Submitted:
10 February 2025
Posted:
11 February 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Optimization of Red Ginger Extraction and Development of Kombucha Ginger Inoculants
2.3. Fermentation of Red Ginger Kombucha
2.4. Analysis of Sugar and Organic Acid Contents by HPLC
2.5. Determination of pH and Total Acidity
2.6. Analysis of Total Polyphenol Content
2.7. Analysis of Total Flavonoid Content
2.8. Measurement of DPPH Free Radical Scavenging Activity
2.9. α-Glucosidase Inhibition Assay
2.10. Statistical Analysis
3. Result and Discussions
3.1. Analysis of Initial Red Ginger Extract Before Fermentation
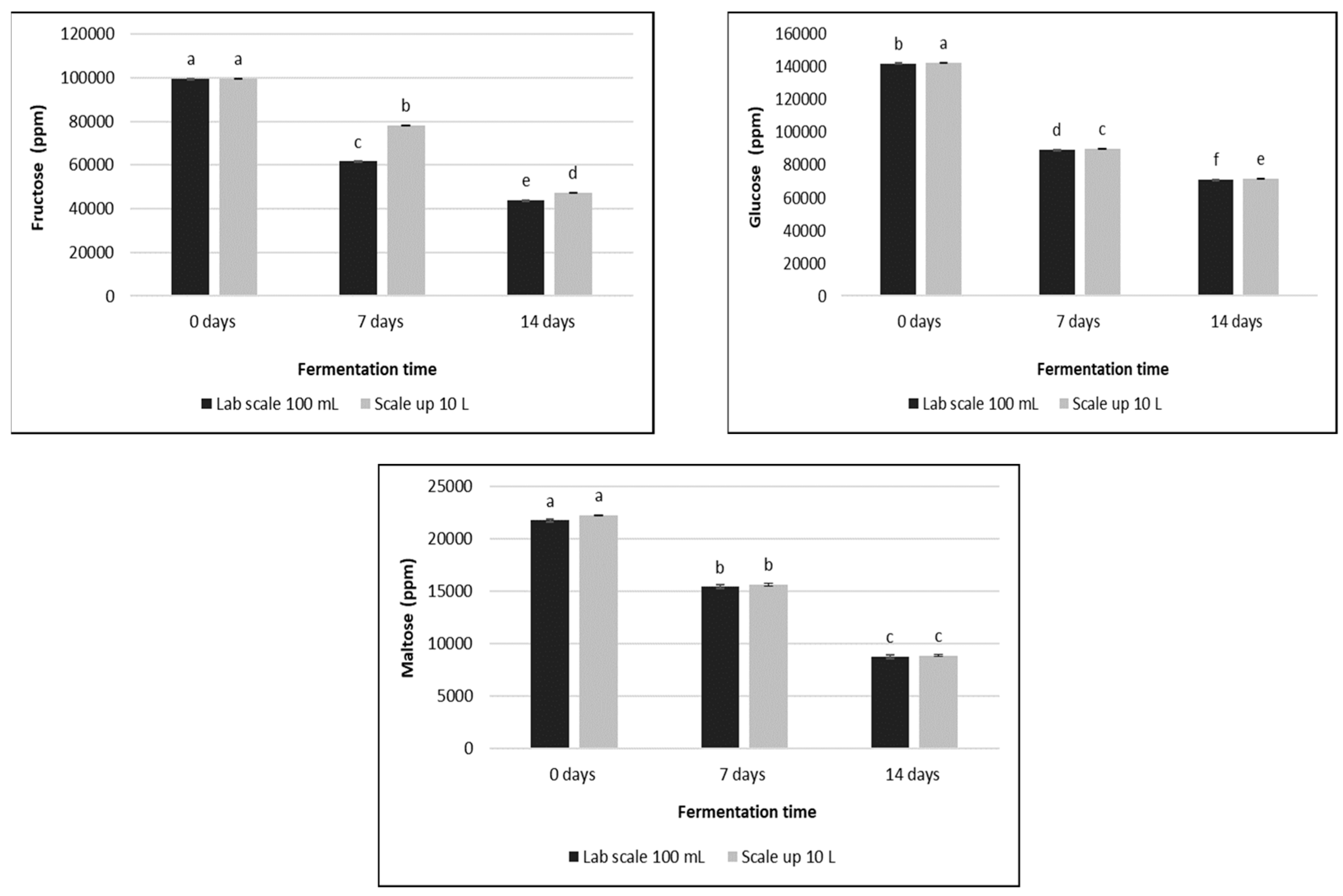
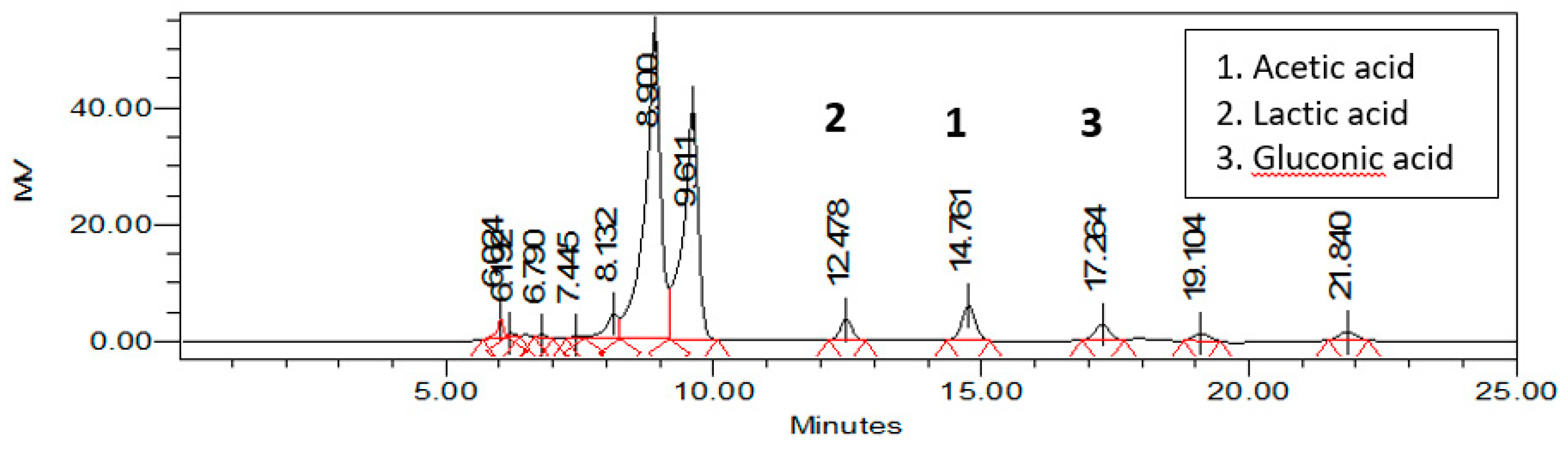
3.2. Sugars and Organic Acids Content
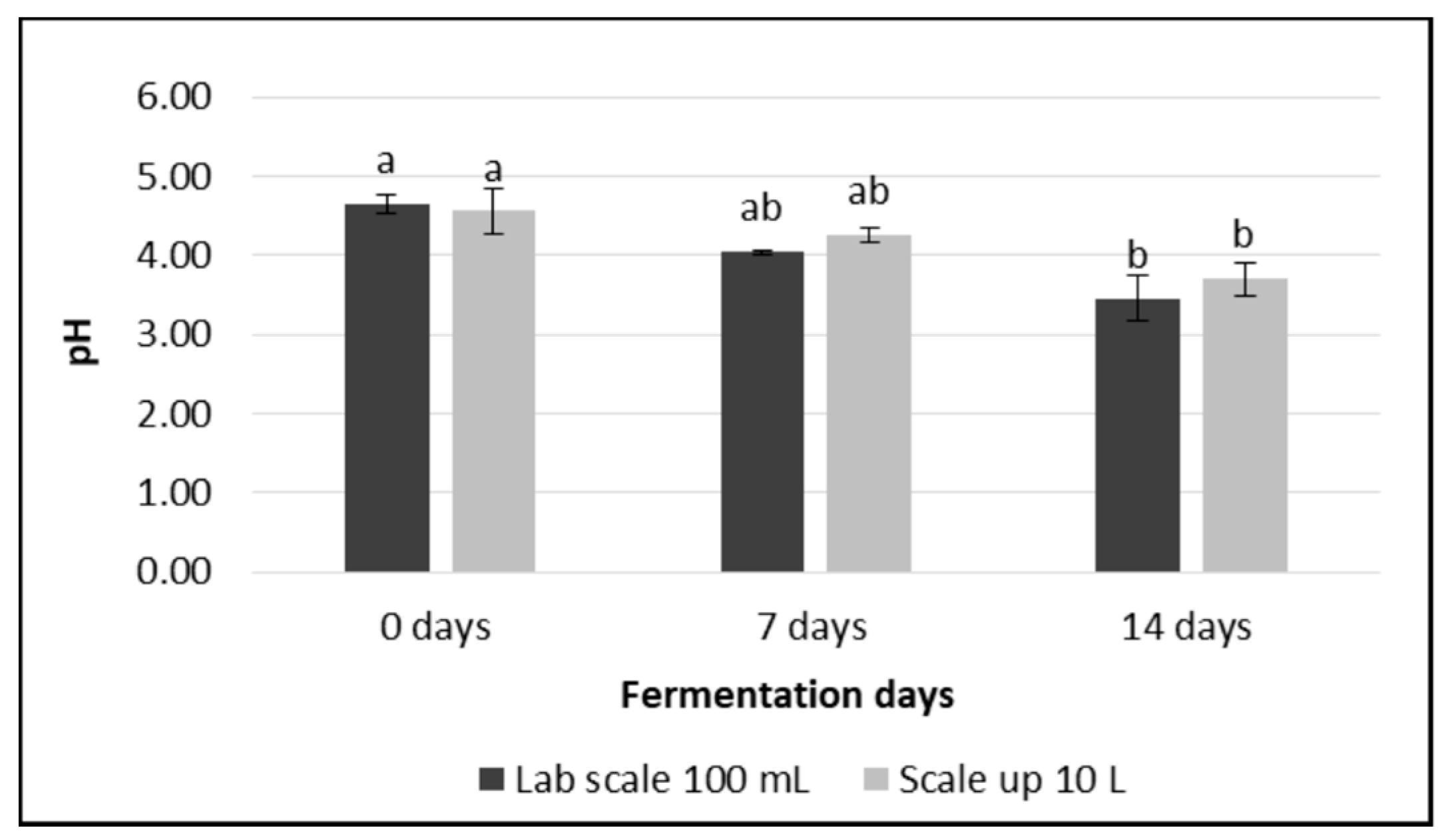
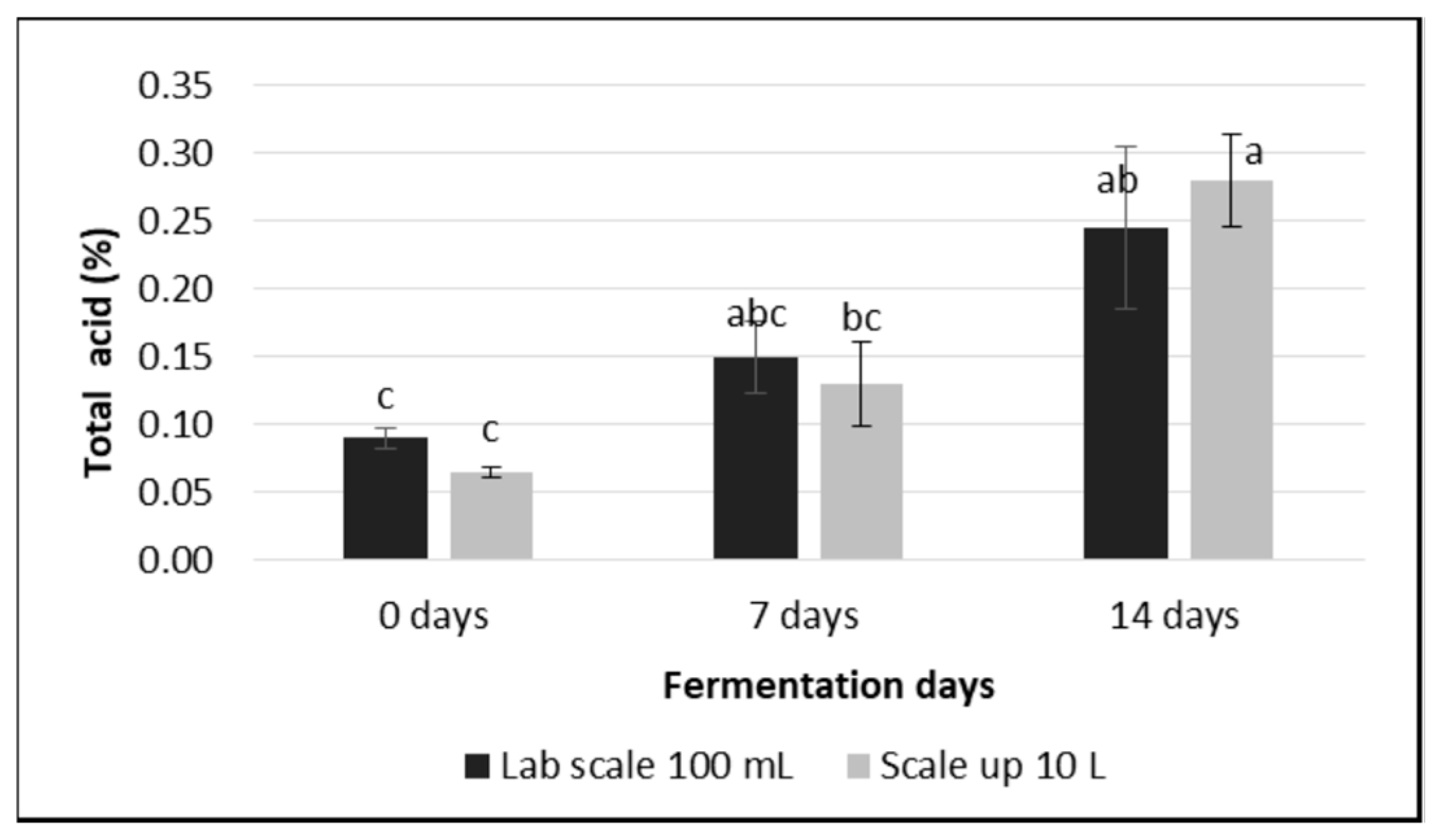
3.3. pH and Total Acid
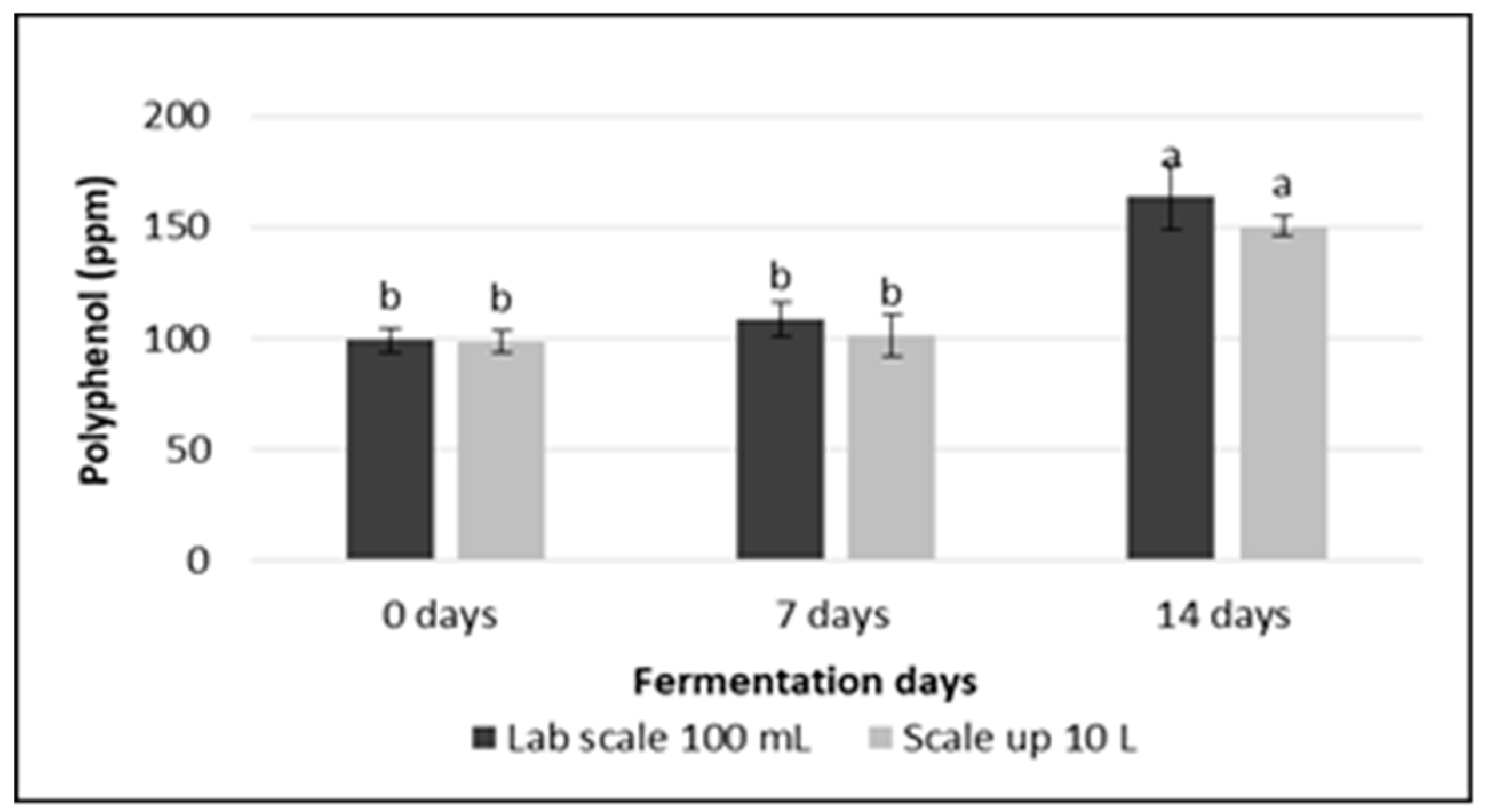
3.4. Total Polyphenol Content
3.5. Total Flavonoid Content
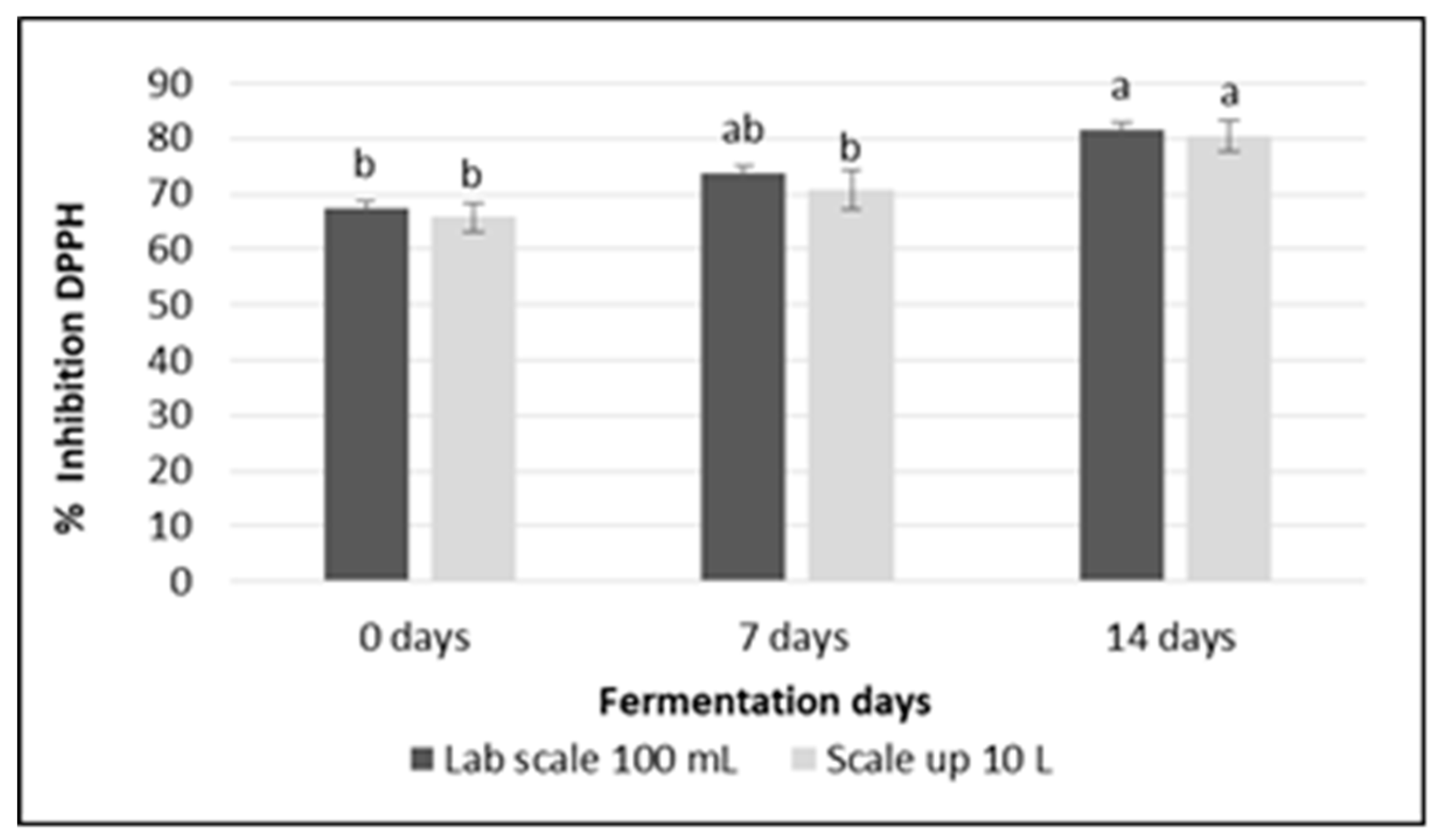
3.6. Free Radical Inhibitory Activity
4. Conclusions
Funding
Ethics information
Acknowledgements
References
- Pratami, M. P.; Anggraeni, A.; Sujarwo, W. Ethnobotany of medicinal plants in Leuwiliang (Bogor), Indonesia. Ethnobotany Res. Appl. 2024, 27, 1–41. http://dx.doi.org/10.32859/era.27.1.1-41. [CrossRef]
- Ali, K.; Flare, A.; Flinn, G. An Overview of the traditional and modern applications of ginger. JHSciRes. 2024, 4, 10–16. DOI: 10.5281/zenodo.13254798. [CrossRef]
- Zhang, S.; Kou, X.; Zhao, H.; Mak, K.-K.; Bali-jepalli M.K., Pichika, M.R. Zingiber officinale var. rubrum: red ginger’s medicinal uses. Molecules 2022, 27, 775. doi: 10.3390/molecules27030775. [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign-score method. Chemistry Central Journal 2018, 12, 82. https://doi.org/10.1186/s13065-018-0445-y. [CrossRef]
- Sharma, S.; Shukla, M.K.; Sharma, K.C.; Tirath, T.; Kumar, L.; Anal, J.M.H.; Upadhyay, S.K.; Bhattacharyya, S.; Kumar, D. Revisiting the therapeutic potential of gingerols against different pharmacological activities. Naunyn Schmiedebergs Arch Pharmacol 2022 396, 633–647. doi: 10.1007/s00210-022-02372-7. [CrossRef]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Park, M.S.; Jeong S.Y., Lee, Y.-G.J.; Shim, H.; Yasmin, T.; Kim, N.W.; Koo, Y.T.; Lee, S.H.; Paik, D.-H.; Jeong, Y.J.; Jeon, H.; Kang, S.C.; Baek, N.-I. Kang, T.H. Steamed ginger may enhance insulin secretion through KATP channel closure in pan-creatic β-cells potentially by increasing 1-dehydro-6-gingerdione content. Nutrients 2020, 12, 324. https://doi.org/10.3390/nu12020324. [CrossRef]
- Alharbi, K.S.; Nadeem, M.S.; Afzal, O.; Alzarea, S.; Altamimi, A.S.A.; Almalki, W.H.; Mubeen, B.; Iftikhar, S.; Shah, L.; Kazmi, I. Gingerol, a natural antioxidant, at-tenuates hyperglycemia and downstream complications. Metabolites 2022, 12, 1274. doi: 10.3390/metabo12121274. [CrossRef]
- William, J.; John, P.; Mumtaz, M.W.; Rashid, A.; Adnan, A.; Mukhtar, H.; Sharif, S.; Raza, S.A.; Akhtar M.T. Antioxidant activity, -glucosidase inhibition and phytochemical profiling of Hyophorbe lagenicaulis leaf extracts. PeerJ 2019, 7, e7022. DOI 10.7717/peerj.7022.
- Salehi, B.; Ata, A.; Kumar, N.V.A.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Ayatollahi, S.A.; Fokou, P.V.T.; Kobarfard, F.; Zakaria, Z.A.; Iriti, M.; Taheri, Y.; Martorell, M.; Sureda, A.; Setzer, W.N.; Du-razzo, A.; Lucarini, M.; Santini, A.; Capasso, R.; Os-trander, E.A.; Ur-Rahman, A.; Choudhary, M.I.; Cho, W.C.; Sharifi-Rad J. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. doi:10.3390/biom9100551. [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. https://doi.org/10.1016/j.annepidem.2018.11.001. [CrossRef]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends in Food Sci Technol 2020, 105, 323–333. https://doi.org/10.1016/j.tifs.2020.09.025. [CrossRef]
- Kitwetcharoen, H.; Phung, L.T.; Klanrit, P.; Thanonkeo, S.; Tippayawat, P.; Yamada, M.; Thanonkeo, P. Kombucha healthy drink—Recent advances in production, chemical composition and health benefits. Fermentation 2023, 9, 48. https://doi.org/10.3390/fermentation9010048. [CrossRef]
- Laureys, D.; Britton, S.J.; De Clippeleer, J. Kombucha tea fermentation: A review. Journal of the American Society of Brewing Chemists 2020, 78, 165–174. https://doi.org/10.1080/03610470.2020.1734150. [CrossRef]
- Selvaraj, S.; Gurumurthy K. An overview of probiotic health booster-kombucha tea. Chin. Herb. Med. 2022, 15, 27–32. doi: 10.1016/j.chmed.2022.06.010. [CrossRef]
- Maryati, Y.; Melanie, H.; Handayani, W.; Yasman Y. Bacterial cellulose production from fermented fruits and vegetables byproducts: A comprehensive study on chemical and morphological properties. Karbala International Journal of Modern Science 2024, 10, 549e563. https://doi.org/10.33640/2405-609X.3376. [CrossRef]
- Mulyani, H.; Artanti, N.; Filailla, E.; Budiari, S.; Maryati, Y.; Melanie, H.; Susilowati, A.; Yuniati, R.; Yasman, Y. Effect of fermented red ginger (Zingiber officinale var. rubrum) using kombucha culture toward free radical scavenging activity. AIP Conf Proc 2023, 2902, 060022. https://doi.org/10.1063/5.0173149. [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem Toxicol 2013, 60, 328–340. https://doi.org/10.1016/j.fct.2013.07.051. [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int J Food Microbiol 2016, 220, 63–72. https://doi.org/10.1016/j.ijfoodmicro.2015.12.015. [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Es-calante-Aburto, A. Recent evidence of the beneficial effects associated with glucuronic acid contained in kombucha beverages. Curr Nutr Rep 2020, 9, 163–170. DOI: 10.19080/AIBM.2017.03.555614. [CrossRef]
- Zahn, J.A. Scale-up and optimization of natural product fermentation processes using mass-guided metabolite fingerprinting. Adv Biotech & Micro 2017, 3, 555614 pp 68–75. DOI: 10.19080/AIBM.2017.03.555614. [CrossRef]
- Schneider, A.; Gerbi, V.; Redoglia, M. A rapid HPLC method for separation and determination of major organic acids in grape musts and wines. Am. J. Enol. Vitic. 1987, 38, 151–155.
- Andreson, M.; Kazantseva, J.; Kuldjarv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.; Vilu, R. Characterisation of chemical, microbial and sensory profiles of commer-cial kombuchas. International Journal of Food Microbiology 2022, 373, 109715. https://doi.org/10.1016/j.ijfoodmicro.2022.109715. [CrossRef]
- Ahmed, R.F.; Hikal, M.S.; Abou-Taleb, K.A. Biological, chemical and antioxidant activities of different types kombucha. Annals of Agricultural Sciences 2020, 65, 35–41. https://doi.org/10.1016/j.aoas.2020.04.001. [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymol. 1999, 299, 152–178. https://doi.org/10.1016/S0076-6879(99)99017-1. [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. Journal of Food and Drug Analysis 2002, 10, 178–182. https://doi.org/10.38212/2224-6614.2748. [CrossRef]
- Sukweenadhi, J.; Yunita, O.; Setiawan, F.; Kartini, K.; Siagian, M.T.; Danduru, A.P.; Avanti, C. Antioxidant activity screening of seven Indonesian herbal extract. Biodiversitas 2020, 21, 2062–2067. DOI:10.13057/biodiv/d210532. [CrossRef]
- Kim, Y.M.; Wang, M.H.; Rhee, H.I. A novel α-glucosidase inhibitor from pine bark. Carbohyd Res 2004, 339, 715–717. https://doi.org/10.1016/j.carres.2003.11.005. [CrossRef]
- Shareef, H.K.; Muhammed, H.J.; Hussein, H.M.; Hameed, I.H. Antibacterial effect of ginger (Zingiber officinale) roscoe and bioactive chemical analysis using gas chromatography mass spectrum. Oriental Journal of Chemistry 2016, 32, 817–837. http://dx.doi.org/10.13005/ojc/320207. [CrossRef]
- Mao, Q.-Q; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. https://doi.org/10.3390/foods8060185. [CrossRef]
- Adriani, L.; Mayasari, N.; Angga, A.; Kartasudjana, R. The effect of feeding fermented kombucha tea on HLD, LDL and total cholesterol levels in the duck bloods. Biotechnology in Animal Husbandry 2011, 27, 1749–1755. https://doi.org/10.2298/BAH1104749A. [CrossRef]
- Wang, S.; Li, C.; Wang, Y.; Wang, S.; Zou, Y.; Sun, Z.; Yuan, L. Changes on physiochemical properties and volatile compounds of Chinese kombucha during fermentation. Food Bioscience 2023, 55, 103029. https://doi.org/10.1016/j.fbio.2023.103029. [CrossRef]
- Aung, T.; Eun, J.B. Production and characterization of a novel beverage from laver (Porphyra dentata) through fermentation with kombucha consortium. Food Chemistry 2021, 350, 129274. https://doi.org/10.1016/j.foodchem.2021.129274. [CrossRef]
- Li, S.; Zhang, Y.; Gao, J.; Li, T.; Li, H.; Mastroyannis, A.; He, S.; Rahaman, A.; Chang, K. Effect of fermentation time on physiochemical properties of kombucha produced from different teas and fruits: Comparative study. Journal of Food Quality 2022, 2022, 2342954. https://doi.org/10.1155/2022/2342954. [CrossRef]
- Yang, L.; Lübeck, M.; Souroullas, K.; Lübeck, P.S. Co-consumption of glucose and xylose for organic acid production by Aspergillus carbonarius cultivated in wheat straw hydrolysate. World J Microbiol Biotechnol 2016, 32, 57. https://doi.org/10.1007/s11274-016-2025-4. [CrossRef]
- Ma, Y.; Li, B.; Zhang, X.; Wang, C.; Chen, W. Production of gluconic acid and its derivatives by microbial fermentation: Process improvement based on integrated routes. Front. Bioeng. Biotechnol. 2022, 10, 864787. doi: 10.3389/fbioe.2022.864787. [CrossRef]
- Ardheniati, M.; Andriani, M.A.M.; Amanto, B.S. Fermentation kinetics in kombucha tea with tea kind variation based on its processing. Jurnal Biofarmasi 2009, 7, 48–55. https://doi.org/10.13057/biofar/f070106. [CrossRef]
- Pratiwi, A.; Elfita, E.; Aryawati, R. Pengaruh waktu fermentasi terhadap sifat fisik dan kimia pada pembuatan minuman kombucha dari rumput laut Sargasssum sp. Maspari Journal: Marine Science Research 2012, 4, 131–136.
- Bhattacharya, S.; Prasenjit, M.; Gachhui, R.; Sil, P.C. Protective effect of kombucha tea against tertiary butyl hydrperoxide induced cytotoxicity and cell death in murine hepatocytes. Indian J Exp Biol 2011, 49, 511–524.
- Santamaría, L.; Reverón, I.; de Felipe, F.L.; de las Rivas, B.; Muñoz, R. Ethylphenol formation by Lactobacillus plantarum: Identification of the enzyme involved in the reduction of vinylphenols. Appl Environ Microbiol 2018, 84, e01064-18. https://doi.org/10.1128/AEM.01064-187. [CrossRef]
- Sinir, G.Ö.; Tamer, C.E.; Suna, S. 10 - Kombucha: A Promising Fermented Functional Beverage, A.M. Grumezescu, A.M. Holban (Eds.), Fermented Beverages, Volume 5: The Science of Beverages, Woodhead Publishing, Sawston UK, 2019: pp 401–432. https://doi.org/10.1016/C2017-0-02379-0. [CrossRef]
- Sova, M.; Saso, L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites, Nutrients 2020, 12, 2190. doi:10.3390/nu12082190. [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D'Al-meida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; de Barros, F.A.R. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Research International 2020, 128, 108782. https://doi.org/10.1016/j.foodres.2019.108782. [CrossRef]
- Martínez-Leal, J.; Ponce-García, N.; Es-calante-Aburto, A. Recent evidence of the beneficial effects associated with glucuronic acid contained in kombucha beverages. Curr Nutr Rep 2020, 9, 163–170.
- Simanjuntak, D.H.; Herpandi, H.; Lestari, S.D. Chemical characteristics and antioxidant activity of water lettuce (Pistia straiotes) leaves kombucha during fermentation. Jurnal Teknologi Hasil Perikanan 2016, 5, 123–133. https://doi.org/10.36706/fishtech.v5i2.3940. [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent advances in the conjugation approaches for enhancing the bioavailability of polyphenols. Food Hydrocolloids 2024, 146, 109221. https://doi.org/10.1016/j.foodhyd.2023.109221. [CrossRef]
- de Oliveira, P.V.; da Silva Júnior, A.H.; de Oliveira, C.R.S.; Assumpção, C.F.; Ogeda, C.H. Kombucha benefits, risks and regulatory frameworks: A review. Food Chemistry Advances 2 (2023) 100288. https://doi.org/10.1016/j.focha.2023.100288. [CrossRef]
- Thummala, S.; Khrisna, M.K.; Natarajan, A. Uppala. S. Antihyperglycaemic efficacy of kombucha in streptozotocin-induced rats. Journal of Functional Foods 2013, 5, 1794–1802. https://doi.org/10.1016/j.jff.2013.08.008. [CrossRef]
- Maryam, G.-D.; Hossein, A.-K.; Zahra, L.; Mahmoud, R.-K. Oxidative stress and antioxidants in diabetes mellitus. Asian Pacific Journal of Tropical Medicine 2020, 13, 431–438. DOI: 10.4103/1995-7645.291036. [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radical Biology and Medicine 2011, 50, 567–575. https://doi.org/10.1016/j.freeradbiomed.2010.12.006. [CrossRef]
- Mendelson, C.; Sparkes, S.; Merenstein, D.J.; Christensen, C.; Sharma, V.; Desale, S.; Auchtung, J.M.; Kok, C.R.; Hallen-Adams, H.E.; Hutkins, R. Kombucha tea as an anti-hyperglycemic agent in humans with diabetes – a randomized controlled pilot investigation. Front Nutr. 2023, 10, 1190248. doi: 10.3389/fnut.2023.1190248. [CrossRef]

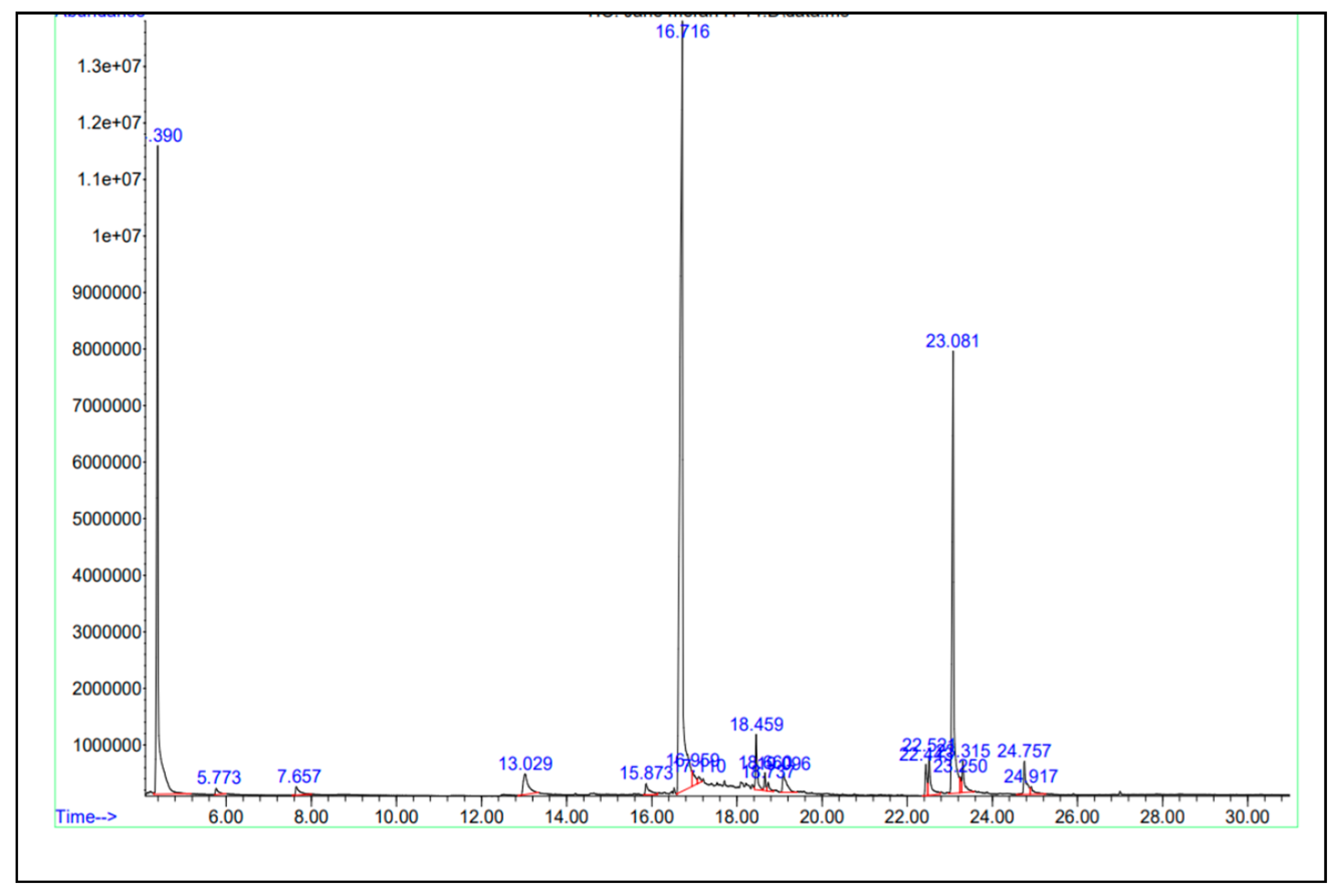
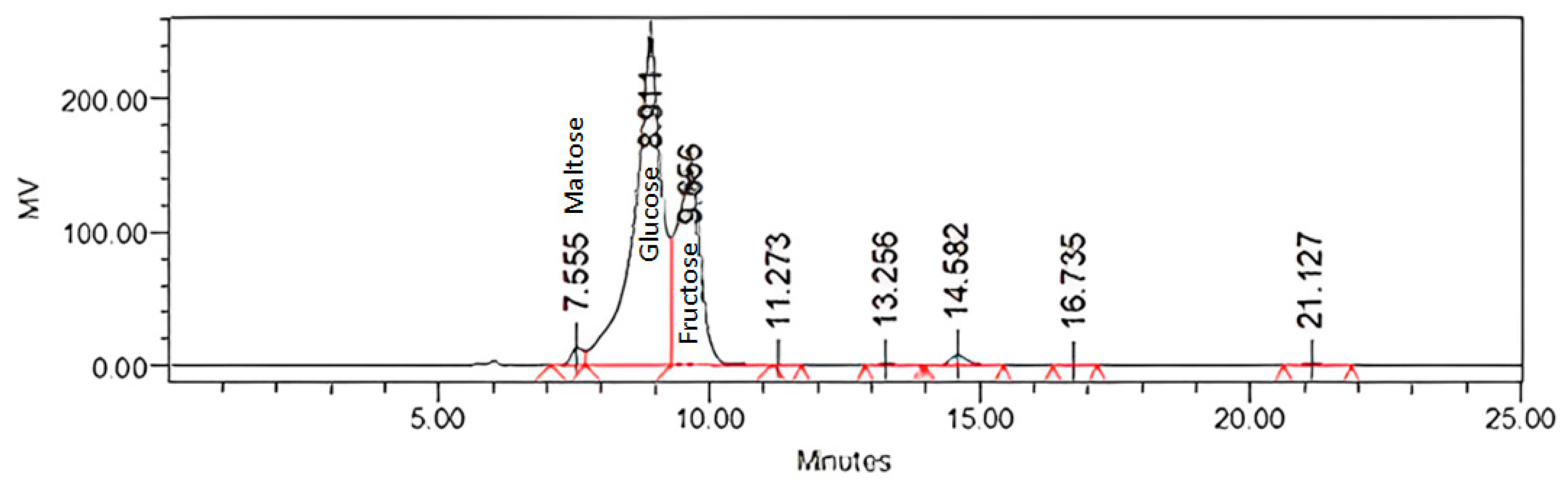



| Peaks | Retention time (minute) |
Area under the peak (%) |
Formula | Molecular weight | compound name | Similarity (%) |
|---|---|---|---|---|---|---|
| 1 | 16.716 | 46.21 | C11H14O3 | 194 | Butan-2-one, 4-(3-hydroxy-2-methoxyphenyl) | 98 |
| 2 | 22.443 | 1.01 | C17H24O3 | 276 | (6)-Isoshogaol | 97 |
| 3 | 22.524 | 1.65 | C17H26O3 | 278 | 6-Paradol | 96 |
| 4 | 23.081 | 18.62 | C17H24O3 | 276 | 6-Shogaol | 99 |
| 5 | 23.315 | 1.60 | C19H28O6 | 352 | (4)-Gingerdiol 3,5-diacetate | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





