1. Introduction to Personalized Medicine and Nanotechnology
1.1. Evolution of Drug Delivery Strategies
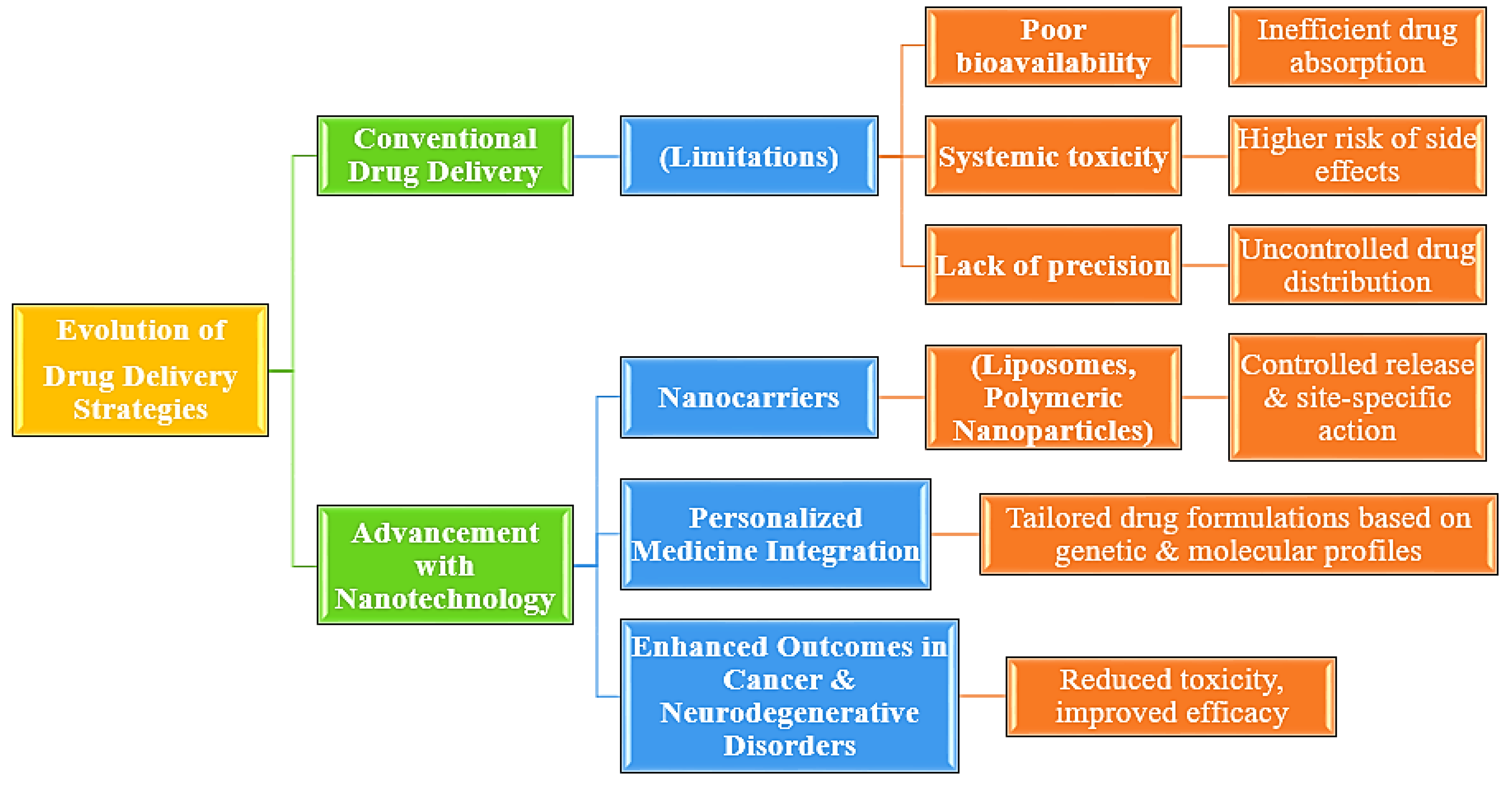
The systems today for drug delivery have developed nanotechnology-based systems that surmount the traditional fears of low bioavailability and systemic toxicity. The oldest formulations of drugs were inaccurate; hence, it led to side effects related to improper delivery of drugs. Drug delivery has advanced with nanocarriers like liposomes and polymeric nanoparticles since it enables the release in a controlled manner toward a site in the body [
1].
Such advancements, for example, in the optimization of drug therapy meant the integration of nanotechnology with personalized medicine-both its formulation was to be designed according to genetic and molecular profiles. Enhanced therapeutic yields could, thus lead to diseases such as cancers and neurodegenerative disorders because their targeted drug delivery would minimize such chances of inducing toxicity along with declining efficacy at hand [
1].
Figure 1.
Evolution of Drug Delivery Strategies[
1]
.
Figure 1.
Evolution of Drug Delivery Strategies[
1]
.
1.2. Targeting Methods and Their Role in Modern Therapeutics
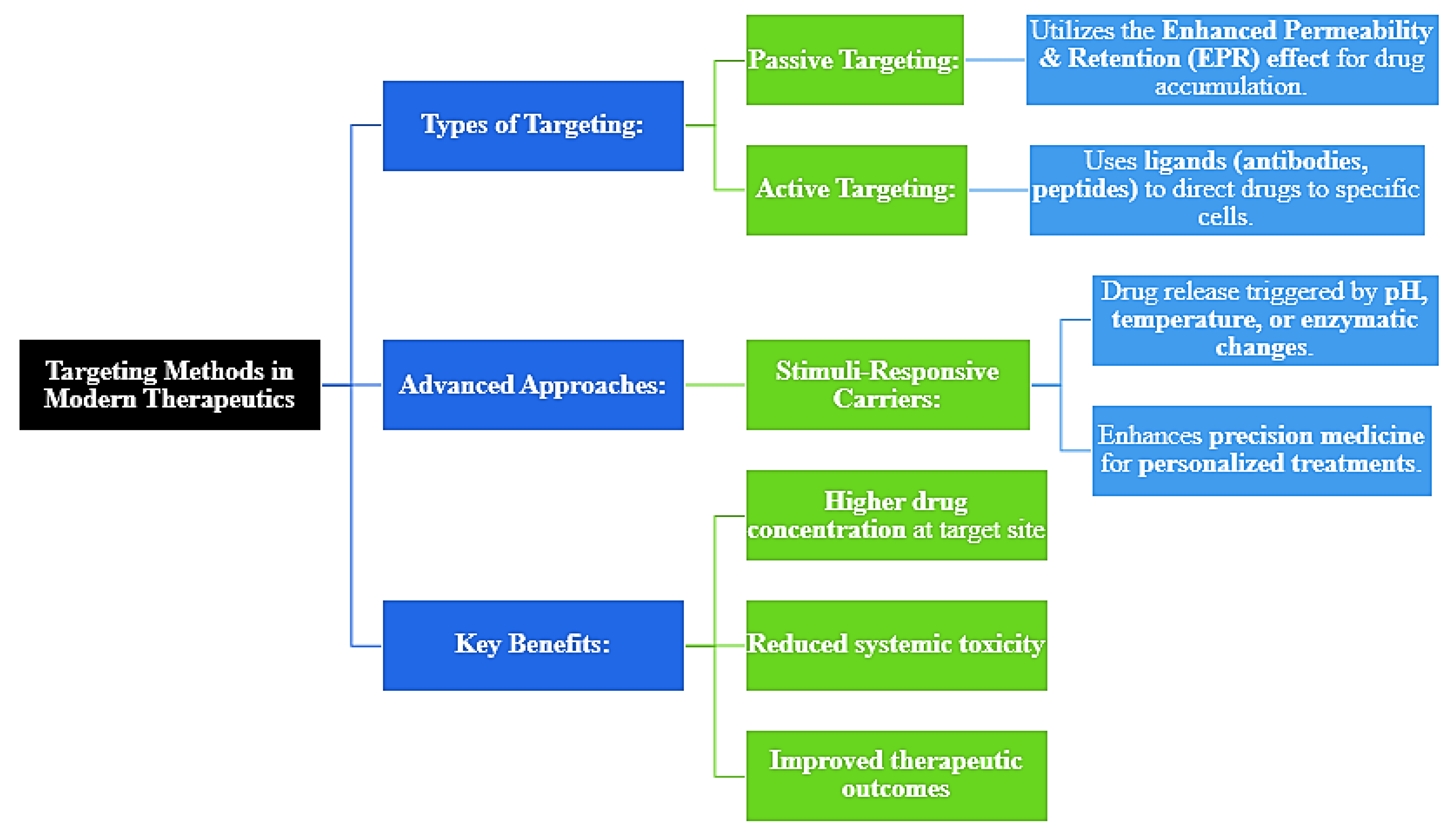
This results in reduced systemic side effects due to targeted delivery of drugs. Targeted drug delivery can be further sub-divided into two categories. First, one is the EPR effect based on the passive targeting, and the other is the active targeting wherein ligands, such as antibodies or peptides, are used for the transport of drugs to targeted cells [
1].
Recent developments in this area are pH-, temperature-, or enzyme-cleavable carriers that deliver drugs according to changes in these variables. This gives the field relevance in precision medicine, which includes personalized treatment focused on improving outcomes but lessening side effects [
1].
Figure 2.
Targeting Methods in Modern Therapeutics.
Figure 2.
Targeting Methods in Modern Therapeutics.
2. Nanocarriers
2.1. Liposomal Vesicular Delivery Systems: Innovations and Mechanisms
Liposomal drug delivery systems revolutionized modern therapeutics with the control of a controlled, site-specific drug release. The enhanced stability of the drugs, lengthening their time in circulation and lowering their toxicities, came through the carriers formed by the vesicular system, which had lipid bilayers. The more recent developments that improved bioavailability and efficacy for treatment are stealth liposomes and ligand-targeted vesicles [
2].
2.2. Nanoparticles in Advanced Drug Delivery
In precision medicine with nanoparticles, drug targeting and controlled release are achieved. The small dimensions can also ensure deep penetration of tissues for treatments of cancers and neurological diseases. Functionalized by polymers or antibodies, increased specificity is achievable in the targeting of drug delivery, ensuring desirable therapeutic benefits without significant adverse effects [
3].
3. Liposomal Drug Delivery in Personalized Therapeutics
3.1. Core Principles and Structural Components
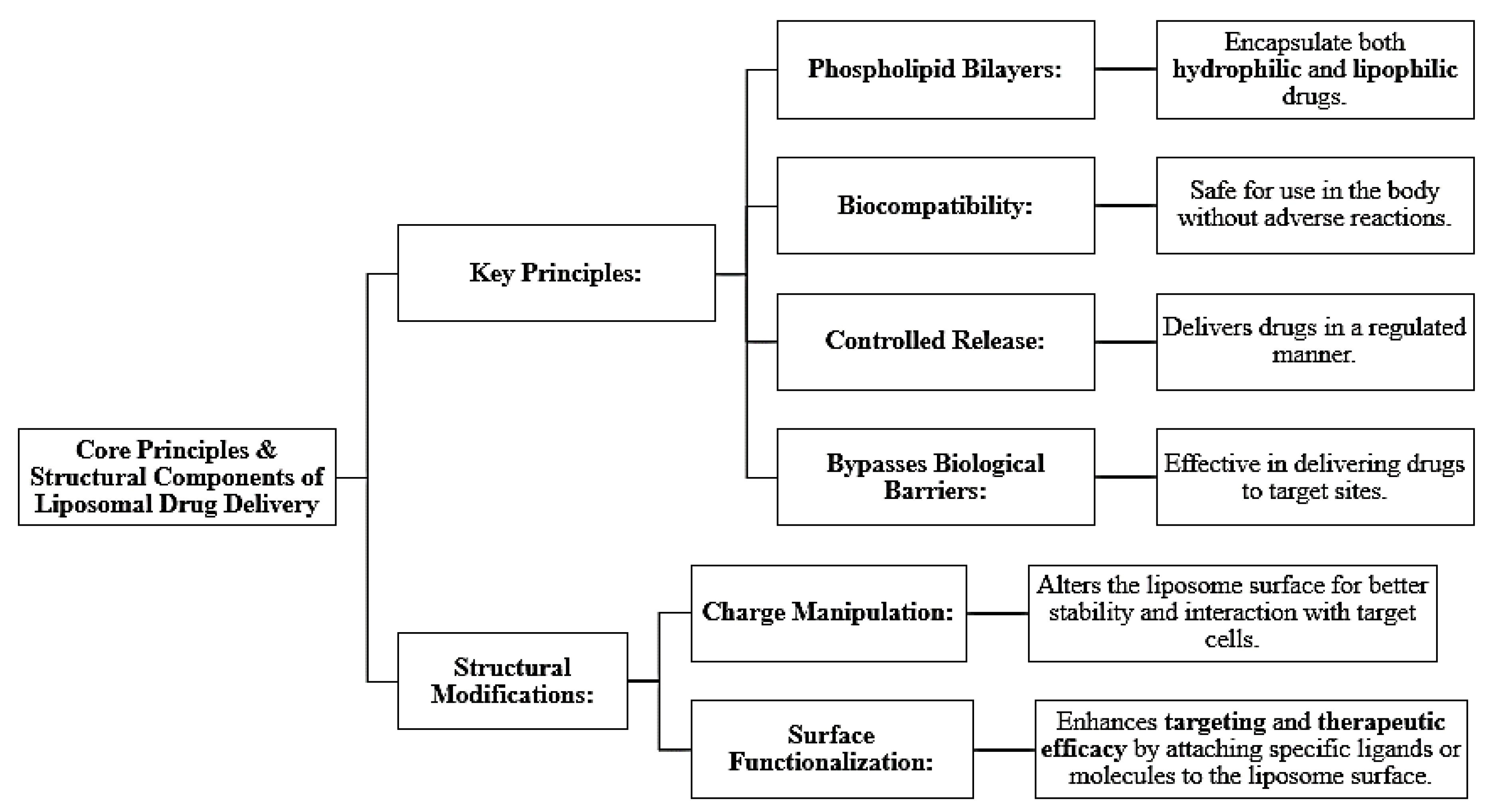
Liposomal drug delivery systems exploit the phospholipid bilayers to encapsulate both hydrophilic and lipophilic drugs. They are biocompatible, have controlled release mechanisms, and can evade biological barriers; thus, they are perfect for targeted therapies. Their therapeutic efficiency is further improved through structural modification, including charge manipulation and surface functionalization [
3].
Figure 3.
Core Principles and Structural Components Liposomal drug delivery systems.
Figure 3.
Core Principles and Structural Components Liposomal drug delivery systems.
3.2. Drug Encapsulation and Release Dynamics
The encapsulation efficiency and controlled release of drugs are some parameters through which the efficacy of carriers can be determined. Lipid composition, pH sensitivity, external triggers involving temperature, and enzymatic activation control the kinetics of release. Recently, site-specific drug delivery, mainly in cancer therapy, has attracted more attention to the amounts of recipients using pHresponsive and enzyme-activated liposomes [
3].
3.3. New Developments in PEGylated Liposomes for Long Circulation Periods
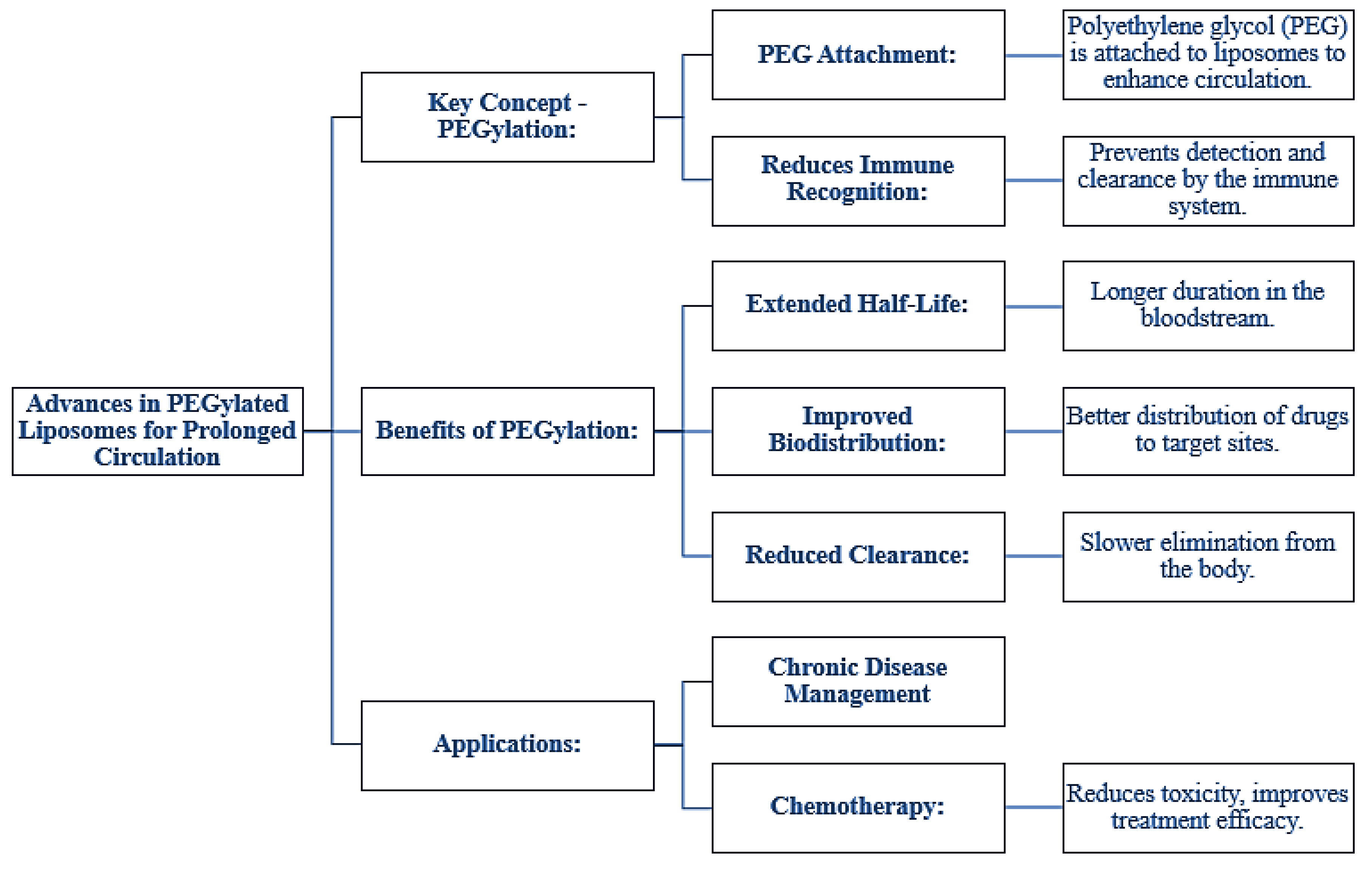
Pegylation, which is the coupling of polyethylene glycol to liposomes, decreases immune recognition, and it increases systemic circulation. The half-life is much longer for the stealth liposomes, the biodistribution is more favorable, and the clearance rate is reduced significantly, which places them uniquely advantageous in chronic diseases and chemotherapy [
4].
Figure 4.
Advances in PEGylated Liposomes for Prolonged Circulation.
Figure 4.
Advances in PEGylated Liposomes for Prolonged Circulation.
4. Naso-Pulmonary and Oral Drug Delivery
4.1. Liposomal Inhalation Therapy for Pulmonary Diseases
Liposomal inhalation therapy offers a non-invasive, targeted delivery of the condition, including asthma, tuberculosis, and pulmonary fibrosis. Inhalable liposomes are an innovative alternative for chronic lung diseases since they have the potential to improve drug retention in the lungs while minimizing systemic side effects [
4].
4.2. New Technologies in Chewable and Effervescent Tablet Technologies
These products are chewable and effervescent, so they improve the compliance of pediatric and geriatric patients. An effervescent formulation increases drug solubility and absorption in the body; a chewable tablet is simply an alternative route to the classic oral intake of solid dosages. Such innovative formulations result in better therapeutic response for conditions in which immediate onset of therapeutic action is required [
5], [
7].
4.3. Mouth-Dissolving Films for Enhanced Bioavailability
Mouth-dissolving films, known as MDFs, is fast-disintegrated drug delivery technology that circumvents the issue of first-pass metabolism and thus increases the bioavailability of drugs. It would be highly useful for patients who have dysphagia and a drug to be absorbed as quickly as possible-for example, cardiovascular or analgesic drugs [
6].
Table 1.
Naso-Pulmonary and Oral Drug Delivery Technologies.
Table 1.
Naso-Pulmonary and Oral Drug Delivery Technologies.
| No. |
Drug Delivery Method |
Mechanism |
Advantages |
Applications |
| 1 |
Liposomal Inhalation Therapy |
Liposomes encapsulate drugs for direct pulmonary delivery |
Targeted drug delivery, prolonged retention, reduced systemic side effects |
Asthma, tuberculosis, pulmonary fibrosis [4] |
| 2 |
Chewable & Effervescent Tablets |
Chewable tablets provide an alternative solid dosage form; effervescent tablets enhance drug solubility |
Improved compliance, rapid drug absorption, better taste |
Pediatric & geriatric patients, immediate-action drugs [5], [7] |
| 3 |
Mouth-Dissolving Films (MDFs) |
Rapid disintegration in the mouth, bypassing first-pass metabolism |
Increased bioavailability, faster onset, useful for dysphagic patients |
Cardiovascular drugs, analgesics, emergency medications [6] |
Nomenclature
Liposomal Inhalation Therapy: Liposome-based drug formulations designed for pulmonary administration to enhance retention and reduce systemic exposure [ 4].
Effervescent Tablets: Solid dosage forms that dissolve in liquid, enhancing drug solubility and absorption [ 5] , [ 7].
Chewable Tablets: Solid oral dosage forms that disintegrate upon chewing for easier ingestion [ 5].
Mouth-Dissolving Films (MDFs): Thin polymeric films that rapidly dissolve in the mouth for fast drug absorption [ 6].
|
5. Proniosomes: A Competitive Alternative to Liposomes
5.1. Fundamentals and Core Composition of Proniosomes
Proniosomes are water-soluble dry carriers, which become niosomes when they contact water. Primarily, these consist of nonionic surfactants, stabilizers, and cholesterol. Proniosomes have more stability in the drugs, easier storage, and controlled release. This self-assembly mechanism is quite effective in drug delivery, especially in both hydrophilic and hydrophobic drugs, and leads to increased bioavailability [
8].
Table 2.
Fundamentals and Core Composition of Proniosomes.
Table 2.
Fundamentals and Core Composition of Proniosomes.
| No. |
Aspect |
Details |
Advantages |
Applications |
| 1 |
Core Composition |
Made of nonionic surfactants, stabilizers, and cholesterol |
Better stability and bioavailability |
Suitable for both hydrophilic & hydrophobic drugs [8] |
| 2 |
Mechanism |
Self-assembly into niosomes upon hydration |
Simplifies drug encapsulation and improves drug release control |
Targeted and controlled drug delivery [8] |
| 3 |
Stability & Storage |
Exists in a dry state; highly stable at room temperature |
Easier storage, longer shelf-life |
Useful in formulations requiring extended storage [8] |
5.2. Comparison with Liposomes
Although both proniosomes and liposomes present promises in terms of biocompatibility and targeted delivery, proniosomes are known for a far longer shelf life and better stability, and hence economical compared to those of liposomes. Proniosomes also foreclose the necessities of specific storing conditions, issues associated with the oxidation and decomposition of lipids in liposomes [
8], [
9].
Table 3.
Comparative Analysis – Proniosomes vs. Liposomes.
Table 3.
Comparative Analysis – Proniosomes vs. Liposomes.
| No. |
Aspect |
Proniosomes |
Liposomes |
Advantages of Proniosomes |
| 1 |
Stability |
More stable due to dry form |
Prone to oxidation and hydrolysis |
Longer shelf-life, minimal degradation [8], [9] |
| 2 |
Storage |
Room temperature storage |
Requires refrigeration, sensitive to temperature |
No need for special storage conditions [8] |
| 3 |
Cost |
Cost-effective and scalable |
Higher production costs |
More economical alternative [9] |
6. Targeted Drug Delivery for Precision Medicine
6.1. Surface-Modified Nanocarriers for Disease-Specific Targeting
Recent advances in the technology of surface modification, especially for attachment and conjugation to antibodies, suggest that nanocarriers will be engineered to specifically recognize diseased tissues. It significantly reduces unwanted secondary effects in the body and enhances the therapeutic action with targeted modifications targeting cancerous cells or inflamed tissues or localized to a cellular organelle [
9].
6.2. Active Targeting Strategies in Liposomal Drug Delivery
Active targeting includes receptor-mediated endocytosis, pH-sensitive drug release, and biomarker-guided delivery. Examples are targeted liposomes toward folate-based cancer therapy, HER2-directed liposomes, and breast cancer treatment, which proved the concept that site-specific action of drugs while enhancing cellular uptake and reducing the systemic toxicity are possible [
10].
7. Liposomal Antibiotics and Herbal Drug Delivery
7.1. Addressing Antibiotic Resistance through Liposomal Nanocarriers
Liposomal formulation is used to strengthen the effect of antibiotics: Increased penetration drug; protection to degradation; weakening the resistance in the bacteria. In addition, another liposome-based formulation containing the amikacin, vancomycin, and ciprofloxacin entrapped within, has also had higher bioavailability and gradual drug release, thereby becoming the primary condition for managing infection caused through multidrugresistant mechanisms [
11], [
12].
7.2. Role of Liposomes and Proniosomes in Herbal Drug Delivery
These liposomal and proniosomal formulations have witnessed an intensive improvement in solubility and bioavailability of the plant drugs along with targeted delivery. The encapsulation of herbal extracts, curcumin, quercetin, and Tribulus terrestris, in vesicular systems showed highly remarkable improvement of their therapeutic activity when applied on chronic diseases of inflammation and metabolic disorders [
13], [
14].
8. Future Perspectives in Personalized Medicine and Nanotechnology
8.1. Hybrid Nanocarrier Systems: Vesicular and Non-Vesicular Platforms
The future of nanomedicine hybrid drug delivery systems includes vesicular and non-vesicular platforms, such as liposomes and proniosomes on one hand, and polymeric nanoparticles, dendrimers, and micelles on the other. This will enhance stability, control release, and multifunctional targeting. Lipid-polymer hybrid nanoparticles have demonstrated good potential in oncology and neurodegenerative therapies by promoting synergistic action of drugs and prolonged circulation [
15].
8.2. Clinical Translation and Future Integration of Nanomedicine
Although there have been tremendous advances, clinical translation remains an issue because of scalability and regulatory concerns, such as long-term evaluation of safety. Both FDA and EMA emphasize biocompatibility, reproducibility, and toxicity assessment in translating nanocarriers to the clinical field. New technologies involving 3D bioprinting, AI-driven formulation design, and patient-specific nano drug development will put within the realm a possibility of translating scientific research into realities and develop a platform that is to be tapped to bridge to accelerate the full integration of the personalized nanomedicine into a mainstream health system [
16].
8.3. New trends in nanotechnology drug delivery
Developments in nanomedicine in future will involve further development of responsive carriers that react to pH/temperature stimuli; enzymatic sensibility and using exosome based drug delivery with gene editing CRISPR-filled nanoparticles. Research is also witnessing the advancement with self-assembling nanostructures coupled with bio-inspiring nanocarriers; virus mimicked nanoparticles, the most efficient would be those bearing cell membrane- coated vesicles for most successful and biomimetic drug translocation. Nanorobotics in its evolving phase, AI-driven nanoformulations, and the in vivo tracking of nanocarriers in real time will revolutionize the field of precision medicine, setting personalized therapeutics closer to a clinical reality [17 - 20].
Conclusion
Convergence of personalized medicine and nanotechnology has dramatically altered the scenario of drug delivery to the most targeted, efficient, and patient-specific therapeutics. Nano-carriers, particularly liposomes, nanoparticles, and proniosomes, have dramatically shifted the scenario to better bioavailability, stability, and precision of drugs. Liposomal drug delivery system presents a higher encapsulation efficiency, controlled release, and increased circulation time; PEGylation and surface modification add these properties to targeting diseases specifically. Naso-pulmonary and oral drug delivery innovation comprises of liposomal inhalation therapy, effervescent tablets, and mouth-dissolving films. Other promising alternatives are proniosomes that show better stability and retention for drugs over liposomes and surpass many of the deficiencies associated with liposomes in practical use. Hybrid nanocarrier systems would best combine the best of two worlds for the approaches-vesicular and non-vesicular-and move multifunctional and precision-based therapeutics to prominence. Overcoming resistance by liposomal antibiotics is excellent, but herbal drug delivery through nanocarriers shows higher bioavailability along with long, arduous journeys towards clinical translation, targeting areas such as scalability, biocompatibility, and frameworks for regulation. The pipeline now includes AI-based formulations, gene-editing nanocarriers, and biomimetic drug transport systems that are certain to catapult personalized nanomedicine to the mainstream of clinical usage.
References
- Sengar, A. (2023). Targeting methods: A short review including rationale, goal, causes, strategies for targeting. International Journal of Research Publication and Reviews, 4(8), 1379-1384. ISSN 2582-7421.
- Jagrati, K. M. , & Sengar, A. (2024). Liposomal vesicular delivery system: An innovative nano carrier. World Journal of Pharmaceutical Research, 13(13), 1155-1169. [CrossRef]
- Prajapati, R. N. , Jagrati, K., Sengar, A., & Prajapati, S. K. (2024). Nanoparticles: Pioneering the future of drug delivery and beyond. World Journal of Pharmaceutical Research, 13(13), 1243-1262.
- Sengar, A. , Jagrati, K., & Khatri, S. (2024). Enhancing therapeutics: A comprehensive review on naso-pulmonary drug delivery systems for respiratory health management. World Journal of Pharmaceutical Research, 13(13), 1112-1140.
- Sengar, A. , Vashisth, H., Chatekar, V. K., Gupta, B., Thange, A. R., & Jillella, M. S. R. S. N. (2024). From concept to consumption: A comprehensive review of chewable tablets. World Journal of Pharmaceutical Research, 13(16), 176-189.
- Sengar, A. , Yadav, S., & Niranjan, S. K. (2024). Formulation and evaluation of mouth-dissolving films of propranolol hydrochloride. World Journal of Pharmaceutical Research, 13(16), 850-861.
- Sengar, A. , Tile, S. A., Sen, A., Malunjkar, S. P., Bhagat, D. T., & Thete, A. K. (2024). Effervescent tablets explored: Dosage form benefits, formulation strategies, and methodological insights. World Journal of Pharmaceutical Research, 13(18), 1424-1435.
- Sengar, A. , Saha, S., Sharma, L., Hemlata, Saindane, P. S., & Sagar, S. D. (2024). Fundamentals of proniosomes: Structure & composition, and core principles. World Journal of Pharmaceutical Research, 13(21), 1063-1071.
- Sengar, A. (2024). Precision in practice: Nanotechnology and targeted therapies for personalized care. International Journal of Advanced Nano Computing and Analytics, 3(2), 56–67.
- Sengar, A. (2025). Innovations and Mechanisms in Liposomal Drug Delivery: A Comprehensive Introduction. Preprints. [CrossRef]
- Sengar, A. (2025). "Liposomal Drug Delivery Systems: An Intro as a Primer for Advanced Therapeutics." Preprints. [CrossRef]
- Sengar, A. (2025). Targeting Strategies in Liposomal Drug Delivery. Preprints. [CrossRef]
- Sengar, A. , Chatekar, V. K., Andhare, S. B., & Sharma, L. (2025). Clinical pharmacology of antibiotics: Mechanisms, therapeutic uses, and resistance patterns. World Journal of Pharmaceutical Research, 14(1), 1531–1545. [CrossRef]
- Sengar, A.; et al. (2025). Advancing urolithiasis treatment through herbal medicine: A focus on Tribulus terrestris fruits. World Journal of Pharmaceutical Research, 14(2), 91–105.
- Sengar, A. Liposomes and beyond: Pioneering vesicular systems for drug delivery. Preprints. https://www.preprints.org/manuscript/202412.2230/v1.
- Sengar, A. (2025). Innovations and mechanisms in liposomal drug delivery: A comprehensive introduction. Journal of Emergency Medicine Open Access (J Emerg Med OA), 3(1), 01-05.
- Sengar, A. (2025). Next-generation liposomal drug delivery systems: Core principles, innovations, and targeting strategies. Preprints. [CrossRef]
- Sengar, A. (2025). Advanced Targeting Strategies and Applications of Liposomal Drug Delivery Systems. Preprints. [CrossRef]
- Sengar, A. (2025). Historical Evolution and Modern Advances in Vesicular Nanocarriers. Preprints. [CrossRef]
- Sengar, A. (2025). The Interplay of Drug Delivery Systems: A Comparative Study of Nanocarriers and Vesicular Formulations. Preprints. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).