Introduction
Precise sequential and spatial activation of the Cdk1 protein kinase is essential for correct entry of cells into mitosis [
1]. This process involves formation of a complex between Cdk1 and its activator Cyclin B1 [
1], and removal of inhibitory phosphates on the kinase residues Threonine 14 and Tyrosine 15 by the phosphatase Cdc25 [
1]. In this respect, Cdk1 can be activated by three Cdc25 isoforms [
1], being Cdc25B the first one that acts on the kinase at the centrosome [
2,
3], and the other two the ones that amplify the centrosomal signal in the cytosol and the nucleus [
1].
Full activation of Cdk1 in the nucleus at late prophase is considered a
point-of-no-return in mitosis [
4]. Here, the nuclear lamina is disassembled as a result of Cdk1 phosphorylation of the Lamin B subunits, triggering the nuclear membrane breakdown (NMBD) [
5,
6,
7]. As a result cytosolic microtubules are able to reach the kinetochores on the chromosomes, coinciding with cells entering prometaphase [
8,
9]. This so-called
mitotic commitment, which requires high levels of Cdk1 activity in the nucleus [
10,
11], when erroneously triggered leads to
mitotic catastrophe and cell death [
12].
On a related aspect of mitosis, it is well known the role of the Survivin protein in establishing proper microtubule-kinetochore attachments in prometaphase as part of the
Chromosome Passenger Complex (CPC) [
13]. Here, errors often lead to multiple mitotic defects and sustained spindle checkpoint [
14,
15,
16,
17] that when overridden, can cause chromosome mis-segregation and aneuploidy, both typical features of many tumors [
13].
Survivin has also been localized at the centrosome [
18] but its role at this non-membranous organelle has not been elucidated yet. In this regard, Survivin depletion causes a
mini spindle phenotype in
Xenopus egg extracts [
17] and HeLa cells [
16,
19], which seems to indicate a failure in centrosome separation, an early mitotic event for which centrosomal Cdk1 activity is required through its effect on Eg5 [
20,
21]. These data, and the fact that Survivin binds to both Cdk1 [
22] and microtubules [
17,
23], seem to suggest a role of Survivin in the correct operation of the main mitotic kinase at the centrosome, which would place the former protein at the hub where decisions to initiate mitosis are made, however, challenge the current model, which envisions Survivin as only acting in mitosis via the CPC [
14,
15]. Surprisingly, despite the interest of this theory, the role of Survivin at an earlier time than prometaphase has not been investigated so far. Therefore, with the intention to unravel the function of Survivin at mitotic onset in cancer cells, I decided to revisit the early mitotic events that follow abrogation of the Survivin protein in HeLa cell cultures.
Discussion
The current model of Survivin function assigns two roles to this protein, namely: i. Its mediation in the correction of faulty microtubule-kinetochore attachments, and ii. the process of cytokinesis, both as part of the CPC [
13]. In agreement with this, it is firmly believed that the first abnormal phenotype, following the interference with Survivin protein, is a prometaphase blockage that responds to errors in the repair of incorrect microtubule-kinetochore connections [
14,
15]. In this regard, before this project started, I was aware of experiments carried out with
Xenopus egg extracts [
17] and HeLa cells [
16,
19], which revealed that, following Survivin abrogation, a peculiar phenotype ensues, consisting of spindles with a very short pole-to-pole distance, also called
mini spindles, which pointed out to a failure in centrosome separation. Here, I believed that these results could poorly be explained by the sole role of the Survivin protein in the CPC since this complex had not been reported to have a function so early in mitosis [
13]. A more plausible explanation here might be a role of Survivin in this organelle’s splitting at early mitosis, an event for which Cdk1 is necessary [
20,
21]. In support of this model, it has been reported that interference with Survivin leads to centrosomal abnormalities, and the disruption of a Survivin-Caspase-3-p21 complex at this location [
18]. In this respect, if Survivin had a role in centrosomal Cdk1 activation, this would place this protein at the hub where decisions to enter mitosis are made, prior to its CPC function, explain why cancer cells show preference for taking over this pathway, and might also provide the explanation for the connection between Survivin and cell death, as a default centrosomal-based apoptotic route [
18] triggered when mitotic onset misfires. This possible new mitotic role of Survivin, however interesting, had never been pursued before, and therefore, I decided to make it the center of my investigation.
Survivin Is Required to Activate Cdk1 in Early Prophase and for Cancer Cells to Commit to Mitosis
To start this project, I first wanted to clarify when cells that lack Survivin first arrest in mitosis. For this purpose, I repeated the RNAi experiments carried out by others in HeLa cells, which led them to conclude that, in the absence of Survivin, a prometaphase blockage ensues [
14,
15]. To my surprise, when I replicated this work, I not only could see less cells entering mitosis (
Figure 1D) but those, which managed to progress into this stage, being blocked at early prophase instead of prometaphase. The evidence supporting this finding in the Survivin-depleted samples was ample, and encompassed: i. The presence of a microtubule ring surrounding their chromatin (
Figure 1B,C, and
Figure 2A,B), ii. the absence of a spindle or the presence of a very faint one (
Figure 1C,
Figure 2A and
Figure S1C), iii. the detection of an intact nuclear lamina (
Figure 2A,B), iv. the partially condensed chromatin (
Figure 1C and
Figure 2A,B), v. the lower amount of Cdk1 activity, and vi. the detection of the microtubule ring and intact nuclear lamina also in cells incubated with the Cdk1 inhibitor Purvalanol (
Figure 2D). From this data, I concluded that Survivin has a role upstream of its CPC function at the level of early Cdk1 activation, an event which occurs in early prophase at the centrosome [
2,
3]. This conclusion was further supported by comparing the activity of Cdk1 in the absence of Survivin, and that of cells incubated with a real prometaphase blocker such as nocodazole [
28] (
Figure 3B).
The results obtained with asynchronous HeLa cultures could be reproduced with synchronous cells. Here, a much more robust mitotic blockage, and declining Cdk1 activity was observed in the cells depleted of Survivin (
Figure 4B,C). From this data, I could easily infer that the mitotic cells that accumulated in the G2/M-phase peak in the absence of Survivin had to be in either G2 or early prophase (i.e., stable checkpoint), as their asynchronous counterparts, since if these cells had committed to mitosis (i.e., prometaphase) in the absence of Cdk1 activity (
Figure 4C), they would have averted the spindle checkpoint due to their low Cdk1 activity, and progressed into a new round of DNA synthesis, as HeLa cells possess very low levels of the p53 tumor suppressor [
41], and therefore lack an effective G1 checkpoint [
42].
The use of the Survivin double mutant in these studies provided a more precise description of the mitotic anomalies that occur when the Survivin’s function is impaired in cells about to enter mitosis. First, these results showed that Survivin is crucial to commit to mitosis (i.e., transition from prophase to prometaphase), as demonstrated by the accumulation of flat or large rounded cells when the SUR D70A/D71A mutant was transfected (
Figure 7A,E,F). Interestingly, these large rounded mitotic cells were already observed in the siRNA studies, and assigned to early prophase (see above explanation). Since in these two cases, this phenotype coincided with a low Cdk1 activity (
Figure 3A and
Figure 7C), and it is well know the need of Cdk1 activation for cells to commit to mitosis, it was only logical to conclude that the impairment in entering mitosis when using either of the above mentioned Survivin antagonists was probably due to the impossibility of the transfected cells to mount a high enough Cdk1 activity with which to transverse the G2/M-phase checkpoint. Second, the Survivin double mutant experiments also showed that in the absence of a functional Survivin protein, and despite their low Cdk1 activity, some cells can still transverse the G2/M-phase checkpoint (33%, see
Figure 7E,
bottom), however, in most cases, they stall or die at this stage. Similar results were also obtained by other researchers in human cells when interfering with their Cdk1 levels via RNAi [
43]. In this case, 20% of cells still could enter mitosis, however, these Cdk1-attenuated cells were impaired in their ability to phosphorylate their mitotic targets. At this point, it is impossible not to think about the targets that Survivin has in cells entering prometaphase and proceeding into cytokinesis [
13], and conclude that all the abnormalities due to the Survivin double mutant seen at this late mitotic stages should be attributed to the role of this protein at the CPC. Focussing on prometaphase however, we should not forget about the extended regulation exerted by Cdk1 on the CPC at this phase [
13,
44,
45,
46], and conclude that maybe, we should start considering the abnormal phenotype caused by Survivin impairment at prometaphase more as a combined outcome due to both CPC malfunction (i.e., Survivin’s traditional role) and Cdk1 misactivation (i.e., new role of Survivin proposed in this paper). Briefly, INCENP is phosphorylated by Cdk1, which regulates the localization and kinase activity of Aurora-B [
44]. Second, Plk1 binds to Haspin in a Cdk1-dependent manner, and reducing Plk1 activity inhibits Haspin phosphorylation of Histone H3 [
45]. Third, Survivin binds to phosphorylated Histone H3, and this step is necessary for CPC accumulation at the centromere [
46]. Interestingly, in this same work, an identical mutant as the one I used in my studies, SUR D70A/D71A, showed no binding to a phosphorylated Histone H3 peptide in vitro, implicating the same two residues, Asp70 and Asp71, in both Cdk1 activation and Survivin centromeric localization, and providing a clever mechanism for Survivin to exert its two functions in a sequential accurate manner. Finally, Borealin, a Survivin-binding partner at the centromere, is phosphorylated by Cdk1, which allows it to bind to Shughosin, and subsequently phosphorylate Histone H2A [
13]. From all the above evidence and the data shown in this paper, it seems fair to conclude that Survivin appears to have an undiscovered role on the CPC via its regulation of the Cdk1 kinase.
Centrosomal Cdk1 Does Not Accumulate in the Absence of Survivin, and it May Cause a Short-Circuit between this Kinase and its Activators
Cdk1 is activated by the dual-specificity phosphatase Cdc25 [
1], with isoform B being the first one that acts on Cdk1 at the centrosome during early prophase [
2,
3], and isoforms A and C being subsequently active at the cytosol and nucleus in a process that amplifies the initial kinase activity [
1], and commits cells to mitosis [
10,
11]. The data in this article now shows that when the Survivin function was compromised in HeLa cells that had not entered mitosis yet, these samples arrested at early prophase (see above discussion), pointing out to a short-circuit in the centrosomal Cdc25B-Cdk1 axis according to the literature. In agreement with this model, the Cdk1 amount and activity at this organelle were greatly reduced in the Survivin-depleted samples (
Figure 8B,C). Interestingly, still a low quantity and activity of centrosomal Cdk1 protein could be seen at early time points that correlated with an also unexpected Cdk1 activity at 12 h in cytosolic extracts from Survivin siRNA-treated samples. This early kinase activity might be due to the initial Cdk1-Cyclin A complex, which escapes Wee1/Myt1-inhibitory phosphorylation, and is required to activate Aurora A [
47], itself the activator of Cdc25B at the centrosome [
48], before Cdk1-Cyclin B1 activity can be triggered in this organelle. The biochemical centrosomal data could be confirmed by immunofluorescence experiments, which also showed a reduced amount of Cdk1 at this location and an unsplitted kinase signal (
Figure 8D), which is indicative of lack of Cdk1 activity at this organelle [
20,
21]. As a conclusion, from all the centrosomal results, I initially envisioned Survivin probably acting as some kind of bridge that could facilitate the activation of Cdk1 via Cdc25B at the centrosome, being that role the reason why, in its absence, cells stalled at early prophase.
Survivin Activates the Cdk1 Kinase via Phosphatase Cdc25B
Apart from the centrosomal Cdk1 only being active in the presence of Survivin, I could detect a fast migrating Cdk1 band, which was previously assigned to the active kinase [
27], being bound to Survivin in the same location (
Figure 8A). This result pointed at Cdc25B being responsible for this effect, and could also be replicated in cytosolic extracts, which also contained centrosomes, of HeLa cells treated with the control siRNA (
Figure 9B). In contrast, similar lysates of Survivin-depleted cells had a much lower amount of the active Cdk1 form and more of the inactive isoform in comparison with the control. The Cdk1 protein in the Survivin-depleted samples had to be cytosolic as hardly any kinase was found in the centrosomes of cells depleted of Survivin (
Figure 8B,C). Interestingly, the putatively Cdc25B-induced shift between the inactive and active Cdk1 forms could be induced in a cell-free assay where Survivin was depleted from mitotic lysates, and then added back (
Figure 9C). Here, I could see that this shift correlated with the recombinant Survivin protein being bound to Cdk1. The accumulation of the faster migrating active Cdk1 protein could also be reproduced in a dose-dependent manner using interphase extracts (
Figure 9D) that phosphorylated Histone H1 (
Figure 9E), a result that might indicate an unknown function of Survivin at interphase that could be behind the cell death observed in cells expressing the Survivin double mutant while progressing towards the G2/M-phase checkpoint (
Figure 7E,
bottom).
Although, I initially supported a role for Survivin as a bridge between Cdc25B and Cdk1 at the centrosome, I discarded this possibility when I could only detect a Cdc25B-Cdk1-Cyclin B1 complex in lysates of HeLa cells treated with the Survivin siRNA (
Figure 10B). As already mentioned above, this complex could only be cytosolic. Also, according to the Histone H1 phosphorylation data (
Figure 3A and
Figure 9A), the Cdc25B bound to Cdk1 in the cytosolic complex had to be inactive. Indeed, when I looked at this immunoprecipitate in detail, I could see the accumulation of the slow-migrating Cdk1 band previously ascribed to the inactive kinase [
27,
35]. The conclusion of Cdc25B being inactive in the absence of Survivin was corroborated by directly measuring the Cdc25B activity in samples where this protein was ablated (
Figure 10D–F). As a consequence of these results, I opted for a new model where Survivin might act as some kind of centrosomal anchor or scaffold for the inactive Cdc25B-Cdk1-Cyclin B1 complex, which by this means, could come close to its centrosomal activator/s.
In the absence of Survivin, Cdc25B somehow accumulated (
Figure 10B,C,F and
Figure 11B), and remained in complex with the Cdk1 protein for a longer period of time (
Figure 10F). This result was very different to what was reported before about this protein’s short stability [
49]. Moreover, Cdc25B normally accumulates at G2, and its activity precedes Cdk1 activity, followed by both events rapidly declining after G2/M-phase [
49,
50,
51] (a result also reproduced in my control experiment (
Figure 10F)), as a consequence of Cdk1-dependent proteasomal degradation [
52].
Overexpression of Cdc25B normally induces mitotic entry [
39,
53]. However, when the recombinant Cdc25B phosphatase was expressed in the Survivin-depleted G2/M-phase blocked HeLa cells, this protein could not rescue the blockage (
Figure 11A,
right), confirming that Survivin is required to activate Cdc25B. In agreement with this theory, when a gain-of-function Cdc25B mutant was expressed in G2/M-phase cells lacking Survivin, they managed to enter mitosis (
Figure 11B), supporting a role for Survivin in signaling through the Cdc25B-Cdk1 axis.
Early Prophase Physiognomy and Reversibility
Following the accumulation of data in this project, it was clear that the Survivin protein is necessary to assemble a functional Cdk1 complex in early prophase. This was a valid conclusion except for the fact that a lower than expected number of rounded cells was observed following the treatment with the different Survivin antagonists. Searching through the literature to find an explanation for this observation, I discovered that early prophase cells do not always look round, but on the contrary, many times remain spread, resembling the physiognomy of interphase or G2 cells [
10,
11]. The choice between these two different appearances seems to rely on the amount of Cdk1 activity in the nucleus. Accordingly, it was quite possible that many of the cells that looked like being in G2, or interphase, following interference with the Survivin function, were actually in early prophase.
Early prophase is a reversible phase, and this reversibility can be triggered by multiple insults. Antephase is the stage to which cells resort following the activation of a G2/M-phase checkpoint branch that responds to stress insults, and is mediated by p38 [
54]. This checkpoint is different to the one triggered by DNA damage, which depends on ATM and/or ATR activity [
55]. Therefore, I would also speculate here that many of the cells that entered mitosis with a low Cdk1 activity as a result of Survivin interference, might have activated their antephase rather than their DNA damage checkpoint, and as a consequence returned to G2, hoping to re-enter mitosis at a better time. This was precisely what could be seen with some of the Survivin double mutant- transfected cells (
Figure 7F). The antephase checkpoint only works while the activity of Cdk1 is not very high, or before nucleolar breakdown [
54]. This would explain why cells that were treated with the Survivin peptide could not return to G2, and continued into the apoptotic program (see below).
Interference with Committed Mitotic Cells via a Cdk1-Binding Survivin Peptide Results in Apoptosis
The Survivin peptide was made with the intention to interfere with the assembly of the Survivin-Cdk1 complex. As expected, incubation of HeLa cell lysates from nocodazole-treated HeLa cells (i.e., prometaphase) with this reagent caused complex disassembly, and loss of Cdk1 activity (
Figure S4B), pointing at Survivin binding being necessary for Cdk1 not only to activate but to remain active.
Interestingly, the Survivin peptide did not cause a G2/prophase blockage, as seen with the other two antagonists of this protein used in this work (i.e., Survivin siRNA and double mutant), and this might have been due to the short half-life of peptides in general, which would have allowed cells approaching mitotic entry in the presence of this reagent to briefly halt at the G2/M-phase checkpoint until the peptide cleared up. A brief G2/M-phase blockage would be difficult to detect by FACS analysis, and this is probably why I could not observe this abnormal phenotype under these conditions. In contrast, once transitioning into prometaphase, a stage at which the Cdk1 kinase is fully active [
10,
11], the presence of the Survivin peptide in cells should have been very troublesome, since at this stage and moving forward both Cdk1 and Survivin are required for events that normally occur fairly rapidly such as spindle formation (17, 20, 21, 56, 57), CPC functioning [
13,
44,
45,
46] and decisions on cell fate [
12,
18]. Here, a short-life potent antagonist of Survivin and Cdk1 function should indeed have a strong effect, and these precisely were the places at which anomalies were detected when committed mitotic HeLa cells were incubated with the Survivin peptide.
Specifically on the cell death caused by the Survivin peptide, it should be noticed that this phenotype was not or minimally observed when the other two Survivin interfering reagents were used in this study. The reason for this might have been the possibility for cells to find refuge in the G2/M-phase checkpoint when subjected to slow-accumulating Survivin antagonists. In support of this theory and the vulnerability of tumoral cells committed to mitosis (i.e., nuclear Cdk1 activation and progression into prometaphase), which are then targeted at their Cdk1 function, cancer cells and tumors in a cancer xenograft model were very sensitive to a combination of taxol and purvalanol A (i.e prometaphase blockage followed by Cdk1 targeting) but not to the reverse order of the treatment (i.e., Cdk1 inhibition leading to G2/M-phase blockage, and therefore lack of effectiveness of a prometaphase drug such as taxol) [
58], indicating that when challenged cancer cells are allowed to rest at the G2/M-phase checkpoint, they can avoid cell death.
More specifically on the cell death observed when HeLa cells were treated with the Survivin peptide, it has been reported that Cdk1-Cyclin B1 activity is required to phosphorylate Procaspase 8 [
59] and Procaspase 9 [
60], and subsequently block the extrinsic [
59] and intrinsic [
60] apoptotic pathways in cancer cell lines. Here, blockage of Cdk1 activity by Cyclin B1 RNAi or the Cdk1 inhibitor RO-3306 [
59], or short-circuit of the kinase by the expression of non-phosphorylatable caspase proforms [
59,
60], led to apoptosis. In agreement with these results, I could also detect Caspase 8 and 9 activity being triggered in HeLa cells after treatment with the Survivin peptide (see
Figure 6C,D). From these results, the Cdk1 kinase rises as some kind of mitotic shield but also, as a factor, which might contribute to tumorigenesis if deregulated. Traditionally, these have been roles assigned to the Survivin protein, and here, a partner in causing these phenotypes may have been found.
There has been a lot of controversy regarding the two seemingly roles of Survivin in cancer regulation, namely: the one in mitosis and the one in apoptosis (for reviews on these two different views see 61 and 62). In this respect, I propose here a model, which attempts to integrate these two areas of research. Herein, Survivin’s initial function would be to initiate mitotic onset by contributing to the activation of Cdk1 at the centrosome. At this stage, interference with Survivin would not lead to fatal consequences, as cells would be able to wait at the G2/M-phase checkpoint until being fit to move on. In contrast, sustained Survivin malfunction in arrested cells resulting in bypass of this checkpoint, or interference with this protein in committed mitotic cells (i.e., prometaphase) that require high Cdk1 activity and a functional CPC, both functions regulated by Survivin, would lead to a myriad of mitotic defects and ultimately cell death.
Survivin Binds to the αC/β4 Loop in Cdk1, a Region Involved in Protein-Protein Interactions, Binding of the Hsp90-Cdc37 Complex and Regulation of Kinases’ Activity
The Survivin peptide that binds to Cdk1 (
Figure S3A and Figure S3C) comprises several negatively-charged residues that reside on the Survivin dimer’s acidic surface [
32]. In fact, even though I used the SUR D70A/D71A double mutant as a Survivin antagonist, both the GST-SUR 71-142 truncated protein, which could not bind the kinase (
Figure S2B), and the alanine point mutant SUR D71, which slightly did (
Figure S5,
bottom), suggested that only the Asp70 residue is crucial for binding to Cdk1. From these observations, I predicted that Survivin should probably recognize a region in the Cdk1 kinase with a net positive charge, and indeed, I was able to narrow down the Survivin-binding sequence in the Cdk1 kinase to amino acids Lys56 through Met85, a region that includes the kinase’s αC/β4 loop, which contains several basic residues (
Figure S3B). Residues 66 through 85 were also part of the original Cdk1 sequence recognized by Survivin, however this kinase region harbors numerous hydrophobic amino acids, and therefore I found it unlikely to be part of the Survivin-binding domain.
The αC/β4 loop is a conserved structural motif present in all eukaryotic protein kinases, which bridges the αC helix in the N lobe and regions at the top of the C lobe [
63]. In most kinases, this loop spans 8 amino acids, which follow the consensus sequence: L-x-H-P-N-T-V-x, where x represents any amino acid [
64]. Functionally, it has been hypothesized that the αC-β4 loop may act as a molecular brake for protein kinases by maintaining auto-inhibitory interactions via hydrogen bonding with the hinge region in the C lobe [
65,
66,
67]. In support of this theory, many mutations have been found at this location, which confer constitutive kinase activity and/or drug resistance, and play a direct role in cancer progression [
64]. The αC-β4 loop is also a site for protein–protein interactions, and as such, it recognizes the molecular chaperone Hsp90 and its co-chaperone Cdc37 [
68,
69], proteins that together promote the proper folding of 60% of the human kinome [
69,
70]. By cryoEM structure of an Hsp90-Cdc37-Cdk4 complex, a general mechanism of Hsp90-Cdc37 action has been postulated [
71], where a conserved H-P-N motif in Cdc37 would mimic the turn within the kinase αC-β4 loop, and push against the kinase αE helix in the C-lobe, preventing the contact between the kinase N and C lobes. As a result of this intermolecular interaction, the kinase would be initially stabilized, and would expose other motifs to which Hsp90 could bind. From all this information, it is tempting to speculate that Survivin might also have a role in the regulation of Cdk1 inter-lobe movement and its activation. In support of this view, Survivin recapitulates some of Cdc37’s features. In effect, apart from binding to the αC-β4 loop region as already stated, Survivin, like Cdc37, binds to the N-terminus of the Hsp90 chaperone [
72,
73]. Also, I could detect a complex between Survivin, Hsp90 and the active Cdk1 protein form at mitosis (
Figure S6). For these reasons, it is plausible to believe that coinciding with mitotic onset, Survivin might function as some kind of Hsp90 co-chaperone. Alternatively, Survivin might bind the chaperone machinery, once the Cdk1 complex has been assembled, and bring it close to its activator/s at the centrosome. Still, a third possibility might be that Survivin unloads the Hsp90-assembled Cdk1 complexes, and delivers them to its centrosomal activator/s. In this regard, this work showed that when Survivin was absent, an inactive cytosolic Cdc25B-Cdk1-Cyclin B1 complex accumulated, and here, it would be interesting to find out whether this complex was bound to Hsp90. A function of Survivin in mediating Cdk1 through Hsp90 is however a bit controversial, as it encounters those who claim that Cdk1 is not a Hsp90 client [
70], and others that support the opposite view [
69,
74,
75].
Conclusions
Survivin has been called a
cancer gene [
76]. This paper now shows that this title may have in part been earned due to an up-to-date unreported role of Survivin in the activation of the Cdc25B-Cdk1 complex. In this regard, there is plenty of research showing a correlation between Cdc25B signaling and tumorigenesis, and it would make perfect sense that cancer cells hijack this pathway in order to control mitotic entry. The connection between Cdc25B and cancer has led to an overwhelming effort trying to develop efficient Cdc25B inhibitors [
77]. These inhibitors however most of the time target the Cdc25B catalytic domain, and here, an exciting alternative might be finding small molecules that disrupt the Survivin-Cdc25B interaction.
In this work, I have also shown that the absence of Survivin leads to an early prophase blockage due to low Cdk1 activity, and propose that this phenotype is very sensitive to events, which override the G2/M-phase checkpoint. Therefore, a combination of drugs that promote mitotic entry, and then inhibit Cdk1 activity might be a very effective way to combat cancer.
Allosteric inhibitors that bind to the αC-β4 loop in Cdks are also on the way [
78], and this paper reinforces the importance of this site by proving that Survivin is another protein that binds to this epitope. From different studies, the αC-β4 loop is now rising as some sort of a hub through which kinases communicate with other proteins, and signals can be sent through a myriad of pathways. Therefore, elucidating the exact function of this region in kinases, and the role of the chaperone machinery at this location, may help refine the design of new drugs against cancer.
To end, I would like to reiterate the connection between Cdk1 activity and CPC’s regulation, and the possible role of Survivin at multitasking between kinases. In this context, Survivin might operate as a coordinator of upstream and downstream mitotic events, which would lead to smooth progression through mitosis.
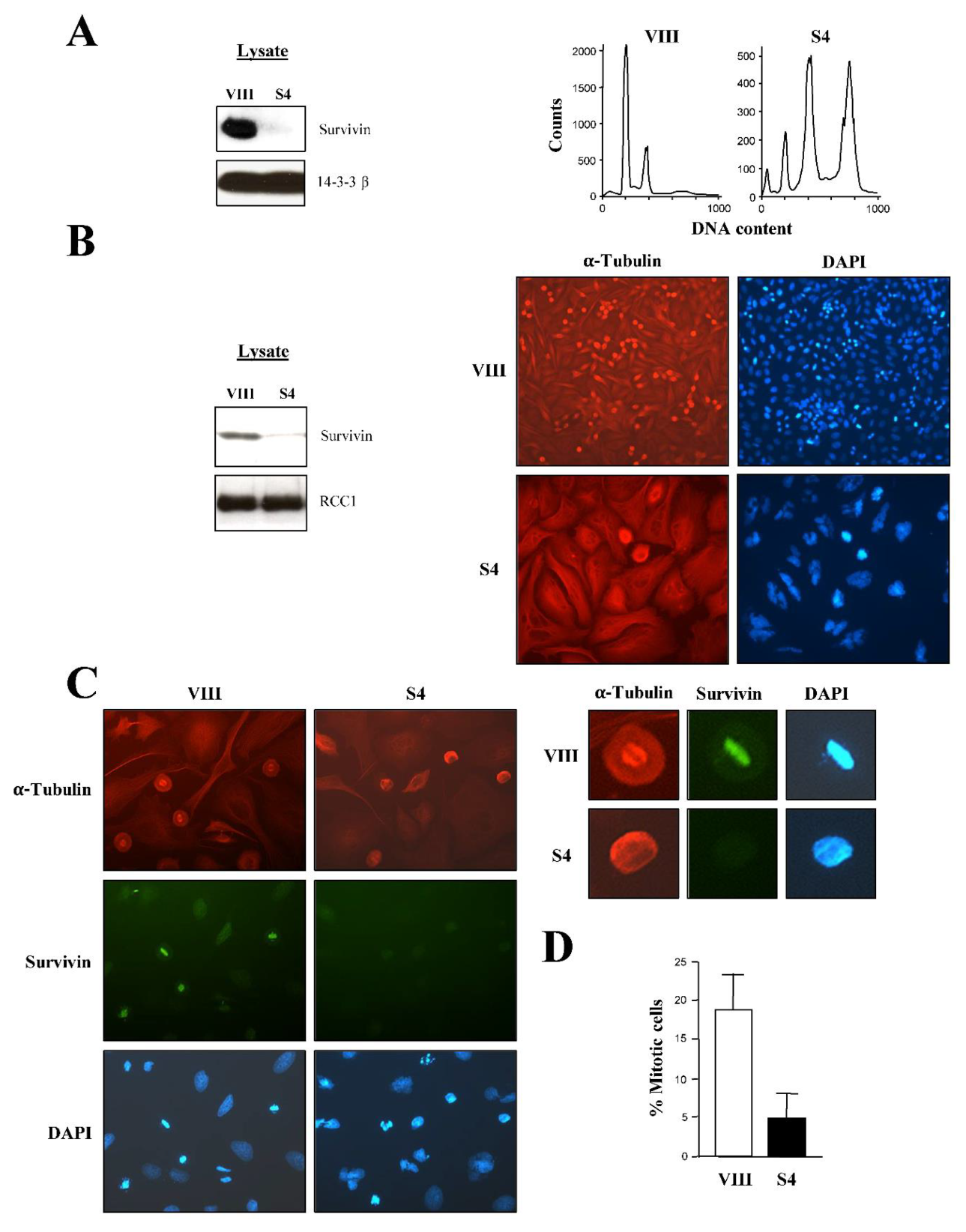
Figure 1.
siRNA-mediated loss of Survivin induces an early prophase blockage in asynchronous HeLa cell cultures. A, Survivin knockdown. Cells transfected with control (VIII) or Survivin (S4) siRNA were analyzed by Western blotting (left) or FACS analysis (right). B, Survivin knockdown cells. Cells transfected with the indicated siRNA were analyzed by Western blotting (left) or fluorescence microscopy using an antibody to α-Tubulin (right). DNA was stained with DAPI. C, Absence of spindle in knockdown cells. Cells transfected with the indicated siRNA were analyzed by fluorescence microscopy (left). Cells were incubated with antibodies against α-Tubulin (top) and Survivin (middle). DNA was stained with DAPI. Right, One cell transfected control (VIII) or Survivin (S4) siRNA at higher magnification. D, Bar graph shows the percentage of mitotic cells in siRNA transfected cultures (n=4).
Figure 1.
siRNA-mediated loss of Survivin induces an early prophase blockage in asynchronous HeLa cell cultures. A, Survivin knockdown. Cells transfected with control (VIII) or Survivin (S4) siRNA were analyzed by Western blotting (left) or FACS analysis (right). B, Survivin knockdown cells. Cells transfected with the indicated siRNA were analyzed by Western blotting (left) or fluorescence microscopy using an antibody to α-Tubulin (right). DNA was stained with DAPI. C, Absence of spindle in knockdown cells. Cells transfected with the indicated siRNA were analyzed by fluorescence microscopy (left). Cells were incubated with antibodies against α-Tubulin (top) and Survivin (middle). DNA was stained with DAPI. Right, One cell transfected control (VIII) or Survivin (S4) siRNA at higher magnification. D, Bar graph shows the percentage of mitotic cells in siRNA transfected cultures (n=4).
Figure 2.
Nuclear lamina stays assembled in Survivin-depleted HeLa cells. A, Nuclear lamina integrity in siRNA-transfected cells. Cells transfected with the indicated siRNA were analyzed by fluorescence microscopy using an antibody to α-Tubulin (left), and Survivin (top middle) or Lamin B (bottom middle). DNA was stained with DAPI. Several cells are labeled with arrow heads (S: Interphase, P: Prophase, PM: Prometaphase, M: Metaphase, T: Telophase, MN: Multinucleated, IL: Intact lamina and SLS: Spindle-like structure). B, Nuclear lamina in interphase or mitotic cells. Cells were transfected with the indicated siRNA, stained with an antibody to Lamin B and α-Tubulin, and analyzed by fluorescence microscopy. DNA was stained with DAPI. Cells are shown at the same scale. Arrows show intact nuclear lamina, and arrow head points out at Lamin B colocalization with the mitotic spindle. C, Percentage of rounded mitotic cells with intact or disassembled nuclear lamina. Rounded mitotic cells transfected and stained as in A were tallied for the presence or absence of an intact lamina (n=3). D, Purvalanol A treatment of cells. Cells were treated with the Cdk1 inhibitor purvalanol A (20 μM), stained with an antibody to Lamin B and α-Tubulin, and analyzed by fluorescence microscopy. DNA was stained with DAPI.
Figure 2.
Nuclear lamina stays assembled in Survivin-depleted HeLa cells. A, Nuclear lamina integrity in siRNA-transfected cells. Cells transfected with the indicated siRNA were analyzed by fluorescence microscopy using an antibody to α-Tubulin (left), and Survivin (top middle) or Lamin B (bottom middle). DNA was stained with DAPI. Several cells are labeled with arrow heads (S: Interphase, P: Prophase, PM: Prometaphase, M: Metaphase, T: Telophase, MN: Multinucleated, IL: Intact lamina and SLS: Spindle-like structure). B, Nuclear lamina in interphase or mitotic cells. Cells were transfected with the indicated siRNA, stained with an antibody to Lamin B and α-Tubulin, and analyzed by fluorescence microscopy. DNA was stained with DAPI. Cells are shown at the same scale. Arrows show intact nuclear lamina, and arrow head points out at Lamin B colocalization with the mitotic spindle. C, Percentage of rounded mitotic cells with intact or disassembled nuclear lamina. Rounded mitotic cells transfected and stained as in A were tallied for the presence or absence of an intact lamina (n=3). D, Purvalanol A treatment of cells. Cells were treated with the Cdk1 inhibitor purvalanol A (20 μM), stained with an antibody to Lamin B and α-Tubulin, and analyzed by fluorescence microscopy. DNA was stained with DAPI.
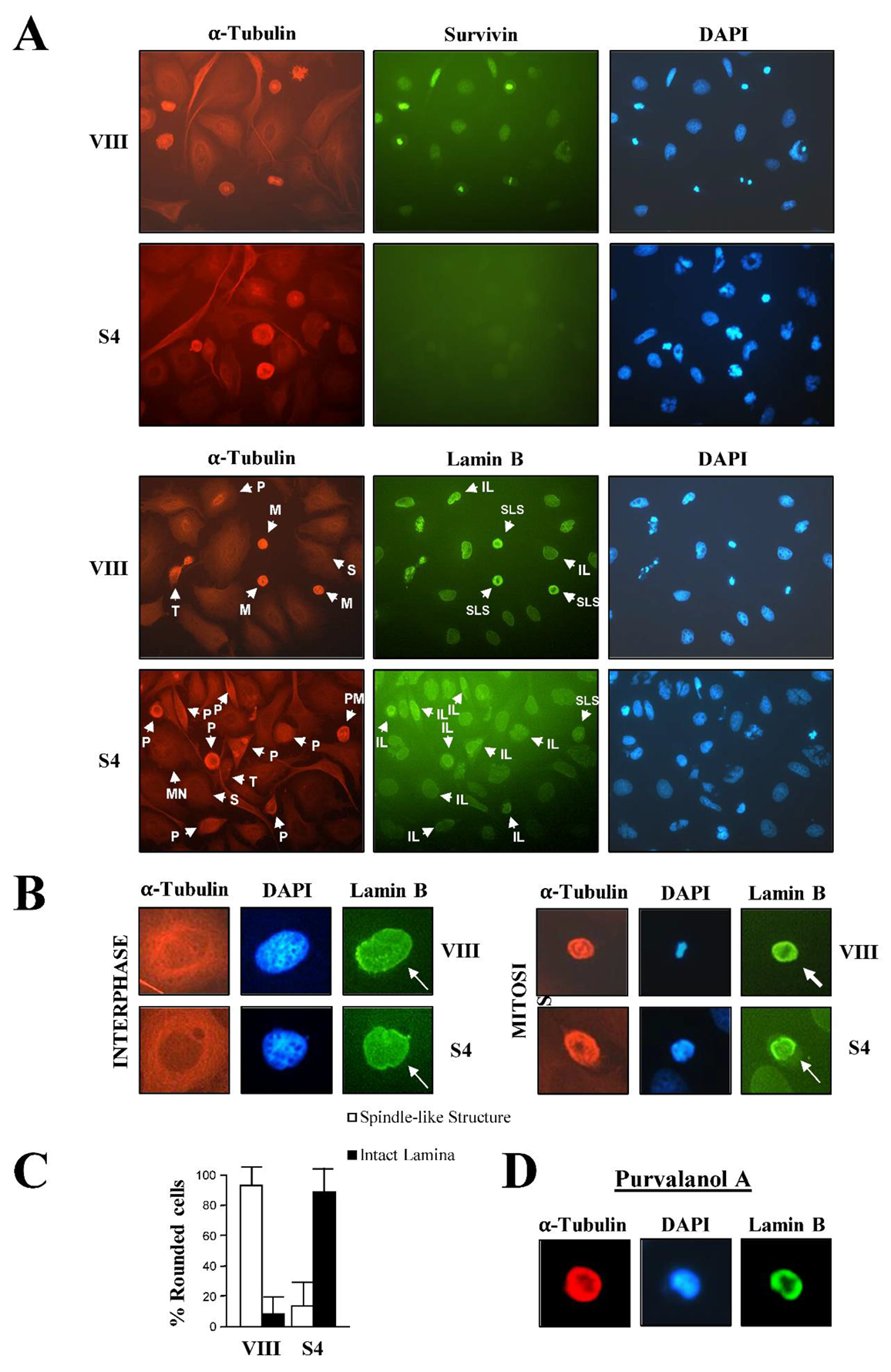
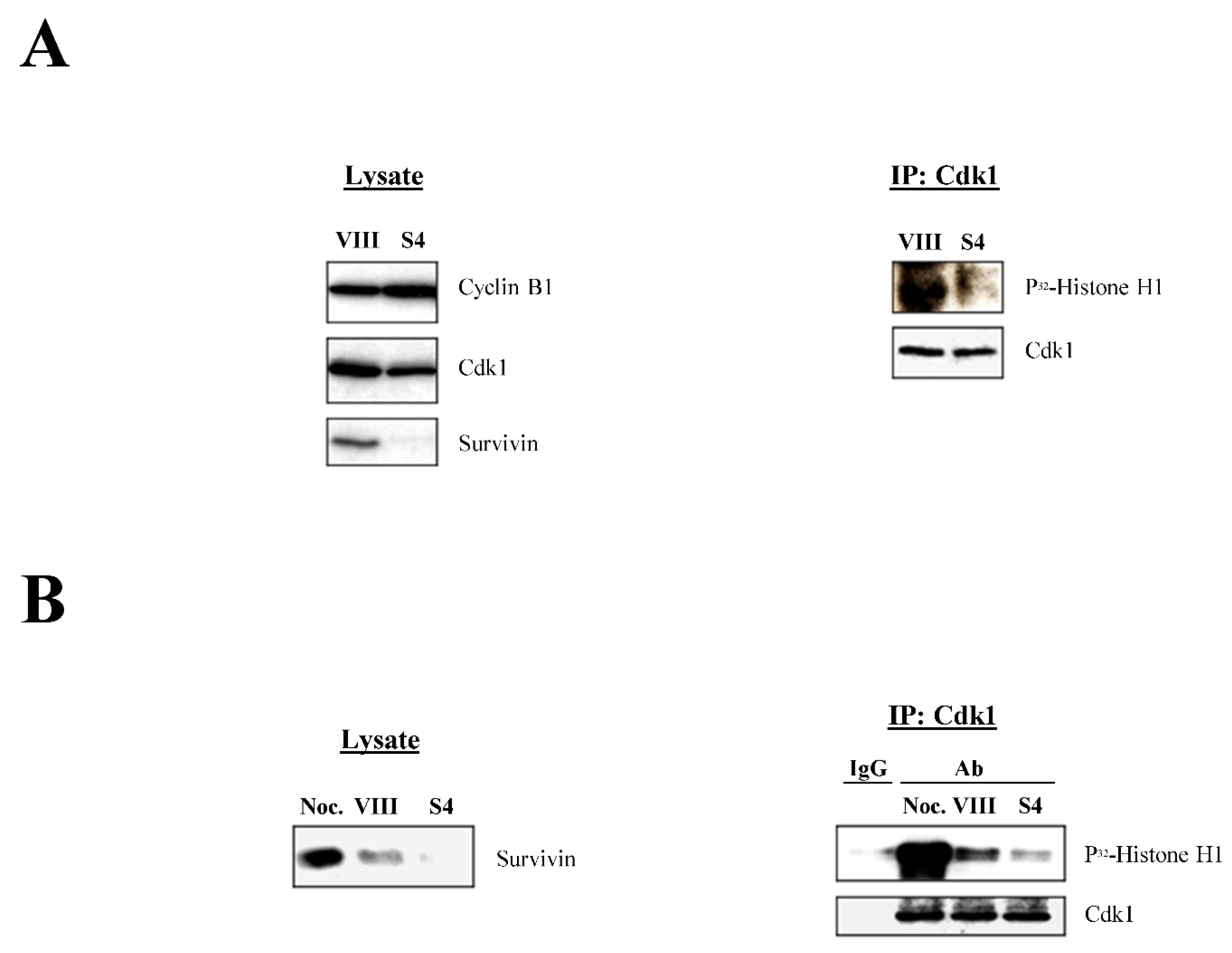
Figure 3.
Cdk1 activity is lower in Survivin-depleted HeLa cells. A, Cdk1 kinase assay. siRNA-transfected asynchronous Cell lysates were used to immunoprecipitate (IP) Cdk1, and the kinase activity was analyzed by a Histone H1 phosphorylation assay (right). Amounts of Cyclin B1, Cdk1 and Survivin were analyzed by Western blotting as a control (left). B, Survivin ablation vs. prometaphase blockage. Cells were treated with 10 μM nocodazole, and their Cdk1 activity was analyzed by an IP and a Histone H1 phosphorylation assay. Survivin siRNA-transfected HeLa cells were used as a control.
Figure 3.
Cdk1 activity is lower in Survivin-depleted HeLa cells. A, Cdk1 kinase assay. siRNA-transfected asynchronous Cell lysates were used to immunoprecipitate (IP) Cdk1, and the kinase activity was analyzed by a Histone H1 phosphorylation assay (right). Amounts of Cyclin B1, Cdk1 and Survivin were analyzed by Western blotting as a control (left). B, Survivin ablation vs. prometaphase blockage. Cells were treated with 10 μM nocodazole, and their Cdk1 activity was analyzed by an IP and a Histone H1 phosphorylation assay. Survivin siRNA-transfected HeLa cells were used as a control.
Figure 4.
Survivin abrogation in synchronous HeLa cells causes a sustained G2/M-phase blockage and diminished Cdk1 activity. A, Cell cycle analysis of untreated synchronous cells. Cells were synchronized with 2 mM thymidine for 48 h, released into fresh medium, and harvested at the indicated time intervals. Collected samples were subjected to an IP using an antibody to Survivin, and analyzed by Western blotting (left) or FACS analysis (right). B, Cell cycle analysis of synchronous cells depleted of Survivin. Cells transfected with control (VIII) or Survivin (S4) siRNA were synchronized as in A, released into fresh medium, and harvested at the indicated time intervals. Collected samples were subjected to Western blotting using a Survivin or Cdk1 antibody (top), or FACS analysis (bottom). C, Cdk1 activity during mitotic transition. Synchronized cells, previously transfected with the indicated siRNA, were harvested when they transitioned through mitosis, and analyzed by FACS (left). Lysates were prepared and used to IP Cdk1, and the immune complexes were analyzed in a Histone H1 phosphorylation assay (top right). The amount of Survivin in the lysates is shown as a control (bottom right).
Figure 4.
Survivin abrogation in synchronous HeLa cells causes a sustained G2/M-phase blockage and diminished Cdk1 activity. A, Cell cycle analysis of untreated synchronous cells. Cells were synchronized with 2 mM thymidine for 48 h, released into fresh medium, and harvested at the indicated time intervals. Collected samples were subjected to an IP using an antibody to Survivin, and analyzed by Western blotting (left) or FACS analysis (right). B, Cell cycle analysis of synchronous cells depleted of Survivin. Cells transfected with control (VIII) or Survivin (S4) siRNA were synchronized as in A, released into fresh medium, and harvested at the indicated time intervals. Collected samples were subjected to Western blotting using a Survivin or Cdk1 antibody (top), or FACS analysis (bottom). C, Cdk1 activity during mitotic transition. Synchronized cells, previously transfected with the indicated siRNA, were harvested when they transitioned through mitosis, and analyzed by FACS (left). Lysates were prepared and used to IP Cdk1, and the immune complexes were analyzed in a Histone H1 phosphorylation assay (top right). The amount of Survivin in the lysates is shown as a control (bottom right).
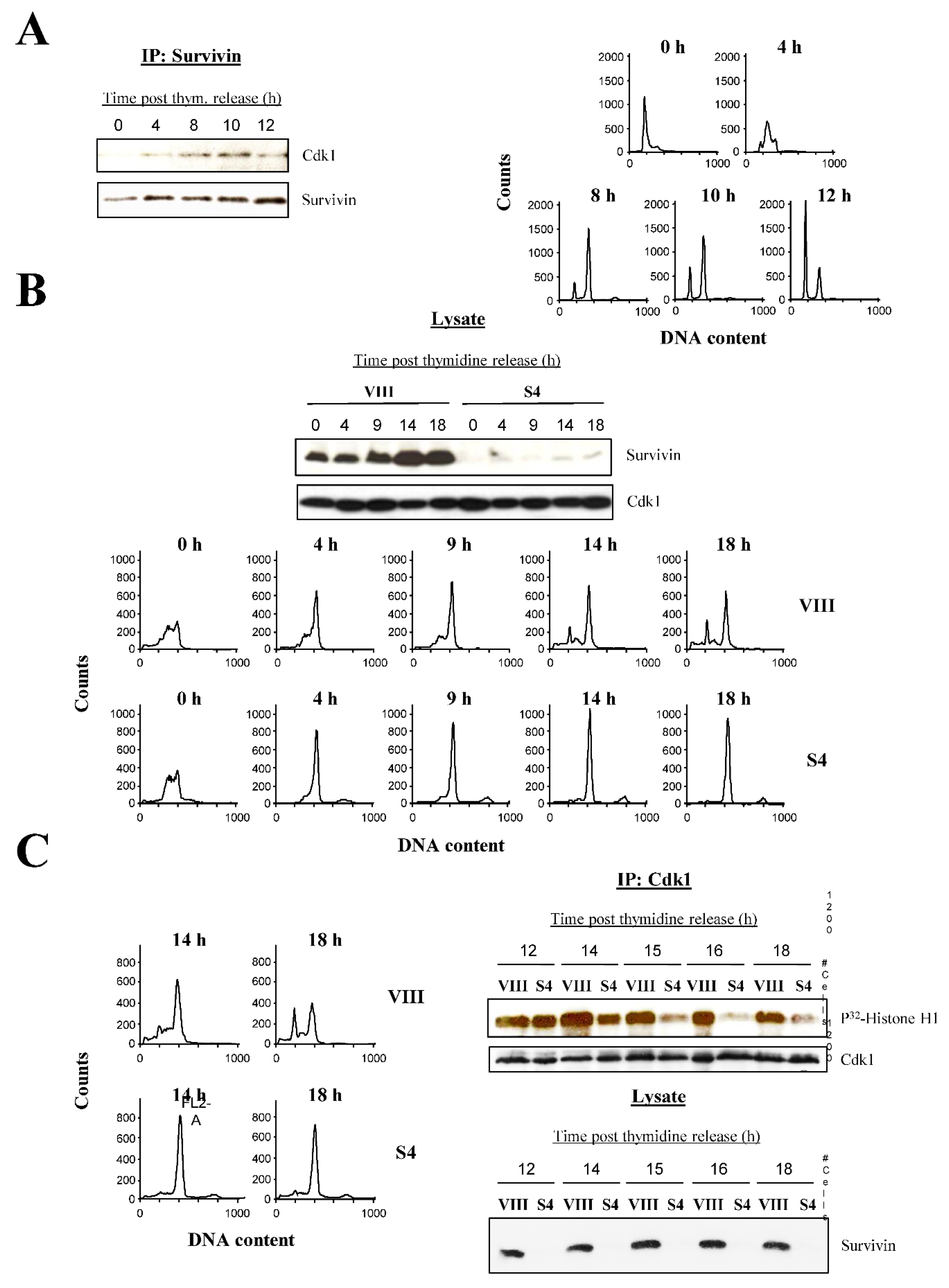
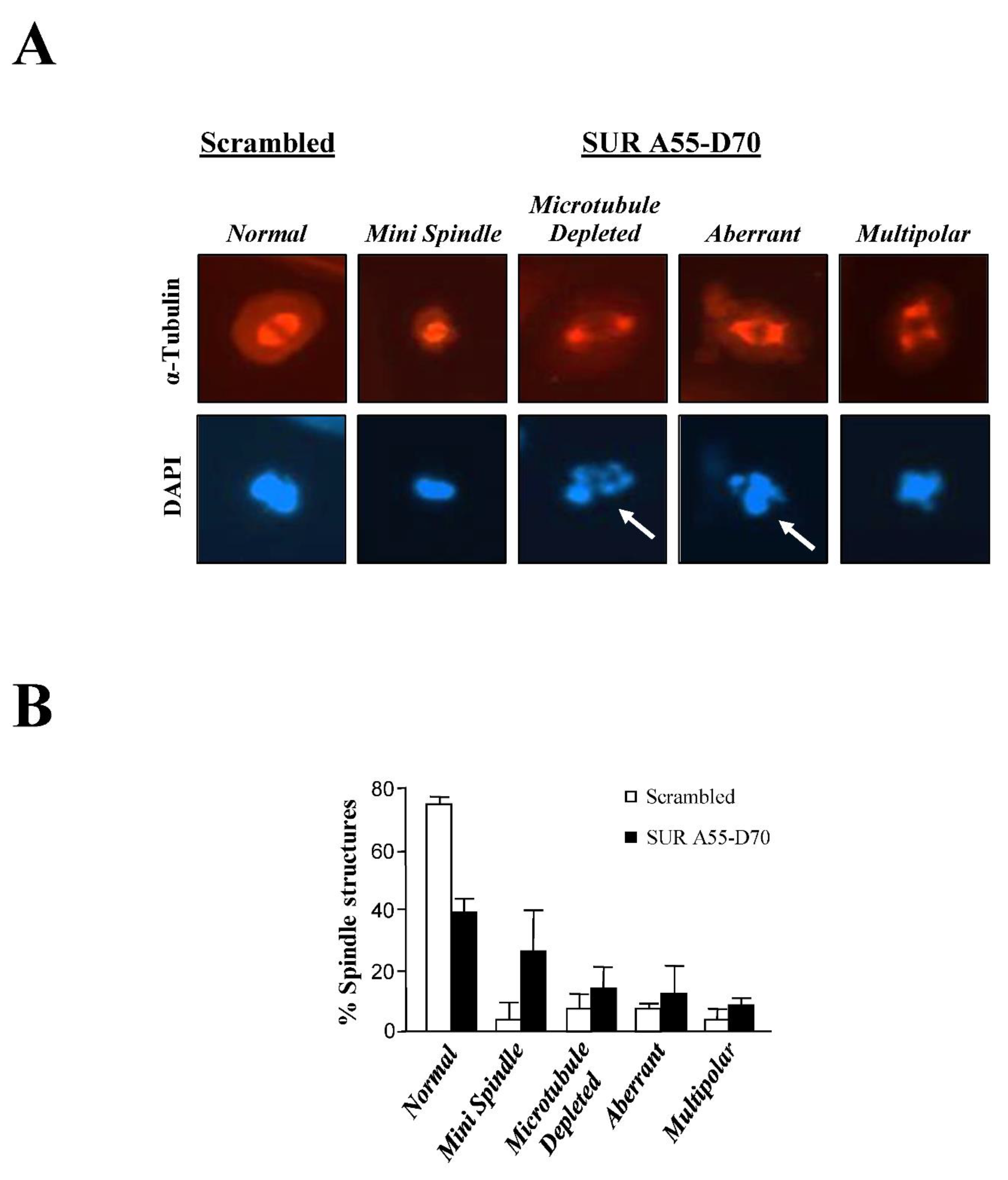
Figure 5.
Treatment of HeLa cells with the SUR A55-D70 peptide causes spindle abnormalities. A, Spindle abnormalities caused by the SUR A55-D70 peptide. Asynchronous cell cultures were transfected with 50 μM scrambled or SUR A55-D70 peptide for 6 h, and cells were stained with an antibody to α-Tubulin. DNA was stained with DAPI. B, Mitotic phenotypes were quantified (n=2).
Figure 5.
Treatment of HeLa cells with the SUR A55-D70 peptide causes spindle abnormalities. A, Spindle abnormalities caused by the SUR A55-D70 peptide. Asynchronous cell cultures were transfected with 50 μM scrambled or SUR A55-D70 peptide for 6 h, and cells were stained with an antibody to α-Tubulin. DNA was stained with DAPI. B, Mitotic phenotypes were quantified (n=2).
Figure 6.
Prolonged incubation of HeLa cells with the SUR A55-D70 peptide causes apoptosis. A, SUR A55-D70 peptide-induced cell death. Asynchronous cell cultures were treated with 50 μM scrambled or SUR A55-D70 peptides for 24 h, and cells were analyzed by FACS (top), or phase-contrast microscopy (bottom). Images represent one of several experiments (n=4). The percentage of apoptotic (<2N), G1 (2N) and G2/M-phase (4N) cells is indicated at top. Dead cells showing some kind of dark material inside are indicated by arrows. B, SUR A55-D70 peptide dose response of transfected HeLa cells. Asynchronous cell cultures were treated with 200 μM scrambled or SUR A55-D70 peptides for 24 h, and cells were analyzed by FACS (left), phase-contrast microscopy (middle) or Trypan Blue staining (right). Images represent one of several experiments (n=4). The percentage of apoptotic (<2N), G1 (2N) and G2/M-phase (4N) cells is indicated on left. Dead cells showing some kind of dark material inside are indicated by arrows, and dead cells looking like bubbles are indicated by arrow heads. C, SUR A55-D70 peptide-induced apoptosis. Asynchronous cell cultures were transfected with 200 μM scrambled or SUR A55-D70 peptides, and cells were analyzed by phase-contrast microscopy (left) and FAM-DEVD-FMK fluorescence microscopy (right) after 24 h. Images represent one of several experiments (n=3). D, SUR A55-D70 peptide activation of caspase 3. Cells treated as in C for 24-48 h were incubated with an antibody to the caspase 3 proform. E, SUR A55-D70 peptide-induced apoptosis during G2/M-phase. G2/M-phase synchronized cells were transfected with 200 μM scrambled or SUR A55-D70 peptides for 12 h, and analyzed by FACS (top), or phase-contrast microscopy (bottom). The percentage of apoptotic (<2N), G1 (2N) and G2/M-phase (4N) cells is indicated.
Figure 6.
Prolonged incubation of HeLa cells with the SUR A55-D70 peptide causes apoptosis. A, SUR A55-D70 peptide-induced cell death. Asynchronous cell cultures were treated with 50 μM scrambled or SUR A55-D70 peptides for 24 h, and cells were analyzed by FACS (top), or phase-contrast microscopy (bottom). Images represent one of several experiments (n=4). The percentage of apoptotic (<2N), G1 (2N) and G2/M-phase (4N) cells is indicated at top. Dead cells showing some kind of dark material inside are indicated by arrows. B, SUR A55-D70 peptide dose response of transfected HeLa cells. Asynchronous cell cultures were treated with 200 μM scrambled or SUR A55-D70 peptides for 24 h, and cells were analyzed by FACS (left), phase-contrast microscopy (middle) or Trypan Blue staining (right). Images represent one of several experiments (n=4). The percentage of apoptotic (<2N), G1 (2N) and G2/M-phase (4N) cells is indicated on left. Dead cells showing some kind of dark material inside are indicated by arrows, and dead cells looking like bubbles are indicated by arrow heads. C, SUR A55-D70 peptide-induced apoptosis. Asynchronous cell cultures were transfected with 200 μM scrambled or SUR A55-D70 peptides, and cells were analyzed by phase-contrast microscopy (left) and FAM-DEVD-FMK fluorescence microscopy (right) after 24 h. Images represent one of several experiments (n=3). D, SUR A55-D70 peptide activation of caspase 3. Cells treated as in C for 24-48 h were incubated with an antibody to the caspase 3 proform. E, SUR A55-D70 peptide-induced apoptosis during G2/M-phase. G2/M-phase synchronized cells were transfected with 200 μM scrambled or SUR A55-D70 peptides for 12 h, and analyzed by FACS (top), or phase-contrast microscopy (bottom). The percentage of apoptotic (<2N), G1 (2N) and G2/M-phase (4N) cells is indicated.
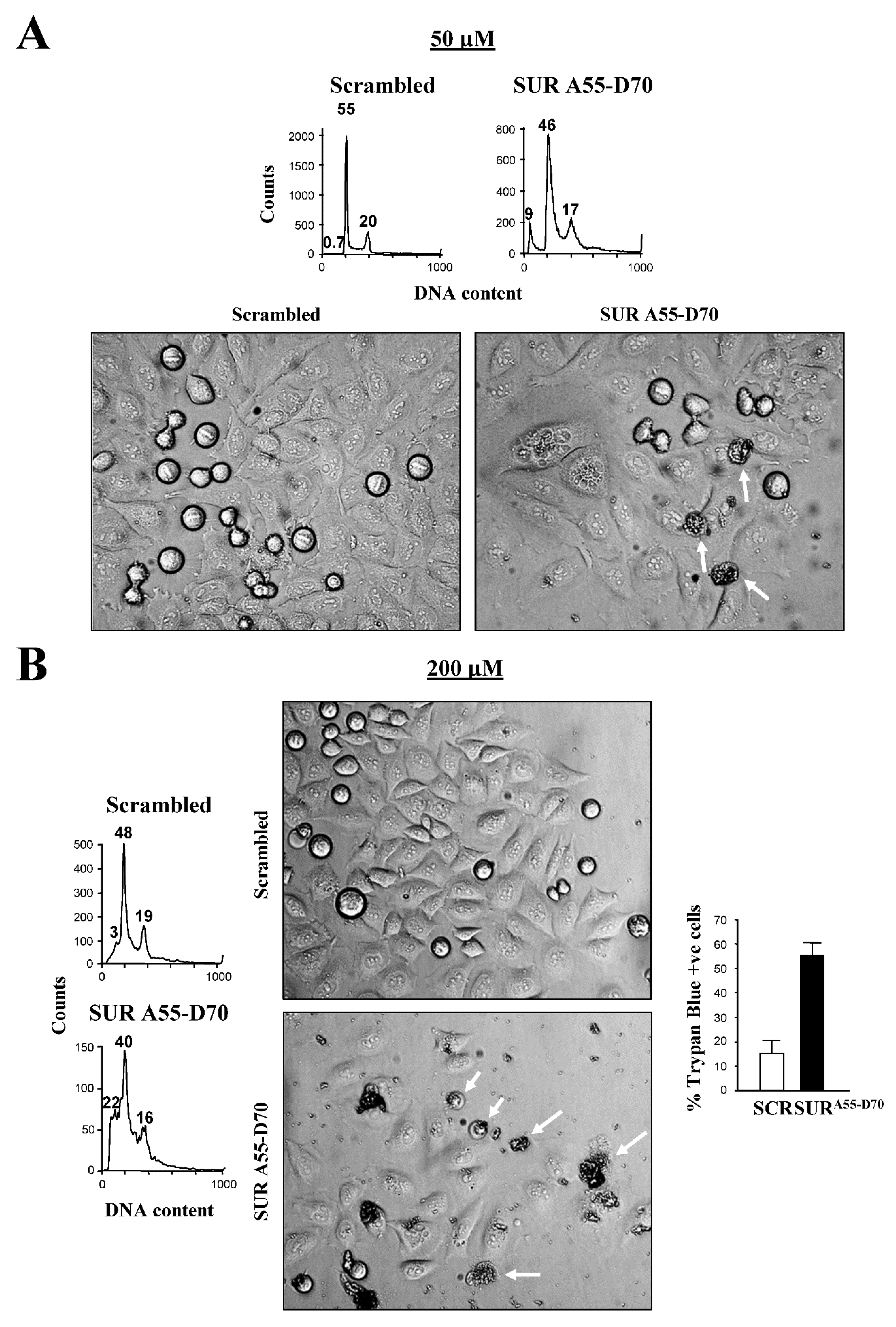
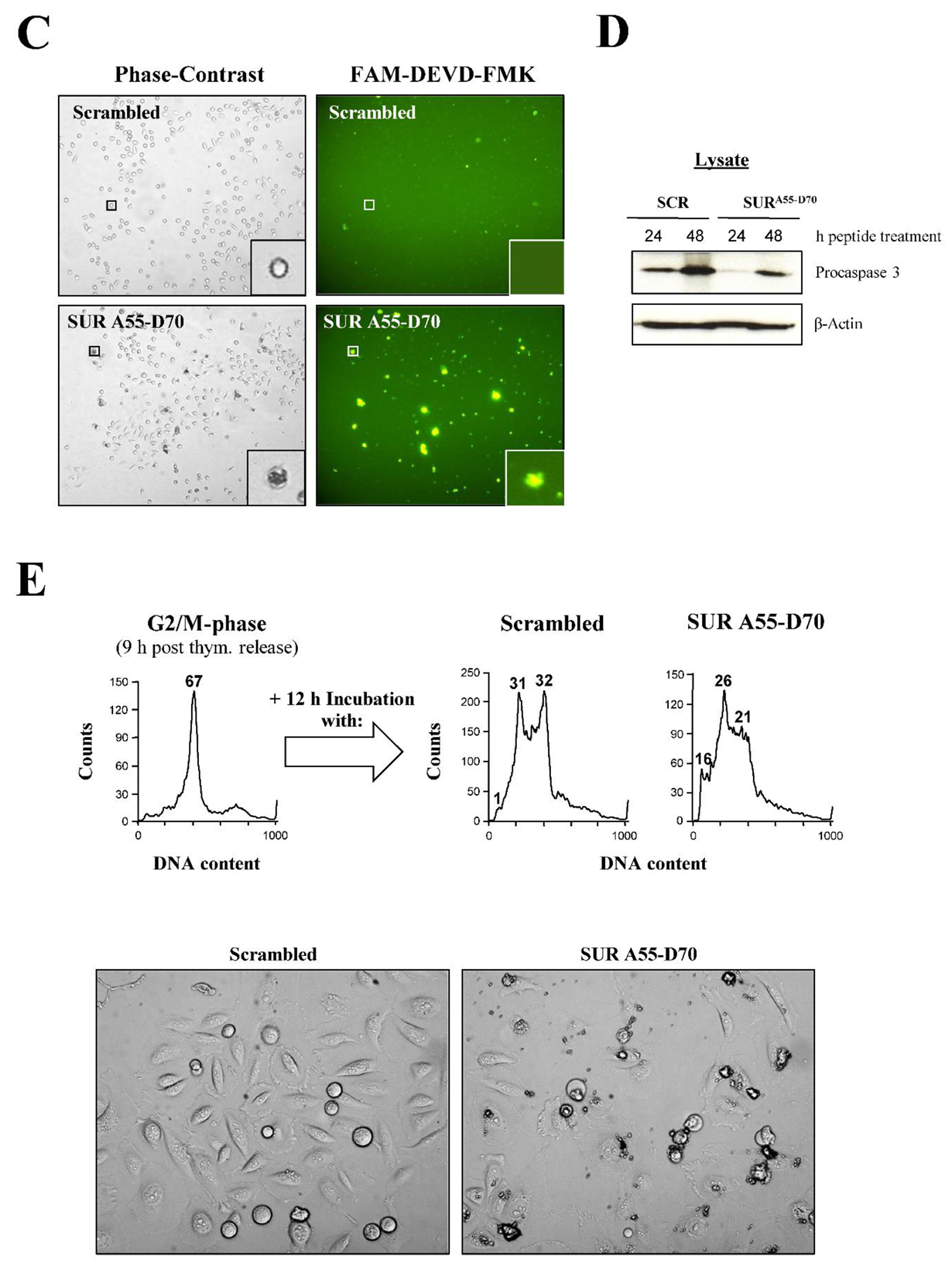
Figure 7.
The Survivin Asp70Ala/Asp71Ala double mutant (SUR D70A/D71A) causes G2/M-phase arrest, mitotic abnormalities and cell death in HeLa cells. A, SUR D70A/D71A causes mitotic abnormalities. Asynchronous cell cultures were transfected with constructs expressing HA-tagged wild type Survivin (HA-SUR WT) or SUR D70A/D71A (HA-SUR D70A/D71A), and subjected to Western blotting using antibodies to HA-Tag, Survivin or β-Actin (left), or analyzed by fluorescence microscopy using antibodies to HA-Tag or α-Tubulin (right). DNA was stained with DAPI. Cells are labeled according to their mitotic phase or chromatin fragmentation. B, Phenotypes observed in SUR D70A/D71A-expressing cells. Graph shows the averages of two independent experiments. C, Cdk1 activity in SUR D70A/D71A-expressing cells. HA-SUR WT- or HA-SUR D70A/D71A-transfected cell lysates were used to IP Cdk1, and pellets were analyzed in a Histone H1 phosphorylation assay (up), or by Western blotting (bottom). D, Detection of phosphorylated inactive Cdk1 form in cells expressing SUR D70A/D71A. Parallel samples as in C were used to determine the level of inactive Cdk1 by Western blotting. E, F, Time-lapse video microscopy of SUR D70A/D71A-expressing cells. Synchronous cell cultures were transfected with GFP-SUR WT or GFP-SUR D70A/D71A, released into fresh medium, and imaged every 10 min continuously for 24 h. Images were taken at the indicated time points. E, Individual cells (bars), transfected with GFP-SUR WT (top) or GFP-SUR D70/D71A mutant (bottom) were quantified for time spent at each indicated mitotic transition and assigned specific phenotypes (see legend). Bottom F, Selected cells undergoing sustained mitotic arrest (magenta and light blue arrows), or cell death after mitosis re-entry (yellow arrow) are shown. Top F, White arrow shows a control-transfected cell that progressed normally through mitosis.
Figure 7.
The Survivin Asp70Ala/Asp71Ala double mutant (SUR D70A/D71A) causes G2/M-phase arrest, mitotic abnormalities and cell death in HeLa cells. A, SUR D70A/D71A causes mitotic abnormalities. Asynchronous cell cultures were transfected with constructs expressing HA-tagged wild type Survivin (HA-SUR WT) or SUR D70A/D71A (HA-SUR D70A/D71A), and subjected to Western blotting using antibodies to HA-Tag, Survivin or β-Actin (left), or analyzed by fluorescence microscopy using antibodies to HA-Tag or α-Tubulin (right). DNA was stained with DAPI. Cells are labeled according to their mitotic phase or chromatin fragmentation. B, Phenotypes observed in SUR D70A/D71A-expressing cells. Graph shows the averages of two independent experiments. C, Cdk1 activity in SUR D70A/D71A-expressing cells. HA-SUR WT- or HA-SUR D70A/D71A-transfected cell lysates were used to IP Cdk1, and pellets were analyzed in a Histone H1 phosphorylation assay (up), or by Western blotting (bottom). D, Detection of phosphorylated inactive Cdk1 form in cells expressing SUR D70A/D71A. Parallel samples as in C were used to determine the level of inactive Cdk1 by Western blotting. E, F, Time-lapse video microscopy of SUR D70A/D71A-expressing cells. Synchronous cell cultures were transfected with GFP-SUR WT or GFP-SUR D70A/D71A, released into fresh medium, and imaged every 10 min continuously for 24 h. Images were taken at the indicated time points. E, Individual cells (bars), transfected with GFP-SUR WT (top) or GFP-SUR D70/D71A mutant (bottom) were quantified for time spent at each indicated mitotic transition and assigned specific phenotypes (see legend). Bottom F, Selected cells undergoing sustained mitotic arrest (magenta and light blue arrows), or cell death after mitosis re-entry (yellow arrow) are shown. Top F, White arrow shows a control-transfected cell that progressed normally through mitosis.
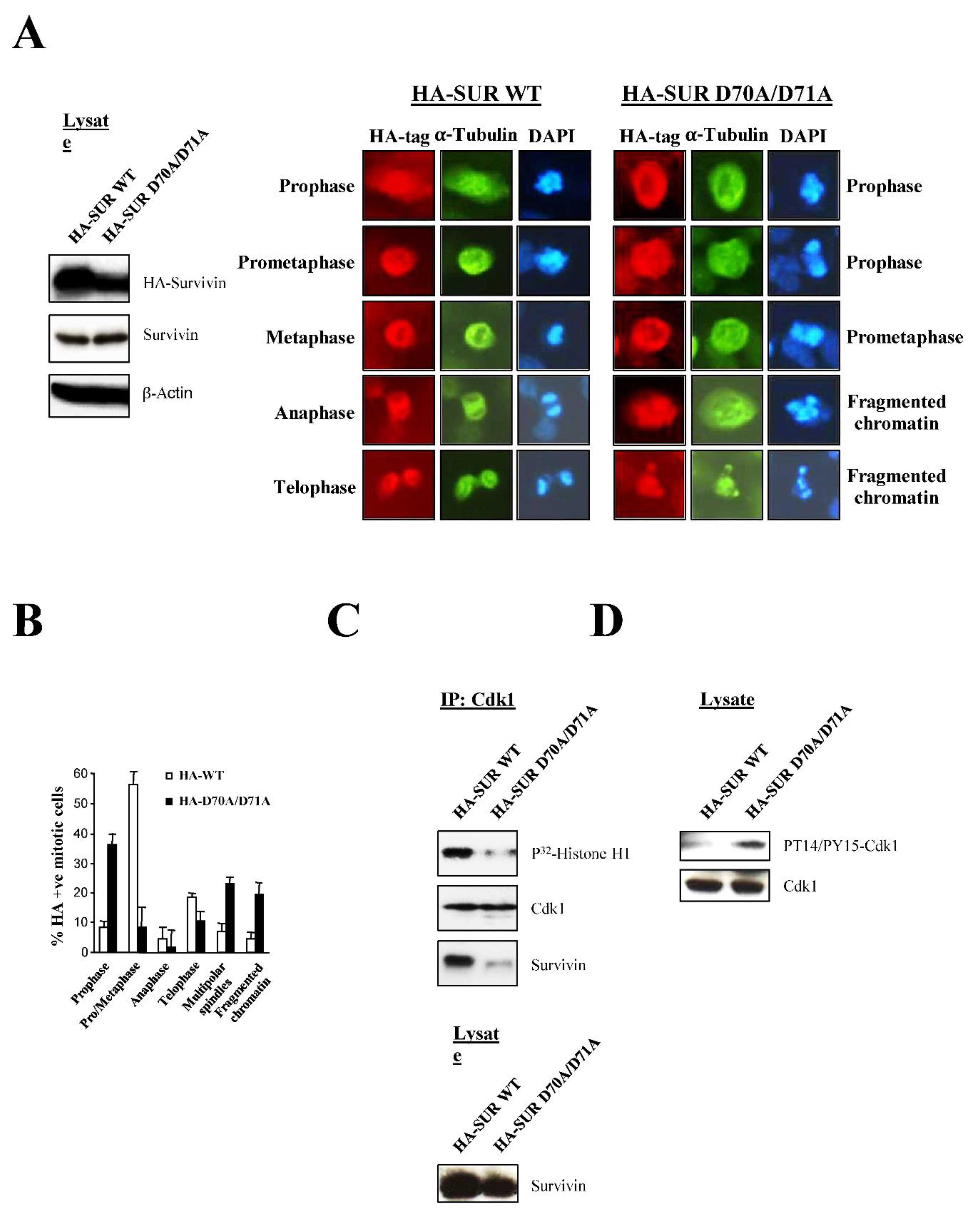
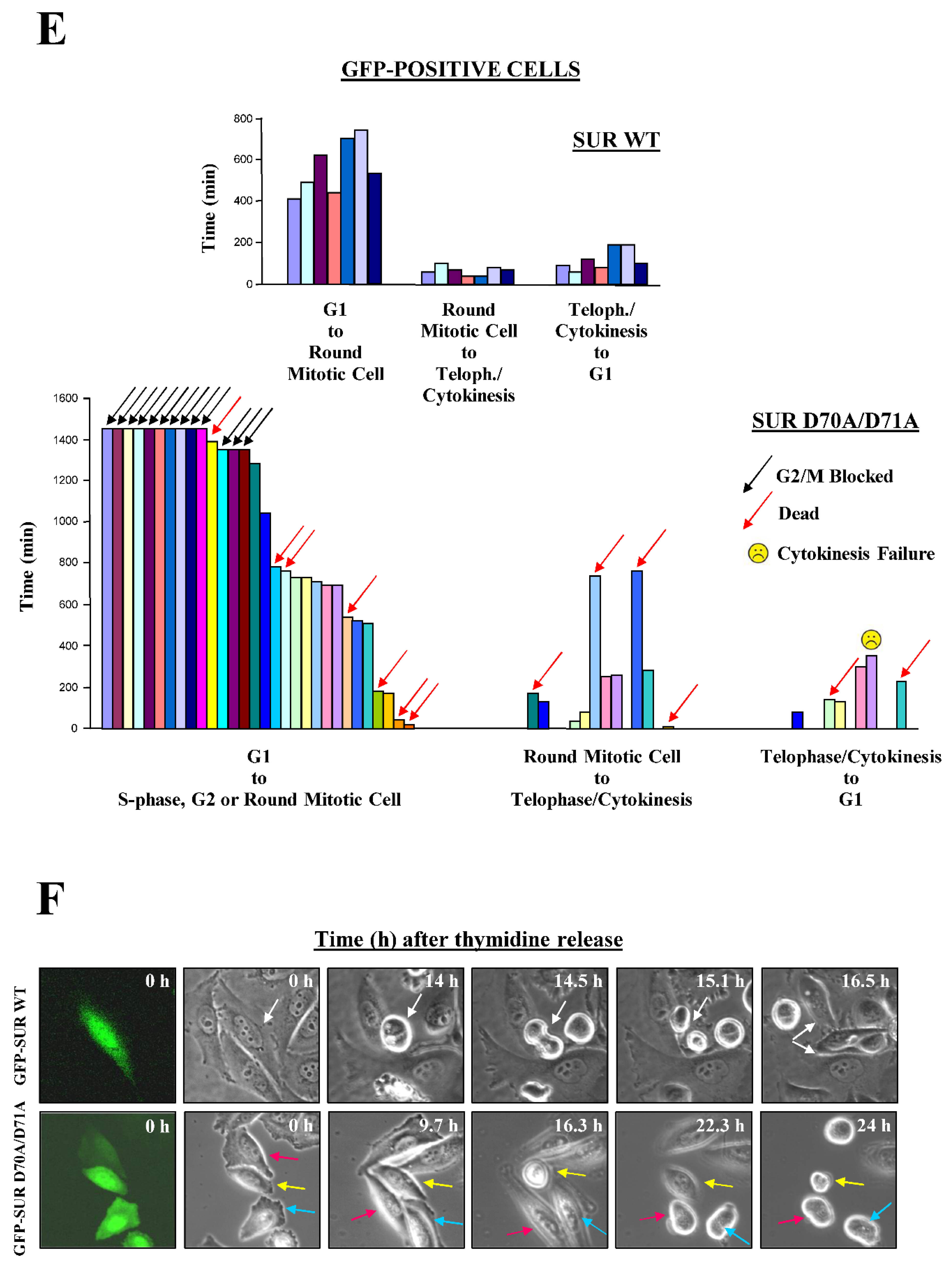
Figure 8.
Survivin is needed to recruit Cdk1 to Hela cell centrosomes. A, Fast-migrating centrosomal Survivin-Cdk1 complex at mitosis. Centrosomes were isolated from lysates of untreated synchronous HeLa cell cultures, subjected to Survivin IP, and analyzed by Western blotting using antibodies to Cdk1 or Survivin (
left).
B, Centrosomal Cdk1 levels in cells depleted of Survivin. Centrosomes were isolated from asynchronous HeLa cells transfected with control (VIII) or Survivin (S4) siRNA, and analyzed by Western blotting using several antibodies (see original data in
Figure S7A).
C, Centrosomal Cdk1 levels and activity in siRNA-treated synchronous HeLa cultures. Centrosomal preparations from synchronized HeLa cells transfected with the indicated siRNA that were collected after release into fresh media at the mentioned times, were analyzed by FACS (
bottom) or IP with an antibody to Cdk1, and pellets were analyzed by Western blotting or a Histone H1 phosphorylation assay (*: 16 h time point was omitted in both treatments due to a loading error (see original data in
Figure S7B). Right panel shows the levels of Cyclin B1, Cdk1 and Survivin in the lysate as a control.
D, Centrosomal Cdk1 signal in Survivin-depleted cells. siRNA-treated cells were analyzed by fluorescence microscopy using an antibody to Cdk1 (
top). DNA was stained with DAPI. Control cells (VIII) were either in early prophase (a, b), prometaphase (c, d) or metaphase (e, f), and Survivin-depleted cells (S4) were in S-phase/G2 (g, h) or early prophase (i-l). Percentage of splitted Cdk1 signal was scored (
bottom) (n=3).
Figure 8.
Survivin is needed to recruit Cdk1 to Hela cell centrosomes. A, Fast-migrating centrosomal Survivin-Cdk1 complex at mitosis. Centrosomes were isolated from lysates of untreated synchronous HeLa cell cultures, subjected to Survivin IP, and analyzed by Western blotting using antibodies to Cdk1 or Survivin (
left).
B, Centrosomal Cdk1 levels in cells depleted of Survivin. Centrosomes were isolated from asynchronous HeLa cells transfected with control (VIII) or Survivin (S4) siRNA, and analyzed by Western blotting using several antibodies (see original data in
Figure S7A).
C, Centrosomal Cdk1 levels and activity in siRNA-treated synchronous HeLa cultures. Centrosomal preparations from synchronized HeLa cells transfected with the indicated siRNA that were collected after release into fresh media at the mentioned times, were analyzed by FACS (
bottom) or IP with an antibody to Cdk1, and pellets were analyzed by Western blotting or a Histone H1 phosphorylation assay (*: 16 h time point was omitted in both treatments due to a loading error (see original data in
Figure S7B). Right panel shows the levels of Cyclin B1, Cdk1 and Survivin in the lysate as a control.
D, Centrosomal Cdk1 signal in Survivin-depleted cells. siRNA-treated cells were analyzed by fluorescence microscopy using an antibody to Cdk1 (
top). DNA was stained with DAPI. Control cells (VIII) were either in early prophase (a, b), prometaphase (c, d) or metaphase (e, f), and Survivin-depleted cells (S4) were in S-phase/G2 (g, h) or early prophase (i-l). Percentage of splitted Cdk1 signal was scored (
bottom) (n=3).
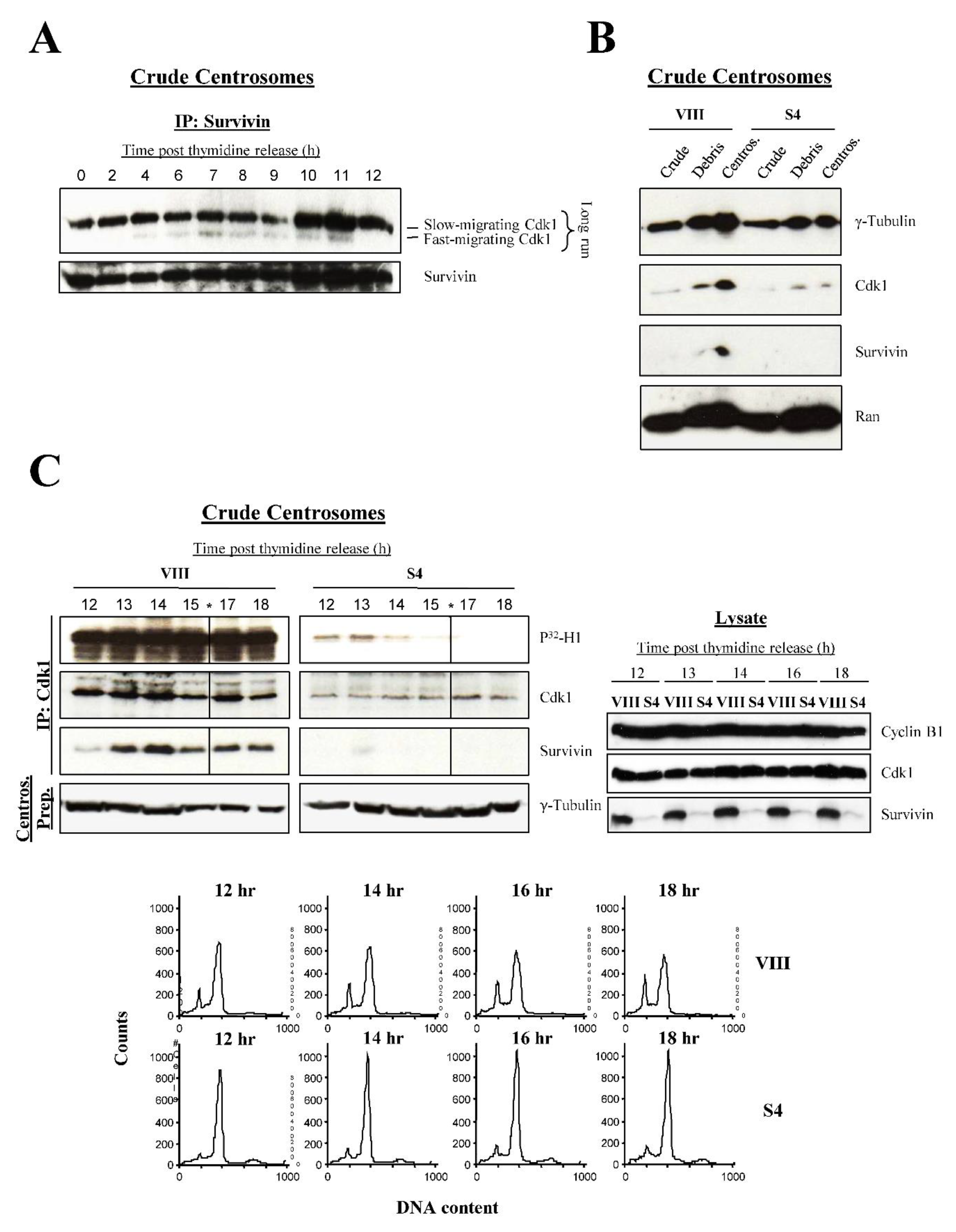
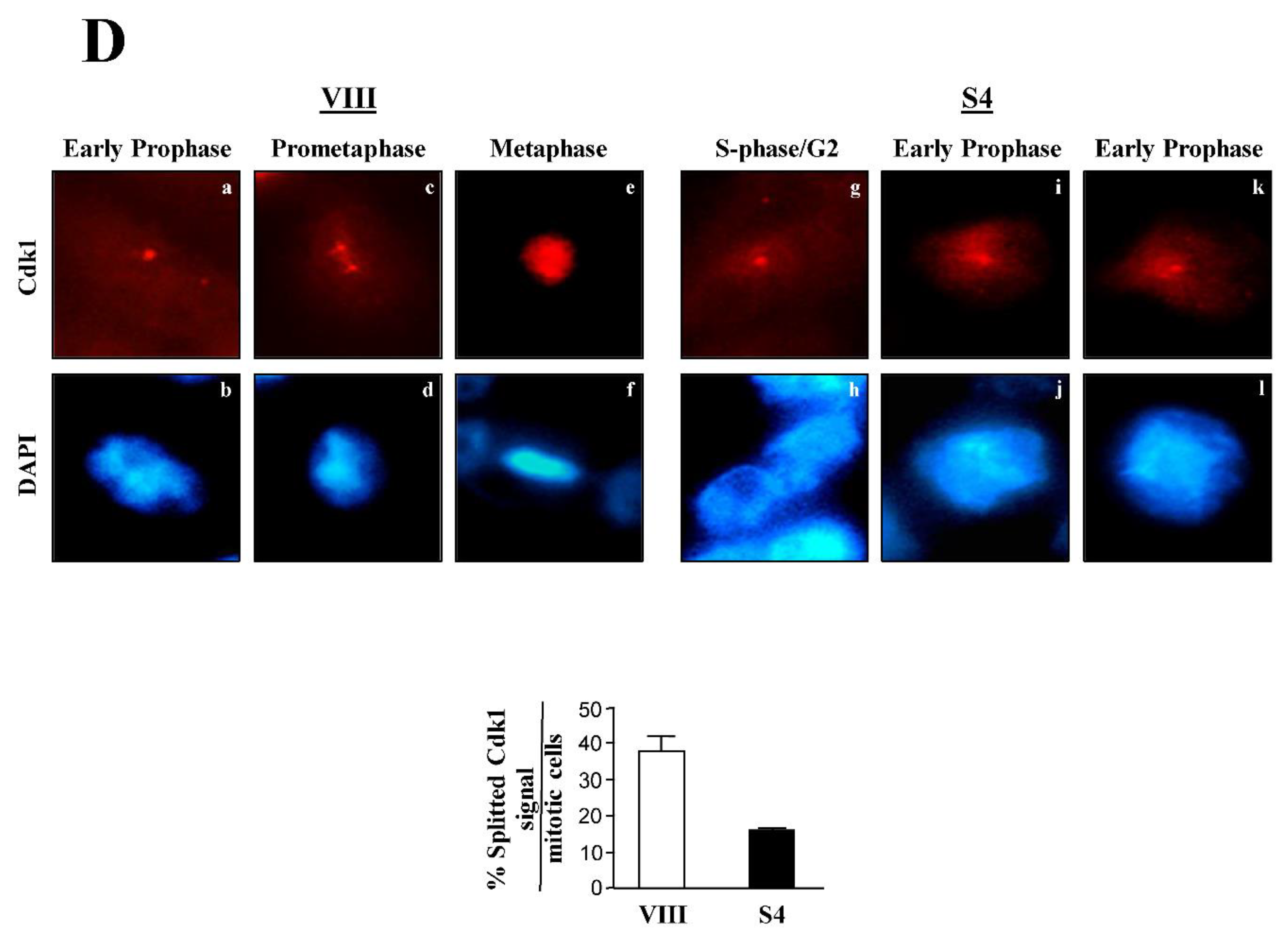
Figure 9.
Cdc25 activity is induced by recombinant Survivin in vitro. A, Phosphorylated inactive Cdk1 isoform in Survivin-depleted Hela cells. Shaken-off cells transfected with control (VIII) or Survivin (S4) siRNA were analyzed by Western blotting using several antibodies (left) or a Histone H1 phosphorylation assay (right). B, Impaired Cdc25 activity in Survivin-knocked down mitotic HeLa cells. Synchronized mitotic cells (14 h) transfected with the indicated siRNA were collected, and analyzed by Western blotting. C, In vitro induction of Cdc25 activity by recombinant Survivin. G2/M-phase HeLa cell lysates were immunodepleted of Survivin (left), supplemented with an ATP-regenerating system, incubated with GST or GST-Survivin, used to IP Cdk1, and analyzed by Western blotting (right up). Binding of the recombinant GST-Survivin protein (*) to the immunoprecipitated Cdk1 was checked by staining the IP membrane with Coomassie Blue. U indicates a nonspecific protein band. D, E, Cdk1 activation by recombinant Survivin in interphase. D, Interphase HeLa cell lysates (top left), depleted of Survivin (bottom left), and supplemented with an ATP-regenerating system, were incubated with different concentrations of GST or GST-Survivin, and analyzed by Western blotting (top right), or a Histone H1 phosphorylation assay (E).
Figure 9.
Cdc25 activity is induced by recombinant Survivin in vitro. A, Phosphorylated inactive Cdk1 isoform in Survivin-depleted Hela cells. Shaken-off cells transfected with control (VIII) or Survivin (S4) siRNA were analyzed by Western blotting using several antibodies (left) or a Histone H1 phosphorylation assay (right). B, Impaired Cdc25 activity in Survivin-knocked down mitotic HeLa cells. Synchronized mitotic cells (14 h) transfected with the indicated siRNA were collected, and analyzed by Western blotting. C, In vitro induction of Cdc25 activity by recombinant Survivin. G2/M-phase HeLa cell lysates were immunodepleted of Survivin (left), supplemented with an ATP-regenerating system, incubated with GST or GST-Survivin, used to IP Cdk1, and analyzed by Western blotting (right up). Binding of the recombinant GST-Survivin protein (*) to the immunoprecipitated Cdk1 was checked by staining the IP membrane with Coomassie Blue. U indicates a nonspecific protein band. D, E, Cdk1 activation by recombinant Survivin in interphase. D, Interphase HeLa cell lysates (top left), depleted of Survivin (bottom left), and supplemented with an ATP-regenerating system, were incubated with different concentrations of GST or GST-Survivin, and analyzed by Western blotting (top right), or a Histone H1 phosphorylation assay (E).
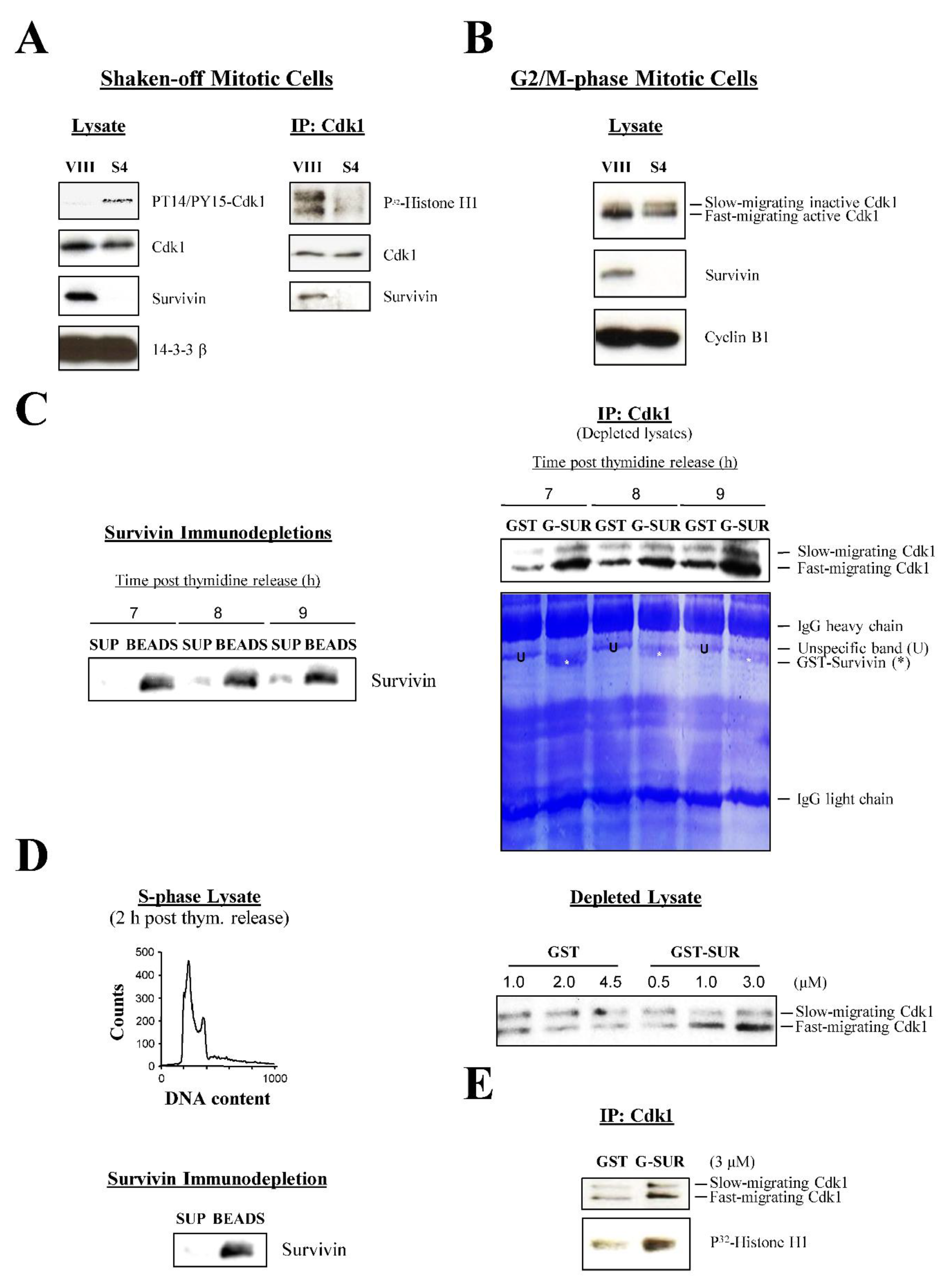
Figure 10.
Survivin regulation of Cdc25B phosphatase activity in HeLa cells. A, Survivin binds directly to Cdc25B. GST or GST-Survivin were mixed with His-Cdc25B, and analyzed by Western blotting. B, Accumulation of a cytosolic Cdc25B-Cdk1-Cyclin B1 complex in the absence of Survivin. Asynchronized cells transfected with control (VIII) or Survivin (S4) siRNA were collected, lysates were used to IP Cyclin B1, and pellets were analyzed by Western blotting. C, Cdc25B accumulation in Survivin-depleted cell cultures. Asynchronous (top) or synchronous (bottom) cells treated with the indicated siRNA were analyzed by Western blotting. D, Cdc25B activity at mitosis onset in siRNA-treated cells. Synchronized HeLa cells transfected with the indicated siRNA were collected at G2/M-phase (top left), analyzed for Survivin expression (bottom left), and used to IP Cdc25B (bottom right). Cdc25B phosphatase activity in the immune complexes was measured by the rate of OMFP hydrolysis (top right). E, Quantification of several experiments as in D (n=4) is shown. F, Cdc25B activity during the cell cycle in siRNA-treated cells. Synchronized cells transfected with the indicated siRNA were collected at the indicated times following thymidine release, analyzed by FACS (bottom), and used to IP Cyclin B1. Immune complexes were analyzed by Western blotting (top left). Cdc25B was IP from the same samples and its activity measured by the rate of OMFP hydrolysis (right).
Figure 10.
Survivin regulation of Cdc25B phosphatase activity in HeLa cells. A, Survivin binds directly to Cdc25B. GST or GST-Survivin were mixed with His-Cdc25B, and analyzed by Western blotting. B, Accumulation of a cytosolic Cdc25B-Cdk1-Cyclin B1 complex in the absence of Survivin. Asynchronized cells transfected with control (VIII) or Survivin (S4) siRNA were collected, lysates were used to IP Cyclin B1, and pellets were analyzed by Western blotting. C, Cdc25B accumulation in Survivin-depleted cell cultures. Asynchronous (top) or synchronous (bottom) cells treated with the indicated siRNA were analyzed by Western blotting. D, Cdc25B activity at mitosis onset in siRNA-treated cells. Synchronized HeLa cells transfected with the indicated siRNA were collected at G2/M-phase (top left), analyzed for Survivin expression (bottom left), and used to IP Cdc25B (bottom right). Cdc25B phosphatase activity in the immune complexes was measured by the rate of OMFP hydrolysis (top right). E, Quantification of several experiments as in D (n=4) is shown. F, Cdc25B activity during the cell cycle in siRNA-treated cells. Synchronized cells transfected with the indicated siRNA were collected at the indicated times following thymidine release, analyzed by FACS (bottom), and used to IP Cyclin B1. Immune complexes were analyzed by Western blotting (top left). Cdc25B was IP from the same samples and its activity measured by the rate of OMFP hydrolysis (right).
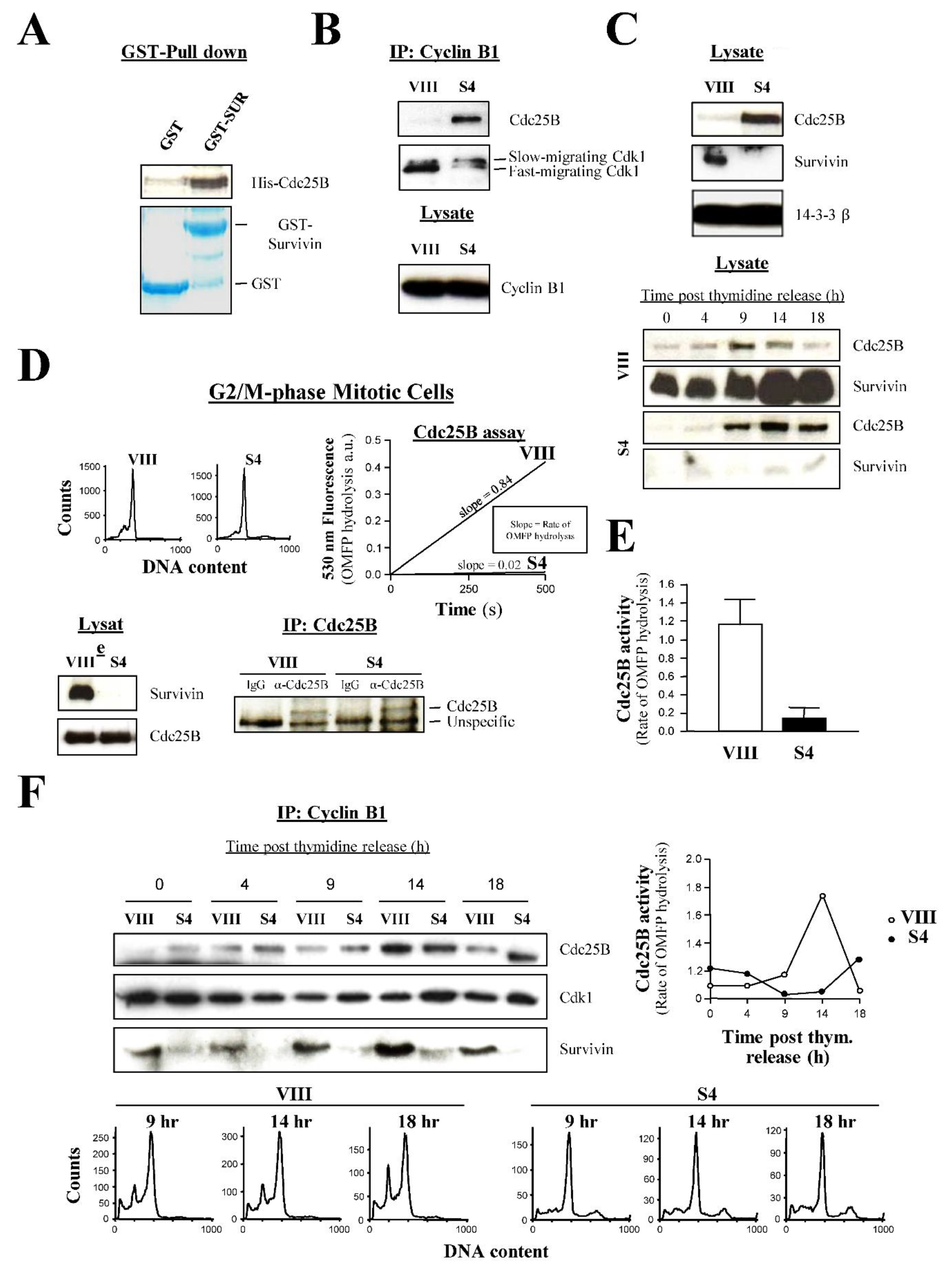
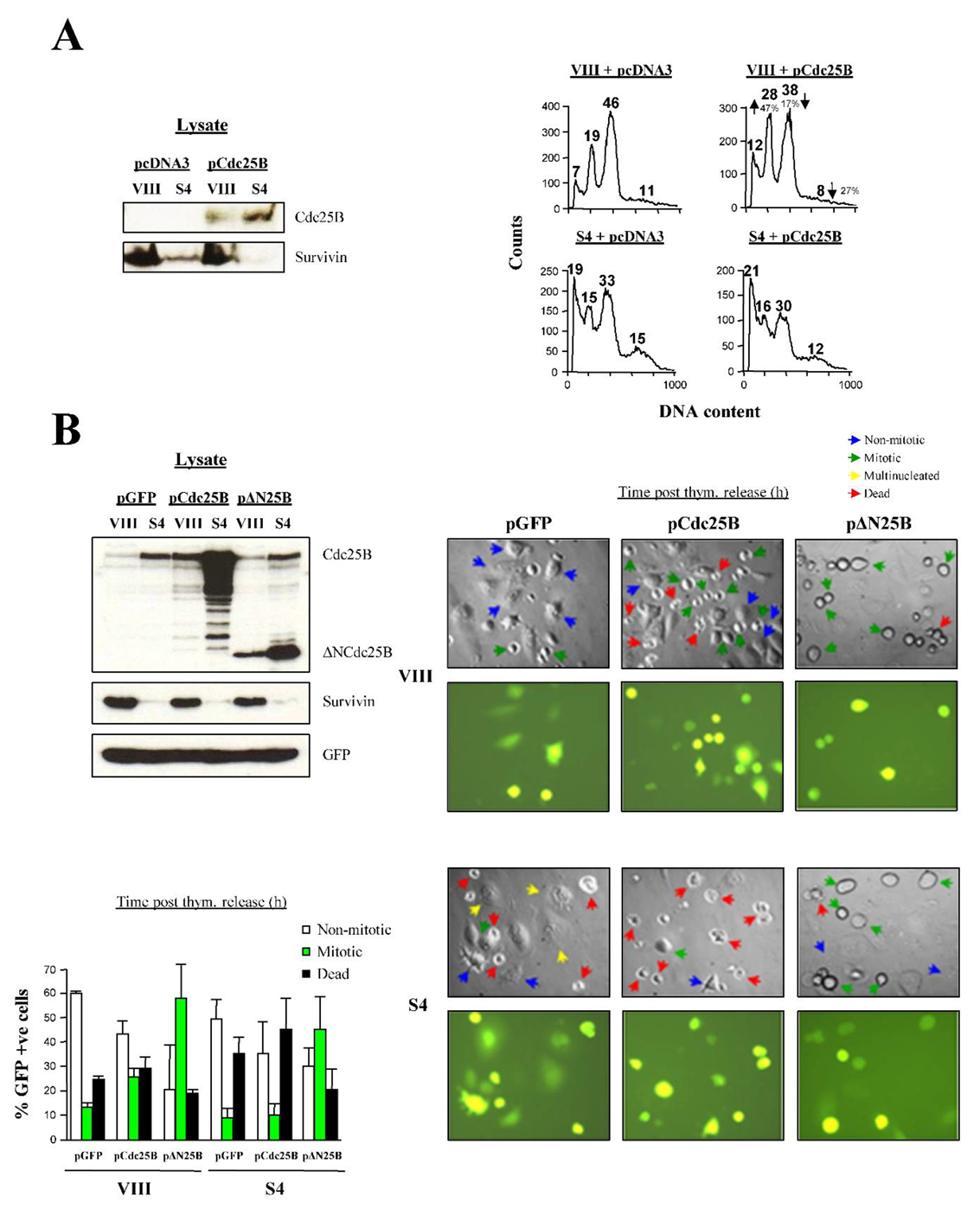
Figure 11.
A gain-of-function Cdc25B mutant can override the blockage induced by Survivin abrogation in HeLa cells. A, Cdc25B-mediated mitotic entry. Cells transfected with control (VIII) or Survivin (S4) siRNA were synchronized for 40 h. Cells were then transfected with a pcDNA3 or pCdc25B plasmid, and placed back in thymidine for another 8 h. Upon release, cells were collected at 16 h, as they transited through G2/M-phase. Cells were analyzed by Western blotting (left) and FACS analysis (right). The percentage of cells in apoptosis (<2N), G1 (2N), G2/M-phase (4N) and polyploid (>4N) is indicated. Arrows and percentages indicate changes in cell populations. B, Gain-of-function ∆NCdc25B mutant overrides blockage induced by Survivin abrogation. siRNA-treated synchronized cells were transfected with a pGFP (control), pCdc25B (wild type Cdc25B) or p∆NCdc25B (constitutively active Cdc25B mutant) plasmid (the last 2 were also transfected with the GFP monitoring plasmid), released into fresh medium, and analyzed by Western blotting (left), or fluorescence and phase-contrast microscopy (right) after 12 h (cells entering mitosis). Averages of 3 independent experiments are shown in bottom left.
Figure 11.
A gain-of-function Cdc25B mutant can override the blockage induced by Survivin abrogation in HeLa cells. A, Cdc25B-mediated mitotic entry. Cells transfected with control (VIII) or Survivin (S4) siRNA were synchronized for 40 h. Cells were then transfected with a pcDNA3 or pCdc25B plasmid, and placed back in thymidine for another 8 h. Upon release, cells were collected at 16 h, as they transited through G2/M-phase. Cells were analyzed by Western blotting (left) and FACS analysis (right). The percentage of cells in apoptosis (<2N), G1 (2N), G2/M-phase (4N) and polyploid (>4N) is indicated. Arrows and percentages indicate changes in cell populations. B, Gain-of-function ∆NCdc25B mutant overrides blockage induced by Survivin abrogation. siRNA-treated synchronized cells were transfected with a pGFP (control), pCdc25B (wild type Cdc25B) or p∆NCdc25B (constitutively active Cdc25B mutant) plasmid (the last 2 were also transfected with the GFP monitoring plasmid), released into fresh medium, and analyzed by Western blotting (left), or fluorescence and phase-contrast microscopy (right) after 12 h (cells entering mitosis). Averages of 3 independent experiments are shown in bottom left.