examine the impact of LINC00261 on PC, Chen et al. (2020) conducted a series of experiments. They initially used qRT-PCR (quantitative reverse transcription polymerase chain reaction) to investigated the expression levels of LINC00261 in 54 PCs and 54 neighboring non-cancerous tissues and found considerable downregulation (fold change = 0.586). This was confirmed by using GEPIA (Gene Expression Profiling Interactive Analysis) bioinformatics analysis, which also showed reduced LINC00261 expression in PC tissues (n=179) against normal tissues (n=171). The authors used Kaplan-Meier survival curves to learn more about its clinical relevance, revealing that decreased LINC00261 expression is associated with poor prognosis and adverse clinical features, such as greater tumor size, advanced TNM (Tumor, Node, Metastasis) stage, and higher metastatic rates.
Furthermore, the study showed that the levels of LINC00261 expression were significantly decreased in the PC cell lines MIA-PaCa2 (Mouse Insulinoma-Associated Pancreatic Cancer-2), Capan-2 (Carcinoma of the Pancreas-2), BXPC-3, PANC-1, CFPAC-1, and AsPC-1 in comparison with the human pancreatic epithelial cells. To further understand its function in vivo, it was discovered that mice injected with PC cells overexpressing LINC00261 had greater survival rates and fewer metastatic foci than mice treated with control cells. In PC cells, LINC00261 mainly targets the molecular level of miR-552-5p. These results from their study highlight the function of LINC00261 in lowering PC metastasis [
33]. Additionally, through TargetScan the authors demonstrated miR-552-5p has binding sites for LINC00261, which have been further validated using dual luciferase reporter assays. It was observed that luciferase activity of LINC00261 diminished in PANC-1 and MIA-PaCa2 cells co-transfected with the miR-552-5p and LINC00261-WT plasmid compared to miR-552-5p negative control in pancreatic epithelial cell line [
33].
Also, miR-552-5p was shown to target FOXO3 (Forkhead box O3). FOXO3 is a tumor suppressor and crucial regulator of the Wnt/β-catenin signaling pathway. It has been identified as a direct target of miR-552-5p [
33]. LINC00261 counteracts miR-552-5p, restoring FOXO3 expression and suppressing the Wnt/β-catenin pathway, as evidenced by reduced levels of β-catenin and transcription factor 4 (TCF4). This inhibition effectively halts epithelial-mesenchymal transition (EMT) and metastasis, which are key processes in PC progression driven by the Wnt/β-catenin pathway. This reactivation of the Wnt pathway increases the expression of β-catenin and TCF4 and enhances EMT markers, highlighting the vital role of the LINC00261/miR-552-5p/FOXO3 axis in PC metastasis (
Figure 1) [
33]. However, these effects are reversed when miR-552-5p is reintroduced into LINC00261 overexpressing PC cells [
33].
In another separate study, Dorn et al. (2020) reported four molecular subtypes of pancreatic ductal adenocarcinoma (PDAC) using the International Cancer Genome Consortium's (ICGC) PDAC dataset: aberrantly differentiated endocrine exocrine (ADEX), immunogenic, pancreatic progenitor, and squamous. This dataset displayed a sample distribution of 25 for ADEX, 26 for immunogenic, 16 for pancreatic progenitor, and 29 for squamous cells. Furthermore, LINC00261 was identified as the most significantly differentially expressed lncRNAs, with a substantial downregulation reported in the squamous subtype compared to the other three [
4]. Further, to investigate the pathways linked to deregulated LINC00261 expression in PDAC samples, the authors used gene set enrichment analysis (GSEA). They identified forkhead box A2 (FOXA2), a chromosomal neighbor of LINC00261, as a direct regulator, with a strong association of (r = 0.72-0.91) across datasets and found a strong positive correlation between LINC00261 and FOXA2, an epithelial marker and EMT inhibitor. Both FOXA2 and LINC00261 showed similar expression patterns across PDAC subtypes. To investigate the regulatory relationship, the authors altered FOXA2 levels in PANC-1 cells [
4]. FOXA2 knockdown reduced LINC00261 transcript levels, while overexpression increased RNA expression and promoter activity (
Figure 1). FOXA2 binding to the LINC00261 promoter was confirmed by ChIP-qPCR (Chromatin Immunoprecipitation followed by quantitative Polymerase Chain Reaction). Further, the authors analyzed datasets such as CCLE (Cancer Cell Line Encyclopedia), PDAC samples (Pancreatic Ductal Adenocarcinoma), and lung adenocarcinoma (LUAD), demonstrating that LINC00261 expression correlated positively with epithelial markers (e.g., CDH1, KRT19, CLDN7 (cadherin 1, cytokeratin 19, claudin 7) and negatively with mesenchymal markers, highlighting its role in regulating differentiation and EMT processes [
4].
Additionally, the researchers further studied the effect of LINC00261 for checking cell migration and invasion in PC cells (PANC-1) and by correlating its expression with CDH1 (Cadherin1) encoding E-cadherin, which is a key EMT marker. Mechanistically, the depletion of LINC00261 was linked to reduced expression of CDH1 (E-cadherin), a crucial protein for maintaining cell-cell adhesion. This suggests that the loss of LINC00261 compromises E-cadherin-mediated adhesion, thereby enhancing cell motility and invasiveness. These results are consistent with clinical data showing that low LINC00261 expression is associated with more aggressive pancreatic cancer (PDAC) phenotypes and worse patient outcomes, underscoring its potential as both a biomarker and a therapeutic target in Pancreatic adenocarcinoma (PDAC) [
4].
Based on GSE16515 (Gene Expression Series number 16515) and GSE32676 dataset analyses, Li Zou et al. (2021) reported that LINC00261 expression is markedly reduced in PC tissues compared to normal controls. Their study identified a positive association between LINC00261 and Inter-Alpha-Trypsin Inhibitor Heavy Chain 5 (ITIH5) expression using ChIPBase (Chromatin Immunoprecipitation Database). Additionally, predictions from lncMAP indicated that LINC00261 might have ITIH5 expression through the transcription factor GATA6 (GATA Binding Protein 6), suggesting an important regulatory function of LINC00261 in the progression of PC (
Figure 1) [
34]. The authors further examined the fact that LINC00261 upregulates ITIH5 expression in PANC-1 cells and stem cells by recruiting the transcription factor GATA6 to a specific binding site (site two) on the ITIH5 promoter. Through a dual-luciferase assay, the authors confirmed that the overexpression of LINC00261 enhances ITIH5 promoter activity while its silencing decreases it. Bioinformatics analyses identified two potential GATA6 binding sites on the promoter, and experimental validation confirmed site two as the functional site for GATA6 binding. ChIP-qPCR assays demonstrated that GATA6 binds effectively to site 2 in a LINC00261-dependent manner, and RIP assays (RNA Immunoprecipitation Assays) established a direct interaction between LINC00261 and GATA6. Silencing GATA6 in LINC00261-overexpressing cells abrogated ITIH5 upregulation, indicating that the effect of LINC00261 is mediated through GATA6 [
34]. These findings suggest that LINC00261 facilitates ITIH5 expression by acting as a scaffold for GATA6 recruitment, highlighting a potential regulatory axis that could be explored for therapeutic interventions in PC. Functional assays demonstrated that increased LINC00261 expression leads to the suppression of key stem cell markers (Nanog, Oct4 (Octamer-binding Transcription Factor 4), Sox2 (SRY-Box Transcription Factor 2), CD133 (Cluster of Differentiation 133), and EpCAM (Epithelial Cell Adhesion Molecule) and reduced cell viability, self-renewal capacity, invasive ability, and tumorigenicity. Additionally, it was observed that PANC-1 stem cells, which had restored LINC00261 expression, exhibited reduced resistance to gemcitabine, the standard chemotherapy drug for PC. The overexpression of LINC00261 in PANC-1 stem cells suggested its potential role as a tumor suppressor in PC. Various assays including sphere formation assays, transwell invasion tests, cytotoxicity assays, and tumor xenograft studies confirmed these effects. This indicates that targeting LINC00261 could help suppress the aggressive and drug-resistant characteristics of pancreatic cancer stem cells, providing a novel therapeutic approach to combat PC progression and improve drug sensitivity [
34]. Moreover, flow cytometry analysis was conducted to check how it regulates the cell cycle, which revealed that overexpression of LINC00261 increased the proportion of cells in the G0/G1 phase, suggesting an interruption in the cell cycle.
To gain more in-depth knowledge, Zou et al. (2021) uncovered that LINC00261 regulates PC progression through its interaction with FOXP3 (Forkhead Box Protein P3). FISH (Fluorescence In Situ Hybridization) assays revealed nuclear localization of LINC00261 and FOXP3 in PC cells, while RIP and ChIP assays confirmed that LINC00261 binds FOXP3, which in turn binds the SCP2 (Sterol Carrier Protein 2) promoter. Overexpression of LINC00261 increased SCP2 expression while reducing FOXP3 levels, disrupting FOXP3-mediated suppression of SCP2. These findings suggest that the LINC00261/FOXP3/SCP2 axis influences PC development, presenting a novel regulatory mechanism and therapeutic target (
Figure 1) [
29].
In the study by Li et al. (2022), the role of LINC00261 in PC was comprehensively analyzed. Utilizing GEO (Gene Expression Omnibus) datasets (GSE15471, GSE16515, and GSE32676), LINC00261 was identified as significantly downregulated in PC tissues, with this reduction correlating to poor patient prognosis (p = 0.014). Survival analysis confirmed that patients with low LINC00261 expression had decreased survival rates [
15]. Functional assays in human PC cell lines revealed lower LINC00261 levels in AsPC-1, Patu8988, MIAPaCa-2, and SW1990 cells than in normal pancreatic cells (HPDE6-C7). Additionally, RNA FISH showed that LINC00261 was localized to both the nucleus and cytoplasm in PANC-1 cells, with a primary concentration in the nucleus. The authors further conclude that low LINC00261 expression is a potential prognostic biomarker in PC, suggesting its tumor-suppressive role [
15].
The authors investigated the potential of LINC00261 as a prognostic biomarker for pancreatic cancer (PC). Initially, they transfected SW1990 cells with the pcDNA3.1-H-LINC00261 plasmid. This process resulted in a significant increase in the levels of LINC00261. Additionally, in order to silence LINC00261 in PANC-1 cells, the authors utilized the pLV3ltr-Puro-U6-LINC00261-i plasmid. This approach led to a reduction in LINC00261 expression by up to 64.2%. It revealed that through transwell assays, LINC00261 expression had no significant effect on cell growth, proliferation, or overall apoptosis rates, overexpression markedly suppressed PC cells' migratory and invasive capabilities. These findings highlight LINC00261's critical role in limiting tumor aggressiveness in PC [
15]. The regulatory role of LINC00261 in EMT and its downstream effects were thoroughly investigated. To demonstrate how LINC00261 regulates PC progression, the authors used qRT-PCR and Western blotting to validate changes in the expression of key EMT markers and transcription factors [
15].
Additionally, the analysis of the GEPIA database revealed that the Twist1 (Twist-related protein1) transcription factor is highly expressed in PC tissues. This Twist1 is known to play a critical role in promoting EMT by activating key target genes such as MMP2 in cancers. It was observed that the downregulation of LINC00261 resulted in increased Twist1 transcription, thereby enhancing EMT processes. The findings reveal LINC00261 as a pivotal regulator in mitigating PC aggressiveness, making it a potential therapeutic target for inhibiting EMT and tumor metastasis in PC. Also, their study discovered that KLF13 upregulates LINC00261 transcription by binding to its promoter. Bioinformatic analyses identified KLF13 as a potential regulator and experimental validation showed that KLF13 co-localized with LINC00261 in the nucleus of PANC-1 cells. Overexpression of KLF13 increased LINC00261 expression significantly, and luciferase assays confirmed that KLF13 directly binds to the LINC00261 promoter, enhancing its activity [
15]. Moreover, the study shows that LINC00261 suppresses mTOR-P70S6K1-S6 (mechanistic Target of Rapamycin- p70 S6 Kinase 1-S6 ribosomal proteins) a signaling pathway activation through KLF13 regulation, which is critical in suppressing PC metastasis. The PI3K/Akt/mTOR (Phosphoinositide 3-Kinase / Protein Kinase B / mechanistic Target of Rapamycin) pathway is known to promote cell migration and metastasis by phosphorylating P70S6K1, leading to actin filament rearrangement and upregulating MMP2 expression. Overexpression of KLF13 suppressed the pathway and decreased metastasis by preventing the activation of mTOR, P70S6K1, and S6. However, when LINC00261 was silenced, this inhibitory effect of KLF13 was reversed, highlighting that LINC00261 plays a pivotal role in modulating this pathway to prevent PC metastasis (
Figure 1) [
15].
Zhai et al.(2023) identified the highest differential expression of LINC00261 between PC and normal cancer through the TCGA database. Additionally, the authors found that the LINC00261 had more potential for coding probability than other proven translatable lncRNAs evaluated by the online tool CPC2 (Coding Potential Calculator 2). Further, the results obtained by ORF Finder (Open Reading Frames) showed that ORF2, ORF7, and ORF12 could be translated [
30]. Additionally, the CCK8 (Cell Counting Kit-8) experiment revealed that only ORF12 overexpression prevented the growth of PC cells, implying that these ORFs may have biological roles. Moreover, the authors found LINC00261 as a key regulator of Notch1 (Notch homolog 1) signaling in PC by encoding the microprotein Notch1 degradation-associated regulatory polypeptide (N1DARP). In normal conditions, Notch1 signaling controls crucial cellular processes like differentiation and apoptosis by promoting the degradation of the Notch1 intracellular domain (N1ICD) [
30].
Moreover, in PC, hyperactivation of this pathway occurs due to the stabilization of N1ICD, driving uncontrolled cell proliferation, stemness, and chemoresistance (
Figure 1). N1DARP, encoded by LINC00261, acts as a tumor suppressor by disrupting the interaction between N1ICD and the deubiquitinase USP10 (Ubiquitin-Specific Protease 10), leading to polyubiquitination and proteasomal degradation of N1ICD confirmed by the Western Blotting analysis in CAPAN1 transfected with a vector or varying concentrations of FLAG-tagged (Fluorescent Antigen) N1DARP plasmid, where overexpressed N1DARP inhibited Notch activation. This results in reduced Notch1 activity, thereby inhibiting tumor growth, stemness traits, and chemoresistance [
30]. The authors also highlighted the therapeutic potential of targeting this disrupted interaction, with the stapled peptide SAH-mAH2-5 derived from N1DARP offering a promising strategy for treating Notch1-activated PC. Zhai et al. validated their findings using both preclinical and clinical samples. Preclinical models included pancreatic cancer organoids (PDAC-1, PDAC-2, PDAC-R) derived from patient samples and genetically engineered KPC (Kras/p53/Cre) and KPNC (Kras/p53/Notch/Cre) mice [
30].
Overall, the results above show that LINC00261 has a tumor-suppressive effect in PC, influencing key pathways such as EMT, mTOR, and Notch signalingas illustrated in (Figure1). Its downregulation is associated with poor prognosis and aggressive tumor phenotypes. However, further research is needed to investigate it as a viable biomarker for the early detection and/or treatment of PC. Despite its potential as a biomarker and therapeutic target, further research is needed to fully understand its mechanisms and validate its clinical application in combating pancreatic cancer progression and improving treatment outcomes.
3.2. LINC00261 and Colorectal Cancer
Colorectal cancer (CRC) is recognized as one of the most prevalent and aggressive malignancies affecting the digestive system, characterized by/known for high morbidity and mortality rates. It continues to pose a significant global challenge [
35,
36,
37]. Despite recent improvements in therapy, patients with metastatic CRC still have a poor 5-year survival rate, which is critically low globally (~10%) despite recent developments in treatment [
36]. Studies have demonstrated that numerous long non-coding RNAs play crucial roles in regulating CRC-related pathways [
38]. Moreover, a poor clinical prognosis and the onset of metastasis in CRC patients are strongly associated with increased expression levels of MALAT1 (Metastasis-Associated Lung Adenocarcinoma Transcript 1), HOTAIR (HOX transcript antisense intergenic RNA), and H19 (H19 Imprinted Maternally Expressed Transcript) [
39]. According to a recent study, the lncRNAs KCNQ1OT1 (KCNQ1 overlapping transcript 1) and WT1-AS (Wilms tumor 1 antisense RNA) have substantial interactions with most miRNAs in the ceRNA network (competing endogenous RNA), indicating that they play important roles in the development of CRC [
39].
In one of the recent studies, Liu et al. (2020) evaluated the clinical and mechanistic role of LINC00261 in CRC. They used RNA expression profiling from the TCGA dataset, which included 459 non-metastatic colorectal CRC samples and 87 metastatic CRC samples, to examine metastasis-specific mRNAs, miRNAs, and lncRNAs in CRC tissue. By comparing non-metastatic and metastatic tissues, the results confirmed 628 differentially expressed (DE) mRNAs, of which 354 were upregulated and 274 were downregulated along with 25 aberrantly expressed miRNAs and 144 dysregulated lncRNAs. Out of 144 dysregulated, 3-lncRNA, such as LINC00114 (long intergenic non-protein coding RNA 114), LINC00261, and HOTAIR, were found to be highly significant in CRC patients compared to normal tissue samples. Additionally, analysis of lncRNA expression and clinical features in CRC showed LINC00261 had a positive correlation with overall survival (OS) (p=0.044 and p=0.0006, respectively), but HOTAIR had a negative correlation with OS (p=0.012). Moreover, the authors observed that LINC00261 expression varies throughout the different stages of colorectal cancer. They noted significant reductions in the expression of lncRNA in stages III and IV of CRC in comparison to stages I and II CRC. Furthermore, the authors did not identify any significant differences when comparing combined CRC tissues to normal colorectal tissues. Their findings emphasize the importance of these lncRNAs as biomarkers in improving CRC prognosis and informing treatment decisions [
39].
In terms of LINC00261, it is found that it exhibits positive coexpression with mRNAs involved in important biological processes such as "GO:0042158~lipoprotein biosynthetic process," (Gene Ontology (GO) "GO:0008152~metabolic process," "Drug metabolism cytochrome P450 (pigment 450)," and "Chemical carcinogenesis" [
39]. The expression of individual P450 variants has been associated with colorectal cancer, where some of these P450s show increased levels. Notably, P450 components showed high expression of CYP51 or CYP2S1 (Cytochrome P450 2S1), which has been correlated with a poor prognosis, with CYP51 (cytochrome P450 14α-sterol demethylase) serving as an independent prognostic indicator (15897573). The findings indicate that LINC00261 is downregulated in colorectal cancer (CRC) and actively influences tumorigenesis and key cancer pathways.
In another study, Xi et al. (2023) investigated whether LINC00261 plays a role in CRC through the miRNAs-mRNA axis. For this, they took the LINC00261-miRNA-148a/WNT10b axis. They established that targeting the LINC00261-miRNA-148a/WNT10b axis could significantly affect CRC cell proliferation and apoptosis, highlighting its importance in tumor growth and programmed cell death. Further, they found that by regulating the miRNA-148a/WNT10b axis, LINC00261 may impact the proliferation of SW480 CRC cells. Further studies reported that transfection of LINC00261-specific siRNAs in CRC cells significantly increased miR-148a and decreased WNT10b (wingless-type MMTV integration site family, member 10B) and β-catenin proteins. The WNT10b is a key molecule of the Wnt/β-catenin signaling pathway, while miR-148a is reported as an oncogenic miRNA in CRC. According to the above findings, LINC00261 may influence the advancement of colon cancer by interfering with the miR-148a/WNT10b axis and disrupting Wnt/β-catenin signaling [
37].
Moreover, the reduced expression of LINC00261 significantly decreased cell viability, increased the apoptosis rate, and caused G1-phase cell cycle arrest in CRC cells. These effects also underscore LINC00261's potential in CRC cancer treatment [
37]. It was also observed that higher expression levels of LINC00261 tend to improve overall survival compared to CRC patients with lower expression levels. LINC00261 is a tumor suppressor in colon cancer, showing that its overexpression inhibits cell growth and migration, and deactivates the Wnt signaling pathway. Both in vitro and xenograft models confirmed LINC00261's role in slowing tumor progression, suggesting it could be a promising therapeutic target for colon cancer. From the above data, we can conclude that LINC00261 could be linked to CRC patients' prognoses, indicating that it could be a useful biomarker for patients with colon cancer [
37].
In another study, Wang et al. (2018) demonstrated that LINC00261 levels markedly down-regulated in tissues and cell lines of colon cancer, with the lowest expression observed in Stage III compared to Stages I and II [
35], which also suggests its prognostic value. Furthermore, the authors observed that LINC00261 significantly decreased cisplatin resistance in colon cancer in vivo while enhancing the drug's efficacy by reducing tumor volume and weight. Their research also utilized the SW480 colon cancer cell line to investigate the relationship between LINC00261 and cisplatin resistance, revealed that the cisplatin-resistant SW480 cell line exhibited markedly lower LINC00261 expression than drug-sensitive cells[
35]. It was observed that when LINC00261 expression was increased in CRC cells through expression vector-based methods, the expression of pro-apoptotic proteins such as BAX (BCL2-associated X protein), FAS (FS-7-associated surface antigen, Fas receptor), Bim (Bcl-2-interacting mediator of cell death), and cleaved caspase-3 increases, which in turn reduced cell viability and migration of CRC cells by increasing E-cadherin and reducing EMT proteins such as MMP2 and MMP9 [
35]. Further, It also blocked β-catenin nuclear translocation, which suppressed Wnt/β-catenin signaling and its target genes such as Myc (Myelocytomatosis oncogene) and CCND1 (Cyclin D1). The in vivo studies further indicated that the overexpression of LINC00261 effectively inhibited both the initiation and progression of colon cancer[
35]. LINC00261 also synergized with cisplatin in the reduction of tumor growth in mice, indicating its potential therapeutic value in overcoming drug resistance and tumor progression. This research underscores that the LINC00261 plays an essential role in cell proliferation, migration, and apoptosis and mediated drug resistance in colon cancer cells. Other studies have indicated that overexpressing LINC00261 could reverse drug resistance, inhibit CRC cell migration and invasion, and identify it as a promising molecule for CRC diagnosis and prognosis. These above data provide a solid foundation for LINC00261-based innovative treatment strategies for CRC [
35].
In another study, Tang et al. (2022) identified an 8-lncRNA prognostic signature, including SNHG7 (small nucleolar RNA host gene 7), ZEB1-AS1 (zinc finger E-box binding homeobox 1 antisense 1), U47924.27, NIFK-AS1 (NIFK Antisense RNA 1), RP1-170O19.17, LINC00261, LINC00925, and CAPN10-AS1 (Calpain 10 Antisense RNA 1) as a potential independent prognostic factor for CRC patients based on the TCGA dataset. The predictive ability of this signature was validated using additional datasets (GSE39582, GSE29621(Gene Expression Omnibus Series) and effectively predicted chemotherapy responses [
36]. Among these lncRNAs, SNHG7, ZEB1-AS1, NIFK-AS1, and others were positively associated with survival risk, whereas LINC00261 was negatively correlated. In a recent study, LINC00261 was significantly downregulated in CRC tissues and linked to lymph node metastasis and clinical stage (
Figure 2). The signature identified high-risk patients with poor survival outcomes, as shown by Kaplan-Meier and ROC analyses (Receiver-operating characteristic curve), where the risk score became an independent prognostic factor. Immune profiling revealed differences in immune cell infiltration and mutational landscapes between risk groups, while IC50 analyses (Half-maximal inhibitory concentration) indicated varying sensitivities to chemotherapy drugs [
36]. Functional studies showed that lncRNAs like ZEB1-AS1 and SNHG7 inhibited CRC cells' growth, migration, and EMT through PI3K/AKT signaling (phosphatidylinositol 3-kinase /protein kinase B), supported by Gene Ontology enrichment and pathway analyses. This indicates that LINC00261 is a prognostic and therapeutic tool for CRC [
36].
Yan et al. (2019) investigated the role of LINC00261 in colon cancer progression and its interaction with miR-324-3p and the Wnt signaling pathway. They found that LINC00261 expression was significantly reduced in (SW620, HT-29, DLD-1, HCT-116, and SW480) colon cancer cells compared to HCEPIC and normal colon cells [
40]. In contrast, miR-324-3p levels were notably elevated in colon cancer cells. Further analysis showed overexpression of LINC00261, using a lentiviral vector (LV-LINC00261) in colon cancer cell lines HCT-116 and DLD-1, inhibits tumor progression by sponging the oncogenic miR-324-3p to activate GSK-3β (glycogen synthase kinase 3β), thus degrading β-catenin and inhibiting the Wnt/β-catenin pathway and resulting in decreased proliferation, migration, invasion, apoptosis enhancement, and decreased tumor growth both in vitro and in vivo. Overexpression of LINC00261 reduced miR-324-3p levels and suppressed colon cancer cell proliferation, migration, and invasion, as demonstrated in HCT-116 and DLD-1 cell lines. Additionally, experiments confirmed that LINC00261 upregulation inhibited cell proliferation [
17].
Mechanistically, LINC00261 repressed colon cancer progression by inactivating the Wnt signaling pathway by modulating miR-324-3p (
Figure 2). These findings suggest that LINC00261 reduces colon cancer by competitively binding itself to miR-324-3p and interfering with oncogenic signaling pathways [
17].
As demonstrated in (Figure2), LINC00261 is significantly downregulated in colon cancer, while miR-324-3p is upregulated. By inactivating the Wnt pathway and negatively regulating miR-324-3p, overexpression of LINC00261 inhibits the spread of colon cancer and could serve as a target for therapeutic intervention [
17].
3.3. Role of LINC00261 in Hepatocellular Cancer
Hepatocellular carcinoma (HCC) is a significant global health issue and is the fourth most prevalent cause of cancer-related deaths globally, ranking sixth in terms of incidence [
8,
19,
41]. Numerous investigations have demonstrated that fatty acid metabolism is a vital metabolic process that supplies energy and signaling molecules, and encourages HCC [
8].
Chen et al. (2022) investigated the role of fatty acid (FA) metabolism-related lncRNAs in HCC. The authors aimed to identify key lncRNAs linked to FA metabolism and assess their prognostic and therapeutic potential. Using datasets from TCGA and Gene Expression Omnibus (GEO), they identified LINC00261, along with SNHG1 (Small Nucleolar RNA Host Gene 1) and SNHG7 (Small Nucleolar RNA Host Gene 7), as pivotal lncRNAs associated with FA metabolism [
8]. To explore these associations, the study employed advanced bioinformatics techniques, such as single-sample gene set enrichment analysis (ssGSEA), to derive FA metabolism scores and correlate them with lncRNA expression. Experimental approaches, including qRT-PCR, FA metabolism PCR arrays, and Western blotting, were utilized to validate their findings and assess the downstream effects of these lncRNAs. Their results demonstrated that LINC00261, a key part of the FA metabolism-related lncRNA signature, plays a critical role in regulating FA metabolism, contributing to immune infiltration and EMT, processes linked to HCC progression and immune evasion. Patients with higher LINC00261 expression exhibited better survival outcomes, underscoring its potential as a prognostic biomarker. Additionally, the study highlighted the significant molecular differences between patient subgroups based on FA metabolism-related lncRNA profiles, such as immune cell heterogeneity, genomic mutations, and EMT activity. These findings suggest that LINC00261 holds promise as a therapeutic target and a predictor of immunotherapy response in HCC [
8].
To comprehensively investigate the role of LINC00261 in HCC, Chen et al. (2022) conducted various experimental analyses, and to see its expression. They used qRT-PCR on HCC tissues and adjacent non-tumorous tissues, revealing significant downregulation of LINC00261 in HCC [
19]. To get into clinical insights, the authors treated HCC cell lines Huh7 and HepG2 with Transforming growth factor-beta 1 (TGF-β1) to induce higher expression of EMT-associated proteins and observed reduced LINC00261 expression [
19]. To know further, the authors investigated the clinical relevance of LINC00261 in HCC and performed immunohistochemistry (IHC) on patient samples to correlate LINC00261 expression and found that low levels of LINC00261 were associated with high levels of p-SMAD3 (phospho-small mothers against decapentaplegic homolog 3), a molecule-activated during TGF-β1 signaling, which promotes EMT, stemness, and metastasis (
Figure 3). Moreover, it is observed that HCC patients having lower expression of LINC00261 and higher level of p-SMAD3 have overall poorer recurrence-free survival [
19]. The above data suggests the combined potential of LINC00261 and p-SMAD3 as useful biomarkers for predicting prognosis outcomes in HCC patients. To better understand LINC00261, the authors constructed LINC00261 knockdown models using MHCC-LM3 (Hepatocellular Carcinoma cell line - Liver Metastatic Cell line 3) and SNU-449 (Seoul National University cell line 449) HCC cell lines. Through these models, they observed that migration and invasion were significantly promoted after LINC00261 knockdown but were suppressed with LINC00261 overexpression of the EMT-associated protein levels. The knockdown of LINC00261 increased ZEB1 (Zinc-finger E-box binding homeobox 1) and Vimentin levels while reducing E-cadherin expression, which indicated EMT promotion (
Figure 3). Conversely, overexpression of LINC00261 reversed these effects, decreasing ZEB1, Slug, and Vimentin while restoring E-cadherin levels. These findings highlighted that LINC00261 suppressed EMT and limited cancer cell viability, suggesting its therapeutic potential in treating cancer by regulating molecular pathways involved in metastasis [
19].
To uncover the mechanisms underlying LINC00261's role in HCC, Chen et al. (2021) conducted a series of well-designed experiments. They performed gain- and loss-of-function studies demonstrating that LINC00261 suppresses migration, invasion, and epithelial-mesenchymal transition (EMT) in HCC cells [
41]. Following this, the researchers investigated the molecular mechanisms involved, using advanced techniques like RNA pull-down assays to identify proteins interacting with LINC00261. The authors revealed that LINC00261 recruits SMAD3 to the promoter region of FOXA2 (Forkhead Box A2), a neighboring tumor suppressor gene, thereby activating its expression [
41]. Further bioinformatics analyses and rescue experiments confirmed that FOXA2 mediates the tumor-suppressive effects of LINC00261 while restoring FOXA2 levels reversed the effects of LINC00261 loss. To explore the silencing of LINC00261, the authors treated HCC cells with GSK126, an inhibitor of Enhancer of Zeste Homolog 2 (EZH2) [
41]. This treatment reduced the trimethylation of histone H3 at lysine 27 (H3K27me3) levels and restored LINC00261 expression. Combining these findings with immunohistochemical staining of patient tissues, they linked high levels of EZH2 and H3K27me3 to low expression of LINC00261 and poorer patient outcomes (
Figure 3). Overall, these experiments provided compelling evidence for the EZH2/LINC00261/FOXA2 axis as a critical regulator of HCC metastasis [
41].
Ma et al. (2021) revealed that fucoidan is a natural polysaccharide derived from brown algae, exhibiting various biological activities, including antioxidant, anti-inflammatory, and antitumor properties. This compound has been shown to inhibit cell growth in vitro and in vivo, decrease invasion and motility, and trigger apoptosis and cell cycle arrest [
42]. The antitumor efficacy of fucoidan has been validated in various cancers, including pancreatic, bladder, and ovarian cancers. Fucoidan inhibits tumor occurrence and development by modulating tumor immunity, obstructing angiogenesis, and disrupting cell cycle processes and apoptosis, indicating its significant potential in tumor therapy [
42]. Fucoidan was found to upregulate LINC00261, which interacted with miR-522-3p and increased the expression of SFRP2 in HCC. Conversely, the down-regulation of LINC00261 increases the expression of miR-522-3p and down-regulated SFRP2 expression and supported cell proliferation (
Figure 3) [
42]. This pathway is crucial in inducing dose-dependent arrest of the cell cycle and induction of apoptosis. It has been previously established that LINC00261 acts as a tumor-suppressor in terms of regulating the expression of miRNA. LINC00261 could also be associated with reduced tumorigenicity of MHCC97H cells through miR-522-3p regulation in HCC. SFRP2 was significantly enhanced in the fucoidan treatment group, suggesting the interaction of LINC00261, miR-522-3p, and SFRP2 in HCC cells [
42].
In another study, Song et al. (2022) investigated the role of the LINC00261/miR105-5p/SELL axis in HCC, particularly its connection to immune cell dysfunction and patient survival. This study highlighted LINC00261 as a critical component of the LINC00261/miR105-5p/SELL axis, which influences immune cell function and patient survival in HCC [
7]. Explore how post-transcriptional regulatory mechanisms involving the LINC00261/miR105-5p/SELL axis influence immune dysfunction and overall survival in HCC patients. They utilized TCGA dataset samples (379 HCC out of these 337 tumor samples and 42 adjacent tissues) to perform comprehensive immune cell profiling and gene expression analysis with CIERSORT (Cell-type Identification by Estimating Relative Subsets of RNA Transcripts). Most of the patients were males with a median age of 61 and were diagnosed at an early stage of HCC as per the TNM staging system. In contrast to the surrounding tissues, tumor tissues exhibited considerably lower expression levels of SELL (L-selectin) (Log2-fold change= −1.14) and LINC00261 (Log2-fold change= −0.90) [
7].
In HCC tissues, the researchers found 201 differentially expressed miRNAs (DEMs), 216 differentially expressed lncRNAs (179 upregulated and 37 downregulated), and 3,705 differentially expressed genes (1628 upregulated and 2077 downregulated). When compared to nearby normal tissues, tumor tissues showed downregulation of SELL (Selectin L). Selectin L is an important immunological regulator with adhesive properties, and its reduced expression contributes to cancer progression by promoting cell proliferation and metastasis [
43]. miR-105-5p plays a role in HCC by influencing immune cells, especially B cells, through the LINC00261/miR-105-5p/SELL pathway. The presence of this miRNA has a direct association with the survival status of patients. Better overall survival (OS) was positively connected with its expression. When LINC00261 attaches itself to miR-105-5p, it functions as a "molecular sponge." Through this interaction, SELL expression is maintained by preventing miR105-5p from binding to the 3' untranslated region (UTR) of the SELL mRNA. In tumor tissues, decreased LINC00261 levels increase miR105-5p activation. Higher levels of LINC002616therapeutic target in HCC [
7]. A more immunosuppressive tumor microenvironment (TME) results from improved immune cell homing and function caused by increased miR-105-5p suppressing SELL. By sponging miR105-5p, LINC00261 functions as a competitive endogenous RNA (ceRNA), stopping miR105-5p from down-regulating SELL. In HCC samples, decreased LINC00261 levels were accompanied by elevated miR105-5p levels, which inhibited SELL (
Figure 3). Survival analysis showed higher SELL and LINC00261 levels were linked to better results, whereas higher miR-105-5p predicted worse overall survival. Kaplan-Meier survival analyses confirmed SELL's positive association with overall survival, further reinforcing its potential as a prognostic marker [
7].
The aboveanalysis reveals significant expression differences between tumor and normal tissues, identifying 3,705 differentially expressed genes (DEGs) vital for cancer research. Upregulated DEGs are linked to cell division and DNA replication, emphasizing their role in tumor growth [
7]. In contrast, downregulated DEGs are associated with immune responses, inflammation, and cell adhesion, suggesting potential therapeutic targets. The study found 201 differentially expressed miRNAs (DEMs) and 216 expressed long non-coding RNAs (DELs), enhancing our understanding of tumor biology and offering new research opportunities. LINC00261 is significantly downregulated in HCC tissues, interfering with its function as a ceRNA (competing endogenous RNA) that protects SELL from miR-105-5p repression, which in turn leads to impairment of immunological functions and metastasis of HCC [
7].
Furthermore, they established a ceRNA network identifying critical interactions influencing survival outcomes. Eleven immune cell types showed alterations in HCC tissues compared to non-cancerous tissues. The LINC00261/miR105-5p/SELL pathway emerged as a key marker, with decreased Selectin L, a molecule essential for immune cell homing and function, was positively correlated with patient survival and markedly downregulated in HCC tissues [
7]. This ceRNA axis implies restoring SELL levels may enhance immune function and outcomes in HCC. SELL expression correlates with improved overall survival. This underscores SELL's importance in immune function and its connection to immunotherapy gene signatures, indicating potential for novel therapeutic approaches. The LINC00261/miR105-5p/SELL axis is a crucial prognostic biomarker and therapeutic target for HCC, substantially improving immunotherapy effectiveness. Further research is necessary to validate these findings and advance targeted treatments [
7].
The above findings indicate that LINC00261 plays a critical role as a tumor suppressor in HCC, and regulates various cancer-associated pathways, including the EZH2/FOXA2 and miRNA axes as shown in (Figure 3). The LINC00261 also affects fatty acid metabolism, immune cell function, and the epithelial-mesenchymal transition. Targeting the mechanisms associated with LINC00261 presents promising opportunities for enhancing immunotherapy and improving clinical outcomes in HCC patients. However, further research is needed to translate these findings into effective treatments.